Abstract
Purpose
CAR-T programs will burden increasingly on healthcare systems, since the implementation of these therapies involves: multidisciplinary team collaboration, post-infusion hospitalization with risk of life-threatening toxicities, frequent in hospital visits and prolonged follow-up which heavily influence patients’ quality of life. In this review we propose an innovative, telehealth-based, model for monitoring CAR-T patients: this method was used for managing a case of COVID-19 infection occurred two weeks after CAR-T cell infusion.
Methods
Several benefits for management of all these aspects of CAR-T programs could be made using telemedicine: for example, telemedicine real-time clinical monitoring could reduce the COVID-19 contagion risks for CAR-T patients.
Results
Our experience confirmed feasibility and utility of this approach in a real-life case. We believe that use of telemedicine for CAR-T patients could improve: the logistics of toxicity monitoring (frequent vital sign checks and neurologic assessments), the multidisciplinary team communication (patient selection, specialists consulting, coordination with pharmacists, etc.), the decrease in hospitalization time and the reduction of ambulatory visits.
Conclusions
This approach will be fundamental for future CAR-T cell program development, enhancing patients’ quality of life and cost-effectiveness for healthcare systems.
Keywords: CAR-T, Cell therapy, Telemedicine, Telehealth, COVID-19, Health system
CAR-T cell therapy is a rapidly evolving option in hemato-oncology, founded on multidisciplinary team collaboration, with several healthcare figures involved in each step: from patient selection to infusion procedure, from early monitoring phase and finally to late follow-up period [1–3]. CAR-T programs will burden increasingly on healthcare systems [4], considering the current guidelines developed by regulatory agencies, which recommend an inpatient CAR-T cells’ administration, followed by a hospitalization of at least 10 days for early detection of signs and symptoms of cytokine release syndrome (CRS), neurological events, and other toxicities [5, 6]. Due to the risk of these possible life-threatening toxicities, after discharge, daily monitoring with ambulatory visits up to 1-month post-infusion is also recommended. In the same period, patients must be instructed to stay near the CAR-T unit (within 30–60 min distance) [5] and those aspects impact on patients’ quality of life when enrolled in a CAR-T program [7]. Worldwide diffusion of CAR-T will be based likely on the ability to reduce the impact of CAR-T therapies on healthcare systems, to minimize treatment waiting times and moreover to improve patients’ well-being. Hence, these desirable advances in CAR-T management could be made through the development of telemedicine programs. WHO defines telemedicine as the delivery of healthcare services from a distance using electronic information and telecommunication [8]. Telehealth allows consultations between patients and clinicians offering a 24/7 real-time clinical support: this is fundamental especially in situations, like CAR-T therapy, where distance and timing are crucial factors. Compared to conventional face to face visits, telemedicine approach could be more useful for well-being of CAR-T patients outside the hospital and at the same time for earlier recognition and treatment of CAR-T toxicities. In addition, new challenges for cellular therapies come from SARS-CoV-2, and its derived COVID-19 pandemic [9, 10]. Patients with hematological malignancies affected by COVID-19 show increased severity of symptoms and an infection-related mortality of 33% after CAR-T therapy [11]. The evidence of higher COVID-19 related mortality in CAR-T patients proves the necessity of new methods to mitigate the risk of infection mostly in this susceptible category of subjects. In this respect, the use of telemedicine for real-time clinical monitoring, without necessity of frequent in-hospital visits, would likely reduce the COVID-19 contagion risks for CAR-T patients. This article shows the development of a telehealth care program in our metropolitan BMT Unit, which is one of the few authorized CAR-T cell therapy treatment centers in Italy. We started from our preexistent outpatient BMT program [12], which was associated with telemedicine tools. In this way we developed an innovative, telehealth-based, CAR-T monitoring model which was used for managing a case of COVID-19 infection occurred 2 weeks after CAR-T cell infusion. We report the case of a 66-year-old man with relapsed DLBCL who underwent CAR-T program in December 2021. The patient was admitted to our BMT unit for treatment with axi-cell construct where he showed persistent mild abdominal pain, B-symptoms, and ECOG performance status 2. Blood tests were in normal range and nasopharyngeal swab resulted negative for SARS-CoV-2, so the patient underwent lymphodepleting chemotherapy followed by axi-cell infusion on January 3, 2022. Since the time of the infusion in the evening the patient developed fever without hypotension or desaturation; laboratory analysis showed mild leukopenia and elevation of IL-6 and CRP. The fever persisted despite the empiric antibiotic therapy and serial blood cultures being persistently negative. Therefore, a diagnosis of CRS was made and the patient was treated with one dose of tocilizumab on day 3 after CAR-T infusion, obtaining a prompt fever’s resolution and rapid normalization of the immune-inflammatory parameters. However, a pre-discharge nasopharyngeal PCR swab performed on day 14 resulted positive for SARS-CoV-2. Despite that, the patient was asymptomatic with normal oxygen saturation, no fever, and normal chest X-ray considering his immunocompromised status; the risks related to a possible hospitalization in a COVID unit and the availability of caregivers’ support, we devised the discharge of the patient at home and started a telemedicine care model with daily monitoring of clinical conditions and vital parameters (twice-a-day tele-visits) and caregivers help (three-times-a-day check of body temperature, pulse rate, blood pressure, oxygen saturation). A weekly video-call with infectious disease specialists was performed as well and the patient and caregivers had the possibility, if necessary, to contact clinicians 24/7 through a dedicated cellular phone. During the post-discharge period, the patient persisted in satisfying clinical status and showed progressive recovery of blood counts. On day 36, nasopharyngeal swab PCR test resulted negative: so, monitoring was continued with ambulatory access once-a-week associated with a tele-visit twice-a-week. A part of this single experience, telemedicine strategy could be certainly implemented by new technologies, such as wearable devices and mobile health-APPs, to screen for fevers and encephalopathy. Consequently, in this paper, we propose a possible, telehealth-based, CAR-T program model: an informatic portal, in the form of website and mobile-APP, could be the hub center in each phase, acting as connection between healthcare professionals and CAR-T patient/caregivers (Fig. 1).
Fig. 1.
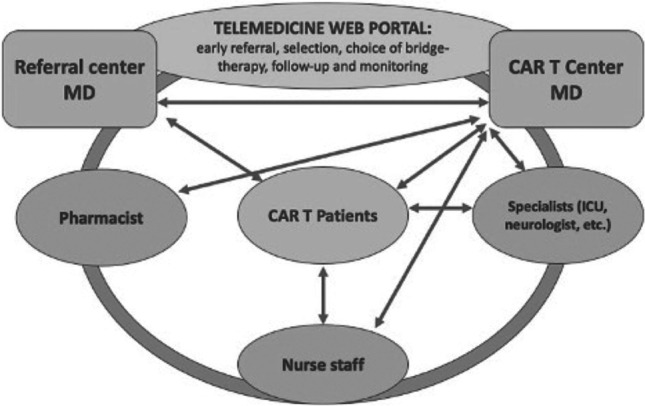
Proposed telehealth care CAR-T program model
Initially, referral center MD contact CAR-T center through the web portal: encrypted laboratory/imaging exams and clinical history are shared through a cloud service for early complete evaluation and selection of CAR-T therapy candidates. During the pre-treatment phase, for initial taking charge, eligible patients are collegially evaluated remotely through videoconferences by the multidisciplinary team. In this way the several specialists involved (neurologist, ICU specialist, etc.) can easily share and estimate the patient-specific risk factors. Moreover, the Web portal allows rapid coordination with pharmacists for drugs’ (e.g., tocilizumab) supply. A nurse figure is assigned to a patient for tele-consultation during pre-admission phase and after discharge. Patients referred to CAR-T therapy, by logging in the APP, can inquire about each step of their planned CAR-T program. Telemedicine allows a reduced hospitalization (10 days in total) for patients not experiencing signs and symptoms of toxicity during the post-infusion period. Early discharge can be followed by remote real-time monitoring: twice-a-day video visits with clinicians associated with three-times-a-day videoconferences between patient, care-givers, and reference nurse. Patient’s vital parameters can be continuously checked by health tools and wearable devices connected to the Web site/mobile-APP. Patients go to the CAR-T unit at least twice-a-week for laboratory and physical examination. If signs of CRS/neurotoxicity occur, the patient is rapidly hospitalized and treated for these complications. During late follow-up, videoconferences between referral centers and CAR-T unit are useful to share information about disease outcome and for a collegial management of possible late toxicities. In conclusion, CAR-T-related toxicities remain an issue of major concern, carrying the risk of ICU admission and therefore requiring huge re-sources for their monitoring, early recognition and treatment. Hence, future development of CAR-T programs will likely depend on a crucial balance between the maintenance of a high level of care and safety after CAR-T cell therapy and the improvement of patients’ quality of life and cost-effectiveness for the healthcare system. Telemedicine will constitute a fundamental pillar of these purposes and can: improve the logistics of toxicity monitoring (frequent vital sign checks and neurologic assessments), engage and facilitate the communication among a multidisciplinary team for patients’ selection, specialists consulting, coordination with pharmacists, etc., reduce the hospitalization time and the number of ambulatory visits. In perspective telemedicine, especially in combination with artificial intelligence, will allow a continuous monitoring of patients’ conditions through the use of wearable devices and specific biosensors [13] to grant an even faster intervention and a prompt resolution of toxicities and complications in CAR-T-treated patients. Our experience confirmed the feasibility and utility of this approach in a real-life case, derived from the necessity of remote patient’s monitoring due to his COVID-19 infection.
Author contributions
All authors contributed to the study conception and design. Supervision has been performed by Claudio Cerchione, Massimo Martino, and Filippo A. Canale. The first draft of the manuscript was written by Massimo Martino and Filippo A. Canale, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research received no external funding. We thank the Italian Ministry of Health within the research line 3 for its support through Ricerca Corrente.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Filippo A. Canale and Massimo Martino are co-first authors.
Massimo Martino and Claudio Cerchione are co-corresponding authors.
Contributor Information
Massimo Martino, Email: massimo.martino@ospedalerc.it.
Claudio Cerchione, Email: claudio.cerchione@irst.emr.it.
References
- 1.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of tox-icities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martino M, Macheda S, Aguglia U, et al. Identifying and managing CAR T-cell-mediated toxicities: on behalf of an Ital-ian CAR-T multidisciplinary team. Expert Opin Biol Ther. 2022;22(3):407–421. doi: 10.1080/14712598.2021.1974394. [DOI] [PubMed] [Google Scholar]
- 3.Martino M, Naso V, Loteta B, Canale FA, Pugliese M, Alati C, Musuraca G, Nappi D, Gaimari A, Nicolini F, Mazza M, Bravaccini S, Derudas D, Martinelli G, Cerchione C. Chimeric antigen receptor T-cell therapy: what we expect soon. Int J Mol Sci. 2022;23(21):13332. doi: 10.3390/ijms232113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heine R, Thielen F, Koopmanschap M, et al. Health economic aspects of chimeric antigen receptor T-cell therapies for hematological cancers: present and future. HemaSphere. 2021;5(2):e524. doi: 10.1097/HS9.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yakoub-Agha I, Chabannon C, Bader P, et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) Haematologica. 2020;105(2):297–316. doi: 10.3324/haematol.2019.229781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://www.ema.europa.eu/documents/product-information/yescarta-epar-product-information_it.pdf.
- 7.Kamal M, Joseph J, Greenbaum U, et al. Patient-reported outcomes for cancer patients with hematological malignancies undergoing chimeric antigen receptor T cell therapy: a systematic review. Transplant Cell Ther. 2021;27(5):390.e1–390.e7. doi: 10.1016/j.jtct.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) NLM classification W 26.5. ISBN9789241564144. ISSN 2220-5462. Geneva: World Health Organization; 2010. Telemedicine: opportunitiesand developments in Member States: report on the sec-ondglobal survey on eHealth. Global Observatory for eHealthseires–2. [Google Scholar]
- 9.Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet [DOI] [PMC free article] [PubMed]
- 10.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spanjaart AM, Ljungman P, de La Camara R, et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: results of a multicenter study on behalf of the European Society for Blood and Marrow Transplan-tation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Leukemia. 2021;35:3585–3588. doi: 10.1038/s41375-021-01466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martino M, Russo L, Martinello T, et al. A home-care, early discharge model after autografting in multiple myeloma: re-sults of a three-arm prospective, non-randomized study. Leuk Lymphoma. 2015;56(3):801–804. doi: 10.3109/10428194.2014.931952. [DOI] [PubMed] [Google Scholar]
- 13.Tagliente I, Solvoll T, Trieste L, De Cecco CN, Murgia F, Bella S. Which indicators for measuring the daily physical ac-tivity? An overview on the challenges and technology limits for Telehealth applications. Technol Health Care. 2016;24(5):665–672. doi: 10.3233/THC-161216. [DOI] [PubMed] [Google Scholar]


