Abstract
Iron chelation therapy (ICT) is the mainstay of treatment in patients with thalassemia requiring blood transfusions. This phase 2 JUPITER study evaluated patient preference between film-coated tablet (FCT) and dispersible tablet (DT) in transfusion-dependent thalassemia (TDT) or non-TDT (NTDT) patients treated with both formulations in a sequential manner. The primary endpoint was patient-reported preference for FCT over DT, while secondary outcomes included patient reported outcomes (PROs) evaluated by overall preference, and by age, thalassemia transfusion status, and previous ICT status. Out of 183 patients screened, 140 and 136 patients completed the treatment periods 1 and 2 of the core study, respectively. At week 48, the majority of patients preferred FCT over DT (90.3 vs. 7.5%; difference of percentage: 0.83 [95% confidence interval (CI), 0.75–0.89; P < 0.0001]). FCT scored better on secondary PROs and showed less severe gastrointestinal symptoms than DT, except in the change of modified Satisfaction with Iron Chelation Therapy (mSICT) preference scores, which were similar for both the formulations. Patients with TDT had stable ferritin levels, while it showed a downward trend up to week 48 in patients with NTDT on deferasirox treatment. Overall, 89.9% of patients reported ≥ 1 adverse event (AE), of which 20.3% experienced ≥ 1 serious AE. The most common treatment-emergent AEs were proteinuria, pyrexia, urine protein/creatinine ratio increase, diarrhea, upper respiratory tract infections, transaminase increase, and pharyngitis. Overall, this study reinforced the observations from the previous study by showing a distinct patient preference for FCT over DT formulation and further supported the potential benefits of life-long compliance with ICT.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00277-023-05240-3.
Keywords: Deferasirox, Dispersible tablets, Film-coated tablet, Patient-reported outcome, Patient preference, Compliance, Adherence, Palatability
Introduction
Self-management is critical in transfusion-dependent thalassemia (TDT), as it requires life-long iron-chelation therapy (ICT). Adherence to long-term therapy for chronic illnesses such as thalassemia is an ongoing clinical challenge across the world [1, 2]. From the patients’ perspective, the following major barriers to treatment adherence exist: the route of administration of ICT, adverse event (AE) profile, including but not limited to gastro-intestinal (GI) tolerability, palatability [3], and overall treatment burden. Patients’ involvement in treatment decision-making plays a pivotal role in improving the adherence rates and overall treatment outcomes [4, 5].
Currently, ICT treatment options include 2 oral agents—deferasirox (DFX) and deferiprone and subcutaneously administered deferoxamine [6]. DFX, a tridentate iron chelator, is approved for the treatment of chronic iron overload in patients with TDT aged 2 years and older and in patients with non–transfusion-dependent thalassemia (NTDT) aged 10 years and older [7, 8]. DFX is available in 2 formulations: dispersible tablets (DTs) and film-coated tablets (FCTs). Both have a well-defined safety and efficacy profile and have been associated with high levels of adherence, patient satisfaction, and quality of life (QOL) [9–13]. However, with the DT formulation, adherence remained a challenge due to unfavorable GI tolerability and palatability [3]. Given the chronic nature of ICT and the importance of patient compliance, the FCT was developed to enhance patient satisfaction by removing the need for faster disintegration and being simpler to administer [14].
In the phase 2 ECLIPSE trial (NCT02125877), DFX FCT demonstrated a comparable safety profile to the DT, with improved compliance and adherence, fewer severe GI-related AEs, and more favorable patient-reported outcomes (PROs), including satisfaction/preference, better palatability, and fewer concerns with FCT than DT [14, 15]. However, in the ECLIPSE study, patients were randomized to either DT or FCT, and the reported outcomes were compared between 2 different patient groups. The current phase 2 JUPITER study (NCT02720536) was designed to evaluate the patient preference between FCT and DT in patients treated with both the formulations, thereby allowing for a more realistic comparison of the 2 DFX formulations from the patients’ standpoint. In addition, patients were recruited from a broader range of countries than in previous studies, as well as including children aged 2 to 9 years, and followed-up patients for a longer period.
Patients and methods
Study design
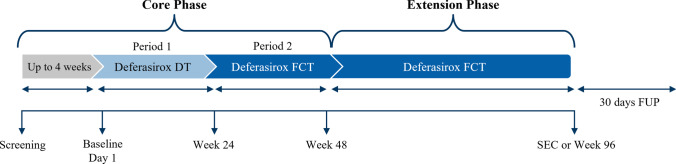
JUPITER was a 48-week, open-label, randomized, multicenter, sequential treatment, phase 2 study in patients with TDT and NTDT evaluating the patient-reported preference between FCT and DT as assessed by a preference questionnaire. The study involved a core phase followed by an optional extension phase (Fig. 1). The core phase consisted of the following 2 periods: treatment period 1 (day 1 to week 24) — patients received the DT formulation; treatment period 2 (week 25–48) — patients were switched to the FCT formulation. Patients who completed the core phase could continue FCT in the extension phase for a maximum of 12 months. At the discretion of the investigator, patients could switch from DT to FCT at any time during treatment period 1, and vice versa, from FCT to DT at any time during treatment period 2 of the core phase.
Fig. 1.
Study design. DT, dispersible tablet; FCT, film-coated tablet; FUP, follow-up; SEC, study evaluation completion
Patients
The study included ICT-naïve as well as pre-treated patients (≥ 6 months with ICT other than DFX), aged ≥ 2 years, with either TDT (serum ferritin [SF] level > 1000 ng/mL at screening, liver iron concentration [LIC] > 3 mg Fe/g dry weight within 6 months prior to screening), or NTDT (SF ≥ 800 ng/mL at screening, LIC ≥ 5 mg Fe/g dry weight within 6 months prior to screening). Patients with creatinine clearance below the contraindication limit in the locally approved prescribing information, serum creatinine > 1.5 × upper limit of normal (ULN), aspartate aminotransferase/alanine aminotransferase > 5 × ULN (unless LIC < 10 mg Fe/g dry weight), urine protein/urine creatinine ratio > 0.5 mg/mg, or impaired GI function were excluded from the study.
After the screening phase, patients pre-treated with deferoxamine were started on the equivalent DFX DT dose, and for those taking deferiprone, the dose was calculated based on their SF level. Chelation-naïve patients were initiated on DT 20 mg/kg/day. At week 25, the dose of FCT was calculated as the equivalent of the current DT dose. Dose adjustments to improve treatment response based on the investigator’s judgment were recommended every 4 weeks for chelation-naïve patients, and every 3 months for pre-treated patients, in the increments of 5 to 10 mg/kg/day for DT or 3.5 to 7 mg/kg/day for FCT, up to a maximum dose of 40 mg/kg/day for DT and 28 mg/kg/day for FCT. Dose adjustments based on safety and dose reductions for patients unable to tolerate the protocol-specified dosing schedule were allowed at any time during the study.
The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki and was approved by independent ethics committees at participating sites. Patients (or parents/guardians) provided written, informed consent prior to enrollment.
Outcomes
The primary endpoint was the patient-reported preference for FCT over DT, calculated as a proportion and measured by preference questionnaire (Suppl. Table 1) at week 48. Key secondary outcomes included the proportion of patient-reported preference for FCT, DT, or previous ICT at week 28; preference of DT over previous ICT at weeks 4 and 24; preference reasons for FCT over DT at weeks 28 and 48; relative consumed pill count during FCT (week 25 to 48) compared with DT (baseline day 1 to week 24); absolute and relative change in domain scores of palatability and modified Satisfaction with Iron Chelation Therapy (mSICT) questionnaire with FCT (week 28 to 48) compared with DT (week 4 to 24) or previous ICT (Screening); overall safety was assessed by frequency and severity of AEs and changes in laboratory parameters during FCT (week 25 to 48) compared with DT (baseline to week 24); and the absolute and relative changes in SF levels from baseline on monthly basis.
PROs were assessed using the patient questionnaire, the mSICT questionnaire, the GI symptom questionnaire, and palatability. For a detailed description of PRO assessments, please refer to the Supplementary Information.
Statistical analysis
The primary analyses of testing patient preference for FCT over DT were conducted via McNemar’s test and P values were provided for the matched pairs. The estimates of proportions (in percentages) of patient preferences and non-preferences for each study treatment were presented, with 95% CI (confidence interval) using Clopper-Pearson method. The type I error was set to 2-sided α = 5% or 1-sided α = 2.5%. Inferential analysis using analysis of covariance (ANCOVA) was performed for comparison between both the treatment groups (FCT and DT) at week 48 for the Full Analysis Set (FAS) and the odds ratio was presented. The ANCOVA model for compliance endpoint included treatment group (FCT and DT), age group (2 to < 6 years, 6 to < 10 years, 10 to < 18 years, and ≥ 18 years), and thalassemia transfusion dependence (TDT and NTDT) as factors. Least squares estimate, 95% CIs, and P-values were presented for each treatment, and the difference between the treatments. The secondary endpoints were analyzed descriptively. Preference was determined overall and by strata of age (< 10 years and ≥ 10 years) and by treatment for the FAS set. For efficacy analysis, change in SF levels by week and treatment is presented for the FAS and by type of thalassemia transfusion status.
Results
Patient disposition
A total of 183 patients from 7 countries (Egypt, Lebanon, Morocco, Saudi Arabia, Thailand, Turkey, and Vietnam) were screened and 148 eligible patients entered the core study; of these, 140 (94.6%) completed treatment period 1, and 136 (91.9%) completed treatment period 2 (Suppl. Figure 1). Of the 116 patients who entered the extension study, 80 patients completed the study; the primary reason for non-completion was accessibility of the FCT formulation outside of the study.
Patient demographics and baseline characteristics
Baseline demographics are presented in Table 1. The median age of the study participants was 12.0 years (range, 2–68 years). The majority of patients identified themselves as “other” ethnic group (96.6%), which included Thai (35.7%), Egyptian (31.8%), Caucasian (11.9%), Vietnamese (8.4%), Turkish (1.4%), and Syrian (0.7%). Most patients had TDT (82.4%) and the median time since diagnosis was 38 months. On an average, patients had received 12.4 units of blood in the preceding 12 months. Despite this, about half of the patients (50.7%) had not received ICT prior to study entry. Among the patients who had received prior ICT, 44 patients (29.7%) received deferiprone, 6 (4.1%) received deferoxamine, and 23 (15.5%) received a combination treatment of both.
Table 1.
Demographics and baseline characteristics
| Characteristics | Total N = 148 |
|---|---|
| Age (years), mean (SD) | 15.32 (13.824) |
| Age groups (years), n (%) | |
| < 18 years (overall) | 93 (62.8) |
| 2–6 (young children) | 61 (41.2) |
| 7–11 (child) | 12 (8.1) |
| 12–17 (adolescent) | 20 (13.5) |
| ≥ 18 years (adult) | 55 (37.2) |
| Gender, n (%) | |
| Male | 66 (44.6) |
| Female | 82 (55.4) |
| Ethnicity | |
| Indian (Indian subcontinent) | 5 (3.4) |
| Other | 143 (96.6) |
| Country of recruitment, n (%) | |
| Egypt | 45 (30.4) |
| Lebanon | 5 (3.4) |
| Morocco | 8 (5.4) |
| Saudi Arabia | 17 (11.5) |
| Thailand | 51 (34.5) |
| Turkey | 10 (6.8) |
| Vietnam | 12 (8.1) |
| Time since diagnosis (months), mean (SD) | 88.4 (113.98) |
| Disease type, n (%) | |
| Transfusion-dependent thalassemia | 122 (82.4) |
| Non-transfusion-dependent thalassemia | 24 (17.6) |
| Patients who are hematologically stable since the examination, n (%) | |
| Yes | 148 (100) |
| No | 0 |
| Total number of transfusion(s) received in last 12 months (units) | 12.4 (7.56) |
| Time since last transfusion received (days) | 39.9 (101.61) |
| Number of units last transfused | |
| Platelets | 0.0 (0.0) |
| PRBC | 1.8 (1.31) |
| Whole blood cells | 0.0 (0.11) |
| Patients received blood transfusion prior to study (both TDT and NTDT) | |
| Yes | 140 (94.6) |
| No | 7 (4.7) |
| Subject received a chelation therapy prior to start of study drug, n (%) | |
| Yes | 73 (49.3) |
| No | 75 (50.7) |
| Time since last iron chelation therapy (from end date), mean (SD) | − 39.4 (175.75) |
| Duration of last iron chelation therapy (days) | 2254.8 (1721.72) |
| No. of patients on DFO, n (%) | 6 (4.1) |
| No. of patients on DFP, n (%) | 44 (29.7) |
| No. of patients on DFO and DFP, n (%) | 23 (15.5) |
DFO, deferoxamine; DFP, deferiprone; PRBC, packed red blood cells
Exposure to treatment and compliance
The mean cumulative planned and the actual dose for DT (treatment period 1) were similar: 6473.2 mg/kg and 6266.1 mg/kg; as was also the case for FCT (treatment period 2) 4451.3 mg/kg and 4364.7 mg/kg. Nearly, 50% of the patients had a single dose change in both treatment periods (treatment period 1, 46.6%; and treatment period 2, 36.4%) while 2 dose changes were required in 18.9% and 23.6% of patients, respectively. The dose was changed on an average after 22.3 days of commencing DT and after 24.7 days for FCT. Apart from protocol-mandated dose changes (treatment period 1, 45.3%; and treatment period 2, 57.1%), lack of efficacy (27.0% and 30.0%) was the most frequently reported reason for changing dose. Compliance, as measured by relative tablet consumption, was slightly higher for DT (98.7%) than for FCT (95.1%). This was reflected in all the age groups except in 7 to 11 years age group (DT, 92.3%; FCT, 93.2%). The highest mean relative tablet consumption was observed in the age group of 2 to 6 years (DT, 102.6%; and FCT, 95.9%) followed by adults (97.5% and 96.4%).
Primary endpoint
A total of 134 of 139 patients responded to question 2 of the preference questionnaire. At week 48, most patients (90.3%) preferred FCT (difference of percentage 0.83, 95% CI 0.75, 0.89; P < 0.0001, Table 2). Sensitivity analysis on patient preference for DT or FCT by age group, by thalassemia transfusion status, or by prior ICT status showed similar results with clear preferences for FCT over DT across all strata (Suppl. Table 2).
Table 2.
Proportion and percentage of patients preferring FCT or DT at week 48 (primary endpoint)
| Treatment (n = 139) |
Type of treatment preferreda (n = 134) |
Comparison | Difference of proportion (difference of percentage) (95% CI; P-value) |
Odds ratio (95% CI; P-value) |
|---|---|---|---|---|
| DFX DT | 10 (7.5%) | vs. DFX FCT | 0.83 (0.75, 0.89; P < 0.001) | 115.42 (48.76, 273.18; P < 0.0001) |
| DFX FCT | 121 (90.3%) | |||
| None of the above | 3 (2.2%) |
Testing patient preference of FCT over DT is conducted via Mc Nemar’s test. Both exact and asymptotic P values are presented for Mc Nemar’s test for the matched pairs. 95% CI of proportion is obtained using Clopper-Pearson method
a Based on answers received for question 2: Among the different medicines you have taken for iron overload, which type of medicine for your iron overload do you (or your child) like the best?
DFX, deferasirox; DT, dispersible tablet; FCT, film-coated tablet; m, the number of patients who have provided answer to question no. 2 of preference questionnaire; n, number of patients selected that particular treatment; N, number of patients in FAS
Secondary endpoints
Patient preference
At week 4, the majority of patients expressed a preference for DT over prior ICT (difference in proportion, 0.66; 95% CI 0.51, 0.77; P < 0.0001) (Table 3). Similar trends were observed at week 24 (difference in proportion, 0.59; 95% CI 0.44, 0.72; P < 0.0001). At week 28, following the change from DT to FCT, the difference of proportion between preference for DT or previous ICT was no longer statistically significant (0.04; 95% CI -0.13, 0.21; P = 0.5078, OR 2.10). Correspondingly, at week 28, most patients preferred FCT (60 out of 69 patients, 87.0%) over DT (6 of 69 patients, 8.7%) or previous ICT (3 of 69 patients, 4.3%). The preference for FCT was statistically significant (95% CI 23.49, 208.58; P < 0.0001).
Table 3.
Proportion and percentage of patients preferring FCT, DT, and previous iron chelation as measured by preference questionnaire at weeks 4, 24, and 28 (secondary endpoint)
| Visit/treatment | Patient preference n/Nm (%) |
Comparison | Difference of proportion (95% CI; P value) |
Odds ratio (95% CI; P value) |
|---|---|---|---|---|
| Week 4 | ||||
| Previous ICT | 11/70 (15.7%) | vs. DFX DT |
0.66 (0.51, 0.77; < 0.0001) |
23.52 (9.74, 56.79; < 0.0001) |
| DFX DT | 57/70 (81.4%) | |||
| Week 24 | ||||
| Previous ICT | 11/69 (15.9%) | vs DFX DT |
0.59 (0.44, 0.72; < 0.0001) |
16.13 (6.92, 37.57; < 0.0001) |
| DFX DT | 52/69 (75.4%) | |||
| Week 28 | ||||
| DFX DT | 6/69 (4.3%) | vs DFX FCT |
0.78 (0.65, 0.88; < 0.0001) |
70.00 (23.49, 208.58; < 0.0001) |
| DFX FCT | 60/69 (87.0%) | |||
| Week 28 | ||||
| Previous ICT | 3/69 (4.3%) | vs DFX FCT |
0.83 (0.71, 0.91; < 0.0001) |
146.67 (37.92, 567.23; < 0.0001) |
| DFX FCT | 60/69 (87.0%) | |||
| Week 28 | ||||
| Previous ICT | 3/69 (4.3%) | vs DFX DT |
0.04 (− 0.13, 0.21; 0.5078) |
2.10 (0.50, 8.74; 0.3028) |
| DFX DT | 6/69 (4.3%) | |||
CI, confidence interval; DFX, deferasirox; DT, dispersible tablet; FCT, film-coated tablet; ICT, iron chelation therapy; Nm, number of patients with previous ICT who were available for analysis
Modified Satisfaction with Iron Chelation Therapy PRO
mSICT scores and observer-reported outcomes (ObsRO) were obtained at baseline (n = 59 and n = 8), week 24 (n = 73 and n = 58), week 28 (n = 79 and n = 58), and week 48 (n = 79 and n = 55) (Fig. 2).
Fig. 2.
Modified SICT scores at baseline, and weeks 24, 28, and 48. (a) Adherence domain. (b) Satisfaction/preference domain. (c) Concern domain. PRO, patient-reported outcomes; SICT, satisfaction with iron chelation therapy; ObsRO, observer reported outcomes. Lower scores indicate better outcomes
Adherence domain
The mean adherence scores for the PRO at baseline, week 24, week 28, and week 48 were similar (Fig. 2a). The primary reason for lack of adherence was forgetting to take the medicine, which improved from 10.8% (15 patients) at baseline to 5.8% (8 patients) at both week 24 and week 48, suggesting better compliance over time. While taste/aftertaste were important reasons for non-adherence during treatment period 1 (week 4: aftertaste [6 patients, 4.3%]; week 24: taste [10 patients, 7.2%]), the inconvenience was reported as more important during the treatment period 2 (week 28, 8 patients, 5.8%; week 48, 6 patients, 4.3%).
Satisfaction/preference
Overall, the mean satisfaction/preference scores reduced from baseline to week 48 for both PRO and ObsRO indicating an improvement in patient satisfaction over time (Fig. 2b). Most patients preferred ICT in the tablet form to be taken 3 times a day (30 patients, 21.6%) at baseline, followed by FCT taken once a day (18 patients, 12.9%) at week 24. At week 4 and week 24 before switching treatment, there was a clear preference for DT (week 4, 85 patients, 61.2%; week 24, 77 patients, 55.4%). However, at week 24 after switching to FCT, the FCT was preferred (5 patients, 3.6%) over the DT (1 patient, 0.7%), and at week 28 and week 48, there was a clear preference for the FCT (week 28, 109 patients, 78.4%; week 48, 107 patients, 77.0%).
Concerns
The mean concerns scores obtained at baseline, week 24, week 28, and week 48 were 10.9, 10.5, 10.9, and 11.0 respectively and for ObsRO scores were 8.9 at baseline and 8.6 at weeks 24, 28, and 48, respectively (Fig. 2c).
Palatability
Overall and across all strata, FCT scored better on both palatability and aftertaste than DT. The majority of patients reported swallowing all the medicines at every time point, regardless of formulation. As expected, FCT had better mean scores for palatability at all time points than DT with the highest scores reported at week 48 (overall, 10.9; < 10 years, 11.0; ≥ 10 years, 10.9). For the aftertaste question, overall, FCT had lower mean scores (better) at every time point than DT with lowest score observed at week 24 (2.7) and week 48 (2.1).
GI symptom diary
Compared with DT, FCT showed less severe GI symptoms over time as measured by the GI symptoms diary score. For both strata (< 10 years and ≥ 10 years), the lowest mean score occurred at week 48 (< 10 years, 2.2; ≥ 10 years, 2.2). The decrease in scores from baseline occurred only in the treatment period 2, except for week 24 in the stratum of patients ≥ 10 years receiving FCT, which had the highest mean score of 9.0 with a mean change from baseline of 5.8. However, as this group had only 4 patients with analyzable data, these results should be interpreted with caution. The “bowel movements” item in the GI symptoms diary did not show a major variation between the 2 formulations over time. The highest mean number of daily bowel movements was 1.9 at seek 4 in the ≥ 10 years stratum and the lowest was 1.3, reported in every stratum at seek 24 in patients receiving FCT.
Serum ferritin level
In patients with TDT, the mean SF levels remained stable over the course of the core phase; the mean SF levels were 2209.50 ± 1128.38 μg/L at baseline, 2431.77 ± 1149.65 μg/L at the end of week 48, and 1845.52 ± 1336.33 μg/L at week 96. During the core phase, the average absolute change in SF values did not increase or decrease significantly, indicating that switching from DT to FCT did not lead to any worsening in SF control. In contrast, the SF levels decreased significantly in the NTDT patient group following treatment; the mean SF levels reduced from 1739.57 ± 1078.82 μg/L at baseline to 1096.57 ± 691.40 μg/L at the end of week 48 and 693.55 ± 272.99 μg/L at week 96.
Safety analysis
Overall, 133 patients (89.9%) reported at least 1 AE, of which 30 patients (20.3%) experienced at least 1 SAE during the study. Five patients (3.4%) discontinued DFX treatment due to an AE and all were non-serious. AEs were more commonly reported in the patients receiving DT (70.3%) than patients receiving FCT during the core (60.7%) or extension (64.7%) study. The most common treatment-emergent AEs (TEAEs) reported in more than 10% of patients in any group were proteinuria, pyrexia, urine protein/creatinine ratio increase, diarrhea, upper respiratory tract infections, transaminase increase, and pharyngitis (Table 4). TEAEs related to DFX were reported in 70 patients (47.3%); most were related to GI, renal, and urinary disorders. Coronavirus disease (COVID-19) of moderate severity was confirmed in 1 patient during the extension phase. The majority of AEs did not show a significant risk difference between treatments; however, there was a statistically significant reduction in GI disorders, infections and infestations, and investigations when patients were receiving FCT (Fig. 3).
Table 4.
Most common AEs (overall; > 5% in any group) regardless of study drug relationship by preferred term and treatment
| Most common AEs | DFX DT (N = 148) |
DFX FCT (N = 140) |
DFX EXFCT (N = 116) |
Total (N = 148) |
|---|---|---|---|---|
| Proteinuria | 9 (6.1) | 10 (7.1) | 12 (10.3) | 26 (17.6) |
| Pyrexia | 8 (5.4) | 9 (6.4) | 7 (6.0) | 23 (15.5) |
| Urine protein/creatinine ratio increase | 13 (8.8) | 12 (8.6) | 7 (6.0) | 22 (14.9) |
| Diarrhea | 16 (10.8) | 5 (3.6) | 2 (1.7) | 21 (14.2) |
| Upper respiratory tract infection | 8 (5.4) | 8 (5.7) | 4 (3.4) | 19 (12.8) |
| Transaminases increase | 9 (6.1) | 9 (6.4) | 5 (4.3) | 17 (11.5) |
| Pharyngitis | 7 (4.7) | 6 (4.3) | 6 (5.2) | 15 (10.1) |
| Urinary tract infection | 9 (6.1) | 3 (2.1) | 3 (3.2) | 13 (8.8) |
| Nasopharyngitis | 6 (4.1) | 7 (5.0) | 3 (2.6) | 13 (8.8) |
| Hepatic enzyme increase | 6 (4.1) | 4 (2.9) | 6 (5.2) | 12 (8.1) |
| Vomiting | 7 (4.7) | 3 (2.1) | 3 (2.6) | 12 (8.1) |
| Nausea | 7 (4.7) | 1 (0.7) | 2 (1.7) | 10 (6.8) |
| Blood bicarbonate increase | 5 (3.4) | 6 (4.3) | 2 (1.7) | 10 (6.8) |
| Serum ferritin increase | 8 (5.4) | 2 (1.4) | 1 (0.9) | 9 (6.1) |
| C-reactive protein increase | 5 (3.4) | 4 (2.9) | 1 (0.9) | 8 (5.4) |
| Hepatomegaly | 8 (5.4) | 0 | 0 | 8 (5.4) |
| Tonsilitis | 4 (2.7) | 2 (1.4) | 5 (4.3) | 8 (5.4) |
AE, adverse event; DFX, deferasirox; DT, dispersible tablet; EXFCT, extension phase; FCT, film-coated tablet
Fig. 3.
Overall adverse events by system organ class sorted by risk difference. CI, conference interval; DT, dispersible tablet; FCT, film-coated tablet
Most SAEs were reported during the extension phase (16 patients in 48 weeks, 13.8%) followed by the DT phase (13 patients in 24 weeks, 8.8%) and the FCT phase (6 patients in 24 weeks, 4.3%). Serious TEAEs that occurred in ≥ 3 patients were pyrexia (4 patients, 2.7%), elevated alanine transaminase (ALT) (4 patients, 2.7%), elevated AST (aspartate transaminase), and proteinuria (each of the 3 patients, 2.0%). No deaths were reported during the study.
Overall, 20 patients (13.5%) required dose adjustments or treatment interruptions due to AEs. These occurred for a higher proportion of patients during the DT treatment period (10 patients, 6.8%) than during FCT (4 patients, 2.9%) or the extension phase with FCT (EXFCT) (6 patients, 4.3%).
In most of the patients, liver enzyme values remained below the ULN throughout the treatment periods. However, a notable increase in the number of patients with fluctuating values for ALT and AST was observed over time. This increase was higher in the DT arm than in FCT or EXFCT arm during the treatment period. Creatinine clearance was ≥ 60 mL/min throughout the study for > 90% of patients.
Discussion
This phase 2, open-label, single-arm JUPITER study evaluated the patient preference as well as overall safety, tolerability, and efficacy for DFX FCT or DT in patients with TDT or NTDT. The study included a broad patient population from Asia and Middle East countries, with a majority of them recruited from Thailand (34.5%) and Egypt (30.4%), allowing additional evidence regarding the use of DFX to be generated in populations representing East-Asian and Mediterranean regions. In addition, children aged 2 to 9 years were included thus allowing further evaluation of the overall safety, tolerability, and efficacy profile of DFX FCT in these unexplored patient groups. This study examined detailed PRO to understand the patient preference overall and also by age, thalassemia transfusion status, and previous ICT status. In general, both overall and across strata, the results of this study reinforced the observations of the previously conducted ECLIPSE study [14] by showing a distinct patient preference for FCT over DT formulation. In line with the ECLIPSE study, this study also reported greater adherence and improved PROs, including increased satisfaction, fewer concerns, and better palatability among patients on FCT compared to those receiving DT [14, 15]. Furthermore, in the current study, despite the protocol allowing patients to resume using DT after the mandated treatment switch to FCT at week 25, none of the patients switched, suggesting that FCT was preferred over DT formulation among the patients.
To enable a direct assessment of patient preferences between the 2 different DFX formulations, this phase 2 study was developed as a single-arm sequential design, thereby exposing the same patients to both formulations (DT and FCT), which allowed a more robust assessment of their treatment preference. This was unlike other studies which used 2 arms to compare DFX formulations and randomized patients who received only one of the DFX formulations [8–10, 14–16].
In patients with TDT and NTDT, ICT is a critical component of the treatment algorithm. However, most patients on ICTs do not adhere to therapy over time, leading to poor treatment outcomes [17]. Good adherence and management of AEs are key strategies for optimizing ICT [16]. Evidence suggests that the treatment regimens that are more convenient and easier to follow tend to have higher adherence rates compared to those requiring lifestyle modifications [18, 19]. In the current study, adherence to study medication was high during the entire study period with no measurable difference between DT and FCT based on pill count, the inferential analysis (ANCOVA model), or the mSICT adherence domain questions. This differed from the ECLIPSE study, where the pill count between DT and FCT was higher in the FCT arm [14, 15]. In addition, the mSICT adherence domain score was noticeably higher than that was observed in the ECLIPSE study [15]. This is likely due to differences in the demographics between these studies. In the current study, the majority of patients were less than 18 years of age, with 41.2% of patients aged from 2 to 6 years. In contrast, the ECLIPSE study only included patients > 10 years and the median age of the study population was 28 years (range 11–81 years) [15]. Expectantly, in the current study, the adherence was highest in patients in the age group of 2 to 6 years based on the mSICT adherence domain, which is also consistent with observations from clinical practice, in which pediatric patient compliance is high due to caregiver/parent supervision. Though forgetfulness regarding the medicine was the main reason for the lack for adherence and was prevalent throughout the entire core part, it reduced from 10.8% at baseline to 5.8% at week 48. The second most reported reason for non-adherence was taste or aftertaste for the DT formulation, and inconvenience for the FCT formulation.
In the current study, the mean changes from baseline in the mSICT preference scores were small and similar for both the formulations, suggesting that the preference domain of the mSICT tool was not as sensitive to a change in medication based on a direct question regarding patient preference. This is in contrast with what was observed in the ECLIPSE study where the mean mSICT preference scores were more in the favor of FCT and the difference in scores between the treatment groups was of a magnitude corresponding to clinically meaningful treatment benefit [15]. In this regard, a group of younger patients (aged 2–9 years) cared for by care giver/parent in this study than those of the ECLIPSE study might confound the mSICT preference scores. Therefore, it is important to keep in mind when designing similar studies in the future using the mSICT tool to evaluate patient preferences.
The efficacy of DFX was as expected, given the protocol-specified dosing guidance with a relatively conservative dose escalation schedule (adjustments permitted every 3 months on the basis of the serum ferritin results). There was a decreasing trend in absolute SF levels over time, which was more pronounced for patients with NTDT than those with TDT. The median change of SF levels from baseline also indicated a decrease, as did the relative change in SF levels. This is also consistent with the previous studies [6, 8, 14, 16].
During the study, there was a lower incidence of TEAEs and fewer GI disturbances with the use of FCT. This was reflected in both the AE profile and in the GI symptoms diary PRO, both of which showed fewer GI pain symptoms for DFX FCT. More patients receiving DT experienced GI AEs (23%) compared to those receiving FCT during the core (13.6%) or extension phase (12.1%). Diarrhea, nausea, vomiting, and upper abdominal pain were the most frequently reported AEs. DFX is associated with mild-to-moderate GI disturbances occurring early during treatment [20]. Given that the DFX FCT formulation is taken with light meals and also lacks the excipients, lactose, and sodium lauryl sulfate, both found in the original DT formulation and thought to be contributing to GI AEs, the FCT formulation was expected to show improved GI tolerability. The results of the current study are in line with the previous studies [14], where FCT showed less severe GI symptoms compared to DT formulation.
In conclusion, the JUPITER study reinforced patient preference for DFX FCT compared to DFX DT in patients with TDT or NTDT. With a broader patient population, including children aged 2 to 9 years, a robust, sequential study design, and longer exposure periods to FCT, the study provided valuable insights into the benefits of the FCT formulation, which in turn could be a major influencing factor toward improved patient experience and better adherence to ICT. Overall, the findings from detailed analyses of responses to various validated PROs suggest that there was a distinct patient preference for FCT over DT across all patient groups. DFX FCT had a comparable safety and efficacy profiles to the DT with fewer severe GI-related AEs and more favorable PROs, including increased satisfaction, fewer concerns, and better palatability. The findings suggest that the recommended dosing of DFX FCT could ensure better tolerability, palatability, and compliance, thus offering a favorable option of ICT for the long-term management of iron overload.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the patients, investigators, and staff at the participating sites for supporting the conduct of the study. The authors also thank Vimal Kumar Muthyala, Suruchi Lele, and Anupama Singh (Novartis Healthcare Private Limited, Hyderabad, India) for providing medical writing support. These services were sponsored by Novartis Pharma AG.
Author contribution
All authors were involved in the critical reviewing of the manuscript content and provided final approval for the submitted version.
Funding
This study was funded by Novartis Pharma AG, Basel, Switzerland. Novartis Pharma AG supported the development of this manuscript, provided data analyses according to the direction of the authors and paid for medical writing support.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics statement
The studies involving human participants were reviewed and approved by relevant institutional review board or ethics committee at each trial site. The patients/participants provided their written informed consent to participate in this study.
Conflict of interest
Vip Viprakasit received research funds and advisory board compensation from Novartis Pharma AG, F. Hoffman-La Roche, Bristol Myers Squibb, Agios, Silence, Ionis Pharmaceuticals, Thermo Fisher Scientific, Qiagen, Roche Diagnostics, and Sebia. Mona M. Hamdy, Hoda M.A. Hassab and Suporn Chuncharunee are Principal Investigators in this phase 2 study and received research grants from Novartis Pharmaceuticals. Ali T. Taher received research grants and consulting fees from Novartis Pharmaceuticals, Bristol Myers Squibb (Celgene), Vifor, Pharmacosmos, and Agios. Ankita Shekhawat, Yamini Sonawane, Laura Torres Perez, and Cassandra Slader are employed by Novartis. Novartis Pharma AG supported the development of this manuscript, provided data analyses according to the direction of the authors, and paid for medical writing support. All authors declare no other competing interests related to the study.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burkhart PV, Sabaté E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh. 2003;35:207. doi: 10.1111/j.1547-5069.2003.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 2.Trachtenberg FL, Gerstenberger E, Xu Y, Mednick L, Sobota A, Ware H, Thompson AA, Neufeld EJ, Thalassemia Clinical Research Network Relationship among chelator adherence, change in chelators, and quality of life in thalassemia. Qual Life Res. 2014;23(8):2277–2288. doi: 10.1007/s11136-014-0671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg SL, Giardina PJ, Chirnomas D, Esposito J, Paley C, Vichinsky E. The palatability and tolerability of deferasirox taken with different beverages or foods. Pediatr Blood Cancer. 2013;60(9):1507–1512. doi: 10.1002/pbc.24561. [DOI] [PubMed] [Google Scholar]
- 4.Chat Chai AS, Draman N, Mohd Yusoff SS, Azman NF, Mohd Zulkifli M, Yaacob NM, Mohamad N, Hassan R, Abdullah WZ, Zilfalil BA. Non-compliance to iron chelation therapy in patients with transfusion-dependent thalassaemia. Pediatr Hematol Oncol. 2021;6(4):207–215. doi: 10.1016/j.phoj.2021.10.005. [DOI] [Google Scholar]
- 5.Chong CC, Redzuan AM, Sathar J, Makmor-Bakry M. Patient perspective on iron chelation therapy: barriers and facilitators of medication adherence. J Patient Exp. 2021;8:2374373521996958. doi: 10.1177/2374373521996958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kontoghiorghe CN, Kontoghiorghes GJ. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des Devel Ther. 2016;10:465–481. doi: 10.2147/DDDT.S79458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Highlights of prescribing information (EXJADE® tablets). Novartis Pharmaceuticals Corporation. July 2020. https://www.novartis.com/us-en/sites/novartis_us/files/exjade.pdf
- 8.Piga A, Galanello R, Forni GL, Cappellini MD, Origa R, Zappu A, Donato G, Bordone E, Lavagetto A, Zanaboni L, Sechaud R, Hewson N, Ford JM, Opitz H, Alberti D. Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006;91(7):873–880. [PubMed] [Google Scholar]
- 9.Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, Aydinok Y, Kattamis A, Kilinc Y, Porter J, Capra M, Galanello R, Fattoum S, Drelichman G, Magnano C, Verissimo M, Athanassiou-Metaxa M, Giardina P, Kourakli-Symeonidis A, Janka-Schaub G, Coates T, Vermylen C, Olivieri N, Thuret I, Opitz H, Ressayre-Djaffer C, Marks P, Alberti D. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107(9):3455–3462. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 10.Cappellini MD, Bejaoui M, Agaoglu L, Porter J, Coates T, Jeng M, Lai ME, Mangiagli A, Strauss G, Girot R, Watman N, Ferster A, Loggetto S, Abish S, Cario H, Zoumbos N, Vichinsky E, Opitz H, Ressayre-Djaffer C, Abetz L, Rofail D, Baladi JF. Prospective evaluation of patient-reported outcomes during treatment with deferasirox or deferoxamine for iron overload in patients with beta-thalassemia. Clin Ther. 2007;29(5):909–917. doi: 10.1016/j.clinthera.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Cappellini MD, Porter J, El-Beshlawy A, Li CK, Seymour JF, Elalfy M, Gattermann N, Giraudier S, Lee JW, Chan LL, Lin KH, Rose C, Taher A, Thein SL, Viprakasit V, Habr D, Domokos G, Roubert B, Kattamis A, EPIC Study Investigators Tailoring iron chelation by iron intake and serum ferritin: the prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias. Haematologica. 2010;95(4):557–566. doi: 10.3324/haematol.2009.014696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappellini MD, Bejaoui M, Agaoglu L, Canatan D, Capra M, Cohen A, Drelichman G, Economou M, Fattoum S, Kattamis A, Kilinc Y, Perrotta S, Piga A, Porter JB, Griffel L, Dong V, Clark J, Aydinok Y. Iron chelation with deferasirox in adult and pediatric patients with thalassemia major: efficacy and safety during 5 years' follow-up. Blood. 2011;118(4):884–893. doi: 10.1182/blood-2010-11-316646. [DOI] [PubMed] [Google Scholar]
- 13.Jordan LB, Vekeman F, Sengupta A, Corral M, Guo A, Duh MS. Persistence and compliance of deferoxamine versus deferasirox in Medicaid patients with sickle-cell disease. J Clin Pharm Ther. 2012;37(2):173–181. doi: 10.1111/j.1365-2710.2011.01276.x. [DOI] [PubMed] [Google Scholar]
- 14.Taher AT, Origa R, Perrotta S, Kourakli A, Ruffo GB, Kattamis A, Goh AS, Cortoos A, Huang V, Weill M, Merino Herranz R, Porter JB. New film-coated tablet formulation of deferasirox is well tolerated in patients with thalassemia or lower-risk MDS: Results of the randomized, phase II ECLIPSE study. Am J Hematol. 2017;92(5):420–428. doi: 10.1002/ajh.24668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taher AT, Origa R, Perrotta S, Kouraklis A, Ruffo GB, Kattamis A, Goh AS, Huang V, Zia A, Herranz RM, Porter JB. Patient-reported outcomes from a randomized phase II study of the deferasirox film-coated tablet in patients with transfusion-dependent anemias. Health Qual Life Outcomes. 2018;16(1):216. doi: 10.1186/s12955-018-1041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartaglione I, Origa R, Kattamis A, Pfeilstöcker M, Gunes S, Crowe S, Fagan N, Vincenzi B, Ruffo GB. Two-year long safety and efficacy of deferasirox film-coated tablets in patients with thalassemia or lower/intermediate risk MDS: phase 3 results from a subset of patients previously treated with deferasirox in the ECLIPSE study. Exp Hematol Oncol. 2020;9:20. doi: 10.1186/s40164-020-00174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidhu S, Kakkar S, Dewan P, Bansal N, Sobti PC. Adherence to iron chelation therapy and its determinants. Int J Hematol Oncol Stem Cell Res. 2021;15(1):27–34. doi: 10.18502/ijhoscr.v15i1.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng WY, Said Q, Hao Y, Xiao Y, Vekeman F, Bobbili P, Duh MS, Nandal S, Blinder M. Adherence to iron chelation therapy in patients who switched from deferasirox dispersible tablets to deferasirox film-coated tablets. Curr Med Res Opin. 2018;34(11):1959–1966. doi: 10.1080/03007995.2018.1470500. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez O, Rodriguez-Cortes H, Robinson N, Lewis N, Pow Sang CD, Lopez-Mitnik G, Paley C. Adherence to deferasirox in children and adolescents with sickle cell disease during 1-year of therapy. J Pediatr Hematol Oncol. 2009;31(10):739–744. doi: 10.1097/MPH.0b013e3181b53363. [DOI] [PubMed] [Google Scholar]
- 20.Highlights of prescribing information (JADENU® tablets). Novartis Pharmaceuticals Corporation. July 2020. https://www.novartis.com/us-en/sites/novartis_us/files/jadenu.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.