Abstract
Small extracellular vesicles (SEVs) secreted by mesenchymal stromal cells (MSCs) are considered one of the most promising biological therapies in recent years. The protective effect of MSCs-derived SEVs on myocardium is mainly related to their ability to deliver cargo, anti-inflammatory properties, promotion of angiogenesis, immunoregulation, and other factors. Herein, this review focuses on the biological properties, isolation methods, and functions of SEVs. Then, the roles and potential mechanisms of SEVs and engineered SEVs in myocardial protection are summarized. Finally, the current situation of clinical research on SEVs, the difficulties encountered, and the future fore-ground of SEVs are discussed. In conclusion, although there are some technical difficulties and conceptual contradictions in the research of SEVs, the unique biological functions of SEVs provide a new direction for the development of regenerative medicine. Further exploration is warranted to establish a solid experimental and theoretical basis for future clinical application of SEVs.
Keywords: Cardiovascular disease, Mesenchymal stomal cells, Small extracellular vesicles, Exosomes, miRNA
Introduction
Cardiomyocytes are permanent and non-renewable cells. Once irreversible myocardial damage occurs, it is difficult for the myocardium to fully recover, leading to heart disorders and end-stage heart failure, which increases the social burden and mortality rate. According to statistics, the number of patients with myocardial injury is expected to exceed 23 million worldwide by 2030 [1]. Therefore, both basic research and clinical studies on myocardial protection are urgently needed. However, current medication and surgical treatments still have limitations, such as adverse drug reactions, postoperative complications, and disease relapse [2]. Therefore, it is still imperative to find novel and effective therapeutic strategies for myocardial protection.
Mesenchymal stromal cells (MSCs) have captured significant attention in the fields of regenerative medicine and translational medicine [3, 4]. MSCs are adult stromal cells that can be cultured from virtually all tissues, including bone marrow, adipose, umbilical cord, and amniotic membrane tissues [5–7]. Due to their capacity for self-renewal and differentiation into tissue-specific cells, MSCs are crucial for tissue repair and functional recovery of organs. There is growing evidence that MSCs-mediated cardioprotection is not dependent on their ability to differentiate into functional cardiomyocytes, but rather is mainly attributed to the effect of MSCs-derived extracellular vesicles (MSCs-EVs) that promote the repair of damaged cardiomyocytes and the recovery of cardiac function. EVs were previously marked as metabolic waste and did not receive much attention. But now, as a representative of cell-free therapeutic options, EVs can exert similar therapeutic effects to MSCs and even circumvent the limitations of stromal cell transplantation, such as tumorigenicity, poor immunogenicity, and low colonization rate [8–10]. According to the International Society for Extracellular Vesicles (ISEV), EVs < 200 nm are referred to as small EVs (SEVs). EVs with a diameter of 30–150 nm, formerly called exosomes, are also included in the category of SEVs. Numerous studies have demonstrated that SEVs derived from MSCs can alleviate myocardial injury and protect cardiac function by delivering cargoes, promoting vascular regeneration, exerting anti-inflammatory effects, and reducing immune rejection [11–13]. In this review, we focused on the protective mechanisms of SEVs derived from MSCs in myocardial protection and discussed the opportunities and challenges of SEVs therapy.
SEVs Derived from MSCs
Characteristics of SEVs
As early as 1971, Aaronson et al. discovered the existence of EVs [14]. Recently, research on EVs has grown dramatically due to the increasing recognition of EVs as disease biomarkers and therapeutics. In 2014, the ISEV proposed Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines [15]. EVs can be classified into three categories: (1) microvesicles (MVs), ranging from 100 to 1000 nm in diameter [16]; (2) apoptotic bodies, which are 1 to 5 μm in diameter and secreted specifically by apoptotic cells [17]; and (3) SEVs, which have diameters ranging approximately from 30 to 150 nm. However, there are still controversies regarding the isolation, nomenclature, characterization, and function of EVs. For instance, the nomenclature for EVs such as exosome and microvesicle has contradictory definition and unclear specific biological functions. Thus, the ISEV updated the guidelines in 2018 to improve the research quality of EVs with input from over 400 international scientists. The new guidelines suggest that researchers name EVs according to their diameter, density, biochemical composition, descriptions of conditions, or cell of origin if they do not have a complete experimental and theoretical basis for granting a new name to specific EVs. Based on this, this review collectively refers to EVs with a diameter < 200 nm as SEVs [18]. Although SEVs were once regarded as waste products during cell metabolism, but now, it is well-accepted that SEVs serve as essential intercellular mediators that participate in cell repair, functional recovery, stromal cell maintenance, and other physiological processes [19].
The process of SEV formation is a complex biological process that involves several steps. It starts with endocytosis on the cell membrane, where the inward budding of early endosomes occurs. Through a series of packaging and transshipment, mature SEVs are released into the extracellular environment via outward budding. Specifically, the secretion of SEVs is regulated by two distinct molecular mechanisms: the endosomal sorting complex required for transport (ESCRT)–dependent mechanism and the ESCRT-independent mechanism. The ESCRT machinery is composed of approximately 20 proteins that assembled into 4 complexes (ESCRT-0, -I, -II, and -III) including vacuolar protein sorting–associated protein 4 (VPS4), vacuolar protein sorting–associated protein (VTA1), and programmed cell death 6–interacting protein (PDCD6IP). These complexes are critical for the recognition and transport of SEVs [20–22]. The ESCRT-0 complex precisely identifies the ubiquitinated cytoplasmic domains of transmembrane proteins and then further sorts them to the endosomal membrane [23]. The ESCRT-I and ESCRT-II complexes bind to the outside of the endosomal membrane, inducing the luminal vesicles of multivesicular bodies (MVBs). The ESCRT-III complex assembles on the outer surface of the endosomal membrane during the generation of MVBs, promoting the formation of MVBs in the nucleus [24]. On the other hand, the biogenesis of SEVs may also occur through the ESCRT-independent pathway. For instance, the classification and assembly of cargoes transported by SEVs occur in a ceramide-dependent manner, such as tetraspanin family proteins (CD81, CD82, and CD9) [25]. Recent evidence has also indicated that Ras-related protein Rab-31 (RAB31) marks an ESCRT-independent pathway in the biogenesis of SEVs [26].
Isolation of SEVs
Since the discovery of SEVs, researchers have faced challenges in stably isolating, purifying, and identifying these nanoscale biological vesicles and their subgroups. A survey published in 2016 found that the most commonly used method for isolating SEVs was ultracentrifugation, with more than 80% of EVs researchers worldwide using this method [27], though other methods such as size-exclusion chromatography are also popular. Other methods for SEV isolation include density gradient separation, precipitation, filtration, size-exclusion chromatography, and immunoaffinity interaction are still being used. Gradient centrifugation for the extraction of SEVs a is simple and low-cost method, but it has poor yield and is time-consuming [28]. SEVs obtained by precipitation and size-exclusion chromatography have low purity and high technical costs [29]. Some researchers also use commercial kits to isolate SEVs. The advantages and disadvantages of various SEV isolation methods are listed in Table 1. MISEV2018 guidelines suggest that a single or a combination of isolation methods can be selected according to the experimental design and the specificity of SEVs. Table 2 provides the details of SEV isolation methods based on recovery and specificity.
Table 1.
Major isolation methods of SEVs
| Method | Principle | Time | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| Ultracentrifugation | Extracellular components are removed by various centrifugal forces | 140–600 min |
Widely used; Low cost; Suitable for large volume samples; Absence of additional chemicals; Great specificity |
Equipment; Time and labor consuming; Contamination; Low recovery; Complicated; Damage of SEVs |
[30–32] |
| Density gradient ultracentrifugation | SEVs are separated into a medium of similar density position by centrifugal force | 250 min–2 days |
High purity; Absence of additional chemicals; Preservation of SEVs |
Equipment; Time and labor consuming; Low production; Complicated |
[32–34] |
| Ultrafiltration | SEVs are separated by using different molecular weight cut-offs ultrafiltration membranes | 130 min |
Low cost; Short time; Suitable for large volume samples; Medium purity |
Filter block; Loss of sample; Contamination; Low production; |
[32, 35] |
| Size-exclusion chromatography | SEVs of different sizes exhibit various elution times passing through porous resin particles | 1 mL/min |
High purity; Easy operation; Preservation of SEVs; Absence of additional chemicals; Reproducibility |
Relatively high cost; Time and labor consuming; Equipment |
[32, 36] |
| Immunoaffinity capture | Binding between biomarkers such as surface antibodies of SEVs and antibody-recognized ligands | Base on antibody |
Easy operation; Preservation of SEVs; High purity |
High cost; Time-consuming; Contamination; Low production |
[33, 35, 37] |
| Polymer precipitation | Reducing the solubility of SEVs | 45–130 min |
Low cost; Easy operation; Preservation of SEVs; High production |
Contamination by non SEVs; Low quantification of SEVs |
[31, 33, 35] |
| Microfluidic technologies | Based on different diameters of microfluidic device with microporous filtration system and immunoaffinity principle | 1–14 µL/min |
High production; Purity; Fast processing time; High sensitivity |
High cost; Equipment; Complicated |
[38–40] |
Table 2.
Considerations for SEVs separation/enrichment
| Validity | Definition | Isolation technique |
|---|---|---|
| High recovery, low specificity | Recover the highest amount of EVs, whatever their nature | Precipitation kits |
| Low molecular weight cutoff centrifugal filters without a further separation step | ||
| Lengthy or very high-speed ultracentrifugation without previous lower-speed steps | ||
| Intermediate recovery, intermediate specificity | Recover mixed EVs along with some amount of free proteins, ribonucleoproteins, and lipoproteins, depending on the matrix |
Size-exclusion chromatography High molecular weight centrifugal filters differential ultracentrifugation using intermediate time/speed with or without wash Tangential flow filtration membrane-affinity columns |
| Low recovery, high specificity | Recover a subtype (or a few subtypes) of EVs with as few non-vesicular components as possible | Subtypes of EVs can be separated by their size, their density upon either flotation or pelleting in a density gradient, their surface protein, sugar, or lipid composition, or other biophysical properties such as surface charge |
Identification of SEVs
The identification of SEVs mainly focuses on their morphology, size, and surface marker proteins. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are commonly used to visualize the morphology of SEVs. Nanoparticle tracking analysis (NTA) can determine the size distribution of SEVs. Western blot and flow cytometry are applied for the identification of the surface markers of SEVs, such as CD9, CD63, and TSG101 [41].
Mechanisms of MSC-SEVs on Myocardial Protection
The transplantation of MSCs has been recognized as a promising strategy for cardiac repair after myocardial injury [42–44]. Increasing experimental studies have suggested that the therapeutic potential of MSCs may arise from their autocrine effects. Notably, recent research has also revealed that MSCs mediate cardiac repair via a paracrine manner, in which SEVs play a vital role. For example, MSCs have been demonstrated to mediate cardioprotection during myocardial ischemia–reperfusion injury (MIRI) by secreting SEVs [45]. MSCs-derived SEVs can be used alone to promote cardiac repair, independent of the biological effects of MSCs. In a rat model of myocardial infarction (MI), MSCs-derived SEVs were even significantly superior to MSCs in attenuating inflammation, inhibiting fibrosis, and improving cardiac function [46]. Here, we review the myocardial protective mechanisms of MSC-SEVs, including the effects of delivering cargo, anti-inflammatory, angiogenesis promotion, and immune regulation, as follows.
microRNAs (miRNAs) Delivered by SEVs
SEVs are known to deliver various biomolecules, including messenger RNAs (mRNAs), miRNAs, proteins, and lipids, to regulate the functions of recipient cells [47]. Among these biomolecules, miRNAs are an evolutionarily conserved class of small single-stranded noncoding RNAs (18–24 nucleotides) that bind to the 3′-untranslated region (3′-UTR) of a specific target mRNA sequence for the post-transcriptional regulation of gene expression [48]. It has been reported that more than 60% of human protein-coding genes are directly regulated by miRNAs, which can suppress gene expression by inhibiting mRNA translation and/or by promoting mRNA degradation [49, 50].
Numerous experiments have shown that SEVs derived from MSCs can alleviate myocardial injury by delivering miRNAs. In a rat model of acute myocardial infarction (AMI), the left ventricular end systolic diameter (LVESD) and left ventricular end diastolic diameter (LVEDD) were significantly increased but intervention with MSC-SEVs reversed this change. While the left ventricular ejection fraction (LVEF) was reduced to 30% in AMI, after treatment with hucMSCs-derived SEVs, LVEF increased to about 60%. Furthermore, MSC-SEVs reduced infarct size from 40 to 20%, indicating a significant myocardial protective effect. Subsequent experiments revealed that miRNA-19a is poorly expressed in myocardial tissues of AMI rats. Injection of hucMSCs-derived SEVs with miRNA-19a inhibitor did not show significant myocardial protective effects, while hucMSCs-derived SEVs with miR-19a enhanced myocardial protection in AMI rats. Further research suggested that hucMSCs-derived SEVs deliver miRNA-19a to target the transcription factor SOX6, activating serine/threonine-protein kinases (AKT), inhibiting mitogen-activated protein kinase 10 (MAPK10)/caspase-3, and ultimately protecting rats from AMI [51]. Similar reports have shown that SEVs secreted by bone marrow–derived MSCs (BM-MSCs) can carry miRNA-19a/19b to injured myocardial tissues, promoting cardiac function recovery and alleviating myocardial fibrosis [52]. Other miRNAs, such as miRNA-125 [53–55], miRNA-19 [56], miRNA-21a [57], miRNA-132 [58], miRNA-182 [59], miRNA-126 [60], miRNA-25 [61], miRNA-210 [62], miRNA-671 [63], miRNA-29c [64], miRNA-486 [65], miRNA-144 [66], miRNA-149 [67], and miRNA-22 [68], have also been experimentally demonstrated to participate in myocardial protection. In these studies, miRNAs delivered into the injured myocardium by SEVs not only alleviate cell injury but also regulate apoptosis, myocardial fibrosis, and pyroptosis. A valuable study analyzed miRNA produced by three clinically grade stromal cells of EVs. While there were some miRNAs in common, each EV had a unique miRNA profiles. All EVs improved cell adhesion/migration, immune response, platelet aggregation, protein translation/stabilization, and RNA processing. This study is beneficial for further clarifying the molecular mechanisms and functions of miRNAs in SEVs [69]. Although the above studies show that SEVs can serve as cargoes for miRNA delivery, lipoproteins have also been proven to deliver miRNAs. Actually, lipoproteins are inevitably mixed with SEVs [70], making further purification of SEVs become one of the major obstacles in current SEVs research. Table 3 lists the mechanistic role of some SEVs-delivered miRNAs in myocardial protection.
Table 3.
miRNAs delivered by MSCs-derived SEVs to regulate cardiac repair
| Disease model | Type of MSCs | Mechanism | Target cell | Dose | Administration | Function | Reference |
|---|---|---|---|---|---|---|---|
| SD rat AMI | HucMSCs | MiR-19a | CM | 400 μg | Intravenous | Targeting SOX6 and activating AKT to protect cardiomyocytes | [51] |
| Mouse MI | BM-MSCs | MiR-19a/b | CM | 0.5 μmol | Intravenous | Enhancing the recovery of cardiac function and reducing cardiac fibrosis | [52] |
| Mouse MI | BM-MSCs | MiR-125b | CM | 50 μg/mL | Intramyocardial | Reducing the infarct size and promoting cardiac repair | [54] |
| SD rat MIRI | BM-MSCs | MiR-125b | CM | 50 μg | Intramyocardial | Protecting against MIRI by targeting SIRT7 | [55] |
| SD rat AMI | BM-MSCs | MiR-19a | CM | 4 × 106 MSCs | Intramyocardial | Enhancing cardiac protection via activation of the AKT and ERK signaling pathways | [56] |
| Mouse MI | BM-MSCs | MiR-21a | CM | 300 μg | Intramyocardial | Contributing to cardiac repair | [57] |
| Mouse MIRI | BM-MSCs | MiR-182 | CM | 50 μg | Intramyocardial | Attenuating MIRI and changing the polarization of macrophages | [59] |
| H/R-cell | BM-MSCs | MiR-126 | HUVECs | / | Co-culture | Enhancing angiogenesis | [60] |
| Mouse MIRI | BM-MSCs | MiR-25-3p | CM | 5 μg in 100 μL PBS | Intramyocardial | Enhancing cardiac protection by targeting pro-apoptotic proteins and EZH2 | [62] |
| SD rat MI | BM-MSCs | MiR-210 | CM | 1 × 106 MSCs | Intramyocardial | Protecting cardiac injury and limiting myocyte apoptosis | [63] |
| Mouse MI | adMSCs | MiR-671 | CM | 100 μg | Intramyocardial | Alleviating myocardial infraction by inactivating TGFBR2/Smad2 | [64] |
| Mouse MIRI | BM-MSCs | MiR-29c | CM | 20 μg | Intramyocardial | Regulating autophagy under IR injury | [65] |
| SD rat MIRI | BM-MSCs | MiR-486-5p | CM | 400 μg | Intravenous | Inhibiting apoptosis and activating the PI3K/AKT pathway | [66] |
| Hypoxia cell | BM-MSCs | MiR-144 | H9C2 | 5 μg/mL | Co-culture | Promoting anti-apoptotic proteins under hypoxic conditions | [67] |
| H/R H9C2 model | BM-MSCs | MiR-149 | H9C2 | 5 μg | Co-culture | Protecting myocardium against H/R injury via miR149/let-7c/Faslg | [68] |
| Mouse MI | BM-MSCs | MiR-22 | CM | 1 μg | Intramyocardial | Reducing cardiac fibrosis and inhibiting apoptosis | [69] |
| Mouse AMI | BM-MSCs | MiR132 | CM | 600 μg | Intramyocardial | Promoting angiogenesis | [70] |
Protein Cargo of MSC-SEVs
MSC-SEVs can directly affect biological processes by regulating the expression of protein levels in damaged myocardium. A study comparing proteomic analyses of myocardial infarction tissue with SEVs-treated tissue showed that MSC-SEVs significantly reduced infarct size by about 15–20% and induced significant changes in inflammatory and apoptosis-related proteins [71]. MSC-SEVs were found to modulate platelet-derived growth factor receptor-β (PDGFR-β) expression immediately and alleviate microvascular dysfunction caused by cardiac ischemia–reperfusion, as well as inhibit fibrosis development [72]. SEVs contain hundreds of proteins associated with cellular communication, inflammation, tissue repair and regeneration, and metabolism [73]. Understanding the biological role of these proteins is crucial for further elucidating the function of SEVs. Proteomic analysis of SEVs derived from bone marrow-MSC (BM-MSC) indicated that the proteins enriched in these vesicles were related to regeneration medicine that include collagen, extracellular matrix, bone regeneration, and muscle regeneration. Proteins in adipose tissue-MSC (AT-MSC) SEVs and umbilical cord-MSC (UC-MSC) SEVs were found to be related to immune response [74]. Vaka et al. reported that there were differences in the protein expression of BM-MSC-SEVs and UC-MSC-SEVs, specifically in proteins involved in actin organization, cadherin binding, and cellular adhesion, indicating the potential of MSC-SEVs proteins in repairing myocardial tissue [69]. Previous studies have also investigated the proteomics of various heart diseases [75–80], providing valuable insights into cardiac pathogenesis and identifying potential therapeutic targets. Correlating these findings with MSC-SEVs proteomics analysis could further elucidate the mechanism of action of MSC-SEVs proteins and facilitate the development of more reliable therapeutic strategies.
Pro-angiogenic
Interstitial cells in myocardial tissues, such as endothelial cells and fibroblasts, interact with myocardial cells to support normal myocardial contractility [81]. During MIRI, vascular regeneration impairment and endothelial dysfunction aggravate irreversible myocardial injury, making revascularization and angiogenesis important mechanisms for protecting the myocardium. Angiogenesis in myocardial tissues is a complex process, which requires the action of pro-angiogenic signal cascades. Some key factors, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and angiogenin (ANG), are closely associated with vascular regeneration [82]. MSCs possess the capacity to promote angiogenesis. MSCs can directly build tubular structures and exhibit significant pro-angiogenic activity on endothelial cells in vitro. The pro-angiogenic function of MSCs is attributed to the secretion of VEGF, ANG, transforming growth factor-beta (TGF-β), nerve growth factor (NGF), and other pro-angiogenic factors by MSCs [83]. SEVs derived from MSCs have been used to improve islet transplantation outcomes due to the secretion of VEGF [84]. In human umbilical vein endothelial cells (HUVECs), the mRNA expression levels of VEGF, ANG-1, and PDGF are decreased following hypoxia injury. However, SEVs derived from BM-MSCs significantly increased the mRNA expression levels of VEGF, PDGF, and ANG-1 in hypoxic HUVECs. Other researchers have found that HUVECs-absorbed SEVs increased the tube formation of endothelial cells in vitro and enhanced the angiogenesis capacity in vivo by downregulated Ras GTPase–activating protein 1 (RASA1) expression [58]. Similarly, cardiac myocyte progenitor cells (CMPCs)–derived SEVs have powerful pro-angiogenic effects due to the presence of extracellular matrix metalloproteinases inducer (EMMPRIN) [85]. MSCs-derived SEVs can certainly have pro-angiogenic effects on repairing myocardial tissues and restoring cardiac function [86].
Anti-inflammatory
Inflammation is a key mechanism that leads to myocardial injury. In various heart diseases, such as acute myocardial infarction, heart failure, and MIRI, the activity of pro-inflammatory factors is increased, and the activation of pathways is not conducive to myocardial repair [87–89]. TNF-α, IL-1, and IL-6 are classical pro-inflammatory factors, and reducing the activity of these factors is beneficial for myocardial repair [90–92]. In a study of AMI in vitro, the levels of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α were significantly increased in cardiomyocytes by oxygen–glucose deprivation (OGD). However, MSC-SEVs reversed this change, obviously improved cell viability, and alleviated apoptosis [61]. Inflammatory response is a critical mechanism by which the heart responds to injury for adaptive remodeling. Dysregulated inflammation leads to the excessive release of inflammatory factors and aggravates myocardial injury. Nucleotide-binding oligomerization domain–like receptor family pyrin domain–containing 3 (NLRP3) is a key factor in the inflammatory cascade, and targeting the NLRP3 inflammasome has become an emerging strategy for anti-inflammatory therapies. MIRI activates NLRP3, leading to the release of inflammatory factors such as IL-1β, IL-18, and caspase1, which eventually aggravating myocardial injury. Inhibition of NLRP3 can reduce the size of myocardial infarction and protect cardiac function [93, 94]. MSCs-derived SEVs can inhibit NLRP3 inflammasome activation and suppress inflammatory cytokine release, thereby protecting cardiomyocytes from hypoxia/reoxygenation (H/R)–induced injury [95]. MSCs-derived SEVs can also enhance the viability of cardiomyocytes in the H/R model by downregulating the levels of NLRP3 and caspase-1 [96].
Immunomodulatory Property
Recent studies have demonstrated the remarkable therapeutic efficacy of MSCs in treating a wide range of autoimmune diseases due to their immunomodulatory properties, such as inhibiting T cell proliferation, increasing the number of Treg cells, inhibiting Th cell expansion, and maintaining the balance between Th1 and Th2 cells [97]. The immunomodulatory effects of MSCs-derived SEVs have also received widespread attention [98]. The microvascular network in myocardial tissue facilitates communication between immune cells and antibodies, making the heart vulnerable to immune-mediated damage. Macrophages play a crucial role in regulating myocardial homeostasis and can be classified into two major polarization states: pro-inflammatory M1 and anti-inflammatory M2. Imbalances between these two phenotypes can lead to inflammatory conditions and cardiac injury. Disruption of macrophage metabolism can exacerbate inflammation and destroy M1/M2 homeostasis. Injections of MSCs-derived SEVs have been shown to restore cardiac function in Sprague–Dawley (SD) rats suffering from MIRI. MSCs-derived SEVs did not affect the total number of macrophages but notably decreased M1 macrophages and increased M2 macrophages. This was manifested by elevated markers of M2 macrophages, such as arginase 1 (Arg1), IL-10, CD206, and TGF-β. Furthermore, MSCs-derived SEVs inhibited the production of lipopolysaccharide (LPS)–induced IL-6 and iNOS but promoted the upregulation of IL-10 and Arg1 in vitro. This study suggested that SEVs facilitated the transformation of macrophages from M1 to M2 phenotype and reduced inflammation in MIRI [59]. MSCs-derived SEVs have inhibitory effects against immune cells, including effector T cells, macrophages, and natural killer cells [99]. Immune rejection is a serious complication of heart transplantation. How to prevent and alleviate immune rejection has always been a challenge for researchers. The biological characteristics of SEVs provide the potential to reduce immune rejection. When combined with immunosuppressive treatment, SEVs derived from donor immature dendritic cells can prolong allograft survival by inhibiting T cell activation [100]. Treatment with MSCs-derived SEVs has been shown to increase the number of Treg cells, decrease the number of CD8 + T cells, suppress the levels of pro-inflammatory factors, and elevate the levels of anti-inflammatory factors in rats receiving heart transplantation. As a result, SEVs not only alleviate immune rejection but also improve the cardiac function of heart allografts [101].
Modified SEVs Derived from Engineered MSCs
MSCs can be selectively modified on demand, and SEVs derived from modified MSCs can precisely target key regulatory pathways and cytokines with biological effects than ordinary SEVs. SEVs produced by MSCs under different culture conditions also have distinct biological functions. SEVs produced by MSCs under hypoxic conditions, for example, can promote the functional recovery of ischemic tissues [102]. In a mouse model of MI induced by ligation of the left anterior descending artery, the effects of MSCs-derived SEVs cultured under normal and hypoxic conditions were compared. MSCs-derived SEVs cultured under hypoxic conditions have a stronger effect on cardiac function recovery, as evidenced by significantly increased cardiac ejection fraction, decreased cardiac left ventricular internal diameter (LVID), and effectively reduced MI size compared with the MSCs-derived SEVs cultured under normal conditions. By comparing the miRNAs transported by normal SEVs and hypo-SEVs, miRNA-125b was found to be significantly enriched in hypo-SEVs. Down-regulation of miRNA-125b weakened the myocardial protection of hypo-SEVs, while overexpression of miRNA-125b-5p inhibited the expression of p53 in ischemic myocardium of MI mice. Blocking the apoptosis of cardiomyocytes is conducive to myocardial protection [54]. Heme oxygenase-1 (HO-1) plays a critical role in myocardial protection by promoting cell survival, repressing cardiomyocyte apoptosis, and enhancing angiogenesis [103]. Hemin is an HO-1 inducer. Researchers had discovered that both SEVs derived from MSCs and SEVs derived from MSCs pre-treated with Hemin could significantly improve cardiac function and reduce fibrosis in MI, but the latter exhibited better myocardial protection. Further miRNA sequencing showed a higher expression pattern of miRNA-183-5p in Hemin-MSCs-SEVs than MSCs-SEVs. miR-183-5p could target high-mobility group box 1 (HMGB1), and miRNA-183-5p knockdown partially reversed the effects of Hemin-MSCs-SEVs in attenuating mitochondrial fission and repressing cell senescence of cardiomyocytes in MI [104].
Angiogenesis is essential for myocardial tissue repair following MI. Hypoxia inducible factor (HIF)–1α, a key transcriptional regulator under hypoxia conditions, regulates the expression of numerous genes related to angiogenesis. HIF-1α promoted the secretion of SEVs by MSCs. SEVs released by HIF-1α-overexpressing MSCs (HIF-MSCs) induced angiogenesis in human endothelial cells. Further study have found that the expression of HES family transcription factor 1(HES1) and helix-loop-helix transcription factor (Hey2) were higher in HUVECs treated with HIF-MSCs-derived SEVs than those treated with MSCs-derived SEVs [105]. Similarly, HIF-1α-overexpressing SEVs were superior to control SEVs in preserving cardiac function and promoting angiogenesis in the SD rat model of AMI [106].
Prospect and Limitation of MSCs-Derived SEVs
The paracrine function of MSCs has made them a promising option for tissue repair and regenerative medicine (Fig. 1). As one of the key paracrine effectors, SEVs are essential for cell-to-cell communication by carrying bioactive substances [107]. MSCs-derived SEVs have unique advantages. Firstly, MSCs may contain mutated DNA that contributes to tumorigenesis, but the existing studies do not find evidence to support the idea that MSCs-derived SEVs lead to the development of tumors. Secondly, SEVs are smaller than MSCs, making them able to easily pass through capillaries, while MSCs are unable to cross the capillary bed and can cause blockage. Thirdly, the infusion dose of MSCs is rapidly reduced after transplantation, whereas MSCs-derived SEVs can attain a larger circulating dose.
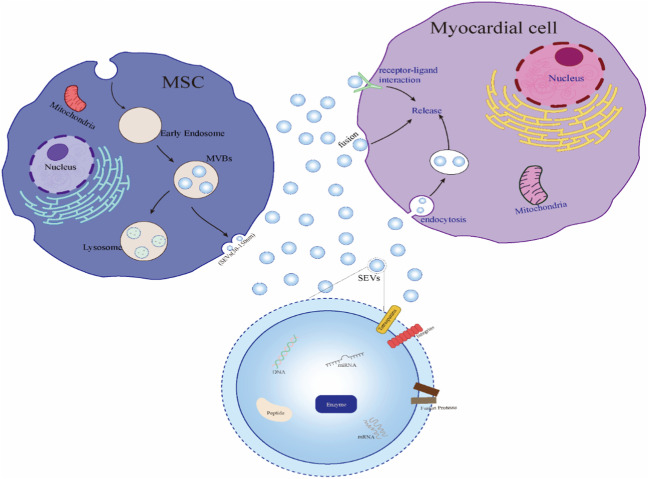
Fig. 1.
Biological characteristics of SEVs. In MSCs, the invaginated cell membrane forms vesicles, and then a variety of proteins, nucleic acids, lipids, and other substances are absorbed by the vesicles to form early endosomes and then MVBs have two pathways: one is to secrete to the extracellular space and release SEVs; another pathway is intracellular lysosomal binding degradation. There are three main ways for cardiomyocytes to absorb SEVs: membrane fusion directly, membrane endocytosis, and receptor binding on the cardiomyocyte membrane. SEVs have a double-membrane structure and are rich in DNA, miRNA, mRNA, polypeptides, enzymes, and other biological active substances. The membrane of SEVs contains tetraspanins, fusion proteins, integrins, etc.
By searching www.ClinnicalTrial.gov, it has been found that there are several clinical studies reporting on the role of MSCs-derived SEVs in other diseases, such as dry eye, T1DM, and periodontitis [99]. However, relatively little is known about the effect of SEVs on cardiovascular diseases. There are two ongoing clinical trials (NCT04356300, NCT05669144) evaluating the effect of MSCs-derived SEVs on cardiovascular diseases, specifically related to aortic dissection and patients undergoing coronary artery bypass grafting (CABG) surgery. The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has become an unprecedented global health crisis [108–110]. Due to the unique biological activity of SEVs, several clinical trials are been conducted to explore the therapeutic effect of SEVs on SARS-CoV-2-infected patients [111, 112].
Currently, there is still controversy regarding the basic research of MSCs-derived SEVs. Unlike MSCs, which have the ability to proliferate, MSCs-derived SEVs cannot be replicated or reproduced. The primary issues that need to be addressed are the potency and therapeutic dose of SEVs. As shown in Table 3, each study used different doses of MSCs-derived SEVs, which may be related to differences in the sources of SEVs, extraction methods, and usage routes. Therefore, it is necessary to establish a unified standard for extracting SEVs. Furthermore, although MSCs-derived SEVs have shown great myocardial protection effects, the specific biological mechanisms still need to be explored. As mentioned earlier, multiple studies have shown that SEVs can alter biological processes in the heart muscle by delivering miRNAs, but some scholars have found that the content of miRNA in SEVs is very low, as little as 0.9% of the total RNA. This means that 100 µg of MSC exosomes only contains 60 ng of miRNA. Therefore, if miRNA from SEVs is a key factor in the biological mechanism, it depends on the high concentration and abundance of SEVs. Additionally, there are many treatments that target miRNA, such as siRNA. Moreover, the cost of extracting SEVs is high. If SEVs are only used as a tool for miRNA delivery, it may result in economic losses and waste. Furthermore, it is speculated that the main biological role of SEVs may be due to the protein cargo they carry [113–115].
Despite the potential of MSCs-derived SEVs in regenerative medicine, several key issues remain to be addressed before extensive clinical application. Firstly, SEVs of different sizes may have different therapeutic effects on cardiac regeneration and repair. And thus, accurately isolating SEVs of the required size is one of the current technical challenges. Secondly, there may be a possibility of mutual invasion and contamination between SEVs from different sources, which could affect their therapeutic efficacy. Thirdly, while studying a single SEV can provide a method to deeply understand the biological characteristics and mechanisms of SEVs, currently, there is a lack of experimental techniques to study a single SEV. Finally, SEVs produced under different conditions can be used to improve cardiac injury under different conditions, And obtaining targeted SEVs accurately is also a challenge for current research.
Conclusion
This review discusses the therapeutic potential of MSCs-derived SEVs for myocardial protection. The publication of numerous studies on the cardioprotective effect of MSCs-derived SEVs has contributed to the understanding of the composition and function of SEVs. At present, new techniques and evaluation criteria for SEVs are urgently needed. Preclinical research on SEVs is still in its preliminary stage, and stronger evidence is required to support the application of SEVs as a novel biological agent in clinical practice.
Author Contribution
Hongkun Wu generated the concept for this review; Xingkai Qian and Guiyou Liang performed the literature search; Hongkun Wu drafted the manuscript; Xingkai Qian and Guiyou Liang critically edited the manuscript. All authors reviewed and approved the final version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 82170286, No. 81960051), Basic Research Program of Guizhou Province (QKHJC-ZK[2022] YB376), The Science and Technology Fund Project of Guizhou Provincial Health Commission (gzwkj2022-088), Project of Guizhou Provincial education Commission, YJSKYJJ[2021]145. YJSKYJJ[2021]146. GSZYQN[2021]NO.13.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Human and animal rights
This article does not contain any studies with animals performed by any of the authors. This article does not contain any studies with human participants or animals performed by any of the authors.
Ethic Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hongkun Wu, Email: maguskovic@gmail.com.
Xingkai Qian, Email: qxkgood@163.com.
Guiyou Liang, Email: guiyou515@163.com.
References
- 1.Smith SC, Jr, Collins A, Ferrari R, et al. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke) Circulation. 2012;126(23):2769–2775. doi: 10.1161/CIR.0b013e318267e99f. [DOI] [PubMed] [Google Scholar]
- 2.Stefanini GG, Holmes DR., Jr Drug-eluting coronary-artery stents. N Engl J Med. 2013;368(3):254–265. doi: 10.1056/NEJMra1210816. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic im-plications. Nat Immunol. 2014;15(11):1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 4.Planat-Benard V, Varin A, Casteilla L. MSCs and inflammatory cells crosstalk in regenerative medicine: concerted actions for optimized resolution driven by energy metabolism. Front Immunol. 2021;30(12):626755. doi: 10.3389/fimmu.2021.626755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mushahary D, Spittler A, Kasper C, et al. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cy-tometry A. 2018;93(1):19–31. doi: 10.1002/cyto.a.23242. [DOI] [PubMed] [Google Scholar]
- 6.Ding DC, Chang YH, Shu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 7.Afari A, Rezaei-Tavirani M, Farhadihosseinabadi B, et al. Human amniotic mesenchymal stem cells to promote/suppress cancer: two sides of the same coin. Stem Cell Res Ther. 2021;12(1):126. doi: 10.1186/s13287-021-02196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou T, Yuan Z, Weng J, et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol On-col. 2021;14(1):24. doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuang WZ, Lin YH, Su LJ, et al. Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J Biomed Sci. 2021;28(1):28. doi: 10.1186/s12929-021-00725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 11.Csöbönyeiová M, Beerová N, Klein M, Debreová-Čeháková M, Danišovič Ľ. Cell-based and selected cell-free therapies for myocardial infarction: how do they compare to the current treatment options? Int J Mol Sci. 2022;23(18):10314. doi: 10.3390/ijms231810314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao L, Qiu F, Cao H, Li H, Dai G, Ma T, Gong Y, Luo W, Zhu D, Qiu Z, Zhu P, Chu S, Yang H, Liu Z. Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics. 2023;13(2):685–703. doi: 10.7150/thno.73568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Chen X, Li P, Lu X, Yan J, Tan H, Zhang C. Exosomes derived from human amniotic fluid mesenchymal stem cells alleviate cardiac fibrosis via enhancing angiogenesis in vivo and in vitro. Cardiovasc Diagn Ther. 2021;11(2):348–361. doi: 10.21037/cdt-20-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aaronson S, Behrens U, Orner R, Haines TH. Ultrastructure of intracellular and extracellular vesicles, membranes, and mye-lin figures produced by Ochromonas danica. J Ultrastruct Res. 1971;35(5):418–430. doi: 10.1016/S0022-5320(71)80003-5. [DOI] [PubMed] [Google Scholar]
- 15.Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sa-hoo S, Tahara H, Wauben MH, Witwer KW, Théry C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;22(3):26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.György B, Módos K, Pállinger E, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117(4):e39–48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019;39(1):BSR20180992. doi: 10.1042/BSR20180992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li FX, Lin X, Xu F, Shan SK, Guo B, Lei LM, Zheng MH, Wang Y, Xu QS, Yuan LQ. The role of mesenchymal stromal cells-derived small extracellular vesicles in diabetes and its chronic complications. Front Endocrinol (Lausanne) 2021;20(12):780974. doi: 10.3389/fendo.2021.780974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21(1):77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Colombo M, Moita C, van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 22.Baietti MF, Zhang Z, Mortier E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Rong Y, Chuang YS, et al. Ubiquitin-dependent lysosomal membrane protein sorting and degradation. Mol Cell. 2015;57(3):467–478. doi: 10.1016/j.molcel.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Hassanzadeh A, Rahman HS, Markov A, et al. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res Ther. 2021;12(1):297. doi: 10.1186/s13287-021-02378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111(9):3100–3110. doi: 10.1111/cas.14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei D, Zhan W, Gao Y, et al. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021;31(2):157–177. doi: 10.1038/s41422-020-00409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardiner C, Di Vizio D, Sahoo S, Théry C, Witwer KW, Wauben M, Hill AF. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;31(5):32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P, Kaslan M, Lee SH, et al. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu LL, Zhu J, Liu JX, et al. A comparison of traditional and novel methods for the separation of exosomes from human samples. Biomed Res Int. 2018;26(2018):3634563. doi: 10.1155/2018/3634563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abramowicz A, Widlak P, Pietrowska M. Proteomic analysis of exosomal cargo: the challenge of high purity vesicle isolation. Mol Biosyst. 2016;12(5):1407–1419. doi: 10.1039/C6MB00082G. [DOI] [PubMed] [Google Scholar]
- 31.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-'t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;27:2. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Möller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;17(4):27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A protocol for exosome isolation and characterization: evaluation of ultra-centrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- 34.Gupta S, Rawat S, Arora V, Kottarath SK, Dinda AK, Vaishnav PK, Nayak B, Mohanty S. An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells. Stem Cell Res Ther. 2018;9(1):180. doi: 10.1186/s13287-018-0923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;1(87):3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 36.An M, Wu J, Zhu J, Lubman DM. Comparison of an optimized ultracentrifugation method versus size-exclusion chromatography for isolation of exosomes from human serum. J Proteome Res. 2018;17(10):3599–3605. doi: 10.1021/acs.jproteome.8b00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai S, Luo B, Jiang P, Zhou X, Lan F, Yi Q, Wu Y. Immuno-modified superparamagnetic nanoparticles via host-guest interactions for high-purity capture and mild release of exosomes. Nanoscale. 2018;10(29):14280–14289. doi: 10.1039/C8NR02871K. [DOI] [PubMed] [Google Scholar]
- 38.Davies RT, Kim J, Jang SC, Choi EJ, Gho YS, Park J. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip. 2012;12(24):5202–5210. doi: 10.1039/c2lc41006k. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Wu HJ, Fine D, Schmulen J, Hu Y, Godin B, Zhang JX, Liu X. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip. 2013;13(15):2879–2882. doi: 10.1039/c3lc41343h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassanpour Tamrin S, Sanati Nezhad A, Sen A. Label-Free Isolation of Exosomes Using Microfluidic Technologies. ACS Nano. 2021;15(11):17047–79. [DOI] [PubMed]
- 41.Yaghoubi Y, Movassaghpour A, Zamani M, et al. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;15(233):116733. doi: 10.1016/j.lfs.2019.116733. [DOI] [PubMed] [Google Scholar]
- 42.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore perfor-mance of infarcted hearts. Nat Med. 2003;9(9):1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 43.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu CB, Huang H, Sun P, et al. Human umbilical cord-derived mesenchymal stromal cells improve left ventricular function, perfusion, and remodeling in a porcine model of chronic myocardial ischemia. Stem Cells Transl Med. 2016;5(8):1004–1013. doi: 10.5966/sctm.2015-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Shao L, Zhang Y, Lan B, et al. MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. Biomed Res Int. 2017;2017:4150705. doi: 10.1155/2017/4150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 48.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 49.Valencia-Sanchez MA, Liu J, Hannon GJ, et al. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20(5):515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 50.Guo H, Ingolia NT, Weissman JS, et al. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang L, Yang L, Ding Y, et al. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle. 2020;19(3):339–353. doi: 10.1080/15384101.2019.1711305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Li L, Liu T, et al. miR-19a/19b-loaded exosomes in combination with mesenchymal stem cell transplantation in a preclinical model of myocardial infarction. Regen Med. 2020;15(6):1749–1759. doi: 10.2217/rme-2019-0136. [DOI] [PubMed] [Google Scholar]
- 53.Chachques JC, Gardin C, Lila N, et al. Elastomeric cardiowrap scaffolds functionalized with mesenchymal stem cells-derived exosomes induce a positive modulation in the inflammatory and wound healing response of mesenchymal stem cell and macrophage. Biomedicines. 2021;9(7):824. doi: 10.3390/biomedicines9070824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu LP, Tian T, Wang JY, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163–6177. doi: 10.7150/thno.28021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Q, Liu Y, Ding X, et al. Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Mol Cell Biochem. 2020;465(1–2):103–114. doi: 10.1007/s11010-019-03671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu B, Kim HW, Gong M, et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;1(182):349–360. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luther KM, Haar L, McGuinness M, et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J Mol Cell Cardiol. 2018;119:125–137. doi: 10.1016/j.yjmcc.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 58.Ma T, Chen Y, Chen Y, et al. MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int. 2018;9(2018):3290372. doi: 10.1155/2018/3290372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao J, Li X, Hu J, et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115(7):1205–1216. doi: 10.1093/cvr/cvz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan Q, Wang Y, Lan Q, et al. Exosomes derived from mesenchymal stem cells ameliorate hypoxia/reoxygenation-injured ECs via transferring microRNA-126. Stem Cells Int. 2019;2(2019):2831756. doi: 10.1155/2019/2831756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng Y, Zhao JL, Peng ZY, et al. L. Exosomal miR-25–3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis. 2020;11(5):317. doi: 10.1038/s41419-020-2545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng H, Chang S, Xu R, et al. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res Ther. 2020;11(1):224. doi: 10.1186/s13287-020-01737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Zhu Y, Wu C, et al. Adipose-derived mesenchymal stem cells-derived exosomes carry microRNA-671 to alleviate myocardial infarction through inactivating the TGFBR2/Smad2 axis. Inflammation. 2021;44(5):1815–1830. doi: 10.1007/s10753-021-01460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li T, Gu J, Yang O, et al. Bone marrow mesenchymal stem cell-derived exosomal miRNA-29c decreases cardiac ischemia/reperfusion injury through inhibition of excessive autophagy via the PTEN/Akt/mTOR signaling pathway. Circ J. 2020;84(8):1304–1311. doi: 10.1253/circj.CJ-19-1060. [DOI] [PubMed] [Google Scholar]
- 65.Sun XH, Wang X, Zhang Y, et al. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res. 2019;177:23–32. doi: 10.1016/j.thromres.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Wen Z, Mai Z, Zhu X, et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11(1):36. doi: 10.1186/s13287-020-1563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zou L, Ma X, Wu B, et al. Protective effect of bone marrow mesenchymal stem cell-derived exosomes on cardiomyoblast hypoxia-reperfusion injury through the miR-149/let-7c/Faslg axis. Free Radic Res. 2020;54(10):722–731. doi: 10.1080/10715762.2020.1837793. [DOI] [PubMed] [Google Scholar]
- 68.Feng Y, Huang W, Wani M, et al. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE. 2014;9(2):e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vaka R, Parent S, Risha Y, Khan S, Courtman D, Stewart DJ, Davis DR. Extracellular vesicle microRNA and protein cargo profiling in three clinical-grade stem cell products reveals key functional pathways. Mol Ther Nucleic Acids. 2023;9(32):80–93. doi: 10.1016/j.omtn.2023.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia C, Dai Z, Jin Y, et al. Emerging antioxidant paradigm of mesenchymal stem cell-derived exosome therapy. Front En-docrinol (Lausanne) 2021;29(12):727272. doi: 10.3389/fendo.2021.727272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kore RA, Wang X, Ding Z, Griffin RJ, Tackett AJ, Mehta JL. MSC exosome-mediated cardioprotection in ischemic mouse heart comparative proteomics of infarct and peri-infarct areas. Mol Cell Biochem. 2021;476(4):1691–1704. doi: 10.1007/s11010-020-04029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Bai L, Liu X, Shen W, Tian H, Liu W, Yu B. Cardiac microvascular functions improved by MSC-derived exosomes attenuate cardiac fibrosis after ischemia-reperfusion via PDGFR-β modulation. Int J Cardiol. 2021;1(344):13–24. doi: 10.1016/j.ijcard.2021.09.017. [DOI] [PubMed] [Google Scholar]
- 73.Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteomics. 2012;2012:971907. doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang ZG, He ZY, Liang S, Yang Q, Cheng P, Chen AM. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):511. doi: 10.1186/s13287-020-02032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henry A, Gordillo-Marañón M, Finan C, Schmidt AF, Ferreira JP, Karra R, Sundström J, Lind L, Ärnlöv J, Zannad F, Mälarstig A, Hingorani AD, Lumbers RT, HERMES and SCALLOP Consortia Therapeutic targets for heart failure identified using proteomics and Mendelian randomization. Circulation. 2022;145(16):1205–1217. doi: 10.1161/CIRCULATIONAHA.121.056663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roselló-Lletí E, Alonso J, Cortés R, Almenar L, Martínez-Dolz L, Sánchez-Lázaro I, Lago F, Azorín I, Juanatey JR, Portolés M, Rivera M. Cardiac protein changes in ischaemic and dilated cardiomyopathy: a proteomic study of human left ventricular tissue. J Cell Mol Med. 2012;16(10):2471–2486. doi: 10.1111/j.1582-4934.2012.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Previs MJ, O’Leary TS, Morley MP, Palmer BM, LeWinter M, Yob JM, Pagani FD, Petucci C, Kim MS, Margulies KB, Arany Z, Kelly DP, Day SM. Defects in the proteome and metabolome in human hypertrophic cardiomyopathy. Circ Heart Fail. 2022;15(6):e009521. doi: 10.1161/CIRCHEARTFAILURE.121.009521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hepponstall M, Konstantinov IE. Proteomics in paediatric cardiac surgery: is a personalised approach feasible? Proteomics Clin Appl. 2014;8(11–12):851–861. doi: 10.1002/prca.201400054. [DOI] [PubMed] [Google Scholar]
- 79.Kim HK, Thu VT, Heo HJ, Kim N, Han J. Cardiac proteomic responses to ischemia-reperfusion injury and ischemic preconditioning. Expert Rev Proteomics. 2011;8(2):241–261. doi: 10.1586/epr.11.8. [DOI] [PubMed] [Google Scholar]
- 80.Coats CJ, Heywood WE, Virasami A, Ashrafi N, Syrris P, Dos Remedios C, Treibel TA, Moon JC, Lopes LR, McGregor CGA, Ashworth M, Sebire NJ, McKenna WJ, Mills K, Elliott PM. Proteomic analysis of the myocardium in hypertrophic obstructive cardiomyopathy. Circ Genom Precis Med. 2018;11(12):e001974. doi: 10.1161/CIRCGENETICS.117.001974. [DOI] [PubMed] [Google Scholar]
- 81.Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14(1):38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lian Q, Zhang Y, Zhang J, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121(9):1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 83.Du WJ, Chi Y, Yang ZX, et al. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res Ther. 2016;7(1):163. doi: 10.1186/s13287-016-0418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keshtkar S, Kaviani M, Sarvestani FS, et al. Exosomes derived from human mesenchymal stem cells preserve mouse islet survival and insulin secretion function. EXCLI J. 2020;3(19):1064–1080. doi: 10.17179/excli2020-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vrijsen KR, Maring JA, Chamuleau SA, et al. Exosomes from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via EMMPRIN. Adv Healthc Mater. 2016;5(19):2555–2565. doi: 10.1002/adhm.201600308. [DOI] [PubMed] [Google Scholar]
- 86.Teng X, Chen L, Chen W, et al. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem. 2015;37(6):2415–2424. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 87.Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, Hausenloy DJ. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73–87. doi: 10.1016/j.pharmthera.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dick SA, Epelman S. Chronic heart failure and inflammation: What Do We Really Know? Circ Res. 2016;119(1):159–176. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 89.Vinten-Johansen J, Jiang R, Reeves JG, Mykytenko J, Deneve J, Jobe LJ. Inflammation, proinflammatory mediators and myocardial ischemia-reperfusion Injury. Hematol Oncol Clin North Am. 2007;21(1):123–145. doi: 10.1016/j.hoc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 90.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93(4):704–711. doi: 10.1161/01.CIR.93.4.704. [DOI] [PubMed] [Google Scholar]
- 91.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173(1):57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eiken HG, Øie E, Damås JK, Yndestad A, Bjerkeli V, Aass H, Simonsen S, Geiran OR, Tønnessen T, Christensen G, Frøland SS, Gullestad L, Attramadal H, Aukrust P. Myocardial gene expression of leukaemia inhibitory factor, interleukin-6 and glycoprotein 130 in end-stage human heart failure. Eur J Clin Invest. 2001;31(5):389–397. doi: 10.1046/j.1365-2362.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- 93.Marchetti C, Chojnacki J, Toldo S, et al. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J Cardiovasc Pharmacol. 2014;63(4):316–322. doi: 10.1097/FJC.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fujisue K, Sugamura K, Kurokawa H, et al. Colchicine improves survival, left ventricular remodeling, and chronic cardiac function after acute myocardial infarction. Circ J. 2017;81(8):1174–1182. doi: 10.1253/circj.CJ-16-0949. [DOI] [PubMed] [Google Scholar]
- 95.Liang C, Liu Y, Xu H, et al. Exosomes of human umbilical cord MSCs protect against hypoxia/reoxygenation-induced pyroptosis of cardiomyocytes via the miRNA-100-5p/FOXO3/NLRP3 pathway. Front Bioeng Biotechnol. 2021;15(8):615850. doi: 10.3389/fbioe.2020.615850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang J, Jin L, Liu Y, et al. Exosomes derived from mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through inhibiting pyroptosis. Drug Des Devel Ther. 2020;16(14):3765–3775. doi: 10.2147/DDDT.S239546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie Q, Liu R, Jiang J, Peng J, Yang C, Zhang W, Wang S, Song J. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res Ther. 2020;11(1):519. doi: 10.1186/s13287-020-02011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang C, Sun J, Tian Y, Li H, Zhang L, Yang J, Wang J, Zhang J, Yan S, Xu D. Immunomodulatory effect of MSCs and MSCs-derived extracellular vesicles in systemic lupus erythematosus. Front Immunol. 2021;16(12):714832. doi: 10.3389/fimmu.2021.714832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee BC, Kang I, Yu KR. Therapeutic features and updated clinical trials of mesenchymal stem cell (MSC)-derived exosomes. J Clin Med. 2021;10(4):711. doi: 10.3390/jcm10040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li X, Li JJ, Yang JY, Wang DS, Zhao W, Song WJ, Li WM, Wang JF, Han W, Zhang ZC, Yu Y, Cao DY, Dou KF. Tolerance in-duction by exosomes from immature dendritic cells and rapamycin in a mouse cardiac allograft model. PLoS ONE. 2012;7(8):e44045. doi: 10.1371/journal.pone.0044045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He JG, Xie QL, Li BB, Zhou L, Yan D. Exosomes derived from IDO1-overexpressing rat bone marrow mesenchymal stem cells promote immunotolerance of cardiac allografts. Cell Transplant. 2018;27(11):1657–1683. doi: 10.1177/0963689718805375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anderson JD, Johansson HJ, Graham CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells. 2016;34(3):601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deng R, Liu Y, He H, et al. Haemin pre-treatment augments the cardiac protection of mesenchymal stem cells by inhibiting mitochondrial fission and improving survival. J Cell Mol Med. 2020;24(1):431–440. doi: 10.1111/jcmm.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng H, Liang X, Han Q, et al. Hemin enhances the cardioprotective effects of mesenchymal stem cell-derived exosomes against infarction via amelioration of cardiomyocyte senescence. J Nanobiotechnology. 2021;19(1):332. doi: 10.1186/s12951-021-01077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gonzalez-King H, García NA, Ontoria-Oviedo I, et al. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells. 2017;35(7):1747–1759. doi: 10.1002/stem.2618. [DOI] [PubMed] [Google Scholar]
- 106.Sun J, Shen H, Shao L, et al. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res Ther. 2020;11(1):373. doi: 10.1186/s13287-020-01881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nguyen-Truong M, Hematti P, Wang Z. Current status of myocardial restoration via the paracrine function of mesenchymal stromal cells. Am J Physiol Heart Circ Physiol. 2021;321(1):H112–H127. doi: 10.1152/ajpheart.00217.2021. [DOI] [PubMed] [Google Scholar]
- 108.Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y, Shen C, Li X, Peng L, Huang D, Zhang J, Zhang S, Wang F, Liu J, Chen L, Chen S, Wang Z, Zhang Z, Cao R, Zhong W, Liu Y, Liu L. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020;6(10):1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Safiabadi Tali SH, LeBlanc JJ, Sadiq Z, Oyewunmi OD, Camargo C, Nikpour B, Armanfard N, Sagan SM, Jahanshahi-Anbuhi S. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 detection. Clin Microbiol Rev. 2021;34(3):e00228–e320. doi: 10.1128/CMR.00228-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Majumder J, Minko T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 2021;23(1):14. doi: 10.1208/s12248-020-00532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jayaramayya K, Mahalaxmi I, Subramaniam MD, Raj N, Dayem AA, Lim KM, Kim SJ, An JY, Lee Y, Choi Y, Raj A, Cho SG, Vellingiri B. Immunomodulatory effect of mesenchymal stem cells and mesenchymal stem-cell-derived exosomes for COVID-19 treatment. BMB Rep. 2020;53(8):400–412. doi: 10.5483/BMBRep.2020.53.8.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lai RC, Tan SS, Yeo RW, Choo AB, Reiner AT, Su Y, Shen Y, Fu Z, Alexander L, Sze SK, Lim SK. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;24(5):29828. doi: 10.3402/jev.v5.29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, Pogosova-Agadjanyan EL, Morrissey C, Stirewalt DL, Hladik F, Yu EY, Higano CS, Tewari M. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A. 2014;111(41):14888–14893. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lai RC, Yeo RW, Tan KH, Lim SK. Mesenchymal stem cell exosome ameliorates reperfusion injury through proteomic complementation. Regen Med. 2013;8(2):197–209. doi: 10.2217/rme.13.4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.