Abstract
Coenzyme Q (CoQ) is important not only as an essential lipid for the mitochondrial electron transport system, but also as an antioxidant. CoQ levels decrease during aging and in various diseases. Orally administered CoQ is not readily taken up in the brain, so it is necessary to develop a method to increase the amount of CoQ in neurons. CoQ is synthesized via mevalonate pathway, like cholesterol. Transferrin, insulin, and progesterone are factors used in the culture of neurons. In this study, we determined the effect of these reagents on cellular CoQ and cholesterol levels. The administration of transferrin, insulin, and progesterone increased cellular CoQ levels in undifferentiated PC12 cells. When serum was removed and only insulin was administered, intracellular CoQ levels increased. This increase was even more pronounced with concurrent administration of transferrin, insulin, and progesterone. Cholesterol level decreased by the administration of transferrin, insulin, and progesterone. Progesterone treatment lowered intracellular cholesterol levels in a concentration-dependent manner. Our findings suggest that transferrin, insulin, and progesterone may be useful in regulating CoQ levels and cholesterol levels, which are products of the mevalonate pathway.
Keywords: coenzyme Q, free cholesterol, transferrin, insulin, progesterone
Introduction
Coenzyme Q (CoQ) is important not only as an essential lipid for the mitochondrial electron transport system to produce ATP, but also as an antioxidant.(1,2) CoQ levels have been reported to decrease with aging and in various diseases, such as Parkinson’s disease.(3–6)
Several other neuronal diseases which are caused by a mutation in CoQ biosynthesis genes, have been reported. Tsuji et al.(7) reported a homozygous mutation in COQ2 in multiple-system atrophy (MSA) patients. They reported that functionally impaired heterozygous COQ2 variants were associated with sporadic MSA. COQ1 is a heterodimer consisting of PDSS1 and PDSS2 proteins. Mutations in the PDSS2 gene cause Leigh syndrome and nephropathy.(8) COQ8 gene mutations are reported to cause progressive neurological disorders with cerebellar atrophy, developmental delay, and hyperlactatemia.(9) In such cases, CoQ10 levels are decreased.
CoQ supplementation as an oral drug has paid attention. In fact, CoQ administration has been reported to be effective in several diseases. For example, in a meta-analysis that reviewed eight randomized controlled trials from one database up to January 2014, CoQ administration in patients undergoing cardiac bypass surgery was associated with a lower rate of inotropic drug use and a lower risk of developing ventricular arrhythmias.(10) In another meta-analysis of three randomized placebo-controlled trials from four databases up to December 2012, CoQ10 administration to infertile men resulted in increased sperm density and motility.(11)
Following its administration, CoQ is primarily taken up by the liver, adrenal gland, and spleen, whereas the levels of CoQ10 taken up by the neurological systems are very low. Yuzuriha et al.(12) reported that following intravenous injection of [14C] CoQ10 into guinea pigs, radioactivity levels were highest in the liver and spleen at 30 min following injection and decreased thereafter. The levels in the blood and kidney peaked at 8 h, whereas those in the heart and brain peaked at 24 h and subsequently decreased. The levels of [14C] CoQ10 in the brain were much lower compared with that in the liver, spleen, and adrenal grand. Bentinger et al.(13) reported that the administration of radioactive CoQ10 ([3H]CoQ) to rats intraperitoneally resulted in its efficient uptake into the circulation and resulted in a high concentration in the spleen, liver, and white blood cells. Lower concentrations were detected in the adrenal glands, ovaries, thymus, and heart whereas essentially no uptake occurred in the kidneys, muscle, or brain. Thus, CoQ is difficult to administer to the brain and a method is needed to increase cellular CoQ10 levels in the nervous system.
CoQ is synthesized intracellularly, and elucidation of the mechanisms regulating CoQ biosynthesis will contribute to the regulation of intracellular CoQ levels. CoQ is produced by the mevalonate pathway.(14,15) The mevalonate pathway also produces cholesterol.(16) Cholesterol homeostasis is essential for cellular function and metabolism. The brain has the highest amount of cholesterol.(17) Approximately 70–80% of cholesterol in the adult brain is partially present in the plasma membrane of neurons and astrocytes, where it influences cell morphology, stabilizes cell surface receptors, and modulates synaptic transmission. Cholesterol is also essential for neurite outgrowth, synapse formation, and the formation of new membranes required for neurotransmitter release, and plays an important role in neuronal differentiation and maturation.(18–20)
We previously evaluated the concentration of CoQ before and after neuronal differentiation. We found that treatment of PC12 cells with nerve growth factor (NGF) significantly increased intracellular CoQ levels during neurite outgrowth and neuronal differentiation.(21) Furthermore, we found that the inhibition of CoQ biosynthesis impaired neurite elongation.(21) Several reports have indicated that a serum-free medium containing transferrin, insulin, and progesterone (TIP) are useful for culturing cells from a variety of nervous system tissues.(22,23) In fact, many commercial culture media additives for neuronal and neural stem cell cultures contain TIP. For example, Thermo Fisher Scientific offers a neuronal cell culture supplement, B-27™ Supplement, which contains TIP. TIP is also contained in the culture medium additives, N2-MAX and N21-MAX Media Supplement, from R and D systems for growing nerve cells.
In this study, we determined the effect of TIP on cellular CoQ and free cholesterol (FC) levels.
Materials and Methods
Cell culture
PC12 established from a rat adrenal medullary tumor (pheochromocytoma) were grown in DMEM/F-12 containing 10% horse serum, 5% fetal bovine serum, and 1% penicillin/streptomycin at 37°C in a 5% CO2 incubator.(21) The cells were seeded at 1 × 105 cells/ml on collagen-coated plates and incubated for 72 h. The culture medium was changed with serum-free DMEM/F-12 medium containing TIP and incubated for 48 h. The medium was changed again and after a 48-h incubation, the cells were collected and analyzed for lipid content and gene expression. The levels of CoQ and FC following TIP treatment were also measured after 2, 4, and 6 days of TIP treatment.
Neurite length measurement
As reported previously, cultured cells were observed at 100× magnification with an inverted phase contrast microscope (#CKX41; OLYMPUS, Tokyo, Japan), photographed with a camera (#IX71; OLYMPUS),(21) and printed. The length of the neurites in the printed picture was measured with a ruler. For the measurement changes in neurite length over time, we used the photographs taken after 4 days of NGF and TIP treatment. For the NGF-treated group, 221 ± 40 neurites were present in the photographs. For the NGF + TIP-treated groups, 217 ± 9 neurite were included in each image. We measured the photographs in triplicate. The results are expressed relative to the NGF-treated group.
Lipid analysis
As reported previously, CoQ and FC levels in cells were analyzed using HPLC-UV, ECD.(21,24–26) Briefly, cells were collected in isopropanol, centrifuged, and the resulting supernatant was analyzed HPLC. Two separation columns (Ascentis® C8, 5 μm, 250 mm × 4.6 mm i.d. and SupelcosilTM LC-18, 3 μm, 5 cm × 4.6 mm i.d.; Supelco Japan, Tokyo, Japan) and a reduction column (RC-10, 15 mm × 4 mm i.d.; IRICA, Kyoto, Japan) were used. The samples were detected by UV and ECD. The mobile phase for the separation was 50 mM NaClO4 in methanol/isopropanol (85/15, v/v) and was run at a flow rate of 0.8 ml/min. The columns were maintained at 25°C.
Quantitative reverse transcription-PCR
Total RNA was prepared from cultured PC12 cells using TRIzol reagent. cDNA was synthesized by reverse transcription using QuantiTect Reverse Transcription Kit (QIAGEN, Venlo, The Netherlands). The PCR primer sequences are shown in Table 1. Quantitative PCR was conducted on a QuantStudio® 5 (Thermo Fisher Scientific) instrument as follows: 95°C for 2 min followed by 40 cycles of 95°C for 5 s and 60°C for 30 s, with a final extension step of 60°C for 1 min. mRNA expression was calculated using the 2−ΔΔCt method.(26,27)
Table 1.
Primer used in real time PCR analysis
| Gene | Forward Sequence (5'-3') | Reverse Sequence (5'-3') |
|---|---|---|
| GAPDH | GTTACCAGGGCTGCCTTCTC | GATGGTGATGGGTTTCCCGT |
| RPL29 | TTGCCAAGAAGCACAACAAG | GGCATCTTGGGCTTGACA |
| PDSS1 | GAAAGGTTTGCCCACTACCT | CATCTGGTCAGAACATGAGGTG |
| PDSS2 | CTTCAGATCTCTCGACACCATC | CAGTGGTAAGCAGTGGGTG |
| coq2 | GATGATGCTCTGATTGGCCT | GGTGTAAATCTGGTGAGCCA |
| coq3 | GGATGAAGATTCTCGACGTTGG | CTCATTCAAGGTCTCCTCCAG |
| coq4 | CGGAGAAGTTGTGGTAAAGTGG | CTCCCAACGCTGTTCATAGTAG |
| coq5 | AGTACCAGAGTAAAGAGGACCC | TGACATTCCGGATCCCAAAG |
| coq6 | CTGCTCAGAGGCCTTGATAATG | CCATCACCTAGGGTAATATGGACC |
| coq7 | CCTCAGGAATCACTTTTGGCTG | GGAATGTCCTATGTAGACCAGG |
| coq8 | GATCTGTCAGAGTGGAGACGTA | CTATGGGGGTCTGTTGCATT |
| coq9 | AGAACTGTTCTCTAGGAGTGGG | CACTATGTGTTGCCTTTGGACC |
| FDFT1 | AGCCACAAGGATGGAGTTCG | GAGTTCCGGTCCATCTTGGG |
| HMGCR | GCTCAGGGTAATCACTTGCT | TAGGCCTGGTTCTTGTTCAC |
| GSS | CTCCAGGGGCTTTAGGGAAG | TTGCCTCAAAGGAGCTTCCA |
| Catalase | TCAGCGTTTGGTGGAGAA | GCCTGGCTCATCTTTATC |
| SOD1 | CCAGTTGTGGTGTCAGGACA | CTCTCTTCATCCGCTGGACC |
| SOD2 | TTCTGGACAAACCTGAGCCC | CCTGAACCTTGGACTCCCAC |
Establishment of CoQ-deficient cells
To decrease CoQ levels in PC12 cells, 4-nitrobenzoate (4-NB), a well-known CoQ biosynthesis inhibitor, was used.(28,29) PC12 cells were cultured with 4-NB that was dissolved in DMSO, and the same volume of DMSO was added to the control cell line. 4-NB was administered for 2 days or 6 months.
Statistical analysis
All results are presented as means and SD. Statistical significance was determined by a Student’s t test and a one-way analysis of variance (ANOVA). Statistical analysis was performed using BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan).
Results
Effect of TIP on cellular CoQ levels
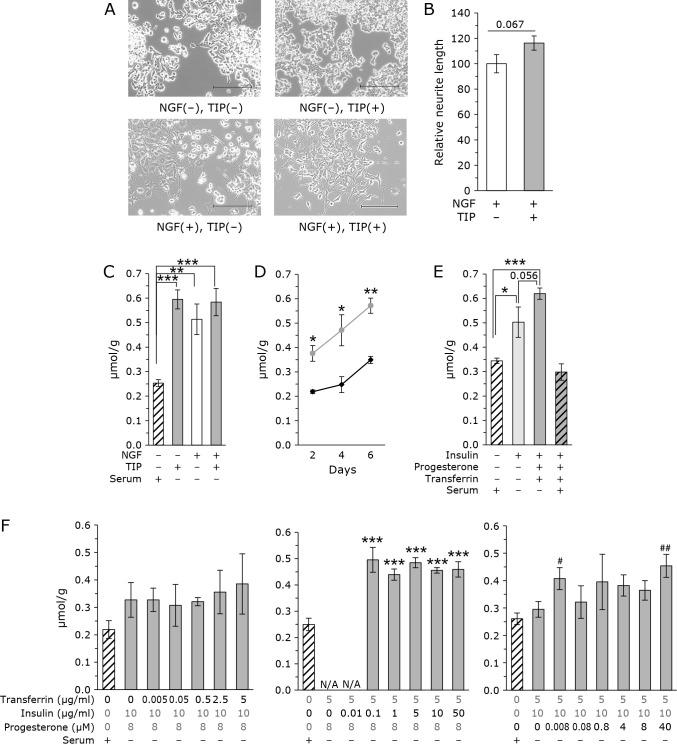
As shown in Fig. 1A, the addition of TIP did not affect cell morphology in PC12 cells in the absence of NGF; however, the addition of NGF significantly affected cell morphology. After administration of NGF, the cells extended into neurites. Based on the cell shape, PC12 cells were considered differentiated into neural cells. NGF-treated cells exhibited enhanced neurite outgrowth following treatment with TIP. Neurite length was measured and as shown in Fig. 1B, the neurites were longer in TIP-treated cells compared with that in TIP untreated cells. PC12 cells contain CoQ9 because it is derived from rats. Figure 1C shows the CoQ levels measured in control and TIP-treated cells. Cellular CoQ levels were significantly increased following the administration of TIP in undifferentiated PC12 cells. CoQ levels increased following NGF treatment as we reported previously.(21) Administration of TIP to NGF-treated differentiated cells increased the average cell CoQ levels, but no significant increase was observed. These results indicate that the addition of TIP increases the levels of CoQ in undifferentiated PC12 cells.
Fig. 1.
Addition of TIP increases cellular CoQ levels in PC12 cells. (A) Microscopic photographs of PC12 cells treated with and without TIP and NGF. Scale bar = 200 μm. The concentration of NGF was 20 ng/ml. TIP consisted of 5 μg/ml transferrin, 10 μg/ml insulin, and 8 μM progesterone. (B) Administration of TIP enhanced the length of neuronal elongation. The concentration of NGF was 20 ng/ml. TIP consisted of 5 μg/ml transferrin, 10 μg/ml insulin, and 8 μM progesterone. The data were analyzed using Student’s t test are expressed as means ± SD (n = 3). (C) CoQ levels corrected for protein in PC12 cells. The concentration of NGF was 20 ng/ml. TIP consisted of 5 μg/ml transferrin, 10 μg/ml insulin, and 8 μM progesterone. The data were analyzed using a one-way ANOVA and are expressed as means ± SD (n = 3). **p<0.01 and ***p<0.001 compared with NGF (−), TIP (−), and serum (+) group. (D) Time course of CoQ levels normalized to protein levels in PC12 cells treated without (black line) or with (gray line) TIP (5 μg/ml transferrin, 10 μg/ml insulin, and 8 μM progesterone). The data were analyzed using a Student’s t test and are expressed as means ± SD (n = 3). *p<0.05 and **p<0.01 compared with and without TIP. (E) Measurement of CoQ levels in the presence insulin. In some experiments, transferrin and progesterone were also administrated. CoQ levels corrected for protein. The data were analyzed using a one-way ANOVA and are expressed as means ± SD (n = 3). *p<0.05 and ***p<0.001 compared with insulin (−). (F) Analysis of the concentration dependence of each factor of TIP. Concentration of 1 factor was changed in the presence of other 2 factors. CoQ levels corrected for protein. White bar: control cells cultured with serum without TIP. Gray bar: Cells cultured without serum with various concentration of TIP. The data were analyzed using a one-way ANOVA and are expressed as means ± SD (n = 3). ***p<0.001 compared with 0 μg/ml transferrin, 0 μg/ml insulin, and 0 μM progesterone. #p<0.05, ##p<0.01 compared with 5 μg/ml transferrin, 10 μg/ml insulin, and 0 μM progesterone.
Next, we analyzed the time-dependent changes in cellular CoQ levels after the addition of TIP in undifferentiated PC12 cells. As shown in Fig. 1C, the CoQ levels increased with time in both control and TIP-treated cells. The levels of CoQ were higher in TIP-treated cells at all time points.
Determination of which factor is important for increasing cellular CoQ levels
To identify which factor increase CoQ levels, we analyzed the change in CoQ levels when only one type of factor was administered. However, when serum was removed and progesterone alone was administered, cells died and could not be analyzed. Cells also died when transferrin alone was administered. When only insulin was administered, cells survived and CoQ levels could be analyzed. As shown in Fig. 1E, insulin-only treatment increased cellular CoQ levels. Interestingly, cellular CoQ levels were tended to be higher when the three were administered simultaneously. Unexpectedly, the effect of TIP was ameliorated when serum was administered at the same time as TIP.
Next, to analyze the concentration dependence of each factor, we analyzed cellular CoQ levels by varying the concentration of only one factor. As shown in Fig. 1F, when the concentration of transferrin was changed, cellular CoQ levels did not change. We failed to evaluate the concentration of insulin at 0 and 0.01 μg/ml, because the cells cannot survive at lower concentrations of insulin without serum. Insulin increased CoQ levels significantly; however, further increases in insulin did not increase CoQ levels. For progesterone, there was a trend toward a concentration-dependent increase in CoQ levels. High progesterone resulted in higher CoQ levels compared with untreated cells.
Effect of TIP on cellular FC levels
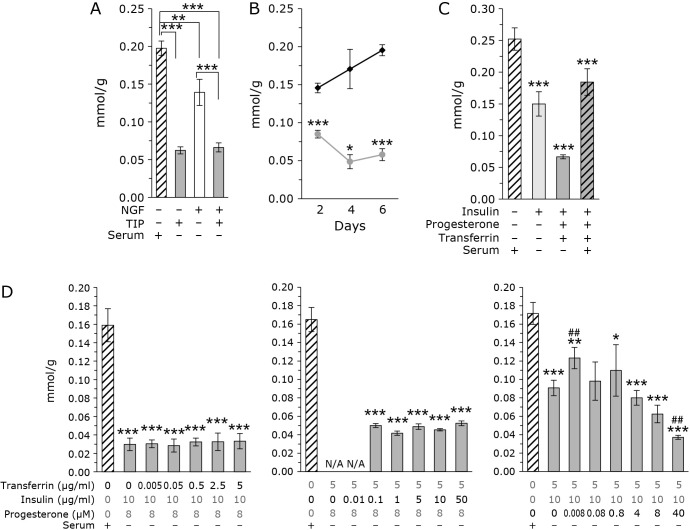
FC is also produced by mevalonate pathway as CoQ. The effect of TIP on the cellular level of FC is studied. Figure 2A shows FC levels in the presence and absence of TIP. FC levels were reduced in the presence of TIP, with and without NGF. Figure 2B shows the time-dependent changes in cellular FC levels. FC levels in TIP-treated cells were lower compared with that in control cells at all time points. FC levels increased with time in control cells, and decreased in TIP-treated cells.
Fig. 2.
Addition of TIP decrease cellular FC levels in PC12 cells. (A) FC levels corrected for protein in PC12 cells. The concentration of NGF was 20 ng/ml. TIP consisted of 5 μg/ml transferrin, 10 μg/ml insulin, and 8 μM progesterone. The data were analyzed using a one-way ANOVA and are expressed as means ± SD (n = 3). (B) Time course of FC levels normalized to protein levels in PC12 cells treated without (black line) or with (gray line) TIP (5 μg/ml transferrin, 10 μg/ml insulin, and 8 μM progesterone). The data were analyzed using a Student’s t test and are expressed as means ± SD (n = 3). *p<0.05 and ***p<0.001 compared with and without TIP. (C) Measurement of FC levels in the presence insulin. In some experiments, transferrin and progesterone were also administrated. FC levels corrected for protein. The data were analyzed using a one-way ANOVA and are expressed as means ± SD (n = 3). ***p<0.001 compared with insulin (−). (D) Analysis of the concentration dependence of each factor of TIP. Concentration of 1 factor was changed in the presence of other 2 factors. FC levels corrected for protein. White bar: control cells cultured with serum without TIP. Gray bar: Cells cultured without serum with various concentration of TIP. The data were analyzed using a one-way ANOVA and are expressed as means ± SD (n = 3). *p<0.05, **p<0.01, and ***p<0.001 compared with 0 μg/ml transferrin, 0 μg/ml insulin, and 0 μM progesterone. #p<0.05, ##p<0.01 compared with 5 μg/ml transferrin, 10 μg/ml insulin, and 0 μM progesterone.
Determination of which factor is important for decreasing cellular FC levels
As shown in Fig. 2C, insulin administration reduced FC levels. The addition of progesterone and transferrin further accelerated this decrease. The addition of serum suppressed the decrease in FC levels. As shown in Fig. 2D, FC levels decreased in a progesterone concentration-dependent manner. The decrease in FC levels may result from the addition of progesterone; however, it should be noted that even at a progesterone concentration of 0 μM, a decrease in FC levels was observed. Taken together, these results indicate that insulin and transferrin exhibit an FC-lowering effect, but progesterone has a concentration-dependent effect on FC levels.
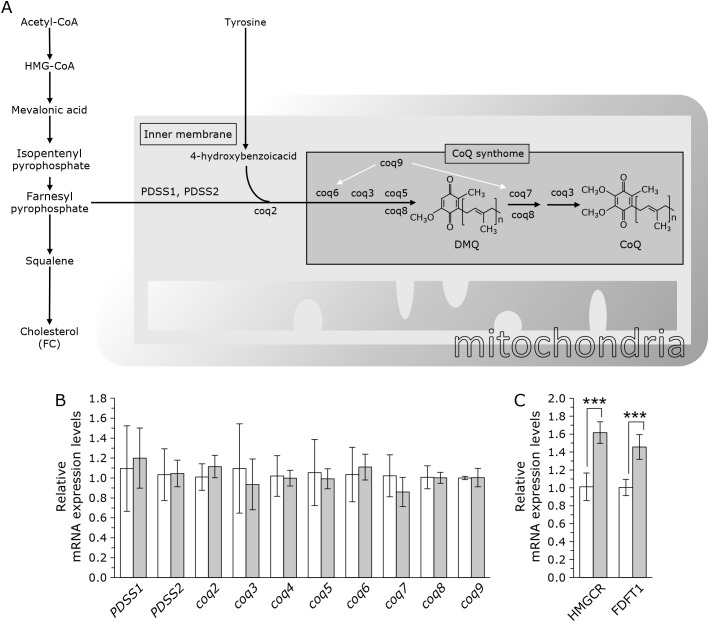
mRNA expression of CoQ and FC biosynthesis enzymes
To elucidate the mechanism of elevated CoQ levels, we analyzed the gene expression of the CoQ synthase gene. Figure 3A illustrates the reported mechanisms for the biosynthesis CoQ. CoQ levels were increased following the administration of TIP; however, FC levels did not increase. Therefore, we measured the expression CoQ synthesis genes after the junction of CoQ synthesis and cholesterol synthesis. As shown in Fig. 3B, the expression levels of these genes were not significantly altered by TIP treatment of PC12 cells. Thus, the increased CoQ levels following the addition of TIP may not be explained simply by change in gene expression. We also analyzed mRNA expressions of FC synthesis genes, HMG-CoA reductase (HMGCR) and farnesyl-diphosphate farnesyltransferase 1 (FDFT1). HMGCR is the rate-limiting enzyme in the mevalonate pathway. FDFT1 is the branch point enzyme between CoQ and cholesterol. As shown in Fig. 3C, the expressions of these genes are upregulated. Therefore, the decreased FC levels following the addition of TIP also may not be explained simply by change in gene expression.
Fig. 3.
Outline of the CoQ synthesis pathway and the expression levels of each gene. (A) Illustrated schemes of the CoQ and cholesterol biosynthetic pathways. The CoQ and cholesterol synthesis pathways share some similarities. (B) The relative mRNA expression levels of coq1 to coq9 were normalized to GAPDH expression. White bar: control, gray bar: with TIP (5 μg/ml transferrin, 10 μg/ml insulin, and 8 μM progesterone). Values are presented as the mean ± SD (n = 3) of the data obtained from three independent experiments. (C) The relative mRNA expression levels of HMGCR and FDFT1 were normalized to GAPDH expression. White bar: control, gray bar: with TIP (5 μg/ml transferrin, 10 μg/ml insulin, and 8 μM progesterone). The data were analyzed using a Student’s t test. The data were analyzed using a Student’s t test and are expressed as means ± SD (n = 6).
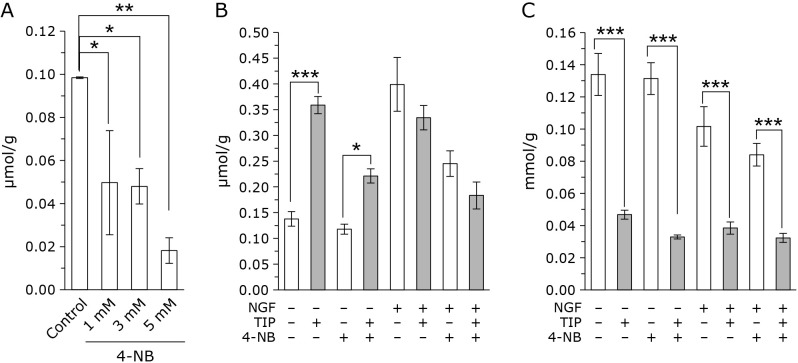
Effect of TIP on 4-NB treated CoQ deficient cells
We next determined whether TIP increases cellular CoQ levels when CoQ synthesis is partially inhibited. The CoQ synthesis inhibitor 4-NB was used to inhibit the synthesis of CoQ. As shown in Fig. 4A, cellular CoQ level decreased by the administration of 4-NB dose dependently. Result shown in Fig. 4A is obtained with 2 day 4-NB treated cells. Administration of 4-NB for longer time (for several months), cells with 5 mM 4-NB died. Therefore, we used 1 mM 4-NB treated cell samples. As shown in Fig. 4B, the addition of TIP to 4-NB-treated for 6 months cell also increased cellular CoQ levels, but this increase was lower compared with that in the control cells. In NGF-treated differentiated cells, 4-NB-treatment reduced cellular CoQ levels. Similar to the control cells, the administration of TIP to 4-NB-treated cells did not increase intracellular CoQ levels in the presence of NGF. FC levels were reduced in both control and 4-NB treated cells following the administration of TIP (Fig. 4C).
Fig. 4.
TIP increases cellular CoQ levels and decreases FC levels in 4-NB-treated PC12 cells. (A) CoQ levels in PC12 cells corrected for protein in the presence of 4-NB (1 mM, 3 mM, and 5 mM) for 2 days. (B) CoQ levels corrected for protein in PC12 cells in the presence and absence of 1 mM 4-NB for 6 months. (C) FC levels corrected for protein in PC12 cells in the presence and absence of 1 mM 4-NB for 6 months. The data were analyzed using a one-way ANOVA and are expressed as means ± SD (n = 3). *p<0.05, **p<0.01, and ***p<0.001 compared with NGF (−), TIP (−), and 4-NB (−) group.
Effect of TIP on antioxidative enzymes
Since TIP treatment increased cellular level of CoQ, which is an important antioxidant, we analyzed the level of antioxidative enzymes. As shown in Fig. 5, mRNA expression level of glutathione synthetase (GSS) does not altered by the administration of TIP. Levels of catalase and superoxide dismutase (SOD) also does not altered.
Fig. 5.
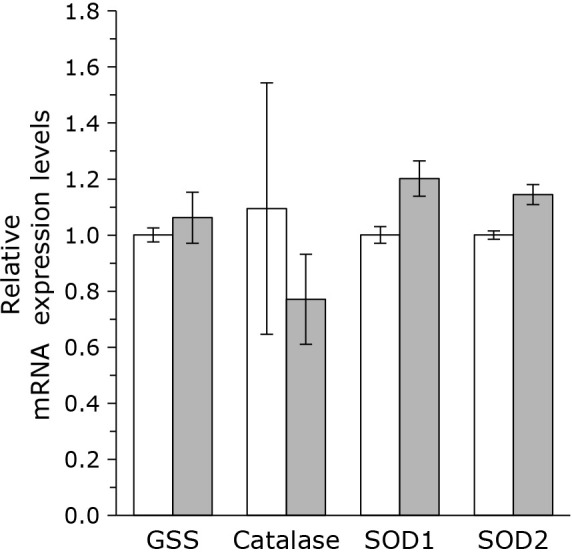
The relative mRNA expression levels of GSS, Catalase, SOD1, and SOD2 reductase were normalized to RPL29 expression. White bar: control, gray bar: with TIP (5 μg/ml transferrin, 10 μg/ml insulin, and 8 μM progesterone). The data are expressed as means ± SD (n = 3).
Discussion
As indicated above, the administration of TIP to undifferentiated PC12 cells increased cellular CoQ and decreased FC levels. CoQ is important to the mitochondrial electron transport system and as an antioxidant. We also previously reported that neurite outgrowth is suppressed by CoQ deficiency.(21) This study shows that administration of TIP increased intracellular CoQ levels. At this time, the neurite outgrowth is increased. Although a more detailed analysis will be done in the future, we found that the administration of TIP increased cellular CoQ levels and increased neurite outgrowth. TIP is added to media for culturing nerve cells and exerts a variety of effects. In the present study, we found that TIP maintains intracellular CoQ and FC levels, which may be one of its key effects.
Several physiological stimuli are known to increase intracellular CoQ levels such as, cold stress.(30) Endurance exercise training reportedly increases CoQ content in red quadriceps, soleus muscles, and adipose tissues.(31) Oxidative stress also increases intracellular CoQ levels.(32–34) Calorie restriction has reported to influence the balance of endogenous CoQ. Long-term calorie restriction increases CoQ in mitochondria from skeletal muscle,(31) liver, heart, and kidney.(35) The administration of dietary omega-3 unsaturated fatty acids increases intracellular CoQ levels.(36) TIP was also found to increase CoQ. TIP contains of protein, peptide hormone, and steroid hormone, the safety of which is assured. Therefore, it may be applied for the treatment of elevated CoQ levels.
The administration of TIP increased the amount of CoQ, which is an antioxidant, but there was no significant change in the expression levels of other antioxidant enzyme-related genes. Moreover, it did not affect the expression levels of enzymes involved in glutathione biosynthesis.
TIP not only increased the intercellular levels of CoQ, but also suppressed the levels of FC in undifferentiated PC12 cells. Progesterone alone suppressed the levels of FC in a dose dependent manner. Metherall et al.(37) previously reported that the administration of progesterone to CHO-7 cells reduced cellular cholesterol levels and increased lanosterol accumulation.(37,38) They also reported that effect of progesterone on reducing cholesterol was observed in HepG2, CHO, Hela, and Caco-2 cells.(37)
An increase in the CoQ levels was observed following the removal of serum and insulin treatment alone. In addition, the administration of progesterone and transferrin tended to promote the insulin-dependent CoQ increase. We examined the putative mechanism of CoQ increase by TIP. A synthesis pathway for CoQ (Fig. 4A) has been proposed.(39) Because TIP increased CoQ levels and decreased FC levels, we focused on CoQ synthase, which functions after the junction between CoQ synthesis and cholesterol synthesis. Specifically, we evaluated PDSS1, PDSS2, and coq2~9. The administration of TIP did not change the expression levels of these CoQ synthetic genes. This suggests that mRNA expression remains the same, whereas the protein content fluctuates or that the protein content also remains the same, but metabolism fluctuates. For the latter, the following hypotheses are possible. Insulin promotes the mevalonate pathway,(40) which may be the result of HMGCR activation. The product, cholesterol, causes negative feedback that suppresses the mevalonate pathway.(41,42) Progesterone inhibits cholesterol synthesis at the stage after lanosterol,(37,38,43) thus suppressing the cholesterol-induced negative feedback loop. As a result, it is possible that the insulin-mediated activation of the mevalonate pathway could further increase CoQ levels. Another possibility is based on recent studies suggesting that CoQ synthase forms a complex called CoQ-synthome, which efficiently synthesizes CoQ.(44) TIP may influence the formation of CoQ-synthome. These possibilities should be studied further.
As shown above, administration of TIP caused changes in CoQ and FC levels, which are products of the mevalonate pathway. Even when serum was removed and only insulin was administered, an increase in CoQ and a decrease in FC were observed, albeit weaker than when TIP was administered. Whether this was due to the effect of insulin administration or the effect of serum removal requires further investigation. Removal of serum from the medium is an important stimulus for neuronal differentiation. For example, N1E-115 cells show neurite outgrowth when serum is removed from the medium.(21,45) Although it is difficult to examine the effect of serum removal alone on PC12 cells used in this experiment because the cells die when the serum is removed, clarification of the effect of serum removal on intracellular CoQ and FC levels is expected to be an issue for future investigation.
The levels of CoQ decrease with age;(3) however, the mechanism is unknown. Also the amount and effects of insulin change with age.(46) Signal transduction following insulin receptor activation decreases with age and progesterone levels fluctuate significantly with aging and menopause,(47–49) especially in women. It has also been reported that transferrin receptors decrease with age.(50) Taken together, these effects may contribute to age-related changes in CoQ levels.
Among the pathological conditions associated with CoQ synthase deficiency, many neurological diseases have been reported.(7–9) CoQ administration may be beneficial for these diseases, but orally administered CoQ is not taken up readily by the brain.(12) Therefore, it is necessary to develop methods to increase CoQ levels in cells by means other than oral supplements. Although we performed in vitro experiments at the level of cultured cells, the administration of TIP increases CoQ levels, even in 4-NB-treated cells. In the future, we will determine whether these effects occur in vivo and anticipate that the administration of TIP will lead to new treatments that increase CoQ levels.
Author Contributions
MK, ST, YY, and AF conceived the project and designed the experiments. AN, YA, MO, AM, AN, and KK performed the experiments. MK, AN, and ST wrote the paper. MK coordinated and directed the project.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 22K11713. The authors would like to thank Enago (www.enago.jp) for the English language review. We thank Ms. S. Sakurai and Ms. H. Jiang for their technical supports.
Abbreviations
- CoQ
coenzyme Q
- FC
free cholesterol
- FDFT1
farnesyl-diphosphate farnesyltransferase 1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GSS
glutathione synthetase
- HMGCR
HMG-CoA reductase
- MSA
multiple-system atrophy
- 4-NB
4-nitrobenzoate
- NGF
nerve growth factor
- SOD
superoxide dismutase
- TIP
transferrin, insulin and progesterone
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Crane FL, Hatefi Y, Lester RL, Widmer C. Isolation of a quinone from beef heart mitochondria. Biochim Biophys Acta 1957; 25: 220–221. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto Y, Komuro E, Niki E. Antioxidant activity of ubiquinol in solution and phosphatidylcholine liposome. J Nutr Sci Vitaminol (Tokyo) 1990; 36: 505–511. [DOI] [PubMed] [Google Scholar]
- 3.Kalén A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989; 24: 579–584. [DOI] [PubMed] [Google Scholar]
- 4.Mischley LK, Allen J, Bradley R. Coenzyme Q10 deficiency in patients with Parkinson’s disease. J Neurol Sci 2012; 318: 72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shults CW, Haas RH, Passov D, Beal MF. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann Neurol 1997; 42: 261–264. [DOI] [PubMed] [Google Scholar]
- 6.Hargreaves IP, Lane A, Sleiman PM. The coenzyme Q10 status of the brain regions of Parkinson’s disease patients. Neurosci Lett 2008; 447: 17–19. [DOI] [PubMed] [Google Scholar]
- 7.Multiple-System Atrophy Research Collaboration. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med 2013; 369: 233–244. [DOI] [PubMed] [Google Scholar]
- 8.López LC, Schuelke M, Quinzii CM, et al. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet 2006; 79: 1125–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagier-Tourenne C, Tazir M, López LC, et al. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am J Hum Genet 2008; 82: 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Frutos F, Gea A, Hernandez-Estefania R, Rabago G. Prophylactic treatment with coenzyme Q10 in patients undergoing cardiac surgery: could an antioxidant reduce complications? A systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2015; 20: 254–259. [DOI] [PubMed] [Google Scholar]
- 11.Lafuente R, González-Comadrán M, Solà I, et al. Coenzyme Q10 and male infertility: a meta-analysis. J Assist Reprod Genet 2013; 30: 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuzuriha T, Takada M, Katayama K. Transport of [14C]coenzyme Q10 from the liver to other tissues after intravenous administration to guinea pigs. Biochim Biophys Acta 1983; 759: 286–291. [DOI] [PubMed] [Google Scholar]
- 13.Bentinger M, Dallner G, Chojnacki T, Swiezewska E. Distribution and breakdown of labeled coenzyme Q10 in rat. Free Radic Biol Med 2003; 34: 563–575. [DOI] [PubMed] [Google Scholar]
- 14.Bentinger M, Tekle M, Dallner G. Coenzyme Q—biosynthesis and functions. Biochem Biophys Res Commun 2010; 396: 74–79. [DOI] [PubMed] [Google Scholar]
- 15.Turunen M, Swiezewska E, Chojnacki T, Sindelar P, Dallner G. Regulatory aspects of coenzyme Q metabolism. Free Radic Res 2002; 36: 437–443. [DOI] [PubMed] [Google Scholar]
- 16.Moutinho M, Nunes MJ, Rodrigues E. The mevalonate pathway in neurons: it’s not just about cholesterol. Exp Cell Res 2017; 360: 55–60. [DOI] [PubMed] [Google Scholar]
- 17.Colardo M, Petraroia M, Lerza L, et al. NGF modulates cholesterol metabolism and stimulates ApoE secretion in glial cells conferring neuroprotection against oxidative stress. Int J Mol Sci 2022; 23: 4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci 2005; 29: 190–201. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki S, Kiyosue K, Hazama S, et al. Brain-derived neurotrophic factor regulates cholesterol metabolism for synapse development. J Neurosci 2007; 27: 6417–6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takamori S, Holt M, Stenius K, et al. Molecular anatomy of a trafficking organelle. Cell 2006; 127: 831–846. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura A, Okamoto M, Maeda A, et al. Cellular level of coenzyme Q increases with neuronal differentiation, playing an important role in neural elongations. J Clin Biochem Nutr 2022; 71: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messer A, Mazurkiewicz JE, Maskin P. Growth of dissociated rat cerebellar cells using serum-free supplemented media and varied transferrin concentrations. Cell Mol Neurobiol 1981; 1: 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A 1979; 76: 514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagase M, Yamamoto Y, Mitsui J, Tsuji S. Simultaneous detection of reduced and oxidized forms of coenzyme Q10 in human cerebral spinal fluid as a potential marker of oxidative stress. J Clin Biochem Nutr 2018; 63: 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto M, Nakamura A, Maeda A, et al. Coenzyme Q10 levels increase with embryonic development in medaka. J Clin Biochem Nutr 2022; 70: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto M, Shimogishi M, Nakamura A, et al. Differentiation of THP-1 monocytes to macrophages increased mitochondrial DNA copy number but did not increase expression of mitochondrial respiratory proteins or mitochondrial transcription factor A. Arch Biochem Biophys 2021; 710: 108988. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 28.Forsman U, Sjöberg M, Turunen M, Sindelar PJ. 4-Nitrobenzoate inhibits coenzyme Q biosynthesis in mammalian cell cultures. Nat Chem Biol 2010; 6: 515–517. [DOI] [PubMed] [Google Scholar]
- 29.Quinzii CM, Tadesse S, Naini A, Hirano M. Effects of inhibiting CoQ10 biosynthesis with 4-nitrobenzoate in human fibroblasts. PLoS One 2012; 7: e30606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aithal HN, Joshi VC, Ramasarma T. Effect of cold exposure on the metabolism of ubiquinone in the rat. Biochim Biophys Acta 1968; 162: 66–72. [DOI] [PubMed] [Google Scholar]
- 31.Gohil K, Rothfuss L, Lang J, Packer L. Effect of exercise training on tissue vitamin E and ubiquinone content. J Appl Physiol (1985) 1987; 63: 1638–1641. [DOI] [PubMed] [Google Scholar]
- 32.Gorman A, McGowan A, Cotter TG. Role of peroxide and superoxide anion during tumour cell apoptosis. FEBS Lett 1997; 404: 27–33. [DOI] [PubMed] [Google Scholar]
- 33.Brea-Calvo G, Rodríguez-Hernández A, Fernández-Ayala DJ, Navas P, Sánchez-Alcázar JA. Chemotherapy induces an increase in coenzyme Q10 levels in cancer cell lines. Free Radic Biol Med 2006; 40: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 34.Brea-Calvo G, Siendones E, Sánchez-Alcázar JA, de Cabo R, Navas P. Cell survival from chemotherapy depends on NF-kappaB transcriptional up-regulation of coenzyme Q biosynthesis. PLoS One 2009; 4: e5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamzalov S, Sohal RS. Effect of age and caloric restriction on coenzyme Q and alpha-tocopherol levels in the rat. Exp Gerontol 2004; 39: 1199–1205. [DOI] [PubMed] [Google Scholar]
- 36.Fernández-Del-Río L, Rodríguez-López S, Gutiérrez-Casado E, et al. Regulation of hepatic coenzyme Q biosynthesis by dietary omega-3 polyunsaturated fatty acids. Redox Biol 2021; 46: 102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metherall JE, Waugh K, Li H. Progesterone inhibits cholesterol biosynthesis in cultured cells. Accumulation of cholesterol precursors. J Biol Chem 1996; 271: 2627–2633. [DOI] [PubMed] [Google Scholar]
- 38.Metherall JE, Li H, Waugh K. Role of multidrug resistance P-glycoproteins in cholesterol biosynthesis. J Biol Chem 1996; 271: 2634–2640. [DOI] [PubMed] [Google Scholar]
- 39.Kawamukai M. Biosynthesis and bioproduction of coenzyme Q10 by yeasts and other organisms. Biotechnol Appl Biochem 2009; 53 (Pt 4): 217–226. [DOI] [PubMed] [Google Scholar]
- 40.Xiao X, Luo Y, Peng D. Updated understanding of the crosstalk between glucose/insulin and cholesterol metabolism. Front Cardiovasc Med 2022; 9: 879355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990; 343: 425–430. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein JL, Rawson RB, Brown MS. Mutant mammalian cells as tools to delineate the sterol regulatory element-binding protein pathway for feedback regulation of lipid synthesis. Arch Biochem Biophys 2002; 397: 139–148. [DOI] [PubMed] [Google Scholar]
- 43.Ono T, Imai Y. Effects of steroid hormones on synthesis of cholesterol in vitro. J Biochem 1971; 70: 45–54. [DOI] [PubMed] [Google Scholar]
- 44.Acosta MJ, Vazquez Fonseca L, Desbats MA, et al. Coenzyme Q biosynthesis in health and disease. Biochim Biophys Acta 2016; 1857: 1079–1085. [DOI] [PubMed] [Google Scholar]
- 45.Richelson E. Regulation of tyrosine hydroxylase activity in mouse neuroblastoma clone N1E-115. J Neurochem 1973; 21: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 46.Møller N, Gormsen L, Fuglsang J, Gjedsted J. Effects of ageing on insulin secretion and action. Horm Res 2003; 60 (Suppl 1): 102–104. [DOI] [PubMed] [Google Scholar]
- 47.Couet C, Delarue J, Constans T, Lamisse F. Age-related insulin resistance: a review. Horm Res 1992; 38: 46–50. [DOI] [PubMed] [Google Scholar]
- 48.Elahi D, Muller DC, McAloon-Dyke M, Tobin JD, Andres R. The effect of age on insulin response and glucose utilization during four hyperglycemic plateaus. Exp Gerontol 1993; 28: 393–409. [DOI] [PubMed] [Google Scholar]
- 49.Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev 1998; 19: 397–428. [DOI] [PubMed] [Google Scholar]
- 50.Moos T, Oates PS, Morgan EH. Expression of the neuronal transferrin receptor is age dependent and susceptible to iron deficiency. J Comp Neurol 1998; 398: 420–430. [PubMed] [Google Scholar]