Abstract
Second-hand smoke exposure is an established cause of several adverse health effects. Tobacco smoke exposure in the environment has been improved by the WHO Framework Convention on Tobacco Control. However, concerns have been raised regarding the health effects of heated tobacco products. Analysis of tobacco smoke biomarkers is critical for assessing the health effects of second-hand tobacco smoke exposure. In this study, nicotine metabolites (nicotine, cotinine, trans-3'-hydroxycotinine) and carcinogenic 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol were analysed in the urine of non-smokers with or without passive exposure to cigarettes and heated tobacco products. In addition, 7-methylguanine and 8-hydroxy-2'-deoxyguanosine were simultaneously measured as DNA damage markers. The results revealed higher levels of urinary nicotine metabolites and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in participants exposed to second-hand tobacco smoke (both cigarettes and heated tobacco products) at home. In addition, the urinary levels of 7-methylguanine and 8-hydroxy-2'-deoxyguanosine tended to be higher in the second-hand tobacco smoke-exposed group. The urinary levels of nicotine metabolites and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol were high in workplaces with no protection against passive smoking. These biomarkers will be useful for evaluating passive exposure to tobacco products.
Keywords: second-hand smoke, passive exposure of tobacco products, heated tobacco products, urinary biomarker, DNA damage
Introduction
Exposure to second-hand smoke (SHS) has been established as a cause of adverse health effects, resulting in 1.2 million deaths annually.(1) Tobacco and tobacco smoke contain more than 9,500 chemicals, including 83 carcinogens.(2) The International Agency for Research on Cancer classified SHS as a Group 1 human carcinogen.(3,4) As a result of a meta-analysis in Japan, SHS exposure at home has been implicated in the statistically significant increase in the risk of lung cancer.(5) Additionally, the revised Health Promotion Act, which requires all workplaces to eliminate SHS by introducing smoke-free spaces inside the building or implementing designated smoking rooms came into effect in 2020 in Japan. In general, precisely evaluating exposure to passive smoking is challenging. Some studies have used urinary cotinine as a marker of SHS,(6) as most of the nicotine absorbed into the body is metabolised to cotinine, the major metabolite of CYP2A6.(7) Because of the relatively long physical half-life of cotinine (approximately 17 h), it remains in the blood for a long time and reaches high concentrations in the blood and urine. However, cotinine is further metabolised to trans-3'-hydroxycotinine (3-HC) by CYP2A6. In addition, inter-individual differences in nicotine metabolism have been observed due to CYP2A6 genetic polymorphisms. Uridine 5'-diphospho-glucuronosyltransferase-catalysed N-glucuronidation is well known as a metabolic pathway of nicotine, cotinine, and 3-HC.(8) Therefore, in this study, we performed enzymatic hydrolysis of glucuronide conjugates prior to urine sample analysis. To evaluate the total nicotine metabolites as biomarkers of passive smoking, the total nicotine equivalents (TNE; nicotine, cotinine, 3-HC, and glucuronide conjugates) were determined in parallel. Furthermore, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and NNAL-glucuronides are metabolites of tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK),(9) and NNK and NNAL are potent carcinogens. Urinary NNAL levels (the sum of NNAL and NNAL glucuronides) have been evaluated in both smokers and second-hand smokers.(10,11) Urinary NNAL has a relatively longer half-life (10–21 days) than urinary cotinine (17 h) and can be detected even a few weeks after tobacco smoke exposure.(12) Total NNAL and NNAL-glucuronides are expected to be a potential biomarker for estimating environmental tobacco exposure. In this study, nicotine-related metabolites, as well as the tobacco smoke-specific carcinogenic metabolite, NNAL was measured.
In addition to tobacco smoke exposure markers, health effect markers at a relatively early stage (DNA methyl adducts and oxidative DNA damage) were determined. Methane diazonium ions, metabolic intermediates of NNAL and NNK, react with DNA to form methyl adducts, such as 7-methylguanine (m7Gua) and O6-methylguanine.(13) In fact, m7Gua has been detected in the tissues of animals treated with NNK, and higher levels of m7Gua have been detected in the lung DNA of current smokers.(14) Moreover, m7Gua in the DNA is excreted into the urine by base excision repair and spontaneous depurination.(15) We focused on urinary m7Gua levels as a biomarker of passive smoking. Tobacco smoke contains toxic, carcinogenic, and mutagenic chemicals, as well as free radicals and reactive oxygen species. These highly reactive substances react with DNA to induce oxidative damage. The levels of 8-hydroxy-2'-deoxyguanosine (8-OHdG) or 8-hydroxygunine, the most widely used biomarkers of oxidative stress,(16,17) have been previously determined in the urine,(18,19) blood,(20) and saliva of cigarette smokers.(21,22) However, urinary m7Gua and 8-OHdG levels may also be useful early health markers of tobacco smoke exposure.
Individuals can be exposed to SHS in their homes, workplaces, and public places. For example, the Centers for Disease Control and Prevention in the United States reported that 19.9% of non-smokers experienced some degree of smoke exposure on the job.(23) According to the National Health and Nutrition Survey in Japan in 2019,(24) individuals could be exposed to passive smoking in the workplace (16.0%), at home (12.5%), on the road (24.1%), and in restaurants (20.8%). The National Institute for Occupational Safety and Health has also determined SHS to be an occupational carcinogen.(25) Therefore, in this study we evaluated passive smoking at home and in the workplace.
The use of heated tobacco products (HTPs) has recently increased. According to the WHO, all forms of tobacco smoking, including HTPs, are harmful.(26) Moreover, the European Respiratory Society concluded that, as with cigarette smoking, HTPs are addictive and carcinogenic to humans.(27) While HTPs are less toxic than conventional cigarettes at 5% dilution, they are cytotoxic to cultured cells.(28) Currently, few studies have reported on the biological monitoring of second-hand exposure to HTPs.(29) Therefore, we also evaluated second-hand exposure to HTPs at home using urinary biomarkers.
Materials and Methods
Urine samples
A total of 746 non-smoking volunteers participated in 2019 and 2021 at the time of periodic medical examinations at the Japanese Red Cross Kumamoto Health Care Center. Urine samples were collected in 5-ml polypropylene tubes and stored at −30°C until analysis. Information regarding smoking status was obtained from the lifestyle questionnaire completed at the time of urine collection. The questionnaire for SHS exposure in the workplace was conducted only in 2021. The number of people analysed [number of subjects, median age (minimum–maximum)] was as follows based on the questionnaire results for each analysis: analysis of passive smokers at home: male [283, 51 (23–83)]; female [393, 51 (19–83)]; analysis of passive smokers at the workplace (countermeasure against SHS): male [181, 50 (23–74)]; female [277, 50 (25–74)]; analysis of passive smokers in the workplace (exposure frequency): male [185, 50 (23–83)]; female [288, 51 (25–75)]. This study was approved by the University of Occupational and Environmental Health, Japan Ethics Committee (R1-037) and Kumamoto University Ethics Committee (1753).
Analysis of tobacco exposure biomarkers
We determined the urinary nicotine, cotinine, 3-HC, and NNAL levels by LC-MS/MS according to the method described by Kawasaki et al.(11)
Analysis of DNA damage biomarkers
Urinary m7Gua and 8-OHdG concentrations were determined using a previously described method employing HPLC-UV/ECD.(11)
Statistical methods
The values for each biomarker were compared using the median values because the data did not follow a normal distribution. Analysis of variance and multiple comparisons were performed using GraphPad Prism, ver. 9.31 (GraphPad Software, San Diego, CA). Two-sided p values <0.05 were considered significant.
Results
Urinary biomarker levels of passive smokers at the home
The urinary levels of nicotine and its metabolites (cotinine, 3-HC, and TNE) were significantly higher in non-smokers with passive exposure to tobacco products, regardless of the type of tobacco (cigarettes or HTPs) (Table 1). The urinary nicotine levels were 1.5 and 1.4 times higher in non-smokers with passive exposure to cigarettes and HTPs than those without passive exposure to cigarettes and HTPs, respectively. Urinary cotinine levels were approximately 2.4 times higher and urinary 3-HC levels were approximately 3 to 2 times higher in non-smokers with passive exposure to cigarettes and HTPs, respectively. As a biomarker of total tobacco smoke exposure, the urinary TNE levels were approximately twice as high in the passive inhalation of cigarette and HTPs smoke group than in the no inhalation group. Urinary NNAL levels were approximately 1.5 times higher in participants with passive exposure to cigarette and HTPs smoke. The m7Gua levels, as a marker of adverse health effects, were also high in the group with passive exposure to cigarette and HTPs smoke. However, urinary 8-OHdG levels were almost the same in each group. Although the number of subjects was limited, the levels of urinary nicotine metabolites, NNAL, m7Gua, and 8-OHdG tended to be high after dual passive exposure to both cigarettes and HTPs.
Table 1.
Urinary biomarker levels of passive exposure of tobacco products among non-smokers at home
| Type of passive exposure | No exposure | Cigarettes | HTPs | Dual | |
|---|---|---|---|---|---|
| Number of participants | 562 | 64 | 46 | 4 | |
| Nicotine (ng/mg creatinine) |
Median | 0.43 | 0.65* | 0.62** | 1.10 |
| Quartile§ | (0.25–0.72) | (0.31–1.10) | (0.42–1.30) | (0.55–1.80) | |
| Ratio | 1 | 1.51 | 1.44 | 2.56 | |
| Cotinine (ng/mg creatinine) |
Median | 0.30 | 0.72** | 0.71** | 2.00 |
| Quartile | (0.17–0.55) | (0.41–2.20) | (0.39–1.50) | (0.42–9.40) | |
| Ratio | 1 | 2.40 | 2.37 | 6.67 | |
| 3-OH cotinine (ng/mg creatinine) |
Median | 0.13 | 0.42** | 0.27** | 1.20 |
| Quartile | (0.07–0.27) | (0.15–1.10) | (0.14–0.86) | (0.14–5.70) | |
| Ratio | 1 | 3.23 | 2.08 | 9.23 | |
| TNE (ng/mg creatinine) |
Median | 0.96 | 2.00** | 2.00** | 6.80 |
| Quartile | (0.60–1.60) | (1.10–4.30) | (1.00–3.40) | (1.11–14.0) | |
| Ratio | 1 | 2.08 | 2.08 | 7.08 | |
| NNAL (pg/mg creatinine) |
Median | 1.60 | 2.30* | 2.50** | 2.60 |
| Quartile | (0.71–2.80) | (1.10–5.00) | (1.40–4.20) | (0.99–8.20) | |
| Ratio | 1 | 1.44 | 1.56 | 1.63 | |
| m7Gua (μg/mg creatinine) |
Median | 8.40 | 10.0* | 10.0** | 9.70 |
| Quartile | (6.60–11.0) | (7.00–13.0) | (8.20–13.0) | (5.00–18.0) | |
| Ratio | 1 | 1.19 | 1.19 | 1.15 | |
| 8-OHdG (ng/mg creatinine) |
Median | 3.8 | 3.9 | 3.7 | 5.6 |
| Quartile | (2.80–5.20) | (2.50–5.00) | (2.60–5.00) | (1.90–11.0) | |
| Ratio | 1 | 1.03 | 0.97 | 1.47 | |
*p<0.05 and **p<0.01 vs no SHS exposure group. §(25% quartile–75% quartile)
Urinary biomarker levels of passive smokers at the workplace
In 2021, a question regarding countermeasures against SHS in the workplace was added to the survey. The results showed that the urinary TNE levels were significantly lower in workplaces with countermeasures against SHS (i.e., no smoking inside the building or a designated smoking room) (Table 2). The urinary NNAL levels, a metabolite of carcinogenic compounds derived from smoking, were significantly higher in the absence of countermeasures against SHS. Finally, the urinary TNE levels were significantly higher in the daily exposure group than in the non-exposure group (Table 3).
Table 2.
Smoking restrictions and urinary biomarker levels of non-smoker at working place
| Smoking restriction | No smoking inside the building |
Designated smoking room |
No restriction | |
|---|---|---|---|---|
| Number of participants | 244 | 193 | 21 | |
| TNE (ng/mg creatinine) |
Median | 1.00 | 1.10 | 2.20** |
| Quartile§ | (0.68–1.70) | (0.65–1.70) | (0.98–8.90) | |
| Ratio | 1 | 1.10 | 2.20 | |
| NNAL (pg/mg creatinine) |
Median | 2.10 | 1.90 | 2.80** |
| Quartile | (1.10–3.70) | (0.91–3.00) | (2.10–7.60) | |
| Ratio | 1 | 0.90 | 1.33 | |
| m7Gua (μg/mg creatinine) |
Median | 9.00 | 8.90 | 10.0 |
| Quartile | (6.90–12.0) | (6.90–11.0) | (6.70–12.0) | |
| Ratio | 1 | 0.99 | 1.11 | |
| 8-OHdG (ng/mg creatinine) |
Median | 4.00 | 3.90 | 4.40 |
| Quartile | (2.70–5.40) | (3.00–5.10) | (3.40–5.70) | |
| Ratio | 1 | 0.98 | 1.10 | |
**p<0.01 vs no SHS exposure group. §(25% quartile–75% quartile)
Table 3.
Frequency of passive smoking and urinary biomarker levels at working place
| Frequency of passive smoking | 0 | 1/month | 1/week | Several/week | Daily | |
|---|---|---|---|---|---|---|
| Number of participants | 347 | 60 | 24 | 22 | 20 | |
| TNE (ng/mg creatinine) |
Median | 1.00 | 1.00 | 1.20 | 1.10 | 2.20** |
| Quartile§ | (0.66–1.80) | (0.65–1.50) | (0.70–1.90) | (0.70–1.80) | (1.20–5.30) | |
| Ratio | 1 | 1.00 | 1.20 | 1.10 | 2.20 | |
| NNAL (pg/mg creatinine) |
Median | 1.80 | 2.50 | 2.30 | 2.10 | 3.30 |
| Quartile | (0.91–3.60) | (1.60–3.50) | (1.60–3.20) | (1.30–2.90) | (1.80–6.30) | |
| Ratio | 1 | 1.38 | 1.28 | 1.17 | 1.83 | |
| m7Gua (μg/mg creatinine) |
Median | 9.10 | 9.00 | 8.60 | 8.60 | 8.30 |
| Quartile | (6.90–12.0) | (7.20–12.0) | (6.20–10.0) | (5.50–10.0) | (6.90–9.80) | |
| Ratio | 1 | 0.99 | 0.95 | 0.95 | 0.91 | |
| 8-OHdG (ng/mg creatinine) |
Median | 4.00 | 4.10 | 3.50 | 3.70 | 3.70 |
| Quartile | (3.00–5.50) | (3.00–5.40) | (2.50–4.50) | (2.90–4.40) | (2.50–5.10) | |
| Ratio | 1 | 1.03 | 0.88 | 0.93 | 0.93 | |
**p<0.01 vs no SHS exposure group. §(25% quartile–75% quartile)
Relationship between nicotine metabolites and NNAL, m7Gua, and 8-OHdG in urine
The total NNAL levels in non-smokers were positively correlated with TNE in urine (p<0.01) (Fig. 1). Urinary m7Gua and 8-OHdG levels showed a weak positive correlation with TNE levels in urine (p<0.05).
Fig. 1.
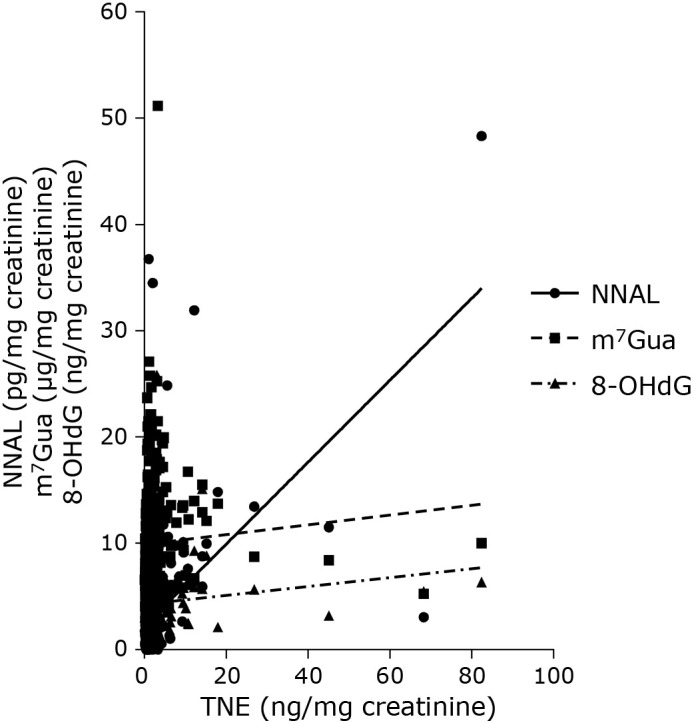
Relationship between TNE levels and NNAL, m7Gua, and 8-OHdG in the urine of non-smokers.
Discussion
Nicotine metabolites (nicotine, cotinine, 3-hydroxycotinine, and TNE) in urine have been measured as biomarkers of SHS exposure. NNAL has recently been used as a biomarker of SHS exposure for non-smokers based on the progress of analytical methods. A major advantage of measuring NNAL is that NNAL and its glucuronides (NNAL-Gluc) are carcinogenic urinary metabolites of the tobacco-specific lung carcinogen, NNK. Currently, most reports on SHS biomonitoring have targeted cigarette smoke exposure. In this study, urinary biomarker levels were significantly higher in non-smokers with passive exposure to cigarette smoke, which is consistent with the results of previous reports. However, despite the increasing number of HTP users, few studies have evaluated the urinary biomarkers of passive exposure to HTPs. Higher levels of total nicotine metabolites (cotinine and 3-HC) have been reported in the spouses and children of fathers who use HTPs.(29) This study showed that the urinary levels of TNE from exposure to cigarettes and HTPs were in the same range. Moreover, the concentration of nicotine in the tobacco filters and mainstream smoke of HTPs was nearly the same as that of cigarettes.(30) The urinary TNE levels observed in this study were reasonable. Thus, even if a smoker switches from cigarettes to HTPs for health reasons, the adverse health effects derived from nicotine on the non-smoking family members will not change significantly.
Regarding NNAL, the concentration in HTP sticks has been found to be one-fifth that of cigarettes.(30) However, in this study, the urinary TNE levels of the HTP-exposed group were in the same range as those in the cigarette smoke-exposed group. Although the reason for this is unclear, the long half-life of urinary NNAL may have affected this outcome. Urinary NNAL levels can accumulate and remain at a certain concentration even after intermittent exposure to passive smoking. Although the number of dual smokers was limited, the median levels of each biomarker were high, and the 25% quartile levels were similar to those of cigarette or HTPs smokers. This may have included heavy smokers in the dual smoker group.
The urinary concentration of total NNAL is positively associated with urinary m7Gua levels in smokers.(31) The urinary levels of m7Gua were significantly positively correlated with cigarettes smoked per day and the Brinkman index.(32) Similarly, the urinary m7Gua levels have been shown to be higher in cigarettes smokers.(11) Moreover, m7Gua levels decreased with smoking cessation.(33,34) In this study, significantly high levels of urinary m7Gua were observed in non-smokers with passive exposure to cigarettes and/or HTPs. This result indicates that passive exposure to HTPs may cause adverse health effects in non-smokers.
Increased levels of 8-OHdG in the lungs and white blood cells of smokers compared with non-smokers have been reported.(20,35) Similarly, 8-OHdG levels in the urine and 8-hydroxyguanine levels in the saliva were found to be significantly higher in smokers.(22) This could be because cigarette smoke contains high levels of free radicals,(36) which can produce oxidative DNA damage, typified by 8-OHdG. Likewise, in a previous study, NNK treatment increased 8-OHdG levels in mouse and rat DNA,(37) which may have resulted from hydroxyl radicals or other reactive oxygen species generated during NNK metabolism. Moreover, SHS-exposed subjects exhibited higher 8-OHdG levels in the DNA of leukocytes,(38) and in another study, the level of urinary 8-OHdG tended to be higher in the SHS-exposed group.(11) In this study, urinary 8-OHdG levels were not significantly different between those exposed and non-exposed to cigarettes or HTPs, which could be explained by the discrepancy between self-reported smokers and non-smokers in this study. In fact, the analysis of all participants showed a statistically positive relationship between TNE and 8-OHdG. Although 8-OHdG is not a specific marker of tobacco smoke exposure, it could be useful for evaluating the adverse health effects of SHS.
At the workplace, urinary TNE and NNAL levels of non-smokers were significantly low due to the anti-smoking measures. Designated smoking rooms are ineffective in protecting against exposure to tobacco smoke, and little difference was observed between the effects of a smoke-free building and designated smoking rooms. Additionally, despite the prohibition of smoking inside buildings, SHS exposure may occur around the building. Thus, a complete ban on smoking may be effective in reducing SHS exposure.
To our knowledge, this is the first study to evaluate NNAL, m7Gua, and 8-OHdG levels in urine as biomarkers of adverse health effects in passive smokers of HTPs. While cotinine is the gold-standard biomarker of tobacco exposure, the simultaneous determination of urinary TNE and other biomarkers, such as NNAL, m7Gua, and 8-OHdG, enables a better evaluation of SHS exposure.
Author Contributions
KKawai, YK, HO, KF, and HY designed and critically discussed the study. HO, AO, KKubota, TY, YN, and MY collected the samples and questionnaires. YK and KKawai analysed the nicotine, cotinine, 3-HC, and NNAL content in the urine. Y-SL and YO analysed the 8-OHdG and m7Gua content in the urine. YK and KKawai statistically analysed the data. All authors have read and approved the final manuscript.
Acknowledgments
This work was supported by the Ministry of Health, Labour and Welfare Sciences Research Grants, Comprehensive Research on Lifestyle Related Diseases including Cardiovascular Diseases and Diabetes Mellitus, Program Grant Number JPMH 20FA1020. We thank Ms. Megumi Taketomi for her assistance with the sample preparation for the measurement of urinary biomarkers.
Abbreviations
- 3-HC
trans-3'-hydroxycotinine
- HTPs
heated tobacco products
- m7Gua
7-methylguanine
- NNAL
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- SHS
second-hand smoke
- TNE
total nicotine equivalents
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Tobacco. World Health Organization. https://www.who.int/health-topics/tobacco#tab=tab_1. Accessed 12 Dec 2022.
- 2.Li Y, Hecht SS. Carcinogenic components of tobacco and tobacco smoke: a 2022 update. Food Chem Toxicol 2022; 165: 113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum 2004; 83: 1–1438. [PMC free article] [PubMed] [Google Scholar]
- 4.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal habits and indoor combustions. IARC Monogr Eval Carcinog Risks Hum 2012; 100 (Pt E): 43–211. [PMC free article] [PubMed] [Google Scholar]
- 5.Hori M, Tanaka H, Wakai K, Sasazuki S, Katanoda K. Secondhand smoke exposure and risk of lung cancer in Japan: a systematic review and meta-analysis of epidemiologic studies. Jpn J Clin Oncol 2016; 46: 942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avila-Tang E, Elf JL, Cummings KM, et al. Assessing secondhand smoke exposure with reported measures. Tob Control 2013; 22: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakajima M, Kwon JT, Tanaka N, et al. Relationship between interindividual differences in nicotine metabolism and CYP2A6 genetic polymorphism in humans. Clin Pharmacol Ther 2001; 69: 72–78. [DOI] [PubMed] [Google Scholar]
- 8.Murphy SE. Biochemistry of nicotine metabolism and its relevance to lung cancer. J Biol Chem 2021; 296: 100722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Smokeless tobacco and some tobacco-specific N-nitrosamines. IARC Monogr Eval Carcinog Risks Hum 2007; 89: 1–626. [PMC free article] [PubMed] [Google Scholar]
- 10.Hecht SS. Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tob Control 2004; 13 Suppl 1: i48–i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki Y, Li YS, Watanabe S, Ootsuyama Y, Kawai K. Urinary biomarkers for secondhand smoke and heated tobacco products exposure. J Clin Biochem Nutr 2021; 69: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob Res 2020; 22: 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecht SS. DNA adduct formation from tobacco-specific N-nitrosamines. Mutat Res 1999; 424: 127–142. [DOI] [PubMed] [Google Scholar]
- 14.Crosbie PA, Harrison K, Shah R, et al. Topographical study of O6-alkylguanine DNA alkyltransferase repair activity and N7-methylguanine levels in resected lung tissue. Chem Biol Interact 2013; 204: 98–104. [DOI] [PubMed] [Google Scholar]
- 15.Loft S, Svoboda P, Kasai H, et al. Prospective study of urinary excretion of 7-methylguanine and the risk of lung cancer: effect modification by mu class glutathione-S-transferases. Int J Cancer 2007; 121: 1579–1584. [DOI] [PubMed] [Google Scholar]
- 16.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res 1997; 387: 147–163. [DOI] [PubMed] [Google Scholar]
- 17.Kasai H, Kawai K. 8-Hydroxyguanine, an oxidative DNA and RNA modification. In: Jurga S, Erdmann VA, Barciszewski J, eds. Modified Nucleic Acids in Biology and Medicine. Springer International Publishing, 2016; 147–185. [Google Scholar]
- 18.Loft S, Vistisen K, Ewertz M, Tjønneland A, Overvad K, Poulsen HE. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis 1992; 13: 2241–2247. [DOI] [PubMed] [Google Scholar]
- 19.Irie M, Tamae K, Iwamoto-Tanaka N, Kasai H. Occupational and lifestyle factors and urinary 8-hydroxydeoxyguanosine. Cancer Sci 2005; 96: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asami S, Hirano T, Yamaguchi R, Tomioka Y, Itoh H, Kasai H. Increase of a type of oxidative DNA damage, 8-hydroxyguanine, and its repair activity in human leukocytes by cigarette smoking. Cancer Res 1996; 56: 2546–2549. [PubMed] [Google Scholar]
- 21.Kawai K, Kasai H, Li YS, et al. Measurement of 8-hydroxyguanine as an oxidative stress biomarker in saliva by HPLC-ECD. Genes Environ 2018; 40: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe S, Kawasaki Y, Kawai K. Salivary 8-hydroxyguanine as a lifestyle-related oxidative stress biomarker in workers. J Clin Biochem Nutr 2020; 66: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su CP, Syamlal G, Tamers S, Li J, Luckhaupt SE. Workplace Secondhand Tobacco Smoke Exposure Among U.S. Nonsmoking Workers, 2015. MMWR Morb Mortal Wkly Rep 2019; 68: 604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The National Health and Nutrition Survey (NHNS) Japan, 2019. The Ministry of Health, Labor and Welfare. https://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html. Accessed 12 Dec 2022. (in Japanese)
- 25.Environmental Tobacco Smoke in the Workplace: Lung Cancer and Other Health Effects. The National Institute for Occupational Safety and Health. https://www.cdc.gov/niosh/docs/91-108/default.html. Accessed 12 Dec 2022.
- 26.Heated Tobacco Products: Information Sheet - 2nd Edition. World Health Organization. https://www.who.int/publications/i/item/WHO-HEP-HPR-2020.2. Accessed 12 Dec 2022.
- 27.ERS Position Paper on Heated Tobacco Products. European Respiratory Society. https://www.ersnet.org/news-and-features/news/ers-position-paper-on-heated-tobacco-products/. Accessed 12 Dec 2022.
- 28.Lyu Q, Jiang L, Zheng H, Hayashi S, Sato K, Toyokuni S. Diluted aqueous extract of heat-not-burn tobacco product smoke causes less oxidative damage in fibroblasts than conventional cigarette. J Clin Biochem Nutr 2022; 71: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onoue A, Inaba Y, Machida K, et al. Association between fathers’ use of heated tobacco products and urinary cotinine concentrations in their spouses and children. Int J Environ Res Public Health 2022; 19: 6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bekki K, Inaba Y, Uchiyama S, Kunugita N. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH 2017; 39: 201–207. [DOI] [PubMed] [Google Scholar]
- 31.Hu CW, Hsu YW, Chen JL, Tam LM, Chao MR. Direct analysis of tobacco-specific nitrosamine NNK and its metabolite NNAL in human urine by LC-MS/MS: evidence of linkage to methylated DNA lesions. Arch Toxicol 2014; 88: 291–299. [DOI] [PubMed] [Google Scholar]
- 32.Tamae K, Kawai K, Yamasaki S, et al. Effect of age, smoking and other lifestyle factors on urinary 7-methylguanine and 8-hydroxydeoxyguanosine. Cancer Sci 2009; 100: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichiba M, Matsumoto A, Kondoh T, Horita M, Tomokuni K. Decreasing urinary PAH metabolites and 7-methylguanine after smoking cessation. Int Arch Occup Environ Health 2006; 79: 545–549. [DOI] [PubMed] [Google Scholar]
- 34.Kawasaki Y, Li YS, Ootsuyama Y, Nagata K, Yamato H, Kawai K. Effects of smoking cessation on biological monitoring markers in urine. Genes Environ 2020; 42: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asami S, Manabe H, Miyake J, et al. Cigarette smoking induces an increase in oxidative DNA damage, 8-hydroxydeoxyguanosine, in a central site of the human lung. Carcinogenesis 1997; 18: 1763–1766. [DOI] [PubMed] [Google Scholar]
- 36.Pryor WA. Biological effects of cigarette smoke, wood smoke, and the smoke from plastics: the use of electron spin resonance. Free Radic Biol Med 1992; 13: 659–676. [DOI] [PubMed] [Google Scholar]
- 37.Chung FL, Xu Y. Increased 8-oxodeoxyguanosine levels in lung DNA of A/J mice and F344 rats treated with the tobacco-specific nitrosamine 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone. Carcinogenesis 1992; 13: 1269–1272. [DOI] [PubMed] [Google Scholar]
- 38.Lodovici M, Caldini S, Luceri C, Bambi F, Boddi V, Dolara P. Active and passive smoking and lifestyle determinants of 8-oxo-7,8-dihydro-2'-deoxyguanosine levels in human leukocyte DNA. Cancer Epidemiol Biomarkers Prev 2005; 14: 2975–2977. [DOI] [PubMed] [Google Scholar]


