Introduction
Dupilumab, which is a fully human anti–interleukin (IL)-4 receptor α monoclonal antibody, inhibits both IL-4 and IL-13 signaling.1 It is approved for the treatment of moderate-to-severe atopic dermatitis (AD), asthma, and chronic rhinosinusitis with nasal polyps, as well as recently for prurigo nodularis, showing efficacy and good long-term safety profile in clinical trials and real-life studies.2, 3, 4 Despite these data, a number of recent publications have reported various adverse events.2 Injection-site reactions are the most commonly reported adverse events, followed by ophthalmic complications (dry eyes, conjunctivitis, blepharitis, and keratitis), paradoxical head and neck erythema (“dupilumab red face”), onset of psoriatic lesions, progression of cutaneous T-cell lymphoma, alopecia areata, and arthritis.2
Vitiligo is an acquired pigmentary disorder of unknown etiology, clinically characterized by the development of white macules due to the loss of functioning melanocytes.5 Herein, we report the case of a patient with AD who developed vitiligo lesions after starting dupilumab therapy.
Case report
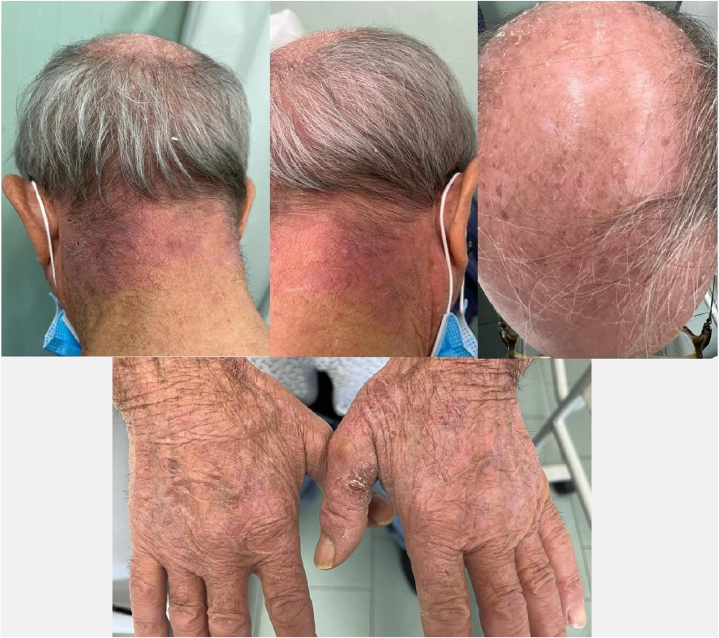
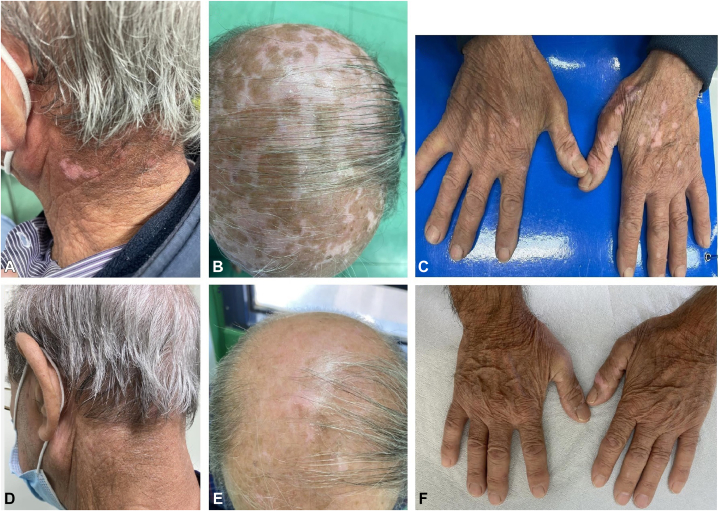
A 79-year-old male patient who has been affected by AD for the past 20 years presented to our attention with eczematous and excoriated patches involving mainly to the scalp, neck, and back of the hands (Fig 1). He had been previously treated with topical corticosteroids, narrow band–UV-B phototherapy, and systemic therapies (methotrexate, cyclosporine), with scant and transient improvement. Other comorbidities included hypertension and benign prostatic hypertrophy. At the time of the first clinical evaluation, Eczema Area and Severity Index score was 25, Pruritus-Numeric Rate Scale score was 9, and Dermatology Life Quality Index score was 20. Hence, we started treatment with dupilumab (600 mg induction dose and then 300 mg every 2 weeks). At 4-week follow-up visit, we observed a complete remission of AD skin lesions (Eczema Area and Severity Index, 0; Dermatology Life Quality Index, 0; and Pruritus-Numeric Rate Scale, 0). Hypopigmented macules all over the scalp, nape of the neck, and back of the hands were visible (Fig 2, A to C). On suspicion of vitiligo triggered by dupilumab treatment, we performed a Wood lamp examination showing fluorescent achromatic patches and a skin biopsy examination revealing the absence of melanocytes in the basal layer of the epidermis and presence of a dermal inflammatory infiltrate of lymphocytes with perivascular distribution.
Fig 1.
Eczematous and excoriated patches involving mainly to the scalp, neck, and back of the hands.
Fig 2.
Hypopigmented macules all over the (A) nape of the neck, (B) scalp, and (C) back of the hands. Complete remission of vitiligo and atopic dermatitis from the (D) nape of the neck, (E) scalp, and (F) back of the hands.
A diagnosis of vitiligo was made. Treatment with topical corticosteroids and narrow band–UV-B phototherapy was started, whereas dupilumab therapy was continued as we aimed to maintain the AD complete clinical response. At 16-week follow-up visit, the clinical examination showed complete remission of vitiligo and AD (Eczema Area and Severity Index, 0; Pruritus-Numeric Rate Scale, 0; and Dermatology Life Quality Index, 0) (Fig 2, D to F). The authors have obtained the consent of the patient for clinical images.
Discussion
To date, there is only one publication on this topic by Takeoka et al5 that described the case of a patient affected by AD with a small achromic patch of vitiligo on his forehead, which enlarged after starting dupilumab. However, to our knowledge, our case appears to be currently the first report in the literature of vitiligo-induced de novo by dupilumab therapy. The molecular drivers of vitiligo occurring during dupilumab treatment are unclear. The pathogenetic mechanism could be related to the imbalance between the T helper (Th)2 and Th1/Th17 pathways.6 It has been supposed that dupilumab-induced IL-4 inhibition causes Th1/Th17 polarization with an increased expression of IL-17, IL-2, tumor necrosis factor-alfa, and interferon-gamma that are involved in the pathogenesis of vitiligo.6 Self-reactive cytotoxic CD8+ T cells, which are recruited by the Th1/Th17 pathway, target melanocytes and promote disease progression through local interferon-gamma production, through a positive feedback mechanism.7 Despite this, in our case, it was not necessary to discontinue dupilumab therapy, and combined treatment with topical corticosteroids and narrow band UV-B resulted in complete recovery from vitiligo, also allowing AD to remain in clinical remission.
However, new studies on this topic are needed to better understand the pathophysiologic mechanisms underlying this rare adverse event during dupilumab therapy.
Conflicts of interest
None disclosed.
Footnotes
Drs Picone and Napolitano contributed equally to this article.
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Napolitano M., Fabbrocini G., Potestio L., et al. A 24-weeks real-world experience of dupilumab in adolescents with moderate-to-severe atopic dermatitis. Dermatol Ther. 2022;35(8) doi: 10.1111/dth.15588. [DOI] [PubMed] [Google Scholar]
- 2.Halling A.S., Loft N., Silverberg J.I., Guttman-Yassky E., Thyssen J.P. Real-world evidence of dupilumab efficacy and risk of adverse events: a systematic review and meta-analysis. J Am Acad Dermatol. 2021;84(1):139–147. doi: 10.1016/j.jaad.2020.08.051. [DOI] [PubMed] [Google Scholar]
- 3.Napolitano M., Nocerino M., Picone V., et al. Dupilumab for the treatment of atopic dermatitis in transplant patients: two case reports and literature review. Dermatol Ther. 2022;35(4) doi: 10.1111/dth.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aschenbrenner D.S. New dupilumab indication for rare skin disease. Am J Nurs. 2023;123(2):26–27. doi: 10.1097/01.NAJ.0000919724.54645.7c. [DOI] [PubMed] [Google Scholar]
- 5.Takeoka S., Kamata M., Yokoi I., Takehara A., Tada Y. Rapid enlargement of vitiligo vulgaris after initiation of dupilumab for atopic dermatitis: a case report. Acta Derm Venereol. 2021;101(10) doi: 10.2340/actadv.v101.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sushama S., Dixit N., Gautam R.K., Arora P., Khurana A., Anubhuti A. Cytokine profile (IL-2, IL-6, IL-17, IL-22, and TNF-α) in vitiligo-New insight into pathogenesis of disease. J Cosmet Dermatol. 2019;18(1):337–341. doi: 10.1111/jocd.12517. [DOI] [PubMed] [Google Scholar]
- 7.Frisoli M.L., Essien K., Harris J.E. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621–648. doi: 10.1146/annurev-immunol-100919-023531. [DOI] [PubMed] [Google Scholar]