Summary
Background
Radium-223, a targeted alpha therapy, is approved to treat bone-dominant metastatic castration-resistant prostate cancer (mCRPC), based on significantly prolonged overall survival versus placebo and a favourable safety profile in the phase 3 ALSYMPCA study. ALSYMPCA was conducted when few other treatment options were available, and prospectively collected data are limited on the use of radium-223 in the current mCRPC treatment landscape. We sought to understand long-term safety and treatment patterns in men who received radium-223 in real-world clinical practice.
Methods
REASSURE (NCT02141438) is a global, prospective, observational study of radium-223 in men with mCRPC. Primary outcomes are adverse events (AEs), including treatment-emergent serious AEs (SAEs) and drug-related AEs during and ≤30 days after radium-223 completion, grade 3/4 haematological toxicities ≤6 months after last radium-223 dose, drug-related SAEs after radium-223 therapy completion, and second primary malignancies.
Findings
Data collection commenced on Aug 20, 2014, and the data cutoff date for this prespecified interim analysis was Mar 20, 2019 (median follow-up 11.5 months [interquartile range 6.0–18.6]), 1465 patients were evaluable. For second primary malignancies, 1470 patients were evaluable, 21 (1%) of whom had a total of 23 events. During radium-223 therapy, 311 (21%) of 1465 patients had treatment-emergent SAEs, and 510 (35%) had drug-related AEs. In the 6 months after completion of radium-223 therapy, 214 (15%) patients had grade 3/4 haematological toxicities. Eighty patients (5%) had post-treatment drug-related SAEs. Median overall survival was 15.6 months (95% confidence interval 14.6–16.5) from radium-223 initiation. Patient-reported pain scores declined or stabilised. Seventy (5%) patients had fractures.
Interpretation
REASSURE offers insight into radium-223 use in global real-world clinical practice with currently available therapies. At this interim analysis, with a median follow-up of almost 1 year, 1% of patients had second primary malignancies, and safety and overall survival findings were consistent with clinical trial experience. Final analysis of REASSURE is due in 2024.
Funding
Bayer HealthCare.
Keywords: Clinical outcomes, Radium-223, Metastatic castration-resistant prostate cancer
Research in context.
Evidence before this study
Radium-223 is a targeted alpha therapy approved for the treatment of castration-resistant prostate cancer (CRPC) with bone metastases based on the phase 3 ALSYMPCA trial, which showed significantly prolonged overall survival compared with placebo. However, ALSYMPCA was conducted between 2008 and 2011, when the only life-prolonging therapy available was docetaxel, and little is known about the long-term safety and effectiveness of radium-223 in the real-world setting with the number of agents now in use. A search of PubMed on Jan 27, 2022, using the terms “radium-223” and “castration-resistant prostate cancer” with “observational study” as a filter, identified one prospective and two retrospective real-world studies involving a total of 673 patients treated between January 2010 and March 2018, whose results were not available when the REASSURE study was designed.
Added value of this study
To our knowledge, REASSURE is the largest prospective observational study of radium-223 treatment in men with mCRPC. We studied radium-223 use in recent real-world settings with a wider range of alternative treatments available compared with ALSYMPCA. In this prespecified interim analysis, the incidence of second primary malignancies was low, and safety and overall survival findings were consistent with clinical trial experience, regardless of use of prior, concomitant, or subsequent newer systemic anticancer therapies.
Implications of all the available evidence
Unlike clinical trials, real-world evidence can demonstrate the effectiveness and safety of treatments in a broad setting, across a wide patient population, and over a long period of time. This prespecified interim analysis of REASSURE provides real-world evidence of safety and survival outcomes for radium-223 therapy in patients treated with multiple other agents, providing robust confirmation of clinical trial experience and the trends reported in smaller real-world cohorts.
Introduction
Radium-223, a targeted alpha therapy, is approved for the treatment of patients with castration-resistant prostate cancer (CRPC) with bone metastases and no known visceral metastases. Regulatory approval was based on findings from the phase 3 ALSYMPCA (Alpharadin in Symptomatic Prostate Cancer; NCT00699751) study, in which radium-223 plus best supportive care demonstrated an overall survival advantage over placebo plus best supportive care.1,2 Radium-223 was well tolerated in ALSYMPCA, with a low incidence of myelosuppression,2 despite its bone-targeted mechanism of action.3 A small number of second primary malignancies were observed in both the radium-223 and the placebo groups during 3-year follow-up.2
Radiation therapy (typically external-beam radiation therapy; EBRT) is known to increase the risk of second primary malignancies in men with prostate cancer, although the risk is low and may not become apparent for several years.4, 5, 6, 7 As more options become available for treatment of metastatic CRPC (mCRPC) and are often used in earlier stages of the disease, it is important to understand the long-term safety, clinical benefit, and treatment patterns in men with mCRPC treated with radium-223 in the current treatment landscape.8,9 REASSURE (Radium-223 alpha Emitter Agent in Safety Study in mCRPC popUlation for long-teRm Evaluation; NCT02141438) was designed to assess safety during and after radium-223 therapy, including the incidence of new second primary malignancies, and clinical effectiveness of radium-223 treatment in patients with mCRPC in real-world global clinical practice. We report findings from an interim analysis of REASSURE prespecified to occur at a point midway between the start of data collection (2014) and the anticipated final results date (2024).
Methods
Study design and participants
REASSURE is an ongoing, global multicentre, prospective, observational, single-arm study of radium-223 use in clinical practice in the USA, Canada, Europe, Israel, and Latin America (appendix p 4). Eligible male sex patients had to have mCRPC with bone metastases and be scheduled to receive radium-223 in accordance with the treating physician. Radium-223 was recommended to be administered according to approved local prescribing information. Concomitant treatment with a bone health agent (BHA; eg, zoledronic acid or denosumab) was optional, according to local practice and applicable guidelines. Patients previously treated with radium-223 or other radiopharmaceuticals were excluded.
The conduct of REASSURE complies with the guidelines and regulations of the European Medicines Agency, US Food and Drug Administration, applicable local laws and regulations, and International Conference on Harmonisation Good Clinical Practice. All patients provided signed informed consent, and approvals were obtained from ethical committees or institutional review boards in the participating countries.
Endpoints and assessments
Primary endpoints are the incidence of second primary malignancies and safety during or after radium-223 therapy. Safety analyses include the incidence of treatment-emergent serious adverse events (SAEs) and drug-related adverse events (AEs) during or up to 30 days after completion of radium-223 therapy, grade 3/4 haematological toxicities up to 6 months after completion of radium-223 treatment, and drug-related SAEs occurring after completion of radium-223 therapy (up to a maximum of 7 years). A drug-related AE is any AE judged by the treating physician to have a reasonable suspected causal relationship to radium-223. All second primary malignancies, defined as any new malignancy unrelated to prostate cancer or other prior cancer, are being recorded for up to 7 years after the last radium-223 dose, regardless of their potential relationship to radium-223 treatment. See appendix p 2 for details of safety assessments. Incidences were calculated as the event counts during the prespecified study period divided by the total number of patients. Cumulative incidence rates, adjusted for exposure, were calculated as the event counts divided by the total time on study of all patients, expressed as patient-years.
Secondary endpoints are overall survival, quantitative pain measures, and the incidence of fractures. Pain scores over time are determined using the Brief Pain Inventory–Short Form (BPI-SF) questionnaire10,11 collected at baseline, during treatment (until 30 days after the final dose), and at the 6-month follow-up visit after completion of radium-223. The proportion of patients with a clinically meaningiful pain response at any time during treatment is reported. A clinically meaningful pain response is defined as a decrease from baseline of at least 2 points in the “pain at its worst” item; in order to see a pain response, this analysis was restricted to patients with a baseline score of 2 or greater (see appendix p 2).
The observation period for each patient is from the start of radium-223 treatment until death, withdrawal of consent, loss to follow-up, or study end (maximum 7 years), whichever occurs first. Information from patient electronic medical records, including use of prior, concomitant, and subsequent systemic life-prolonging therapies (appendix p 3), is recorded at baseline, at each radium-223 treatment cycle, approximately 3, 6, and 12 months after treatment for the first year, and then yearly.
Statistical analysis
A sample size of 1334 patients was planned to provide 1200 evaluable patients, assuming a 10% loss to follow-up. With 1200 patients, if the observed incidence of second primary malignancies at any time during the study (maximum planned follow-up 7 years) was between 1.1% and 6.9% (values based on data from the ALSYMPCA study 3-year follow-up and from the US National Cancer Institute's Surveillance, Epidemiology, and End Results [SEER] database with >10 years of follow-up2,12), the 95% confidence interval (CI) for the rate of second primary malignancies (based on the exact binomial distribution) would be approximately 1.3–3.0.
The analysis population in this prespecified interim analysis included all patients who had received at least one radium-223 injection and had data entered into the study database at the time of the data cutoff. For the primary analysis of second primary malignancies, data are included from an additional five patients who had received at least one radium-223 injection but whose data were not entered into the database before the data cutoff date.
All statistical analyses are descriptive. Data are reported as summary statistics (medians and interquartile ranges [IQR]) for continuous variables, and frequencies and percentages for categorical variables. Overall survival is estimated using the Kaplan–Meier method. Patients alive (or patients whose death is not confirmed) at the data cutoff are censored at the last date known to be alive. Statistical evaluations were done using SAS 9.2 or higher (SAS Institute Inc., Cary, NC, USA).
Role of the funding source
In collaboration with the investigators, the funder of the study, Bayer HealthCare, had a role in the study design, data collection, analysis, interpretation, and writing of the report.
Results
Data collection commenced on Aug 20, 2014, and the data cutoff date for this prespecified interim analysis was Mar 20, 2019, after a median follow-up of 11.5 months (IQR 6.0–18.6) from the first radium-223 injection.
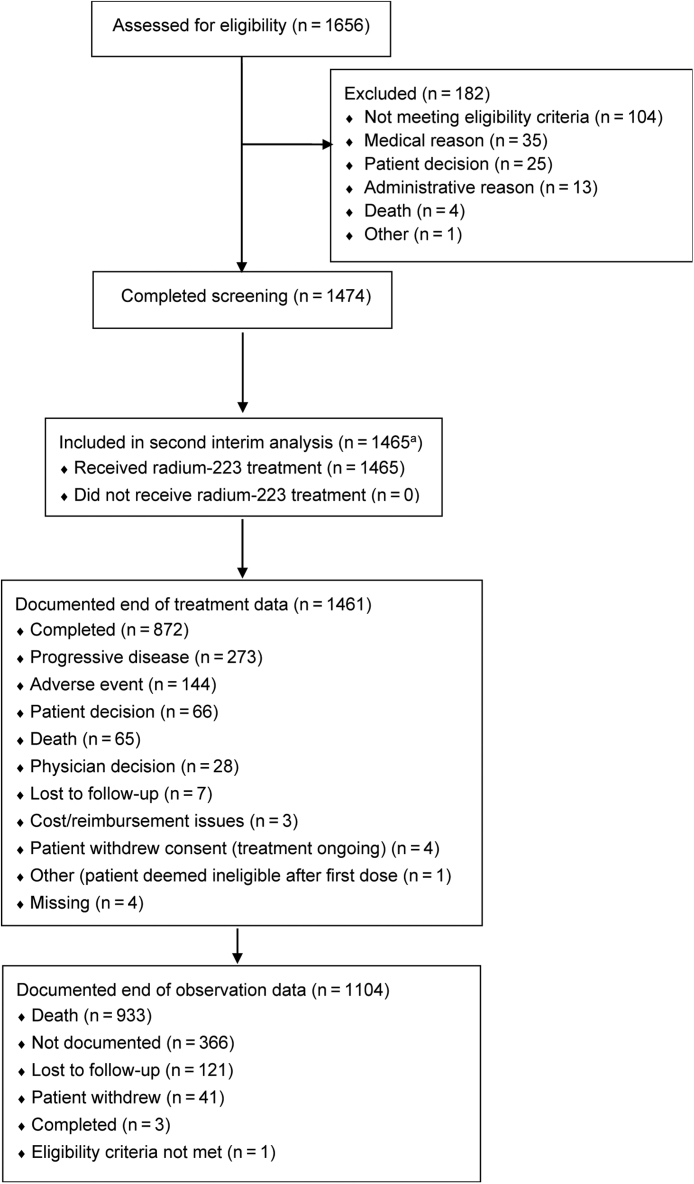
Among 1474 patients who completed screening, nine did not receive radium-223 with data recorded before the cutoff date for this interim analysis. The remaining 1465 patients, who comprised the safety population, were enrolled at 189 sites in Europe (n = 899), the USA (n = 530), and other countries (n = 36). Of these 1465 patients, 1461 (99.7%) had documented end-of-treatment data, of whom 273 (19%) stopped radium-223 treatment as a result of progressive disease and 144 (10%) stopped radium-223 treatment as a result of an AE. No patient was still on radium-223 treatment at the time of this analysis (Fig. 1). In total, 868 (59%) of 1465 patients completed all six planned cycles of radium-223 treatment.
Fig. 1.
CONSORT diagram of patient disposition. aFive additional patients are included in the analysis of second primary malignancies.
Documented end-of-observation data were available for 1104 (75%) of 1465 patients. The most common primary reason for end of observation was death (993 [90%] of 1104 patients).
Patient characteristics at initial diagnosis are shown in Table 1 and baseline demographics and disease characteristics at study entry are shown in Table 2. All patients were male sex (gender was not recorded). Malignancies diagnosed before study entry, other than prostate cancer, included malignant skin cancers in 37 (3%) of 1465 patients and other solid tumours or haematological malignancies in 90 (6%) patients. Thirty (2%) patients had fractures before radium-223 therapy. Patients may have had more than one prior malignancy or fracture before enrolment.
Table 1.
Patient clinical characteristics at initial diagnosis.
| Characteristic | Patients (n = 1465) |
|---|---|
| Gleason score | |
| ≤6 | 186 (13%) |
| 7 | 359 (25%) |
| 8–10 | 709 (48%) |
| Unknown | 196 (13%) |
| Missing | 15 (1%) |
| Risk factors for cancer | |
| Benign, malignant, or unspecified neoplasms | 12 (1%) |
| Family history of cancer | 697 (48%) |
| Prior radiation therapy to prostate or bone | 808 (55%) |
| Former or current smoker | 739 (50%) |
| Moderate or heavy alcohol intake | 105 (7%) |
| Other risk factors for cancer | 78 (5%) |
| Metastatic status | |
| Localised (M0) | 776 (53%) |
| Metastatic (M1) | 380 (26%) |
| Metastatic status not reported (MX) | 81 (6%) |
| Missing | 228 (16%) |
Data are n (%) or median (IQR). Percentages may not total 100 because of missing data and/or rounding. IQR = interquartile range.
Table 2.
Patient demographics and clinical characteristics at study entry.
| Characteristic | Patients (n = 1465) |
|---|---|
| Age, years | 73 (67–79) |
| ECOG PS | |
| 0 | 441 (30%) |
| 1 | 727 (50%) |
| ≥2 | 220 (15%) |
| Missing | 77 (5%) |
| Time from diagnosis of CRPC to study entry, months (n = 744) | 13 (3–27) |
| Time from initial diagnosis of bone metastases to study entry, months (n = 994) | 23 (12–42) |
| Prior interventions | |
| Radiation therapy to prostate | 486 (33%) |
| Surgery | 291 (20%) |
| Systemic anticancer therapya | 900 (61%) |
| No therapy to prostate | 709 (48%) |
| Missing resection status | 26 (2%) |
| Extent of disease (n = 1349 with bone scan at baseline)b | |
| <6 lesions | 259 (19%) |
| 6–20 lesions | 636 (47%) |
| >20 lesions | 270 (20%) |
| Superscan | 81 (6%) |
| Extent of disease not documented | 73 (5%) |
| Metastases | |
| Bone metastases only | 1193 (81%) |
| Bone metastases plus lymph nodes | 183 (13%) |
| Bone metastases plus lymph nodes plus other metastases | 25 (2%) |
| Bone metastases plus other metastases, excluding lymph nodes | 64 (4%) |
| Non-bone metastasis location (in >1% of patients)c | |
| Lymph node | 208 (14%) |
| Retroperitoneal | 64 (4%) |
| Pelvic | 49 (3%) |
| Para-aortic | 40 (3%) |
| Iliac | 37 (3%) |
| Mediastinal | 29 (2%) |
| Lung | 37 (3%) |
| Bladder | 17 (1%) |
| Liver | 16 (1%) |
| Laboratory valuesd | |
| Alkaline phosphatase, U/L (n = 1048) | 135 (82–263) |
| Haemoglobin, g/dL (n = 1300) | 12 (11–13) |
| Lactate dehydrogenase, U/L (n = 555) | 269 (200–419) |
| Prostate-specific antigen, ng/mL (n = 1053) | 59 (16–201) |
| Mean BPI-SF score | |
| Pain severity (n = 1312) | 3.0 (2.2) |
| Pain at its worst (n = 1320) | 4.1 (2.9) |
| BPI-SF “pain at its worst” score (n = 1320) | |
| 0 | 217 (16%) |
| 1 | 76 (6%) |
| 2 | 134 (10%) |
| 3 | 150 (11%) |
| 4 | 130 (10%) |
| 5 | 166 (13%) |
| 6 | 119 (9%) |
| 7 | 123 (9%) |
| 8 | 130 (10%) |
| 9 | 45 (3%) |
| 10 | 30 (2%) |
Data are median (IQR), mean (SD), or n (%). Percentages may not total 100 because of missing data and/or rounding. BPI-SF = Brief Pain Inventory Short Form. CRPC = castration-resistant prostate cancer. ECOG PS = Eastern Cooperative Oncology Group performance status. IQR = interquartile range. SD = standard deviation.
Systemic anticancer therapies included docetaxel, cabazitaxel, abiraterone acetate, enzalutamide, and sipuleucel-T.
The total includes 30 patients who had a bone scan with negative findings; these patients had a history of bone metastases identified on other imaging.
Location of other visceral metastases: abdomen (one), adrenal gland (seven), brain (two), breast (one), kidney (three), pelvis (one), peritoneum (one), pleura (one), prostate (one), rectum (four), skull base (one), soft tissue (two), spinal cord (two), T10 vertebra (one), thyroid gland (one), urethra (one).
Laboratory tests were done locally, with varying normal ranges.
The analysis of second primary malignancies included the 1465 patients in the safety population and five additional patients who had been treated before the data cutoff date but whose data were entered into the study database after the cutoff date. Of these 1470 patients, 21 (1%) had a total of 23 new second primary malignancies recorded during or after they received radium-223 (median six injections, IQR 4–6), with a cumulative incidence of 1.04 per 100 patient-years. Reported malignancies included five lung, four skin, four urinary tract, three gastrointestinal, three haematological (one each of monocytic leukaemia, non-Hodgkin lymphoma, and recurrent plasma cell myeloma), two neuroendocrine, and two pancreatic cancers. Fifteen of the 21 patients had received prior radiotherapy, and one received radiotherapy concomitantly with radium-223. The second primary malignancies were recorded over a range of 8–860 days from start of radium-223 therapy; three new malignancies were recorded during radium-223 therapy, nine occurred up to 6 months after the last radium-223 dose, eight occurred 6–18 months after radium-223 completion, and three occurred after >18 months. Twenty-two malignancies were assessed as unrelated to radium-223. One patient, who received four radium-223 injections, had monocytic leukaemia with onset 4 months after start of radium-223 treatment that was assessed by the investigator as related to radium-223.
Overall, AEs were recorded in 701 (48%) of the 1465 patients in the safety population (Table 3). During and up to 30 days after the last radium-223 administration, treatment-emergent SAEs occurred in 311 (21%) patients and drug-related treatment-emergent AEs in 510 (35%) patients. The most common drug-related treatment-emergent AEs were diarrhoea in 157 (11%) patients (four [0.3%] grade 3/4), nausea in 127 (9%) patients (two [0.1%] grade 3/4), and anaemia in 122 (8%) patients (87 [6%] grade 3/4) (appendix p 5). Nine deaths during radium-223 treatment or follow-up were reported as drug related (three thrombocytopenia, three anaemia, and one each pancytopenia, cardiac failure, and monocytic leukaemia).
Table 3.
Summary of adverse events.
| Patients (n = 1465) | |
|---|---|
| Any AE of interesta | 701 (48%) |
| Treatment-emergent AEs occurring during treatment or ≤30 days after last radium-223 dose | |
| SAEs | 311 (21%) |
| SAEs resulting in death | 92 (6%) |
| Drug-related AEs | |
| Any grade | 510 (35%) |
| Grade ≥3 | 155 (11%) |
| Resulting in radium-223 discontinuation | 82 (6%) |
| Resulting in death | 9 (1%) |
| Post-treatment AEs occurring >30 days after completion of radium-223 | |
| Grade 3/4 haematological toxicities ≤6 months after completion of radium-223 | |
| Grade 3 | 203 (14%) |
| Grade 4 | 26 (2%) |
| Drug-related SAEs ≤7 years after completion of radium-223 | |
| Any SAEs | 28 (2%) |
| Resulting in death | 2 (<1%) |
AE = adverse event; SAE = serious adverse event.
AEs of interest were treatment-emergent SAEs and drug-related AEs during and ≤30 days after radium-223 completion, grade 3/4 haematological toxicities ≤6 months after last radium-223 dose, and drug-related SAEs after radium-223 therapy completion.
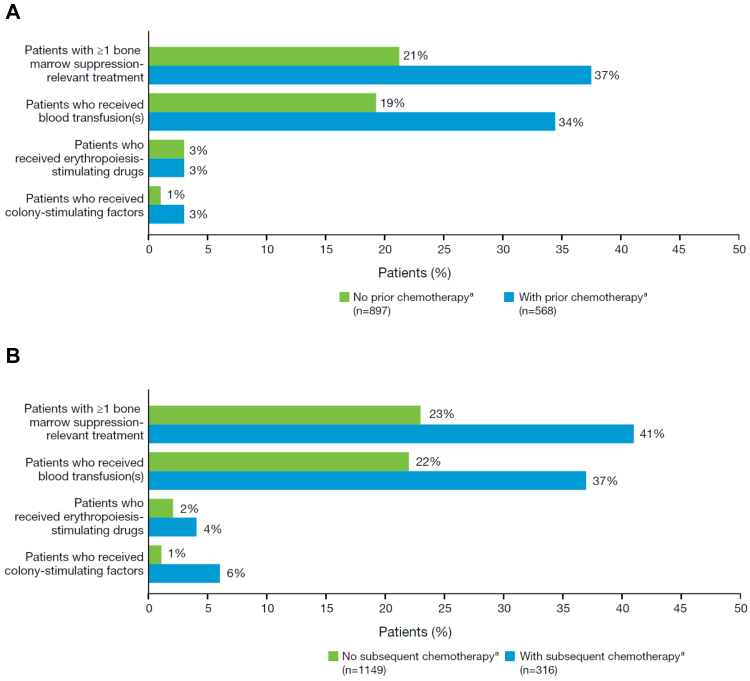
In the 6 months after completion of radium-223 therapy, 214 (15%) of 1465 patients had grade 3/4 haematological toxicities, namely anaemia in 177 (12%), thrombocytopenia in 52 (4%), neutropenia in 14 (1%), pancytopenia in eight (<1%), and leucopenia not further specified in seven (<1%). In total, 397 (27%) patients received therapeutic or preventive treatments for bone-marrow suppression (eg, blood transfusions or growth factors) after the start of radium-223 treatment. Of these 397 patients, 209 (53%) had received chemotherapy before radium-223 treatment and 128 (32%) received chemotherapy after radium-223 completion. The proportions of patients requiring red blood cell transfusions, erythropoiesis-stimulating drugs, and colony-stimulating factors are shown in Fig. 2. Abnormal laboratory values related to bone-marrow suppression are shown in the appendix (p 6).
Fig. 2.
Use of therapeutic or preventive treatments for bone-marrow suppression (n = 1465). (A) After start of radium-223 treatment in patients who did or did not receive prior chemotherapy. (B) After completion of radium-223 treatment in patients who did or did not receive subsequent chemotherapy. aPatients may have received chemotherapy at other times.
After completion of radium-223 therapy, 80 (5%) of 1465 patients had drug-related SAEs, primarily anaemia in 25 (2%) patients (appendix p 7). These SAEs occurred after a median of 4.1 months (IQR 1.8–5.9). Eleven patients died as a result of post-treatment SAEs that were deemed to be drug related (four anaemia, four thrombocytopenia, and one each pancytopenia, cardiac failure, general physical health deterioration, monocytic leukaemia, and decreased white blood cell count). Some patients had more than one SAE listed as cause of death. The SAEs resulting in death occurred after a median of 2.3 months (IQR 1.2–4.1) after the last dose of radium-223.
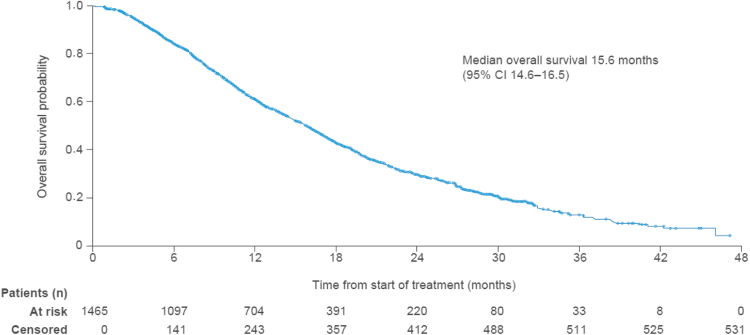
Median overall survival was 15.6 months (95% CI 14.6–16.5; Fig. 3). Of the 1465 patients, 934 (64%) had died by the data cutoff date (Mar 20, 2019) and 531 (36%) who were alive or had no confirmed death were censored at the last known date alive; 171 of the 531 patients were permanently lost to follow-up. Sixty-five (4%) patients died within 30 days after the last dose of radium-223, and 869 (59%) died more than 30 days after the last radium-223 dose. Disease progression was the main cause of death, reported in 718 (49%) of all 1465 patients, or 77% of the 934 patients who died.
Fig. 3.
Kaplan–Meier estimate of overall survival (n = 1465). Of the 531 censored patients at month 48, 171 were permanently lost to follow-up. CI = confidence interval.
Overall, 1320 patients had data for the BPI-SF “pain at its worst” score at baseline, 1027/1320 (78%) had a baseline score of 2 or greater and 293/1320 [22%] had a baseline score of <2. During treatment 566/1027 patients (55%) had a clinically meaningful pain response (a decrease of ≥2 points in the “pain at its worst” item; appendix p 8).
Fractures occurred in 70 (5%) of 1465 patients (4.34 per 100 person-years; appendix p 9). Sixteen (2%) of 678 patients who started BHAs before radium-223 and 19 (3%) of 566 patients who received concomitant BHAs had a fracture, compared with 54 (7%) of 787 patients and 51 (6%) of 899 patients who did not receive any prior or concomitant BHAs, respectively. Twenty-two (2%) of 1465 patients had fractures that occurred during or after concomitant radium-223 and abiraterone/prednisone or enzalutamide treatment.
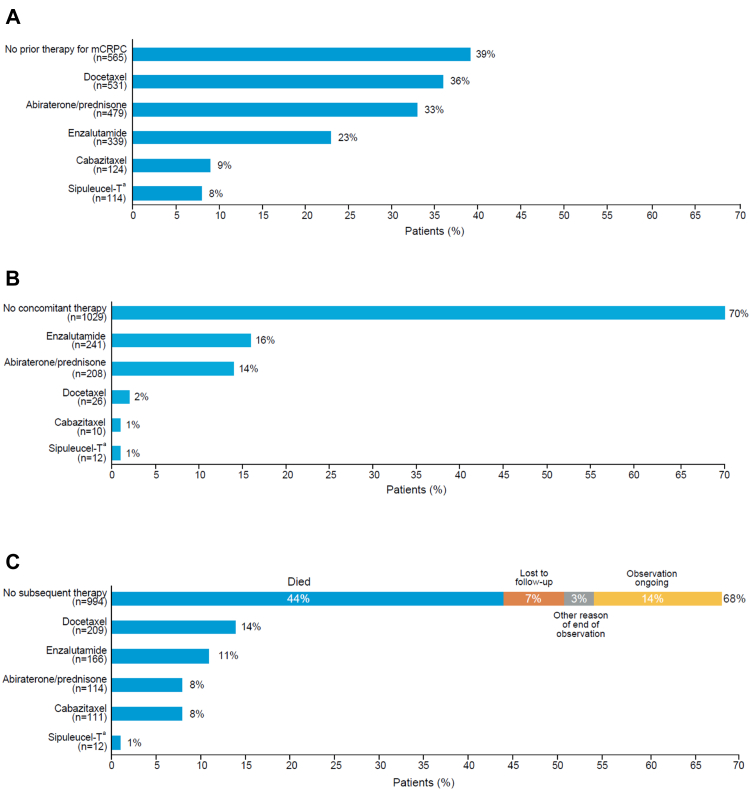
Systemic life-prolonging anticancer treatments for mCRPC that were completed before the start of radium-223 therapy are shown in Fig. 4A. Patients received radium-223, either as monotherapy or in combination with other anticancer therapies, as their first treatment for mCRPC in 565 of 1465 cases (39%) or after completion of one mCRPC treatment (435/1465, 30%), two mCRPC treatments (286/1465, 20%), or three or more mCRPC treatments (179/1465, 12%). In total, 160 (11%) of 1465 patients were treated with both abiraterone/prednisone and enzalutamide before starting radium-223 therapy.
Fig. 4.
Systemic anticancer therapies (n = 1465). (A) Prior therapies for mCRPC (completed before first radium-223 injection). (B) Concomitant therapies (started before or during radium-223 therapy, ongoing during radium-223 therapy, and completed during or after radium-223 therapy). (C) Subsequent therapies (started after completion of radium-223 therapy). Patients may have received more than one systemic anticancer therapy at each stage. mCRPC = metastatic castration-resistant prostate cancer. aSipuleucel-T is licensed in the USA only.
Of the 1465 patients, 436 (30%) received radium-223 concomitantly with systemic life-prolonging anticancer treatments. Most of these treatments were non-myelosuppressive (abiraterone, enzalutamide, and sipuleucel-T), although a small proportion of men received chemotherapy with docetaxel or cabazitaxel, or both (Fig. 4B).
Systemic anticancer therapy started after the last dose of radium-223 is shown in Fig. 4C. Overall, 471 (32%) of 1465 patients received at least one subsequent systemic life-prolonging anticancer therapy. Myelosuppressive therapy (docetaxel and/or cabazitaxel) was administered in 284 (19%) patients and treatments targeting the androgen receptor pathway in 262 (18%). After they finished radium-223 treatment, 994 (68%) patients did not receive further therapy, primarily because 649 (65%) of the 994 patients had died (Fig. 4C). Of the 994 patients, 364 (37%) received radium-223 as their first therapy, 284 (29%) completed one prior therapy, 203 (20%) completed two prior therapies, and 143 (14%) completed three or more prior therapies.
Overall, 706 (48%) of 1465 patients never received taxane therapy.
Before they started radium-223, 678 (46%) of 1465 patients had received at least one BHA, including 197 (13%) who stopped BHA therapy before radium-223. During radium-223 treatment, 566 (39%) patients continued or initiated BHA therapy. After completion of radium-223 therapy, 64 (4%) patients initiated BHA therapy for the first time (appendix p 10).
Before radium-223 initiation, 808 (55%) of 1465 patients had received radiotherapy (486 [33%] to prostate; 477 [33%] to bone). During radium-223 therapy, 91 (6%) patients received radiotherapy (18 [1%] to prostate, 76 [5%] to bone).
Discussion
Data collection for REASSURE started in 2014 when cabazitaxel, abiraterone, enzalutamide, and sipuleucel-T were new treatment options for mCRPC. None of these agents was available to patients when the radium-223 phase 3 ALSYMPCA trial was done from 2008 to 2011.1 Thus, REASSURE gives a more current real-world assessment of radium-223 safety and effectiveness in the context of the growing number of systemic therapies available for prostate cancer.
At this prespecified interim analysis of REASSURE, after a median follow-up of almost 1 year, no new safety signals were identified compared with the established safety profile of radium-223 in ALSYMPCA and an earlier interim analysis of REASSURE.2,13 The proportion of patients who completed all six planned cycles of radium-223 treatment was similar in both studies (59% for REASSURE and 58% for ALSYMPCA).1 Among the 1470 participants in REASSURE who had been treated with radium-223 by the data cutoff date, 21 (1%) patients had a total of 23 new malignancies, most of which occurred within 6 months after completion of radium-223 treatment. One new malignancy was assessed by the investigator to be related to radium-223. The final analysis, with longer follow-up, should provide a better indication of the incidence of second primary malignancies, as radiation-related malignancies, such as bladder and colorectal cancer, tend to occur several years after exposure.4, 5, 6, 7 In the 3-year follow-up of the ALSYMPCA trial, 4 second primary malignancies were reported among 572 men treated with radium-223, compared with three malignancies in the placebo group.2 No second primary malignancies were reported in a phase 2 study of radium-223 in 49 men with mCRPC after 3 years of follow-up.14 In men with prostate cancer who received EBRT, a low but significantly increased risk of other malignancies has been reported up to 20 years after treatment.4, 5, 6, 7 In an observational study of 2234 men with mCRPC (most with bone metastases) in the US SEER–Medicare database, an incidence rate of 5.9 second primary malignancies per 100 patient-years was reported; this rate was three times the rate of any cancers observed in the SEER population in men of a similar age without prostate cancer.15
Comparisons between studies must of course consider differences in patient eligibility criteria and baseline clinical characteristics. For example, patients with visceral metastases or with a malignant lymphadenopathy greater than 3 cm in the short-axis diameter were excluded from ALSYMPCA but not from REASSURE, whereas patients treated with radium-223 in ALSYMPCA had higher median PSA than patients in REASSURE (146 ng/mL vs 59 ng/mL, respectively) and a greater tumour burden (>20 metastases or Superscan in 41% of patients vs 26%, respectively).2 These factors might be attributed to the fact that patients in ALSYMPCA were enrolled between 2008 and 2011, when many of the newer systemic therapies were not available; the greater number of treatment options available to patients in REASSURE might have affected their baseline clinical characteristics and might potentially affect their tolerance to other treatments and/or efficacy outcomes. Nevertheless, consistent with ALSYMPCA, anaemia and diarrhoea were among the most commonly reported AEs during radium-223 treatment in REASSURE.1,2 Thrombocytopenia was the most common haematological toxicity associated with radium-223 administration. Post-treatment haematological toxicity occurred in a higher proportion of patients who received chemotherapy before compared with after radium-223. These data suggest that chemotherapy can be given after radium-223 therapy without increased toxicity, although the proportion of patients requiring growth factors was higher in those who were treated with chemotherapy than in those who were not.
The secondary endpoints of REASSURE were selected for their clinical relevance to physicians and patients: overall survival, quantitative pain response, and fractures. The median overall survival from the start of radium-223 treatment in this interim analysis is consistent with median durations reported in ALSYMPCA, other clinical trials, and retrospective real-world studies of radium-223 in men with mCRPC.16, 17, 18, 19, 20, 21 To our knowledge, REASSURE is the largest study to quantify a clinically meaningful pain response to radium-223 (defined as a decrease from baseline of ≥2 points in the “pain at its worst” item in the BPI-SF questionnaire). Pain was an entry criterion in ALSYMPCA, and a decline in pain-related quality of life was reported; however, patient-reported pain responses to radium-223 were not quantitatively assessed in that study.22 The prospective observational ROTOR registry (Registry of Treatment Outcomes in a Non-study Population of Symptomatic mCRPC Patients Treated With Radium-223; NCT03223727) evaluated clinical outcomes, including pain response to radium-223, in patients with mCRPC, both symptomatic and asymptomatic, who had been extensively pretreated. In 105 men with data, clinically meaningful improvements in quality of life (measured using the Functional Assessment of Cancer Therapy-Prostate questionnaire) and pain (measured using the BPI-SF “pain at its worst” item) were reported in 31% and 50% of patients, respectively. Pain improvement was achieved whether or not patients had pain at baseline.23 These findings are consistent with those from the current REASSURE interim analysis, in which 34% of patients had a clinically meaningful pain response, although, as noted earlier, differences in baseline characteristics between patient populations make cross-trial comparisons challenging.
The incidence of fracture in REASSURE was somewhat higher than the 4% or less incidence reported in ALSYMPCA,1,2 but lower than that reported in other studies and real-world analyses.16,18 In REASSURE, the low use of concomitant BHAs was surprising, given that BHA use in men with mCRPC and bone metastases is recommended in international guidelines.24,25 Although reasons for not using BHAs were not collected, clinicians may have elected to avoid a BHA during radium-223 treatment because of the bone-targeted mechanism of action of radium-223. This issue should be addressed and clarified in guidelines and educational symposia.
In REASSURE, a substantial proportion of patients had not completed another systemic anticancer therapy for mCRPC before they initiated radium-223, although they might have received prior systemic therapy for hormone-sensitive or non-metastatic disease. In another real-world analysis of treatment patterns in 2259 men with mCRPC in the US Flatiron database treated between 2013 and 2017, only 2% of all men in the analysis, or 15% of men who were ever treated with radium-223, received radium-223 as first-line therapy.26 This discrepancy could be related to the slightly later time frame of REASSURE (2014–2019), as well as the underlying populations (all men with mCRPC in the US database compared with only men with mCRPC who ever received radium-233 in a global setting). In comparison, patients in ALSYMPCA had to have been treated with docetaxel, or be unfit for or declined docetaxel, before receiving radium-223 treatment. Use of other prior or concomitant therapies was not reported in ALSYMPCA.1 In the ERA-223 study of radium-223 in combination with abiraterone acetate and prednisone in men with mCRPC and bone metastases, patients were excluded if they had previously received chemotherapy or abiraterone for CRPC before receiving radium-223. Again, use of other prior or concomitant therapies was not reported.27
It is surprising that a small proportion of patients were treated with chemotherapy concurrently with radium-223, given the US prescribing information for radium-223 warning against such practice outside clinical trials because of overlapping haematological toxicity profiles.2 Concomitant use of either abiraterone or enzalutamide was also recorded in some patients, likely because of a desire to reduce prostate-specific antigen levels, which would not necessarily be achieved with radium-223, as seen in ALSYMPCA.1 The US Food and Drug Administration and European Medicines Agency issued warnings about the concurrent use of radium-223 with abiraterone acetate + prednisone in late 2017, as a result of an increase in fracture rate of uninvolved bone observed in patients treated with the combination in the ERA-223 trial.27 The incidence of concomitant EBRT use in REASSURE, mainly for pain palliation in 72 (5%) patients, is lower than the 16% incidence within 12 weeks of screening in the radium-223 arm of the ALSYMPCA trial, but all patients in ALSYMPCA were symptomatic at study entry.1
After radium-223 treatment, almost half of the patients died before they could receive subsequent therapy. Of the remainder, some received subsequent systemic anticancer therapy, including docetaxel, cabazitaxel, enzalutamide, or abiraterone. Approximately half of the REASSURE population never received a taxane at any time, reflecting the benefits of having a range of treatment options now available, as many patients are concerned about taxane toxicity.28
Potential limitations of any non-interventional real-world study include the lack of blinding and randomisation, the heterogeneity of the patient population and treatments administered, differences in treatment pathways across centres and countries, and the lack of consistency in data collection during follow-up. However, REASSURE was specifically designed to capture real-world experience with radium-223 and long-term safety. The heterogeneous patient population and multinational scope may be viewed as strengths in reflecting the real-world situation for patients with mCRPC who are treated with radium-223. Although the median follow-up duration in this interim analysis is relatively short, most patients had reached end of observation, indicating that the data are mature enough to allow a good assessment of radium-223 safety in a real-world setting.
In summary, this planned interim analysis of REASSURE demonstrated that, in real-world clinical practice, radium-223 has a safety profile consistent with clinical trial experience, which does not appear to be affected by prior, concomitant, or subsequent use of other systemic anticancer therapies or radiotherapy. The risk of second primary malignancies was low, albeit over a short follow-up period. The final analysis of REASSURE, after longer follow-up, will provide additional information on the long-term safety and clinical outcomes associated with radium-223 in men with mCRPC and bone metastases treated in the real world. A graphical abstract and plain language summary are available in the appendix (pp 11–12).
Contributors
CSH verifies that this study was done per protocol and vouches for data accuracy and completeness. CSH, DJG, NDS, OS, KM, PSC, CNS, FS, JPS, JB, MRS, and BT contributed to the concept and design of the study in collaboration with Bayer HealthCare. CSH, DJG, NDS, OS, KM, PSC, CNS, FS, JPS, JB, MRS, and BT acquired and had access to the study data. CSH, DJG, NDS, OS, KM, PSC, CNS, FS, JPS, JB, MRS, KC, PS, FV, and BT participated in interpreting the data and developing or reviewing the manuscript and provided final approval to submit the manuscript for publication. CSH was responsible for the final decision to submit the manuscript for publication.
Data sharing statement
Availability of the data underlying this publication will be determined according to Bayer's commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, time point, and process of data access.
As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after Jan 1, 2014.
Interested researchers can use www.vivli.org to request access to anonymised patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the member section of the portal.
Data access will be granted to anonymised patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Declaration of interests
All authors report support for the present manuscript from Bayer for medical writing funding to Open Health.
Celestia S. Higano reports support for the present manuscript from Bayer for clinical trial funding to institution; grants or contracts from AbbVie (Contract to develop unbranded educational slide set), Vaccitech (Clinical trial consulting contract, no payments yet), and Verity; consulting fees from the Prostate Cancer Clinical Trials Consortium (Consulting PCCCTC medical monitor for trials sponsored by ESSA and Bayer), Prostate Cancer Supportive Care Program (Consulting Medical Director), and Astellas, AstraZeneca, Ferring, Genentech, Merck Sharp & Dohme, Myovant, Pfizer, Tolmar, and Vaccitech (all advisory boards); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Tolmar (medical writing); payment for expert testimony from Ferring; participation on a Data Safety Monitoring Board or Advisory Board for AstraZeneca, Exelixis, Advantagene/Candel, and Alliance Foundation (all DSMBs).
Daniel J. George reports grants or contracts from Astellas, AstraZeneca, BMS, Calithera, Exelixis, J&J, Pfizer, Novartis, and Sanofi (all paid to his institution); royalties or licenses from Up-to-Date (paid to self); consulting fees from Bayer, Ideo Oncology, Merck, Michael J Hennessey, Myovant, Propella, RevHealth, Seattle Genetics, WebMD, and Xcures (all paid to self); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer, Exelixis, and Sanofi (all paid to self); payment for expert testimony from Wilmer Hale (paid to self); support for attending meetings and/or travel from Bayer, Exelixis, and Sanofi (paid to self); participation on a Data Safety Monitoring Board or Advisory Board for Janssen and AstraZeneca (both paid to self); and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid, for AACR (senior editor), Millennium Med Pub, and CAHO (Co-Editor-in-Chief) (all paid to self).
Neal D. Shore reports consulting fees from AbbVie, Alessa Therapeutics, Akido, Arquer, Asieris, Astellas, Astra Zeneca, Bayer, BMS, Boston Scientific, Clarity, Cold Genesys, Dendreon, Exact Images, Ferring, Foundation Medicine, ImmunityBio, Incyte, Invitae, Janssen, Lantheus, Lilly, MDX, Merck, Minomic, Myovant, Myriad, NGM, Nonagen, Novartis, NYMOX, Photocure, Pfizer, PlatformQ, Profound, Promaxo, Protara, Sanofi, SesenBio, Speciality Networks, Telix, Tolmar, Vaxiion; payment for expert testimony from Ferring.
Oliver Sartor reports grants or contracts from Advanced Accelerator Applications, Amgen, AstraZeneca, Bayer, Constellation, Endocyte, Invitae, Janssen, Lantheus, Merck, Progenics, and Tenebio; consulting fees from Advanced Accelerator Applications (AAA), Astellas, AstraZeneca, Bayer, Blue Earth Diagnostics, Inc., Bavarian Nordic, Bristol Myers Squibb, Clarity Pharmaceuticals, Clovis, Constellation, Dendreon, EMD Serono, Fusion, Isotopen Technologien, Merck, Meunchen, Janssen, Myovant, Myriad, Noria Therapeutics, Inc., NorthStar, Novartis, Noxopharm, Progenics, POINT Biopharma, Pfizer, Sanofi, Tenebio, Telix, Theragnostics; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Advanced Accelerator Applications (AAA), Astellas, AstraZeneca, Bayer, Blue Earth Diagnostics, Inc., Bavarian Nordic, Bristol Myers Squibb, Clarity Pharmaceuticals, Clovis, Constellation, Dendreon, EMD Serono, Fusion, Isotopen Technologien, Merck, Meunchen, Janssen, Myovant, Myriad, Noria Therapeutics, Inc., NorthStar, Novartis, Noxopharm, Progenics, POINT Biopharma, Pfizer, Sanofi, Tenebio, Telix, Theragnostics; patents planned, issued or pending: Koochekpour, Sartor AO, inventors. Saposin C and receptors as targets for treatment of benign and malignant disorders. US patent awarded January 23, 2007 (patent no. 7,166,691); participation on a Data Safety Monitoring Board or Advisory Board for AstraZeneca, Jannsen, Pfizer, and Myovant; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for Louisiana Cancer Research Consortium – Board, LA. Cancer and Lung Trust; and stock or stock options in Clarity Pharmaceuticals, Noria Therapeutics, Inc., Lilly, Clovis, Glaxo Smith Kline, Abbvie, Cardinal Health, and United Health Group.
Kurt Miller reports consulting fees from Accord, Janssen, Novartis, Pfizer, and Roche; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Accord, Janssen, Novartis, Pfizer, and Roche; and participation on a Data Safety Monitoring Board or Advisory Board for Myovant.
Peter S. Conti reports no other conflicts to declare.
Cora N. Sternberg reports consulting fees from Pfizer, MSD, AZ, Astellas, Sanofi, Genzyme, Roche-Genentech, BMS, Bayer, Foundation Medicine, Gilead, Medscape, UroToday, CCN Clinical, Janssen, NCI (all ad boards over at least 5 years).
Fred Saad reports grants or contracts from Astellas, AstraZeneca, Janssen, Myovant, Merck, Novartis, Sanofi, and Pfizer (payment to institution); consulting fees from Amgen, Astellas, AstraZeneca, Bayer, Janssen, Myovant, Merck, Novartis, Sanofi, Pfizer, Tolmar (payment to self); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen, Astellas, AstraZeneca, Bayer, Janssen, Myovant, Merck, Novartis, Sanofi, Pfizer, Tolmar (payment to self).
Juan Pablo Sade reports no other conflicts to declare.
Joaquim Bellmunt reports royalties or licenses from UpToDate; consulting fees from Pfizer/MSD, Genentech, AstraZeneca, and Merck; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Pfizer/MSD, Genentech, AstraZeneca, and Merck; support for attending meetings and/or travel from Pfizer; participation on a Data Safety Monitoring Board or Advisory Board from Pfizer/MSD, Genentech, AstraZeneca, and Merck.
Matthew R. Smith reports grants or contracts from Bayer for the present study (paid to self and institution) and from Astellas, Bayer, Janssen, Lilly, and Pfizer (all paid to institution); consulting fees from Astellas, Bayer, Janssen, Lilly, and Pfizer (all paid to self); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astellas, Bayer, Janssen, Lilly, and Pfizer (all paid to self); and participation on a Data Safety Monitoring Board or Advisory Board for Bayer, Janssen, and Lilly (all paid to self).
Kumari Chandrawansa reports stock or stock options in Bayer; other financial or non-financial interests: employee of Bayer.
Per Sandström reports other financial or non-financial interests: employee of Bayer.
Frank Verholen reports other financial or non-financial interests: employee of Bayer.
Bertrand Tombal reports support for the present manuscript from Bayer (support of study); grants or contracts from Bayer (grant for studies); consulting fees from Astellas, Bayer, Novartis, Janssen, Accor, and MSD (all payments to his company: UROADVISE); and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer (paid to his company: UROADVISE) and Astellas.
Acknowledgements
This study was supported by Bayer HealthCare (Whippany, NJ, USA). The authors thank Jeffrey Meltzer of Bayer HealthCare and Peter Hassenpflug of Cerner Enviza for statistical support. Medical writing support in the preparation of this manuscript was provided by Yvonne E. Yarker, PhD, ISMPP CMPP™, and Sara Black, ISMPP CMPP™, of OPEN Health Communications (London, UK), with financial support from Bayer HealthCare. Editorial assistance in the preparation of this manuscript was provided by Lila Adnane (Bayer HealthCare).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101993.
Appendix A. Supplementary data
References
- 1.Parker C., Nilsson S., Heinrich D., et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 2.Parker C.C., Coleman R.E., Sartor O., et al. Three-year safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases from phase 3 randomized Alpharadin in Symptomatic Prostate Cancer Trial. Eur Urol. 2018;73:427–435. doi: 10.1016/j.eururo.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Bruland O.S., Nilsson S., Fisher D.R., Larsen R.H. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. 2006;12:6250s–6257s. doi: 10.1158/1078-0432.CCR-06-0841. [DOI] [PubMed] [Google Scholar]
- 4.Davis E.J., Beebe-Dimmer J.L., Yee C.L., Cooney K.A. Risk of second primary tumors in men diagnosed with prostate cancer: a population-based cohort study. Cancer. 2014;120:2735–2741. doi: 10.1002/cncr.28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hegemann N.S., Schlesinger-Raab A., Ganswindt U., et al. Risk of second cancer following radiotherapy for prostate cancer: a population-based analysis. Radiat Oncol. 2017;12:2. doi: 10.1186/s13014-016-0738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray L., Henry A., Hoskin P., Siebert F.-A., Venselaar J. Second primary cancers after radiation for prostate cancer: a systematic review of the clinical data and impact of treatment technique. Radiother Oncol. 2014;110:213–228. doi: 10.1016/j.radonc.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallis C.J., Mahar A.L., Choo R., et al. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ. 2016;352:i851. doi: 10.1136/bmj.i851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Sullivan J.M., Carles J., Cathomas R., et al. Radium-223 within the evolving treatment options for metastatic castration-resistant prostate cancer: recommendations from a European Expert Working Group. Eur Urol Oncol. 2020;3:455–463. doi: 10.1016/j.euo.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Lorente D., Fizazi K., Sweeney C., de Bono J.S. Optimal treatment sequence for metastatic castration-resistant prostate cancer. Eur Urol Focus. 2016;2:488–498. doi: 10.1016/j.euf.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Cleeland C.S., Ryan K.M. Pain assessment: global use of the Brief pain inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 11.M D Anderson Cancer Center Brief pain inventory (short Form) https://www.mdanderson.org/documents/Departments-and-Divisions/Symptom-Research/BPI-SF_English-24h_Original_SAMPLE.pdf
- 12.Brenner D.J., Curtis R.E., Hall E.J., Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer. 2000;88:398–406. doi: 10.1002/(sici)1097-0142(20000115)88:2<398::aid-cncr22>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Dizdarevic S., Petersen P.M., Essler M., et al. Interim analysis of the REASSURE (Radium-223 alpha Emitter Agent in non-intervention Safety Study in mCRPC popUlation for long-teRm Evaluation) study: patient characteristics and safety according to prior use of chemotherapy in routine clinical practice. Eur J Nucl Med Mol Imag. 2019;46:1102–1110. doi: 10.1007/s00259-019-4261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uemura H., Uemura H., Nagamori S., et al. Three-year follow-up of a phase II study of radium-223 dichloride in Japanese patients with symptomatic castration-resistant prostate cancer and bone metastases. Int J Clin Oncol. 2019;24:557–566. doi: 10.1007/s10147-018-01389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saltus C.W., Vassilev Z.P., Zong J., et al. Incidence of second primary malignancies in patients with castration-resistant prostate cancer: an observational retrospective cohort study in the United States. Prostate Cancer. 2019;2019 doi: 10.1155/2019/4387415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sternberg C.N., Saad F., Graff J.N., et al. A randomised phase II trial of three dosing regimens of radium-223 in patients with bone metastatic castration-resistant prostate cancer. Ann Oncol. 2020;31:257–265. doi: 10.1016/j.annonc.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Matsubara N., Nagamori S., Wakumoto Y., et al. Phase II study of radium-223 dichloride in Japanese patients with symptomatic castration-resistant prostate cancer. Int J Clin Oncol. 2018;23:173–180. doi: 10.1007/s10147-017-1176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higano C., Tombal B., Miller K., et al. Clinical outcome with radium-223 (Ra-223) in patients (pts) previously treated with abiraterone (Abi) or enzalutamide (Enza): a retrospective study of real-world (RW) data from pts with metastatic castration-resistant prostate cancer (mCRPC) Ann Oncol. 2018;29(Suppl 8):288–289. [Google Scholar]
- 19.Badrising S.K., Louhanepessy R.D., van der Noort V., et al. A prospective observational registry evaluating clinical outcomes of radium-223 treatment in a nonstudy population. Int J Cancer. 2020;147:1143–1151. doi: 10.1002/ijc.32851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boni G., Mazzarri S., Cianci C., et al. (223)Ra-chloride therapy in men with hormone-refractory prostate cancer and skeletal metastases: real-world experience. Tumori. 2018;104:128–136. doi: 10.1177/0300891618765571. [DOI] [PubMed] [Google Scholar]
- 21.Kuppen M.C., Westgeest H.M., van der Doelen M.J., et al. Real-world outcomes of radium-223 dichloride for metastatic castration resistant prostate cancer. Future Oncol. 2020;16:1371–1384. doi: 10.2217/fon-2020-0039. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson S., Cislo P., Sartor O., et al. Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann Oncol. 2016;27:868–874. doi: 10.1093/annonc/mdw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badrising S.K., Louhanepessy R.D., van der Noort V., et al. Integrated analysis of pain, health-related quality of life, and analgesic use in patients with metastatic castration-resistant prostate cancer treated with Radium-223. Prostate Cancer Prostatic Dis. 2022;25:248–255. doi: 10.1038/s41391-021-00412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mottet N., Cornford P., van den Bergh R.C.N., et al. 2022. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer.https://uroweb.org/guideline/prostate-cancer/ [DOI] [PubMed] [Google Scholar]
- 25.Gillessen S., Choudhury A., Rodriguez-Vida A., et al. Decreased fracture rate by mandating bone protecting agents in the EORTC 1333/PEACEIII trial combining Ra223 with enzalutamide versus enzalutamide alone: an updated safety analysis. J Clin Oncol. 2021;39(Suppl):5002. [Google Scholar]
- 26.George D.J., Sartor O., Miller K., et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer. 2020;18:284–294. doi: 10.1016/j.clgc.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Smith M., Parker C., Saad F., et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:408–419. doi: 10.1016/S1470-2045(18)30860-X. [DOI] [PubMed] [Google Scholar]
- 28.Eliasson L., de Freitas H.M., Dearden L., Calimlim B., Lloyd A.J. Patients' preferences for the treatment of metastatic castrate-resistant prostate cancer: a discrete choice experiment. Clin Ther. 2017;39:723–737. doi: 10.1016/j.clinthera.2017.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.