Abstract
Background
In the endovascular era, most of vertebral artery (VA) and posterior inferior cerebellar artery (PICA) aneurysms were mainly treated with endovascular procedures. This study aimed to demonstrate the microsurgical treatment via the far-lateral approach without C1 laminectomy and its clinical outcomes.
Methods
Forty-eight patients with VA and proximal PICA aneurysms treated by microsurgery through the far-lateral approach without C1 laminectomy, between January 2016 and June 2021, were retrospectively evaluated.
Results
Most patients (87.5%) presented with subarachnoid hemorrhage. Grading at presentation was poor in 41.7%. The rates of VA dissecting aneurysms, saccular aneurysms of the VA–PICA junction, and true PICA saccular aneurysms were 54.2, 18.7, and 14.6%, respectively. All aneurysms were located above the lower margin of the foramen magnum. The far-lateral approach without C1 laminectomy was successfully used in all patients without residual aneurysms. Various surgical strategies were applied depending on the characteristics of the aneurysm. Good outcomes 3 months postoperatively were achieved in 77.1% and 89.3% for the overall and good-grade groups, respectively.
Conclusions
Microsurgery is a safe and effective treatment of VA and proximal PICA aneurysms. Moreover, the far-lateral approach without C1 laminectomy was adequate and effective for aneurysms located above the lower border of the foramen magnum.
Keywords: Far-lateral approach, C1 laminectomy, Vertebral artery aneurysm, PICA aneurysm
1. Introduction
Owing to the evolution of endovascular techniques and devices and the higher rates of microsurgical complications, vertebral artery (VA) and posterior inferior cerebellar artery (PICA) aneurysms are predominantly treated with endovascular procedures.1, 2, 3, 4, 5, 6, 7
Proximal PICA and VA aneurysms have a close relationship with the lower cranial nerves and brainstem and are reachable via the far-lateral or lateral suboccipital approach.8, 9, 10, 11 With the lower rates of aneurysm remnants and recurrences, the functional outcomes of microsurgery were favorable with endovascular treatment. However, the most important drawback of microsurgery was the higher rates of lower cranial nerve palsy.1,7 The relatively high surgical morbidity and mortality mainly resulted from the complicated surgical approach to the VA and proximal PICA, which are blocked by vital structures such as lower cranial nerves and brainstem.1,12
With well-developed microsurgical techniques and treatment strategies, microsurgery remains important in the treatment of VA and proximal PICA aneurysms with an acceptable rate of surgical complications and higher rates of aneurysm obliteration.7,12,13 The key surgical exposure for VA and proximal PICA aneurysms is the far-lateral approach, which has numerous variants. Matsushima et al suggested indications for drilling the condylar fossa and occipital condyle, which depends on the level of the lesion related to the hypoglossal canal, but the clear indication for C1 laminectomy was not established.14,15 This study aimed to demonstrate the surgical outcomes and reveal that C1 laminectomy is not necessary for VA and proximal PICA aneurysms in specific location.
2. Material and methods
We retrospectively reviewed data from patients who underwent microsurgery for the treatment of VA and proximal PICA (from the PICA origin to the caudal loop) aneurysms at the Faculty of Medicine Vajira Hospital, Navamindradhiraj University, between January 2016 and June 2021. We enrolled patients with ruptured and unruptured aneurysms. The main indications for open surgery were the financial aspect of endovascular devices. The exclusion criteria were as follows: distal PICA aneurysms (distal to the caudal loop), giant aneurysms, vertebrobasilar junction aneurysms, treatment with endovascular techniques, supportive treatment because of any problems, and follow-up loss. Data regarding the location of the aneurysms, aneurysm characteristics, surgical techniques, surgical outcomes, and Glasgow outcome score (GOS) at 3 months postoperatively were collected and analyzed.
2.1. Operative techniques
With the semiprone park-bench position, a paramedian linear or “L”-shaped skin incision was made. The occipital artery (OA) was harvested with an “L”-shaped skin incision in cases that need OA–PICA bypass. Suboccipital muscles were dissected in a layer-by-layer fashion. The V3 segment of the VA was exposed through the suboccipital triangle.16, 17, 18, 19 After retrosigmoid craniotomy was made, the posterolateral part of the foramen magnum was removed while preserving the posterior arch of C1. After the posterior condylar emissary vein was coagulated and cut, the condylar fossa was exposed, and the sigmoid magnum triangle, which is the posterior part of the jugular tubercle, was identified and drilled until the blue line of the hypoglossal canal was partially seen. The posterior part of the jugular tubercle, which is located above the hypoglossal canal, was drilled as much as possible (Video 1). At this step, the transcondylar fossa approach was completed (Fig. 1A). This approach was sufficient for lesions located above the hypoglossal canal. If the lesion is located below the hypoglossal canal, the occipital condyle, which is located below the blue line, should be partially drilled (transcondylar approach).20 After the dura was opened, the ipsilateral cerebellar hemisphere and tonsils were retracted superomedially to expose the V4 segment of the VA, proximal PICA, and lower cranial nerves (Fig. 1B). If necessary, OA–PICA bypass was performed first.18,21 Different aneurysm clipping techniques (direct clipping, trapping, proximal occlusion, distal occlusion, or blind-alley formation) was performed depending on the aneurysm characteristics.13,21
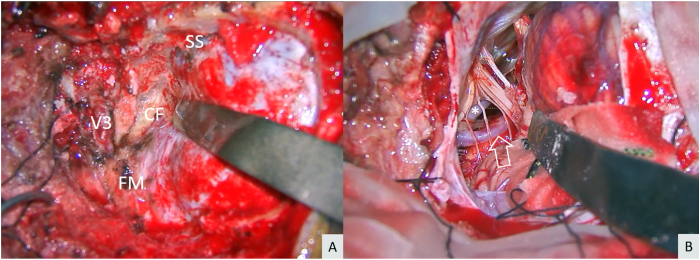
Fig. 1.
Left far-lateral transcondylar fossa approach (A) After the third segment of the vertebral artery (V3) and sigmoid sinus (SS) were exposed and the foramen magnum (FM) and condylar fossa (CF) were drilled, the transcondylar fossa approach was completed (B) After dural opening and elevation of the cerebellar tonsil, the lower cranial nerves, vertebral artery (asterisk), and posterior inferior cerebellar artery (arrow) were identified.
2.2. Outcome assessment
Outcomes were evaluated using GOS 3 months postoperatively, obliteration of the aneurysm, and new neurological deficits. GOS of 4 and 5 were defined as good outcomes, whereas GOS of 1–3 were defined as poor outcomes.
3. Results
3.1. Patient characteristics and clinical presentation
The study included 48 patients with VA and proximal PICA aneurysms. The average age was 56 years. Female patients were twice as many as male patients. The most frequent presentations were headache and altered consciousness (41.7%). Most patients (87.5%) experienced subarachnoid hemorrhage (SAH) at the initial presentation. The grading at the initial presentation was poor (World Federation of Neurosurgical Societies [WFNS] grades 4 and 5) in 41.7% of the patients. Patients with unruptured aneurysms were included in the good-grade group. VA dissecting aneurysms were the most common types (54.2%), followed by saccular aneurysms of the VA–PICA junction (18.7%). By aneurysm characteristics, dissecting, saccular, and fusiform types were found in 28 (58.3%), 19 (39.6%), and 1 (2.1%) patient, respectively. By location, VA, VA–PICA junction, and true proximal PICA aneurysms were found in 30 (62.5%), 9 (18.75%), and 9 (18.75%) patients, respectively, and all aneurysms are located above the lower margin of the foramen magnum. VA dissecting aneurysms were classified as pre-PICA, PICA, post-PICA, and non-PICA types13 (Table 1).
Table 1.
Patient characteristics and clinical presentation.
| Data | Total (n = 48) |
|---|---|
| Female:Male | 32:16 |
| Mean age in years | 56 |
| Presentation | |
| Headache | 15 (31.2%) |
| Alteration of consciousness | 10 (20.8%) |
| Headache with AOC | 20 (41.7%) |
| Seizure | 2 (4.2%) |
| Hemiparesis | 1 (2.1%) |
| Subarachnoid hemorrhage | |
| Yes | 42 (87.5%) |
| No | 6 (12.5%) |
| Grading at presentation | |
| Good grade | 28 (58.3%) |
| Poor grade | 20 (41.7%) |
| Type and location of aneurysms | |
| VA dissecting aneurysm | 26 (54.2%) |
| Pre-PICA type | 6 |
| PICA type | 8 |
| Post-PICA type | 8 |
| Non-PICA type | 4 |
| VA saccular aneurysm | 3 (6.2%) |
| VA fusiform aneurysm | 1 (2.1%) |
| VA-PICA saccular aneurysm | 9 (18.7%) |
| True PICA saccular aneurysm | 7 (14.6%) |
| True proximal PICA dissecting aneurysm | 2 (4.2%) |
AOC, alteration of consciousness; PICA, posterior inferior cerebellar artery; VA, vertebral artery.
3.2. Treatment and outcomes
The transcondylar or transcondylar fossa approach without C1 laminectomy was performed in all patients, of which 46 patients went through the ipsilateral approaches and two patients with the contralateral approach. For saccular aneurysms of the VA, VA–PICA, and true PICA, direct clipping and OA–PICA bypass with clipping were performed in 18 and 1 patient, respectively. For VA dissecting aneurysms without PICA involvement, proximal occlusion, distal occlusion, and complete trapping were performed in 15, 1, and 2 patients, respectively. For VA dissecting aneurysms with PICA involvement, OA–PICA bypass was performed in all patients (n = 8). The blind-alley formation and complete trapping were used in 6 and 2 patients, respectively. In one patient, fusiform VA aneurysm was obliterated with proximal occlusion. Two patients with proximal PICA dissecting aneurysms were treated with OA–PICA with trapping (Table 2). The average operative times were 5 and 6 h for non-bypass and bypass cases, respectively. The average temporary clip times were 8 and 29 min for non-bypass and bypass cases, respectively.
Table 2.
Aneurysm characteristics, surgical techniques and complete aneurysm obliteration.
| Aneurysm characteristics | Surgical technique | Number of patients | Complete obliteration |
|---|---|---|---|
| VA, VA-PICA and true PICA saccular aneurysms | Clipping | 18 | 18 |
| OA-PICA bypass with clipping | 1 | 1 | |
| VA dissecting aneurysm | Proximal occlusion (V4) | 14 | 14 |
| Proximal occlusion (V3) | 1 | 1 | |
| Distal occlusion (V4) | 1 | 1 | |
| Trapping | 2 | 2 | |
| OA-PICA bypass with blind-alley formation | 6 | 6 | |
| OA-PICA bypass with trapping | 2 | 2 | |
| VA fusiform aneurysm | Proximal occlusion (V4) | 1 | 1 |
| True PICA dissecting aneurysm | OA-PICA bypass with trapping | 2 | 2 |
| Total | 48 | 48 |
OA, occipital artery; PICA, posterior inferior cerebellar artery; V3, third segment of the vertebral artery; V4, fourth segment of the vertebral artery.
Complete aneurysm obliteration was achieved in all patients. If OA–PICA bypass (n = 11) was performed, good bypass patency was accomplished in all cases. Three months postoperatively, good outcomes were achieved in 37 (77.1%) patients for the overall and 25 (89.3%) patients for the good-grade groups. Three patients in the good-grade group had poor surgical outcomes. One 80-year-old patient with ruptured VA–PICA aneurysm developed severe sepsis from pneumonia postoperatively and died of respiratory failure. A 52-year-old patient with ruptured VA–PICA aneurysm developed aspiration pneumonia postoperatively. Another 76-year-old patient with ruptured proximal true PICA aneurysm developed postoperative hydrocephalus and ventriculoperitoneal shunt malfunction. After shunt revision, GOS 4 was achieved 10 months postoperatively. All patients with VA dissecting aneurysms achieved good outcomes (Table 3). No postoperative neck pain was detected in all patients at the last follow-up.
Table 3.
Presenting grade and clinical outcomes 3 months postoperatively.
| Good outcomes (GOS 4,5) | Poor outcomes (GOS 1–3) | Total | |
|---|---|---|---|
| Good grade (WFNS 1–3 + unruptured cases) | 25 (89.3%) | 3 | 28 |
| Poor grade (WFNS 4,5) | 12 | 8 | 20 |
| Total | 37 (77.1%) | 11 | 48 |
GOS, Glasgow outcome score; WFNS, World Federation of Neurosurgical Societies.
A new onset of dysphagia was detected in 2 (8%) patients with good grades who suffered from ruptured right VA–PICA aneurysms treated with direct clipping and ruptured left VA dissecting aneurysms with PICA involvement treated with OA–PICA bypass and blind-alley formation. For the latter patient, the high position of the caudal loop of the PICA concealed in the cerebellomedullary fissure was detected intraoperatively. The lateral medullary segment of the PICA was used as the recipient artery for bypass after intentionally sacrificing two roots of the spinal accessory nerve to access this segment of the artery. In both patients, the dysphagia spontaneously improved over time and completely recovered within 6 months postoperatively.
4. Illustrative cases
4.1. Illustrative case 1
An 84-year-old female patient presented with incidental finding of a right VA aneurysm. A 4-mm saccular aneurysm of the right VA without PICA involvement was detected by computed tomography angiography (CTA) (Fig. 2A). A right anterior inferior cerebellar artery (AICA)–PICA loop was found. The aneurysm was located just above the hypoglossal canal. The right far-lateral transcondylar approach (Fig. 2B and C) and aneurysm clipping were performed with the standard curve clip (Fig. 2D and E). Complete obliteration of the aneurysm with good patency of the VA was confirmed by CTA immediately after the surgery (Fig. 2F). No further neurological deficits were detected. The patient achieved a GOS of grade 5 at discharge date and 3 months after the surgery (Video 2).
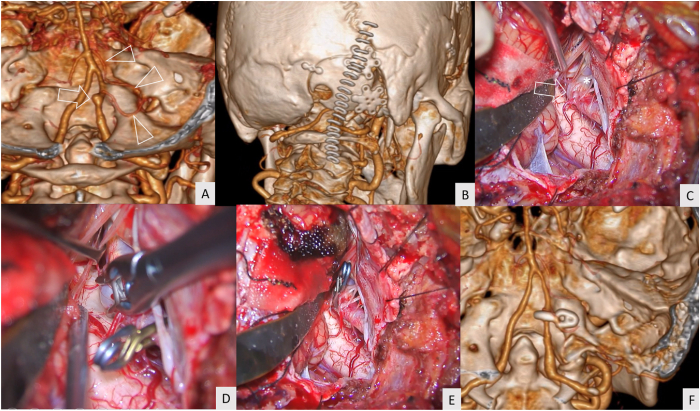
Fig. 2.
Illustrative case 1 (A) Computed tomography angiography (CTA) demonstrated a saccular aneurysm of the right vertebral artery (arrow) located just above the hypoglossal canal. The anterior inferior cerebellar artery–posterior inferior cerebellar artery (AICA–PICA) loop was identified (arrowhead) (B) The linear paramedian incision (staples) was used for the right far-lateral transcondylar approach (C) A saccular aneurysm (arrow) and the hypoglossal nerve (asterisk) were identified through the right far-lateral transcondylar approach (D) The aneurysm clip was applied to the aneurysm neck (E) Operative field after aneurysm clipping (F) Postoperative CTA revealed complete aneurysm obliteration and good patency of the right vertebral artery.
4.2. Illustrative case 2
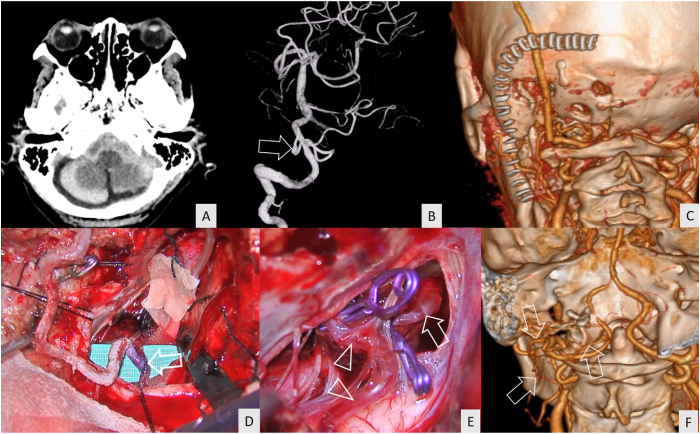
A 63-year-old female patient presented with sudden alteration of consciousness. The WFNS grade was 5 at the first hospital. CT showed a diffuse SAH with predominance in the right cerebellomedullary cistern (Fig. 3A). CTA detected a right VA–PICA aneurysm located below the hypoglossal canal (Fig. 3B). The right far-lateral transcondylar approach (Fig. 3C) and aneurysm clipping were performed (Fig. 3D and E). Complete obliteration of the aneurysm with PICA preservation was confirmed by CTA immediately after the surgery (Fig. 3F). The postoperative course was uneventful. The patient recovered and achieved GOS grade of 3 at 3 months after the surgery (Video 3-1, 3-2).
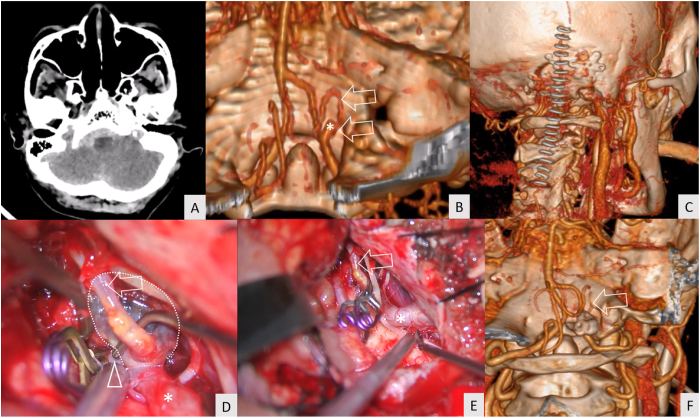
Fig. 3.
Illustrative case 2 (A) Computed tomography revealed thick subarachnoid clot at the right cerebellomedullary cistern (B) Computed tomography angiography (CTA) demonstrated a saccular aneurysm of the right vertebral artery–posterior inferior cerebellar artery (VA–PICA) junction (asterisk) located below the hypoglossal canal. The arrow shows the course of the posterior inferior cerebellar artery (PICA) (C) The linear paramedian incision (staples) was used for the right far-lateral transcondylar approach (D) A saccular aneurysm (dotted line), PICA (arrow), proximal vertebral artery (asterisk), and distal vertebral artery (arrow head) were identified after proximal and distal control (clip) (E) Aneurysm clips were applied to the aneurysm neck. The PICA (arrow) and proximal vertebral artery (asterisk) were identified after aneurysm clipping (clips) (F) The postoperative CTA revealed complete aneurysm obliteration and good patency of the right vertebral artery and PICA (arrow).
4.3. Illustrative case 3
A 39-year-old female patient had an incidental finding of a left true PICA aneurysm located just proximal to the caudal loop (Fig. 4A and B). A linear paramedian incision was used for the left far-lateral transcondylar fossa approach (Fig. 4C). After the aneurysm was identified intraoperatively (Fig. 4D), the aneurysm was occluded while preserving the PICA (Fig. 4E). Complete obliteration of the aneurysm was confirmed by CTA (Fig. 4F). No further neurological deficits were detected. A GOS of 5 was achieved at discharge and 3 months after the surgery (Video 4).
Fig. 4.
Illustrative case 3 (A, B) Computed tomography angiography (CTA) demonstrated a saccular aneurysm of the left true PICA (arrow) located just proximal to the caudal loop (arrow head) (C) A linear paramedian incision (staples) was used for the left far-lateral transcondylar fossa approach (D) A saccular aneurysm (arrow) was identified through the left far-lateral transcondylar approach (E) The operative field after aneurysm clipping (F) The postoperative CTA revealed complete aneurysm obliteration.
4.4. Illustrative case 4
A 62-year-old female patient presented with a sudden severe headache and initial WFNS grade 2. Posterior fossa SAH was detected by CT (Fig. 5A). A dissecting aneurysm at the lateral medullary segment of the left PICA was demonstrated on digital subtraction angiography (DSA) (Fig. 5B). An “L”-shaped incision was performed for OA harvesting and left far-lateral transcondylar approach (Fig. 5C). OA–PICA bypass (Fig. 5D) and aneurysm trapping (Fig. 5E) were performed. The CTA performed immediately after the surgery showed complete obliteration of the aneurysm with patent OA–PICA bypass (Fig. 5F). No further neurological deficits were detected postoperatively. The patient achieved a GOS of 4 at discharge and 3 months after the surgery (Video 5-1, 5-2).
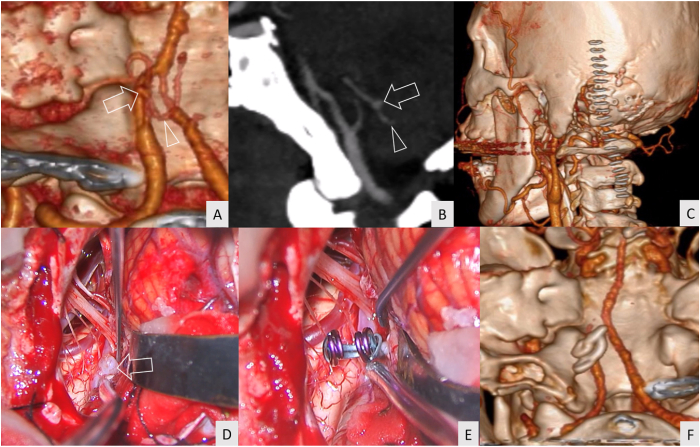
Fig. 5.
Illustrative case 4 (A) Computed tomography revealed thick subarachnoid hemorrhage at the posterior fossa (B) Digital subtraction angiography demonstrated a fusiform shape aneurysm (arrow) at the lateral medullary segment of the left posterior inferior cerebellar artery (PICA) (C) An “L”-shaped skin incision (staples) was used for occipital artery (OA) harvesting and a left far-lateral transcondylar approach (D) OA–PICA bypass (arrow) was completed (E) The aneurysm was obliterated by complete trapping with the preservation of the proximal (arrow) and distal PICA (arrowhead) (F) Postoperative computed tomography angiography revealed the patency of the OA (arrow).
5. Discussion
5.1. Endovascular treatment for VA and PICA aneurysms
Endovascular treatment is effective for VA and PICA aneurysms and provides good functional outcomes. Because of the relatively high morbidity and mortality from the complicated surgical approach to the posterior circulation, the treatment of posterior circulation aneurysms was changing from open surgery to endovascular treatment, especially for fusiform or dissecting aneurysms.1,3, 4, 5,7
For nondissecting aneurysms, various outcomes were revealed. Spetzler et al reported the 6-year results of 500 patients with ruptured cerebral aneurysms. In total, 21 patients with PICA aneurysms were treated with clipping (n = 18) and coiling (n = 3). Although the aneurysm obliteration rates were significantly lower and retreatment rates were significantly higher in patients undergoing coiling than in those undergoing clipping, the outcomes for posterior circulation aneurysms continued to favor coiling.1 Mericle et al reported 31 patients with proximal PICA aneurysms treated with endovascular techniques. Most patients (84%) presented with SAH with a high percentage of poor grades (88%). Favorable outcome was achieved in 68% with 100% complete aneurysm obliteration.22 Chalouhi et al reported 76 patients with PICA aneurysms treated with endovascular techniques. Favorable outcomes were achieved in 93% and 78.7% of the cases in the unruptured and ruptured groups, respectively, but the residual aneurysm rate was 36.6%.23 Yin et al reported the 4-year results of 55 patients with PICA aneurysms (n = 13 rupture; n = 42 unruptured). Endovascular treatment and surgical clipping were performed in 26 and 29 patients, respectively. They found that endovascular treatment achieved better outcomes (modified Rankin scale score ≤2) than surgical treatment (96.2% vs. 89.7%).24 Juszkat et al reported the endovascular treatment for 38 patients with ruptured PICA saccular aneurysms. Aneurysms were located at the proximal PICA in 32 patients (84%). Good outcomes were achieved in 60.5% of the patients, with 29.4% recurrent rate.25
For dissecting aneurysms, studies have reported treatment results. Liu et al reported 56.7–100% aneurysm obliteration in 196 patients (13% SAH) with VA dissecting aneurysms treated with various endovascular techniques. Favorable outcomes (modified Rankin score 0.5 ± 1.1) were achieved in this series. The most challenging case for endovascular therapy was a ruptured vertebral dissecting aneurysm with PICA involvement.26 Catapano et al reported 91 patients with VA dissecting aneurysms treated with endovascular techniques; 32% of the patients had a poor neurological status at presentation. Favorable outcomes were achieved in 68%. The rates of procedural complications (8%) and retreatment (13%) were significantly higher with stent/coil treatment than with vessel sacrifice or flow diversion devices.2
5.2. Open surgery for VA and PICA aneurysms
For patients with limitations for endovascular treatment, such as tortuosity or atherosclerosis of the VA, allergies to contrast agents, or financial considerations, open surgery was inevitable and should be performed with the appropriate surgical strategy to decrease surgical complications, especially lower cranial nerve deficits.27,28
Many case series of microsurgical treatment with various clinical characteristics and outcomes have been presented. Matsushima et al reported the success of microsurgical treatment of eight patients with VA–PICA saccular aneurysms (six ruptured and two unruptured ones) via seven transcondylar fossa approaches and one transcondylar approach. Transient postoperative lower cranial nerve deficit was displayed in three (37.5%) patients: two for hypoglossal nerve palsy and one for dysphagia.20 Al-khayat et al reported 52 patients with VA and PICA aneurysms treated with microsurgery. Favorable outcomes were accomplished in 90.3%, but postoperative lower cranial nerve palsy was high (48%).29 Rodríguez-Hernández et al reported 51 (64% SAH) patients with PICA aneurysms treated with various microsurgical techniques via the far-lateral approach. Proximal PICA aneurysms (located inside the vagoaccessory triangle) were found in 43 (84%) patients. Good outcomes were achieved in 80% of the patients, with 100% obliteration rate and 10% complication rate.12
Spetzler et al reported 6-year results of 500 patients with ruptured cerebral aneurysms, of which 21 patients with PICA aneurysms were treated with clipping (n = 18) and coiling (n = 3). For PICA aneurysms, clipping had a significantly higher incidence of poor outcomes than coiling at 3-year (58.3% vs. 26.9%) and 6-year (62.1% vs. 29.6%) follow-up.1 Williamson et al reported the 4-year results of 21 patients with ruptured PICA aneurysms (saccular aneurysms, n = 14; fusiform and dissecting aneurysms, n = 8) treated with microsurgery (n = 19) and endovascular methods (n = 2). They found that patients with PICA aneurysms were more likely to have a fusiform aneurysm (36% vs. 12%) and had a higher incidence of lower cranial nerve dysfunction than patients without PICA aneurysms (50% vs. 16%). Patients with PICA aneurysms had a significantly higher incidence of poor outcome than all other patients with aneurysm at discharge. Both the high rates of lower cranial nerve dysfunction and fusiform aneurysms in this cohort may help explain this poor outcome.30 Ota et al reported the outcomes of 59 patients with VA aneurysms accessed by the transcondylar fossa approach. They focused on the drainage pattern of the posterior condylar emissary vein, but treatment outcome was not described.16 Seoane et al reported 52 patients with proximal PICA aneurysms treated with the far-lateral approach without drilling the occipital condyle. Favorable outcome was accomplished in 92.8%, but postoperative lower cranial nerve palsy was relatively high (25%).31 Palanisamy et al reported 20 patients with unruptured VA and VA–PICA aneurysms successfully treated by the transcondylar fossa approach while preserving the C1 lamina. One patient (5%) with wrapping of the aneurysm showed aneurysms of the same size postoperatively. One patient (5%) developed transient postoperative lower cranial nerve deficit, which completely recovered at 6 months postoperatively and one patient suffered from CSF rhinorrhea postoperatively, which needs ventriculoperitoneal shunt. No postoperative clinical outcome was described in this study.12 Fatehi et al reported 50 (70% rupture) patients with PICA aneurysms treated with microsurgery (n = 39) and endovascular therapy (n = 11). Residual neck or residual aneurysms were detected in 4 (10.2%) patients treated with microsurgery. They concluded no significant difference in outcomes between the microsurgical and endovascular treatment groups at short- and long-term follow-ups.32 Del Maestro et al reported 25 (60% rupture) patients with PICA aneurysms treated with microsurgery (92% by direct clipping and 8% by PICA–PICA bypass with trapping) via the far-lateral approach, and 60% of the patients achieved good outcomes.33
For dissecting aneurysms, three surgical series were found. Frisoli et al reported 42 patients with vertebrobasilar dissecting aneurysms treated with various microsurgical techniques. Of these patients, 34 (81%) presented with SAH and 38% of this group was classified to have poor clinical grade at presentation. The complete aneurysm obliteration rate was 95%, and the surgical complication rate was 7%. Good outcomes were observed in 20 (48%) of the 42 patients.9 Wongsuriyanan et al reported seven patients with ruptured unclippable VA aneurysms (include dissecting aneurysms) with PICA involvement (71.4% had poor grades) treated with OA–PICA bypass and blind-alley formation (clip occlusion of the proximal VA and PICA origin). The complete obliteration rate of aneurysms was 100%, and favorable outcomes were achieved in 42.9% of the cases 1 month after the surgery. OA–PICA bypass was patent in all patients, and no PICA infarction was detected in this series.21 Durongwatana et al reported 22 patients with VA dissection (91% rupture) treated with pure microsurgery. Four dissections types (PICA, pre-PICA, post-PICA, and non-PICA type) were employed with various methods according to their proposed treatment algorithm. Complete obliteration was accomplished in 100%, and favorable outcomes were achieved in 86.4 and 100% for the overall and good-grade groups, respectively.13
5.3. Open surgery and endovascular treatment for VA and PICA aneurysms
For saccular PICA aneurysms, a meta-analysis demonstrates that microsurgical clipping results in superior angiographic outcomes, comparable functional outcomes, but higher rates of lower cranial nerve palsy than endovascular treatment.7
For dissecting aneurysms of the VA and proximal PICA, two meta-analyses have demonstrated that endovascular treatment was associated with high rates of complete occlusion, good long-term neurologic outcomes, and low recurrence and mortality rates. Deconstructive techniques are associated with higher occlusion rates. PICA involvement may lower the efficacy of the endovascular treatment.26,34,35 With open surgery, high occlusion rates and good outcomes can be achieved by an appropriate surgical strategy and adequate skull-based approach. Appropriate PICA revascularization and optimal clip occlusion were needed for dissecting aneurysms with PICA involvement with high rates of favorable outcomes.13,21 No meta-analysis compared the effectiveness and safety of open surgery and endovascular treatment for dissecting aneurysms.
In the present study, we collected 48 patients with VA and proximal PICA aneurysms (including saccular, dissecting, and fusiform aneurysms) treated with pure microsurgical techniques via the far-lateral approach. The outcomes of this study were comparable with those of a large case series (n ≥ 30) of microsurgery and endovascular treatment (Table 4).2,8, 9, 10, 11,22,23,25,26,29,31
Table 4.
Treatment outcomes of the large clinical studies involving vertebral artery and posterior inferior cerebellar artery aneurysms.
| Series (year) | Treatment | No. Of patients | SAH | Poor grade | Type of aneurysms | Overall favorable outcomes | Residual aneurysms | New deficits/procedural complications |
|---|---|---|---|---|---|---|---|---|
| Al-khayat et al29 (2005) | Open surgery | 52 | 65% | 53% | PICA aneurysm, n = 47 VA aneurysm, n = 5 |
90.3 | NA | 48% |
| Mericle et al22 (2006) | Endovascular technique | 31 | 84% | 88% | Proximal PICA | 68% | 0% | 10% |
| Rodriguez-Hernandez et al11 (2011) | Open surgery | 43 (proximal PICA) | 64% (all PICA) | 38% (all PICA) | PICA aneurysm | 80% (all PICA) | 0% | 10% (all PICA) |
| Chalouhi et al23 (2013) | Endovascular technique | 76 | 80% | 47% | PICA aneurysms -Dissecting, n = 9 -Saccular, n = 67 |
Unruptured, 93% Ruptured, 78.7% |
36.6% | 12.7% |
| Lehto et al8 (2014) | Open surgery | 30 (proximal PICA) | 93% (all PICA) | 62% (all PICA) | Saccular 57% Fusiform 43% |
69% (all PICA) | Saccular, 6% Fusiform, 12% (all PICA) |
19% (all PICA) |
| Bohnstedt et al10 (2015) | Open surgery and endovascular technique | 79 (VA + proximal PICA) | 78% (VA + proximal PICA) | NA | Open surgery: VA and proximal PICA, n = 50 (63.3%) -Saccular, n = 40 (80%) -Dissecting, n = 2 (4%) -Fusiform, n = 8 (16%) Endovascular: VA and proximal PICA, n = 29 (36.7%) -Saccular, n = 22 (76%) -Dissecting, n = 6 (21%) -Fusiform, n = 1 (3%) |
NA | Rebleed -Open surgery = 0 -Endo = 1 |
Open surgery, 22 (44%) Endovascular, 3 (10%) |
| Juszkat et al25 (2016) | Endovascular technique | 38 | 100% | 47% | Proximal PICA saccular, n = 32 (84%) | 60.5% (all PICA) | Recurrent 29.4% (all PICA) | 4 (10%) |
| Seoane et al31 (2017) | Open surgery | 56 (proximal + distal PICA) | 60.7% | NA | Proximal PICA, n = 52 (93%) | 92.8% (all PICA) | 0% (all PICA) | 25% |
| Frisoli et al9 (2021) | Open surgery | 42 | 63% | NA | Vertebrobasilar dissecting aneurysm | 48% | 5% | 7% |
| Catapano et al2 (2022) | Endovascular technique | 91 | 59% | 44% | VA dissecting aneurysm | 68% | 13% | 18% |
| Liu et al26 (2022) | Endovascular technique | 196 | 13% | NA | VA dissecting aneurysm | NA | 0–43% | NA |
| The current study | Open surgery | 48 | 87.5 | 41.7% | VA and proximal PICA: -Dissecting aneurysm, n = 28 (58.3%) -Saccular aneurysm, n = 19 (39.6%) -Fusiform aneurysm, n = 1 (2.1%) |
77.1% | 0% | 8% |
Endo, endovascular treatment; NA, not available; PICA, posterior inferior cerebellar artery; SAH, subarachnoid hemorrhage; VA, vertebral artery.
To the best of our knowledge, this study presents a large series of patients with VA and proximal PICA aneurysms with the highest percentage of SAH treated by pure microsurgery using the far-lateral approach in a single institute.
5.4. Far-lateral approach and variants
Many variations of the posterolateral skull-based approach to VA and PICA aneurysms were developed (Table 5). In 1986, Heros36 described the modified lateral suboccipital approach (extreme-lateral removal of the foramen magnum rim toward the condylar fossa, just posterior to the occipital condyle, with C1 laminectomy) for treating VA and proximal basilar trunk aneurysms without resecting the condyle. In 1990, Sen and Sekhar37 described the extreme-lateral approach to intradural lesions of the cervical spine and foramen magnum. This approach was the same as the modified lateral suboccipital approach described by Heros36 but added the removal of the posterior part of occipital condyle and lateral mass of C1. In 1991, Bertalanffy et al38 described the transcondylar approach, which is same as the extreme-lateral approach described by Sen and Sekhar,37 but added the removal of the jugular tubercle.
Table 5.
Posterolateral approach to the anterolateral foramen magnum and extent of bone resection.
| Extent of bone resection | Posterolateral rim of the foramen magnum | Posterior arch of C1 | Posterior part of the occipital condyle | Jugular tubercle |
|---|---|---|---|---|
| Modified lateral suboccipital approach (Heros36 1986) | Y | Y | N | N |
| Extreme lateral approach (Sen and Sekhar37 1990) | Y | Y | Y | N |
| Transcondylar approach (Bertalanffy et al38 1991) | Y | Y | Y | Y |
| Extreme-lateral inferior transtubercular exposure (ELITE) (Day et al40 1997) | Y | Y/N | Y | Y |
| Transcondylar fossa approach (Matsushima et al41 1998) | Y | Y/N | N | Y |
| Far-lateral approach without drilling the occipital condyle (Seoane et al31 2017) | Y | Y | N | N |
| Modified transcondylar fossa approach (Palanisamy et al12 2018) | Y | N | N | Y |
| This study | Y | N | Y/N | Y |
N, no; Y, yes.
With the extreme-lateral approach and transcondylar approach, the atlanto-occipital joint is partially damaged, leading to instability and postoperative cervical pain.28,39 In 1997, Day et al40 described the extreme-lateral inferior transtubercular exposure (ELITE), which involved the removal of the anterolateral rim of the foramen magnum, jugular tubercle, and posterior one-third of the occipital condyle. The lateral portion of the C1 posterior arch was removed in selected cases that require more inferior exposure. They emphasized that the removal of the anterolateral rim of the foramen magnum and jugular tubercle is the key step for the anterior exposure of the lower brainstem. In 1998, Matsushima et al41 described the transcondylar fossa (supracondylar transjugular tubercle) approach to the foramen magnum. This approach is almost the same as the transcondylar approach described by Bertalanffy et al38; however, the occipital condyle and atlanto-occipital joint were preserved. With this approach, the condylar fossa is removed laterally to the sigmoid sinus and anteriorly to the hypoglossal canal. The posterior part of the jugular tubercle above the hypoglossal canal is also drilled away to the anterolateral foramen magnum. The posterior arch of the C1 was added when the inferior approach was required. This approach is suitable for the lesion located above the hypoglossal canal. In 2017, Seoane et al31 reported the far-lateral approach without drilling the occipital condyle to treat VA–PICA aneurysms. The lateral rim of the foramen magnum was removed with C1 hemilaminectomy. The condylar fossa and occipital condyle were preserved. This approach was the same as the modified lateral suboccipital approach described by Heros.36 In 2018, Palanisamy et al12 reported the modified transcondylar fossa approach (transcondylar fossa approach while preserving the C1 lamina) for the treatment of VA and VA–PICA aneurysms.
Matsushima et al20 described the important anatomical landmarks for the transcondylar fossa approach. The landmarks of the condylar fossa were the 1) posterior condylar canal and emissary vein in the canal and 2) sigmoid magnum triangle which is a part of the condylar fossa. The condylar fossa is the posterior portion of the jugular tubercle, and the tip of the sigmoid magnum triangle is the jugular tubercle.42 If the lesion is located below the hypoglossal canal, the medial parts of the occipital condyle and lateral mass of C1 are removed in addition to the transcondylar fossa approach described above (transcondylar approach).20
This study reported the successful far-lateral approach without C1 laminectomy to access VA and proximal PICA aneurysms located above the lower border of the foramen magnum. The condylar fossa was drilled in all cases (transcondylar fossa approach). The drilling of the posteromedial part of the occipital condyle was added (transcondylar approach) when the aneurysm is located at or below the level of the hypoglossal canal. According to the results of this study, C1 laminectomy was not necessary for VA and proximal PICA aneurysms located above the lower border of the foramen magnum. When the aneurysm is located below the foramen magnum, C1 laminectomy should be added to the transcondylar approach.
5.5. Limitations of the current study
This study has some limitations. First, it was a retrospective descriptive study. Second, postoperative outcomes depend on many factors, including aneurysm characteristics (saccular or dissecting type), aneurysm location (PICA or non-PICA involvement), and preoperative clinical status of the patients (particularly in ruptured cases). Third, the follow-up period was relatively short because most patients were referred from a hospital far from our institute. Finally, CTA was routinely used for preoperative and postoperative vascular imaging, which provides lower-quality images of vascular structures, compared with DSA.
6. Conclusions
Microsurgery is a safe and effective treatment of VA and proximal PICA aneurysms. The far-lateral transcondylar or transcondylar fossa approach without C1 laminectomy was adequate and effective for aneurysms located above the lower border of the foramen magnum.
CRediT authorship contribution statement
Kitiporn Sriamornrattanakul: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. Nasaeng Akharathammachote: Supervision, Methodology. Areeporn Chonhenchob: Supervision, Conceptualization. Atithep Mongkolratnan: Investigation, Conceptualization. Nattawut Niljianskul: Visualization, Conceptualization. I-sorn Phoominaonin: Investigation, Conceptualization. Chanon Ariyaprakai: Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- AICA
anterior inferior cerebellar artery
- CTA
computed tomography angiography
- GOS
Glasgow outcome score
- OA
occipital artery
- PICA
posterior inferior cerebellar artery
- SAH
subarachnoid hemorrhage
- VA
vertebral artery
- WFNS
World Federation of Neurosurgical Societies
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.wnsx.2023.100216.
Appendix ASupplementary data
The following are the Supplementary data to this article:
References
- 1.Spetzler R.F., McDougall C.G., Zabramski J.M., et al. The barrow ruptured aneurysm trial: 6-year results. J Neurosurg. 2015;123(3):609–617. doi: 10.3171/2014.9.JNS141749. [DOI] [PubMed] [Google Scholar]
- 2.Catapano J.S., Ducruet A.F., Cadigan M.S., et al. Endovascular treatment of vertebral artery dissecting aneurysms: a 20-year institutional experience. J Neurointerventional Surg. 2022;14(3):257–261. doi: 10.1136/neurintsurg-2020-017089. [DOI] [PubMed] [Google Scholar]
- 3.Aboukaïs R., Zairi F., Boustia F., Bourgeois P., Leclerc X., Lejeune J.P. Vertebral artery-posterior inferior cerebellar artery convergence aneurysms treated by endovascular or surgical treatment: mid- and long-term outcome. Neurochirurgie. 2016;62(2):72–77. doi: 10.1016/j.neuchi.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Awad A.J., Mascitelli J.R., Haroun R.R., De Leacy R.A., Fifi J.T., Mocco J. Endovascular management of fusiform aneurysms in the posterior circulation: the era of flow diversion. Neurosurg Focus. 2017;42(6):E14. doi: 10.3171/2017.3.FOCUS1748. [DOI] [PubMed] [Google Scholar]
- 5.Eller J.L., Dumont T.M., Mokin M., et al. Endovascular treatment of posterior circulation aneurysms. Neurol Res. 2014;36(4):339–343. doi: 10.1179/1743132814Y.0000000323. [DOI] [PubMed] [Google Scholar]
- 6.Starnoni D., Maduri R., Al Taha K., et al. Ruptured PICA aneurysms: presentation and treatment outcomes compared to other posterior circulation aneurysms. A Swiss SOS study. Acta Neurochir. 2019;161(7):1325–1334. doi: 10.1007/s00701-019-03894-5. [DOI] [PubMed] [Google Scholar]
- 7.Ali A.M.S., Hannan C.J., Islim A.I., Mascitelli J.R., Javadpour M. Surgical and endovascular treatment of saccular posterior inferior cerebellar artery aneurysms: systematic review and meta-analysis. World Neurosurg. 2022;162:e168–e177. doi: 10.1016/j.wneu.2022.02.103. [DOI] [PubMed] [Google Scholar]
- 8.Lehto H., Harati A., Niemelä M., et al. Distal posterior inferior cerebellar artery aneurysms: clinical features and outcome of 80 patients. World Neurosurg. 2014;82(5):702–713. doi: 10.1016/j.wneu.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Frisoli F.A., Srinivasan V.M., Catapano J.S., et al. Vertebrobasilar dissecting aneurysms: microsurgical management in 42 patients. J Neurosurg. 2021:1–9. doi: 10.3171/2021.9.JNS21397. [DOI] [PubMed] [Google Scholar]
- 10.Bohnstedt B.N., Ziemba-Davis M., Edwards G., et al. Treatment and outcomes among 102 posterior inferior cerebellar artery aneurysms: a comparison of endovascular and microsurgical clip ligation. World Neurosurg. 2015;83(5):784–793. doi: 10.1016/j.wneu.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Hernández A., Lawton M.T. Anatomical triangles defining surgical routes to posterior inferior cerebellar artery aneurysms. J Neurosurg. 2011;114(4):1088–1094. doi: 10.3171/2010.8.JNS10759. [DOI] [PubMed] [Google Scholar]
- 12.Palanisamy D., Yasuhiro Y., Kyosuke M., Tsukasa K., Katsumi T., Kato Y. Transcondylar fossa approach to unruptured vertebral artery and vertebral artery-posterior inferior cerebellar artery aneurysms: surgical outcome. World Neurosurg. 2018;119:e783–e791. doi: 10.1016/j.wneu.2018.07.268. [DOI] [PubMed] [Google Scholar]
- 13.Durongwatana N., Sriamornrattanakul K., Wongsuriyanan S., Akharathammachote N. Microsurgical treatment of vertebral artery dissection: surgical strategies and treatment outcomes. World Neurosurg. 2022;159:e375–e388. doi: 10.1016/j.wneu.2021.12.057. [DOI] [PubMed] [Google Scholar]
- 14.Wen H.T., Rhoton A.L., Jr., Katsuta T., de Oliveira E. Microsurgical anatomy of the transcondylar, supracondylar, and paracondylar extensions of the far-lateral approach. J Neurosurg. 1997;87(4):555–585. doi: 10.3171/jns.1997.87.4.0555. [DOI] [PubMed] [Google Scholar]
- 15.Salas E., Sekhar L.N., Ziyal I.M., Caputy A.J., Wright D.C. Variations of the extreme-lateral craniocervical approach: anatomical study and clinical analysis of 69 patients. J Neurosurg. 1999;90(2 Suppl):206–219. doi: 10.3171/spi.1999.90.2.0206. [DOI] [PubMed] [Google Scholar]
- 16.Ota N., Tanikawa R., Yoshikane T., et al. Surgical microanatomy of the posterior condylar emissary vein and its anatomical variations for the transcondylar fossa approach. Oper Neurosurg (Hagerstown). 2017;13(3):382–391. doi: 10.1093/ons/opw038. [DOI] [PubMed] [Google Scholar]
- 17.Hatano Y., Ota N., Noda K., et al. Surgical microanatomy of the occipital artery for suboccipital muscle dissection and intracranial artery reconstruction. Surg Neurol Int. 2019;10:127. doi: 10.25259/SNI-16-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsuno M., Tanikawa R., Uemori G., Kawasaki K., Izumi N., Hashimoto M. Occipital artery-to-posterior inferior cerebellar artery anastomosis with multiple-layer dissection of suboccipital muscles under a reverse C-shaped skin incision. Br J Neurosurg. 2015;29(3):401–405. doi: 10.3109/02688697.2015.1004300. [DOI] [PubMed] [Google Scholar]
- 19.Sriamornrattanakul K., Akharathammachote N., Chonhenchob A., et al. Course of the V3 segment of the vertebral artery relative to the suboccipital triangle as an anatomical marker for a safe far lateral approach: a retrospective clinical study. Surg Neurol Int. 2022;13:304. doi: 10.25259/SNI_346_2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushima T., Matsukado K., Natori Y., Inamura T., Hitotsumatsu T., Fukui M. Surgery on a saccular vertebral artery-posterior inferior cerebellar artery aneurysm via the transcondylar fossa (supracondylar transjugular tubercle) approach or the transcondylar approach: surgical results and indications for using two different lateral skull base approaches. J Neurosurg. 2001;95(2):268–274. doi: 10.3171/jns.2001.95.2.0268. [DOI] [PubMed] [Google Scholar]
- 21.Wongsuriyanan S., Sriamornrattanakul K. blind-alley formation and occipital artery-posterior inferior cerebellar artery bypass for the treatment of unclippable vertebral artery aneurysms with posterior inferior cerebellar artery involvement. World Neurosurg. 2020;138:e539–e550. doi: 10.1016/j.wneu.2020.02.174. [DOI] [PubMed] [Google Scholar]
- 22.Mericle R.A., Reig A.S., Burry M.V., Eskioglu E., Firment C.S., Santra S. Endovascular surgery for proximal posterior inferior cerebellar artery aneurysms: an analysis of Glasgow Outcome Score by Hunt-Hess grades. Neurosurgery. 2006;58(4):619–625. doi: 10.1227/01.NEU.0000204127.81249.28. discussion 619-25. [DOI] [PubMed] [Google Scholar]
- 23.Chalouhi N., Jabbour P., Starke R.M., et al. Endovascular treatment of proximal and distal posterior inferior cerebellar artery aneurysms. J Neurosurg. 2013;118(5):991–999. doi: 10.3171/2012.12.JNS121240. [DOI] [PubMed] [Google Scholar]
- 24.Yin Q., Guo H., Wei J., Liu P., Lv M., Li Y. Posterior inferior cerebellar artery aneurysms: comparison of results of surgical and endovascular managements at one single center. Neurol India. 2020;68(5):1115–1124. doi: 10.4103/0028-3886.294555. [DOI] [PubMed] [Google Scholar]
- 25.Juszkat R., Kram P., Stanisławska K., et al. Ten years of experience in endovascular treatment of ruptured aneurysms of the posterior inferior cerebellar artery. Intervent Neuroradiol. 2016;22(2):129–137. doi: 10.1177/1591019915622164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu P., Li Z., Hu L., et al. Clinical characteristics, endovascular choices, and surgical outcomes of intracranial vertebral artery dissecting aneurysms: a consecutive series of 196 patients. J Neurosurg. 2022:1–8. doi: 10.3171/2022.4.JNS22609. [DOI] [PubMed] [Google Scholar]
- 27.Murai Y., Matano F., Yokobori S., Onda H., Yokota H., Morita A. Treatment strategies of subarachnoid hemorrhage from bilateral vertebral artery dissection: a case report and literature review focusing on the availability of stent placement. World Neurosurg. 2017;106 doi: 10.1016/j.wneu.2017.06.167. 1050.e11-1050.e20. [DOI] [PubMed] [Google Scholar]
- 28.Nanda A., Konar S., Bir S.C., Maiti T.K., Ambekar S. Modified far lateral approach for posterior circulation aneurysms: an institutional experience. World Neurosurg. 2016;94:398–407. doi: 10.1016/j.wneu.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 29.Al-khayat H., Al-Khayat H., Beshay J., Manner D., White J. Vertebral artery-posteroinferior cerebellar artery aneurysms: clinical and lower cranial nerve outcomes in 52 patients. Neurosurgery. 2005;56(1):2–10. discussion 11. [PubMed] [Google Scholar]
- 30.Williamson R.W., Wilson D.A., Abla A.A., et al. Clinical characteristics and long-term outcomes in patients with ruptured posterior inferior cerebellar artery aneurysms: a comparative analysis. J Neurosurg. 2015;123(2):441–445. doi: 10.3171/2014.10.JNS141079. [DOI] [PubMed] [Google Scholar]
- 31.Seoane P., Kalb S., Clark J.C., et al. Far-lateral approach without drilling the occipital condyle for vertebral artery-posterior inferior cerebellar artery aneurysms. Neurosurgery. 2017;81(2):268–274. doi: 10.1093/neuros/nyw136. [DOI] [PubMed] [Google Scholar]
- 32.Fatehi M., Rizzuto M.A., Prakash S., Haw C., Gooderham P.A., Redekop G.J. Functional outcomes after treatment of posterior inferior cerebellar artery aneurysms. Cureus. 2020;12(11) doi: 10.7759/cureus.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Maestro M., Luzzi S., Galzio R. Microneurosurgical management of posterior inferior cerebellar artery aneurysms: results of a consecutive series. Acta Neurochir Suppl. 2021;132:33–38. doi: 10.1007/978-3-030-63453-7_5. [DOI] [PubMed] [Google Scholar]
- 34.Sönmez Ö., Brinjikji W., Murad M.H., Lanzino G. Deconstructive and reconstructive techniques in treatment of vertebrobasilar dissecting aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36(7):1293–1298. doi: 10.3174/ajnr.A4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan J., Li G., Kong X., et al. Endovascular treatment for ruptured and unruptured vertebral artery dissecting aneurysms: a meta-analysis. J Neurointerventional Surg. 2017;9(6):558–563. doi: 10.1136/neurintsurg-2016-012309. [DOI] [PubMed] [Google Scholar]
- 36.Heros R.C. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64:559–562. doi: 10.3171/jns.1986.64.4.0559. [DOI] [PubMed] [Google Scholar]
- 37.Sen C.N., Sekhar L.N. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery. 1990;27:197–204. doi: 10.1097/00006123-199008000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Bertalanffy H., Seeger W. The dorsolateral, suboccipital, transcondylar approach to the lower clivus and anterior portion of the craniocervical junction. Neurosurgery. 1991;29:815–821. doi: 10.1097/00006123-199112000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Nanda A., Vincent D.A., Vannemreddy P.S., Baskaya M.K., Chanda A. Far-lateral approach to intradural lesions of the foramen magnum without resection of the occipital condyle. J Neurosurg. 2002;96:302–309. doi: 10.3171/jns.2002.96.2.0302. [DOI] [PubMed] [Google Scholar]
- 40.Day J.D., Fukushima T., Giannotta S.L. Cranial base approaches to posterior circulation aneurysms. J Neurosurg. 1997;87(4):544–554. doi: 10.3171/jns.1997.87.4.0544. [DOI] [PubMed] [Google Scholar]
- 41.Matsushima T., Natori Y., Katsuta T., Ikezaki K., Fukui M., Rhoton A.L. Microsurgical anatomy for lateral approaches to the foramen magnum with special reference to transcondylar fossa (supracondylar transjugular tubercle) approach. Skull Base Surg. 1998;8(3):119–125. doi: 10.1055/s-2008-1058570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsushima T., Kawashima M., Masuoka J., Mineta T., Inoue T. Transcondylar fossa (supracondylar transjugular tubercle) approach: anatomic basis for the approach, surgical procedures, and surgical experience. Skull Base. 2010;20(2):83–91. doi: 10.1055/s-0029-1242193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.