Abstract
Background
This study tested whether combined dapagliflozin and entresto treatment would be superior to either one alone for preserving the left-ventricular ejection-fraction (LVEF) in rat after ischemia-reperfusion (IR) injury.
Methods
Cell culture using H9C2 cells and IR injury in rat with dapagliflozin-entresto treatment were conducted in the present study.
Results
In vitro flow-cytometric result showed that the intracellular and mitochondrial reactive oxygen species and mitochondrial permeability transition pore, and protein levels of oxidative-stress/DNA-damaged markers [NADPH-oxidase-1 (NOX-1)/NOX-2/oxidized-protein/γ-H2A-histone-family member X (γ-H2AX)] were significantly higher in hydrogen peroxide (H2O2) (300μM)-treated H9C2 cells as compared with the controls that were significantly reversed in sacubitril/valsartan and dapagliflozin therapy in the same H2O2-treated condition, whereas the protein expressions of antioxidants [Sirtuin-1 (SIRT1)/SIRT3/superoxide dismutase/catalase/glutathione peroxidase) exhibited an opposite pattern among the groups (all p<0.001). Adult-male-Sprague-Dawley rat (n=40) were equally categorized into group 1 (sham-operated control), group 2 (IR), group 3 (IR+dapagliflozin/20mg/kg/orally at 3h and post-days 1/2/3 after IR), group 4 (IR+entresto/100mg/kg/orally at 3h and post-days 1/2/3 after IR) and group 5 (IR+dapagliflozin+entresto) and the hearts were harvested by day 3 after IR. The 3rd day’s LVEF was highest in group 1, lowest in group 2 and significantly higher in group 5 than in groups 3/4, but it was similar between the latter two groups (p<0.001). The protein expressions of oxidative-stress (NOX-1/NOX-2/oxidized protein), fibrotic (transforming-growth factor-ß/phosphorylated-Smad3), apoptotic [mitochondrial-Bax/cleaved-caspase-3/cleaved-poly (ADP-ribose) polymerase], mitochondria/DNA damaged (cytosolic-cytochrome-c/γ-H2AX), pressure-overload/heart-failure [brain natriuretic peptide (BNP)/ß-myosin heavy chain] and autophagic (ratio of meiotic cyclins CLB3-II/CLB3-I) biomarkers, and the upstream (high-mobility group box 1/Toll-like receptor-4/MyD88/phosphorylated-nuclear factor-κB and downstream [interleukin (IL)-1ß/IL-6/tumor necrosis factor-α] inflammatory signalings revealed an antithetical features of LVEF among the groups (all p<0.0001). The cellular levels of inflammatory (myeloperoxidase+/CD68+), pressure-overload/heart-failure (BNP+) and DNA-damage (γ-H2AX+) biomarkers as well as infarct area demonstrated an opposite pattern of LVEF among the groups (all p<0.0001).
Conclusion
Incorporated entresto-dapagliflozin treatment was superior to either one alone on protecting the heart against IR injury.
Keywords: Ischemia-reperfusion injury, Entresto, Dapagliflozin, Inflammation, Oxidative stress
Graphical abstract

At a glance commentary
Scientific background on the subject
Myocardial ischemia-reperfusion (IR) injury is one of the leading causes of mortality in different settings ischemic heart diseases. This study tested whether dapagliflozin- entresto treatment would preserving the heart function and improving outcomes in IR rodent.
What this study adds to the field
Our results clearly clarified those of oxidative-stress and innate inflammatory mediators fundamentally contributed to myocardial damage in setting of IR and combined entresto and dapagliflozin treatment effectively protected the heart architecture and its function against IR injury.
Introduction
Myocardial ischemia-reperfusion (IR) injury is one of the most important issues that commonly occur in clinical settings of myocardial ischemic events/infarction, open-heart surgical intervention, cardiogenic shock, or cardiac arrest of different etiologies [[1], [2], [3], [4], [5]] which contributes to great adverse cardiac events and unfavorable prognostic outcomes. In fact, the patients with acute ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) always suffers from “double injury” to the heart [6,7], i.e., the first injury comes from an occlusion of the infarcted-related artery (IRA) and the secondary injury is from the opening the IRA by primary PCI, followed by restoring the blood flow in the IRA, which, in turn, elicits a so-called “IR injury”, resulting in jeopardized myocardium and heart function as well as worsened heart failure (HF) [[6], [7], [8]]. Regrettably, despite state-of-the-art advancement in pharmacomodulation, a mature technique for coronary intervention, good development in circulatory mechanical support and intensive care to the primary PCI patients [[9], [10], [11], [12]], IR injury to myocardium still plays an extremely important role on increasing morbidity and mortality [2,6].
Notably, restoring blood flow followed by re-opening the IRA is identified to frequently generate free radicals, reactive oxygen species (ROS), and overwhelming inflammation and immunogenic response [[13], [14], [15], [16], [17]]. Of distinctive importance is that the generated ROS interact with critical molecules/peptides/proteins, for example: ion voltage-channels, sarcoendoplasmic reticulum, calcium-releasing conduits, and myofilament proteins which are linked with the excitation-contraction couplings. Altering these structures of molecules/peptides/proteins would alter their dynamic characters or susceptibility to proteolytic enzymes. Basic research has further revealed that ROS are the fundamental contributors to the opening of the mitochondrial permeability transition pore (MPTP), resulting in the release of cytochrome c from mitochondria into cytosol along with other complex elements that is able to cause hyper-contracture and cell death [18]. This reaction finally leads to loss of myocardial contractile function [19,20]. These complicated mechanisms involving in IR injury could at least in part explain why after decades of keen investigations in advancement of pharmaco-therapeutic management and refinement in PCI procedure, there is still lacking an effective therapy for myocardial IR damage.
Sacubitril/valsartan (Sac/Val), also called Entresto, is recognized as a distinctive drug for therapy of chronic HF. Randomized placebo-controlled clinical trials have demonstrated that entresto treatment is much better than angiotensin converting enzyme inhibitor (ACEI) or angiotensin II type I receptor blockers (ARBs) for ameliorating the symptom/sign of HF, left ventricular ejection fraction (LVEF) and prognostic outcome in patients with HF [[21], [22], [23], [24]]. However, whether the entresto treatment could attenuate the HF and preserve the heart function and prognostic outcome in the setting of IR has not yet been assessed.
Cardiac pump function always depends on the constant and persistent energy supply with the circulatory glucose to supply a critical metabolic fuel. Glucose transporters are categorized into two main subgroups, i.e., the glucose transport facilitators (GLUT) and sodium-glucose cotransporters (SGLT) [25]. Human cardiomyocytes express high grades of SGLT-1 gene expression [25]. SGLT-1 is a fundamental myocardium glucose transporter that is bothered by different states of disease and SGLT-2 is mainly allocated in renal tubules [26,27].
Empagliflozin, one kind of SGLT-2 inhibitor, is a new medicine for type 2 diabetic mellitus (DM) [28,29]. A clinical trial has recently shown that type 2 DM patients at high hazard for cardiovascular events who were treated by empagliflozin [30] had substantially lower percentage of primary composite end point (i.e., cardiovascular outcome and of death from any cause) than patients in the control group [30]. Additionally, empagliflozin treatment offered notably lower risk of death from cardiovascular reasons [30,31]. More recently, a phase III randomized double-blinded placebo-control trial [32] has shown that dapagliflozin, another new SGLT-2 inhibitor, has pleotropic effects on preserving LVEF, improving the HF and the long-term outcomes in type II diabetic myocardial infarction (MI) patients beyond the glucose-lowering effect. However, the involving mechanistic basis with regard to how the empagliflozin or dapagliflozin treatment ameliorated primary composite cardiovascular outcomes and reduced death from any cause in the setting of myocardial IR injury remains unclear.
Materials and methods
Ethical issue
Every animal procedure was authorized by the Institute of Animal Care and Use Committee at KCGMH (Affidavit of Approval of Animal Use Protocol No. 2019092601).
Animal grouping and acute myocardial IR injured animal model
Adult male Sprague-Dawley rats (n = 40) weighing 320–350 g were equally categorized into group 1 (sham-operated control, i.e., just opening the chest wall, followed by closing the skin and muscle layers), group 2 (acute IR), group 3 [IR + dapagliflozin (20 mg/kg) orally at 3 h and by days 1, 2 and 3 after IR procedure], group 4 [IR + entresto (100 mg/kg) orally at 3 h and by days 1, 2 and 3 after IR procedure] and group 5 (IR + dapagliflozin + entresto). The dosages of entresto and dapagliflozin were based on our previous reports [33,34].
The procedure and protocol for acute myocardial IR injury were based on our previous report [35]. Under sterile conditions, the heart of each animal was exposed via a left thoracotomy. IR injury was conducted by ligating flow within the left coronary artery for 40 min with a 7-0 prolene suture, 3 mm distal to the margin of the left atrium. The knot was then released after 40-min ischemia, followed by reperfusion for a period of 3 days. After the procedure, the animals recovered in a portable animal intensive care unit (ThermoCare®) for 24 h. The rats were euthanized by day 3 after IR induction, and hearts were removed for investigation.
Echocardiographic examination
Transthoracic echocardiographic study (TES) has been introduced in our previous study [33]. Briefly, TES was carried out in each animal before and on day 3 after myocardial IR procedure utilizing an ultrasound machine (Vevo 2100, Visualsonics). LVEF was calculated as follows: LVEF (%) = [left ventricular end diastolic dimension (LVEDD3)-left ventricular end systolic dimension (LVESD3)/LVEDD3] × 100%.
Western blot analysis of left ventricular (LV) myocardium
The procedure and protocol have been described in detail in our previous reports [[33], [34], [35]]. Briefly, sections were incubated with specific primary antibodies. Signals were investigated with horseradish peroxidase (HRP)-conjugated goat anti-mouse, goat anti-rat, or goat anti-rabbit IgG.
Immunohistochemical (IHC) and immunofluorescent (IF) examinations
The procedure and protocol for IHC and IF studies have been described in our previous studies [[33], [34], [35]]. Briefly, for IHC and IF staining, rehydrated paraffin sections were first conducted with 3% hydrogen peroxide (H2O2) for 30 min and incubated with Immuno-Block reagent (BioSB, Santa Barbara, CA, USA) for 30 min at room temperature. Three sections of heart specimens from each rat were analyzed.
Histological quantification of myocardial fibrosis and infarct area
The procedures and protocols have been described in our previous reports [[33], [34], [35]]. Briefly, the integrated areas (μm2) of infarct area and fibrosis on each section were calculated using the Image Tool 3 (IT3) image analysis software (University of Texas, Health Science Center, San Antonio, UTHSCSA; Image Tool for Windows, Version 3.0, USA). The mean pixel number per high-power field (HPF) for each animal was then determined by summing all pixel numbers and divided by 9. The mean integrated area (μm2) of myocardial fibrosis per HPF was collected using a conversion factor of 19.24 (1 μm2 corresponded to 19.24 pixels).
Flow cytometric analyses for apoptosis, intracellular ROS and mitochondrial permeability transitional pore (MPTP).
The percentages of viable/apoptotic cells were measured using flow cytometry and double staining of annexin V and propidium iodide (PI).
Additionally, flow cytometric analyses were conducted for total cellular ROS (i.e., by H2DCFDA assay), mitochondrial ROS (i.e., by MitoSOX assay) and MPTP (i.e., by calcein assay), respectively.
Statistical analysis
Variables were expressed as mean ± standard deviation (SD). Statistical analysis was conducted by ANOVA, followed by Bonferroni multiple-comparison post hoc test. SAS statistical software for Windows version 8.2 (SAS Institute, Cary, NC, USA) was utilized. A probability value < 0.05 was considered statistically significant.
Results
Sac/Val and dapagliflozin therapy downregulated the ROS, MPTP and apoptosis in H9C2 cells [Fig. 1]
Fig. 1.
Sacubitril (Sac)/valsartan (Val) and dapagliflozin therapy suppressed the expressions of ROS, MPTP and apoptosis in H9C2 cells. A) Illustrating the flow cytometric analysis of total intracellular reactive oxygen species (ROS). B) Analytical result of mean fluorescent intensity (MIF) of DCFDA, ∗ vs. other groups with different symbols (†, ‡), p < 0.0001. C) Illustrating the cytometric analysis of mitochondrial ROS. D) Analytical result of MIF of MitoSOX, ∗ vs. other groups with different symbols (†, ‡), p < 0.0001. E) Illustrating the flow cytometric analysis of mitochondrial permeability transitional pore (MPTP). F) Analytical result of MFI of MPTP (i.e., by calcein-AM assay), ∗ vs. other groups with different symbols (†, ‡), p < 0.0001. G) Flow cytometric analysis of early apoptosis (annexin V+/PI-), ∗ vs. other groups with different symbols (†, ‡), p < 0.001. H) Flow cytometric analysis of late apoptosis (annexin V+/PI+), ∗ vs. other groups with different symbols (†, ‡), p < 0.001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 6). Symbols (∗, †, ‡) indicate significance (at 0.05 level). Group A = H9C2 only; group B = H9C2 + H2O2 (300 μM); group C = H9C2 + H2O2 (300 μM) + Sac/Val (12.5 μM); group D = H9C2 + H2O2 (300 μM) + dapagliflozin (100 μM).
To elucidate whether the therapeutic impact of Sac/Val and dapagliflozin therapy would suppress the expressions of ROS, MPTP and apoptosis in H9C2 cells undergoing oxidative stress. The H9C2 cells were categorized into group A (H9C2 only), group B [H9C2 + H2O2 (300 μM) treated for 6 h], group C [H9C2 + H2O2 (300 μM) treated for 6 h, then washed and followed by Sac/Val (12.5 μM) treated for 16 h] and group D [H9C2 + H2O2 (300 μM) treated for 6 h, then washed and followed by dapagliflozin (100 μM) treated for 16 h], respectively. The flow cytometric result demonstrated that the fluorescent intensity of 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), an indicator of total intracellular ROS, and fluorescent intensity of MitoSOX, an indicator of mitochondrial ROS, were highest in group B as compared with group A, and those were reversed in groups C and D, but they were similar between the latter two groups.
Additionally, fluorescent intensity of calcein, an indicator of MPTP expression, and early and late apoptosis exhibited an identical pattern of oxidative stress among the four groups.
Sac/Val and dapagliflozin therapy downregulated the oxidative stress and DNA-damaged biomarkers and upregulated the antioxidants in H9C2 cells [Fig. 2]
Fig. 2.
Sac/Val and dapagliflozin therapy downregulated the oxidative stress and DNA-damaged biomarkers and upregulated the antioxidants in H9C2 cells. A) Protein expression of NOX-1, ∗ vs. other groups with different symbols (†, ‡), p < 0.001. B) Protein expression of NOX-2, ∗ vs. other groups with different symbols (†, ‡), p < 0.001. C) Protein expression of γ-H2AX, ∗ vs. other groups with different symbols (†, ‡), p < 0.001. D) The oxidized protein expression, ∗ vs. other groups with different symbols (†, ‡), p < 0.001 (Note: the left and right lanes shown on the upper panel represent protein molecular weight marker and control oxidized molecular protein standard, respectively). M.W. = molecular weight; DNP = 1–3 dinitrophenylhydrazone. E) Protein expression of SIRT1, ∗ vs. other groups with different symbols (†, ‡), p < 0.001. F) Protein expression of SIRT3, ∗ vs. other groups with different symbols (†, ‡), p < 0.001. G) Protein expression of superoxide dismutase (SOD), ∗ vs. other groups with different symbols (†, ‡), p < 0.001. H) Protein expression of catalase (CAT), ∗ vs. other groups with different symbols (†, ‡), p < 0.001. I) Protein expression of glutathione peroxidase (GPX), ∗ vs. other groups with different symbols (†, ‡), p < 0.001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 3). Symbols (∗, †, ‡) indicate significance (at 0.05 level). Group A = H9C2 only; group B = H9C2 + H2O2 (300 μM); group C = H9C2 + H2O2 (300 μM) + Sac/Val (12.5 μM); group D = H9C2 + H2O2 (300 μM) + dapagliflozin (100 μM).
To assess whether Sac/Val and dapagliflozin therapy would downregulate the oxidative stress and DNA damage, and upregulate the antioxidants, the Western blot was performed with the identical cell grouping as shown in Fig. 1. The results showed that the protein levels of NADPH-oxidase-1 (NOX-1), NOX-2 and oxidized protein, three indices of oxidative stress, and the protein expression of γ-H2A-histone-family member X (γ-H2AX), an indicator of DNA-damaged biomarker, were significantly higher in group B than in other groups, significantly higher in groups C and D than in group A, but they were similar between groups C and group D. On the other hand, the protein expressions of Sirtuin 1(SIRT1), SIRT3, superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX), five fundamental roles in the entire antioxidant defense grids, exhibited an opposite pattern of oxidative stress among the four groups.
Entresto and dapagliflozin therapy preserved heart function and attenuated infarct size by day 3 after acute IR induction [Fig. 3]
Fig. 3.
Entresto and dapagliflozin treatment effectively preserved heart function and attenuated infarct size in LV myocardium by day 3 after acute IR induction. A) The baseline of left ventricular ejection fraction (LVEF), p > 0.5. By day 3 after ischemia-reperfusion (IR) induction, the analytical result of LVEF, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. B to F) Illustrating the H.E. stain for identification of infarction area (yellow dotted line). G) Analytical result of infarction area, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 8 for LVEF; n = 6 for H.E. stain). Symbols (∗, †, ‡, §) indicate significance (at 0.05 level). Abbreviations: LV: left ventricular; SC: sham-operated control; IR: ischemia reperfusion; DAPA: dapagliflozin; En: entresto.
To evaluate the heart function, the 2-D echo was utilized in the present study. As we expected, the baseline LVEF did not differ among the five groups. However, by day 3 after acute left ventricular IR procedure, the LVEF was highest in group 1 (SC), lowest in group 2 (IR), significantly lower in group 3 (IR + dapagliflozin) and group 4 (IR + entresto) than in group 5 (IR + dapagliflozin + entresto), but it was similar between groups 3 and 4.
To clarify whether the infarct size was inversely correlated to the LVEF, the microscopic finding of H.E. stain was performed. The result showed that the infarct area exhibited an opposite pattern of LVEF among the groups.
Entresto and dapagliflozin therapy suppressed the fibrosis and DNA-damaged marker by day 3 after acute IR induction [Fig. 4]
Fig. 4.
Entresto and dapagliflozin therapy suppressed the fibrosis and DNA-damaged marker in LV myocardium by day 3 after acute IR induction. A to E) Illustrating the microscopic finding (100x) for identification of fibrosis in LV myocardium (blue color). All scale bars in lower right corner represent 100 μm. F) Analytical result of fibrotic area, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. G to K) Illustrating immunofluorescent microscopic finding (400x) for identification of γ-H2AX + cells (pink color). All scale bars in lower right corner represent 20 μm. L) Analytical result of number of positively stained γ-H2AX cells, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 6). Symbols (∗, †, ‡, §) indicate significance (at 0.05 level). Abbreviations: LV: left ventricular; SC: sham-operated control; IR: ischemia reperfusion; DAPA: dapagliflozin; En: entresto.
To assess whether the fibrosis was increased in LV myocardium after IR induction, IHC stain was conducted in our study. The result of the Masson's trichrome stain showed that the fibrotic area of LV myocardium was lowest in group 1, highest in group 2 and significantly lower in group 5 than in groups 3 and 4, but it was similar between the latter two groups. Additionally, the number of positively stained γ-H2AX, an indicator of DNA-damage, exhibited an identical pattern of fibrosis among the five groups.
Entresto and dapagliflozin therapy inhibited the inflammatory cell infiltration in LV myocardium by day 3 after acute IR induction [Fig. 5]
Fig. 5.
Entresto and dapagliflozin therapy ameliorated inflammatory cell infiltration in LV myocardium by day 3 after acute IR induction. A to E) Illustrating the immunohistochemical microscopic finding (400x) for identification of myeloperoxidase (MPO)+ cells (gray color). F) Analytical result of number of positively stained MPO cells, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. G to K) Illustrating the immunofluorescent microscopic finding for identification of CD68+ cells (pink color). L) Analytical result of number of positively stained CD68 cells, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. All scale bars in lower right corner represent 20 μm. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 6). Symbols (∗, †, ‡, §) indicate significance (at 0.05 level). Abbreviations: LV: left ventricular; SC: sham-operated control; IR: ischemia reperfusion; DAPA: dapagliflozin; En: entresto.
To verify whether these two regimens would have therapeutic impact on downregulating the cellular level of inflammation, the IHC and IF stains were used in the present study. The results demonstrated that the cellular expressions of MPO and CD68, two indices of inflammation, were lowest in group 1, highest in group 2 and significantly lower in group 5 than in groups 3 and 4, but they did not differ between the latter two groups.
Entresto and dapagliflozin therapy ameliorated the expression of BNP and preserved connexin43 expression in LV myocardium by day 3 after acute IR procedure [Fig. 6]
Fig. 6.
Entresto and dapagliflozin therapy ameliorated the expression of BNP and preserved connexin43 expression in LV myocardium by day 3 after acute IR induction. A to E) Illustrating the immunohistochemical microscopic finding (400x) for identification of cellular expression of brain natriuretic peptide (BNP) (gray color). F) Analytical result of the expression of BNP, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. G to K) Illustrating the cellular expression of connexin43 (Cx43) (green strip-like band) (red arrows). L) Analytical result of Cx43 expression, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. All scale bars in lower right corner represent 20 μm. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 6). Symbols (∗, †, ‡, §) indicate significance (at 0.05 level). Abbreviations: LV: left ventricular; SC: sham-operated control; IR: ischemia reperfusion; DAPA: dapagliflozin; En: entresto.
The cellular expression of brain natriuretic peptide (BNP), a pressure overload/heart failure biomarker, was lowest in group 1, highest in group 2 and significantly lower in group 5 than in groups 3 and 4, but it did not differ between the latter two groups. On the other hand, the cellular expression of connexin43 (Cx43), a gap junction for cell-to-cell communication, demonstrated an opposite pattern of BNP among the groups.
Entresto and dapagliflozin therapy inhibited oxidative stress and enhanced antioxidants in LV myocardium by day 3 after acute IR procedure [Fig. 7]
Fig. 7.
Entresto and dapagliflozin therapy inhibited oxidative stress and enhanced antioxidants in LV myocardium by day 3 after acute IR induction. A) Protein expression of NADPH-oxidase-1 (NOX-1), ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. B) Protein expression of NOX-2, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. C) Oxidized protein expression, ∗ vs. other groups with different symbols (†, ‡), p < 0.001 (Note: the left and right lanes shown on the upper panel represent protein molecular weight marker and control oxidized molecular protein standard, respectively). M.W. = molecular weight; DNP = 1–3 dinitrophenylhydrazone. D) Protein expression of SIRT1, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. E) Protein expression of SIRT3, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 6). Symbols (∗, †, ‡, §) indicate significance (at 0.05 level). Abbreviations: LV: left ventricular; SC: sham-operated control; IR: ischemia reperfusion; DAPA: dapagliflozin; En: entresto.
The western blot analysis revealed that the protein levels of NOX-1, NOX-2 and oxidized protein, three indicators of oxidative stress, were lowest in group 1, highest in group 2 and significantly lower in group 5 than in groups 3 and 4, but they were similar between the latter two groups. On the other hand, the protein expression of SIRT1 and SIRT3, two indicators of antioxidants, displayed an opposite pattern of oxidative stress.
Entresto and dapagliflozin therapy inhibited apoptosis, fibrosis and autophagy in LV myocardium by day 3 after acute IR procedure [Fig. 8]
Fig. 8.
Entresto and dapagliflozin therapy inhibited apoptosis, fibrosis and autophagy in LV myocardium by day 3 after acute IR induction. A) Protein expression of mitochondrial (mito)-Bax, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. B) Protein expression of cleaved caspase 3 (c-Casp3), ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. C) Protein expression of cleaved Poly (ADP-ribose) polymerase (c-PARP), ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. D) Protein expression of phosphorylated (p)-Smad3, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. E) Protein expression of transforming growth factor (TGF)-β, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. F) Protein ratio of CL3B-II to CL3B-I, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 6). Symbols (∗, †, ‡, §) indicate significance (at 0.05 level). Abbreviations: LV: left ventricular; SC: sham-operated control; IR: ischemia reperfusion; DAPA: dapagliflozin; En: entresto.
The protein levels of mitochondrial Bax, cleaved caspase 3 and cleaved poly (ADP-ribose) polymerase (c-PARP), three indictors of apoptosis, and phosphorylated (p)-Smad3 and transforming growth factor beta (TGF-β), two indices of fibrosis, were lowest in group 1, highest in group 2 and significantly lower in group 5 than in groups 3 and 4, but they revealed a similar pattern between the latter two groups. Additionally, the protein ratio of CL3B-II to CL3B-I, an index of autophagy, was similar to the apoptosis among the groups.
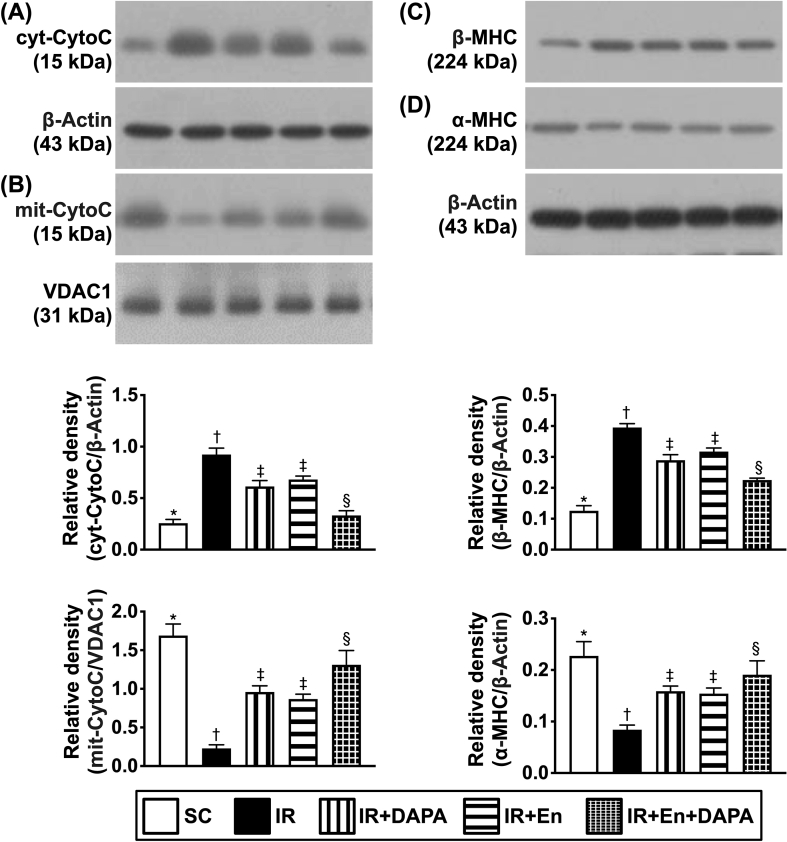
Entresto and dapagliflozin therapy preserved the mitochondrial integrity and inhibited myocardial hypertrophy and DNA damage in LV myocardium by day 3 after acute IR procedure [Fig. 9]
Fig. 9.
Entresto and dapagliflozin therapy preserved the mitochondrial integrity and attenuated myocardial hypertrophy and DNA damage in LV myocardium by day 3 after acute IR induction. A) Protein expression of cytosolic cytochrome c (cyt-CytoC), ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. B) Protein expression of mitochondrial cytochrome c (mit-CytoC), ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. C) Protein expression of β-myosin heavy chain (β-MHC), ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. D) Protein expression of α-MHC, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 6). Symbols (∗, †, ‡, §) indicate significance (at 0.05 level). Abbreviations: LV: left ventricular; SC: sham-operated control; IR: ischemia reperfusion; DAPA: dapagliflozin; En: entresto.
The Western blot analysis demonstrated that cytosolic cytochrome c, an indicator of mitochondrial damage, was lowest in group 1, highest in group 2 and significantly lower in group 5 than in groups 3 and 4, but it showed no difference between the latter two groups. On the other hand, the protein expression of mitochondrial cytochrome c, an index of mitochondrial integrity, displayed a similar pattern of cytosolic cytochrome c among the five groups.
Additionally, the protein level of β-myosin heavy chain (MHC), a pressure overload biomarker [36], revealed a similar pattern compared to that of cytosolic cytochrome c among the five groups. On the other hand, the protein level of α-MHC, a reverse myocardial hypertrophic marker [36], displayed an opposite pattern to that of β-MHC among the five groups.
Entresto and dapagliflozin therapy suppress the upstream and downstream inflammatory signalings [Fig. 10]
Fig. 10.
Entresto and dapagliflozin therapy suppressed the upstream and downstream inflammatory signalings. A) Protein expression of high-mobility group protein 1 (HMG-1), ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. B) Protein expression of toll-like receptor (TLR)-4, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. C) Protein expression of Myeloid differentiation primary response 88 (MYD88), ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. D) Protein expression of phosphorylated nuclear factor (p-NF)-κB, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. E) Protein expression of tumor necrosis factor (TNF)-α, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. F) Protein expression of interleukin (IL)-1β, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. G) Protein expression of IL-6, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. H) Protein expression of IKB-α, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. I) Protein expression of IKB-β, ∗ vs. other groups with different symbols (†, ‡, §), p < 0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 6). Symbols (∗, †, ‡, §) indicate significance (at 0.05 level). Abbreviations: LV: left ventricular; SC: sham-operated control; IR: ischemia reperfusion; DAPA: dapagliflozin; En: entresto.
To ascertain whether these two therapeutic regimens would suppress the inflammatory signaling pathways, the Western blot analysis was utilized once again in the present study. The result demonstrated that the protein expressions of high-mobility group box 1 (HGMB1), toll-like receptor (TLR)-4, MyD88 and phosphorylated nuclear factor (p-NF)-κB, four indicators of upstream inflammation, were lowest in group 1, highest in group 2 and significantly lower in group 5 than those of groups 3 and 4, but they revealed a similar pattern between the latter two groups.
Additionally, the protein expressions of tumor necrosis factor (TNF)-α, IL-1β and IL-6, three indicators of downstream inflammatory signaling, exhibited a similar pattern of upstream inflammatory signaling among the five groups. On the other hand, the protein expressions of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IKB-α) and IKB-β, two guardians for inhibiting the upstream inflammatory signaling transmitted into the downstream inflammatory signaling, exhibited an opposite pattern of inflammatory proteins among the five groups.
Discussion
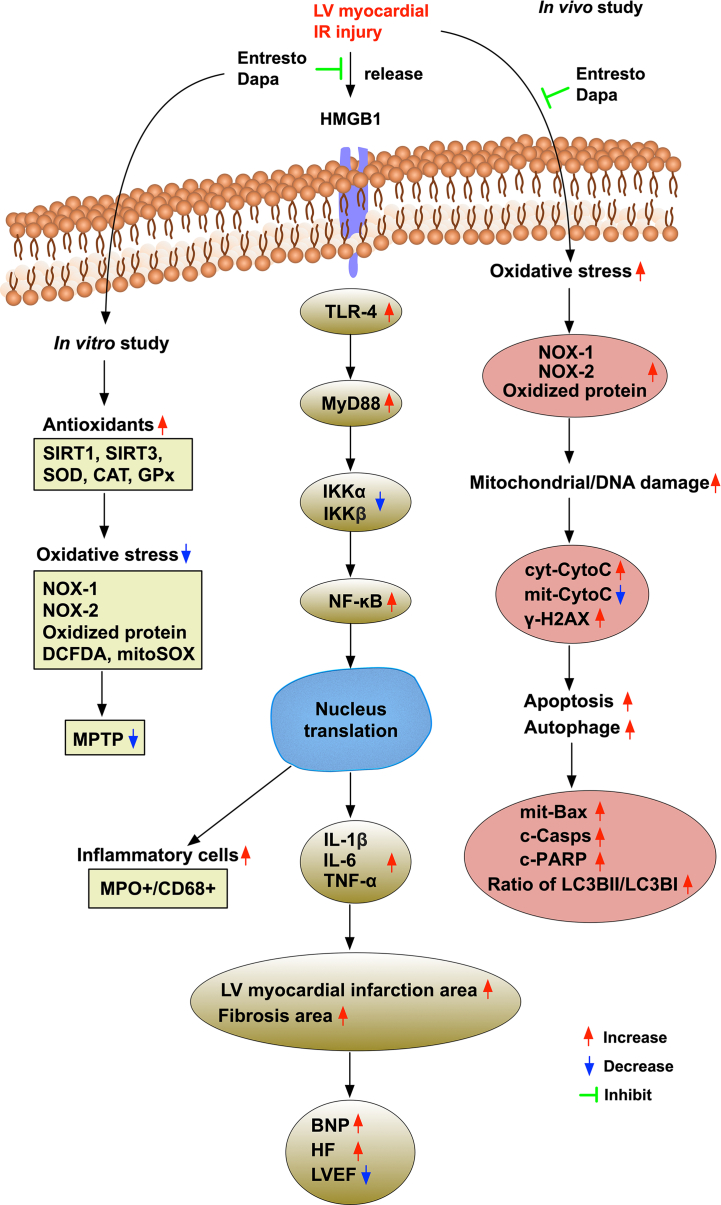
This study investigated the therapeutic impact of entresto and dapagliflozin on protecting the LV myocardium and preserving the heart function against IR injury yielded several preclinical striking implications. First, dapagliflozin therapy was comparable to entresto on preserving the heart function in setting of IR. Second, combining these two regimens was better than either one regimen alone on protecting the heart function and the integrity of heart architecture as well as away from pressure overload/heart failure (i.e., reduced BNP/β-MHC) against IR injury, suggesting that these benefits could be attributed to a synergic effect of entresto plus dapagliflozin therapy. Third, both in vitro and in vivo studies consistently demonstrated that entresto-dapagliflozin therapy on protecting the heart against IR injury dominantly via inhibiting the inflammatory reaction and oxidative stress as well as upregulating the antioxidants [refer to Fig. 11].
Fig. 11.
Illustrating the underlying mechanisms of entresto-dapagliflozin treatment for preserving the heart function and integrity of cardiac structure. Abbreviations: LVEF: left ventricular ejection fraction; IR: ischemia-reperfusion; DAPA: dapagliflozin; cyt-CytoC: cytosolic cytochrome c; mit-CytoC: cytosolic cytochrome c.
To verify whether entresto and dapagliflozin treatment would protect the cardiomyocytes against H2O2 (i.e., oxidative stress stimulation), we first designed the in vitro study. The result of flow cytometry showed that the total intracellular and mitochondrial ROS and MPTP were significantly upregulated in H2O2-treated H9C2 cells than those of the controls. However, these parameters were significantly suppressed in the same oxidative-stress condition regardless of treatment by entresto or dapagliflozin, implicating that these drugs could avoid the cells and mitochondrial damage far away from H2O2. Another important finding from in vitro study was that when looked at the protein levels of oxidative stress and antioxidant levels, we found that the protein expressions of NOX-1, NOX-2 and oxidized protein were remarkably increased whereas the protein expressions of antioxidants (i.e., SIRT1, SIRT3, SOD, CAT, and GPX) were remarkably lower in H2O2-treated H9C2 cells than in the control group that those were notably reversed by entresto and dapagliflozin, implicating that these two drugs could protect the heart from IR injury which might be at least in part through other signaling pathways of antioxidants. Based on our in vitro study, we further performed an animal model of IR to clarify the reality of the therapeutic effect of these two drugs in setting of in vivo.
The most important finding in the present study was that as compared with IR only animals, the LVEF was significantly preserved in entresto- or dapagliflozin-treated and further significantly preserved in combined entresto- and dapagliflozin-treated IR animals. Interestingly, our previous study has showed that empagliflozin therapy effectively protected the heart and kidney against cardiorenal syndrome injury [34]. Our another previous study has also demonstrated that entresto treatment validly protected the heart-lung organs against transverse aortic stricture induced cardiopulmonary syndrome damage in rat [37]. Accordingly, our result corroborated with the findings of our previous studies [34,37]. Additionally, our findings also demonstrated that the fibrotic area and infarct area were also remarkably upregulated in IR rats than in those of the sham-operated control (SC) rats that were significantly reduced by entresto or dapagliflozin therapy and further substantially reduced in combined entresto and dapagliflozin therapy. In this way, our findings, in addition to strengthening the results of our previous studies [34,37], could, partially, account for why the LVEF was significantly preserved in those of IR animals treated with both entresto and dapagliflozin than in those without.
Situations of ischemia and tissue necrosis developed in any tissue and organs always draw forth inflammation and oxidative stress, which in turn, commonly elicit a cascade of cell apoptosis and death and organ dysfunction [[33], [34], [35], [36], [37]]. A principal finding in the present study was that oxidative stress and mitochondrial/DNA-damaged biomarkers in LV myocardium were notably augmented in IR than in SC animals. Additionally, not only the upstream but also the downstream inflammatory signaling pathways were clearly clarified to be remarkably enhanced in IR than in SC animals. Our finding, corroborating with the findings of the previous reports [[33], [34], [35], [36], [37]], could probably account for the two signalings (i.e., oxidative stress and inflammation) might be the major contributors for the cardiac ultrastructural damage and heart dysfunction in setting of acute heart IR injury. Of distinctive importance was that these molecular-cellular perturbations were significantly reversed by entresto and dapagliflozin treatments and further significantly reversed by combining these two treatment regimens.
It is well recognized that plasma level of BNP is a useful biomarker for predicting severity of acute heart failure, pressure overload and prognostic outcome [38]. Additionally, the fetal gene expression of β-MHC is also a good biomarker correlated with the pressure overload [36], whereas the gene expression of α-MHC just reflects the opposite intrinsic expression of β-MHC. Furthermore, our previous preclinical studies have revealed that the BNP biomarker was substantially increased in rodent LV myocardium in the settings of dilated cardiomyopathy (DCM) [36] and cardiorenal syndrome (CRS) [34]. Moreover, our previous studies have also demonstrated that the expression of Cx43 was substantially inhibited in DCM [36] and CRS [34] in rodent myocardium, suggesting that it is a novel parameter of unfavorable prognostic outcome. An essential finding in the present study was that not only the cellular expression of BNP but also the protein expression of β-MHC was remarkably upregulated, whereas the cellular expression of Cx43 and protein expression of α-MHC were significantly downregulated in LV myocardium of IR animals. In this way, the results of our present study, in addition to corroborating with the findings of the previous studies [34,36,38], could once again explain why the LVEF and integrity of heart ultrastructure were markedly diminished in IR animals. Auspiciously, these molecular-cellular perturbations were eminently reversed in IR animals treated by entresto-dapagliflozin.
Study limitation
This study has limitations. First, despite the short-term (i.e., study period was only 3 days) outcomes were captivating and promising, the long-term outcome of these treatment is currently unclear. Second, we did not stepwise titrate up the dosage of the entresto and dapagliflozin in the present study. Thus, we could not provide information regarding the optimal dosages of these two drugs.
In conclusion, in light of both oxidative stress and innate inflammation were the two major contributors for myocardial damage in setting of IR, the result of this study suggested that combination of entresto and dapagliflozin therapies was better than just one therapy for protecting the heart architecture and its function against IR injury.
Funding
This study was funded by a program grant from Chang Gung Memorial Hospital, Chang Gung University (Grant number: CMRPG8K0401).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We thank Chang Gung Memorial Hospital, Chang Gung University for the research support.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Chen Y.S., Chao A., Yu H.Y., Ko W.J., Wu I.H., Chen R.J., et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2003;41:197–203. doi: 10.1016/s0735-1097(02)02716-x. [DOI] [PubMed] [Google Scholar]
- 2.Ito H., Maruyama A., Iwakura K., Takiuchi S., Masuyama T., Hori M., et al. Clinical implications of the 'no reflow' phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–228. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- 3.Kloner R.A., Ellis S.G., Lange R., Braunwald E. Studies of experimental coronary artery reperfusion. Effects on infarct size, myocardial function, biochemistry, ultrastructure and microvascular damage. Circulation. 1983;68:I8–I15. [PubMed] [Google Scholar]
- 4.Rokos I.C., French W.J., Koenig W.J., Stratton S.J., Nighswonger B., Strunk B., et al. Integration of pre-hospital electrocardiograms and ST-elevation myocardial infarction receiving center (SRC) networks: impact on Door-to-Balloon times across 10 independent regions. JACC Cardiovasc Interv. 2009;2:339–346. doi: 10.1016/j.jcin.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Rokos I.C., Larson D.M., Henry T.D., Koenig W.J., Eckstein M., French W.J., et al. Rationale for establishing regional ST-elevation myocardial infarction receiving center (SRC) networks. Am Heart J. 2006;152:661–667. doi: 10.1016/j.ahj.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Yip H.K., Chen M.C., Chang H.W., Hang C.L., Hsieh Y.K., Fang C.Y., et al. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and no-reflow phenomenon. Chest. 2002;122:1322–1332. doi: 10.1378/chest.122.4.1322. [DOI] [PubMed] [Google Scholar]
- 7.Yip H.K., Chen M.C., Chang H.W., Kuo F.Y., Yang C.H., Chen S.M., et al. Transradial application of PercuSurge GuardWire device during primary percutaneous intervention of infarct-related artery with high-burden thrombus formation. Catheter Cardiovasc Interv. 2004;61:503–511. doi: 10.1002/ccd.10685. [DOI] [PubMed] [Google Scholar]
- 8.Sheu J.J., Tsai T.H., Lee F.Y., Fang H.Y., Sun C.K., Leu S., et al. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med. 2010;38:1810–1817. doi: 10.1097/CCM.0b013e3181e8acf7. [DOI] [PubMed] [Google Scholar]
- 9.Jolly S.S., James S., Dzavik V., Cairns J.A., Mahmoud K.D., Zijlstra F., et al. Thrombus aspiration in ST-segment-elevation myocardial infarction: an individual patient meta-analysis: thrombectomy trialists collaboration. Circulation. 2017;135:143–152. doi: 10.1161/CIRCULATIONAHA.116.025371. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi T., Shiomi H., Morimoto T., Watanabe H., Ono K., Shizuta S., et al. Incidence and prognostic impact of heart failure hospitalization during follow-up after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. Am J Cardiol. 2017;119:1729–1739. doi: 10.1016/j.amjcard.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Wallentin L., Becker R.C., Budaj A., Cannon C.P., Emanuelsson H., Held C., et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 12.Wiviott S.D., Braunwald E., McCabe C.H., Montalescot G., Ruzyllo W., Gottlieb S., et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 13.De Scheerder I., Vandekerckhove J., Robbrecht J., Algoed L., De Buyzere M., De Langhe J., et al. Post-cardiac injury syndrome and an increased humoral immune response against the major contractile proteins (actin and myosin) Am J Cardiol. 1985;56:631–633. doi: 10.1016/0002-9149(85)91024-0. [DOI] [PubMed] [Google Scholar]
- 14.Frangogiannis N.G. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangogiannis N.G., Smith C.W., Entman M.L. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 16.Lambert J.M., Lopez E.F., Lindsey M.L. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130:147–158. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lange L.G., Schreiner G.F. Immune mechanisms of cardiac disease. N Engl J Med. 1994;330:1129–1135. doi: 10.1056/NEJM199404213301607. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsui H., Kinugawa S., Matsushima S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc Res. 2009;81:449–456. doi: 10.1093/cvr/cvn280. [DOI] [PubMed] [Google Scholar]
- 19.Chuah S.C., Moore P.K., Zhu Y.Z. S-allylcysteine mediates cardioprotection in an acute myocardial infarction rat model via a hydrogen sulfide-mediated pathway. Am J Physiol Heart Circ Physiol. 2007;293:H2693–H2701. doi: 10.1152/ajpheart.00853.2007. [DOI] [PubMed] [Google Scholar]
- 20.Misra M.K., Sarwat M., Bhakuni P., Tuteja R., Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–RA219. [PubMed] [Google Scholar]
- 21.Desai A.S., McMurray J.J., Packer M., Swedberg K., Rouleau J.L., Chen F., et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36:1990–1997. doi: 10.1093/eurheartj/ehv186. [DOI] [PubMed] [Google Scholar]
- 22.Gori M., Senni M. Sacubitril/valsartan (LCZ696) for the treatment of heart failure. Expert Rev Cardiovasc Ther. 2016;14:145–153. doi: 10.1586/14779072.2016.1128827. [DOI] [PubMed] [Google Scholar]
- 23.Jhund P.S., Fu M., Bayram E., Chen C.H., Negrusz-Kawecka M., Rosenthal A., et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015;36:2576–2584. doi: 10.1093/eurheartj/ehv330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMurray J.J., Packer M., Desai A.S., Gong J., Lefkowitz M.P., Rizkala A.R., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 25.von Lewinski D., Gasser R., Rainer P.P., Huber M.S., Wilhelm B., Roessl U., et al. Functional effects of glucose transporters in human ventricular myocardium. Eur J Heart Fail. 2010;12:106–113. doi: 10.1093/eurjhf/hfp191. [DOI] [PubMed] [Google Scholar]
- 26.DeFronzo R.A., Davidson J.A., Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5–14. doi: 10.1111/j.1463-1326.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhou L., Cryan E.V., D'Andrea M.R., Belkowski S., Conway B.R., Demarest K.T. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1) J Cell Biochem. 2003;90:339–346. doi: 10.1002/jcb.10631. [DOI] [PubMed] [Google Scholar]
- 28.Fala L. Jardiance (empagliflozin), an SGLT2 inhibitor, receives FDA approval for the treatment of patients with type 2 diabetes. Am Health Drug Benefits. 2015;8:92–95. [PMC free article] [PubMed] [Google Scholar]
- 29.Grempler R., Thomas L., Eckhardt M., Himmelsbach F., Sauer A., Sharp D.E., et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83–90. doi: 10.1111/j.1463-1326.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 30.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 31.Fitchett D., Zinman B., Wanner C., Lachin J.M., Hantel S., Salsali A., et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiviott S.D., Raz I., Sabatine M.S. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. Reply. N Engl J Med. 2019;380:1881–1882. doi: 10.1056/NEJMc1902837. [DOI] [PubMed] [Google Scholar]
- 33.Yang C.C., Chen Y.T., Chen C.H., Li Y.C., Shao P.L., Huang T.H., et al. The therapeutic impact of entresto on protecting against cardiorenal syndrome-associated renal damage in rats on high protein diet. Biomed Pharmacother. 2019;116 doi: 10.1016/j.biopha.2019.108954. [DOI] [PubMed] [Google Scholar]
- 34.Yang C.C., Chen Y.T., Wallace C.G., Chen K.H., Cheng B.C., Sung P.H., et al. Early administration of empagliflozin preserved heart function in cardiorenal syndrome in rat. Biomed Pharmacother. 2019;109:658–670. doi: 10.1016/j.biopha.2018.10.095. [DOI] [PubMed] [Google Scholar]
- 35.Chua S., Lee F.Y., Tsai T.H., Sheu J.J., Leu S., Sun C.K., et al. Inhibition of dipeptidyl peptidase-IV enzyme activity protects against myocardial ischemia-reperfusion injury in rats. J Transl Med. 2014;12:357. doi: 10.1186/s12967-014-0357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y.L., Chung S.Y., Chai H.T., Chen C.H., Liu C.F., Chen Y.L., et al. Early administration of carvedilol protected against doxorubicin-induced cardiomyopathy. J Pharmacol Exp Ther. 2015;355:516–527. doi: 10.1124/jpet.115.225375. [DOI] [PubMed] [Google Scholar]
- 37.Lu H.I., Tong M.S., Chen K.H., Lee F.Y., Chiang J.Y., Chung S.Y., et al. Entresto therapy effectively protects heart and lung against transverse aortic constriction induced cardiopulmonary syndrome injury in rat. Am J Transl Res. 2018;10:2290–2305. [PMC free article] [PubMed] [Google Scholar]
- 38.Santaguida P.L., Don-Wauchope A.C., Oremus M., McKelvie R., Ali U., Hill S.A., et al. BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart Fail Rev. 2014;19:453–470. doi: 10.1007/s10741-014-9442-y. [DOI] [PubMed] [Google Scholar]