Abstract
Introduction:
In this study, we prospectively investigated changes in serum interleukin-6 (IL-6), high-sensitivity C-reactive protein (hsCRP) and full white blood cell (WBC) counts during the diagnosis and treatment of paediatric patients with appendicitis. We also investigated the effects of the COVID-19 pandemic on the diagnosis and treatment processes of paediatric appendicitis patients.
Materials and Methods:
A non-perforated appendicitis group (n = 110), a perforated appendicitis group (n = 35) and an appendicitis + COVID-19 group (n = 8) were formed. Blood samples were taken upon admission and every day until the three studied parameters returned to normal values. To investigate the effects of the COVID-19 pandemic on paediatric appendicitis patients, the perforated appendicitis rates and the times from the onset of the first symptoms to the operation before and during the pandemic were compared.
Results:
WBC, IL-6, and hsCRP dropped below the upper limits on the second postoperative day in the non-perforated appendicitis group, four to six days postoperatively in the perforated appendicitis group, and three to six days postoperatively in the appendicitis + COVID-19 group. These parameters were not within normal range in patients who developed complications during follow-up. The time from the onset of abdominal pain to the surgery was significantly longer during than before the pandemic in both the non-perforated appendicitis group and the perforated appendicitis group.
Conclusions:
Our results show that WBC, IL-6, and hsCRP are useful laboratory parameters that can complete clinical examinations in the diagnosis of appendicitis in paediatric patients and the identification of complications that may develop postoperatively.
Keywords: COVID-19 pandemic, high-sensitivity C-reactive protein, interleukin 6, paediatric appendicitis, white blood cell
INTRODUCTION
Appendicitis is a serious abdominal disorder that requires emergency abdominal surgery in children.[1] Interleukin 6 (IL-6) is an important biomarker of inflammation.[2,3] Similarly, high-sensitivity C-reactive protein (hsCRP) and white blood cell (WBC) counts are reliable laboratory markers that can be used to diagnose intra-abdominal inflammation.[4-6]
The aim of this study was to prospectively investigate changes in IL-6, hsCRP and WBC levels during the diagnosis and treatment of paediatric appendicitis patients. Since the COVID-19 outbreak occurred after we started this study,[7] we also investigated the effects of the pandemic on the diagnosis and treatment processes of paediatric appendicitis patients. In this study, we also examined whether pandemic conditions led to an increase in perforation rate in paediatric appendicitis patients.
MATERIALS AND METHODS
This single-centre prospective study was conducted between December 2018 and May 2021 in the Paediatric Surgery Clinic Clinic of Turgut Ozal Medical Centre, Malatya, Turkiye.
Ethical consideration
Ethics approval was obtained from the Clinical Research Ethics Committee of the Faculty of Medicine of Inonu University, Malatya, Turkiye (2018/152). As of March 2020, appendicitis patients with COVID-19 infection were also included after obtaining ethics approval. Informed consent was obtained from the legal guardians of all patients included in the study. This study was carried out in compliance with the Helsinki Declaration. These documents are ready in the archives of our clinic.
Study groups
We made two different analyses in our study. In the first analysis, we evaluated the course of the laboratory parameters (IL-6, hsCRP and WBC) in the diagnosis and follow-up process of appendicitis patients. For this pupose a total number of 3 groups were designed and formed: A non-perforated appendicitis group, a perforated appendicitis group, and a COVID-19 + appendicitis group. In the second analysis, we evaluated the effects of the COVID-19 pandemic on paediatric appendicitis patients. A total of four groups were created for this. These groups were consisted of perforated and no perforated appendicitis patients before the pandemic and perforated and non-perforated appendicitis patients after the pandemic, respectively. The inclusion criteria were (i) clinical diagnosis of appendicitis, (ii) a patient age of 4–17 years, (iii) laboratory tests necessary for pre- and post-operative studies, (iv) no chronic diseases, such as diabetes and epilepsy, (v) parental informed consent and (vi) open surgical appendectomy. Based on these criteria, 110 non-perforated appendicitis (catarrhal, phlegmonous and gangrenous) patients (75 patients before the pandemic, 35 patients after the pandemic), 35 perforated appendicitis patients (20 patients before the pandemic, 15 patients after the pandemic), and 8 patients with COVID-19 infection of them with no perforated had 7 appendicitis and one of them with perforated appendicitis which were treated during the study period were included. We did not form a control group. Instead, we used our laboratory reference values.
We also divided patients into four groups to analyse the effects of Covid-19 pandemic conditions on perforation rate. For this purpose: A non-perforated appendicitis patients group before the pandemic (n = 75) a non-perforated appendicitis group after the pandemic (n = 42 (Covid-19 negative (n = 35), Covid-19 positive (n = 7)), a perforated appendicitis group before the pandemic (n = 20) and a perforated appendicitis group after the pandemic (n = 16 (Covid-19 negative (n = 15), Covid-19 positive (n = 1)) were formed.
Referral to the emergency room and diagnosis
The parents of children who referred to the emergency room 48 h or more after the onset of abdominal pain were asked why they had delayed referral. If the reasons for the delay were related to the fear of getting COVID-19 infection or to the denied admission because of the overwhelmed emergency room, it was considered a delay due to the pandemic related conditions. Reasons for delay besides these reasons were considered “other” reasons.
Our basic evaluation protocol consisted of Abdominal and Lung radiographs, Laboratory parameters and Alvarado score system for abdominal pain patients.
Routine laboratory tests were performed on all patients. The same IL-6, hsCRP, and WBC reference values were used during diagnosis and follow-up for both non-perforated and perforated appendicitis patients. Also, the same physical and radiological examinations were performed. Abdominal and lung radiography were performed in all patients. We performed abdominal radiography to find free air and chest X-ray to evaluate right lower lobe pneumonia. Radiographic examination of the patients in this study not revealed free air and right lower lobe pneumonia. Abdominal ultrasound was performed only for the girl patients, who were over 10 years of age, in order to rule out tubal, ovarian and uterine pathologies, and for girl and boy patients whose symptoms lasted longer than 48 hours. USG findings were not included in this study because the USG user radiologist was not the same person and was not performed on all patients. All patients were assigned Alvarado scores during the diagnosis. We have been using the Alvarado scoring system (ASS) in our clinic forlong and we were more experienced in using this scoring system. Therefore, in our study, we used ASS for diagnosis in the emergency department. Only patients with scores of 7 or higher were considered acute abdomen patients and underwent surgery.[8] The Alvarado scoring system is shown in Table 1. The time duration from the onset of abdominal pain to surgery was calculated and recorded in hours. In the preoperative period, all patients were given sulbactam-ampicillin IV (Ampicillin 50 mg/kg single dose) as a prophylactic antibiotic.
Table 1.
The Alvarado scoring system
| Symptoms | Score |
|---|---|
| Migratory right iliac fossa (RIF) abdominal pain | 1 |
| Anorexia | 1 |
| Nausea/vomiting | 1 |
| Tenderness: RIF | 2 |
| Rebound tenderness RIF | 1 |
| Elevated temperature | 1 |
| Leucocytosis | 2 |
| Shift to the left of neutrophils | 1 |
| Total score | 10 |
Pre-operative anaesthetic procedures of all patients including appendicitis with COVID-19 infection
Patients with American Society of Anaesthesiology scores of I–IV were undergoing surgery. The preoperative fasting periods for all patients appendicitis were six hours for solid foods and two hours for clear fluids. Preoperative anaesthetic evaluation was achieved in the COVID-19 area outside the operation room. Moreover, we made all preparations including an additional suction machine, anaesthetic and resuscitation drugs, spinal needles, disposable face masks, laryngoscopes with disposable blades, stylets, and a transparent sheet to cover the patient during the endotracheal intubation.[9] High-level personal protective equipment consisting of N95 masks, impermeable body suits, glasses, face visors, shoe covers, and double-layered medical gloves was used by the entire team, including the paediatric surgeon, anaesthesiologist, nursing staff, and assistants. Wearing N95 masks, the patients were then transferred to the operation room through a corridor in which all other patient and personnel movement was stopped to minimise the risk of contamination.
General anaesthesia protocol
General anaesthesia with rapid-sequence induction and intubation was administered according to a standardised protocol by an experienced anaesthesiologist after low-flow pre-oxygenation.[10] Propofol (0.5–2 mg/kg), rocuronium (0.8–1 mg/kg) and fentanyl (0.1 μg/kg) were administered intravenously in doses calculated according to the actual body weight. We used double gloves during intubation and changed the outer gloves immediately after laryngoscopy procedure. COVID-19 patients were ventilated only after confirming a closed system throughout these manoeuvres, with a tidal volume of 5–10 mL/kg based on the actual body weight and an appropriate frequency of 12–26 breaths per minute using a Dräger Primus ventilator (Dräger AG, Lübeck, Germany). All personnel wore appropriate personal protective equipment during the operation. Non-anaesthesia personnel left the room before extubation. The patients were then extubated, and a surgical mask was placed over their airways. Analgesia was administered to all patients using appropriate doses of tramadol (0.5–1 mg/kg, intravenous [IV]) and paracetamol (10 mg/kg, IV) at the time of skin suturing.
Regional anaesthesia protocol of all eligible patients
For those patients, whose fasting period is not sufficient for the surgery neuraxial anaesthesia was recommended.[10] In addition, neuraxial anaesthesia was recommended to the patients with Covid-19 and Appendicitis to prevent the risk of contamination to the anaesthesia team during mask ventilation, laryngeal intubation and extubation. Neuraxial anaesthesia was performed with a 20-G or 22-G needle, and 5–15 mg of 5% bupivacaine was injected into the subarachnoid space for the spinal block. Neuraxial anaesthesia was performed as a single dose and performed within 2-3 minutes.
Surgical procedures
All surgeries were performed by the same surgical and anaesthesiology teams. All appendicitis patients received the standard appendicitis surgical (open appendectomy) and medical treatment proposed by Dunn.[1] When operating on patients with COVID-19 infection, the surgical team used double surgical masks. No member of the surgical team tested positive for COVID-19 during the study period. The peritoneal cavities of all perforated appendicitis patients were drained using a Penrose drain.[1] The drain was removed after 24–48 h.
Post-operative treatment
In the postoperative period, patients with non-perforated appendicitis were given sulbactam-ampicillin (Ampicillin 50 mg/kg per dose every 6 hours)[11], treatment for an average of 2 to 5 days, the first two days IV and then orally, until the laboratory parameters were normalized. Antibiotic therapy in this group was discontinued at the day in which the laboratory parameters normalized. Patients with perforated appendicitis underwent an intravenous ceftriaxone (50 mg/kg per dose every 12 hours) -metronidazole (10 mg/kg per dose every 8 hours) treatment combination for two to five days until fever decreased, then ceftriaxone treatment alone for two to five days until laboratory parameters returned to normal levels, then oral Cefixime (8mg/kg/single dose) for 5 to 10 days until clinically complete recovery has reached.[11] For postoperative pain control a dose of 10 mg/kg IV paracetamol was given 3 times a day.[11] Appendectomy tissue samples were evaluated histopathologically to confirm the diagnosis of appendicitis.
Post-operative treatment of appendicitis patients with COVID-19 infection and multisystem inflammatory syndrome in children
Reverse transcriptase–polymerase chain reaction (PCR) positivity for COVID-19 RNA, clinical findings such as fever, cough, headache, diarrhoea, and sore throat in the case of COVID-19 exposure within the previous two weeks, or bilateral mild ground-glass opacities were defined as COVID-19 infection.[12,13] A fever of ≥38°C for more than 24 hours, evidence of COVID-19 (PCR positivity, serology positivity, antigen positivity, or COVID-19 exposure within the previous four weeks), involvement of at least two systems, and laboratory evidence of inflammation (increased hsCRP, IL-6, fibrinogen, D-dimer, ferritin, lactate dehydrogenase, and neutrophil levels and reduced albumin and lymphocytes) were defined as multisystem inflammatory syndrome in children (MIS-C).[12,13] To the patients with acute COVID-19 infection, only supportive treatments (fluid support and paracetamol) were administered; antiviral treatment was not administered. All MIS-C patients received intravenous immunoglobulin (2 g/kg) and methylprednisolone (2 mg/kg). [12,13] Teicoplanin + ceftriaxone was also administered because MIS-C might be confused with toxic shock syndrome. This treatment protocol was not applied to non-Covid-19 patients. In our study, we diagnosed MIS-C in all patients in the appendicitis + COVID-19 group. To patients those were with perforated appendicitis, also received metronidazole as a treatment against anaerobic bacteria.[12,13]
Blood sample collection
Blood samples were collected from all patients during the initial evaluation and diagnosis in period in the emergency room. Samples were also taken at 10:00 a.m. every day postoperatively until the three studied parameters dropped below the upper reference values. The blood samples were centrifuged at 4000 rpm for 7 min, and serum samples were collected and stored at −80°C until analysis.
Biochemical analysis
Serum IL-6 levels were determined by using a Roche E170 system (Roche Diagnostics, Osaka, Japan) using the electrochemiluminescence method. WBC counts and hsCRP levels were measured using routine blood biochemistry tests. The reference ranges of the laboratory parameters are shown in Table 2.
Table 2.
Laboratory Tests
| Parameter | Method | Reference range |
|---|---|---|
| White blood cell count (WBC) | Haematological Counter (Sysmex XN-1000, Istanbul, Turkey) | 4.3-10.3 X103/μl |
| Interleukin-6 (IL-6) | Electrochemiluminescent (Roche E 170 system, R&D, Osaka, JAPAN) | <7 pg/ml |
| High sensitive C-reactive protein (hCRP) | Siemens BN-II (nephalometric) | <0,35 mg/Dl |
Statistical analyses
Histograms, q-q plots and the Shapiro–Wilk test were used to assess data normality. Levene’s test was used to assess variance homogeneity. One-way analysis of variance (ANOVA), Welch’s ANOVA, Kruskal–Wallis H-test and Mann–Whitney U-test were used for intergroup comparisons continuous variables. Pearson’s Chi-squared test was used for comparisons between categorical variables. Siegel–Castellan test was conducted for post hoc comparisons. The analyses were performed using TURCOSA statistical software (Turcosa Analitik Ltd. Co., Kayseri/Turkey). A P value of less than 0.05 was considered as statistically significant.
RESULTS
A total of 153 paediatric patients were evaluated in this study. There were a total of 3 groups in our study. These groups were designed as non-perforated Appendicitis group [n = 110], [before the Covid-19 pandemic n = 75, after the Covid-19 pandemic n = 35], Perforated Appendicitis group [n = 35], [before the Covid-19 pandemic n = 20, after the Covid-19 pandemic n = 15] and Covid-19 + Appendicitis group [n = 8, 7 Non-perforated, 1 perforated]. The clinical characteristics of the three patient groups are shown in Table 3. There was no statistically significant difference between the groups in terms of age and gender. The Alvarado scores in the perforated appendicitis group were significantly higher than in the other groups. The mean operation time in the perforated appendicitis group was significantly longer than in the other groups. The mean time duration of hospitalization and intensive care in the non-perforated appendicitis group was significantly shorter than in the other groups.
Table 3.
Patient characteristics
| Variable | Groups | P | ||
|---|---|---|---|---|
|
| ||||
| Non- Perforated Appendicitis (n=110) | Perforated Appendicitis (n=35) | Covid-19 + Appendicitis (n=8 (7 Non-perforated, 1 perforated)) | ||
| Age (years) | 10.6±3.3 | 11.2±3.8 | 12.8±2.7 | 0.185 |
| Gender (male) | 59 (53.6) | 25 (71.4) | 3 (37.5) | 0.095 |
| Preop Alvarado score | 8 (5-9)a | 9 (8-10)b | 8 (8-9)ab | <0.001 |
| Operation time (min) | 63 (30-120)a | 95 (55-120)b | 63 (30-120)a | <0.001 |
| Lenght of hospital stay (days) | 2 (1-5)a | 6 (5-12)b | 5 (5-7)b | <0.001 |
| Length of intensive care unit stay (days) | 0 (0-3)a | 2 (0-4)b | 0 (0-5)b | <0.001 |
Values are expressed as n(%), mean±SD or median (min-max). Different superscripts in the same row indicate a statistically significant difference among groups
Only two patients developed postoperative complications. When these complications occurred in these two patients, our study was not ended yet. So we didn’t know when these laboratory parameters should returned to normal levels in patients with perforated appendicitis. One of them was a perforated appendicitis patient with COVID-19 infection. This patient was 17 years old, male. This patient of preoperative Alvarado score of this patient was 9. The total duration of hospitalization of this patient was 14 days. The Penrose drain was removed on the second postoperative day. Because the patient did not have leak from the drain on the 2nd postoperative day. But the laboratory parameters were still high, and we thought it was due to MIS-C which was related to Covid-19 infection. Until then, we did not exactly know how the disease progresses in the case of Covid-19 + perforated Appendicitis. Therefore, we did not consider the possibility that the reason for the high laboratory parameters in this patient might be an overlooked surgical complication. However, when the feces leakaged from the surgical wound, we understood the gravity of the situation. We guess that the surgical stump may have opened after the 5th postoperative day. Because after this date, the patient›s abdominal pain severity has increased. Since the remaining stump after the appendectomy was left shorter than usual, it opened, and leakage developed. Feces leakage from the surgical wound was detected on the eighth postoperative day. The patient underwent repair surgery at the same day, and the stump was primarily closed. The other patient, in whom perforation in the cecum developed postoperatively, had perforated appendicitis without COVID-19 infection. This patient was a 10 years old, male. Preoperative Alvarado score of this patient was 8. For this patient the total duration of hospitalization was 11 days. The Penrose drain was removed on the second postoperative day. Feces leakage from the surgical wound was observed on the third postoperative day. The patient underwent repair surgery on the same day, and the cecum was primarily closed.
The statistical analysis of laboratory values is summarized in Table 4. The preoperative ratio of polymorph nuclear leukocytes was significantly higher in the perforated appendicitis group than in the other two groups.
Table 4.
Comparison of preoperative and postoperative laboratory parameters among patient groups
| Laboratory parameter | Groups | P | ||
|---|---|---|---|---|
|
| ||||
| Non-Perforated Appendicitis (n=110) | Perforated Appendicitis (n=35) | Covid-19 Appendicitis (n=8, 7 non-perforated, 1 perforated) | ||
| IL-6 (pg/mL) | ||||
| Preop | 28.17 (3.67-274.30)a | 356.40 (7.78-5000.00)b | 68.94 (35.22-244.32)b | <0.001 |
| Postop Day-1 | 13.50 (1.50-22.10)a | 148.40 (18.40-1172.40)b | 36.20 (10.20-82.40)b | <0.001 |
| Postop Day-2 | 2.25 (1.20-9.20)a,* | 92.80 (13.20-532.40)b | 23.80 (7.40-40.40)b | <0.001 |
| Postop Day-3 | 1.80 (1.10-4.60)a | 44.80 (9.60-144.30)b | 17.30 (5.10-22.90)b | <0.001 |
| Postop Day-4 | - | 16.00 (4.00-86.00) | 10.00 (3.00-18.00) | 0.046 |
| Postop Day-5 | - | 7.00 (2.00-19.00)* | 5.00 (2.00-11.00)* | 0.350 |
| Postop Day-6 | - | 5.00 (3.00-9.00) | 5.00 (2.00-5.00) | 0.240 |
| Postop Day-7 | 5.00 (5.00-5.00) | - | - | |
| WBC (cell/mm3) | ||||
| Preop | 15.40 (8.40-24.48)a | 19.70 (13.40-28.29)b | 13.05 (5.03-16.20)a | <0.001 |
| Postop Day-1 | 10.20 (6.20-15.70)a,* | 17.30 (11.30-19.60)b | 10.77 (6.80-14.90)a | <0.001 |
| Postop Day-2 | 6.85 (4.70-10.50)a | 12.90 (10.50-16.30)b | 9.70 (6.80-13.20)a,* | <0.001 |
| Postop Day-3 | 6.30 (4.50-10.50)a | 11.70 (10.40-14.50)b | 7.75 (5.50-11.50)a | <0.001 |
| Postop Day-4 | 8.30 (7.30-9.20)* | |||
| HsCRP (mg/dL) | ||||
| Preop | 1.20 (0.25-32.50)a | 7.85 (0.79-32.50)b | 4.40 (0.31-17.80)ab | <0.001 |
| Postop Day-1 | 0.55 (0.21-5.50)a | 5.20 (0.52-23.50)b | 3.00 (0.35-22.10)b | <0.001 |
| Postop Day-2 | 0.32 (0.11-2.50)a,* | 4.20 (0.49-18.50)b | 1.85 (0.31-16.80)b | <0.001 |
| Postop Day-3 | 0.32 (0.22-0.36)a | 2.10 (0.40-11.20)b | 1.10 (0.32-8.50)b | <0.001 |
| Postop Day-4 | - | 1.00 (0.00-9.00) | 1.00 (0.00-5.00) | 0.482 |
| Postop Day-5 | - | 1.00 (0.00-6.00) | 1.00 (0.00-1.00) | 0.905 |
| Postop Day-6 | - | 0.00 (0.00-3.00)* | 0.00 (0.00-0.00)* | 0.101 |
| Postop Day-7 | - | 0.00 (0.00-1.00) | 0.00 (0.00-0.00) | 0.500 |
| Postop Day-8 | - | 0.00 (0.00-1.00) | - | - |
| Postop Day-9 | - | 0.00 (0.00-0.00) | - | - |
| Preop PMN ratio (%) | 81.60 (55.00-93.90)a,* | 87.20 (67.60-95.10) b,* | 77.35 (71.00-89.60)a,* | 0.004 |
*The first time point at which the median statistic falls below the reference value in the relevant group. Values are expressed as median (min-max). Different superscripts in the same row indicate a statistically significant difference among groups
The changes in WBC count, IL-6 and hsCRP parameters over time are shown in Figures 1-3.
Figure 1.
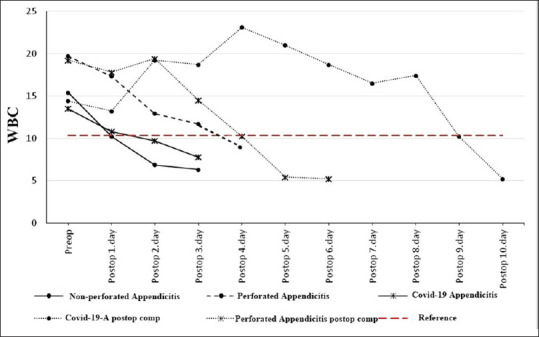
Changeover time in WBC across the three groups and two complicated patients. WBC: White blood cell
Figure 3.
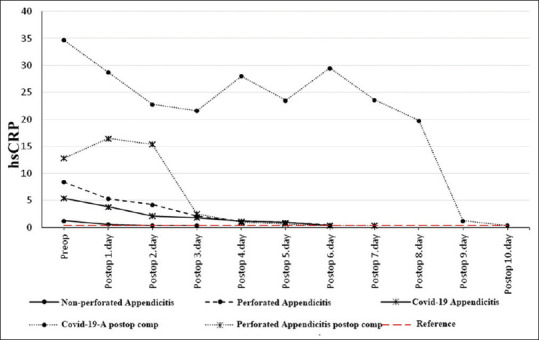
Changeover time in serum hsCRP levels across the three groups and two complicated patients. hsCRP: High-sensitivity C-reactive protein
The WBC count dropped below the upper reference value on the first postoperative day in the non-perforated appendicitis group, on the fourth postoperative day in the Perforated appendicitis group, and on the second postoperative day in the COVID-19 + appendicitis group [Figure 1].
The IL-6 value dropped below the upper reference value on the second postoperative day in the non-perforated appendicitis group, on the fifth postoperative day in the Perforated appendicitis group and on the fifth postoperative day in the COVID-19 + appendicitis group [Figure 2].
Figure 2.
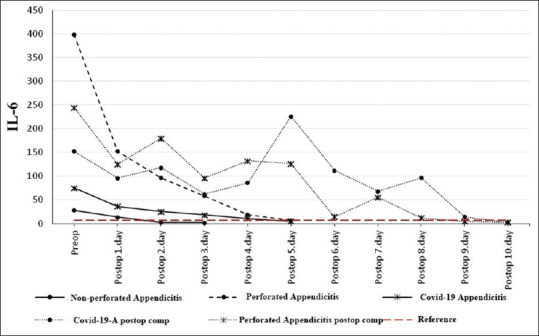
Changeover time in serum IL-6 levels across the three groups and two complicated patients. IL-6: Interleukin-6
The hsCRP value dropped below the upper reference value on the second postoperative day in the non-perforated appendicitis group, on the sixth postoperative day in the Perforated appendicitis group and on the sixth postoperative day in the COVID-19 + appendicitis group [Figure 3].
In two patients who developed postoperative complications, the levels of all three parameters remained above the upper reference limits until the complications were surgically corrected. We thought that the abnormal condition in these three parameters was due to Covid-19 infection in the Covid-19 patient and due to generalized peritonitis in the perforated appendicitis patient. In the perforated appendicitis patient without COVID-19 infection, the WBC count dropped below the upper reference value one day after the second operation [Figure 1], hsCRP three days after the second operation [Figure 3], and IL-6 five days after the second operation [Figure 2]. In the perforated appendicitis patient with COVID-19 infection, the levels of all three parameters dropped below the upper reference values one day after the second operation [Figures 1-3].
The statistical analysis of the effects of the pandemic on appendicitis patients is summarized in Tables 5 and 6. The time duration from the onset of abdominal pain to the surgery was significantly longer during than before the pandemic in both the non-perforated appendicitis group and the perforated appendicitis group [Table 5]. While the perforation rate was 21.1% [20/95 patients] before the Covid-19 pandemic, we found it to be 27.6% [16/58 patients] after the pandemic. Although the rate of perforated appendicitis has slightly increased, this increase was not found to be statistically significant [Table 6].
Table 5.
The relationship between pandemic conditions and time until surgery after abdominal pain begins
| Groups | Time until surgery after abdominal pain begins (hours) |
|---|---|
| Non- perforated Appendicitis | |
| Before the Covid-19 pandemic (n=75) | 18 (9-25) |
| After the Covid-19 pandemic (n=35) | 20 (17-40) |
| P | <0.001 |
| Perforated Appendicitis | |
| Before the Covid-19 pandemic (n=20) | 105 (57-122) |
| After the Covid-19 pandemic (n=15) | 195 (125-300) |
| P | <0.001 |
| Covid-19-Apendicitis (n=8 , 7 Non-perforated, 1 perforated ) | 38 (25-50) |
Values are expressed as median (min-max).
Table 6.
Effect of pandemic conditions on perforation rate
| Groups | Before the Covid-19 pandemic | After the Covid-19 pandemic | P |
|---|---|---|---|
| Non-Perforated Appendicitis | 75 (78.9) | 42 (72.4) (n=7 Covid-19+) | |
| Perforated Appendicitis | 20 (21.1) | 16 (27.6) (n=1 Covid-19+) | 0.420 |
DISCUSSION
The development of surgical complications after appendicitis surgery in patients is a rare but important condition.[14,15] Clinical evaluation alone may be inadequate, especially in perforated appendicitis patients with peritonitis.[14,15] Therefore, knowing when the laboratory parameters return to normal values might be useful for the diagnosis and treatment of those complications. There are a limited number of prospective studies that provide information on this subject. We found that the WBC, IL-6, and hsCRP levels dropped below the upper limits on the first postoperative day in non-perforated appendicitis patients, four to six days postoperatively in perforated appendicitis patients, and three to six days postoperatively in appendicitis patients with COVID-19 infection. These results may be useful in the postoperative follow-up of the appendicitis patients, as we found that these parameters did not drop below the upper limits until postoperative complications were surgically corrected.
IL-6 is a cytokine with a long chain composed of 212 amino acids.[8] It plays a key role in the acute phase of inflammation,[16] in immune cell maturation,[17] being involved in the differentiation of B and T cells, and in immunoglobulin production.[17] C-reactive protein (CRP) is an acute-phase protein with important pro-inflammatory functions in opsonisation, phagocytises and classical complement pathway activation.[18] The hsCRP test can detect extremely low CRP concentrations. The WBC count is a laboratory marker of intra-abdominal inflammation.[4]
IL-6, CRP and WBC are among many laboratory biomarkers those have been explored for the diagnosis and prediction of the severity and complications of appendicitis in paediatric patients. Yang et al. reported that CRP and WBC were useful not only for the diagnosis of appendicitis but also for the detection of perforation.[5] De Dios et al. also found that CRP is useful in detecting perforated appendicitis,[19] as visceral adipose tissue increases the CRP level in perforated appendicitis. Similarly, Snyder et al.[20] and Anderson et al.[21] have suggested the importance of CRP and WBC tests for diagnosis of appendicitis. Mohammed et al. found that a CRP value higher than 0.8 mg/dL and a WBC count of more than 11,000 cells/mm3 are reliable indicators of appendicitis.[22] Özozan et al. have suggested a preoperative hsCRP cut-off of 0.35 mg/dL for the detection of perforated appendicitis.[6] Moreover, a retrospective study by Van den Worm et al. found that the CRP level upon admission is a reliable predictor of the severity of appendicitis.[23] However, in a prospective study involving patients with acute appendicitis and nonspecific abdominal pain, Groslj-Grenc et al. found that among IL-6, WBC, CRP, and lipopolysaccharide-binding protein, only IL-6 has diagnostic value.[24] Similarly, Destek et al. suggested that it is important to look at IL-6 levels for the early diagnosis of acute appendicitis.[25] Branescu et al. prospectively investigated postoperative IL-6 levels in patients with non-perforated appendicitis and found that they returned to normal levels in the this group 72 hours after surgery.[26] A systematic review by Acharya et al. found that the traditional WBC test most commonly used for the diagnosis of appendicitis is inexpensive but has moderate diagnostic accuracy, whereas the newer IL-6 test is more expensive but has higher diagnostic accuracy.[27] However, Shogilev et al. suggested that a single lab marker has low a diagnostic accuracy, and it increases when markers such as WBC and CRP are used together.[28] Similarly, Yildirim et al. found that investigating IL-6, IL-10, CRP, and WBC together is more beneficial for diagnosing appendicitis and avoiding negative laparotomies.[29] In a retrospective study 850 paediatric appendicitis patients were evaluated and 10.3% of the patients developed complications that required re-operation. Complications in these patients were diagnosed with clinical evaluation, radiological evaluation and CRP level and WBC.[14] As a result of our study, we believe that the most valuable parameter in recognizing postoperative surgical complications is the clinical evaluation of a surgeon. However, we believe that serum IL-6 and hsCRP levels and WBC are helpful parameters together with clinical evaluation in the recognition of surgical complications in complex conditions such as generalized peritonitis and COVID-19 infection + MIS-C.
The COVID-19 pandemic has affected paediatric appendicitis patients.[30-32] In a retrospective study on the effects of the pandemic on the treatment of paediatric appendicitis patients, Velayos et al. found higher complication rates and longer hospital stays during than before the pandemic but no significant difference in the time duration between the onset of the first symptoms and the operation. 30 In another retrospective study, Fisher et al. found an increase in number of perforated appendicitis patients during the pandemic.[31] Similarly, Place et al. reported that the rate of perforated appendicitis patients was 19% in the one-year period before the COVID-19 outbreak and increased to 39% during the pandemic.[32] In our clinic, there was no delay in the hospitalization and surgical treatment of paediatric appendicitis patients during the pandemic, and the medical staff and equipment were adequate. However, we found that the time duration from start of the abdominal pain to the surgery has increased during the pandemic in all groups (Perforated and non-perforated appendicitis groups). Also we think that the number of perforated appendicitis patients slightly increased during the pandemic, although the increase was not statistically significant.
The results of this study need to be confirmed by researches with a greater number of patients having postoperative complication.
Limitations
This study has certain limitations. First, the number of patients with postoperative complications was not sufficient to form an independent group. Second, the numbers of patients in the groups were not equal, although there were no statistically significant differences in terms of age and gender. Third, because the patients were naturally admitted at different times of the day, the preoperative blood samples were accordingly taken at different times. One of the limitations of our study was that the non-perforated appendicitis group was not considered as separate groups (catarrhal, phlegmonous and gangrenous).
CONCLUSIONS
Our findings show that the most valuable parameter in recognizing postoperative surgical complications is the clinical evaluation of a surgeon and WBC, IL-6, and hsCRP are valuable laboratory parameters that can complete clinical examinations in the diagnosis of paediatric appendicitis and the identification of postoperative complications. Furthermore, the significant increase in the time duration between the onset of the first symptoms and the operation observed during the COVID-19 pandemic could be attributed to conditions related to the pandemic.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to thank Yücel DUMAN for his valuable contribution to this study.
REFERENCES
- 1.Dunn JC. Appendicitis. In: Grosfeld JL, O'Neil JA, Coran AG, Fonkalsrud EW, editors. Textbook of Pediatric Surgery. 6th. 98. Vol. 2. Philadelphia: Mosby Elsevier; 2006. pp. 1501–13. [Google Scholar]
- 2.Germolec DR, Shipkowski KA, Frawley RP, Evans E. Markers of inflammation. Methods Mol Biol. 2018;1803:57–79. doi: 10.1007/978-1-4939-8549-4_5. [DOI] [PubMed] [Google Scholar]
- 3.Pritts T, Hungness E, Wang Q, Robb B, Hershko D, Hasselgren PO. Mucosal and enterocyte IL-6 production during sepsis and endotoxemia-role of transcription factors and regulation by the stress response. Am J Surg. 2002;183:372–83. doi: 10.1016/s0002-9610(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 4.Xiao Z, Wilson C, Robertson HL, Roberts DJ, Ball CG, Jenne CN, et al. Inflammatory mediators in intra-abdominal sepsis or injury –A scoping review. Crit Care. 2015;19:373. doi: 10.1186/s13054-015-1093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Liu C, He Y, Cai Z. Laboratory markers in the prediction of acute perforated appendicitis in children. Emerg Med Int. 2019;2019:4608053. doi: 10.1155/2019/4608053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Özozan ÖV, Vural V. High C-reactive protein level as a predictor for appendiceal perforation. Ulus Travma Acil Cerrahi Derg. 2020;26:63–6. doi: 10.14744/tjtes.2019.14799. [DOI] [PubMed] [Google Scholar]
- 7.Hassan SA, Sheikh FN, Jamal S, Ezeh JK, Akhtar A. Coronavirus (COVID-19):A review of clinical features, diagnosis, and treatment. Cureus. 2020;12:e7355. doi: 10.7759/cureus.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalan M, Talbot D, Cunliffe WJ, Rich AJ. Evaluation of the modified Alvarado score in the diagnosis of acute appendicitis:A prospective study. Ann R Coll Surg Engl. 1994;76:418–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Velly L, Gayat E, Quintard H, Weiss E, De Jong A, Cuvillon P, et al. Guidelines:Anaesthesia in the context of COVID-19 pandemic. Anaesth Crit Care Pain Med. 2020;39:395–415. doi: 10.1016/j.accpm.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palas A, Raval J, Aiyer RG, Arunlal B. Pediatric E. N. T. emergencies during COVID-19 pandemic:Our experience. [cited 2021 Jan 4];Indian J Otolaryngol Head Neck Surg. 2021 4:1–5. doi: 10.1007/s12070-020-02357-z. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7781556 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St Peter SD, Tsao K, Spilde TL, Holcomb GW, 3rd, Sharp SW, Murphy JP, et al. Single daily dosing ceftriaxone and metronidazole vs standard triple antibiotic regimen for perforated appendicitis in children:a prospective randomized trial. J Pediatr Surg. 2008;43:981–5. doi: 10.1016/j.jpedsurg.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sankar J, Dhochak N, Kabra SK, Lodha R. COVID-19 in children:Clinical approach and management. Indian J Pediatr. 2020;87:433–42. doi: 10.1007/s12098-020-03292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS–CoV-2 and hyperinflammation in pediatric COVID-19:Version 2. Arthritis Rheumatol. 2021;73:e13–e29. doi: 10.1002/art.41616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frongia G, Mehrabi A, Ziebell L, Schenk JP, Günther P. Predicting postoperative complications after pediatric perforated appendicitis. J Invest Surg. 2016;29:185–94. doi: 10.3109/08941939.2015.1114690. [DOI] [PubMed] [Google Scholar]
- 15.Wee JJ, Park CJ, Lee YT, Cheong YL, Rai R, Nah SA. A simple classification of peritoneal contamination in perforated appendicitis predicts surgery-related complications. J Paediatr Child Health. 2020;56:272–5. doi: 10.1111/jpc.14591. [DOI] [PubMed] [Google Scholar]
- 16.Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Analytic review:Interleukin-6 in surgery, trauma, and critical care:Part I:Basic science. J Intensive Care Med. 2011;26:3–12. doi: 10.1177/0885066610395678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6:From basic science to medicine. Arthritis Res. 2002;4(3):S233–42. doi: 10.1186/ar565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004;279:48487–90. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 19.de Dios O, Gavela-Pérez T, Aguado-Roncero P, Pérez-Tejerizo G, Ricote M, González N, et al. C-reactive protein expression in adipose tissue of children with acute appendicitis. Pediatr Res. 2018;84:564–7. doi: 10.1038/s41390-018-0091-z. [DOI] [PubMed] [Google Scholar]
- 20.Snyder MJ, Guthrie M, Cagle S. Acute appendicitis:Efficient diagnosis and management. Am Fam Physician. 2018;98:25–33. [PubMed] [Google Scholar]
- 21.Andersson M, Rubér M, Ekerfelt C, Hallgren HB, Olaison G, Andersson RE. Can new inflammatory markers improve the diagnosis of acute appendicitis?World J Surg. 2014;38:2777–83. doi: 10.1007/s00268-014-2708-7. [DOI] [PubMed] [Google Scholar]
- 22.Mohammed AA, Daghman NA, Aboud SM, Oshibi HO. The diagnostic value of C-reactive protein, white blood cell count and neutrophil percentage in childhood appendicitis. Saudi Med J. 2004;25:1212–5. [PubMed] [Google Scholar]
- 23.Van den Worm L, Georgiou E, De Klerk M. C-reactive protein as a predictor of severity of appendicitis. S Afr J Surg. 2017;55:14–7. [PubMed] [Google Scholar]
- 24.Groselj-Grenc M, Repse S, Dolenc-Strazar Z, Hojker S, Derganc M. Interleukin-6 and lipopolysaccharide-binding protein in acute appendicitis in children. Scand J Clin Lab Invest. 2007;67:197–206. doi: 10.1080/00365510601010397. [DOI] [PubMed] [Google Scholar]
- 25.Destek S, Gül VO, Menteş MÖ, Çiçek AF. Diagnostic efficacy of serum procalcitonin, IL-6, IL-2, and D-dimer levels in an experimental acute appendicitis model. Turk J Gastroenterol. 2019;30:641–7. doi: 10.5152/tjg.2019.18534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brănescu C, Serban D, Dascălu AM, Oprescu SM, Savlovschi C. Interleukin 6 and lipopolysaccharide binding protein –Markers of inflammation in acute appendicitis. Chirurgia (Bucur) 2013;108:206–14. [PubMed] [Google Scholar]
- 27.Acharya A, Markar SR, Ni M, Hanna GB. Biomarkers of acute appendicitis:Systematic review and cost-benefit trade-off analysis. Surg Endosc. 2017;31:1022–31. doi: 10.1007/s00464-016-5109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shogilev DJ, Duus N, Odom SR, Shapiro NI. Diagnosing appendicitis:Evidence-based review of the diagnostic approach in 2014. West J Emerg Med. 2014;15:859–71. doi: 10.5811/westjem.2014.9.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yildirim O, Solak C, Koçer B, Unal B, Karabeyoğlu M, Bozkurt B, et al. The role of serum inflammatory markers in acute appendicitis and their success in preventing negative laparotomy. J Invest Surg. 2006;19:345–52. doi: 10.1080/08941930600985686. [DOI] [PubMed] [Google Scholar]
- 30.Velayos M, Muñoz-Serrano AJ, Estefanía-Fernández K, Sarmiento Caldas MC, Moratilla Lapeña L, López-Santamaría M, et al. Influence of the coronavirus 2 (SARS-Cov-2) pandemic on acute appendicitis. An Pediatr (Engl Ed) 2020;93:118–22. doi: 10.1016/j.anpede.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher JC, Tomita SS, Ginsburg HB, Gordon A, Walker D, Kuenzler KA. Increase in pediatric perforated appendicitis in the New York City metropolitan region at the epicenter of the COVID-19 outbreak. Ann Surg. 2021;273:410–5. doi: 10.1097/SLA.0000000000004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Place R, Lee J, Howell J. Rate of pediatric appendiceal perforation at a children's hospital during the COVID-19 pandemic compared with the previous year. JAMA Netw Open. 2020;3:e2027948. doi: 10.1001/jamanetworkopen.2020.27948. [DOI] [PMC free article] [PubMed] [Google Scholar]


