Abstract
Evidence supports the notion that metabolic pathways are major regulators of organismal aging, and that metabolic perturbations can extend health- and lifespan. For this reason, dietary interventions and compounds perturbing metabolism are currently explored as anti-aging strategies. A common target for metabolic interventions delaying aging is cellular senescence, a state of stable growth arrest that is accompanied by various structural and functional changes including the activation of a pro-inflammatory secretome. Here, we summarize the current knowledge on the molecular and cellular events associated with carbohydrate, lipid and protein metabolism, and define how macronutrients can regulate induction or prevention of cellular senescence. We discuss how various dietary interventions can achieve prevention of disease and extension of healthy longevity by partially modulating senescence-associated phenotypes. We also emphasize the importance of developing personalized nutritional interventions that take into account the current health and age status of the individual.
Keywords: Aging, Macronutrients, Metabolism, Senescence
Introduction
Dietary interventions and aging
Aging is a major risk factor for non-communicable pathologies such as cancer, cardiovascular diseases, musculoskeletal dysfunctions and neurodegeneration. The aging process is characterized by complex molecular, cellular and tissue-level changes which lead to a gradual decline in functional capacity [1]. Studies in model organisms have demonstrated that diet has profound influence on aging rates, and that the nutritional status is directly correlated with the risk of age-associated morbidities [2]. Macronutrients are the primary constituents of foods and the source of calories which organisms consume in large amounts to meet energy demands and to promote growth. Macronutrients comprise three elements: carbohydrates, proteins, and fats. Through various metabolic pathways, macronutrients are digested into their basic building blocks: amino acids from protein, saccharides from carbohydrates, and fatty acids from fats [3]. Limiting general caloric intake without malnutrition by reduction of macronutrients intake, also known as calorie restriction, is a robust intervention shown to extend the health- and lifespan of several experimental animals, including rodents and non-human primates [4]. However, it is becoming more evident that not only the total amount, but also quality and relative proportions, of macronutrients play an important role in long-term metabolic health.
Aging and cellular senescence
Cellular senescence is a state of stable cell cycle arrest activated in response to stressors or developmental signals [5]. Although senescent cells do not divide, they remain metabolically active, continue to grow in size and develop a secretory phenotype termed senescence-associated secretory phenotype (SASP) [6]. Cellular senescence functions as a potent mechanism against tumorigenesis in a cell-autonomous manner [7]. In addition, senescent cells can play a pivotal role in optimal tissue regeneration, repair and embryonic development [[8], [9], [10]].
In contrast to these beneficial functions, chronic presence of senescent cells with a SASP can cause low grade sterile inflammation, leading to tissue dysfunction and degeneration [11]. In accordance with the contribution to the loss of tissue homeostasis by altering the function of neighboring cells, the clearance of senescent cells delays aging and increases lifespan in mice [12]. Cellular senescence may therefore be viewed as an elegant mechanism that preserves tissue function early in life but that becomes detrimental in older organisms [13]. Despite the considerable increase in studies that focus on cellular senescence in the last decade, there is no single marker that can be used for all types of senescent cells [14]. Multiple markers and molecular tools are available, and a combination of these markers is a preferred method for the identification of senescent cells [5,15]. The most commonly used markers are: high activity of the lysosomal senescence-associated beta-galactosidase (SA-β-gal) [16]; enhanced expression of cyclin-dependent kinase (CDK) inhibitors CDKN1A (p21) and CDKN2A (p16) [17], loss of the nuclear lamina protein lamin B1 [18]; secretion of biomolecules included in the SASP [6], and persistent DNA damage response which is preferentially located at telomeres [19].
Dietary macronutrients in longevity and cellular senescence
There is substantial evidence supporting the concept that metabolic pathways are major regulators of tissue and organismal aging, and that certain metabolic perturbations can lead to prolonged health and longevity. Interestingly, various dietary regimens and metabolic perturbations influence the accumulation of senescent cells and the development of the SASP. In the next sections, we provide an overview of the molecular and cellular events by which macronutrients modulate the induction of senescence-associated phenotypes, and outline the relevance of balancing macronutrient intake to reduce detrimental senescence-mediated effects on disease and aging.
Carbohydrates
Carbohydrates are used in different cellular processes, including cellular signaling and energy production. Glucose, which is the end product of many types of carbohydrates, has been extensively studied in terms of metabolism and influence on the aging process. In yeasts and nematodes glucose-enriched diets shorten lifespan [20]. In humans, the consumption of high glycemic index foods is correlated to the increased risk of different age-related diseases like type 2 diabetes (T2D) and cardiovascular diseases. For instance, a meta-analysis study concluded that diets with higher glycemic index (GI) and load are associated with a higher risk for developing T2D [21]. Mortality risk increases with high sugar intake [22], and glucose dysregulation is a major risk factor for many diseases associated with aging [23]. T2D is a significant risk factor for many age-related diseases (e.g., kidney, cardiovascular and neurodegenerative diseases) [[24], [25], [26]], suggesting that glucose dysregulation might accelerate biological aging. Notably consumption of a diet with low GI late in life extends the lifespan of mice [27]. Therefore, consumption of low GI food might be even more important at later stages in life as the body capacity to regulate glucose levels progressively declines with age [28].
One common hallmark of senescent cells is dysfunctional mitochondria, a state which leads to increased glycolysis as an adaptive process [29]. Many studies have shown that glucose consumption, glycolysis and lactate production are increased in senescent cells [[30], [31], [32]]. Because a major energy-consuming process of senescent cells is the SASP, it is likely that SASP levels are proportional to glucose availability. In agreement with this concept, circulating cytokines are acutely induced by hyperglycemia through oxidative stress mechanisms [33], and high GI carbohydrates increase the activation of NF-κB, a major inducer of the pro-inflammatory arm of the SASP [34]. p53 represses both glucose transport, by inhibiting a subset of glucose transporters, and lactate transport, by suppressing MCT1, and glycolysis through the induction of TIGAR or the inhibition of PGM [35]. Additionally, p53 suppresses several components of the SASP [6], suggesting that the SASP might be regulated, in part, by glycolysis.
Despite glycolysis being an important mechanism for glucose metabolism, it can potentially cause cellular injury due to increased production of toxic products, including methylglyoxal. Methylglyoxal is a dicarbonyls, and a potent glycating agent that can react with proteins, lipids and nucleic acids to form advanced glycation-end products (AGEs) [36]. Production of AGEs aberrantly increases during hyperglycemia [36] (Fig. 1). Since the senescence phenotype is accompanied by increased glycolysis, the aberrant accumulation of senescent cells might lead to the accumulation of methylglyoxal, and subsequent increased production of AGEs – a mechanism by which senescent cells could promote systemic dysfunctions and multimorbidity.
Fig. 1.
Potential mechanisms through which hyperglycemia, as well as certain compounds (dashed border), may modulate the induction of cellular senescence.
Abbreviations: ROS: reactive oxygen species; NO: nitric oxide; SASP: senescence-associated secretory phenotype; AGEs: advanced glycation end products.
Glucose levels might not only modulate senescence-associated phenotypes, but also promote entry into a senescence state, as multiple pathways are implicated in hyperglycemia induced-senescence [37]. Direct induction of cellular senescence by hyperglycemia might be particularly relevant for cells able to uptake glucose without the action of insulin. For example, in endothelial cells glucose uptake mainly occurs via glucose transporter 1 (GLUT-1) in an insulin-independent manner [38]. High intracellular glucose concentration can stimulate the production of mitochondrial superoxide and ROS via glucose autoxidation, leading to cellular damage and damage-induced senescence [39]. Moreover, some studies have shown that hyperglycemia reduces nitric oxide (NO) production, which is known to interfere with the induction of cellular senescence [40,41]. Numerous experiments have also shown that downregulation of sirtuins (SIRTs) could be implicated in hyperglycemia-induced cellular damage. In accordance, hyperglycemia has been shown to downregulate the expression of SIRT3 and, consequently, promotes the induction of senescence [42] (Fig. 1). Interestingly, downregulation of SIRT3 leads to a type of senescence mediated by mitochondrial dysfunction termed “MiDAS” (Mitochondrial Dysfunction-Associated Senescence) which has a less pro-inflammatory signature [43]. Another sirtuin, SIRT1, was reported to protect endothelial cells from hyperglycemia-induced senescence, partially through the reduction of oxidative stress [44].
Glucose fluctuations with intermittent high glucose (IHG), which can occur after the consumption of high GI foods (i.e., postprandial spikes in blood glucose) and/or in diabetes, is detrimental for the integrity of endothelial tissue, and has been shown to induce senescence more robustly compared to constant hyperglycemia [45,46]. Hyperglycemia is associated with high AGEs production, and AGEs (e.g. glycated collagen) and dicarbonyls (e.g. Glyoxal and Methylglyoxal) induce senescence in endothelial cells [47,48] and kidney epithelial cells [49]. As a result, hyperglycemia can increase the number of senescent cells which can favor insulin resistance and type 2 diabetes further increasing a hyperglycemic state. This might create a vicious cycle where hyperglycemia and diabetes cause premature accumulation of senescent cells, and aberrant cellular senescence sustains pathology and accelerates tissue degeneration [[50], [51], [52], [53]].
Some dietary carbohydrates are known to be indigestible but can be processed by the gut microbiome to produce different bioactive molecules with health-promoting effects [54]. Butyrate, a short chain fatty acid (SCFA) by-product of dietary fiber fermentation, was shown to downregulate inflammatory pathways in different conditions, including aging [55,56]. The potential effect of SCFA on senescence is described in the lipids section.
As glucose metabolism is a key player in the aging process, modulation of glucose consumption and metabolism has the potential to prevent tissue dysfunction and delay age-related decline. Based on human observational studies, the first-line drug for T2D, metformin, has gained interest as pharmacological intervention to extend human health and longevity [57], and it is currently under preparation for the first clinical trial on aging called TAME [58]. Interestingly, Metformin was shown to inhibit the SASP through interfering with NF-κB signaling [59]. However, many questions need to be answered concerning potential adverse effects, non-responders, and whether metformin has any positive effect on healthy individuals, including reducing the SASP [58]. Another diabetic control drug with an anti-aging potential is the α-glucosidase inhibitor acarbose. Acarbose and other α-glucosidase inhibitors alter the absorption of carbohydrates through the inhibition of their conversion into simple sugars in the intestine, leading to a reduction in postprandial glucose blood level [60]. Acarbose increases the levels of butyrate in both the colon and in serum [61,62], and its concentrations are associated with increased survival [63]. Additionally, acarbose was shown to reduce mTOR activity in different tissues [64,65]. In a recent study, acarbose was able to extend median and maximal lifespan and to improve various health parameters in genetically heterogeneous male and female mice [66]. As mentioned previously, glycolysis might promote tissue aging via promoting the formation of AGEs. Remarkably, d-glucosamine, which can act as a glycolysis inhibitor, was shown to possess longevity effects in aging mice [67]. Furthermore, it will also be interesting to assess whether the removal of senescent cells from aging organisms achieves health benefits due to the restoration of glucose homeostasis. Importantly, genetic and pharmacological removal of senescent cells was shown to alleviate the progression of both type 1 and type 2 diabetes [51,52,68].
Proteins
Proteins are complex molecules that assume a plethora of structural, metabolic, and signaling functions that are essential for cellular viability. Protein intake is emerging as a critical player in longevity. Moderate reduction of protein intake can extend healthspan and lifespan in different species [69]. The importance of dietary protein intake for longevity has been evaluated by the Geometric Framework for Nutrition [70]. Mice fed with a diet that is low in protein-to-carbohydrate (P:C) ratio showed enhanced insulin sensitivity, improved metabolic health, slower aging, and extended lifespan, regardless of caloric intake. In contrast, diets with high P:C ratio worsen metabolic health, and negatively impact longevity [71,72]. In humans, a 6-week protein restriction (7–9% of energy intake) randomized controlled study in overweight adults resulted in improved metabolic parameters [73]. Additionally, in individuals aged between 50 and 65, low protein intake (less than 10% of calories from protein) has been linked to a significant decrease in the risk of all-cause and cancer-related mortality [74]. Okinawans, the longest living population in the world, are used to a dietary regimen with only 9% of calories derived from proteins, with a 9:85 ratio of protein-to-carbohydrate [75]. Although the restriction of proteins by a 1:10 ratio of protein-to-carbohydrate has been associated with extended longevity and prevention of various age-related diseases across many species [76], the type of dietary carbohydrate consumed can have an important impact on the metabolic health effects induced by a low protein diet [77]. In addition, individuals older than 65 consuming a low protein diet (<10%) exhibit increased mortality and old mice are not able to maintain their weight on a low protein diet, indicating that protein utilization is negatively affected by aging [74]. These results suggest that protein intake should be adjusted to life stage and balanced with the other macronutrients in order to achieve optimal health.
In addition to protein intake, protein source (i.e., animal or plant) might also have an impact on healthspan. Animal proteins are associated with a higher risk of mortality, especially from cardiovascular diseases, compared to plant proteins, which are associated with lower all-cause mortality [78]. In animal studies, casein was associated with a 5-fold increase in the risk of developing atherosclerosis compared to soy protein [79]. In a prospective study, postmenopausal women showed a 30% reduction in the risk for coronary artery disease mortality following an isocaloric replacement of animal with plant proteins [80]. The “Nurses' Health Study” and the “Health Professionals Follow-Up Study” showed that increased intake of animal proteins is linked to higher all-cause, cardiovascular, and cancer-related mortalities, whereas plant proteins were linked to lower all-cause and cardiovascular mortalities. These findings could be, at least in part, explained by the proportion of amino acids in animal versus plant proteins [78]. The restriction of certain amino acids, such as methionine and branched chain amino acids (BCAAs), can have a significant impact on health and lifespan in different experimental models. A randomized controlled study showed that a 6-week intervention of diets with a moderate protein restriction (7–9% of energy intake) was correlated with decreased BCAAs levels in plasma and improved metabolic parameters [73]. Mice fed a diet low in the BCAAs isoleucine and valine had an improvement in body composition and glucose tolerance [73,81]. Likewise, methionine restriction has been shown to extend lifespan in different model organisms from yeast to rodents [82]. With regard to protein composition, vegan and plant-based diets are naturally low in BCAAs and methionine, suggesting that such diets might extend healthy longevity [83].
The evolutionarily conserved insulin/IGF-1 signaling pathway plays an essential role in controlling lifespan [84]. Interestingly, low protein diets were associated with low circulating levels of IGF-1, and decreased mortality and the incidence of cancer in subjects younger than 65 years [74]. The restriction of a single amino acid, such as methionine, was also shown to reduce plasma IGF-1 in mice [85]. Of note, prolonged exposure of cells to IGF-1 can regulate the SIRT1-p53 pathway and promote senescence [86], suggesting a potential delay in the accumulation of senescent cells in methionine-restricted dietary conditions (Fig. 2). Interestingly, we have recently shown that higher protein dietary content correlates with an increase in different senescence-associated markers in mouse liver [87].
Fig. 2.
An overview of how an increased intake of dietary proteins may induce cellular senescence, with particular focus on oxidation response and certain amino acids.
Abbreviations: BCAAs: branched-chain amino acids; mTOR: mechanistic target of rapamycin; FGF21: fibroblast growth factor 21; SIRT1: sirtuin 1; SASP: senescence-associated secretory phenotype.
mTOR signaling is known to have a major role in the aging process, and availability of branched-chain amino acids, in particular leucine and methionine, regulates mTORC1 activity [88] (Fig. 2). Interestingly, it has been suggested that mTOR signaling is a primary mediator of the conversion from a quiescent to a senescent state, a process that is defined as ‘geroconversion’ [89]. In addition, mTOR acts as a promoter of the secretory phenotype (SASP) in at least two ways. On one side, it increases the translation of IL-1α mRNA, which elevates inflammatory genes regulated by the transcription factor NF-κB [90]. On the other side, it interacts with MAPK leading to an increase in the translation of the MK2 kinase, which consequently prevents the degradation of different SASP factor transcripts by ZFP36 ring finger protein-like 1 [91].
Rapamycin, an mTOR inhibitor, is able to significantly increase longevity in mice even when administered at a late-stage in life [[92], [93], [94]], in addition to reducing the SASP [90]. The Dog Aging Project, a longitudinal study of aging in companion dogs, has started a large-scale clinical trial involving the use of rapamycin in dogs for health and lifespan extension. The large-scale intervention study is based on the promising results of a short-term pilot study that showed an improved heart function in rapamycin-treated middle-aged dogs, compared to placebo [95]. Overall, these observations are encouraging the use of mTOR inhibitors to enhance human longevity. Conversely, prolonged rapamycin treatment may lead to potential adverse effects, such as glucose intolerance and immunosuppression. For this reason, intermittent dosing regimens have been proposed to reduce the adverse effects of rapamycin [96]. An alternative strategy can be the use of molecules that can specifically target mTORC1, given that many rapamycin-driven side effects are caused by its inhibition of mTORC2.
Another mechanism by which moderate protein restriction can enhance longevity is through the increase in the production of hydrogen sulfide (H2S) [69]. Mammalian cells produce H2S through the reverse trans-sulfuration pathway, where sulfur is transferred from homocysteine to cysteine, producing several sulfur metabolites in the process, including H2S [97]. Homocysteine is produced from dietary methionine, and the former is used to generate cysteine through the action of the cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE, encoded by CTH gene) enzymes. Interestingly, CSE deficiency was shown to stimulate cellular senescence in mouse embryonic fibroblast (MEFs) [98]. MEFs isolated from CTH-KO mice showed elevated oxidative stress, reduced proliferative capacity, higher number SA-β-gal cells and increased levels of p53 and p21 compared to wild-type controls [98]. Sodium hydrosulfide (NaHS), a H2S donor, reportedly reduced the protein levels of p53 and p21, levels of ROS, and the percentage of SA-β-gal-positive cells. These effects were attributed to the sulfhydration of Keap1 protein and the activation of Nrf2, which is a transcription factor that mediates the expression of antioxidant genes [98]. Another study showed that H2S prevents H2O2-induced senescence in HUVECs by enhancing the activity, but not the levels, of SIRT1 [99] (Fig. 2). Similar findings were reported in the Hcy-induced neuronal senescence model using HT22 cells [100]. Interestingly, it was shown that the intracellular levels of H2S are increased during cellular senescence due to an increased transcription of CTH [101]. Although H2S could potentially suppress NF-κB by reducing the phosphorylation of IκBα and p65 subunit, it is yet to be determined whether the induction of H2S in senescent cells can have a direct effect on senescence-associated phenotypes other than inflammation.
Fibroblast growth factor 21 (FGF21) is an insulin-sensitizing hormone associated with a better metabolic health and longevity [102]. Because FGF21 is induced by protein, BCAAs or methionine restriction, it has been suggested that the health-promoting effects of such interventions are in part due to the increase of circulating FGF21 [69]. It is interesting to note that vegan diets -which are intrinsically low in total proteins and in methionine and BCAAs- are associated with increased levels of FGF21 [83]. FGF21 can prevent or delay senescence induction in osteoarthritis via the increased expression of SIRT1 and subsequent inhibition of mTOR [103]. Additionally, it was shown that in endothelial cells, FGF21 is able to delay and prevent both replicative and premature senescence, respectively [104] (Fig. 2).
Lipids
Lipids constitute a broad category of essential water-insoluble biomolecules with crucial functions as energy sources, cellular membrane constituents and signaling molecules. Lipids are obtained primarily in the form of fatty acids, which normally occur in food sources in their esterified form (i.e., fats). After intake, fatty acids can either be used for energy production through mitochondrial β-oxidation or serve as building blocks for more complex lipid classes [105].
Excessive fat intake in humans is strongly associated with the increased prevalence of obesity and metabolic diseases. Studies on dietary fats have mainly focused on the total amount consumed or on the relative contribution to caloric intake. In animal models, high fat diets are frequently employed to study obesity, metabolic dysregulation and subsequent development of pathologies otherwise associated with aging such as diabetes, atherosclerosis or hepatic steatosis [[106], [107], [108], [109]]. However, it is becoming increasingly clear that the quality of dietary fats and the balance between various macronutrients play a larger role in determining health outcomes than the absolute amount of fat consumed [110].
Reported negative health effects of high fat diets in humans broadly correspond to the issues generally associated with hypercaloric feeding and energy imbalance, with very little evidence that excess calories obtained from fat instead than from proteins or carbohydrates would have a more detrimental impact [110]. Foods with high fat content can be overconsumed due to high palatability and limited satiating capacity, and the high energy density of fats can lead to hypercaloric intake [111]. A comparative analysis of various experimental rodent diets indicated that the weight gain conventionally observed in mice maintained on a HF diet partly results from compensatory overfeeding elicited by limited intake of protein and carbohydrate [71]. However, medium chain triglycerides such as those found in coconut oil may be able to contribute to satiety and limit appetite [112].
Supporting the claim that HF diets are not detrimental per se is the positive health outcome of ketogenesis during aging. In conditions of limited carbohydrate availability, metabolism shifts towards increased beta oxidation with consequent production of beneficial ketone bodies such as beta-hydroxybutyrate [113]. Mice maintained on a ketogenic diet from middle age display an extended median lifespan [114], and male mice intermittently fed an isocaloric ketogenic diet exhibit improvements in longevity and limited cognitive and motor decline at old age [115,116]. Pilot studies have recently shown that ketogenesis is linked to improved cognition in aged patients suffering from HIV-associated neurocognitive impairment [117], and in individuals with mild cognitive impairment [118].
The qualitative composition of the lipid fraction of the diet is probably the most important factor pertaining to fat consumption, as different fatty acids have clearly distinct properties and physiological effects. The risk of habitual overfeeding and consequent weight gain is highest in the context of a diet rich in saturated fatty acids, which promote hypothalamic inflammation and dysregulation of feeding behavior [119]. Conversely, unsaturated fatty acids can reverse inflammatory processes in the hypothalamus and limit passive food intake [120]. Male Wistar rats fed diets with similar amounts of differently sourced fats exhibit profound differences in health and longevity. In this case, a sunflower-oil based diet resulted in a shorter medial lifespan and increased bone loss and liver fibrosis at old age compared to a virgin olive oil based (MUFA rich) or fish oil based (n-3 PUFA rich) diet [121]. Epidemiological studies indicate that a substitution of saturated fatty acid (SFA) with mono or polyunsaturated (MUFA or PUFA) without reduction of total fat intake, such as by adoption of a Mediterranean diet, is the best approach for addressing multiple cardiovascular risk factors (LDL-C, ApoB, ApoA-I and circulating triglyceride levels) [122]. Additionally, excess SFA can induce generalized inflammation via Tlr4-dependent macrophage polarization [123], while increasing MUFA levels may be able to counteract this phenomenon through PPARγ activation [124].
Different organismal lipid profiles are associated with age and health status, and their manipulation could alleviate age-related pathophysiological processes [125]. Nevertheless, it is difficult to estimate the extent to which dietary interventions are capable of such manipulation. Individual lipid profiles depend not only on nutritional fat input, but also on de novo lipogenesis and expression of enzymes acting on fatty acid chains in terms of saturation, elongation and incorporation into more complex lipid forms. PUFAs stand out in this regard, because their biosynthesis is impossible in mammalian cells and therefore must be obtained solely from the diet. Interconversion between different n-3 PUFA forms is possible but rate-limited, and ability to synthesize EPA from ALA declines with age [126]. It could therefore be argued that changes in the intake of PUFAs stand the highest chance of modulating lipid profiles through dietary means. Because de novo lipogenesis in mammals primarily results in the production of palmitic acid, it might be expected that excessive reliance on this process would shift the organismal lipid profile towards an unfavorable state. In line with this idea, a few studies indicate that diets with very low-fat composition lead to increased all-cause mortality [122].
During aging, the capacity of subcutaneous adipose tissue to store fat declines, and fat deposits migrate to ectopic locations with suboptimal abilities to control fatty acid release [127]. As a result, the levels of circulating free fatty acids increase with age and create a predisposition towards lipotoxicity. In this context, the aged organism could be particularly reliant on a good dietary balance between different fatty acids, since increased circulating levels, due to impaired storage in adipose tissue, can exacerbate both their beneficial and detrimental effects. High fat diet-induced obesity and dyslipidemia correlate with increased accumulation of multiple senescent cell types in various animal models [128,129], as well as in human patients [130]. It was shown that a high fat diet can induce senescence in mice, mainly in visceral adipose tissue, and that the elimination or prevention of senescent cells alleviates obesity-induced metabolic dysfunctions [51,131]. The need to store excess fat exerts mitotic stress on preadipocyte populations, ultimately leading to a massive increase in senescent cell burden in adipose tissue [127]. An upregulation of senescence markers was also shown in renal tubular cells of obese mice maintained on HF nutrition [132]. In the rodent brain, excessive exposure to dietary fat (and particularly palmitic acid) induces senescence and inflammation through an increase in oxidative stress paired with impairment of autophagy [133]. In a non-human primate model (baboons), a HF diet was able to induce premature endothelial cell senescence after only 7 weeks [134], indicating that development of obesity is not a prerequisite for the occurrence of detrimental health effects of high fat exposure. Excess palmitate exposure was also shown to induce senescence in endothelial cells in different studies [135,136]. Moreover, senescent adipocyte progenitor cells seem to impair adipogenesis via cell-autonomous mechanisms — by losing adipogenic capacity, triglyceride storage, and secretion of adipokines [137], and in a non-cell autonomous fashion through the secretion of activin A [138].
Due to the fact that lipid storage in adipocytes seems to be an important protective mechanism against lipotoxicity and metabolic disease [139], senescence-driven impairment of adipose tissue might play an important role in promoting metabolic dysregulations. Due to dysfunctional mitochondria, some types of senescent cells have also decreased capacity to efficiently catabolize fatty acids by β-oxidation. As a consequence, some senescent cells, such as hepatocytes and fibroblasts, accumulate excessive fat and become pathogenic [140] (Fig. 3).
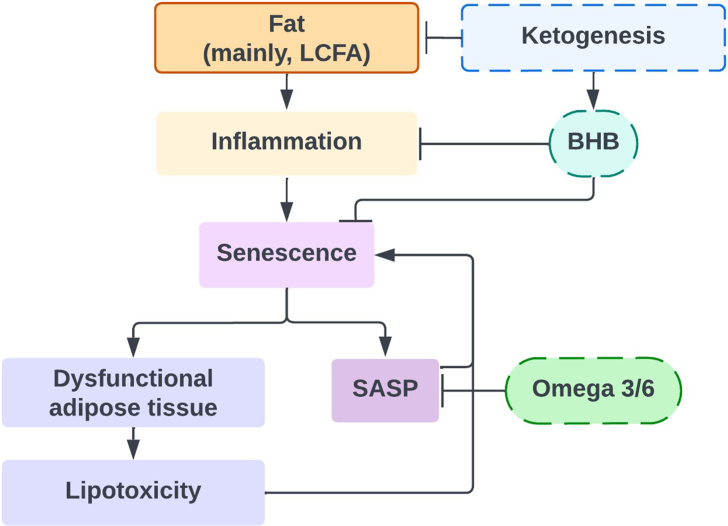
Fig. 3.
A diagram showing how the intake of dietary fats, as well as certain interventions (dashed border), may induce inflammation and cellular senescence.
Abbreviations: LCFA: long-chain fatty acid; BHB: β-hydroxybutyrate; SASP: senescence-associated secretory phenotype.
The secretory output of senescent cells contributes significantly to the systemic pro-inflammatory milieu of the aged organism. The modulatory effects of individual fatty acids on inflammation have been studied primarily in terms of mechanisms involving immune cells. Nevertheless, it could be argued that in tissues and organisms with a high senescence burden, large shifts in inflammatory status would not be easily achievable without involvement of senescent cell responses. Detailed studies are still needed to explore the mechanistic influence of different fatty acids on SASP regulation, while some indicators are already arising from present research endeavors. As mentioned previously, senescence markers were increased in mice liver fed diets enriched in protein, but pro-inflammatory secretory markers were further enhanced in diets rich in fat content, suggesting the possible involvement of fatty acids in SASP regulation [87]. The intense secretory activity of some senescent cells is partly sustained through increased β-oxidation [141,142]. Based on transcriptional data indicating an upregulation of fatty acid synthase in senescent cells, concomitant with increased expression of enzymes participating in fatty acid oxidation and downregulation of stearoyl-CoA desaturase, it has been suggested that saturated fatty acids represent the major energetic substrate in senescence [142]. It is possible that high availability of SFA could support an elevated production of SASP components, which would be compatible with the described pro-inflammatory effects of high SFA consumption. Interestingly, PUFAs constitute the backbone for synthesis of oxylipins, representing the lipid fraction of the SASP [143]. It is unlikely that oxylipin production would be significantly responsive to overall nutritional availability of PUFAs, since senescent cells employ mechanisms to increase their efficiency of PUFA uptake (CD36 upregulation) [144] and PUFA release from cellular membranes (increased phospholipase A2 activity) [145]. In this context, a reduction of PUFA intake would be more likely to exert detrimental effects on neighboring healthy cells rather than limiting senescent cell activity. Nevertheless, the ratios between n-6/n-3 PUFAs might be able to modulate the composition of the senescent secretome in ways similar to those described for healthy cells, by entering different metabolic pathways. In recipients of kidney transplant, supplementation with marine n-3 PUFAs decreases plasma levels of multiple SASP factors including GCSF, IL1α, MMP-1 and MMP-13 [146]. n-3 PUFAS have been shown to limit the inflammatory responsiveness of macrophages by regulating the recruitment of Toll-like receptors to lipid rafts [147,148]. It is conceivable that similar mechanisms might operate in senescent cells, where Tlr2 participates in SASP regulation.
In contrast to saturated long-chain fatty acids, short-chain fatty acids (e.g., butyrate) were shown to have a plethora of beneficial effects, including anti-inflammatory properties [149]. Intriguingly, a related molecule with a strong chemical similarity, β-hydroxybutyrate (BHB), is the most abundant ketone body found in humans. BHB is mainly produced in the liver via fatty acid oxidation, and its production is enhanced in conditions where glucose is insufficient, such as fasting and exercise, in order to be used as an alternative energy source [150]. BHB is thought to have anti-aging properties [151]: on one side, it has a protective effect against the induction of senescence through upregulation of OCT4 and subsequent increase in Lamin B1 [152], which is an important protein that protects cells from damage by inducing a quiescent state [153]; on the other side, it is able to suppress oxidative stress [154] and attenuate inflammation [132,155], suggesting a potential role in modulating the pro-inflammatory arm of the SASP. Notably, some studies have demonstrated the beneficial properties of ketogenic diets on longevity [114,115] which might be, at least in part, driven by high BHB levels (Fig. 3).
Future directions
As discussed in this review, cellular senescence can be considered both a cause and a consequence of metabolic dysregulations, and perturbations affecting macronutrients metabolism can influence aging and longevity partly through modulating either the entry into a senescence state or different senescence-associated phenotypes, in particular the pro-inflammatory arm of the SASP. Dietary interventions represent a promising measure to prevent cellular and organismal aging, thereby preventing or delaying a plethora of age-related diseases. Nevertheless, the design of such interventions, and whether they can be combined with anti-senescence strategies, is a poorly understood area of research.
Based on the current literature, some links between certain dietary choices, age-associated mechanisms and the consequences for health and longevity are emerging. For example, high protein intake is linked to increased mortality possibly due to premature induction of pro-aging and pro-SASP mechanisms such as the IGF1 and the mTOR pathways [71]. Simultaneously, protein intake is crucial for the preservation of muscle mass and adequate intake is required to prevent age-associated muscle loss. The source of protein may also be important in this context, as protein derived from plants contain a composition of amino acids that can promote longevity compared to protein derived from animal sources [83]. Foods with high GI can cause elevated levels of blood glucose and insulin, specifically postprandial [66], and high fat diets with abundant intake of carbohydrates can cause lipotoxicity, which can lead to various metabolic dysfunctions and the premature induction of senescence [51,131,156].
Interventions such as calorie restriction, ketogenic diet, and fasting might affect many of the aforementioned pathways and promote health [157]. Interestingly, calorie restriction can limit the accumulation of senescent cells in both mice and humans, suggesting that its pro-longevity properties might be, at least in part, due to reduced burden of senescence [158,159]. From a practical point of view, however, neither calorie restriction nor ketogenic diets are easy to follow. While fasting might represent a more feasible approach, the effects of specific fasting regimens on cellular and organismal senescence is still unknown in humans [160]. Studies in model organisms have suggested that treatment with certain regulators of metabolic pathways, such as metformin, acarbose, and rapamycin, represents a promising strategy to achieve pro-longevity effects, partially by modulating senescence-associated phenotypes, and could possibly be applied to humans [58,96]. Doubtlessly, the best results can be obtained by combining different interventions, whether targeting metabolism or senescence, in a personalized manner. Therefore, there is an urgent requirement in the field for developing strategies and biomarkers that can be used to measure biological age and to assess the efficacy of the applied interventions.
Conflict of interest
MD is the scientific co-founder of Cleara Biotech and is member of the scientific advisory board of Oisin Biotechnologies.
Acknowledgments
M.D. laboratory is funded by grants from the Dutch Cancer Foundation (KWF), Impetus Longevity and Dutch Research Council (NWO).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontana L., Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161(1):106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prentice A.M. Macronutrients as sources of food energy. Public Health Nutr. 2005;8(7A):932–939. doi: 10.1079/phn2005779. [DOI] [PubMed] [Google Scholar]
- 4.Green C.L., Lamming D.W., Fontana L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol. 2022;23(1):56–73. doi: 10.1038/s41580-021-00411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorgoulis V., Adams P.D., Alimonti A., Bennett D.C., Bischof O., Bishop C., et al. Cellular senescence: defining a path forward. Cell. 2019;179(4):813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Coppé J.P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J., et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas J., Feltes B.C., Poloni Jde F., Lenz G., Bonatto D. Senescence; an endogenous anticancer mechanism. Front Biosci. 2012;17(7):2616–2643. doi: 10.2741/4074. [DOI] [PubMed] [Google Scholar]
- 8.Demaria M., Ohtani N., Youssef S.A., Rodier F., Toussaint W., Mitchell J.R., et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosteiro L., Pantoja C., Alcazar N., Marión R.M., Chondronasiou D., Rovira M., et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science. 2016;354(6315) doi: 10.1126/science.aaf4445. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz-Espín D., Cañamero M., Maraver A., Gómez-López G., Contreras J., Murillo-Cuesta S., et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155(5):1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Ovadya Y., Krizhanovsky V. Senescent cells: SASPected drivers of age-related pathologies. Biogerontology. 2014;15(6):627–642. doi: 10.1007/s10522-014-9529-9. [DOI] [PubMed] [Google Scholar]
- 12.Baker D.J., Childs B.G., Durik M., Wijers M.E., Sieben C.J., Zhong J., et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calcinotto A., Kohli J., Zagato E., Pellegrini L., Demaria M., Alimonti A. Cellular senescence: aging, cancer, and injury. Physiol Rev. 2019;99(2):1047–1078. doi: 10.1152/physrev.00020.2018. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Segura A., Nehme J., Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28(6):436–453. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Kohli J., Wang B., Brandenburg S.M., Basisty N., Evangelou K., Varela-Eirin M., et al. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat Protoc. 2021;16(5):2471–2498. doi: 10.1038/s41596-021-00505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee B.Y., Han J.A., Im J.S., Morrone A., Johung K., Goodwin E.C., et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5(2):187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 17.Stein G.H., Drullinger L.F., Soulard A., Dulić V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19(3):2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freund A., Laberge R.M., Demaria M., Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell. 2012;23(11):2066–2075. doi: 10.1091/mbc.E11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hewitt G., Jurk D., Marques F.D., Correia-Melo C., Hardy T., Gackowska A., et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee D., Son H.G., Jung Y., Lee S.V. The role of dietary carbohydrates in organismal aging. Cell Mol Life Sci. 2017;74(10):1793–1803. doi: 10.1007/s00018-016-2432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livesey G., Taylor R., Livesey H.F., Buyken A.E., Jenkins D.J.A., Augustin L.S.A., et al. Dietary glycemic index and load and the risk of type 2 diabetes: assessment of causal relations. Nutrients. 2019;11(6):1436. doi: 10.3390/nu11061436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramne S., Alves Dias J., González-Padilla E., Olsson K., Lindahl B., Engström G., et al. Association between added sugar intake and mortality is nonlinear and dependent on sugar source in 2 Swedish population-based prospective cohorts. Am J Clin Nutr. 2019;109(2):411–423. doi: 10.1093/ajcn/nqy268. [DOI] [PubMed] [Google Scholar]
- 23.Brewer R.A., Gibbs V.K., Smith D.L., Jr. Targeting glucose metabolism for healthy aging. Nutr Healthy Aging. 2016;41(1):31–46. doi: 10.3233/NHA-160007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatterjee S., Peters S.A., Woodward M., Mejia Arango S., Batty G.D., Beckett N., et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300–307. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma R.C. Genetics of cardiovascular and renal complications in diabetes. J Diabetes Investig. 2016;7(2):139–154. doi: 10.1111/jdi.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pop-Busui R., Lu J., Lopes N., Jones T.L. BARI 2DInvestigators. Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J Peripher Nerv Syst. 2009;14(1):1–13. doi: 10.1111/j.1529-8027.2009.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nankervis S.A., Mitchell J.M., Charchar F.J., McGlynn M.A., Lewandowski P.A. Consumption of a low glycaemic index diet in late life extends lifespan of Balb/c mice with differential effects on DNA damage. Longev Healthspan. 2013;21(1):4. doi: 10.1186/2046-2395-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chia C.W., Egan J.M., Ferrucci L. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circ Res. 2018;123(7):886–904. doi: 10.1161/CIRCRESAHA.118.312806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman J., Fielder E., Passos J.F. Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett. 2019;593(13):1566–1579. doi: 10.1002/1873-3468.13498. [DOI] [PubMed] [Google Scholar]
- 30.James E.L., Michalek R.D., Pitiyage G.N., de Castro A.M., Vignola K.S., Jones J., et al. Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. J Proteome Res. 2015;14(4):1854–1871. doi: 10.1021/pr501221g. [DOI] [PubMed] [Google Scholar]
- 31.Liao E.C., Hsu Y.T., Chuah Q.Y., Lee Y.J., Hu J.Y., Huang T.C., et al. Radiation induces senescence and a bystander effect through metabolic alterations. Cell Death Dis. 2014;5(5):e1255. doi: 10.1038/cddis.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moiseeva O., Bourdeau V., Roux A., Deschênes-Simard X., Ferbeyre G. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol Cell Biol. 2009;29(16):4495–4507. doi: 10.1128/MCB.01868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esposito K., Nappo F., Marfella R., Giugliano G., Giugliano F., Ciotola M., et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 34.Dickinson S., Hancock D.P., Petocz P., Ceriello A., Brand-Miller J. High-glycemic index carbohydrate increases nuclear factor-kappaB activation in mononuclear cells of young, lean healthy subjects. Am J Clin Nutr. 2008;87(5):1188–1193. doi: 10.1093/ajcn/87.5.1188. [DOI] [PubMed] [Google Scholar]
- 35.Itahana Y., Itahana K. Emerging roles of p53 family members in glucose metabolism. Int J Mol Sci. 2018;19(3):776. doi: 10.3390/ijms19030776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thornalley P.J. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269(1):1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ksiazek K., Korybalska K., Jörres A., Witowski J. Accelerated senescence of human peritoneal mesothelial cells exposed to high glucose: the role of TGF-beta1. Lab Invest. 2007;87(4):345–356. doi: 10.1038/labinvest.3700519. [DOI] [PubMed] [Google Scholar]
- 38.Artwohl M., Brunmair B., Fürnsinn C., Hölzenbein T., Rainer G., Freudenthaler A., et al. Insulin does not regulate glucose transport and metabolism in human endothelium. Eur J Clin Invest. 2007;37(8):643–650. doi: 10.1111/j.1365-2362.2007.01838.x. [DOI] [PubMed] [Google Scholar]
- 39.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shosha E., Xu Z., Narayanan S.P., Lemtalsi T., Fouda A.Y., Rojas M., et al. Mechanisms of diabetes-induced endothelial cell senescence: role of arginase 1. Int J Mol Sci. 2018;19(4):1215. doi: 10.3390/ijms19041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi T., Matsui-Hirai H., Miyazaki-Akita A., Fukatsu A., Funami J., Ding Q.F., et al. Endothelial cellular senescence is inhibited by nitric oxide: implications in atherosclerosis associated with menopause and diabetes. Proc Natl Acad Sci USA. 2006;103(45):17018–17023. doi: 10.1073/pnas.0607873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang B., Cui S., Bai X., Zhuo L., Sun X., Hong Q., et al. SIRT3 overexpression antagonizes high glucose accelerated cellular senescence in human diploid fibroblasts via the SIRT3-FOXO1 signaling pathway. Age(Dordr) 2013;35(6):2237–2253. doi: 10.1007/s11357-013-9520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiley C.D., Velarde M.C., Lecot P., Liu S., Sarnoski E.A., Freund A., et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metabol. 2016;23(2):303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H., Wan Y., Zhou S., Lu Y., Zhang Z., Zhang R., et al. Endothelium-specific SIRT1 overexpression inhibits hyperglycemia-induced upregulation of vascular cell senescence. Sci China Life Sci. 2012;55(6):467–473. doi: 10.1007/s11427-012-4329-4. [DOI] [PubMed] [Google Scholar]
- 45.Maeda M., Hayashi T., Mizuno N., Hattori Y., Kuzuya M. Intermittent high glucose implements stress-induced senescence in human vascular endothelial cells: role of superoxide production by NADPH oxidase. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu S.L., Wang M.L., He Y.T., Guo S.W., Li T.T., Peng W.J., et al. Capsaicin ameliorates intermittent high glucose-mediated endothelial senescence via the TRPV1/SIRT1 pathway. Phytomedicine. 2022;100 doi: 10.1016/j.phymed.2022.154081. [DOI] [PubMed] [Google Scholar]
- 47.Chen J., Brodsky S.V., Goligorsky D.M., Hampel D.J., Li H., Gross S.S., et al. Glycated collagen I induces premature senescence-like phenotypic changes in endothelial cells. Circ Res. 2002;90(12):1290–1298. doi: 10.1161/01.res.0000022161.42655.98. [DOI] [PubMed] [Google Scholar]
- 48.Navarrete Santos A., Jacobs K., Simm A., Glaubitz N., Horstkorte R., Hofmann B. Dicarbonyls induce senescence of human vascular endothelial cells. Mech Ageing Dev. 2017;166:24–32. doi: 10.1016/j.mad.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Liu J., Yang J.R., Chen X.M., Cai G.Y., Lin L.R., He Y.N. Impact of ER stress-regulated ATF4/p16 signaling on the premature senescence of renal tubular epithelial cells in diabetic nephropathy. Am J Physiol Cell Physiol. 2015;308(8):C621–C630. doi: 10.1152/ajpcell.00096.2014. [DOI] [PubMed] [Google Scholar]
- 50.Palmer A.K., Tchkonia T., LeBrasseur N.K., Chini E.N., Xu M., Kirkland J.L. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64(7):2289–2298. doi: 10.2337/db14-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer A.K., Xu M., Zhu Y., Pirtskhalava T., Weivoda M.M., Hachfeld C.M., et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18(3) doi: 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson P.J., Shah A., Ntranos V., Van Gool F., Atkinson M., Bhushan A. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metab. 2019;29(5):1045–1060. doi: 10.1016/j.cmet.2019.01.021. e10. [DOI] [PubMed] [Google Scholar]
- 53.Testa R., Ceriello A. Pathogenetic loop between diabetes and cell senescence. Diabetes Care. 2007;30(11):2974–2975. doi: 10.2337/dc07-1534. [DOI] [PubMed] [Google Scholar]
- 54.Suter P.M. Carbohydrates and dietary fiber. Handb Exp Pharmacol. 2005;(170):231–261. doi: 10.1007/3-540-27661-0_8. [DOI] [PubMed] [Google Scholar]
- 55.Matt S.M., Allen J.M., Lawson M.A., Mailing L.J., Woods J.A., Johnson R.W. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi: 10.3389/fimmu.2018.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vinolo M.A., Rodrigues H.G., Nachbar R.T., Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell J.M., Bellman S.M., Stephenson M.D., Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31–44. doi: 10.1016/j.arr.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Soukas A.A., Hao H., Wu L. Metformin as anti-aging therapy: is it for everyone? Trends Endocrinol Metab. 2019;30(10):745–755. doi: 10.1016/j.tem.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moiseeva O., Deschênes-Simard X., St-Germain E., Igelmann S., Huot G., Cadar A.E., et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell. 2013;12(3):489–498. doi: 10.1111/acel.12075. [DOI] [PubMed] [Google Scholar]
- 60.Caspary W.F., Graf S. Inhibition of human intestinal alpha-glucosidehydrolases by a new complex oligosaccharide. Res Exp Med (Berl) 1979;175(1):1–6. doi: 10.1007/BF01851228. [DOI] [PubMed] [Google Scholar]
- 61.Weaver G.A., Tangel C.T., Krause J.A., Parfitt M.M., Jenkins P.L., Rader J.M., et al. Acarbose enhances human colonic butyrate production. J Nutr. 1997;127(5):717–723. doi: 10.1093/jn/127.5.717. [DOI] [PubMed] [Google Scholar]
- 62.Wolever T.M., Chiasson J.L. Acarbose raises serum butyrate in human subjects with impaired glucose tolerance. Br J Nutr. 2000;84(1):57–61. [PubMed] [Google Scholar]
- 63.Smith B.J., Miller R.A., Ericsson A.C., Harrison D.C., Strong R., Schmidt T.M. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019;19(1):130. doi: 10.1186/s12866-019-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen Z., Hinson A., Miller R.A., Garcia G.G. Cap-independent translation: a shared mechanism for lifespan extension by rapamycin, acarbose, and 17α-estradiol. Aging Cell. 2021;20(5) doi: 10.1111/acel.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrison D.E., Strong R., Allison D.B., Ames B.N., Astle C.M., Atamna H., et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harrison D.E., Strong R., Alavez S., Astle C.M., DiGiovanni J., Fernandez E., et al. Acarbose improves health and lifespan in aging HET3 mice. Aging Cell. 2019;18(2) doi: 10.1111/acel.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weimer S., Priebs J., Kuhlow D., Groth M., Priebe S., Mansfeld J., et al. D-Glucosamine supplementation extends life span of nematodes and of ageing mice. Nat Commun. 2014;5:3563. doi: 10.1038/ncomms4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L., Wang B., Gasek N.S., Zhou Y., Cohn R.L., Martin D.E., et al. Targeting p21(Cip1) highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell Metabol. 2022;34(1):186. doi: 10.1016/j.cmet.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitada M., Ogura Y., Monno I., Koya D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine. 2019;43:632–640. doi: 10.1016/j.ebiom.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simpson S.J., Le Couteur D.G., James D.E., George J., Gunton J.E., Solon-Biet S.M., et al. The Geometric Framework for Nutrition as a tool in precision medicine. Nutr Healthy Aging. 2017;4(3):217–226. doi: 10.3233/NHA-170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solon-Biet S.M., McMahon A.C., Ballard J.W., Ruohonen K., Wu L.E., Cogger V.C., et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metabol. 2014;19(3):418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Solon-Biet S.M., Mitchell S.J., Coogan S.C., Cogger V.C., Gokarn R., McMahon A.C., et al. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Rep. 2015;11(10):1529–1534. doi: 10.1016/j.celrep.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fontana L., Cummings N.E., Arriola Apelo S.I., Neuman J.C., Kasza I., Schmidt B.A., et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16(2):520–530. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levine M.E., Suarez J.A., Brandhorst S., Balasubramanian P., Cheng C.W., Madia F., et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metabol. 2014;19(3):407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willcox B.J., Willcox D.C., Todoriki H., Fujiyoshi A., Yano K., He Q., et al. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci. 2007;1114:434–455. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- 76.Le Couteur D.G., Solon-Biet S., Wahl D., Cogger V.C., Willcox B.J., Willcox D.C., et al. New horizons: dietary protein, ageing and the okinawan ratio. Age Ageing. 2016;45(4):443–447. doi: 10.1093/ageing/afw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wali J.A., Milner A.J., Luk A.W.S., Pulpitel T.J., Dodgson T., Facey H.J.W., et al. Impact of dietary carbohydrate type and protein-carbohydrate interaction on metabolic health. Nat Metabol. 2021;3(6):810–828. doi: 10.1038/s42255-021-00393-9. [DOI] [PubMed] [Google Scholar]
- 78.Song M., Fung T.T., Hu F.B., Willett W.C., Longo V.D., Chan A.T., et al. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;176(11):1453–1463. doi: 10.1001/jamainternmed.2016.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brandhorst S., Longo V.D. Protein quantity and source, fasting-mimicking diets, and longevity. Adv Nutr. 2019;10(Suppl_4):S340–S350. doi: 10.1093/advances/nmz079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kelemen L.E., Kushi L.H., Jacobs D.R., Jr., Cerhan J.R. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol. 2005;161(3):239–249. doi: 10.1093/aje/kwi038. [DOI] [PubMed] [Google Scholar]
- 81.Yu D., Richardson N.E., Green C.L., Spicer A.B., Murphy M.E., Flores V., et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metabol. 2021;33(5):905–922 e6. doi: 10.1016/j.cmet.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kitada M., Ogura Y., Monno I., Xu J., Koya D. Effect of methionine restriction on aging: its relationship to oxidative stress. Biomedicines. 2021;9(2) doi: 10.3390/biomedicines9020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Norman K., Klaus S. Veganism, aging and longevity: new insight into old concepts. Curr Opin Clin Nutr Metab Care. 2020;23(2):145–150. doi: 10.1097/MCO.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 84.Fontana L., Partridge L., Longo V.D. Extending healthy life span - from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller R.A., Buehner G., Chang Y., Harper J.M., Sigler R., Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4(3):119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tran D., Bergholz J., Zhang H., He H., Wang Y., Zhang Y., et al. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell. 2014;13(4):669–678. doi: 10.1111/acel.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nehme J., Yang D., Altulea A., Varela-Eirin M., Wang L., Hu S., et al. High dietary protein and fat contents exacerbate hepatic senescence and SASP in mice. FEBS J. 2023 Mar;290(5):1340–1347. doi: 10.1111/febs.16292. [DOI] [PubMed] [Google Scholar]
- 88.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169(2):361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 89.Blagosklonny M.V. Geroconversion: irreversible step to cellular senescence. Cell Cycle. 2014;13(23):3628–3635. doi: 10.4161/15384101.2014.985507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laberge R.M., Sun Y., Orjalo A.V., Patil C.K., Freund A., Zhou L., et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17(5):1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herranz N., Gallage S., Mellone M., Wuestefeld T., Klotz S., Hanley C.J., et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015;17(9):1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Flynn J.M., O’Leary M.N., Zambataro C.A., Academia E.C., Presley M.P., Garrett B.J., et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging cell. 2013;12(5):851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herrera J.J., Pifer K., Louzon S., Leander D., Fiehn O., Day S.M., et al. Early or late-life treatment with acarbose or rapamycin improves physical performance and affects cardiac structure in aging mice. J Gerontol Biol Med Sci. 2022 doi: 10.1093/gerona/glac221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Urfer S.R., Kaeberlein T.L., Mailheau S., Bergman P.J., Creevy K.E., Promislow D.E.L., et al. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. GeroScience. 2017;39(2):117–127. doi: 10.1007/s11357-017-9972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arriola Apelo S.I., Neuman J.C., Baar E.L., Syed F.A., Cummings N.E., Brar H.K., et al. Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging cell. 2016;15(1):28–38. doi: 10.1111/acel.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sbodio J.I., Snyder S.H., Paul B.D. Regulators of the transsulfuration pathway. Br J Pharmacol. 2019;176(4):583–593. doi: 10.1111/bph.14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang G., Zhao K., Ju Y., Mani S., Cao Q., Puukila S., et al. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxidants Redox Signal. 2013;18(15):1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 99.Suo R., Zhao Z.Z., Tang Z.H., Ren Z., Liu X., Liu L.S., et al. Hydrogen sulfide prevents H(2)O(2)-induced senescence in human umbilical vein endothelial cells through SIRT1 activation. Mol Med Rep. 2013;7(6):1865–1870. doi: 10.3892/mmr.2013.1417. [DOI] [PubMed] [Google Scholar]
- 100.Kang X., Li C., Xie X., Zhan K.B., Yang S.Q., Tang Y.Y., et al. Hydrogen sulfide inhibits homocysteine-induced neuronal senescence by up-regulation of SIRT1. Int J Med Sci. 2020;17(3):310–319. doi: 10.7150/ijms.38602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gupta K., Mathew A.B., Chakrapani H., Saini D.K. H(2)S contributed from CSE during cellular senescence suppresses inflammation and nitrosative stress. Biochim Biophys Acta, Mol Cell Res. 2022;1870(2) doi: 10.1016/j.bbamcr.2022.119388. [DOI] [PubMed] [Google Scholar]
- 102.Xie T., Leung P.S. Fibroblast growth factor 21: a regulator of metabolic disease and health span. Am Journal of physiology Endocrinology and metabolism. 2017;313(3):E292–E302. doi: 10.1152/ajpendo.00101.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu H., Jia C., Wu D., Jin H., Lin Z., Pan J., et al. Fibroblast growth factor 21 (FGF21) alleviates senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the SIRT1-mTOR signaling pathway. Cell Death Dis. 2021;12(10):865. doi: 10.1038/s41419-021-04157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan J., Wang J., Huang H., Huang Y., Mi T., Zhang C., et al. Fibroblast growth factor 21 delayed endothelial replicative senescence and protected cells from H(2)O(2)-induced premature senescence through SIRT1. Am J Transl Res. 2017;9(10):4492–4501. [PMC free article] [PubMed] [Google Scholar]
- 105.Aon M.A., Bhatt N., Cortassa S.C. Mitochondrial and cellular mechanisms for managing lipid excess. Front Physiol. 2014;5:282. doi: 10.3389/fphys.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buettner R., Scholmerich J., Bollheimer L.C. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15(4):798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 107.Winzell M.S., Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53:S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 108.Nakamura A., Terauchi Y. Lessons from mouse models of high-fat diet-induced NAFLD. Int J Mol Sci. 2013;14(11):21240–21257. doi: 10.3390/ijms141121240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Getz G.S., Reardon C.A. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(5):1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.San-Cristobal R., Navas-Carretero S., Martinez-Gonzalez M.A., Ordovas J.M., Martinez J.A. Contribution of macronutrients to obesity: implications for precision nutrition. Nat Rev Endocrinol. 2020;16(6):305–320. doi: 10.1038/s41574-020-0346-8. [DOI] [PubMed] [Google Scholar]
- 111.Blundell J.E., MacDiarmid J.I. Fat as a risk factor for overconsumption: satiation, satiety, and patterns of eating. J Am Diet Assoc. 1997;97(7 Suppl):S63–S69. doi: 10.1016/s0002-8223(97)00733-5. [DOI] [PubMed] [Google Scholar]
- 112.Maher T., Clegg M.E. Dietary lipids with potential to affect satiety: mechanisms and evidence. Crit Rev Food Sci Nutr. 2019;59(10):1619–1644. doi: 10.1080/10408398.2017.1423277. [DOI] [PubMed] [Google Scholar]
- 113.Longo R., Peri C., Cricri D., Coppi L., Caruso D., Mitro N., et al. Ketogenic diet: a new light shining on old but gold biochemistry. Nutrients. 2019;11(10) doi: 10.3390/nu11102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberts M.N., Wallace M.A., Tomilov A.A., Zhou Z., Marcotte G.R., Tran D., et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metabol. 2018;27(5):1156. doi: 10.1016/j.cmet.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Newman J.C., Covarrubias A.J., Zhao M., Yu X., Gut P., Ng C.P., et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metabol. 2017;26(3):547–557 e8. doi: 10.1016/j.cmet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roberts M.N., Wallace M.A., Tomilov A.A., Zhou Z., Marcotte G.R., Tran D., et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metabol. 2017;26(3):539–546 e5. doi: 10.1016/j.cmet.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morrison S.A., Fazeli P.L., Gower B., Willig A.L., Younger J., Sneed N.M., et al. Cognitive effects of a ketogenic diet on neurocognitive impairment in adults aging with HIV: a pilot study. J Assoc Nurses AIDS Care. 2020;31(3):312–324. doi: 10.1097/JNC.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fortier M., Castellano C.A., St-Pierre V., Myette-Cote E., Langlois F., Roy M., et al. A ketogenic drink improves cognition in mild cognitive impairment: results of a 6-month RCT. Alzheimer’s Dementiathe. 2021;17(3):543–552. doi: 10.1002/alz.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sergi D., Williams L.M. Potential relationship between dietary long-chain saturated fatty acids and hypothalamic dysfunction in obesity. Nutr Rev. 2020;78(4):261–277. doi: 10.1093/nutrit/nuz056. [DOI] [PubMed] [Google Scholar]
- 120.Cintra D.E., Ropelle E.R., Moraes J.C., Pauli J.R., Morari J., Souza C.T., et al. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PloS One. 2012;7(1) doi: 10.1371/journal.pone.0030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ramirez-Tortosa C.L., Varela-Lopez A., Navarro-Hortal M.D., Ramos-Pleguezuelos F.M., Marquez-Lobo B., Ramirez-Tortosa M., et al. Longevity and cause of death in male wistar rats fed lifelong diets based on virgin olive oil, sunflower oil, or fish oil. J Gerontol Biol Med Sci. 2020;75(3):442–451. doi: 10.1093/gerona/glz091. [DOI] [PubMed] [Google Scholar]
- 122.Wu J.H.Y., Micha R., Mozaffarian D. Dietary fats and cardiometabolic disease: mechanisms and effects on risk factors and outcomes. Nat Rev Cardiol. 2019;16(10):581–601. doi: 10.1038/s41569-019-0206-1. [DOI] [PubMed] [Google Scholar]
- 123.Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pararasa C., Ikwuobe J., Shigdar S., Boukouvalas A., Nabney I.T., Brown J.E., et al. Age-associated changes in long-chain fatty acid profile during healthy aging promote pro-inflammatory monocyte polarization via PPARgamma. Aging cell. 2016;15(1):128–139. doi: 10.1111/acel.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schroeder E.A., Brunet A. Lipid profiles and signals for long life. Trends Endocrinol Metab (TEM) 2015;26(11):589–592. doi: 10.1016/j.tem.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burdge G.C., Calder P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev. 2005;45(5):581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- 127.Tchkonia T., Morbeck D.E., Von Zglinicki T., Van Deursen J., Lustgarten J., Scrable H., et al. Fat tissue, aging, and cellular senescence. Aging cell. 2010;9(5):667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sone H., Kagawa Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia. 2005;48(1):58–67. doi: 10.1007/s00125-004-1605-2. [DOI] [PubMed] [Google Scholar]
- 129.Minamino T., Orimo M., Shimizu I., Kunieda T., Yokoyama M., Ito T., et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nature medicine. 2009;15(9):1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 130.Conley S.M., Hickson L.J., Kellogg T.A., McKenzie T., Heimbach J.K., Taner T., et al. Human obesity induces dysfunction and early senescence in adipose tissue-derived mesenchymal stromal/stem cells. Front Cell Dev Biol. 2020;8:197. doi: 10.3389/fcell.2020.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schafer M.J., White T.A., Evans G., Tonne J.M., Verzosa G.C., Stout M.B., et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes. 2016;65(6):1606–1615. doi: 10.2337/db15-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim S.R., Jiang K., Ogrodnik M., Chen X., Zhu X.Y., Lohmeier H., et al. Increased renal cellular senescence in murine high-fat diet: effect of the senolytic drug quercetin. Transl Res. 2019;213:112–123. doi: 10.1016/j.trsl.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hou J., Jeon B., Baek J., Yun Y., Kim D., Chang B., et al. High fat diet-induced brain damaging effects through autophagy-mediated senescence, inflammation and apoptosis mitigated by ginsenoside F1-enhanced mixture. J Ginseng Res. 2022;46(1):79–90. doi: 10.1016/j.jgr.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shi Q., Hubbard G.B., Kushwaha R.S., Rainwater D., Thomas C.A., 3rd, Leland M.M., et al. Endothelial senescence after high-cholesterol, high-fat diet challenge in baboons. Am J Physiol Heart Circ Physiol. 2007;292(6):H2913–H2920. doi: 10.1152/ajpheart.01405.2006. [DOI] [PubMed] [Google Scholar]
- 135.Li Y., Peng Z., Wang C., Li L., Leng Y., Chen R., et al. Novel role of PKR in palmitate-induced Sirt1 inactivation and endothelial cell senescence. Am J Physiol Heart Circ Physiol. 2018;315(3):H571–H580. doi: 10.1152/ajpheart.00038.2018. [DOI] [PubMed] [Google Scholar]
- 136.Venable M.E., Yin X. Ceramide induces endothelial cell senescence. Cell Biochem Funct. 2009;27(8):547–551. doi: 10.1002/cbf.1605. [DOI] [PubMed] [Google Scholar]
- 137.Mitterberger M.C., Lechner S., Mattesich M., Zwerschke W. Adipogenic differentiation is impaired in replicative senescent human subcutaneous adipose-derived stromal/progenitor cells. J Gerontol Biol Med Sci. 2014;69(1):13–24. doi: 10.1093/gerona/glt043. [DOI] [PubMed] [Google Scholar]
- 138.Xu M., Palmer A.K., Ding H., Weivoda M.M., Pirtskhalava T., White T.A., et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4 doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Unger R.H., Scherer P.E. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. TEM (Trends Endocrinol Metab) 2010;21(6):345–352. doi: 10.1016/j.tem.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ogrodnik M., Miwa S., Tchkonia T., Tiniakos D., Wilson C.L., Lahat A., et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8 doi: 10.1038/ncomms15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Quijano C., Cao L., Fergusson M.M., Romero H., Liu J., Gutkind S., et al. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle. 2012;11(7):1383–1392. doi: 10.4161/cc.19800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wiley C.D., Campisi J. The metabolic roots of senescence: mechanisms and opportunities for intervention. Nat Metabol. 2021;3(10):1290–1301. doi: 10.1038/s42255-021-00483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wiley C.D., Sharma R., Davis S.S., Lopez-Dominguez J.A., Mitchell K.P., Wiley S., et al. Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis. Cell Metabol. 2021;33(6):1124–1136. doi: 10.1016/j.cmet.2021.03.008. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lizardo D.Y., Lin Y.L., Gokcumen O., Atilla-Gokcumen G.E. Regulation of lipids is central to replicative senescence. Mol Biosyst. 2017;13(3):498–509. doi: 10.1039/c6mb00842a. [DOI] [PubMed] [Google Scholar]
- 145.Wiley C.D., Brumwell A.N., Davis S.S., Jackson J.R., Valdovinos A., Calhoun C., et al. Secretion of leukotrienes by senescent lung fibroblasts promotes pulmonary fibrosis. JCI Insight. 2019;4(24) doi: 10.1172/jci.insight.130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chan J., Eide I.A., Tannaes T.M., Waldum-Grevbo B., Jenssen T., Svensson M. Marine n-3 polyunsaturated fatty acids and cellular senescence markers in incident kidney transplant recipients: the omega-3 fatty acids in renal transplantation (ORENTRA) randomized clinical trial. Kidney Med. 2021;3(6):1041–1049. doi: 10.1016/j.xkme.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wong S.W., Kwon M.J., Choi A.M., Kim H.P., Nakahira K., Hwang D.H. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284(40):27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takashima A., Fukuda D., Tanaka K., Higashikuni Y., Hirata Y., Nishimoto S., et al. Combination of n-3 polyunsaturated fatty acids reduces atherogenesis in apolipoprotein E-deficient mice by inhibiting macrophage activation. Atherosclerosis. 2016;254:142–150. doi: 10.1016/j.atherosclerosis.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 149.Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 150.Rojas-Morales P., Tapia E., Pedraza-Chaverri J. beta-Hydroxybutyrate: a signaling metabolite in starvation response? Cell Signal. 2016;28(8):917–923. doi: 10.1016/j.cellsig.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 151.Edwards C., Copes N., Bradshaw P.C. D-ss-hydroxybutyrate: an anti-aging ketone body. Oncotarget. 2015;6(6):3477–3478. doi: 10.18632/oncotarget.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Han Y.M., Bedarida T., Ding Y., Somba B.K., Lu Q., Wang Q., et al. Beta-hydroxybutyrate prevents vascular senescence through hnRNP A1-mediated upregulation of Oct4. Molecular Cell. 2018;71(6):1064–10678 e5. doi: 10.1016/j.molcel.2018.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shimi T., Butin-Israeli V., Adam S.A., Hamanaka R.B., Goldman A.E., Lucas C.A., et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011;25(24):2579–2593. doi: 10.1101/gad.179515.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shimazu T., Hirschey M.D., Newman J., He W., Shirakawa K., Le Moan N., et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Youm Y.H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D., et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nature Med. 2015;21(3):263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yazici D., Sezer H. Insulin resistance, obesity and lipotoxicity. Adv Exp Med Biol. 2017;960:277–304. doi: 10.1007/978-3-319-48382-5_12. [DOI] [PubMed] [Google Scholar]
- 157.Mirzaei H., Di Biase S., Longo V.D. Dietary interventions, cardiovascular aging, and disease: animal models and human studies. Circ Res. 2016;118(10):1612–1625. doi: 10.1161/CIRCRESAHA.116.307473. [DOI] [PubMed] [Google Scholar]
- 158.Fontana L., Nehme J., Demaria M. Caloric restriction and cellular senescence. Mech Ageing Dev. 2018;176:19–23. doi: 10.1016/j.mad.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 159.Fontana L., Mitchell S.E., Wang B., Tosti V., van Vliet T., Veronese N., et al. The effects of graded caloric restriction: XII. Comparison of mouse to human impact on cellular senescence in the colon. Aging Cell. 2018;17(3) doi: 10.1111/acel.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Patterson R.E., Laughlin G.A., LaCroix A.Z., Hartman S.J., Natarajan L., Senger C.M., et al. Intermittent fasting and human metabolic health. J Acad Nutr Diet. 2015;115(8):1203–1212. doi: 10.1016/j.jand.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]