Key Points
Question
What effect does incision-site injection of microdosed antibiotics have on the rate of surgical site infection (SSI) before skin cancer surgery?
Findings
In this randomized clinical trial, among 681 patients undergoing skin cancer surgery, intra-incisional microdosed clindamycin delivered along with local anesthetic significantly reduced the rate of SSI (defined as a postoperative wound infection score of 5 or more at any postoperative visit) compared with control (local anesthetic alone). In contrast, intra-incisional microdosed flucloxacillin had no significant effect on the SSI rate.
Meaning
These data provide robust evidence to inform guidelines regarding SSI prophylaxis before skin cancer surgery, which are currently lacking.
This randomized clinical trial evaluates whether microdosed incisional antibiotics reduce the rate of surgical site infections before skin cancer surgery.
Abstract
Importance
Surgical site infections (SSIs) represent a costly and preventable complication of cutaneous surgery. However, there is a paucity of randomized clinical trials investigating antibiotic prophylaxis for reducing SSIs in skin cancer surgery, and evidence-based guidelines are lacking. Incisional antibiotics have been shown to reduce the rate of SSIs before Mohs micrographic surgery, but this represents a small subset of skin cancer surgery.
Objective
To determine whether microdosed incisional antibiotics reduce the rate of SSIs before skin cancer surgery.
Design, Setting, and Participants
In this double-blind, controlled, parallel-design randomized clinical trial, adult patients presenting to a high-volume skin cancer treatment center in Auckland, New Zealand, for any form of skin cancer surgery over 6 months from February to July 2019 were included. Patient presentations were randomized to one of 3 treatment arms. Data were analyzed from October 2021 to February 2022.
Interventions
Patients received an incision site injection of buffered local anesthetic alone (control), buffered local anesthetic with microdosed flucloxacillin (500 µg/mL), or buffered local anesthetic with microdosed clindamycin (500 µg/mL).
Main Outcomes and Measures
The primary end point was the rate of postoperative SSI (calculated as number of lesions with SSI per total number of lesions in the group), defined as a standardized postoperative wound infection score of 5 or more.
Results
A total of 681 patients (721 total presentations; 1133 total lesions) returned for postoperative assessments and were analyzed. Of these, 413 (60.6%) were male, and the mean (SD) age was 70.4 (14.8) years. Based on treatment received, the proportion of lesions exhibiting a postoperative wound infection score of 5 or greater was 5.7% (22 of 388) in the control arm, 5.3% (17 of 323) in the flucloxacillin arm, and 2.1% (9 of 422) in the clindamycin arm (P = .01 for clindamycin vs control). Findings were similar after adjusting for baseline differences among arms. Compared with lesions in the control arm (31 of 388 [8.0%]), significantly fewer lesions in the clindamycin arm (9 of 422 [2.1%]; P < .001) and flucloxacillin (13 of 323 [4.0%]; P = .03) arms required postoperative systemic antibiotics.
Conclusions and Relevance
This study evaluated the use of incisional antibiotics for SSI prophylaxis in general skin cancer surgery and compared the efficacy of flucloxacillin vs clindamycin relative to control in cutaneous surgery. The significant reduction in SSI with locally applied microdosed incisional clindamycin provides robust evidence to inform treatment guidelines in this area, which are currently lacking.
Trial Registration
anzctr.org.au Identifier: ACTRN12616000364471
Introduction
Skin cancer represents the most common cancer worldwide,1 and surgical excision is the most common treatment approach.2 However, surgical site infections (SSIs) can complicate cutaneous surgery procedures.3 Infections are painful, delay recovery, increase resource requirements (eg, follow-up visits and secondary interventions, including systemic antibiotics, wound debridement, topical wound care, and secondary wound closure), and are associated with a worse cosmetic outcome.4 It has been estimated that up to 60% of SSIs could be prevented with evidence-based measures.5
High-quality evidence supports the use of prophylactic antibiotics in other surgical domains, including colorectal and orthopedic surgery.6,7,8 Conversely, literature regarding prophylactic systemic antibiotics in other clean soft tissue surgery (eg, breast, thyroid, hernia) is controversial and less conclusive or unsupported.9,10,11 However, there is a lack of randomized clinical trial evidence to determine the role of antibiotic prophylaxis for reducing SSIs in skin cancer surgery. All 3 existing consensus statements—from the World Health Organization,12 the US Centers for Disease Control and Prevention,13 and the joint American College of Surgeons and Surgical Infection Society14—lack guidelines for SSI prevention specific to cutaneous surgery.
Systemic antibiotic prophylaxis is not usually recommended before clean cutaneous surgery.15 Furthermore, systemic antibiotic utilization without clear indication constitutes poor antibiotic stewardship and contributes to antimicrobial resistance.16 Localized delivery of microdosed incisional antibiotics represents an alternative to systemic antibiotics and has been shown to significantly reduce the rate of SSIs in patients undergoing Mohs micrographic surgery.17,18 Mohs surgery reflects a relatively small proportion of skin cancer management and is characterized by unique procedural features that limit extension to general skin surgery, including longer duration of open wounds subject to multiple rounds of incisional injections prior to definitive closure and disproportionate application on the head and neck. While original and well conducted, these studies are also 2 decades old without published replication and did not include direct comparison between antibiotic drug classes. By far the most common form of skin cancer management worldwide is surgical excision with immediate closure. To our knowledge, there are no generally applicable or contemporary randomized clinical trials reporting evidence for prophylactic antibiotics (either systemic or incisional) for significantly reducing SSIs in general skin cancer surgery.
Localized administration of microdosed antibiotics within local anesthetic has a number of advantages. These include immediate and targeted delivery to the operative site, no additional punctures or injection volume requirements, guaranteed compliance with therapy, substantial reduction in systemic antibiotic selection pressure (particularly within gut flora), minimized risk of drug interactions, low cost, and a feasible and scalable applicability to high-volume skin cancer treatment centers.19
The aim of the current Prophylactic Incisional Antibiotics in Skin Cancer Surgery (PICASSo) trial was to evaluate the safety and efficacy of a single preoperative microdose of locally infiltrated flucloxacillin or clindamycin for reducing SSIs in cutaneous surgery for skin cancer. We hypothesized that a significant reduction in SSI could be demonstrated with a single microdose of locally applied antibiotic relative to controls without adverse effects.
Methods
Trial Design
This prospective, double-blind, parallel-design randomized clinical trial was conducted at one of the highest-volume skin cancer treatment centers in New Zealand (Middlemore Hospital, Auckland, New Zealand) over a 6-month period (February to July 2019). The study protocol was approved by the New Zealand Central Health and Disability Ethics Committee15; the study protocol can be found in Supplement 1, and the statistical analysis plan can be found in Supplement 2. Written informed consent was obtained preoperatively in parallel with routine surgical consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Study Participants
All adult patients presenting for skin cancer surgery under local anesthetic were eligible. Exclusion criteria were allergies to both penicillin and clindamycin, preoperative systemic intake of any antibiotic within 7 days before surgery, or inability to return for face-to-face postoperative wound assessment. Patients could be enrolled and randomized more than once if they underwent additional procedures within the study period, assuming all eligibility criteria remained met. Race and ethnicity were recorded according to patient self-identification, with options defined by the investigator as follows: Asian; European; Māori; Middle Eastern, Latin American, or African; and Pasifika.
Study Treatments
Patient presentations were randomized to receive incision site injection of buffered local anesthetic alone (control group; lidocaine, 1%, plus adrenaline 1:100 000 standard solution [AstraZeneca] buffered 1:10 with sodium bicarbonate, 8.4% [Hospira]), buffered local anesthetic with microdosed flucloxacillin (500 µg/mL; Pfizer), or buffered local anesthetic with microdosed clindamycin (500 µg/mL; Pfizer). Antibiotic concentrations were extrapolated from existing data on peak serum levels with standard systemic dosing and designed to ensure minimum inhibitory concentrations for common skin flora.20,21 Randomizations were made (1:1:1) using a custom database running a blinded schedule that managed randomization with random block sizes. Patients randomized to the flucloxacillin arm who had a penicillin allergy were automatically reallocated to the clindamycin arm (also blinded, assuming no clindamycin allergy), and patients randomized to clindamycin who had a clindamycin allergy were automatically reallocated to the control arm.
Lesions were treated using a blinded prefilled infiltration syringe corresponding to allocation that had been prepared by a trial pharmacist in 10-mL aliquots. To promote blinding integrity, syringes were labeled in a random order within each batch. Syringes were stored at 4 °C, protected from light, and replaced with a fresh batch every 48 hours. Patients and all members of operative and follow-up teams were blinded to allocation until study conclusion. If more than 1 site was treated, the original allocation arm was maintained for that patient presentation. Participants enrolled more than once were independently randomized at each presentation. Any deviations to treatment received compared with protocol were recorded.
Procedures and Assessments
Surgical excision, wound closure/reconstruction, and postoperative management were performed according to standard of care. Patients were monitored for adverse reactions perioperatively and questioned about sensitivity reactions at each follow-up visit. Safety was monitored by an independent data monitoring committee throughout the trial.
All assessments were performed by clinical staff masked to allocation. All participants were invited for standardized postoperative assessment by a consistent trial nursing team between 7 and 21 days postoperatively. Additional assessments were performed opportunistically during any further encounters (for example, in case of wound healing concerns) as required. Each assessment included a patient questionnaire eliciting any adverse effects experienced, adverse events, and/or antibiotics prescribed for any reason (eMethods in Supplement 3). A standardized wound assessment was also performed for each operative site using a previously validated postoperative wound infection (POWI) scoring system (Table 1).18 When more than 1 postoperative assessment was made, the highest POWI score recorded for each lesion was used for analysis. For wounds closed directly, length of closure was recorded.
Table 1. Postoperative Wound Infection (POWI) Scalea.
| Score | Condition |
|---|---|
| 0 | Normal healing |
| 1 | Normal healing but with 1 of the following signs of infection: erythema, edema, or increased pain |
| 2 | Normal healing but with 2 of the following signs of infection: erythema, edema, or increased pain |
| 3 | Normal healing but with 3 of the following signs of infection: erythema, edema, and increased pain |
| 4 | Hemoserous discharge combined with 2 of the following: erythema, edema, or increased pain |
| 5 | Pus combined with 1 of the following: erythema, edema, or increased pain; or hemoserous discharge combined with all of erythema, edema, and increased pain |
| 6 | Pus combined with 2 of the following: erythema, edema, or increased pain |
| 7 | Pus combined with all of erythema, discharge, and increased pain |
The POWI scale as defined by Heuther et al.15
Patient-specific factors were recorded for each participant, including medical immunosuppression, diabetes, history of prior SSI, and smoking status. In any case of overt infection, culture swabs were obtained for microbiological analysis to guide tailored antibiotic treatment.
Outcomes
The primary end point was the rate of SSI (defined as POWI score of 5 or more at any postoperative assessment), calculated as the number of lesions with SSI per total number of lesions in the group. Secondary end points included safety and patient, lesion, and infection factors associated with SSI.
Sample Size
A prerecruitment power analysis calculation was performed based on a retrospective analysis of skin cancer operations undertaken at the same unit over a 6-month period between February and July 2015. In total, 2088 lesions were excised from 938 patients in 1053 procedures; rates of documented and possible SSI were 3.3% and 11.3%, respectively. These rates were averaged to estimate the expected SSI rate (7%); the anticipated SSI rate in the treatment group was estimated to be 2%. To achieve 80% power with a 5% level of significance (to which a false discovery rate correction was applied to allow comparison between each active treatment and control), it was calculated that 987 lesions would need to be recruited.
Statistical Analysis
Outcomes analysis was performed on all patients who had at least 1 postoperative assessment in both the intention-to-treat (per protocol) population and on the basis of actual treatment received (as treated). Descriptive statistics (means with SD and ranges with ranges and IQRs) were used to describe continuous variables. Categorical measurements are reported as counts and frequencies of nonmissing observations and proportion in each valid category.
The multinomial counts for POWI as well as the binomial counts for SSI were analyzed with (multiple) logistic regression using the lme4 package,22 with treatment as a fixed model term. Residual analysis was done using the DHARMAa package. A mixed-effects model with patient identifier as the random term was compared with the model without a random term. The models without random effects had lower Akaike information criterion values as well as nonsignificant model assumptions.23 The random term was therefore dropped in the final analysis.24 Pairwise comparisons were calculated after the model fit using the estimated parameters and covariance matrices assuming multivariate normal distributions. Statistical significance was defined as a P value less than .05, and all P values were 2-tailed. Statistical analyses were performed using R version 4.0.3 (The R Foundation).
Results
Study Population and Treatments
A total of 906 patients were screened over 986 presentations, and 735 patients (782 presentations; 1215 lesions) were randomized and completed treatment (Figure 1). Some patients presented more than once and were assessed for eligibility at each presentation, with 28 patients randomized to multiple treatment arms over the recruitment period (eFigure in Supplement 3). Most patients had more than 1 lesion excised per presentation (mean [SD] lesions, 1.6 [1.13]; maximum, 9 lesions).
Figure 1. CONSORT Diagram.
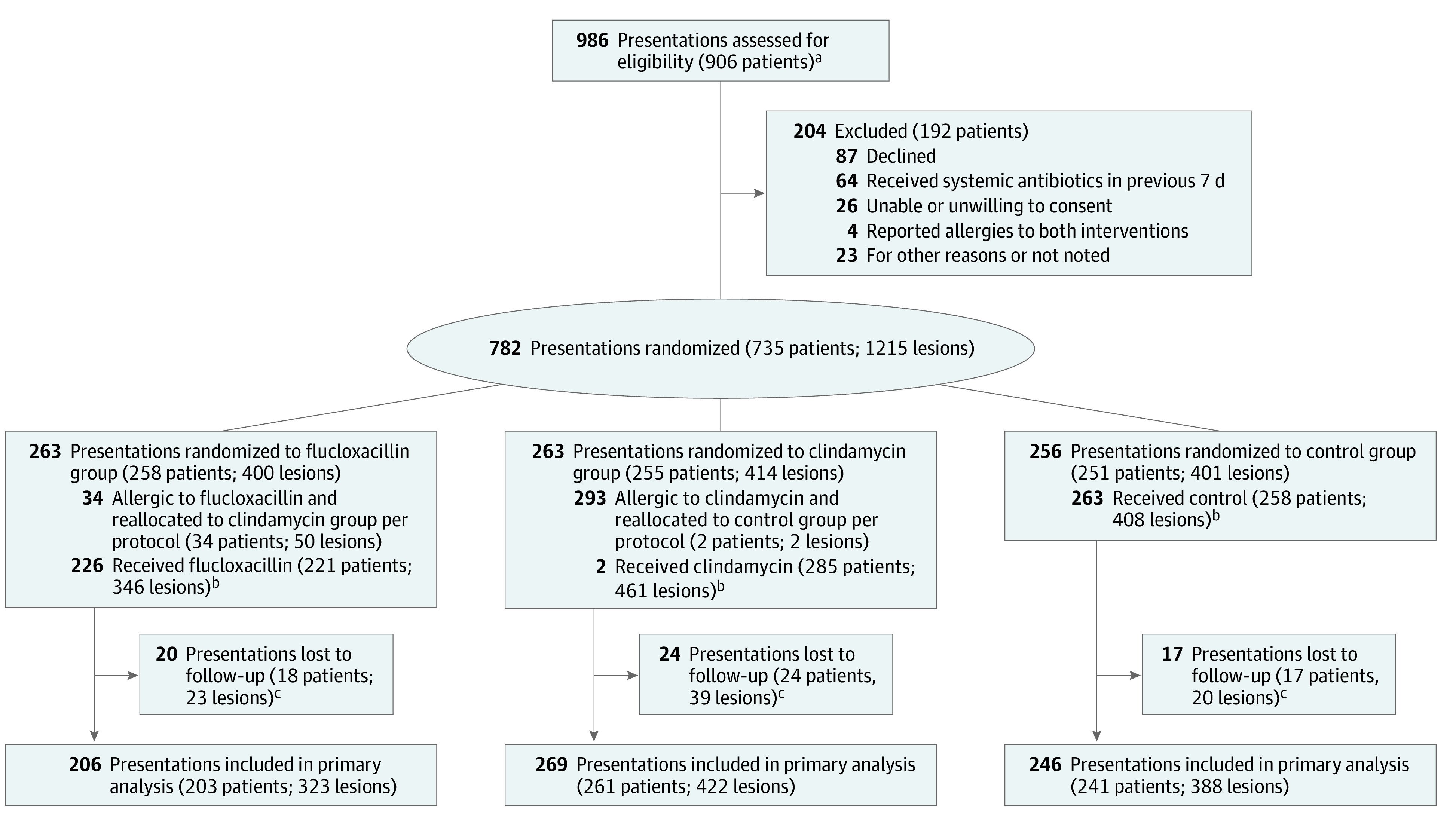
aSome patients presented more than once over the study period and were therefore eligible for enrollment and randomization on more than 1 occasion. These patients could be randomized to different treatment arms at each presentation.
bA total of 11 lesions (11 of 1215 [0.9%]; 9 patients in 9 presentations) erroneously received a treatment that differed from the randomized allocation; of these, 7 (7 of 1133 lesions [0.6%]; 5 patients in 5 presentations) completed follow-up and were included in the as-treated analysis.
cFollow-up included patients who completed at least 1 postoperative assessment.
A total of 681 patients (1133 lesions [93.3%] over 721 operative presentations) returned for at least 1 postoperative assessment and were included in outcome analyses. Of these, 413 (60.6%) were male, and the mean (SD) age was 70.4 (14.8) years. European individuals predominated overall (634 [93.1%]), followed by Pasifika individuals (15 [2.2%]), Māori individuals (14 [2.1%]), Asian individuals (12 [1.8%]), and Middle Eastern, Latin American, or African individuals (6 [0.9%]). Patient characteristics are detailed in Table 2. The proportion of lesions completing treatment but failing to return for postoperative assessment (ie, lost to follow-up) was 8.5% (39 of 461), 6.6% (23 of 346), and 4.9% (20 of 408) in the clindamycin, flucloxacillin, and control groups, respectively.
Table 2. Participant Characteristics.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Overall (N = 681) | Control group (n = 241) | Flucloxacillin group (n = 203) | Clindamycin group (n = 261) | |
| Sex | ||||
| Female | 268 (39.4) | 84 (22.7) | 86 (51.5) | 105 (40.2) |
| Male | 413 (60.6) | 157 (77.3) | 117 (48.5) | 156 (59.8) |
| Age, y | ||||
| Mean (SD) | 70.4 (14.8) | 71.9 (14.0) | 70.7 (14.5) | 69.3 (15.5) |
| Median (IQR) | 73 (62-81) | 74 (65-82) | 73 (64-80) | 71 (60-81) |
| Age group, y | ||||
| <40 | 24 (3.5) | 7 (2.9) | 5 (2.5) | 12 (4.6) |
| 40-49 | 42 (6.2) | 10 (4.9) | 13 (5) | 20 (8.3) |
| 50-59 | 70 (10.3) | 20 (7.7) | 20 (8.3) | 31 (15.3) |
| 60-69 | 147 (21.6) | 54 (22.4) | 42 (20.7) | 56 (21.5) |
| 70-79 | 207 (30.4) | 81 (39.9) | 68 (26.1) | 65 (27) |
| ≥80 | 190 (27.9) | 69 (26.4) | 55 (22.8) | 76 (37.4) |
| Missing | 1 (0.1) | 0 | 0 | 1 (0.4) |
| Race and ethnicitya | ||||
| Asian | 12 (1.8) | 6 (2.5) | 4 (1.5) | 2 (1) |
| European | 634 (93.1) | 223 (109.9) | 192 (79.7) | 243 (93.1) |
| Māori | 14 (2.1) | 4 (1.5) | 3 (1.5) | 7 (2.9) |
| Middle Eastern, Latin American, or African | 6 (0.9) | 0 | 1 (0.4) | 5 (2.5) |
| Pasifika | 15 (2.2) | 8 (3.9) | 3 (1.2) | 4 (1.5) |
| Current smoker | ||||
| Yes | 48 (7.0) | 13 (6.4) | 16 (6.6) | 20 (7.7) |
| No | 633 (93.0) | 228 (94.6) | 187 (92.1) | 241 (92.3) |
| Immunosuppressed | 12 (1.8) | 19 (9.4) | 17 (7.1) | 16 (6.1) |
| Diabetic | 47 (6.9) | 13 (6.4) | 16 (6.6) | 20 (7.7) |
| History of prior SSI | ||||
| Yes | 37 (5.4) | 16 (6.1) | 9 (3.4) | 17 (6.5) |
| No | 643 (94.4) | 225 (93.4) | 194 (80.5) | 243 (100.8) |
| Missing | 1 (0.1) | 0 | 0 | 1 (0.5) |
| Self-reported drug allergy | ||||
| Flucloxacillin | 65 (9.5) | NA | NA | NA |
| Clindamycin | 4 (0.6) | NA | NA | NA |
| Presentations per patient | ||||
| 1 | 644 (94.6) | NA | NA | NA |
| 2 | 35 (5.1) | NA | NA | NA |
| 3 | 1 (0.1) | NA | NA | NA |
| 4 | 1 (0.1) | NA | NA | NA |
| Lesions per patient | ||||
| 1 | 424 (62.3) | NA | NA | NA |
| 2 | 153 (22.5) | NA | NA | NA |
| 3 | 54 (7.9) | NA | NA | NA |
| 4 | 27 (4.0) | NA | NA | NA |
| 5 | 15 (2.2) | NA | NA | NA |
| 6 | 2 (0.3) | NA | NA | NA |
| 7 | 3 (0.4) | NA | NA | NA |
| 8 | 2 (0.3) | NA | NA | NA |
| 9 | 1 (0.1) | NA | NA | NA |
Abbreviations: NA, not applicable; SSI, surgical site infection.
Ethnicity was recorded according to patient self-identification, with options defined by the investigator as follows: Asian; European; Māori; Middle Eastern, Latin American, or African; and Pasifika.
The rate of self-reported allergy to flucloxacillin and clindamycin was 9.5% (65 of 681) and 0.6% (4 of 681), respectively. A total of 34 patients (50 lesions) and 2 patients (2 lesions) with conflicting allergies to flucloxacillin or clindamycin were blindly reallocated to clindamycin or control, respectively, per study protocol. Five patients (7 lesions [0.6%]) were administered nonintended treatment due to protocol errors (eTable 1 in Supplement 3).
Based on actual treatment received, 388 of 1133 lesions analyzed (34.2%) were in the control group, 323 (28.5%) were in the flucloxacillin group, and 422 (37.2%) were in the clindamycin group (Table 3). There were more ulcerated lesions in the control arm (86 [22.1%]) than the flucloxacillin and clindamycin arms (46 [14.2%] and 52 [12.3%], respectively). The most common surgery type was excision and direct closure (approximately 80% across all arms). The mean (SD) volume injected per length of direct closure was 1.5 (1.0) mL/cm. The most common location was the head and neck (approximately 55% across arms), followed by the trunk and extremities.
Table 3. Lesion and Intervention Characteristics by Treatment Group.
| Characteristic | No. (%) | ||||
|---|---|---|---|---|---|
| Control (n = 388 lesions) | Flucloxacillin (n = 323 lesions) | P value vs control | Clindamycin (n = 422 lesions) | P value vs control | |
| Sex | |||||
| Female | 141 (36.3) | 130 (40.2) | .29 | 163 (38.6) | .50 |
| Male | 247 (63.7) | 193 (59.8) | 259 (61.4) | ||
| Lesion ulceration | |||||
| Yes | 86 (22.2) | 46 (14.2) | .007 | 52 (12.3) | <.001 |
| No | 301 (77.6) | 275 (85.1) | .01 | 368 (87.2) | <.001 |
| Missing | 1 (0.3) | 2 (0.6) | .47 | 2 (0.5) | .62 |
| Surgery type | |||||
| Excision and direct closure | 319 (82.2) | 255 (79.0) | .03 | 347 (82.2) | >.99 |
| Length of closure, mean (SD), cm | 3.5 (2.4) | 3.4 (2.5) | .37 | 3.6 (2.4) | .27 |
| Volume injected, mean (SD), mL | 5.1 (4.6) | 5.2 (4.8) | .37 | 5.6 (4.7) | .12 |
| Excision and grafting | 50 (12.9) | 59 (18.3) | .048 | 63 (14.9) | .40 |
| Excision and local tissue rearrangement | 19 (4.9) | 9 (2.8) | .16 | 12 (2.8) | .13 |
| Surgery location | |||||
| Head | 207 (53.3) | 179 (55.4) | .54 | 229 (54.3) | .74 |
| Neck | 14 (3.6) | 14 (4.3) | .62 | 17 (4.0) | .76 |
| Trunk | 67 (17.3) | 49 (15.2) | .45 | 95 (22.5) | .06 |
| Upper extremity | 44 (11.3) | 26 (8.0) | .14 | 31 (7.3) | .05 |
| Lower extremity | 55 (14.2) | 55 (17.0) | .30 | 47 (11.1) | .19 |
| Other | 1 (0.3) | 0 | .97 | 3 (0.7) | .38 |
| Postoperative antibiotics | |||||
| Yes | 31 (8.0) | 13 (4.0) | .03 | 9 (2.1) | <.001 |
| No | 355 (91.5) | 310 (96.0) | .02 | 413 (97.9) | <.001 |
| Missing | 2 (0.5) | 0 | .98 | 0 | .98 |
SSI
Based on actual treatment received, the proportion of lesions with a clinically significant SSI (POWI score of 5 or higher at any postoperative assessment) was 5.7% (22 of 388) in the control group, 5.3% (17 of 323) in the flucloxacillin group, and 2.1% (9 of 422) in the clindamycin group (Figure 2A). Results in the intention-to-treat population were almost identical (eTable 2 in Supplement 3). Statistically significant differences in SSI rate persisted after adjustment for baseline differences in lesion ulceration (Figure 2B). The proportion of lesions with a POWI score of 0 was highest in the flucloxacillin group, but this difference was no longer significant after adjustment for lesion ulceration (Figure 2). Significantly fewer lesions in both treatment arms were prescribed postoperative antibiotics during follow-up compared with controls (clindamycin, 2.1% [9 of 422]; flucloxacillin, 4.0% [13 of 323]; control, 8% [31 of 388]) (Table 3).
Figure 2. Maximal Postoperative Wound Infection (POWI) Scores As Treated and After Correction for Differences in Number of Ulcerated Lesions Among Study Groups.
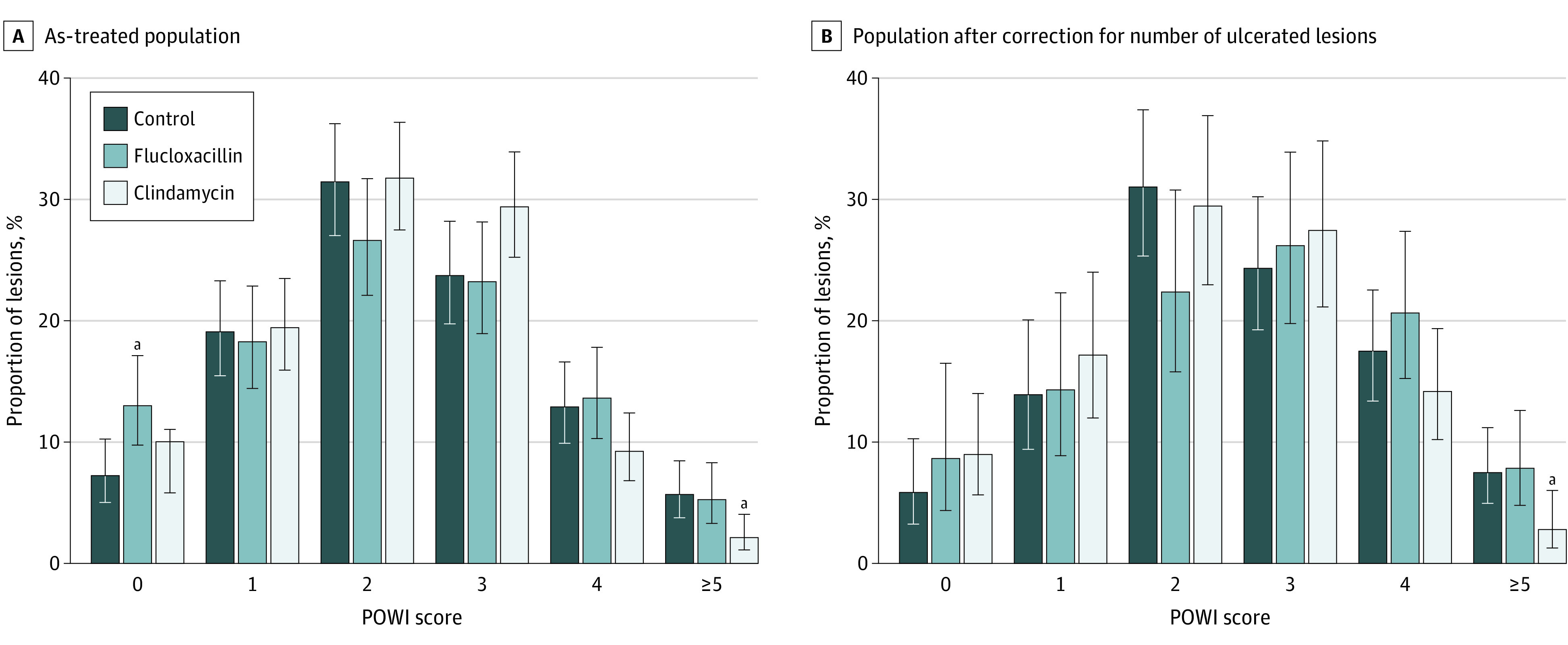
Error bars indicate 95% CIs.
aP < .05 vs control.
Adverse Events
No adverse events, including abnormal patient-reported pain, wound complications, or anaphylaxis, were observed.
Discussion
This double-blind, prospective randomized clinical trial showed that microdosed incisional antibiotic treatment with clindamycin significantly reduced the rate of SSI before skin cancer surgery. The use of postoperative systemic antibiotics significantly reduced with incisional antibiotics by one-half (flucloxacillin) or one-quarter (clindamycin) relative to the control group. Treatment with locally infiltrated microdosed incisional flucloxacillin and clindamycin was safe and well tolerated. Findings were consistent in the intention-to-treat and as-treated analyses. Based on these results, we recommend the routine adoption of incisional microdosed clindamycin for patients undergoing skin cancer surgery. This strategy appears suitable for widespread implementation because of the magnitude of the effect observed and the absence of adverse events.
Evidence-based antibiotic prophylaxis poses several benefits in skin cancer surgery. SSIs represent one of the most serious potentially avoidable complications affecting patient experience, cost, and surgical outcome in skin cancer surgery. Both melanoma and nonmelanoma skin cancers are frequently chronically ulcerated and colonized.25,26 Operative sites are subject to altered perfusion with gravity dependency as well as tension and undermining that can impair immunity during wound healing.27 Finally, tumor sites may experience fundamentally impaired local immunity, potentiating localized tumorigenesis and subsequent neoplasia.28
Despite this, to our knowledge, there have been no evidence-based recommendations on antibiotic prophylaxis in skin cancer surgery to date. Although guidelines can be extrapolated from other surgical settings,9,10,11,12,13,14 they are not specific to skin cancer surgery. Preoperative infusion of systemic antibiotics is not necessarily warranted or practical in high-throughput, local anesthetic skin surgery. Oral clindamycin is an option for the ambulatory setting, although nonessential systemic antibiotic administration constitutes a recognized risk for antibiotic resistance,16 which has been declared a global public health threat by the World Health Organization.29,30 Nevertheless, evidence for alternative approaches to the prevention of SSIs in patients undergoing skin cancer surgery has been lacking. The Centers for Disease Control and Prevention guidelines currently state that nonparenteral antimicrobial prophylaxis of SSIs represents an unresolved issue.13 Finally, to our knowledge, no guidelines advocate postoperative antibiotics for uncomplicated dermatologic surgery.31
This study confirms safety and efficacy reported in patients undergoing Mohs surgery17,18 and substantially extends evidence supporting the use of incisional antibiotics to patients undergoing standard excisional skin cancer surgery. Mohs surgery reflects a specialized form of skin cancer excision using microscopic analysis of tissue margins in steps until no remaining cancer is seen.32 However, standard surgical excision is more available, less costly, and represents the most common management approach for skin cancer worldwide.
Flucloxacillin and clindamycin were chosen for the intervention arms in this study because of their activity against common skin flora, generic availability, solubility, and amenability for codelivery with standard lidocaine-based local anesthesia using standard injection equipment, reported efficacy in Mohs SSI prophylaxis,17,18 and clinically invisible/blindable incorporation into existing practice. Penicillin-class antibiotics are inexpensive and readily available with time-honored utility in skin infections, but their use is limited by a 10% to 20% rate of self-reported penicillin allergy33,34 (9.5% in our study). Clindamycin provides an equivalently priced, well-tolerated alternate antibiotic for prophylactic use and is commonly recognized as a primary alternative with penicillin allergy.35,36
Clindamycin was significantly more effective at preventing SSI than flucloxacillin in our study. Possible reasons include slightly broader coverage of commonly cultured bacteria in skin and soft tissue infections, including community-associated methicillin-resistant Staphylococcus aureus,35,36 efficacy against anaerobic bacteria that may be relevant to chronically ulcerated skin lesions, and lesser local tissue inflammation compared with flucloxacillin. Clindamycin’s lower allergic contraindication (0.6% in our study) facilitates practical implementation as a first-line SSI prophylaxis agent in skin cancer surgery.
The technique of locally administered microdosed clindamycin with local anesthetic enables several benefits, including easy standardization and patient inclusion, perfect compliance, immediate targeted delivery to the operative site, low cost vs systemic antibiotic administration, economy of scale in high-volume treatment centers, limited drug-drug interactions, and decreased potentiation of antibiotic resistance. In terms of pharmacokinetics, local administration achieves predictable and immediate effective concentrations at the cutaneous operative site, including equal or better penetration into zones of impaired circulation relative to systemic delivery routes.37 Additional factors relevant to implementation include cost of antibiotic (approximately ¢1.2 to ¢4.9 per 10-mL syringe of local anesthetic, based on average wholesale price),21,36 and pharmacist time for preparation.
Limitations
This study has limitations. While the study provides high-quality evidence that responsibly improves clinically important outcomes for an extremely common indication where there was previously a lack of evidence, limitations include single-center recruitment and the inability to analyze wounds in patients who did not return for postoperative assessment. However, the lost to follow-up population was small (81 of 1214 randomized lesions [6.7%]) and least likely to include patients requiring management of postoperative complications, thus biasing toward overestimation of the true rate of SSI in our study. Notably, lost assessments were highest in the clindamycin group (39 of 461 lesions [8.5%]) vs the flucloxacillin and control groups (23 of 346 lesions [6.6%] and 20 of 408 lesions [4.9%], respectively).
Another limitation was the presence of significantly fewer ulcerated lesions in the treatment groups relative to control, creating a potential source of bias toward a higher rate of SSI in the control group. However, the decrease in SSIs with incisional clindamycin vs control remained significant after adjusting for lesion ulceration.
Patients receiving systemic antibiotics for any indication within 7 days before surgery (eg, for coincidental urinary or upper respiratory tract infection) were excluded from eligibility. This was done to avoid any confounding influence on skin flora during the perioperative period that might have an impact on intervention effect, but this would not contraindicate incisional antibiotics in standard practice.
This was a single-center trial performed within a predominantly European study population, in a country bearing one of the highest incidences of skin cancer worldwide1 and in a publicly funded health care system with universal access to skin cancer surgery, which may influence generalizability to other environments. While the significant decrease in postoperative antibiotic use after incisional antibiotics tracks with the primary outcome measure, its translational validity would benefit from further prospective study.
European individuals predominated the study population, mirroring the demographic characteristics of patients with skin cancer overall. However, it has been shown that racial and ethnic minority groups and socioeconomically deprived populations demonstrate higher rates of community-associated methicillin-resistant S aureus both regionally and internationally.38,39,40,41,42,43,44 Therefore, there may be a role for further personalizing incisional antimicrobial choice for maximal clinical benefit based on race and ethnicity or even individual cutaneous microbiomes, and this represents an opportunity for further study.
Conclusions
To our knowledge, this is the first report on the safety and efficacy of incisional antibiotics for SSI prophylaxis in routine excisional skin cancer surgery. In a double-blinded, prospective randomized clinical trial, microdosed clindamycin delivered simultaneously with local anesthetic significantly reduced both the rate of SSI overall and the prescription of systemic antibiotics during postoperative follow-up. The intervention is safe, easily implemented in routine practice, and substantially reduces unintended antimicrobial selection outside the targeted operative field. These results establish evidence-based guidelines for antibiotic prophylaxis in one of the most common surgical interventions performed worldwide, where they have been previously absent.
Trial Protocol
Statistical Analysis Plan
eMethods. Patient Questionnaire (Elicited at Each Postoperative Visit With Trial Nurse)
eFigure. Overlap of Patients Between Assessment (A) and Treatment (B) Groups
eTable 1. Lesion Distribution Across Arms
eTable 2. Rate of Surgical Site Infection (SSI) Based on a Postoperative Wound Infection (POWI) Score of ≥5 by Treatment Group in the Intention-to-Treat (Per Protocol) and Actual Treatment (As Treated) Populations
Data Sharing Statement
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.PDQ Adult Treatment Editorial Board . Skin cancer treatment (PDQ): health professional version. Accessed February 15, 2021. https://www.ncbi.nlm.nih.gov/books/NBK65928/
- 3.Heal CF, Buettner PG, Drobetz H. Risk factors for surgical site infection after dermatological surgery. Int J Dermatol. 2012;51(7):796-803. doi: 10.1111/j.1365-4632.2011.05189.x [DOI] [PubMed] [Google Scholar]
- 4.Del Rosso JQ. Wound care in the dermatology office: where are we in 2011? J Am Acad Dermatol. 2011;64(3)(suppl):S1-S7. doi: 10.1016/j.jaad.2010.10.038 [DOI] [PubMed] [Google Scholar]
- 5.Anderson DJ, Podgorny K, Berríos-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605-627. doi: 10.1086/676022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosco JA, Tejada PRR, Catanzano AJ, Stachel AG, Phillips MS. Expanded gram-negative antimicrobial prophylaxis reduces surgical site infections in hip arthroplasty. J Arthroplasty. 2016;31(3):616-621. doi: 10.1016/j.arth.2015.09.051 [DOI] [PubMed] [Google Scholar]
- 7.Nelson RL, Glenny AM, Song F. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev. 2009;(1):CD001181. [DOI] [PubMed] [Google Scholar]
- 8.Wukich DK, Dikis JW, Monaco SJ, Strannigan K, Suder NC, Rosario BL. Topically applied vancomycin powder reduces the rate of surgical site infection in diabetic patients undergoing foot and ankle surgery. Foot Ankle Int. 2015;36(9):1017-1024. doi: 10.1177/1071100715586567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher M, Jones DJ, Bell-Syer SV. Prophylactic antibiotics to prevent surgical site infection after breast cancer surgery. Cochrane Database Syst Rev. 2019;(9):CD005360. doi: 10.1002/14651858.CD005360.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medas F, Canu GL, Cappellacci F, et al. Antibiotic prophylaxis for thyroid and parathyroid surgery: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2021;164(3):482-488. doi: 10.1177/0194599820947700 [DOI] [PubMed] [Google Scholar]
- 11.Orelio CC, van Hessen C, Sanchez-Manuel FJ, Aufenacker TJ, Scholten RJ. Antibiotic prophylaxis for prevention of postoperative wound infection in adults undergoing open elective inguinal or femoral hernia repair. Cochrane Database Syst Rev. 2020;(4):CD003769. doi: 10.1002/14651858.CD003769.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Global guidelines for the prevention of surgical site infection. Accessed February 15, 2020. http://apps.who.int/iris/bitstream/10665/250680/1/9789241549882-eng.pdf?ua=1
- 13.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. ; Healthcare Infection Control Practices Advisory Committee . Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784-791. doi: 10.1001/jamasurg.2017.0904 [DOI] [PubMed] [Google Scholar]
- 14.Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: surgical site infection guidelines, 2016 update. J Am Coll Surg. 2017;224(1):59-74. doi: 10.1016/j.jamcollsurg.2016.10.029 [DOI] [PubMed] [Google Scholar]
- 15.Rosengren H, Heal C, Smith S. An update on antibiotic prophylaxis in dermatologic surgery. Curr Dermatol Rep. 2012;1(2):55-63. doi: 10.1007/s13671-012-0012-z [DOI] [Google Scholar]
- 16.World Health Organization . Antibiotic resistance. Accessed February 15, 2020. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance
- 17.Griego RD, Zitelli JA. Intra-incisional prophylactic antibiotics for dermatologic surgery. Arch Dermatol. 1998;134(6):688-692. doi: 10.1001/archderm.134.6.688 [DOI] [PubMed] [Google Scholar]
- 18.Huether MJ, Griego RD, Brodland DG, Zitelli JA. Clindamycin for intraincisional antibiotic prophylaxis in dermatologic surgery. Arch Dermatol. 2002;138(9):1145-1148. doi: 10.1001/archderm.138.9.1145 [DOI] [PubMed] [Google Scholar]
- 19.Barich DH, Zell MT, Munson EJ. Physicochemical properties, formulation, and drug delivery. In: Wang B, Hu L, Siahaan J, eds. Drug Delivery: Principles and Applications. 2nd ed. Wiley; 2016:35-48. doi: 10.1002/9781118833322.ch3 [DOI] [Google Scholar]
- 20.Sutherland R, Croydon EA, Rolinson GN. Flucloxacillin, a new isoxazolyl penicillin, compared with oxacillin, cloxacillin, and dicloxacillin. BMJ. 1970;4(5733):455-460. doi: 10.1136/bmj.4.5733.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merative Micromedex. Homepage. Accessed April 3, 2022. https://www.micromedexsolutions.com/
- 22.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1-48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 23.Dziak JJ, Coffman DL, Lanza ST, Li R, Jermiin LS. Sensitivity and specificity of information criteria. Brief Bioinform. 2020;21(2):553-565. doi: 10.1093/bib/bbz016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnham KP, Anderson DR. Multimodel Inference: A Practical Information-Theoretic Approach. 2nd ed. Springer Verlag; 2002. [Google Scholar]
- 25.Samarasinghe V, Madan V. Nonmelanoma skin cancer. J Cutan Aesthet Surg. 2012;5(1):3-10. doi: 10.4103/0974-2077.94323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bønnelykke-Behrndtz ML, Steiniche T. Ulcerated melanoma: aspects and prognostic impact. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017. [PubMed] [Google Scholar]
- 27.Krishnan NM, Brown BJ, Davison SP, et al. Reducing wound tension with undermining or imbrication—do they work? Plast Reconstr Surg Glob Open. 2016;4(7):e799. doi: 10.1097/GOX.0000000000000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georgescu SR, Tampa M, Mitran CI, et al. Tumour microenvironment in skin carcinogenesis. Adv Exp Med Biol. 2020;1226:123-142. doi: 10.1007/978-3-030-36214-0_10 [DOI] [PubMed] [Google Scholar]
- 29.Toner E, Adalja A, Gronvall GK, Cicero A, Inglesby TV. Antimicrobial resistance is a global health emergency. Health Secur. 2015;13(3):153-155. doi: 10.1089/hs.2014.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization . Antimicrobial resistance. Accessed February 12, 2021. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
- 31.Johnson-Jahangir H, Agrawal N. Perioperative antibiotic use in cutaneous surgery. Dermatol Clin. 2019;37(3):329-340. doi: 10.1016/j.det.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 32.Etzkorn JR, Alam M. What is Mohs surgery? JAMA Dermatol. 2020;156(6):716. doi: 10.1001/jamadermatol.2020.0039 [DOI] [PubMed] [Google Scholar]
- 33.MacFadden DR, LaDelfa A, Leen J, et al. Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis. 2016;63(7):904-910. doi: 10.1093/cid/ciw462 [DOI] [PubMed] [Google Scholar]
- 34.Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. is this patient allergic to penicillin? an evidence-based analysis of the likelihood of penicillin allergy. JAMA. 2001;285(19):2498-2505. doi: 10.1001/jama.285.19.2498 [DOI] [PubMed] [Google Scholar]
- 35.Smieja M. Current indications for the use of clindamycin: a critical review. Can J Infect Dis. 1998;9(1):22-28. doi: 10.1155/1998/538090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johns Hopkins Medicine . Clindamycin. Accessed April 4, 2022. https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540131/all/Clindamycin
- 37.Edmondson HT. Parenteral and oral clindamycin therapy in surgical infections: a preliminary report. Ann Surg. 1973;178(5):637-642. doi: 10.1097/00000658-197311000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abeysekera N, Wong S, Jackson B, Buchanan D, Heiss-Dunlop W, Mathy JA. Evolving threat of community acquired methicillin resistant Staphylococcus aureus upper extremity infections in the South Pacific: 2011-2015. J Hand Surg Asian Pac Vol. 2019;24(2):129-137. doi: 10.1142/S2424835519500164 [DOI] [PubMed] [Google Scholar]
- 39.Buchanan D, Heiss-Dunlop W, Mathy JA. Community acquired methicillin resistant Staphylococcus aureus hand infections: a South Pacific perspective—characteristics and implications for antibiotic coverage. Hand Surg. 2012;17(3):317-324. doi: 10.1142/S0218810412500244 [DOI] [PubMed] [Google Scholar]
- 40.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616-687. doi: 10.1128/CMR.00081-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gualandi N, Mu Y, Bamberg WM, et al. Racial disparities in invasive methicillin-resistant Staphylococcus aureus infections, 2005-2014. Clin Infect Dis. 2018;67(8):1175-1181. doi: 10.1093/cid/ciy277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loewen K, Schreiber Y, Kirlew M, Bocking N, Kelly L. Community-associated methicillin-resistant Staphylococcus aureus infection: literature review and clinical update. Can Fam Physician. 2017;63(7):512-520. [PMC free article] [PubMed] [Google Scholar]
- 43.Ritchie SR, Fraser JD, Libby E, Morris AJ, Rainey PB, Thomas MG. Demographic variation in community-based MRSA skin and soft tissue infection in Auckland, New Zealand. N Z Med J. 2011;124(1332):21-30. [PubMed] [Google Scholar]
- 44.See I, Wesson P, Gualandi N, et al. Socioeconomic factors explain racial disparities in invasive community-associated methicillin-resistant staphylococcus aureus disease rates. Clin Infect Dis. 2017;64(5):597-604. doi: 10.1093/cid/ciw808 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods. Patient Questionnaire (Elicited at Each Postoperative Visit With Trial Nurse)
eFigure. Overlap of Patients Between Assessment (A) and Treatment (B) Groups
eTable 1. Lesion Distribution Across Arms
eTable 2. Rate of Surgical Site Infection (SSI) Based on a Postoperative Wound Infection (POWI) Score of ≥5 by Treatment Group in the Intention-to-Treat (Per Protocol) and Actual Treatment (As Treated) Populations
Data Sharing Statement


