Key Points
Question
What are the safety and efficacy of empagliflozin in patients with heart failure and preserved ejection fraction (HFpEF) with background diuretic use?
Findings
In this post hoc analysis of a randomized clinical trial, empagliflozin was associated with comparable improvements in time to cardiovascular death or heart failure hospitalization, first and total heart failure hospitalizations, rate of decline in estimated glomerular filtration rate, and health status, regardless of background diuretic use or dose. Empagliflozin was also associated with a reduced likelihood of diuretic initiation or dose escalation and an increased likelihood of diuretic de-escalation and discontinuation after randomization.
Meaning
The findings suggest that treatment with empagliflozin in patients with HFpEF should be independent of diuretic therapy and may result in reduced need for diuretics.
This post hoc analysis of a randomized clinical trial evaluates the safety and efficacy of empagliflozin in patients with heart failure and preserved ejection fraction who are taking diuretics.
Abstract
Importance
The diuretic effect of sodium-glucose cotransporter 2 inhibitors may result in interaction with background diuretic therapy in patients with heart failure and preserved ejection fraction (HFpEF).
Objective
To assess the safety and efficacy of empagliflozin in combination with background diuretic therapy and the association of empagliflozin with the need for conventional diuretics.
Design, Setting, and Participants
This was a post hoc analysis of the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved). EMPEROR-Preserved was a phase 3, randomized, placebo-controlled, double-blind clinical trial conducted from March 2017 to April 2021. Patients with class II to IV heart failure and left ventricular ejection fraction greater than 40% were included. Of 5988 patients enrolled, 5815 (97.1%) had baseline data on diuretic use and were included in this analysis, which was conducted from November 2021 to August 2022.
Interventions
Participants in EMPEROR-Preserved were randomized to empagliflozin or placebo. In this analysis, participants were divided into 4 subgroups: no diuretics and furosemide-equivalent diuretic dose of less than 40 mg, 40 mg, and greater than 40 mg at baseline.
Main Outcomes and Measures
The main outcomes of interest were first hospitalization for heart failure (HHF) or cardiovascular death (CV death) and its components. Association of empagliflozin vs placebo with outcomes by baseline diuretic status (no diuretic vs any dose) and dose (no diuretic, <40 mg, 40 mg, and > 40mg) was assessed. Association of empagliflozin use with changes in diuretic therapy was also studied.
Results
Among 5815 patients (mean [SD] age, 71.9 [9.4] years; 2594 [44.6%] female) with known baseline diuretic use, 1179 (20.3%) were not taking diuretics, 1725 (29.7%) were taking less than 40 mg, 1772 (30.5%) were taking 40 mg, and 1139 (19.6%) were taking greater than 40 mg. In the placebo arm, patients with higher diuretic doses had worse outcomes. Empagliflozin decreased the risk of HHF or CV death, regardless of background diuretic status (hazard ratio [HR], 0.81; 95% CI, 0.70-0.93] for the diuretic group vs HR, 0.72; 95% CI, 0.48-1.06 for the nondiuretic group; P for interaction = .58). Similarly, diuretic status was not associated with changes in improvements in first HHF, total HHF, rate of decline in estimated glomerular filtration rate, and Kansas City Cardiomyopathy Questionnaire 23 clinical summary score with empagliflozin. Findings were consistent when patients were categorized by diuretic dose. Empagliflozin was associated with a decreased likelihood of diuretic dose escalation (HR, 0.74; 95% CI, 0.65-0.84) and an increased likelihood of de-escalation (HR, 1.15; 95% CI, 1.02-1.30). Empagliflozin was associated with an increased risk of volume depletion in patients taking diuretics (HR, 1.34; 95% CI, 1.13-1.59).
Conclusion
In this study, treatment with empagliflozin was similar regardless of diuretic use or dose. Empagliflozin use was associated with decreased conventional diuretic dosing.
Trial Registration
ClinicalTrials.gov Identifier: NCT03057951
Introduction
The Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) evaluated the efficacy of empagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, for the treatment of heart failure with preserved ejection fraction (HFpEF).1 Compared with placebo, empagliflozin was found to significantly reduce the risk of first hospitalization for heart failure (HHF) or cardiovascular (CV) death, slow the decline in estimated glomerular filtration rate (eGFR), and improve health-related quality of life (HRQoL).
SGLT2 inhibitors prevent the absorption of sodium and glucose in the proximal renal tubule, resulting in natriuresis, glucosuria, and increased urine output.2 In addition, several other mechanisms likely mediate the benefit from SGLT2 inhibitors, including increased autophagy, reduced inflammation, improvement of energy metabolism, and prevention of adverse cardiac remodeling.3,4 It has been suggested that SGLT2 inhibitors primarily act through a diuretic mechanism and that their benefit may be attenuated in patients already taking other diuretics.5 It is also possible that combined use of SGLT2 inhibitors with conventional diuretics may increase the risk of volume depletion events, acute kidney injury, and other adverse effects. Use of SGLT2 inhibitors may also impact the need for conventional diuretic therapy.
The aim of this post hoc analysis was to assess the safety and efficacy of empagliflozin in relation to background diuretic therapy. An additional objective was to study the association of empagliflozin with the use of conventional diuretics over time.
Methods
Study Design and Eligibility Criteria
The design and primary results of the EMPEROR-Preserved trial have been previously published.1,6 The trial protocol is in Supplement 1. The ethics committee at each center approved the trial, and all patients provided written informed consent. EMPEROR-Preserved was a double-blind, randomized, placebo-controlled, event-driven trial conducted from March 2017 to April 2021, which aimed to assess the safety and efficacy of empagliflozin for the treatment of HFpEF. Individuals were eligible for inclusion in the trial if they had chronic heart failure (HF), New York Heart Association (NYHA) class II to IV, with left ventricular ejection fraction greater than 40%. Individuals had to meet the following 2 criteria: an N-terminal prohormone B-type natriuretic peptide (NT-proBNP) level greater than 300 pg/mL (greater than 900 pg/mL for patients with atrial fibrillation) and HHF in the past 6 months or left atrial or left ventricular structural changes on echocardiography. Race was collected via electronic case report form based on patient-reported information in order to monitor diversity in the trial and ensure the recruitment of a representative patient population. A total of 5988 patients were enrolled and randomized to receive either empagliflozin, 10 mg, or placebo. The median (IQR) follow-up time was 26.2 (18.1-33.1) months.
Baseline Diuretic Use
Of the 5988 original patients, 5815 (97.1%) had data on baseline diuretic use and were included in the present analysis. For the current study, torsemide, 20 mg; bumetanide, 1 mg; azosemide, 60 mg; and ethacrynic acid, 100 mg, were considered equivalent to furosemide, 40 mg intravenously or 80 mg orally (all doses refer to total daily prescribed). This classification is in line with a previous analysis from the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial.7 Patients were categorized into the following subgroups according to baseline diuretic therapy: no diuretic use and furosemide-equivalent doses of less than 40 mg, 40 mg, and greater than 40 mg. Patients treated with only a nonloop diuretic agent were included in the group of patients with less than 40 mg furosemide-equivalent doses. Mineralocorticoid receptor antagonists were not classified as diuretics for the current analysis.
Outcomes
The following clinical outcomes were studied: the composite end point of first HHF or CV death, total (first and recurrent) HHF, first HHF, CV death, all-cause death, rate of decline in eGFR, and the composite kidney end point (chronic dialysis or kidney transplant or sustained reduction of 50% or greater in eGFR or sustained eGFR less than 15 mL/min/1.73 m2 or renal death). Change in HRQoL was assessed using Kansas City Cardiomyopathy Questionnaire 23 (KCCQ-23), which was completed by patients at baseline and at 12, 32, and 52 weeks’ follow-up, and includes the total symptom score, the clinical summary score, and the overall summary score. Changes in key physiologic outcomes, including glycated hemoglobin, hematocrit, NT-proBNP, weight, systolic blood pressure, and uric acid, were also studied. Adverse events of interest included adverse events leading to trial drug discontinuation (including fatal events), hyperkalemia, acute kidney failure, and volume depletion events. Volume depletion was a composite of various preferred terms, including hypotension, orthostatic hypotension, hypovolemic shock, circulatory collapse, syncope, presyncope, dehydration, and hypovolemia.
Statistical Analysis
Baseline characteristics of patients in each subgroup (no diuretics, any dose, and furosemide-equivalent doses <40, 40, and >40 mg) were analyzed descriptively. Categorical variables were summarized as frequencies and percentages and compared across categories with the χ2 test, while continuous variables were summarized as means and standard deviations and compared using the t test. To evaluate a trend across doses, an ordinal regression likelihood ratio test was used. Clinical outcomes and changes in diuretic therapy (initiation, increase in dose, de-escalation, and permanent discontinuation) were analyzed in a time-to-first–event fashion. Time-to-first-event outcomes were analyzed using a multivariable Cox regression model to obtain hazard ratios (HRs) and 95% CIs. Total HHF was analyzed using a joint frailty model together with cardiovascular death to obtain HRs and 95% CIs. In both cases, the multivariable models adjusted for the following baseline characteristics: left ventricular ejection fraction, age, and eGFR (as continuous covariates) as well as sex, diabetes status, and region. The variables used in the regression model were the same as those used in the prespecified model for the primary analysis of the EMPEROR-Preserved trial.
The eGFR slope was analyzed based on receiving-treatment data, using a random coefficient model allowing intercept and slope to vary randomly between patients. The analysis model included age, left ventricular ejection fraction, and baseline eGFR as linear covariates and sex, region, diabetes status, baseline-by-time interaction, and treatment-by-time interaction as fixed covariates. Changes in KCCQ summary scores and physiologic outcomes were also analyzed using a mixed model with repeated measures. The analysis model included age, eGFR, and left ventricular ejection fraction as linear covariates, and sex, region, diabetes, sex, interaction of visit by treatment by baseline dose of diuretics, and interaction of baseline value by visit as fixed effects.
Outcomes were studied in the placebo group alone to characterize the natural history of patients by baseline diuretic dose. For this analysis, the group receiving no diuretics at baseline was treated as a reference against which outcomes in other subgroups were compared. Next, the treatment effect of empagliflozin (vs placebo) by baseline diuretic therapy was assessed for each outcome. Two comparisons were done: according to baseline diuretic status (no diuretics vs any dose) and according to baseline dose of diuretics (none, <40 mg, 40 mg and >40 mg). HRs and mean differences across subgroups were compared by adding subgroup-by-treatment interaction terms to the models. Data were analyzed from November 2021 to August 2022.
Results
Baseline Characteristics
Of 5815 patients (mean [SD] age, 71.9 [9.4] years; 2594 [44.6%] female; [817 Asian (14.0%); 253 Black (4.4%); 4381 White (75.3%); and 362 other or mixed race (6.2%), including American Indian or Alaska Native, Native Hawaiian or Pacific Islander, and self-reported mixed race, consolidated due to very low prevalence in the trial population]), 1179 (20.3%) were not taking diuretics, 1725 (29.7%) were taking less than 40 mg, 1772 (30.5%) were taking 40 mg, and 1139 (19.6%) were taking greater than 40 mg furosemide-equivalent doses. Patient characteristics in the empagliflozin and placebo arms were generally balanced in the diuretic and the nondiuretic group (eTable 1 in Supplement 2). Patients taking diuretics were more likely to be older and female; less likely to be Asian; had a higher body mass index, heart rate, higher NYHA class, and NT-proBNP level; had a lower KCCQ clinical summary score, had a greater frequency of recent HHF; and had a higher burden of comorbidities, including atrial fibrillation, hypertension, chronic kidney disease, and diabetes. (Table).
Table. Baseline Characteristics of Patients in the EMPEROR-Preserved Trial According to Baseline Diuretic Therapy.
| Furosemide-equivalent dose | P value for interaction (no diuretic vs any dose)a | P value for trend (by dose)b | |||||
|---|---|---|---|---|---|---|---|
| No diuretic (n = 1179) | <40 mg (n = 1725) | 40 mg (n = 1772) | >40 mg (n = 1139) | Any dose (n = 4636) | |||
| Characteristics of diuretic use | |||||||
| Furosemide-equivalent dose | |||||||
| Median (IQR), mg | NA | 20 (20-20) | 40 (40-40) | 80 (80-125) | 40 (40-75) | NA | NA |
| Loop diuretics only, No. (%) | NA | 882 (51.1) | 1519 (85.7) | 945 (83.0) | 3346 (72.2) | NA | <.001 |
| Nonloop diuretics only, No. (%) | NA | 755 (43.8) | 0 | 0 | 755 (16.3) | NA | <.001 |
| Both loop and nonloop diuretics, No. (%) | NA | 88 (5.1) | 253 (14.3) | 194 (17.0) | 535 (11.5) | NA | <.001 |
| Hydrochlorothiazide, No. (%) | NA | 530 (30.7) | 126 (7.1) | 92 (8.1) | 748 (16.1) | NA | .003 |
| Chlorthalidone, No. (%) | NA | 55 (3.2) | 11 (0.6) | 7 (0.6) | 73 (1.6) | NA | .16 |
| Indapamide, No. (%) | NA | 205 (11.9) | 64 (3.6) | 22 (1.9) | 291 (6.3) | NA | .02 |
| Demographic characteristics and vitals | |||||||
| Age, mean (SD), y | 70.9 (9.4) | 72.4 (8.9) | 71.7 (9.6) | 72.3 (10.0) | 72.1 (9.4) | <.001 | .02 |
| Female, No. (%) | 452 (38.3) | 836 (48.5) | 795 (44.9) | 511 (44.9) | 2142 (46.2) | <.001 | .04 |
| Male, No. (%) | 727 (61.7) | 889 (51.5) | 977 (55.1) | 628 (55.1) | 2494 (53.8) | ||
| Race, No. (%)c | |||||||
| Asian | 247 (20.9) | 305 (17.7) | 172 (9.7) | 93 (8.2) | 570 (12.3) | <.001 | <.001 |
| Black | 31 (2.6) | 58 (3.4) | 97 (5.5) | 67 (5.9) | 222 (4.8) | ||
| White | 803 (68.1) | 1290 (74.8) | 1372 (77.4) | 916 (80.4) | 3578 (77.2) | ||
| Other or mixed raced | 98 (8.3) | 71 (4.1) | 130 (7.3) | 63 (5.5) | 264 (5.7) | ||
| Region, No. (%) | |||||||
| Asia | 218 (18.5) | 268 (15.5) | 122 (6.9) | 71 (6.2) | 461 (9.9) | <.001 | <.001 |
| Europe | 402 (34.1) | 825 (47.8) | 782 (44.1) | 554 (48.6) | 2161 (46.6) | ||
| North America | 115 (9.8) | 179 (10.4) | 183 (10.3) | 211 (18.5) | 573 (12.4) | ||
| Latin America | 383 (32.5) | 389 (22.6) | 562 (31.7) | 175 (15.4) | 1126 (24.3) | ||
| Heart rate, mean (SD), bpm | 69.1 (11.4) | 70.0 (11.6) | 71.1 (11.9) | 71.0 (12.6) | 70.7 (12.0) | <.001 | <.001 |
| Systolic blood pressure, mean (SD), mm Hg | 132.4 (14.9) | 132.6 (15.2) | 131.6 (15.8) | 130.5 (16.8) | 131.7 (15.8) | .17 | <.001 |
| Diastolic blood pressure, mean (SD), mm Hg | 76.8 (10.5) | 76.2 (10.1) | 76.0 (10.5) | 73.5 (11.2) | 75.4 (10.6) | <.001 | <.001 |
| Weight, mean (SD), kg | 77.0 (17.7) | 79.7 (18.5) | 83.0 (19.1) | 87.6 (21.3) | 82.9 (19.7) | <.001 | <.001 |
| BMI, mean (SD)e | 27.9 (5.2) | 29.3 (5.6) | 30.4 (5.8) | 31.6 (6.3) | 30.3 (5.9) | <.001 | <.001 |
| Medical history, No. (%) | |||||||
| Atrial fibrillation | 461 (39.1) | 868 (50.3) | 944 (53.3) | 666 (58.5) | 2478 (53.5) | <.001 | <.001 |
| Hypertension | 1017 (86.3) | 1585 (91.9) | 1621 (91.5) | 1043 (91.6) | 4249 (91.7) | <.001 | <.001 |
| CAD | 474 (40.2) | 590 (34.2) | 584 (33.0) | 405 (35.6) | 1579 (34.1) | <.001 | .0121 |
| CKD | 484 (41.1) | 844 (48.9) | 1009 (56.9) | 766 (67.3) | 2619 (56.5) | <.001 | <.001 |
| BMI ≥30, No. (%)e | 377 (32.0) | 707 (41.0) | 877 (49.5) | 650 (57.1) | 2234 (48.2) | <.001 | <.001 |
| Diabetes | 487 (41.3) | 807 (46.8) | 888 (50.1) | 684 (60.1) | 2379 (51.3) | <.001 | <.001 |
| Laboratory measurements | |||||||
| Estimated GFR, mL/min/1.73 m2 | 66.6 (18.7) | 62.5 (18.2) | 59.4 (20.2) | 53.5 (20.5) | 59.1 (19.9) | <.001 | <.001 |
| Estimated GFR <60 mL/min/1.73 m2, No. (%) | 438 (37.2) | 792 (45.9) | 939 (53.0) | 729 (64.0) | 2460 (53.1) | <.001 | <.001 |
| Creatinine, mean (SD), mg/dL | 1.09 (0.33) | 1.11 (0.33) | 1.21 (0.41) | 1.34 (0.48) | 1.21 (0.41) | <.001 | <.001 |
| Hematocrit, % | 41.7 (4.7) | 41.1 (4.5) | 40.8 (4.8) | 40.1 (5.0) | 40.7 (4.8) | <.001 | <.001 |
| Heart failure history | |||||||
| NYHA functional classification, No. (%) | |||||||
| IIf | 1061 (90.0) | 1469 (85.2) | 1430 (80.7) | 803 (70.5) | 3702 (79.9) | <.001 | <.001 |
| III | 115 (9.8) | 252 (14.6) | 338 (19.1) | 326 (28.6) | 916 (19.8) | ||
| IV | 1 (0.1) | 2 (0.1) | 4 (0.2) | 10 (0.9) | 16 (0.3) | ||
| Principal cause of heart failure, No. (%) | |||||||
| Ischemic | 506 (42.9) | 572 (33.2) | 616 (34.8) | 364 (32.0) | 1552 (33.5) | <.001 | <.001 |
| Nonischemic | 672 (57.0) | 1153 (66.8) | 1156 (65.2) | 775 (68.0) | 3084 (66.5) | ||
| NT-proBNP, mean (SD), pg/mL | 1098.6 (1502.9) | 1341.8 (1633.3) | 1589.6 (2312.1) | 1824.1 (2209.5) | 1554.9 (2066.0) | <.001 | <.001 |
| KCCQ clinical summary score, mean (SD) | 77.7 (18.9) | 73.1 (19.9) | 68.1 (21.1) | 62.3 (22.2) | 68.6 (21.3) | <.001 | <.001 |
| Left ventricular ejection fraction, mean (SD), % | 54.3 (9.1) | 55.0 (8.7) | 53.3 (8.6) | 54.8 (8.7) | 54.3 (8.7) | .98 | .22 |
| Hospitalization for heart failure in past 12 mo, No. (%) | 140 (11.9) | 306 (17.7) | 485 (27.4) | 395 (34.7) | 1186 (25.6) | <.001 | <.001 |
| Device therapy, No. (%) | |||||||
| Implantable cardioverter defibrillatorg | 62 (5.3) | 44 (2.6) | 68 (3.8) | 52 (4.6) | 164 (3.5) | .006 | .99 |
| Cardiac resynchronizationh | 4 (0.3) | 8 (0.5) | 4 (0.2) | 8 (0.7) | 20 (0.4) | .66 | .43 |
| Other heart failure therapy, No. (%) | |||||||
| ACEi/ARB/ARNi | 927 (78.6) | 1436 (83.2) | 1474 (83.2) | 851 (74.7) | 3761 (81.1) | .05 | .03 |
| β-Blocker | 1008 (85.5) | 1454 (84.3) | 1552 (87.6) | 1004 (88.1) | 4010 (86.5) | .37 | .005 |
| MRA | 354 (30.0) | 514 (29.8) | 770 (43.5) | 529 (46.4) | 1813 (39.1) | <.001 | <.001 |
| ARNi | 32 (2.7) | 26 (1.5) | 43 (2.4) | 28 (2.5) | 97 (2.1) | .20 | .70 |
| ACEi/ARB/ARNi, β-blocker, and MRA | 268 (22.7) | 383 (22.2) | 567 (32.0) | 350 (30.7) | 1300 (28.0) | <.001 | <.001 |
| Glucose-lowering medication, No. (%)i | |||||||
| Biguanide | 294 (60.4) | 440 (54.5) | 486 (54.7) | 318 (46.5) | 1244 (52.3) | .001 | <.001 |
| Sulphonamide | 102 (20.9) | 171 (21.2) | 193 (21.7) | 141 (20.6) | 505 (21.2) | .89 | .94 |
| DPP-4 inhibitor | 67 (13.8) | 128 (15.9) | 96 (10.8) | 90 (13.2) | 314 (13.2) | .74 | .13 |
| GLP-1 receptor agonist | 8 (1.6) | 11 (1.4) | 17 (1.9) | 22 (3.2) | 50 (2.1) | .51 | .02 |
| Insulin | 113 (23.2) | 186 (23.0) | 268 (30.2) | 275 (40.2) | 729 (30.6) | .001 | <.001 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor-neprilysin inhibitor; BMI, body mass index; bpm, beats per min; CAD, coronary artery disease; CKD, chronic kidney disease; DPP-4, dipeptidyl peptidase-4; GFR, glomerular filtration rate; GLP-1, glucagonlike peptide-1; KCCQ, Kansas City Cardiomyopathy Questionnaire; MRA, mineralocorticoid receptor antagonists; NA, not applicable; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
P value for interaction for comparison between diuretic vs nondiuretic groups.
P value for trend for comparison of no diuretic, <40 mg, 40 mg, and >40 mg.
Race was collected via electronic case report form based on patient-reported information in order to monitor diversity in the trial and ensure the recruitment of a representative patient population.
Other or mixed race included American Indian or Alaska Native, Native Hawaiian or Pacific Islander, and self-reported mixed race, which were consolidated to 1 category due to very low prevalence in the trial population.
Calculated as weight in kilograms divided by height in meters squared.
Includes 4 patients with NYHA I, 2 in the diuretics group and 2 in the no diuretics group.
Includes all the patients with an implantable cardioverter-defibrillator regardless of the presence or absence of cardiac resynchronization therapy.
Includes all the patients who were receiving cardiac resynchronization therapy regardless of the presence or absence of a defibrillator.
Only includes patients with type 2 diabetes at baseline.
Outcomes and Adverse Events in the Placebo Arm
In the placebo arm, compared with the nondiuretic group (reference), the diuretic group had a higher risk of HHF or CV death (HR, 1.81; 95% CI, 1.38-2.39; P < .001), total HHF (HR, 3.21; 2.15-4.80; P < .001), first HHF (HR, 2.75; 95% CI, 1.85-4.07; P < .001), and all-cause mortality (HR, 1.40; 95% CI, 1.06-1.85; P = .02). Findings were similar after stratification by diuretic dose, with higher diuretic doses associated with a stepwise increase in the risk of all aforementioned clinical outcomes (eFigure 1 in the Supplement). In the placebo arm, neither baseline diuretic use status nor dose was associated with the risk of the composite kidney end point (eFigure 1 in the Supplement) or eGFR slope. Patients taking higher doses of diuretics in the placebo arm experienced greater increases in NT-proBNP and greater reductions in body weight at 52 weeks. Neither diuretic status nor dose was associated with the magnitude of change in any of the other physiologic outcomes studied (eTable 2 in Supplement 2).
In the placebo arm, diuretic use was associated with a lesser improvement in KCCQ clinical summary score at all time points (12, 32, and 52 weeks). When analyzed by dose, a stepwise decrease in the magnitude of KCCQ clinical summary score improvement was seen with increasing diuretic dose. The findings were similar for KCCQ total symptom score and KCCQ overall summary score (eFigure 2 in Supplement 2).
Patients in the placebo arm who were receiving a diuretic had a numerically higher risk of adverse events leading to placebo discontinuation (10.5 vs 7.9 events per 100 patient-years), volume depletion events (5.6 vs 4.1 events per 100 patient-years), and acute kidney failure (7.9 vs 4.9 events per 100 patient-years). Findings were similar when studied by diuretic dose. The risk of hyperkalemia did not vary considerably by diuretic status (4.1 vs 4.1 events per 100 patient-years) or dose.
Association of Empagliflozin With Clinical Outcomes by Baseline Diuretic Therapy
Baseline diuretic status was not associated with changes in the benefit of empagliflozin for the primary end point (HR, 0.81; 95% CI, 0.70-0.93 for the diuretic group; HR, 0.72; 95% CI, 0.48-1.06 for the nondiuretic group; P for interaction = .58), total HHF, or first HHF. No treatment by diuretic status interaction was noted for CV death or all-cause death end points (Figure 1). The association of empagliflozin with the kidney end point was not calculated due to a small number of events in the nondiuretic group. When participants were categorized by diuretic dose, the association with empagliflozin appeared consistent across dose categories for all aforementioned outcomes.
Figure 1. Comparison of Empagliflozin vs Placebo on Clinical Outcomes by Baseline Diuretic Use.
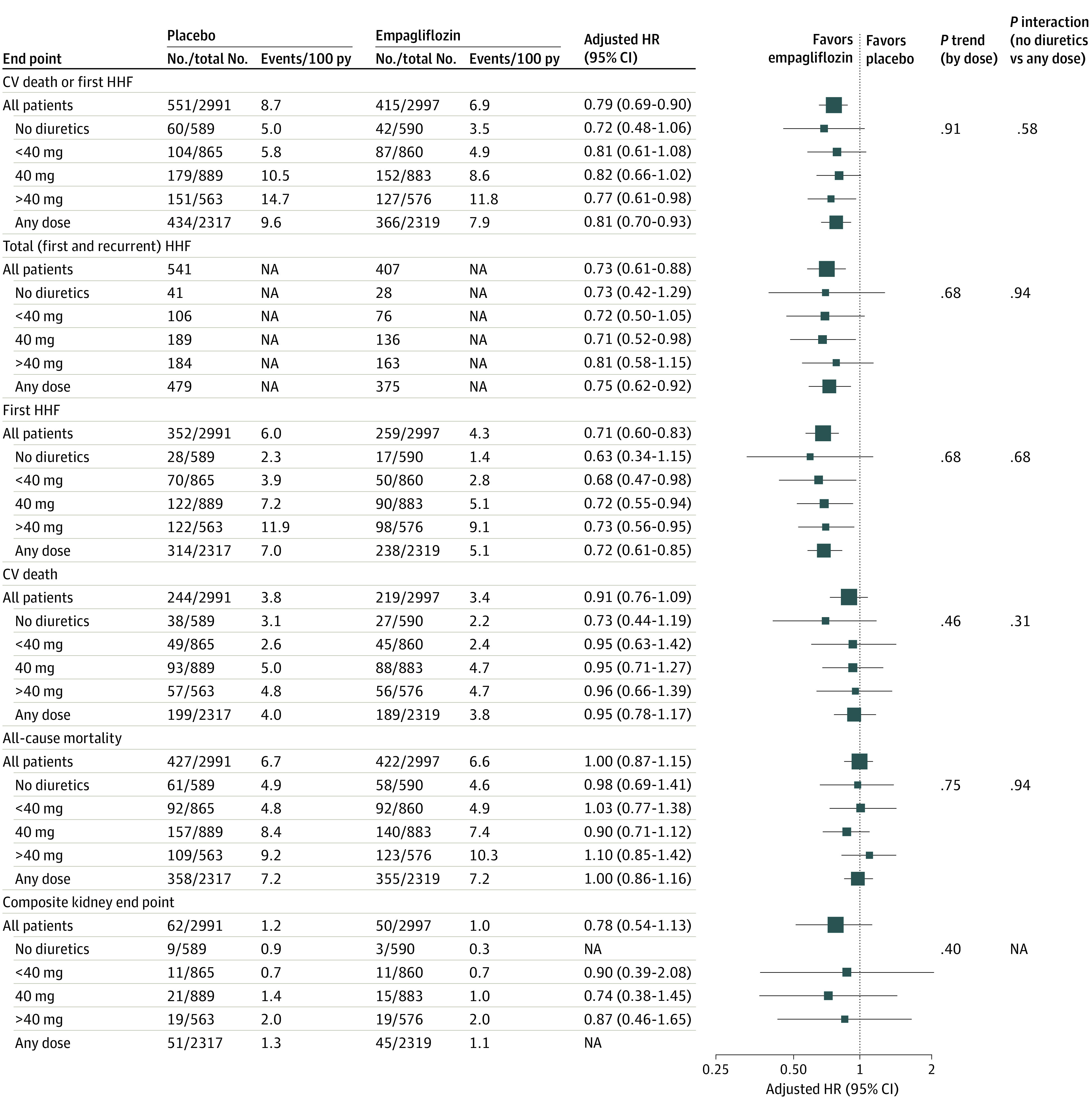
CV indicates cardiovascular; HHF, hospitalization for heart failure; HR, hazard ratio; NA, not applicable; py, patient-year.
Compared with placebo, empagliflozin was associated with a slower rate of decline in the estimated glomerular filtration rate, regardless of baseline diuretic use or dose (eTable 3 in Supplement 2). Empagliflozin was also associated with improved KCCQ clinical summary scores similarly in both the diuretic and nondiuretic groups at 12-week, 32-week, and 52-week follow-up. These findings were consistent when patients were categorized by diuretic dose. Findings were similar for KCCQ total symptom score and KCCQ overall summary score (Figure 2).
Figure 2. Comparison of Empagliflozin vs Placebo on Kansas City Cardiomyopathy Questionnaire Subdomain Scores According to Baseline Diuretic Therapy.
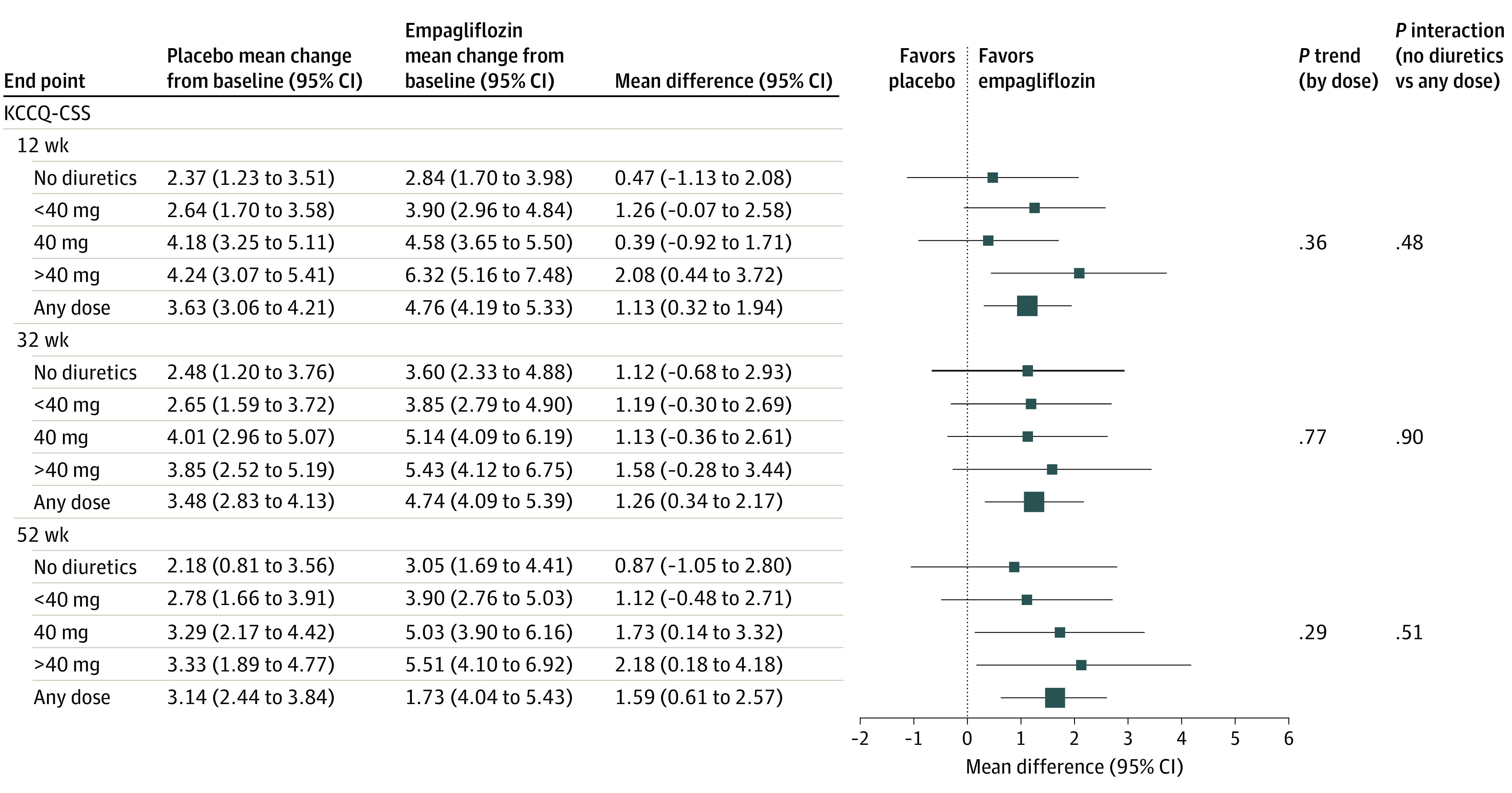
KCCQ-CSS indicates Kansas City Cardiomyopathy Questionnaire clinical summary score.
Compared with placebo, empagliflozin was associated with decreased NT-proBNP levels similarly in the diuretic and nondiuretic groups (geometric mean ratio [95% CI] at week 52: 0.95 [0.91-1.00] in the diuretic group; 0.91 [0.83-0.99] in the nondiuretic group; P for interaction = .40). Findings were consistent when patients were categorized by diuretic dose (eTable 3 in Supplement 2).
Empagliflozin was associated with reduced weight and hemoglobin A1c, with no significant interaction by diuretic status. However, categorization by diuretic dose revealed that patients taking higher doses of diuretics were significantly less likely to experience weight loss or a decrease in hemoglobin A1c at week 52. Empagliflozin was associated with increased hematocrit and decreased systolic blood pressure and uric acid, with no effect modification by diuretic status or dose.
Association of Empagliflozin With Adverse Effects by Baseline Diuretic Therapy
Empagliflozin was associated with a higher incidence of volume depletion events in the diuretic group (7.5 vs 5.6 events per 100 patient-years; HR, 1.34; 95% CI, 1.13-1.59) but not the nondiuretic group (4.3 vs 4.1 events per 100 patient-years; HR, 1.06; 95% CI, 0.70-1.61; P for interaction = .32) (eTable 4 in Supplement 2). Among the preferred terms grouped under volume depletion, the most commonly reported was hypotension, followed by syncope and dehydration (eTable 5 in Supplement 2). In both groups, the treatment arms did not differ in the frequency of acute kidney failure, hyperkalemia, or adverse events leading to trial drug discontinuation (including fatal events).
Association of Empagliflozin With Changes in Diuretic Therapy After Randomization
Among patients who were not taking diuretics at baseline, empagliflozin was associated with a lesser likelihood of diuretic initiation (HR, 0.73; 95% CI, 0.59-0.90; P = .004) compared to placebo (Figures 3 and 4). In those who were treated with diuretics at baseline, empagliflozin use was associated with a significantly greater probability of diuretic discontinuation (HR, 1.43; 95% CI, 1.15-1.78; P = .002) (Figures 2 and 4), and de-escalation (HR, 1.15; 95% CI, 1.02-1.30; P = .02) and a decreased likelihood of diuretic dose escalation (HR, 0.74; 95% CI, 0.65-0.84; P < .001). Likelihood of diuretic discontinuation, de-escalation, and escalation did not vary by baseline diuretic dose.
Figure 3. Cumulative Incidence Curves Showing Time to Initiation of Diuretics in Patients Not Receiving Diuretics at Baseline and Time to Permanent Discontinuation of Diuretics in Patients Receiving Diuretics at Baseline.
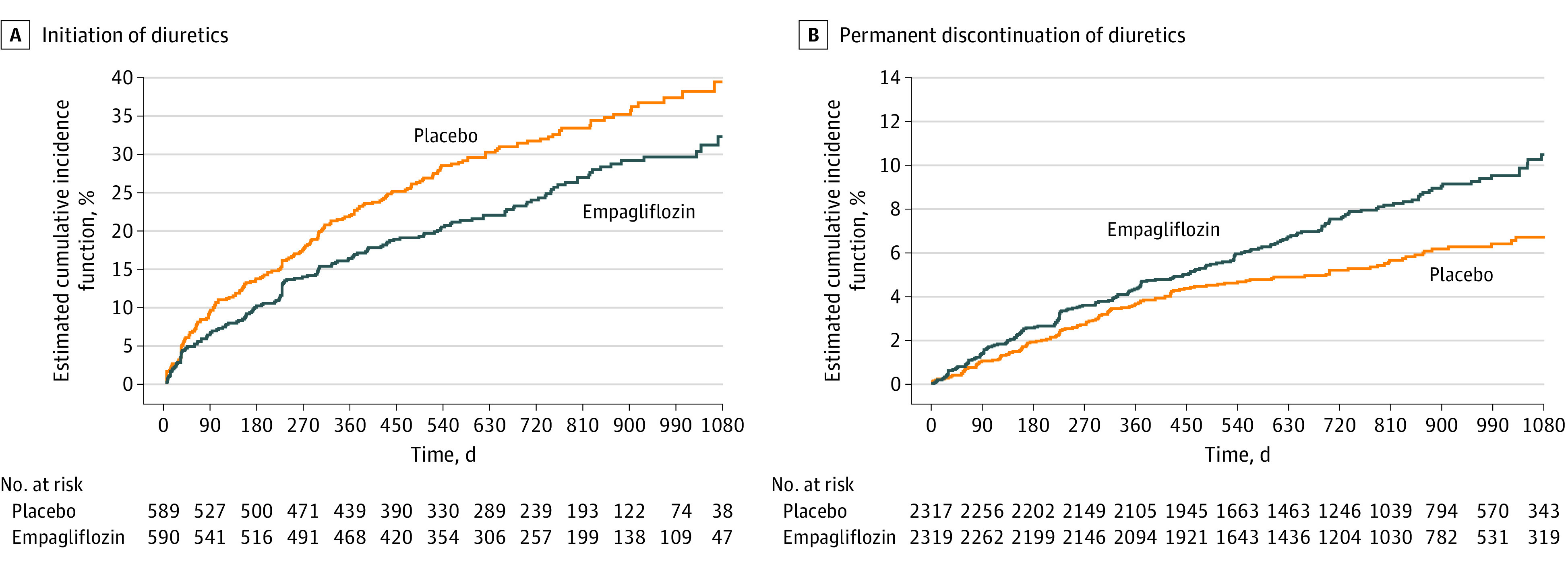
Figure 4. Change in Diuretic Therapy in Empagliflozin vs Placebo Arms.
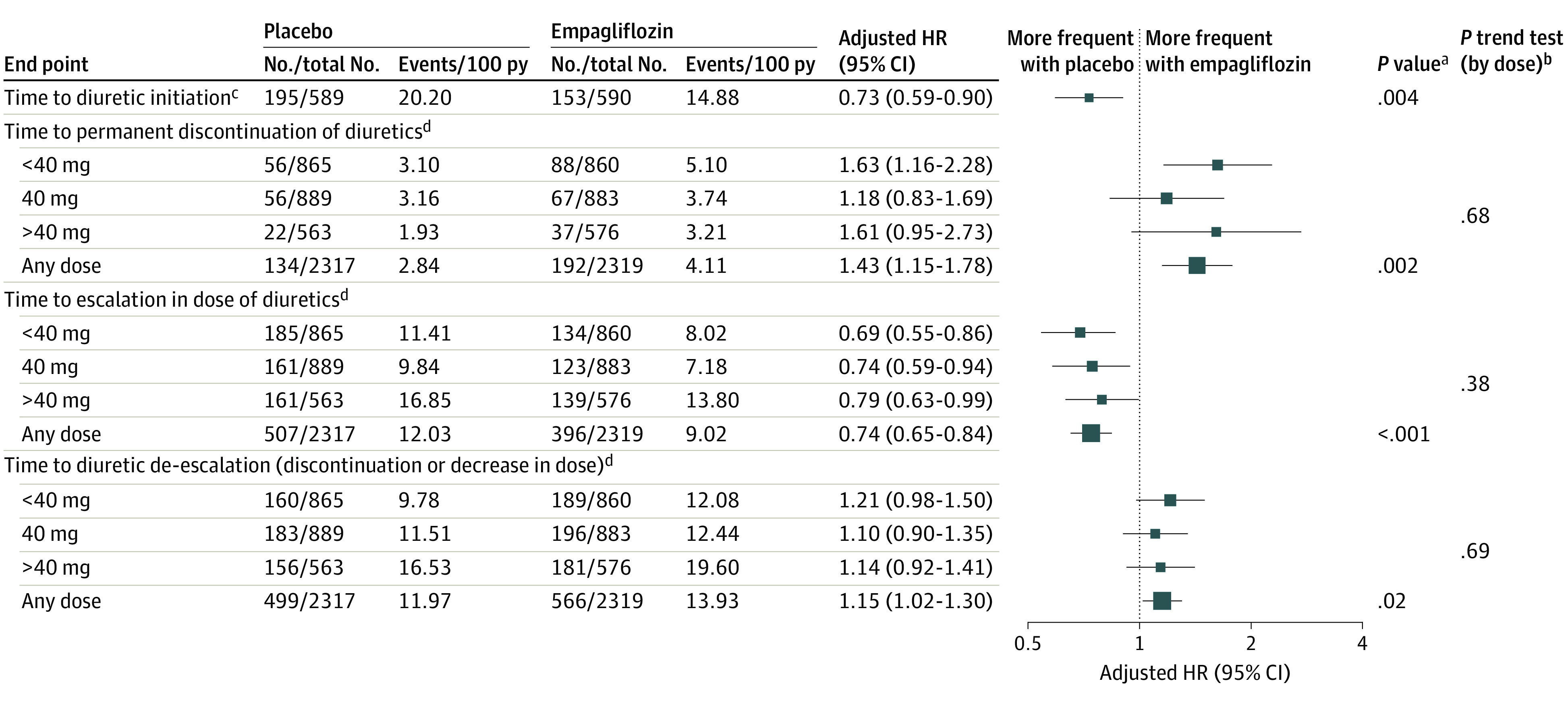
HR indicates hazard ratio; py, patient-year.
aP value for comparison of empagliflozin vs placebo.
bP trend for comparison of baseline diuretic doses of <40mg, 40mg, and >40mg.
cFor patients not receiving diuretic therapy at baseline.
dFor patients receiving diuretic therapy at baseline.
Discussion
This study yields several notable findings. First, analysis of the placebo arm of the EMPEROR-Preserved trial showed that diuretic therapy was a risk marker for adverse outcomes in HFpEF, with higher doses associated with greater risk. Second, the benefit of empagliflozin on first HHF or CV death, first HHF, and total HHF was consistent regardless of background diuretic therapy or diuretic dose. Third, patients taking higher doses of diuretics were less likely to experience weight loss or decrease in hemoglobin A1c (in patients with diabetes) with empagliflozin. Fourth, among patients taking diuretics, the addition of empagliflozin was associated with an increased risk of volume depletion events but not of hyperkalemia or acute kidney failure. Fifth, patients randomized to empagliflozin (vs placebo) had a lesser likelihood of diuretic initiation and dose escalation and a greater likelihood of diuretic de-escalation and permanent discontinuation.
In the EMPEROR-Preserved trial, approximately 80% of the patients were treated with diuretics at baseline, which is similar to other contemporary HFpEF and HF with reduced ejection fraction (HFrEF) cohorts.7,8,9,10,11 To our knowledge, the current study is the first to delineate characteristics of diuretic use in patients with HFpEF. The most common furosemide-equivalent dose of diuretics in EMPEROR-Preserved was 40 mg, similar to patients with HFrEF in the DAPA-HF trial.7 Patients who were not treated with diuretics at baseline had the most favorable clinical profile and better outcomes. Higher baseline doses of diuretics were associated with more severe HF, poorer HRQoL, greater burden of comorbidities, and a higher risk of adverse outcomes. However, the benefit of empagliflozin on the primary outcome (first HHF or CV death), first HHF, total HHF, eGFR slope, and HRQoL was consistent regardless of baseline diuretic status or dose. This is in line with findings from HFrEF patients in the DAPA-HF trial,7 where baseline diuretic therapy did not modify the benefit of dapagliflozin on these outcomes.
Several potential mechanisms of benefit have been proposed with SGLT2 inhibitors.12 SGLT2 inhibitors have been shown to activate a transcriptional paradigm that mimics oxygen and nutrient deprivation, which in turn upregulates autophagy of damaged organelles.4 This results in decreased inflammasome activation, which lessens cardiomyocyte dysfunction and coronary microvascular injury. SGLT2 inhibitors also improve cardiac energy metabolism, specifically by enhancing oxidation of long-chain fatty acids, which is the primary fuel of the heart.13 Overall, SGLT2-inhibitors improve cardiomyocyte physiology and metabolism and decrease inflammation and oxidative stress, 2 important mechanisms in the pathophysiology of HFpEF. SGLT2 inhibitors also reduce the mass and proinflammatory state of epicardial adipose tissue, which can act as a transducer of systemic inflammatory process onto the heart.14
SGLT2 inhibitors inhibit sodium and glucose reabsorption in the proximal renal tubule and thus promote both natriuretic and osmotic diuresis. However, the diuretic effects are short lived and, even if durable, it is not clear whether these effects contribute in any way to the heart failure benefits seen with these drugs. The finding that statistical significance was achieved in the EMPEROR-Preserved trial as early as 18 days following randomization is a function of the number of events noted at that time and does not imply any specific mechanism.15
The favorable association of empagliflozin with decreased diuretic use also does not provide specific mechanistic insights. Changes in the use of diuretics often precedes and follows hospitalization for heart failure and thus any drug that reduces admissions for worsening heart failure would be expected to shift diuretic use in a favorable manner. Furthermore, between-group differences in the use of diuretics in EMPEROR-Preserved began to emerge 60 to 90 days following randomization, a time course that is inconsistent with an immediate natriuretic effect of SGLT2 inhibitors. It is therefore noteworthy that a reduced requirement for diuretics has been noted with other disease-modifying HF drugs, including drugs that have no diuretic effects. Specifically, patients with HFrEF who were treated with sacubitril/valsartan (vs enalapril) were more likely to have their loop diuretic dose reduced and less likely to have it increased.16 Sacubitril/valsartan was also associated with a modestly lower frequency of diuretic initiation in patients with HFpEF compared with valsartan alone.17
The results of this analysis have important clinical implications. In patients who are not taking diuretics, SGLT2 inhibitors should not be withheld due to concerns of destabilizing the euvolemic status, as empagliflozin improved clinical outcomes without increasing volume depletion events in this population. At the time an SGLT2 inhibitor is initiated, most patients with HFpEF would not require a change in diuretic dose. However, as in every patient with heart failure, physicians should be prepared to adjust the dose of diuretics according to each patient’s needs. Doing so will minimize the small risk of volume depletion when SGLT2 inhibitors and loop diuretics are combined. Patient education, daily weights, and monitoring for volume depletion is advisable. In the longer term, as HF status improves in patients taking empagliflozin, the need for diuretics may be reduced.
Limitations
The results of this study should be interpreted with certain limitations in mind. This analysis was not prespecified. Although several possible confounding factors were adjusted for, residual confounding is possible, particularly when comparing prognoses across diuretic dose categories in the placebo arm. Markers of diuresis and natriuresis, such as urine volumes and urinary sodium excretion, were not assessed.
Conclusions
In this analysis, empagliflozin use was associated with improvement in HHF or CV death, first HHF, total HHF, eGFR slope, and KCCQ scores in patients with HFpEF, regardless of background diuretics use or dose. Empagliflozin was associated with a slightly increased risk of volume depletion in patients taking diuretics. Empagliflozin also reduced the likelihood of diuretic initiation and dose escalation and increased the likelihood of diuretic de-escalation and permanent discontinuation.
Trial protocol
eTable 1. Characteristics of patients in the empagliflozin and placebo groups in patients with and without diuretic use at baseline
eTable 2. Change from baseline in estimated glomerular filtration rate slope and physiologic variables (at 52 weeks) in the placebo arm, according to baseline diuretic status and dose
eTable 3. Effect of empagliflozin versus placebo on eGFR slope and physiologic outcomes (at 52 weeks), according to baseline diuretic therapy
eTable 4. Adverse effects in the empagliflozin versus placebo arm according to baseline diuretic use
eTable 5. Frequency of each preferred term grouped under ‘volume depletion’
eFigure 1. Event rates for primary and secondary outcomes in placebo arm according to baseline diuretic status
eFigure 2. Change in Kansas City Cardiomyopathy Questionnaire subdomain scores KCCQ-CSS (A), KCCQ-TSS (B) and KCCQ-OSS (C) in the placebo arm according to baseline diuretic status and dose
Data sharing statement
References
- 1.Anker SD, Butler J, Filippatos G, et al. ; EMPEROR-Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 2.Tang J, Ye L, Yan Q, Zhang X, Wang L. Effects of sodium-glucose cotransporter 2 inhibitors on water and sodium metabolism. Front Pharmacol. 2022;13:800490. doi: 10.3389/fphar.2022.800490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020;5(6):632-644. doi: 10.1016/j.jacbts.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Packer M. Autophagy stimulation and intracellular sodium reduction as mediators of the cardioprotective effect of sodium-glucose cotransporter 2 inhibitors. Eur J Heart Fail. 2020;22(4):618-628. doi: 10.1002/ejhf.1732 [DOI] [PubMed] [Google Scholar]
- 5.Verma A, Patel AB, Waikar SS. SGLT2 inhibitor: not a traditional diuretic for heart failure. Cell Metab. 2020;32(1):13-14. doi: 10.1016/j.cmet.2020.06.014 [DOI] [PubMed] [Google Scholar]
- 6.Anker SD, Butler J, Filippatos GS, et al. ; EMPEROR-Preserved Trial Committees and Investigators . Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved trial. Eur J Heart Fail. 2019;21(10):1279-1287. doi: 10.1002/ejhf.1596 [DOI] [PubMed] [Google Scholar]
- 7.Jackson AM, Dewan P, Anand IS, et al. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA-HF. Circulation. 2020;142(11):1040-1054. doi: 10.1161/CIRCULATIONAHA.120.047077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitt B, Pfeffer MA, Assmann SF, et al. ; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383-1392. doi: 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, Vaduganathan M, Claggett BL, et al. Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction: DELIVER trial. JACC Heart Fail. 2022;10(3):184-197. doi: 10.1016/j.jchf.2021.11.006 [DOI] [PubMed] [Google Scholar]
- 10.Zannad F, McMurray JJ, Krum H, et al. ; EMPHASIS-HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11-21. doi: 10.1056/NEJMoa1009492 [DOI] [PubMed] [Google Scholar]
- 11.Solomon SD, McMurray JJV, Claggett B, et al. ; DELIVER Trial Committees and Investigators . Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089-1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 12.Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(4):422-434. doi: 10.1016/j.jacc.2019.11.031 [DOI] [PubMed] [Google Scholar]
- 13.Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17(12):761-772. doi: 10.1038/s41569-020-0406-8 [DOI] [PubMed] [Google Scholar]
- 14.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71(20):2360-2372. doi: 10.1016/j.jacc.2018.03.509 [DOI] [PubMed] [Google Scholar]
- 15.Butler J, Siddiqi TJ, Filippatos G, et al. Early benefit with empagliflozin in heart failure with preserved ejection fraction: insights from the EMPEROR-Preserved trial. Eur J Heart Fail. 2022;24(2):245-248. doi: 10.1002/ejhf.2420 [DOI] [PubMed] [Google Scholar]
- 16.Vardeny O, Claggett B, Kachadourian J, et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM-HF trial. Eur J Heart Fail. 2019;21(3):337-341. doi: 10.1002/ejhf.1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatur S, Claggett BL, Vardeny O, et al. Sacubitril/valsartan and loop diuretic requirement in heart failure with preserved ejection fraction in the PARAGON-HF trial. Eur J Heart Fail. 2023;25(1):87-94. doi: 10.1002/ejhf.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Characteristics of patients in the empagliflozin and placebo groups in patients with and without diuretic use at baseline
eTable 2. Change from baseline in estimated glomerular filtration rate slope and physiologic variables (at 52 weeks) in the placebo arm, according to baseline diuretic status and dose
eTable 3. Effect of empagliflozin versus placebo on eGFR slope and physiologic outcomes (at 52 weeks), according to baseline diuretic therapy
eTable 4. Adverse effects in the empagliflozin versus placebo arm according to baseline diuretic use
eTable 5. Frequency of each preferred term grouped under ‘volume depletion’
eFigure 1. Event rates for primary and secondary outcomes in placebo arm according to baseline diuretic status
eFigure 2. Change in Kansas City Cardiomyopathy Questionnaire subdomain scores KCCQ-CSS (A), KCCQ-TSS (B) and KCCQ-OSS (C) in the placebo arm according to baseline diuretic status and dose
Data sharing statement


