Key Points
Questions
What is the subsequent risk of mental disorders among individuals with SARS-CoV-2 infection, and are the associations specific for COVID-19?
Findings
In this cohort study including the total adult population of Denmark and covering all SARS-CoV-2 polymerase chain reaction tests, the overall risk of new-onset mental disorders was increased in SARS-CoV-2–positive individuals compared with individuals not tested; however, the risk did not exceed that of SARS-CoV-2–negative individuals. Patients hospitalized with COVID-19 had markedly increased risk compared with the general population, although the risk was similarly elevated compared with patients hospitalized for non–COVID-19 infections.
Meaning
These findings suggest that deterioration of mental health after hospitalization for COVID-19 is common but no more frequent than after other infections with similar severity.
This nationwide cohort study assesses the association of SARS-CoV-2 infection and subsequent risk of mental disorders and use of psychotropic medication among the adult population of Denmark.
Abstract
Importance
Psychiatric outcomes after COVID-19 have been of high concern during the pandemic; however, studies on a nationwide level are lacking.
Objective
To estimate the risk of mental disorders and use of psychotropic medication among individuals with COVID-19 compared with individuals not tested, individuals with SARS-CoV-2–negative test results, and those hospitalized for non–COVID-19 infections.
Design, Setting, and Participants
This nationwide cohort study used Danish registries to identify all individuals who were alive, 18 years or older, and residing in Denmark between January 1 and March 1, 2020 (N = 4 152 792), excluding individuals with a mental disorder history (n = 616 546), with follow-up until December 31, 2021.
Exposures
Results of SARS-CoV-2 polymerase chain reaction (PCR) testing (negative, positive, and never tested) and COVID-19 hospitalization.
Main Outcomes and Measures
Risk of new-onset mental disorders (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, codes F00-F99) and redeemed psychotropic medication (Anatomical Therapeutic Chemical classification codes N05-N06) was estimated through survival analysis using a Cox proportional hazards model, with a hierarchical time-varying exposure, reporting hazard rate ratios (HRR) with 95% CIs. All outcomes were adjusted for age, sex, parental history of mental illness, Charlson Comorbidity Index, educational level, income, and job status.
Results
A total of 526 749 individuals had positive test results for SARS-CoV-2 (50.2% men; mean [SD] age, 41.18 [17.06] years), while 3 124 933 had negative test results (50.6% women; mean [SD] age, 49.36 [19.00] years), and 501 110 had no tests performed (54.6% men; mean [SD] age, 60.71 [19.78] years). Follow-up time was 1.83 years for 93.4% of the population. The risk of mental disorders was increased in individuals with positive (HRR, 1.24 [95% CI, 1.17-1.31]) and negative (HRR, 1.42 [95% CI, 1.38-1.46]) test results for SARS-CoV-2 compared with those never tested. Compared with individuals with negative test results, the risk of new-onset mental disorders in SARS-CoV-2–positive individuals was lower in the group aged 18 to 29 years (HRR, 0.75 [95% CI, 0.69-0.81]), whereas individuals 70 years or older had an increased risk (HRR, 1.25 [95% CI, 1.05-1.50]). A similar pattern was seen regarding psychotropic medication use, with a decreased risk in the group aged 18 to 29 years (HRR, 0.81 [95% CI, 0.76-0.85]) and elevated risk in those 70 years or older (HRR, 1.57 [95% CI, 1.45-1.70]). The risk for new-onset mental disorders was substantially elevated in hospitalized patients with COVID-19 compared with the general population (HRR, 2.54 [95% CI, 2.06-3.14]); however, no significant difference in risk was seen when compared with hospitalization for non–COVID-19 respiratory tract infections (HRR, 1.03 [95% CI, 0.82-1.29]).
Conclusion and Relevance
In this Danish nationwide cohort study, overall risk of new-onset mental disorders in SARS-CoV-2–positive individuals did not exceed the risk among individuals with negative test results (except for those aged ≥70 years). However, when hospitalized, patients with COVID-19 had markedly increased risk compared with the general population, but comparable to risk among patients hospitalized for non–COVID-19 infections. Future studies should include even longer follow-up time and preferentially include immunological biomarkers to further investigate the impact of infection severity on postinfectious mental disorder sequelae.
Introduction
The COVID-19 pandemic has severely impacted human health worldwide. COVID-19, besides being an infectious respiratory illness, can affect multiple organ systems,1 including the brain,2,3 with high prevalence of persisting neuropsychiatric symptoms after initial SARS-CoV-2 infection.4 In the beginning of the pandemic, a SARS-CoV-2 infection of the brain was initially suspected as a potential mechanism for the neuropsychiatric complications; but, increasing evidence shows that indirect immune-mediated mechanisms and sequelae from critical illness are more important.5 Thus, the effects on the brain might be similar to those observed after other types of infections of similar disease severity, as severe non–COVID-19 infections are also linked to an increased risk of mental disorders.6,7,8
Two large-scale studies using data from electronic health care records report increased risk of new-onset mental disorders after COVID-19 compared with influenza,9,10 while another large-scale study found no difference between patients hospitalized with COVID-19 and patients admitted for other severe acute respiratory tract infections.11 Furthermore, studies investigating patients in contact with primary care found no difference in incident mental illness between those with positive and negative test results for SARS-CoV-2,12 while another study found that those with mild COVID-19 even had lower rates of mental disorders compared with those with negative test results.13 Data from hospital electronic health care records only capture individuals in contact with the health care system, while missing the majority of individuals with positive test results for SARS-CoV-2 without a hospital contact. This might introduce a surveillance bias,14 as patients with COVID-19 were more vigilantly monitored during the pandemic. Moreover, prior studies that include information about primary and secondary care are based on selected cohorts from US veterans10 or specific health care organizations,9 which reduces generalizability of their findings. Additionally, study results could be biased by the lack of adjustment for important confounding measures when investigating psychiatric outcomes, such as socioeconomic factors and family history of mental illness. Currently, comprehensive nationwide studies that can capture all individuals tested for SARS-CoV-2 and adjust for important confounding measures are lacking.
Therefore, we conducted the first nationwide study to date, to our knowledge, covering all SARS-CoV-2 polymerase chain reaction (PCR) tests performed in the whole of Denmark and all psychiatric hospitalizations and prescriptions for psychotropic medication. We aimed to study the association between COVID-19 and subsequent risk of mental disorders and use of psychotropic medication. Specifically, the study objective was to compare the risk of incident mental disorders and psychotropic medication use in (1) individuals with positive test results for SARS-CoV-2 compared with those who had negative test results and those not tested at all, and (2) patients hospitalized for COVID-19 compared with patients hospitalized for non–COVID-19 infections and with individuals not hospitalized.
Methods
Study population
Figure 1 provides a schematic overview of the study population and design. Data were obtained by linkage of the nationwide Danish registries using unique personal registration numbers, which are assigned to every resident in Denmark at the time of birth.15 All register-based personal information was anonymized for research purposes without requiring informed consent, and the project was approved by the Danish Data Protection Agency. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Figure 1. Schematic Presentation of Study Design and Structure of Data Analysis .
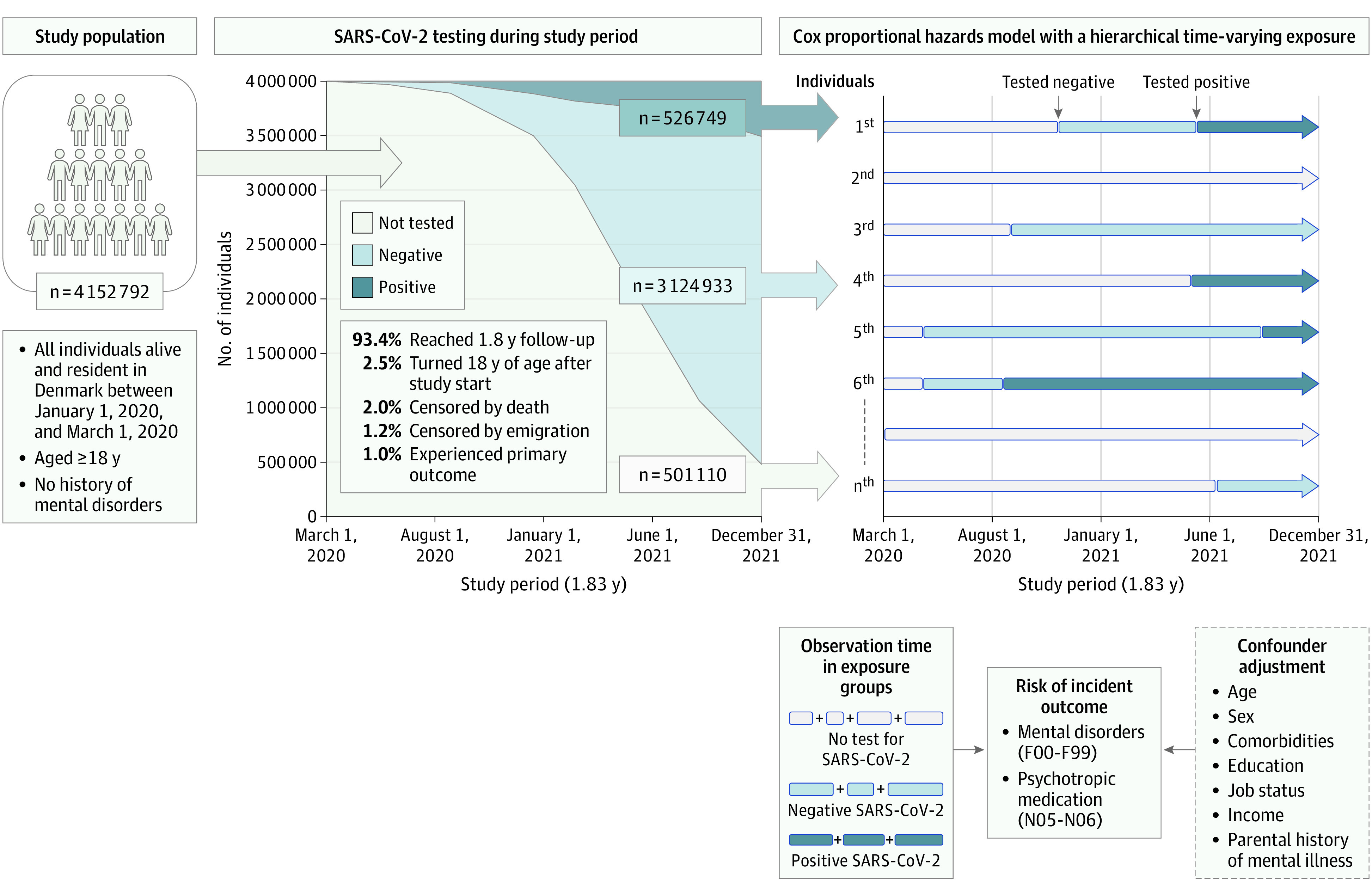
SARS-CoV-2 polymerase chain reaction testing of study population during the COVID-19 pandemic and how each individual contributes to observation time in potentially 3 different exposure groups are shown. Exposure is time-varying and hierarchical (ie, status can change from not tested to negative or positive and from negative to positive but not vice versa). Individual numbers 1st, 2nd, 3rd, etc, illustrate examples of each individual in the study contributing observation time to different groups of exposure.
The population was defined via the Danish Civil Registration System,16 providing information on age, sex, and parents of all individuals born in Denmark. All individuals alive and resident in Denmark between January 1 and March 1, 2020, and 18 years or older were included. Individuals with a history of mental disorders (International Classification of Diseases, Eighth Revision [ICD-8] codes 290-315; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] codes F00-F99) before March 1, 2020, were excluded from the study population for analyses of new-onset mental disorder, and we excluded all individuals with a prior prescription of a psychotropic medication (Anatomical Therapeutic Chemical classification [ATC] codes N05-N06) for analyses of new-onset first redemption of any psychotropic medication. Individuals were followed from March 1, 2020, until outcome, censoring, or end of follow-up on December 31, 2021 (eFigure 1 in Supplement 1).
Registers
Study populations were linked via the personal unique registration number to the following registers: the Danish Psychiatric Central Research Register,17 which contains data on all inpatient and outpatient contacts with Danish psychiatric facilities; the Danish Microbiology Database,18 with all SARS-CoV-2 PCR tests performed in Denmark; the Danish National Hospital Registry,19 which contains records of all inpatient, outpatient, and emergency department visits at Danish hospitals; the Danish National Prescription Registry,20 containing complete information from all pharmacies in Denmark with redeemed prescriptions since 1995; and the Database for Integrated Labour Market Research,21 containing data on most recent educational level completed, income level, and association with the job market. Diagnostic information was based on the ICD-8 from 1977 to 1993 and on the ICD-10 from 1994. Treatment in Danish hospitals is free of charge for all residents, ensuring that all psychiatric admissions are represented in the Danish Psychiatric Central Research Register.
Exposures: SARS-CoV-2 Testing and COVID-19 Hospitalization
All SARS-CoV-2 PCR testing records were retrieved from the Danish Microbiology Database. Multiple tests per individual were available with unique dates indicating when testing was performed (eFigure 2 in Supplement 1). The exposure variable consisted of 3 groups: not tested, negative test results, or positive test results defined in a hierarchical and time-varying manner. Individuals were included in the not-tested group until tested and moved to the negative or positive groups, depending on the result of the SARS-CoV-2 test on the date of the test. At the date of the first positive SARS-CoV-2 test result, individuals moved to the positive group and remained there (Figure 1). Severity of the primary exposure was further analyzed in several variations: COVID-19 hospitalization defined as hospital admission with ICD-10 codes B34.2 or B97.2, admission within 2 days before to 14 days after a positive SARS-CoV-2 PCR test result, and admission duration of at least 12 hours; admission to the intensive care unit (ICU) were captured using the Danish national ICU database procedural codes NABB and/or NABE22; total COVID-19–related admission days; total number of positive SARS-CoV-2 test results (2 positive test results separated by 30 days); and time from first positive test result to outcome.
Exposure of COVID-19 hospitalization was also a time-varying exposure and compared with exposure for hospitalization for other infections, including any respiratory tract infection, and separately for pneumonia and influenza (see eTable 1 in Supplement 1 for specific ICD-10 codes). A washout period was introduced excluding all individuals who had been hospitalized for any infection between 2010 to the start of study period (March 1, 2020) to ensure that the hospital contact for other infections was for a new-onset and not a recurrent condition.
Outcomes: Mental Disorders and Psychotropic Medication Use
Primary outcomes were any mental disorder using ICD-10 codes F00 to F99 and first redemption of any psychotropic medication using ATC codes N05 to N06. Secondary outcomes were subclassified diagnoses in organic mental disorders (ICD-10 codes F00-F09); schizophrenia spectrum disorders (ICD-10 codes F20-F29); mood disorders (ICD-10 codes F30-F39); neurotic, stress-related, and somatoform disorders (ICD-10 codes F40-F48); and specific medication in the groups of antidepressants (ATC code N06A), antipsychotics (ATC code N05A), anxiolytics (ATC code N05B), and antidementia drugs (ATC code N06D). Date of onset was defined as first psychiatric contact or first redemption of psychotropic medication (eTable 1 in Supplement 1).
Confounding Variables
Basic confounders were defined as age in years and sex. Confounders of susceptibility for any diagnosis were parental history of mental illness (any ICD-10 diagnosis code of F00-F99 or ICD-8 code of 290-315) and the Charlson Comorbidity Index23 (0, 1, 2, 3, or ≥4 chronic disorders). Socioeconomic confounders were educational level (elementary school, vocational training or high school, and short- and long-cycle higher education), income (divided in quintiles), and work status (working, unemployed, retired, not in the workforce, or student).
Statistical Analysis
A Cox proportional hazards model was used for survival analysis and hazard rate ratios (HRR) with 95% CIs were reported as measures of relative risk. Calendar time rather than age was used as the underlying time scale, as the degree of exposure to SARS-CoV-2 and the outcome of mental disorders were highly dependent on the specific calendar time, while exposure and outcome were relatively constant when stratifying the result in age groups. It was recently shown that using calendar time improves precision and reduces bias for modeling during the COVID-19 pandemic,24 and this ensured that abrupt changes in outcome rate on the baseline hazard time scale are controlled for. All analyses were adjusted for age in a time-varying manner, whereas adjustment for the variables of sex, parental history of mental illness, Charlson Comorbidity Index, educational level, income, and job status were defined at start of follow-up. Primary and secondary outcomes were investigated in groups with and without SARS-CoV-2 testing, in SARS-CoV-2–positive vs –negative groups, in patients hospitalized with COVID-19 vs the general population, and in patients hospitalized with COVID-19 vs other infections. The effect of COVID-19 exposure severity was only investigated with the primary outcome. We performed several sensitivity analyses to investigate the robustness of our findings. First, we analyzed incidence rates of primary outcome at different time periods over the total study period to see if incidence rates were markedly increased during specific time periods. Second, in contrast to our primary model where all individuals were included at March 1, 2020, sensitivity analyses were performed with a dynamic population that only included individuals at the date of their first SARS-CoV-2 test. Third, we investigated effect modification of all confounder variables by testing their interaction with the exposure variable. Two-tailed P < .05 indicated statistical significance. Statistical analyses were conducted by 1 investigator (R. H. B. C.) using R, version 4.2.2, with survival package version 3.4-0 (R Project for Statistical Computing).
Results
Demographics
The study population consisted of 4 152 792 individuals with a mean [SD] age of 48.84 [19.02] years (50.5% women, 49.5% men) who were living in Denmark between January 1 and March 1, 2020, and were 18 years or older before the end of follow-up on December 31, 2021. A total of 526 749 individuals (12.68%) had positive test results for SARS-CoV-2 (49.8% women and 50.2% men; mean [SD] age, 41.18 [17.06] years), while 3 124 933 (75.25%) only had negative SARS-CoV-2 test results (50.6% women and 49.4% men; mean [SD] age, 49.36 [19.00] years), and 501 110 (12.07%) had no SARS-CoV-2 tests performed (45.4% women and 54.6% men; mean [SD] age, 60.71 [19.78] years) during the study period. Follow-up time was 1.83 years for 93.4% of the population, and a total of 39 528 002 SARS-CoV-2 PCR tests were analyzed. eFigure 3 in Supplement 1 depicts a study flowchart and eTable 2 in Supplement 1 summarizes demographic characteristics.
Testing for SARS-CoV-2 and the Risk of New-Onset Mental Disorders
Overall, the risk of mental disorders wase increased in individuals with positive (HRR, 1.24 [95% CI, 1.17-1.31]) and negative (HRR, 1.42 [95% CI, 1.38-1.46]) SARS-CoV-2 test results compared with those never tested for SARS-CoV-2 (Figure 2). There were no significant temporal effects on the increased risk of new-onset mental disorders with time since a positive SARS-CoV-2 test (eTable 3 in Supplement 1).
Figure 2. Risk of New-Onset Mental Disorder and Psychotropic Medication Use Among Individuals Tested for SARS-CoV-2 and Patients Hospitalized for COVID-19 or Other Respiratory Tract Infections.
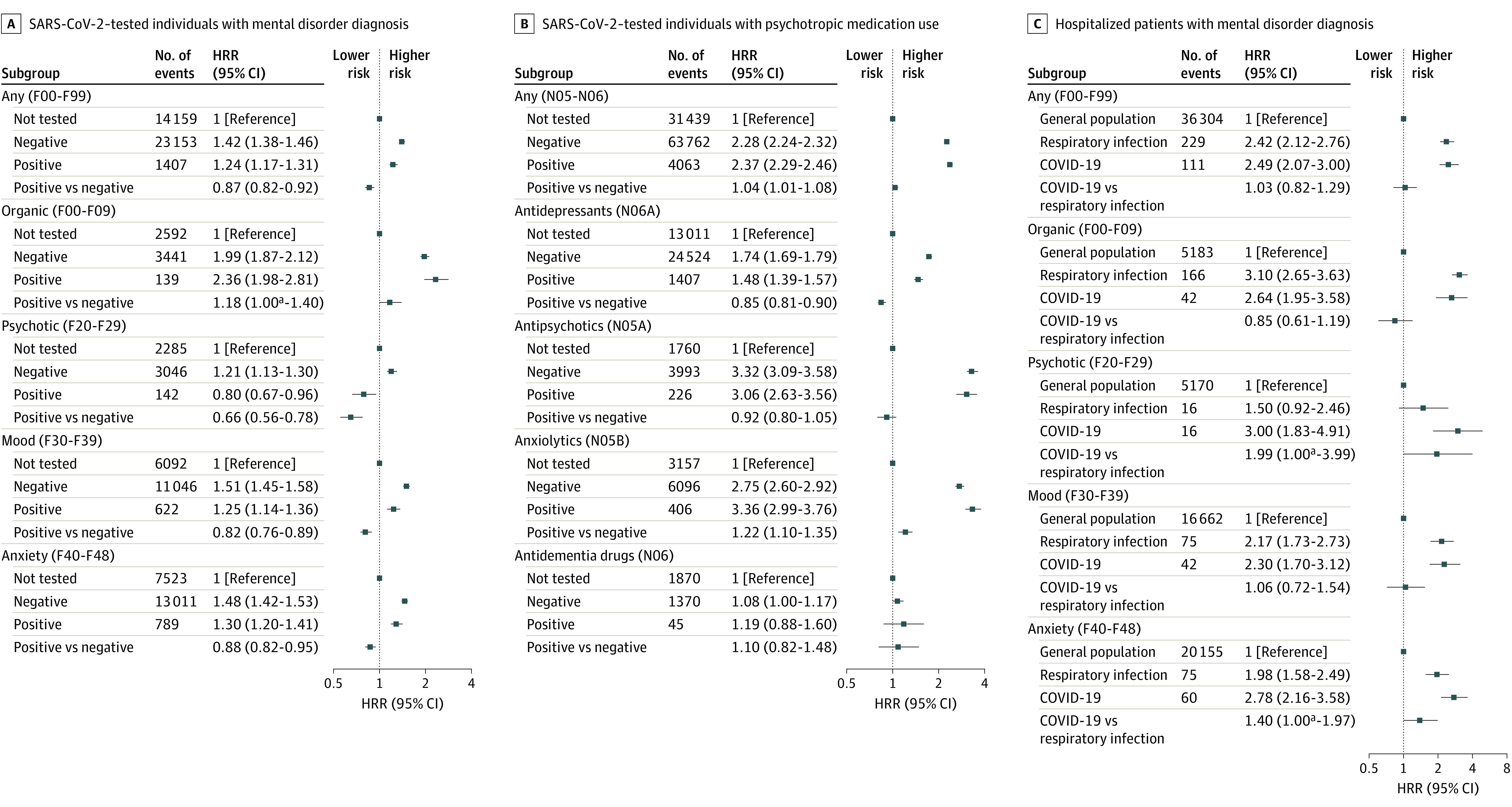
Includes any and specific incident mental disorders defined as International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, codes F00 to F99 among individuals undergoing SARS-CoV-2 testing (A); any redeemed newly prescribed psychotropic medication defined as Anatomical Therapeutic Chemical classification codes N05 to N06 among individuals undergoing SARS-CoV-2 testing (B); and patients hospitalized for COVID-19 or other non–COVID-19 respiratory tract infections (C). Error bars indicate 95% CIs. Individuals with a prior hospitalization for a respiratory tract infection between 2010 and March 2020 were excluded prior to analysis to ensure that the hospital contact for other respiratory tract infections was new onset and not a recurrent condition. All analyses were adjusted for age, sex, parental history of mental illness, Charlson Comorbidity Index, educational level, income, and work status. HRR indicates hazard rate ratio.
aThe lower limit of the 95% CI was smaller than 1.00 (P = .05).
Positive vs Negative SARS-CoV-2 Test Results and the Risk of New-Onset Mental Disorders
Compared with those with negative test results, individuals with positive SARS-CoV-2 test results had an overall lower risk of a new-onset mental disorder (HRR, 0.87 [95% CI, 0.82-0.92]), primarily seen among individuals aged 18 to 29 years (HRR, 0.75 [95% CI, 0.69-0.81]), whereas among individuals 30 years and older, no significant difference was observed (HRR, 1.03 [95% CI, 0.96-1.11]). However, in individuals 70 years and older, there was an increased risk of mental disorders when compared with individuals with negative test results (HRR, 1.25 [95% CI, 1.05-1.50]) (Figure 3).
Figure 3. Risk of New-Onset Mental Disorder and Psychotropic Medication Use Among Individuals Tested for SARS-CoV-2 and Patients Hospitalized for COVID-19 Stratified in Age Groups.
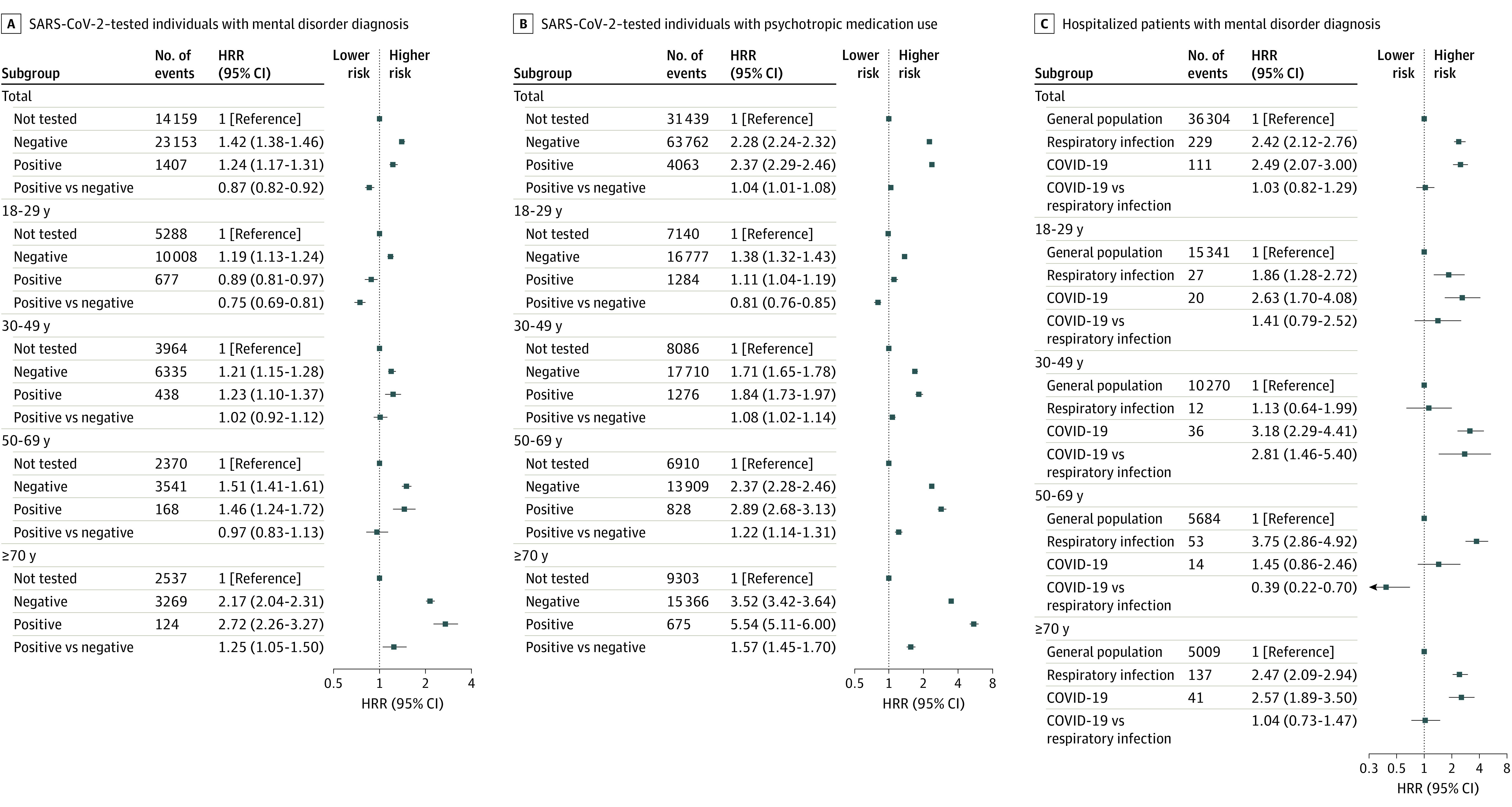
Includes any incident mental disorder defined as International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, codes F00 to F99 in individuals undergoing SARS-CoV-2 testing (A); any redeemed newly prescribed psychotropic medication defined as Anatomical Therapeutic Chemical classification codes N05 to N06 in individuals undergoing SARS-CoV-2 testing (B); and patients hospitalized for COVID-19 or other non–COVID-19 respiratory tract infections (C). Error bars indicate 95% CIs. Individuals with a prior hospitalization for a respiratory tract infection between 2010 and March 2020 were excluded prior to analysis to ensure that the hospital contact for other respiratory tract infections was new onset and not a recurrent condition. All analyses were adjusted for age, sex, parental history of mental illness, Charlson Comorbidity Index, educational level, income, and work status. HRR indicates hazard rate ratio.
Furthermore, individuals with multiple SARS-CoV-2 infections (≥2) had an increased risk of new-onset mental disorders compared with individuals with only negative test results (HRR, 1.66 [95% CI, 1.10-2.50]) (eTable 4 and eFigure 4 in Supplement 1). Last, an increasing number of comorbidities was associated with increased risk of mental disorders in both SARS-CoV-2–positive and –negative individuals compared with those not tested (eFigure 5 in Supplement 1). The risks of specific mental disorder categories are detailed in Figure 2.
SARS-CoV-2 Testing and the Risk of First-Time Use of Psychotropic Medication
Individuals with positive test results for SARS-CoV-2 had a slightly increased risk of redeeming psychotropic medication compared with those with negative test results (HRR, 1.04 [95% CI, 1.01-1.08]), with lower risk in the group aged 18 to 29 years (HRR, 0.81 [95% CI, 0.76-0.85]) and increased risks in those 30 years or older (HRR, 1.20 [95% CI, 1.16-1.25]). Overall, the risk of subsequently needing treatment with psychotropic medication increased with increasing age and was highest among individuals 70 years or older (HRR, 1.57 [95% CI, 1.45-1.70]) (Figure 3). Compared with those who had negative test results, individuals with positive test results for SARS-CoV-2 had an increased risk of redeeming anxiolytics (HRR, 1.22 [95% CI, 1.10-1.35]), a lower risk of using antidepressants (HRR, 0.85 [95% CI, 0.81-0.90]), and no significant difference regarding antipsychotics and antidementia medication (Figure 2).
COVID-19 Hospitalization and the Risk of New-Onset Mental Disorders
Hospitalization for COVID-19 was associated with an increased risk of a new-onset mental disorder with HRR of 2.33 (95% CI, 1.94-2.80) compared with the general population not hospitalized for COVID-19, which was even higher among patients with COVID-19 admitted to the ICU (HRR, 3.61 [95% CI, 2.18-5.99]). However, no significant difference was seen between patients with COVID-19 with short (1-2 days) compared with longer (>5 days) hospitalizations (HRR, 1.07 [95% CI, 0.61-1.87]) (eTable 5 in Supplement 1). Regarding the risk of specific mental disorder categories, patients hospitalized for COVID-19 had an increased risk of organic mental disorders (HRR, 2.64 [95% CI, 1.95-3.58]), including dementia (HRR, 2.20 [95% CI, 1.52-3.20]), schizophrenia spectrum disorders (HRR, 3.00 [95% CI, 1.83-4.91]), affective disorders (HRR, 2.30 [95% CI, 1.70-3.12]), and neurotic, stress-related, and somatoform disorders (HRR, 2.78 [95% CI, 2.16-3.58]) (Figure 2).
COVID-19 Hospitalization Compared With Hospitalization for Other Infections
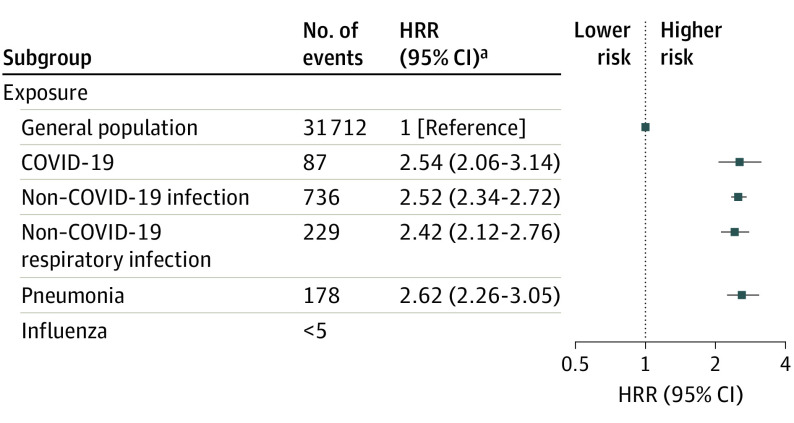
Compared with individuals without hospitalizations, the risk of new-onset mental disorders was similarly increased (all P < .001) among individuals hospitalized for COVID-19 (HRR, 2.54 [95% CI, 2.06-3.14]), any other non–COVID-19 infection (HRR, 2.52 [95% CI, 2.34-2.72]), any respiratory tract infection (HRR, 2.42 [95% CI, 2.12-2.76]), and pneumonia (HRR, 2.62 [95% CI, 2.26-3.05]) (Figure 4 and eTable 6 in Supplement 1). No significant difference was seen in the risk for specific mental disorders between hospitalized patients with COVID-19 and those hospitalized for non–COVID-19 respiratory tract infections (HRR, 1.03 [95% CI, 0.82-1.29]) (Figure 2 and eTable 6 in Supplement 1).
Figure 4. Risk of New-Onset Mental Disorders After Hospitalization for COVID-19 or Non–COVID-19 Infections.
Any incident mental disorder is defined as International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, codes F00 to F99. Due to Danish General Data Protection Regulation rules applied to the use of the nationwide registers, outcome results from groups with fewer than 5 individuals are prohibited to be displayed to ensure data privacy; thus, risk was not calculated in the group with influenza. Error bars indicate 95% CIs. Individuals with a prior hospitalization for a respiratory tract infection between 2010 and March 2020 were excluded prior to analysis to ensure that the hospital contact for other respiratory tract infections was new onset and not a recurrent condition. All analyses were adjusted for age, sex, parental history of mental illness, Charlson Comorbidity Index, educational level, income, and work status. HRR indicates hazard rate ratio.
aNo statistical difference between COVID-19 and all other non–COVID-19 infections.
Sensitivity Analysis
During the total study period, outcome events between those tested and not tested for SARS-CoV-2 (eFigure 6 in Supplement 1) and incidence rate ratios stratified in age groups between those with positive and negative test results (eFigure 7 in Supplement 1) were stable. We created a second dynamic population (starting with 0) only including those tested for SARS-CoV-2 while excluding those never tested (ending with 3 651 682). The findings were similar between SARS-CoV-2–positive vs SARS-CoV-2–negative individuals regarding primary and secondary outcomes as our original analysis, except for the risk of new-onset organic mental disorders (ICD-10 codes F00-F09), which was increased in those with positive test results (HRR, 1.21 [95% CI, 1.02-1.43]) (eTable 7 in Supplement 1). eTables 8 to 13 in Supplement 1 provide details on effect modification.
Discussion
This nationwide cohort study of all SARS-CoV-2 PCR tests performed in Denmark investigated the association of COVID-19 with the subsequent risk of mental disorders and use of psychotropic medication. Overall, individuals who were tested for SARS-CoV-2 had a higher risk of new-onset mental disorders compared with individuals who were never tested. However, when compared with those with negative test results, the risk of new-onset mental disorders was not further increased except among older individuals. Regarding psychotropic medication use in individuals with positive test results compared with those with negative test results for SARS-CoV-2, there was a slightly greater risk that increased with age and was highest among individuals 70 years and older. Patients hospitalized for COVID-19 had a substantially increased risk of new-onset mental disorders compared with the general population, but the risks were similarly increased as after hospitalization for non–COVID-19 infections.
Previous large-scale studies have been hampered by lack of nationwide coverage that is present in the Danish registers, which might explain some of the conflicting results, particularly in hospitalized patients, for whom some studies showed increased risk in patients with COVID-19 compared with influenza and other respiratory tract infections,9,10 while others,11 including our study, found that COVID-19 was not associated with further risk increase than observed after other respiratory tract infections. The databases used for study analysis might influence study outcomes, as both the US-based TriNetX database (TriNetX LLC)9 and the US Veterans Health Administration10 are based on electronic health records, and the UK-based Qresearch11 database links primary care data with hospital records, whereas our study uses the Danish registers with nationwide coverage of all PCR tests conducted, including all hospitalizations and prescriptions on a nationwide scale in a tax-funded universal health care system with extensive adjustments for potential confounders.
Differences in testing behavior and test availability between countries could result in different patients with COVID-19 captured in the respective databases. In the US, it was estimated that between February 2020 and September 2021, only 1 in 4 people with a SARS-CoV-2 infection were detected,25 while individuals with mild or no symptoms went undetected,26 as the incentive for being tested increases with increasing disease severity. Contrary to the US, from May 2021, all SARS-CoV-2 PCR testing in Denmark was free of charge and available for all,27 which removed economic barriers for lower socioeconomic groups, leading to a larger detection rate of mild cases (ensuring capture of almost all cases as those with positive test results on lateral flow antigen tests had to confirm this with a PCR test). Furthermore, Denmark introduced a nationwide “Corona passport” in May 2021 that gave access to social and educational activities if one could provide a negative SARS-CoV-2 test result. This could partly explain the lower risk in young (aged 18-29 years) individuals with positive test results as access to social life, which correlates with mental well-being,28 increases the risk of getting infected with SARS-CoV-2. Additionally, the increased risk in individuals with negative SARS-CoV-2 test results could to some extent be explained by an underlying non–COVID-19 respiratory tract infection that would motivate the individual to get a PCR test and concurrently increase the risk of a new-onset mental disorder.7,8 Nonetheless, a substantial proportion of our study cohort (12.07%) had no testing recorded. Our study and others12 show that these 2 groups (negative test results and never tested) are dissimilar regarding mental disorder outcomes and therefore a control group based on the absence of SARS-CoV-2 detection will result in a different control group than that based on the presence of a negative SARS-CoV-2 test result.
It seems that COVID-19 severity plays a crucial role in the subsequent risk of mental disorders. An isolated episode of SARS-CoV-2 infection without hospitalization had a small increase in risk compared with those not tested, those with multiple SARS-CoV-2 infections had a slightly higher risk, whereas hospitalization for COVID-19 was associated with a large risk increase and greatest among patients in the ICU, but similar to that for patients hospitalized for non–COVID-19 infections. This finding suggests that disease severity is associated with increased risk of mental health outcomes rather than the underlying microbiological agent and is not a specific effect that is only seen after a SARS-CoV-2 infection. Non–COVID-19 specific mechanisms behind this association of increased mental disorder risk in severe respiratory tract infections could include hyperinflammatory states6,7,8 seen in patients hospitalized with COVID-1929,30,31 and other respiratory tract infections,32 autoimmune reactions,33 neuropsychiatric symptoms associated with the post-ICU syndrome,2,34 and preexisting poorer health among hospitalized patients with COVID-19, which influence future risk of mental disorders. In contrast, viral neurotropism is unlikely to be an important mechanism due to its rarity.35
Strength and Limitations
The strengths of our study include that it is a nationwide study of a universally tax-funded health care system that allowed us to follow up all individuals with PCR testing for SARS-CoV-2, regardless of health care access or COVID-19 severity, and all inpatient and outpatient diagnoses of mental disorders adjusting for a comprehensive set of confounders. This greatly reduces selection bias regarding study exposure and vastly increases the validity of the outcome and the generalizability of our findings.
This study also has some limitations. Behavioral patterns in response to fear of COVID-19 and incentive to get a SARS-CoV-2 PCR test could affect the risk of exposure and outcome, factors that are not entirely captured in the registries (eg, fear of getting COVID-19 urges increased PCR testing driven by underlying anxiety that increases the risk of being diagnosed with an anxiety disorder). Furthermore, the outcome of psychotropic medication cannot be solely attributed to treatment for a mental disorder, as these drugs can also be used for treating insomnia or pain and in a palliative setting, and we only included mental disorder diagnoses categorized in the F-chapter of the ICD-10.
Conclusions
The findings of this cohort study suggest that there is an overall higher risk of incident mental disorders among individuals who are positive for SARS-CoV-2 compared with those never tested but lower compared with those with negative test results, although moderated by age, as the lower risk was observed among individuals aged 18 to 29 years while those 70 years or older had an increased risk. Furthermore, psychotropic medication use was slightly increased among individuals positive for SARS-CoV-2 compared with those with negative test results, especially among individuals 70 years or older. Additionally, the risk increased with increasing severity and was highest among critically ill hospitalized patients with COVID-19 compared with the general population, but similarly elevated among patients hospitalized for non–COVID-19 infections. Future studies should include even longer follow-up time and preferentially include immunological biomarkers to further investigate the impact of infection severity on potential long-term sequelae; furthermore, there is a clear need to disentangle the molecular brain mechanisms leading to neuropsychiatric symptoms after severe infections to improve the treatments and elucidate molecular underpinnings to mental disorders.
eTable 1. Diagnosis and Medication Codes Used for Study Exposures and Outcomes Based on the International Classification Diseases (ICD) Codes and Anatomical Therapeutic Chemical (ATC) Codes
eTable 2. Demographic Characteristics of Study Population
eTable 3. Temporal Effect of Time Since Positive SARS-CoV-2 Test on the Risk of New-Onset Mental Disorder
eTable 4. Risk of New-Onset Mental Disorder After Multiple SARS-CoV-2 Infections
eTable 5. Number of Admission Days During Hospitalization for COVID-19 and the Risk of New-Onset Mental Disorders
eTable 6. Risk of Any Mental Disorder in Patients Hospitalized for COVID-19 and Other Infections
eTable 7. Risk of Mental Disorders and Redeeming Psychotropic Medication in SARS-CoV-2–Positive Compared With –Negative Tested Individuals—Sensitivity Analysis
eTable 8. Summary of Findings on Effect Modification Analyses on Primary Outcome of Study
eTable 9. Effect of Immigration Status and Country of Origin on the Risk of New-Onset Mental Disorders
eTable 10. Effect of Parental History of Mental Illness on the Risk of New-Onset Mental Disorders
eTable 11. Effect of Charlson Comorbidity Index on the Risk of New-Onset Mental Disorders
eTable 12. Effect of Sex on the Risk of New-Onset Mental Disorders
eTable 13. Effect of Income, Education, and Working Status on the Risk of New-Onset Mental Disorders
eFigure 1. Cumulative Incidence (Risk) of Emigration, Death, and Primary Outcome Using the Aalen-Johansen Estimator to Account for Competing Risk for the Study Population
eFigure 2. Cumulative (A) and Daily Number (B) of SARS-CoV-2 PCR Tests Performed During the Study Period
eFigure 3. Flowchart Over Study Population for Primary Outcome
eFigure 4. Risk of New-Onset Mental Disorder After Multiple SARS-CoV-2 Infections
eFigure 5. Effect of Increasing Comorbidities and the Risk for New-Onset Mental Disorders
eFigure 6. Overview of Primary Outcome Events in Total Population and in Those Tested or not Tested for SARS-CoV-2
eFigure 7. Incidence Rate of Primary Outcome in Individuals not Tested or Tested Positive or Negative for SARS-CoV-2 During Study Observation Period Stratified in Age Groups
Data Sharing Statement
References
- 1.Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi: 10.1038/s41591-022-01909-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nersesjan V, Fonsmark L, Christensen RHB, et al. Neuropsychiatric and cognitive outcomes in patients 6 months after COVID-19 requiring hospitalization compared with matched control patients hospitalized for non–COVID-19 illness. JAMA Psychiatry. 2022;79(5):486-497. doi: 10.1001/jamapsychiatry.2022.0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nersesjan V, Amiri M, Lebech AM, et al. Central and peripheral nervous system complications of COVID-19: a prospective tertiary center cohort with 3-month follow-up. J Neurol. 2021;268(9):3086-3104. doi: 10.1007/s00415-020-10380-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badenoch JB, Rengasamy ER, Watson C, et al. Persistent neuropsychiatric symptoms after COVID-19: a systematic review and meta-analysis. Brain Commun. 2021;4(1):fcab297. doi: 10.1093/braincomms/fcab297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spudich S, Nath A. Nervous system consequences of COVID-19. Science. 2022;375(6578):267-269. doi: 10.1126/science.abm2052 [DOI] [PubMed] [Google Scholar]
- 6.Köhler-Forsberg O, Petersen L, Gasse C, et al. A Nationwide study in Denmark of the association between treated infections and the subsequent risk of treated mental disorders in children and adolescents. JAMA Psychiatry. 2019;76(3):271-279. doi: 10.1001/jamapsychiatry.2018.3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168(12):1303-1310. doi: 10.1176/appi.ajp.2011.11030516 [DOI] [PubMed] [Google Scholar]
- 8.Benros ME, Waltoft BL, Nordentoft M, et al. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70(8):812-820. doi: 10.1001/jamapsychiatry.2013.1111 [DOI] [PubMed] [Google Scholar]
- 9.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416-427. doi: 10.1016/S2215-0366(21)00084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with COVID-19: cohort study. BMJ. 2022;376:e068993. doi: 10.1136/bmj-2021-068993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clift AK, Ranger TA, Patone M, et al. Neuropsychiatric ramifications of severe COVID-19 and other severe acute respiratory infections. JAMA Psychiatry. 2022;79(7):690-698. doi: 10.1001/jamapsychiatry.2022.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abel KM, Carr MJ, Ashcroft DM, et al. Association of SARS-CoV-2 infection with psychological distress, psychotropic prescribing, fatigue, and sleep problems among UK primary care patients. JAMA Netw Open. 2021;4(11):e2134803. doi: 10.1001/jamanetworkopen.2021.34803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund LC, Hallas J, Nielsen H, et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis. 2021;21(10):1373-1382. doi: 10.1016/S1473-3099(21)00211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haut ER, Pronovost PJ. Surveillance bias in outcomes reporting. JAMA. 2011;305(23):2462-2463. doi: 10.1001/jama.2011.822 [DOI] [PubMed] [Google Scholar]
- 15.Frank L. Epidemiology. The epidemiologist’s dream: Denmark. Science. 2003;301(5630):163. doi: 10.1126/science.301.5630.163 [DOI] [PubMed] [Google Scholar]
- 16.Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB. The Danish Civil Registration System: a cohort of eight million persons. Dan Med Bull. 2006;53(4):441-449. [PubMed] [Google Scholar]
- 17.Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011;39(7)(suppl):54-57. doi: 10.1177/1403494810395825 [DOI] [PubMed] [Google Scholar]
- 18.Voldstedlund M, Haarh M, Mølbak K; MiBa Board of Representatives . The Danish Microbiology Database (MIBA) 2010 to 2013. Euro Surveill. 2014;19(1):20667. doi: 10.2807/1560-7917.ES2014.19.1.20667 [DOI] [PubMed] [Google Scholar]
- 19.Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register: a valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263-268. [PubMed] [Google Scholar]
- 20.Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7)(suppl):38-41. doi: 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 21.Petersson F, Baadsgaard M, Thygesen LC. Danish registers on personal labour market affiliation. Scand J Public Health. 2011;39(7)(suppl):95-98. doi: 10.1177/1403494811408483 [DOI] [PubMed] [Google Scholar]
- 22.Blichert-Hansen L, Nielsson MS, Nielsen RB, Christiansen CF, Nørgaard M. Validity of the coding for intensive care admission, mechanical ventilation, and acute dialysis in the Danish National Patient Registry: a short report. Clin Epidemiol. 2013;5:9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 24.Lund LC, Støvring H, Pottegård A, Andersen M, Hallas J. Cox regression using a calendar time scale was unbiased in simulations of COVID-19 vaccine effectiveness & safety. J Clin Epidemiol. Published online February 16, 2023. doi: 10.1016/j.jclinepi.2023.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention . Estimated COVID-19 burden. Updated August 12, 2022. Accessed December 22, 2022. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html
- 26.Wu SL, Mertens AN, Crider YS, et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun. 2020;11(1):4507. doi: 10.1038/s41467-020-18272-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pottegård A, Kristensen KB, Reilev M, et al. Existing data sources in clinical epidemiology: the Danish COVID-19 cohort. Clin Epidemiol. 2020;12:875-881. doi: 10.2147/CLEP.S257519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51(suppl):S54-S66. doi: 10.1177/0022146510383501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campochiaro C, Dagna L. The conundrum of interleukin-6 blockade in COVID-19. Lancet Rheumatol. 2020;2(10):e579-e580. doi: 10.1016/S2665-9913(20)30287-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gustine JN, Jones D. Immunopathology of Hyperinflammation in COVID-19. Am J Pathol. 2021;191(1):4-17. doi: 10.1016/j.ajpath.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryabkova VA, Churilov LP, Shoenfeld Y. Influenza infection, SARS, MERS and COVID-19: cytokine storm: the common denominator and the lessons to be learned. Clin Immunol. 2021;223:108652. doi: 10.1016/j.clim.2020.108652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartley CM, Johns C, Ngo TT, et al. Anti–SARS-CoV-2 and autoantibody profiles in the cerebrospinal fluid of 3 teenaged patients with COVID-19 and subacute neuropsychiatric symptoms. JAMA Neurol. 2021;78(12):1503-1509. doi: 10.1001/jamaneurol.2021.3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith S, Rahman O. Post Intensive Care Syndrome. StatPearls; 2022. [Google Scholar]
- 35.Lewis A, Frontera J, Placantonakis DG, et al. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J Neurol Sci. 2021;421:117316. doi: 10.1016/j.jns.2021.117316 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Diagnosis and Medication Codes Used for Study Exposures and Outcomes Based on the International Classification Diseases (ICD) Codes and Anatomical Therapeutic Chemical (ATC) Codes
eTable 2. Demographic Characteristics of Study Population
eTable 3. Temporal Effect of Time Since Positive SARS-CoV-2 Test on the Risk of New-Onset Mental Disorder
eTable 4. Risk of New-Onset Mental Disorder After Multiple SARS-CoV-2 Infections
eTable 5. Number of Admission Days During Hospitalization for COVID-19 and the Risk of New-Onset Mental Disorders
eTable 6. Risk of Any Mental Disorder in Patients Hospitalized for COVID-19 and Other Infections
eTable 7. Risk of Mental Disorders and Redeeming Psychotropic Medication in SARS-CoV-2–Positive Compared With –Negative Tested Individuals—Sensitivity Analysis
eTable 8. Summary of Findings on Effect Modification Analyses on Primary Outcome of Study
eTable 9. Effect of Immigration Status and Country of Origin on the Risk of New-Onset Mental Disorders
eTable 10. Effect of Parental History of Mental Illness on the Risk of New-Onset Mental Disorders
eTable 11. Effect of Charlson Comorbidity Index on the Risk of New-Onset Mental Disorders
eTable 12. Effect of Sex on the Risk of New-Onset Mental Disorders
eTable 13. Effect of Income, Education, and Working Status on the Risk of New-Onset Mental Disorders
eFigure 1. Cumulative Incidence (Risk) of Emigration, Death, and Primary Outcome Using the Aalen-Johansen Estimator to Account for Competing Risk for the Study Population
eFigure 2. Cumulative (A) and Daily Number (B) of SARS-CoV-2 PCR Tests Performed During the Study Period
eFigure 3. Flowchart Over Study Population for Primary Outcome
eFigure 4. Risk of New-Onset Mental Disorder After Multiple SARS-CoV-2 Infections
eFigure 5. Effect of Increasing Comorbidities and the Risk for New-Onset Mental Disorders
eFigure 6. Overview of Primary Outcome Events in Total Population and in Those Tested or not Tested for SARS-CoV-2
eFigure 7. Incidence Rate of Primary Outcome in Individuals not Tested or Tested Positive or Negative for SARS-CoV-2 During Study Observation Period Stratified in Age Groups
Data Sharing Statement