Abstract
Cerebrovascular lesions are prevalent in late life and frequently co-occur but the relationship to cognitive impairment is complicated by the lack of consensus around which lesions represent hallmark pathologies for vascular impairment, particularly in the presence of Alzheimer’s disease (AD). We developed an easily applicable model of cerebrovascular disease (CVD), defined as the presence of two or more lesions: moderate to severe cerebral amyloid angiopathy, moderate to severe arteriolosclerosis, infarcts (large, lacunar, or micro), and/or hemorrhages. AD was defined as intermediate or high AD neuropathologic change. The contribution of vascular risk factors such as atherosclerosis and/or a health history of heart disease, hyperlipidemia, stroke events, diabetes, or hypertension was also assessed. Logistic regression analysis reported the association of CVD with increasing age, vascular risk factors, AD, and cognitive impairment in this study of 1,485 autopsied individuals. Cerebrovascular lesions were present in 48% and 16% had CVD. Increasing age associated with all lesions (p<0.001), except hemorrhages (p=0.41). CVD was more likely in individuals with vascular risk factors or AD (p<0.01). CVD, but not individual cerebrovascular lesions, associated with impairment in cases without AD (p<0.01), but not in cases with AD (p>0.61). From this, we conclude that a simple, additive model of CVD is 1) age and AD-associated, 2) is associated with vascular risk factors, and 3) clinically correlates with cognitive decline independent of AD.
Keywords: Cerebrovascular disease, Alzheimer’s disease, Infarcts, Amyloid angiopathy, Arteriolosclerosis, Vascular risk factors, Ageing
Introduction
Alzheimer’s disease (AD) and cerebrovascular disease (CVD) are the two most common causes of late-life dementia [1]. AD is best described as the widespread distribution of β-amyloid deposits, tau-positive neurofibrillary tangles, and cortical neuritic plaques as primary pathologies along with the cerebral amyloid angiopathy (CAA), Lewy bodies, and TDP-43 inclusions also prevalent as secondary pathologies [2],[3]. CVD is also considered the accumulation of multiple pathologies including infarcts (large, lacunar, and micro), arteriolosclerosis, perivascular space dilation, perivascular hemosiderin leakage, myelin loss, CAA (leptomeningeal, parenchymal and capillary), hemorrhages (micro and larger), fibrinoid necrosis, and microaneurysms [4]. Unfortunately, while consensus criteria exists to determine the level of AD neuropathologic change by the examination of three hallmark pathologies, it is not clear which cerebrovascular lesions relate to impairment and which accumulate due to conditions such as cardiac atherosclerosis, monogenic stroke disorders, embolic disease, vasculopathies, haematological disorders, or metabolic disorders or simply ‘brain ageing’ [4],[5],[6],[7].
To address this, the VCING study reported seven pathologies that reproducibly correlate with cognitive impairment to varying degrees in a study of 113 individuals (55 to 100 years) including leptomeningeal CAA, large infarcts, lacunar infarcts, microinfarcts, arteriolosclerosis, perivascular space dilation and myelin loss [8]. A simplified model was proposed highlighting just three cerebrovascular lesions: large infarcts, moderate/severe occipital leptomeningeal CAA, and moderate/severe arteriolosclerosis in the occipital white matter.
Inspired by this attempt at consensus, we screened for three lesions in the occipital cortex – moderate to severe leptomeningeal CAA, moderate to severe white matter arteriolosclerosis, and microinfarcts – in a cohort of 1,485 individuals (31 to 103 years old). We then compiled available autopsy data for the presence of moderate to severe CAA in other cortical areas, global infarcts (large, lacunar and micro), and large hemorrhages. We describe an additive model of CVD, defined as a presence of multiple cerebrovascular lesions. The goal of this study is to test the hypothesis that our easily replicable, histological definition of CVD associates with increasing age, with cognitive impairment and/or dementia, and with vascular risk factors such as atherosclerosis and/or a health history of heart disease, hyperlipidemia, stroke events, diabetes, or hypertension.
Materials and Methods
Cohort
The cohort consisted of individuals with known ages at death of ≥30 years, and available cerebrovascular and AD pathology measures who participated in the autopsy program at the Center for Neurodegenerative Disease Research (CNDR) at the University of Pennsylvania. Of 1,785 individuals age ≥30 at time of death, AD pathology measures were known for 1,659. As of December 2020, cerebrovascular measures were assessed in 1,512 of these individuals. 7 were excluded for having rare clinical diagnoses and 20 were excluded for rare neuropathological diagnoses, resulting in a cohort of 1,485 individuals. Clinical diagnoses included no impairment (NI) for individuals without a neurodegenerative disease, AD (Probable and Possible), amyotrophic lateral sclerosis (Suspected, Possible, Probable, and Definite), corticobasal syndrome, cognitive impairment (including amnestic and non-amnestic mild cognitive impairment), frontotemporal degeneration (including behavioral variant, primary progressive aphasias, and not otherwise specified), multiple system atrophy, Parkinson’s disease (with and without cognitive impairment), Parkinson’s disease dementia or dementia with Lewy bodies, progressive supranuclear palsy, schizophrenia, and vascular dementia. The frequency of each is listed in Table 1. Neuropathologically, the cohort includes neuropathologic AD (intermediate or high level of AD neuropathologic change), low AD (low level of AD neuropathologic change), not AD (including primary age related tauopathy and unremarkable cases), amyotrophic lateral sclerosis, corticobasal degeneration, frontotemporal lobar degeneration, Lewy body disease, Pick disease, and progressive supranuclear palsy, with the frequency of each is listed in Table 2.
Table 1. Frequency of cases by primary clinical diagnosis.
1 defined as intermediate or high AD neuropathologic change
2 includes mild cognitive impairment (MCI), amnestic MCI, and cognitively impaired (not MCI)
3 includes Probable and Possible AD
4 includes suspected, possible, probable, and definite ALS
5 includes FTLD-bvFTD, FTLD-PPA (sementia dementia), FTLD-PPA (PNFA), FTLD-PPA (logopenic), and FTLD-FTD (NOS)
6 includes PD with MCI
7 includes PD with dementia, DLB, and DLB with PD
| Clinical Diagnosis | n | % | Female | Age at onset mean (y) | Late onset | Age at death mean (y) | Cognitive Impairment or dementia | npAD1 | CVD (single) | CVD (multiple) |
|---|---|---|---|---|---|---|---|---|---|---|
| All | 1485 | 100% | 44% | 64.9 | 51% | 74.5 | 66% | 48% | 32% | 16% |
| Not impaired | 129 | 9% | 51% | - | - | 73.5 | 0% | 16% | 27% | 14% |
| Cognitive impairment2 | 30 | 2% | 47% | 76.5 | 79% | 82.6 | 100% | 80% | 37% | 27% |
| AD3 | 430 | 29% | 54% | 68.7 | 68% | 78.2 | 100% | 94% | 41% | 24% |
| Vascular dementia | 25 | 2% | 48% | 75.3 | 96% | 83.4 | 100% | 64% | 28% | 44% |
| ALS4 | 149 | 10% | 39% | 59.0 | 27% | 64.0 | 21% | 11% | 21% | 1% |
| Frontotemporal disease5 | 178 | 12% | 45% | 61.4 | 35% | 70.5 | 100% | 40% | 29% | 10% |
| MSA | 42 | 3% | 36% | 57.1 | 18% | 66.2 | 5% | 12% | 26% | 7% |
| PD6 | 136 | 9% | 26% | 63.6 | 47% | 77.0 | 35% | 18% | 32% | 13% |
| PDD/DLB7 | 185 | 12% | 24% | 63.7 | 44% | 76.8 | 100% | 44% | 34% | 15% |
| CBS | 72 | 5% | 56% | 64.7 | 47% | 71.6 | 26% | 46% | 35% | 21% |
| PSP | 79 | 5% | 38% | 66.5 | 57% | 72.7 | 6% | 13% | 29% | 9% |
| Schizophrenia | 30 | 2% | 73% | - | - | 83.1 | 100% | 3% | 20% | 20% |
| Logistic regression association with CVD | ||||||||||
| Predictor | OR | 95% CI | p-value | |||||||
| Age at Death | 1.20 | (1.14, 1.27) | <0.001 | |||||||
| Cognitive impairment2 | 1.77 | (0.76, 4.12) | 0.18 | |||||||
| AD3 | 2.24 | (1.50, 3.36) | <0.001 | |||||||
| Vascular dementia | 2.54 | (0.98, 6.56) | 0.06 | |||||||
| ALS4 | 0.54 | (0.32, 0.92) | 0.02 | |||||||
| Frontotemporal disease5 | 1.00 | (0.63, 1.59) | 0.99 | |||||||
| MSA | 0.90 | (0.43, 1.89) | 0.78 | |||||||
| PD6 | 0.96 | (0.59, 1.58) | 0.88 | |||||||
| PDD/DLB7 | 1.19 | (0.75, 1.86) | 0.48 | |||||||
| CBS | 1.90 | (1.05, 3.41) | 0.03 | |||||||
| PSP | 0.88 | (0.49, 1.56) | 0.65 | |||||||
| Schizophrenia | 0.65 | (0.29, 1.48) | 0.31 |
Table 2. Frequency of cases by primary neuropathological diagnosis.
1 includes unremarkable, PART and AGD
2 defined as intermediate or high AD neuropathologic change
3 includes ALS with cognitive decline or dementia
| Neuropathological Diagnosis | n | % | Female | Age at onset mean(y) | Late onset | Age at death mean(y) | Cognitive impairment or dementia | npAD2 | CVD (single) | CVD (multiple) |
|---|---|---|---|---|---|---|---|---|---|---|
| All | 1485 | 100% | 44% | 64.9 | 51% | 74.5 | 66% | 48% | 32% | 16% |
| not AD1 | 53 | 4% | 42% | 69.6 | 26% | 71.5 | 16% | 0% | 25% | 15% |
| low AD | 122 | 8% | 51% | 74.5 | 56% | 76.8 | 25% | 0% | 23% | 20% |
| AD2 | 569 | 38% | 55% | 67.8 | 64% | 77.6 | 94% | 100% | 43% | 24% |
| ALS3 | 130 | 9% | 40% | 58.8 | 26% | 63.8 | 13% | 11% | 21% | 0% |
| FTLD-TDP | 108 | 7% | 44% | 61.3 | 32% | 69.0 | 93% | 9% | 18% | 6% |
| LBD | 282 | 19% | 23% | 63.8 | 46% | 77.3 | 75% | 33% | 32% | 15% |
| MSA | 47 | 3% | 38% | 57.8 | 24% | 65.7 | 4% | 6% | 32% | 9% |
| CBD | 41 | 3% | 54% | 63.5 | 49% | 68.3 | 39% | 7% | 29% | 5% |
| Pick | 24 | 2% | 38% | 57.7 | 21% | 67.7 | 92% | 0% | 29% | 4% |
| PSP | 109 | 7% | 36% | 68.1 | 64% | 75.6 | 26% | 16% | 26% | 11% |
| Logistic regression association with CVD | ||||||||||
| Predictor | OR | 95% CI | p-value | |||||||
| Age at Death | 1.19 | (1.13, 1.26) | <0.001 | |||||||
| low AD | 0.98 | (0.50, 1.91) | 0.94 | |||||||
| AD | 2.59 | (1.44, 4.67) | <0.01 | |||||||
| ALS | 0.51 | (0.25, 1.04) | 0.06 | |||||||
| FTLD-TDP | 0.52 | (0.25, 1.06) | 0.07 | |||||||
| LBD | 1.09 | (0.59, 2.01) | 0.78 | |||||||
| MSA | 1.28 | (0.57, 2.89) | 0.55 | |||||||
| CBD | 0.88 | (0.37, 2.09) | 0.78 | |||||||
| Pick | 0.86 | (0.31, 2.41) | 0.78 | |||||||
| PSP | 0.77 | (0.39, 1.53) | 0.46 |
Since our brain bank consists of such a diverse population of neurodegenerative diseases, two subgroups were defined to assess the clinical impact of AD and cerebrovascular pathology (Table 3). The AD spectrum subgroup (n=743) is comprised of cases with underlying AD neuropathology (from not AD to high AD) as the primary neuropathological diagnosis regardless of clinical symptoms. The NI to AD subgroup (n=566) is comprised of cases in the AD spectrum subgroup but excludes clinical frontotemporal syndromes and other atypical AD presentations. Informed consent for autopsy was obtained in accordance with state laws and protocols approved by the University of Pennsylvania.
Table 3. Cohort description.
1 all cases with a primary neuropathologic diagnosis of Not AD or low, intermediate or high AD neuropathologic change.
2 all AD spectrum cases excluding atypical presentations such as frontotemporal syndromes.
3 onset of clinical symptoms ≥65 years.
4 moderate to severe atherosclerosis, available for n=1,480.
5 available for n=867.
6 includes aortic stenosis, atrial fibrillation, cardiac arrhythmia, coronary artery disease, and cardiac murmur; available for n=867.
7 available for n=846.
8 includes hyperlipidemia and hypercholesterolemia; available for n=577.
9 includes transient ischemic attack; available for n=845.
10 includes probable AD, possible AD, dementia not otherwise specified, and cognitive impairment.
| Cohort | All | AD spectrum1 | NI to AD2 |
|---|---|---|---|
| n | 1485 | 743 | 566 |
| Age of onset, years (SD) | 64.9 (10.9) | 68.1 (10.3) | 69.1 (10.0) |
| Late onset3 | 51% | 60% | 63% |
| Age at death, years (SD) | 74.5 (11.0) | 77.0 (10.7) | 77.3 (10.8) |
| Female | 44% | 53% | 54% |
| APOE ε4 | 40% | 54% | 55% |
| No impairment | 9% | 18% | 24% |
| Cognitive impairment/dementia | 66% | 78% | 76% |
| Vascular risk factors | |||
| Atherosclerosis4 | 34% | 40% | 39% |
| Diabetes5 | 8% | 10% | 9% |
| Heart6 | 18% | 27% | 28% |
| Hypertension7 | 36% | 43% | 48% |
| Lipid8 | 38% | 42% | 43% |
| Stroke9 | 5% | 10% | 8% |
| Any risk factor | 64% | 73% | 77% |
| Multiple risk factors | 35% | 46% | 50% |
| CVD | |||
| None | 48% | 60% | 61% |
| Single | 32% | 38% | 38% |
| Multiple | 16% | 22% | 23% |
| Neuropathologic AD | |||
| All | 48% | 76% | 78% |
| AD impairment/dementia10 | 28% | 56% | 74% |
| No impairment | 1% | 3% | 4% |
| Atypical | 8% | 17% | 0% |
| Other disease | 10% | 0% | 0% |
Vascular risk factors
Vascular risk factors included atherosclerosis and/or a history of heart disease, hyperlipidemia, stroke events, diabetes, or hypertension, extracted from health records spanning three decades. Heart disease (n=867) included diagnoses of atrial fibrillation, aortic stenosis, cardiac arrhythmia, coronary artery disease, and/or cardiac murmur. Stroke events (n=845) included diagnoses of transient ischemic attacks and/or cerebrovascular accidents. Hyperlipidemia (n=577) included diagnoses of hyperlipidemia or hypercholesterolemia. Diabetes (n=867) and hypertension (n=846) status were also obtained. Atherosclerosis was defined as moderate to severe atherosclerosis observable in the circle of Willis at autopsy and was available for the majority of cases (n=1,480). 846 cases had enough health history data to define them as having any single risk factor or multiple risk factors.
Pathology
Sixteen brain regions are routinely examined in the CNDR neuropathology evaluations [9]. Each region was assigned a semi-quantitative score, i.e. none, rare, mild, moderate, or severe for neurofibrillary tangles based on immunohistochemistry against tau (mouse antibody PHF1, a gift from Dr. Peter Davies), or for plaques and CAA based on immunohistochemistry against amyloid-β (mouse antibody NAB228, generated in CNDR). Furthermore, arteriolosclerosis scores were determined based on hematoxylin and eosin histology. Moderate to severe CAA and arteriolosclerosis was assessed as per Deramecourt et al [10]. All cases were reviewed by a board-certified neuropathologist (JQT and/or EBL) for quality assurance and accurate grading. The level of AD neuropathology was assessed by the amount of tau-positive neurofibrillary tangles, tau-positive neuritic plaques, and amyloid-β positive plaques according to consensus criteria [2]. Neuropathologic AD was defined as an intermediate or high level of AD neuropathologic change.
Cerebrovascular lesions were assessed by examination of histological and immunohistochemically stained slides and compiled from post-autopsy gross and microscopic reports and as described below. Data on large infarcts were collected from reports detailing infarcts ≥1 cm in diameter visible at autopsy. Lacunar infarcts were also determined from records that reported grossly visible infarcts <1 cm in diameter or that were described as lacunar infarcts at time of autopsy. Microinfarcts were defined as infarcts <0.5cm in diameter that were not observed grossly and were collected from microscopic reports. In addition, the occipital hematoxylin and eosin slides were examined for all cases for the presence of microinfarcts. Hemorrhages refers to large hemorrhages described grossly. Arteriosclerosis in the occipital white matter was assessed for all cases. CAA severity was primarily assessed in the occipital cortex and was reported as a maximum of scores from three additional neocortical regions if available: middle frontal cortex, superior temporal cortex, and angular gyrus. Four neocortical CAA scores were available for the majority of cases (n=1,258).
Statistical analysis
R 3.6.2 was used for all regression analyses. Univariate analysis was performed to quantify the association between variables. Logistic regression analysis was used to build a model of dementia to assess each measure’s contribution. Odds ratios and effect sizes for age were assessed for each 5-year increase with confidence intervals reported at 95%. Likelihood ratio tests for nested models were used to assess the effect of variables with multiple categories, such as clinical diagnosis. We investigated the effect of missing vascular risk factor data through inverse probability weighting [11]. This procedure aims to correct potential bias incurred by considering only subjects with available risk factor data. Inverse probability weighting results were very similar to those using available data, illustrating the robustness of results. As such, complete case analyses are presented. Unadjusted analysis of cases with cognitive impairment or dementia, and with no CVD, with a single cerebrovascular lesion and with CVD, between participants with and without dementia was performed using Pearson’s χ2 tests using two degrees of freedom. All statistical tests were two-sided with statistical significance set at <0.05 level.
Results
Full cohort demographics
Demographic and neuropathological data was available for 1,485 cases (Table 3). NI individuals, comprising 9% of the cohort, were without a history of clinical neurodegenerative disease. Of the remaining individuals, 51% were clinically late-onset cases with an average age of onset of 64.9 years and an average age of death of 74.5 years. 66% had cognitive impairment or dementia including AD, frontotemporal syndrome, and Parkinson’s disease dementia or Dementia with Lewy bodies (see Table 1 for the breakdown by primary clinical diagnosis). Pathologically, over ten primary neuropathological diagnoses were represented, with AD and Lewy body disease representing the most frequent underlying neurodegenerative diseases (see Table 2 for the breakdown by primary neurodegenerative disease). Vascular risk factors – such as the presence of moderate to severe atherosclerosis or a health history that included diabetes, heart problems, hypertension, high levels of lipids, or stroke events – were present in 64% of individuals. Atherosclerosis, hypertension, and high lipids were the most common risk factors. These risk factors frequently co-occurred. Individually, diabetes, heart disease, and stroke had a >90% co-occurrence with other vascular risk factors while atherosclerosis, hypertension, and high lipids had a >60% co-occurrence with other vascular factors. Overall, multiple risk factors were present in 35% of individuals. Neuropathologic AD, defined as the presence of intermediate or high AD neuropathologic change, occurred in 48% of all cases, representing 28% with AD dementia, 8% with atypical AD clinical phenotypes such as frontotemporal degeneration, 10% of cases as a co-pathology, and 1% of NI cases.
Cerebrovascular lesions
The presence and severity of cerebrovascular lesions were histologically assessed (Figure 1, Table 4). The most common lesions affected blood vessels including the presence of moderate to severe CAA or arteriolosclerosis which affected 29% and 18% of the overall cohort, respectively. Infarcts and hemorrhages were less common, but when present, the majority occurred when concurrent cerebrovascular lesions were described. Large infarcts, for instance, were noted in only 5% of all cases, but 86% of cases with large infarcts had other cerebrovascular lesions. Similarly, the majority of cerebrovascular lesions co-occurred 52-86% of the time, with only CAA occurring with other lesions in a minority of cases 38% of the time. Given the preponderance of concurrent cerebrovascular lesions, CVD was defined when more than one lesion was present. Overall, cerebrovascular lesions were observed in 48% (n=720) of cases, including 32% (n=482) with a single lesion and 16% (n=238) with multiple lesions.
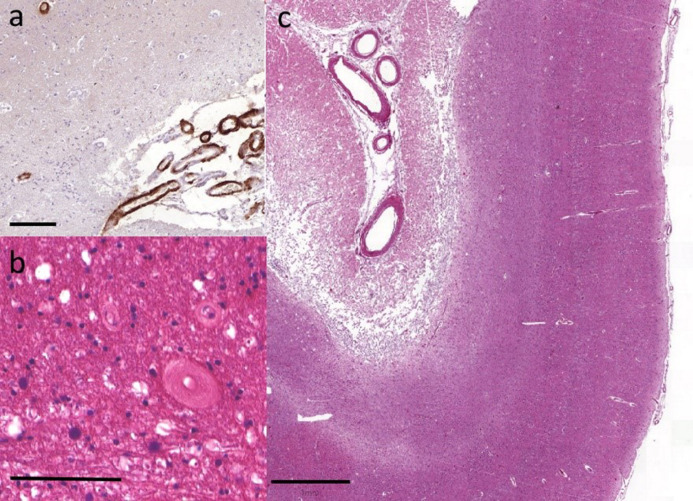
Figure 1. Representative cerebrovascular lesions.
Moderate to severe (a) leptomeningeal CAA was frequently observed in the occipital cortex. (b) Severe white matter arteriolosclerosis was not uncommon particularly in the occipital white matter adjacent to the ventricle. (c) Infarcts were observed in all subcortical and cortical regions, including this large infarct affecting occipital cortex. Scale bar is 100um in (a) and (b) and 1mm in (c).
Table 4. Cerebrovascular lesion frequency.
1 Proportion of lesion-positive cases with each lesion.
| Overall | Single | Multiple | |
| Cerebrovascular lesion | n (%) | n (%)1 | n (%)1 |
| Large infarct | 73 (5) | 10 (14) | 63 (86) |
| Lacunar infarct | 86 (6) | 22 (26) | 64 (74) |
| Microinfarct | 108 (7) | 42 (39) | 66 (61) |
| Hemorrhage | 36 (2) | 17 (47) | 19 (53) |
| Moderate/severe arteriolosclerosis | 261 (18) | 126 (48) | 135 (52) |
| Moderate/severe CAA | 427 (29) | 266 (62) | 161 (38) |
| Any lesion | 720 (48) | 482 (67) | 238 (33) |
| None | 765 (52) |
We next assessed, which, if any, neuropathological or clinical groups had elevated frequencies of CVD. As age at death significantly associated with the presence of either single or multiple cerebrovascular lesions (OR 1.27, 1.21-1.34, p<0.001), we adjusted for age in our analysis. Neuropathologically, the cases with a primary diagnosis of amyotrophic lateral sclerosis, corticobasal degeneration, frontotemporal lobar degeneration, Lewy body disease, Pick disease, and progressive supranuclear palsy, low AD , and neuropathologic AD were compared to the cases with ‘not AD’ as their primary diagnosis. Only neuropathologic AD had an increased prevalence of CVD (OR 2.59, 1.44-4.67, p<0.01). Other neurodegenerative diseases were not more likely to have CVD (Table 2). Similarly, clinical AD dementia cases were twice as likely as NI cases to have either single or multiple cerebrovascular lesions (OR 2.24, 1.50 - 3.36, p<0.001). Other clinical groups were not more likely to have CVD (Table 1) except for corticobasal syndrome cases (OR 1.90, 1.05-3.41, p=0.03). Since many of the corticobasal syndrome cases were neuropathologically diagnosed as AD, to further assess the impact of clinical diagnoses, we performed a likelihood ratio test comparing the fit of a model including both age and neuropathologic AD to a model including age, neuropathologic AD and clinical diagnoses. In this analysis, the model including clinical diagnoses did not improve the model fit (p=0.33). Thus, it appears that only cases with neuropathologic AD had an increased likelihood for CVD.
Age and AD associations with CVD
Since an increased incidence of CVD only occurred with neuropathologic AD and the level of AD neuropathologic change was assessed in all cases, we next fit logistic regression models using neuropathologic AD and age as predictors for each cerebrovascular lesion (Table 5). In this analysis, increasing age associated with an increasing likelihood of each lesion except hemorrhage (p<0.001) and neuropathologic AD associated with an increasing likelihood of the presence of CAA (p<0.001) but not the other cerebrovascular lesions (p>0.07). Overall, single cerebrovascular lesions were not age associated (p=0.09), but were neuropathologic AD-associated (p<0.001), while CVD associated with both increasing age and neuropathologic AD (p<0.001). We conclude that infarcts and arteriolosclerosis are more likely with increasing age, while CAA is more likely when AD pathology is present, and CVD is associated with both increasing age and neuropathologic AD.
Table 5. Age and AD associations with CVD .
(No legend)
| Age | Neuropathologic AD | ||||||
|---|---|---|---|---|---|---|---|
| Lesion | Effect | 95% CI | p-value | Effect | 95% CI | p-value | |
| Infarct, large | 1.25 | (1.11, 1.41) | <0.001 | 0.98 | (0.60, 1.60) | 0.94 | |
| Infarct, lacunar | 1.26 | (1.13, 1.42) | <0.001 | 0.76 | (0.48, 1.20) | 0.24 | |
| Infarct, micro | 1.20 | (1.08, 1.33) | <0.001 | 1.28 | (0.85, 1.94) | 0.24 | |
| Hemorrhage | 0.94 | (0.80, 1.09) | 0.41 | 1.06 | (0.53, 2.13) | 0.86 | |
| Moderate/severe arteriolosclerosis | 1.21 | (1.13, 1.29) | <0.001 | 1.29 | (0.98, 1.72) | 0.07 | |
| Moderate/severe CAA | 1.12 | (1.05, 1.19) | <0.001 | 7.42 | (5.61, 9.82) | <0.001 | |
| Any single lesion | 1.05 | (0.99, 1.11) | 0.09 | 2.29 | (1.82, 2.89) | <0.001 | |
| CVD (multiple) | 1.29 | (1.19, 1.39) | <0.001 | 2.25 | (1.65, 3.06) | <0.001 |
Vascular risk factors and CVD
Vascular risk factor information was available for a majority of the cohort (57%; n=846). Unsurprisingly, the presence of any or multiple vascular factors increased with age (any risk factor = OR 1.48, 1.37-1.59, p<0.01; multiple risk factors = OR 1.32, 1.23-1.42, p<0.01). To better understand the role of vascular risk factors in the development of cerebrovascular pathology, we next performed logistic regression analysis incorporating vascular risk factors, age and AD in a model of CVD (Table 6). When any vascular risk factors are present, there is an association with CVD (p<0.001) but not single cerebrovascular lesions (p=0.37). Vascular risk factors had the strongest association with the presence of infarcts (p<0.001), and also associated with arteriolosclerosis (p<0.01), but did not associate with CAA (p=0.55). In these analyses, age consistently associated with all cerebrovascular lesions (p<0.01), while neuropathologic AD strongly associated with CAA (p<0.001), arteriolosclerosis (p=0.001), but not infarcts (p=0.23). In all cases, multiple risk factors were not significantly more likely to associate with cerebrovascular lesions in the presence of any risk factor.
Table 6. Vascular risk factor associations with CVD.
(No legend)
| Risk factor | Age | Neuropathologic AD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cerebrovascular lesion | Vascular risk factors | OR | p-value | OR | p-value | OR | p-value | |||
| Infarcts, any | Single | 3.31 | <0.001 | 1.20 | 0.001 | 0.78 | 0.23 | |||
| Multiple | 2.25 | <0.001 | 1.23 | <0.001 | 0.77 | 0.21 | ||||
| Moderate/severe arteriosclerosis | Single | 3.02 | <0.01 | 1.28 | <0.01 | 3.64 | 0.001 | |||
| Multiple | 1.89 | 0.05 | 1.33 | <0.001 | 2.07 | 0.01 | ||||
| Moderate/severe CAA | Single | 0.90 | 0.56 | 1.43 | <0.001 | 8.63 | <0.001 | |||
| Multiple | 1.10 | 0.57 | 1.40 | <0.001 | 8.46 | <0.001 | ||||
| Any single lesion | Single | 0.86 | 0.37 | 1.17 | <0.01 | 2.56 | <0.001 | |||
| Multiple | 0.87 | 0.38 | 1.16 | <0.01 | 2.57 | <0.001 | ||||
| CVD | Single | 2.33 | 0.001 | 1.55 | <0.001 | 2.81 | <0.001 | |||
| Multiple | 2.01 | 0.001 | 1.57 | <0.001 | 2.75 | <0.001 |
AD spectrum and NI to AD demographics
Because cognitive impairment or dementia due to causes other than neuropathologic AD was common in our cohort, we hypothesized that the clinical impact of CVD would only be apparent in cases with AD or cerebrovascular pathology as their primary pathology. These cases are the AD spectrum and NI to AD subgroups (Table 3). The AD spectrum subgroup includes approximately half of the full cohort (n=743) including 56% with AD dementia, 17% with atypical dementias and 3% with neuropathologic AD in the absence of cognitive impairment. The NI to AD subgroup (n=566) is the AD spectrum subgroup excluding the atypical AD cases and includes 74% with AD dementia. The AD spectrum and NI to AD groups shared similar demographics. Both were older at onset (68.1 and 69.1 respectively) and at death (77.0 and 77.3 respectively) compared to the overall cohort. More women were present in the AD spectrum and NI to AD groups (53% and 54% compared to 44%). CVD was elevated in these groups. CVD was present at 22% and 23% respectively compared to 16% for the overall cohort. The presence of one or more risk factors was also higher in the AD spectrum and NI to AD groups. Any vascular risk factor was present at 73% and 77% respectively compared to 64% for the full cohort, while multiple risk factors were present in 46% and 50% respectively compared to 35% for the overall cohort.
Clinical relevance of CVD
We next asked if the presence of CVD associated with cognitive impairment in the AD spectrum and NI to AD subgroups (Table 7). In the full cohort, CVD did not correlate with a higher incidence of impairment in cases with (p=0.13) or without (p=0.77) neuropathologic AD. In the AD spectrum subgroup, neuropathologic AD cases with CVD did not differ by their incidence of impairment (p=0.90) compared to neuropathologic AD cases without CVD. However, cases without neuropathologic AD but with CVD were substantially more likely to have impairment than cases without neuropathologic AD or CVD (p<0.001). In the absence of neuropathologic AD, the incidence of impairment was 56% if definite CVD was present, but only 19% if limited CVD was present, and 12% if CVD was absent. A similar result was observed in the NI to AD cohort. Neuropathologic AD cases, with and without CVD, did not differ by their incidence of impairment (p=0.61), but cases without neuropathologic AD, but with CVD, were substantially more likely to have impairment than cases without CVD (p<0.01). In the NI to AD cohort, in the absence of neuropathologic AD, the incidence of impairment was 30% if CVD was present, but only 6% if a single cerebrovascular lesion was present, and 5% if no cerebrovascular lesions were observed.
Table 7. Association of CVD by level of npAD with cognitive impairment.
(No legend)
| Cohort | CVD | n | Cognitive impairment or dementia | χ² | p-value |
|---|---|---|---|---|---|
| Full cohort | |||||
| Not/low AD | None | 522 | 42% | 4.02 | 0.13 |
| Single | 181 | 44% | |||
| Multiple | 73 | 55% | |||
| Neuropathologic AD | None | 243 | 88% | 0.54 | 0.77 |
| Single | 301 | 89% | |||
| Multiple | 165 | 90% | |||
| AD Spectrum | |||||
| Not/low AD | None | 101 | 12% | 30.23 | <0.001 |
| Single | 41 | 19% | |||
| Multiple | 33 | 56% | |||
| Neuropathologic AD | None | 187 | 95% | 0.98 | 0.61 |
| Single | 244 | 94% | |||
| Multiple | 137 | 92% | |||
| NI to AD | |||||
| Not/low AD | None | 75 | 5% | 11.60 | <0.01 |
| Single | 31 | 6% | |||
| Multiple | 20 | 30% | |||
| Neuropathologic AD | None | 148 | 95% | 0.22 | 0.90 |
Discussion
That cerebrovascular disease is the second most common cause of dementia after Alzheimer’s disease is well known [17], but the term “cerebrovascular disease” has various meanings depending on the clinical, neuroimaging or neuropathological context. Even within the field of neuropathology, dozens of lesions have been reported, many of which are dependent on laboratory-specific sampling and histological techniques [4],[6]. Furthermore, while understanding of individual lesions has improved [18],[12],[19],[20],[21], the co-occurrence of lesions confounds efforts to correlate the lesion-specific effects with cognitive impairment [8],[14]. Thus, it is important to define a model of CVD that is straightforward and easily reproduced (Figure 2).
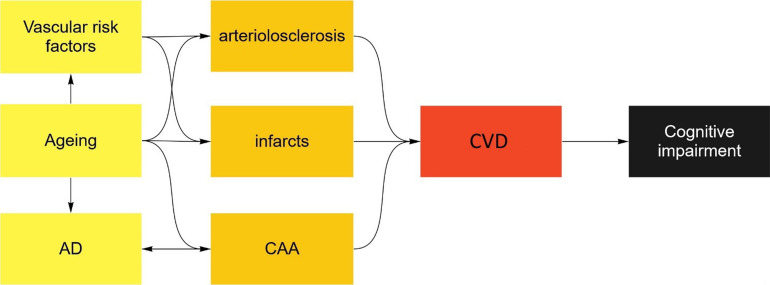
Figure 2. CVD associates with cognitive impairment.
The possibility of either AD (OR 1.11, 95% CI 1.02-1.20, p<0.01) or vascular co-morbidities (OR 1.46, 95% CI 1.30-1.64, p<0.01) is more likely with increasing age. Individual CVD lesions frequently occur when AD or vascular risk factors are present (see Table 3and Table 4). Vascular risk factors also increase the likelihood of multiple CVD lesions (OR 1.94, 95% CI 1.07-3.52, p=0.03) as does ageing itself (OR 1.15, 95% CI 1.02-1.30, p = 0.03). Multiple CVD lesions (OR 2.36, 95% CI 1.23-4.52, p<0.01), but not single lesions (OR 0.93, 95% CI 0.54-1.60, p=0.80), associate with cognitive impairment or dementia. Logistic regression modelling the effect of age, AD pathology, vascular risk factors and CVD on cognitive impairment for AD spectrum cases with available vascular risk factors (n=421). Similar results were obtained when modelling for dementia (not shown).
Our approach examined six CVD pathologies including moderate/severe cortical CAA, moderate/severe white matter arteriolosclerosis, large infarcts, lacunar infarcts, microinfarcts and hemorrhages, and defined a moderate to high level of CVD as the presence of two or more of these pathologies. The VCING study’s model of three pathologies weighted large infarcts twice as strongly as moderate/severe occipital leptomeningeal CAA or moderate/severe occipital white matter arteriolosclerosis and defined a moderate to high level of CVD as the presence of either large infarcts, both CAA and arteriolosclerosis, or large infarcts with one or both other lesions. Since large infarcts were associated with other CVD lesions 86% of the time, our additive model of CVD is functionally similar to the VCING model that defines all cases with large infarcts as having a moderate level of CVD. Indeed, the correspondence between the VCING model and our study was high (Pearson’s correlation coefficient = 0.803). Cases with a moderate or high level of CVD by VCING were also defined as a moderate to high level of CVD by CNDR with 91-100% agreement (Table 8). However, with the inclusion of additional lesions in our model, many more cases have cerebrovascular pathology than are defined by the VCING model, so 36-44% of our moderate to high level CVD cases would not be captured by the VCING model (Table 8).
Table 8. Comparison with VCING guidelines.
(No legend)
| a. by VCING preferred model level of CVD | b. by CNDR level of CVD | |||||||
|---|---|---|---|---|---|---|---|---|
| Level | VCING n | CNDR n | Agreement | Level | CNDR n | VCING n | Agreement | |
| None | 955 | 765 | 80% | None | 765 | 765 | 100% | |
| Low | 391 | 312 | 80% | Low | 482 | 312 | 65% | |
| Moderate | 109 | 99 | 91% | Moderate | 166 | 73 | 44% | |
| High | 30 | 30 | 100% | High | 72 | 26 | 36% |
Defining CVD as the presence of multiple cerebrovascular lesions correlated well with cognitive impairment in the absence of definite AD, while single cerebrovascular lesions did not (Table 7). Other studies have also reported that a moderate or high burden of cerebrovascular lesions is a better correlate of cognitive decline than low levels [19],[20],[21]. By age 90, for instance, close to 50% of individuals without dementia have microinfarcts, while just over 50% of individuals with dementia have infarcts [22]. However, three or more microinfarcts were present in 26% of those with dementia compared to 7% without, indicating that it is the presence of multiple infarcts that associated with dementia, while one or two were tolerated without concomitant dementia. Similar results have been reported for younger cohorts [21],[23], suggesting that a more severe CVD burden is a better correlate of impairment. It should also be pointed out that an additive model of CVD is functionally similar to the consensus criteria for AD [2]. In AD, an individual with only β-amyloid plaques has a low level of AD neuropathologic change, but an individual with additional tau-positive neurofibrillary tangles who also develop tau-positive cortical neuritic plaques is more likely to have an intermediate or high level of AD neuropathologic change. Most older individuals without cognitive impairment have a low level of AD neuropathologic change but most individuals with dementia have a high level of AD neuropathologic change [3].
Our study attempted to disentangle the contribution of ageing itself or AD pathology to the development of cerebrovascular lesions (Table 5). Individual lesions such as infarcts, moderate/severe arteriolosclerosis, and moderate/severe CAA associated with increasing age, but only moderate/severe CAA associated with the presence of AD pathology. However, it should be noted that AD and CVD rarely occur in isolation. Not only is the common co-morbid lesion frequency estimated at >90% for CAA, ~50% for arteriolosclerosis, >30% for microinfarcts, >10% for larger infarcts and >10% for hemorrhages [14], but both AD and cerebrovascular pathologies may accumulate a decade or even decades before clinical symptoms [24],[25],[26],[27]. Recent imaging studies have even suggested that hypoperfusion [28] and blood brain barrier dysfunction [13] are early events in the development of AD, much as they are speculated to be in the development of CVD. Nonetheless, our data is consistent with ageing as a risk factor for infarcts and arteriolosclerosis, and AD as a risk factor for CAA.
CVD also associated with vascular risk factors in our study (Table 6). Both CVD and AD are ageing-related diseases, but cerebrovascular lesions such as infarcts or moderate/severe arteriolosclerosis were more likely when co-morbidities such as hypertension, diabetes or atherosclerosis were present. While these associations between vascular risk factors, cerebrovascular pathology, and cognitive impairment are widely assumed to exist, they are rarely reported [7],[11],[14]. There are multiple reasons for this, but the largest confound may be the overlap between risk factors associated with both CVD and AD. Indeed, several ‘vascular’ risk factors are also AD risk factors and previous studies have suggested that up to 35% of late-life dementia may be preventable by targeting multiple risk factors such as diabetes, hypertension, obesity, smoking, depression, cognitive inactivity, and physical inactivity [21],[29],[30]. Hypertension and diabetes are potentially modifiable risk factors for clinical AD [13], atherosclerosis is increasingly seen as contributing to dementia in a mode similar to AD [30], and vascular risk factors have correlated with increasing AD biomarkers in preclinical AD [3]. In our study, multiple vascular risk factors were often present in the same individual, but, surprisingly, the presence of multiple risk factors was not more likely than the presence of a single risk factor to associate with CVD pathology.
The present work is best understood in the context of the strengths and limitations of our study design. For this study, as recommended in the preferred model of the VCING study [8], we screened the occipital cortex for CAA, arteriolosclerosis and micro-infarcts. It should be noted that our model is minimalistic, and other models of cerebrovascular disease also deserve closer attention for their relevance to the study of dementia in older adults. Strozyk et al examined a 6-point vascular score (involving large infarcts, lacunar infarcts and leukoencephalopathy) that associated with dementia [15]; Deramecourt et al reported a 20-point vascular score (involving arteriolosclerosis; amyloid angiopathy, perivascular hemosiderin leakage; perivascular space dilation, myelin loss and cortical micro-infarcts and large infarcts in multiple regions) that correlated well with vascular and mixed dementia [10]; the Newcastle categorization describes cerebrovascular disease as heterogeneous and proposes six subtypes to best describe the presence and distribution of cerebrovascular lesions [31]. One obvious limitation is that we graded leptomeningeal, parenchymal and capillary CAA as a single cortical CAA score when each type of CAA may have differential effects in a CVD model of cognitive impairment [32],[16]. Relatedly, reporting infarcts by subtype – i.e. strategic infarcts versus multiple small lacunes versus cortical infarcts – may have revealed which type of infarct most correlated with cognitive impairment [15],[31]. The primary benefit of screening the occipital cortex for arteriolosclerosis, micro-infarcts and CAA is that it appears to be susceptible to both CAA [5] and micro-infarcts [22]. Nonetheless, the inclusion of additional regions or stains may have also affected the overall picture of CVD in our cohort, as the differences in postmortem sampling and staining methods is a well-known problem in the field [4]. In our analysis of vascular risk factors, we describe atherosclerosis in the circle of Willis as a risk factor, while other studies report atherosclerosis as a neuropathologic change [26],[33]. In fact, both views on atherosclerosis are valid. Atherosclerosis as an intracranial pathologic change may be a consequence of other risk factors such as hyperlipidemia, and atherosclerosis as a risk factor may contribute to the intracerebral parenchymal lesions in our study. Finally, the majority of cases in our brain bank are composed of individuals with a neurodegenerative disease who were enrolled for brain tissue donation through tertiary referral clinics, and therefore our cohort has a younger age at death than many community- or population-based cohorts, with an overall lower incidence of cerebrovascular lesions than is observed in older cohorts [34]. As a consequence, the number of AD-associated CVD lesions is perhaps overrepresented compared to the number of age-associated CVD lesions.
In conclusion, we report an additive model of CVD that accounts for the presence of global infarcts, moderate/severe CAA, and moderate/severe arteriolosclerosis. When multiple cerebrovascular lesions are present, CVD associates with cognitive impairment or dementia in the absence of other neurodegenerative diseases and, importantly, CVD also associates with vascular risk factors. As a straightforward to apply definition of CVD, our model could be easily reproduced in community-based cohorts for a more complete understanding of the frequency of CVD in the population. Future studies may be better able to disentangle the overlap between risk factors, ageing, and AD pathology with cerebrovascular pathology and thus improve the detection and treatment of vascular cognitive impairment and dementia.
Author contributions
JQT conceived and designed the study. JLR and EBL also contributed to the study design. JLR, BA and NL performed immunohistochemistry and semi-quantitative grading which were verified by JQT and EBL. HR and SXX performed the statistical analysis. JQT, HR, SXX, VMYL, EBL and JLR contributed to data analysis and interpretation. JLR wrote the initial draft of the manuscript, with all authors contributing to the final version. All authors read and approved the final manuscript.
Potential conflicts of interest
EBL is an editorial board member but was not involved in the editorial handling of this manuscript. JLR (no conflict); HR (no conflict); SXX (no conflict); BA ((no conflict), NL (no conflict); VMYL (no conflict); JQT (no conflict)
Funding
US National Institute on Aging (National Institutes of Health U19 AG062418, P30 AG010124, PI: JQT; P01 AG017586, PI: VMYL; P01 AG066597, P30 AG072979, PI: EBL).
Acknowledgements
We are indebted to patients and families who donated tissue to the CNDR. We also thank all past and current referring neurologists, pathology assistants and pathologists for making this research possible. In addition, we would like to thank Theresa Schuck for helping us procure and stain all samples.
References
- Dementia prevention, intervention, and care. Livingston Gill, Sommerlad Andrew, Orgeta Vasiliki, Costafreda Sergi G., Huntley Jonathan, Ames David, Ballard Clive, Banerjee Sube, Burns Alistair, Cohen-Mansfield Jiska, Cooper Claudia, Fox Nick, Gitlin Laura N., Howard Robert, Kales Helen C., Larson Eric B., Ritchie Karen, Rockwood Kenneth, Sampson Elizabeth L., Samus Quincy, Schneider Lon S., Selbæk Geir, Teri Linda, Mukadam Naaheed. The Lancet. 2017 Dec;390(10113) doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Montine Thomas J., Phelps Creighton H., Beach Thomas G., Bigio Eileen H., Cairns Nigel J., Dickson Dennis W., Duyckaerts Charles, Frosch Matthew P., Masliah Eliezer, Mirra Suzanne S., Nelson Peter T., Schneider Julie A., Thal Dietmar Rudolf, Trojanowski John Q., Vinters Harry V., Hyman Bradley T. Acta Neuropathologica. 2012 Jan;123(1) doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The development and convergence of co-pathologies in Alzheimer’s disease. Robinson John L., Richardson Hayley, Xie Sharon X., Suh EunRan, Van Deerlin Vivianna M., Alfaro Brian, Loh Nicholas, Porras-Paniagua Matias, Nirschl Jeffrey J., Wolk David, Lee Virginia M.-Y., Lee Edward B., Trojanowski John Q. Brain. 2021 Apr 12;144(3) doi: 10.1093/brain/awaa438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Kalaria Raj N. Acta Neuropathologica. 2016 May;131(5) doi: 10.1007/s00401-016-1571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Review: Vascular dementia: clinicopathologic and genetic considerations. Vinters H. V., Zarow C., Borys E., Whitman J. D., Tung S., Ellis W. G., Zheng L., Chui H. C. Neuropathology and Applied Neurobiology. 2018 Apr;44(3) doi: 10.1111/nan.12472. [DOI] [PubMed] [Google Scholar]
- Postmortem Examination of Vascular Lesions in Cognitive Impairment: A Survey Among Neuropathological Services. Pantoni Leonardo, Sarti Cristina, Alafuzoff Irina, Jellinger Kurt, Munoz David G., Ogata Jun, Palumbo Vanessa. Stroke. 2006 Apr;37(4) doi: 10.1161/01.STR.0000206445.97511.ae. [DOI] [PubMed] [Google Scholar]
- Small vessel disease: mechanisms and clinical implications. Wardlaw Joanna M., Smith Colin, Dichgans Martin. The Lancet Neurology. 2019 Jul;18(7) doi: 10.1016/S1474-4422(19)30079-1. [DOI] [PubMed] [Google Scholar]
- Vascular cognitive impairment neuropathology guidelines (VCING): The contribution of cerebrovascular pathology to cognitive impairment. Skrobot Olivia A., Attems Johannes, Esiri Margaret, Hortobágyi Tibor, Ironside James W., Kalaria Rajesh N., King Andrew, Lammie George A., Mann David, Neal James, Ben-Shlomo Yoav, Kehoe Patrick G., Love Seth. Brain. 2016 Nov;139(11) doi: 10.1093/brain/aww214. [DOI] [PubMed] [Google Scholar]
- A platform for discovery: The university of pennsylvania integrated neurodegenerative disease biobank. Toledo Jon B., Van Deerlin Vivianna M., Lee Edward B., Suh EunRan, Baek Young, Robinson John L., Xie Sharon X., McBride Jennifer, Wood Elisabeth M., Schuck Theresa, Irwin David J., Gross Rachel G., Hurtig Howard, McCluskey Leo, Elman Lauren, Karlawish Jason, Schellenberg Gerard, Chen-Plotkin Alice, Wolk David, Grossman Murray, Arnold Steven E., Shaw Leslie M., Lee Virginia M.‐Y., Trojanowski John Q. Alzheimer's & Dementia. 2014 Jul;10(4) doi: 10.1016/j.jalz.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staging and natural history of cerebrovascular pathology in dementia. Deramecourt Vincent, Slade Janet Y., Oakley Arthur E., Perry Robert H., Ince Paul G., Maurage Claude-Alain, Kalaria Raj N. Neurology. 2012 Apr 03;78(14) doi: 10.1212/WNL.0b013e31824e8e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Review of inverse probability weighting for dealing with missing data. Seaman Shaun R., White Ian R. Statistical Methods in Medical Research. 2013 Jun;22(3) doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- Vascular dementia. O'Brien John T., Thomas Alan. The Lancet. 2015 Oct;386(10004) doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- Brain arteriolosclerosis. Blevins Brittney L., Vinters Harry V., Love Seth, Wilcock Donna M., Grinberg Lea T., Schneider Julie A., Kalaria Rajesh N., Katsumata Yuriko, Gold Brian T., Wang Danny J. J., Ma Samantha J., Shade Lincoln M. P., Fardo David W., Hartz Anika M. S., Jicha Gregory A., Nelson Karin B., Magaki Shino D., Schmitt Frederick A., Teylan Merilee A., Ighodaro Eseosa T., Phe Panhavuth, Abner Erin L., Cykowski Matthew D., Van Eldik Linda J., Nelson Peter T. Acta Neuropathologica. 2021 Jan;141(1) doi: 10.1007/s00401-020-02235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classification of covert brain infarct subtype and risk of death and vascular events. Gutierrez Jose, Gil-Guevara Andrea, Ramaswamy Srinath, DeRosa Janet, Di Tullio Marco R., Cheung Ken, Rundek Tatjana, Sacco Ralph L., Wright Clinton B., Elkind Mitchell S.V. Stroke. 2020 Jan;51(1) doi: 10.1161/STROKEAHA.119.026068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vascular risk factors and Alzheimer's disease: are these risk factors for plaques and tangles or for concomitant vascular pathology that increases the likelihood of dementia? An evidence-based review. Chui Helena C., Zheng Ling, Reed Bruce R., Vinters Harry V., Mack Wendy J. Alzheimer's Research & Therapy. 2011;3(6) doi: 10.1186/alzrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CNS small vessel disease: A clinical review. Cannistraro Rocco J., Badi Mohammed, Eidelman Benjamin H., Dickson Dennis W., Middlebrooks Erik H., Meschia James F. Neurology. 2019 Jun 11;92(24) doi: 10.1212/WNL.0000000000007654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watershed microinfarct pathology and cognition in older persons. Kapasi Alifiya, Leurgans Sue E., James Bryan D., Boyle Patricia A., Arvanitakis Zoe, Nag Sukriti, Bennett David A., Buchman Aron S., Schneider Julie A. Neurobiology of Aging. 2018 Oct;70 doi: 10.1016/j.neurobiolaging.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The overlap between vascular disease and Alzheimer’s disease - lessons from pathology. Attems Johannes, Jellinger Kurt A. BMC Medicine. 2014 Dec;12(1) doi: 10.1186/s12916-014-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Microinfarcts are common and strongly related to dementia in the oldest-old: The 90+ study. Corrada María M., Sonnen Joshua A., Kim Ronald C., Kawas Claudia H. Alzheimer's & Dementia. 2016 Aug;12(8) doi: 10.1016/j.jalz.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathological correlates of dementia in a longitudinal, population-based sample of aging. Sonnen Joshua A., Larson Eric B., Crane Paul K., Haneuse Sebastien, Li Ge, Schellenberg Gerald D., Craft Suzanne, Leverenz James B., Montine Thomas J. Annals of Neurology. 2007 Aug 20;62(4) doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Jack Clifford R., Knopman David S., Jagust William J., Shaw Leslie M., Aisen Paul S., Weiner Michael W., Petersen Ronald C., Trojanowski John Q. The Lancet Neurology. 2010 Jan;9(1) doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerebral microinfarcts: A systematic review of neuropathological studies. Brundel Manon, Bresser Jeroen, Dillen Jeroen J, Kappelle L. Jaap, Biessels Geert Jan. Journal of Cerebral Blood Flow & Metabolism. 2012 Mar;32(3) doi: 10.1038/jcbfm.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vascular contributions to cognitive impairment, clinical Alzheimer's disease, and dementia in older persons. Kapasi Alifiya, Schneider John A. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2016 May;1862(5) doi: 10.1016/j.bbadis.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevalence and severity of cerebral amyloid angiopathy: A population-based study on very elderly Finns (Vantaa 85+): Cerebral amyloid angiopathy in very elderly Finns. Tanskanen M., Mäkelä M., Myllykangas L., Notkola I.-L., Polvikoski T., Sulkava R., Kalimo H., Paetau A. Neuropathology and Applied Neurobiology. 2012 Jun;38(4) doi: 10.1111/j.1365-2990.2011.01219.x. [DOI] [PubMed] [Google Scholar]
- Cerebrovascular disease in ageing and Alzheimer’s disease. Love Seth, Miners J. Scott. Acta Neuropathologica. 2016 May;131(5) doi: 10.1007/s00401-015-1522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Montagne Axel, Nation Daniel A., Sagare Abhay P., Barisano Giuseppe, Sweeney Melanie D., Chakhoyan Ararat, Pachicano Maricarmen, Joe Elizabeth, Nelson Amy R., D’Orazio Lina M., Buennagel David P., Harrington Michael G., Benzinger Tammie L. S., Fagan Anne M., Ringman John M., Schneider Lon S., Morris John C., Reiman Eric M., Caselli Richard J., Chui Helena C., Tcw Julia, Chen Yining, Pa Judy, Conti Peter S., Law Meng, Toga Arthur W., Zlokovic Berislav V. Nature. 2020 May 07;581(7806) doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High rates of co-occurrence of hypertension, elevated low-density lipoprotein cholesterol, and diabetes mellitus in a large managed care population. Selby Joe V., Peng Tiffany, Karter Andrew J., Alexander Mark, Sidney Stephen, Lian Jean, Arnold Amy, Pettitt Dan. The American Journal of Managed Care. 2004 Feb;10(2 Pt 2) [PubMed] [Google Scholar]
- The projected effect of risk factor reduction on Alzheimer's disease prevalence. Barnes Deborah E., Yaffe Kristine. The Lancet Neurology. 2011 Sep;10(9) doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contribution of vascular pathology to the clinical expression of dementia. Strozyk Dorothea, Dickson Dennis W., Lipton Richard B., Katz Mindy, Derby Carol A., Lee Sunhee, Wang Cuiling, Verghese Joe. Neurobiology of Aging. 2010 Oct;31(10) doi: 10.1016/j.neurobiolaging.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Kalaria Raj N. Acta Neuropathologica. 2016 May;131(5) doi: 10.1007/s00401-016-1571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Yarchoan Mark, Xie Sharon X., Kling Mitchel A., Toledo Jon B., Wolk David A., Lee Edward B., Van Deerlin Vivianna, Lee Virginia M.- Y., Trojanowski John Q., Arnold Steven E. Brain. 2012 Dec 01;135(12) doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenchymal and vascular Aβ-deposition and its effects on the degeneration of neurons and cognition in Alzheimer's disease. Thal Dietmar Rudolf, Griffin W. Sue T., Braak Heiko. Journal of Cellular and Molecular Medicine. 2008 Oct;12(5b) doi: 10.1111/j.1582-4934.2008.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerebral amyloid angiopathy burden and cerebral microbleeds: Pathological evidence for distinct phenotypes. Graff-Radford Jonathan, Lesnick Timothy G., Mielke Michelle M., Constantopoulos Eleni, Rabinstein Alejandro A., Przybelski Scott A., Vemuri Prashanthi, Botha Hugo, Jones David T., Ramanan Vijay K., Petersen Ronald C., Knopman David S., Boeve Bradley F., Murray Melissa E., Dickson Dennis W., Jack Clifford R., Kantarci Kejal, Reichard R. Ross. Ikram M. Arfan., editor. Journal of Alzheimer's Disease. 2021 May 04;81(1) doi: 10.3233/JAD-201536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risk of incident clinical diagnosis of Alzheimer's disease–type dementia attributable to pathology‐confirmed vascular disease. Dodge Hiroko H., Zhu Jian, Woltjer Randy, Nelson Peter T., Bennett David A., Cairns Nigel J., Fardo David W., Kaye Jeffrey A., Lyons Deniz-Erten, Mattek Nora, Schneider Julie A., Silbert Lisa C., Xiong Chengjie, Yu Lei, Schmitt Frederick A., Kryscio Richard J., Abner Erin L. Alzheimer's & Dementia. 2017 Jun;13(6) doi: 10.1016/j.jalz.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]