Abstract
Confocal laser endomicroscopy (CLE) represents a new non-invasive in vivo imaging technique that holds considerable promise in neurosurgery and neuropathology. CLE is based on the principle of optical sectioning which uses pinholes placed in the light path to selectively image photons of a specific focal plane by filtering out photons above and below the focal plane. Potential indications of CLE in neurosurgery and neuropathology include intraoperative tumor diagnosis and staging as well as assessment of tumor resection margins notably in the case of diffusely infiltrating gliomas. CLE-based tumor analysis in near-real time may also have a significant impact on future tumor resection strategies. We here discuss the technical features of CLE, its potential for wide-field imaging, its role in comparison to established histological techniques for intraoperative tumor assessment and its position in digital pathology and telepathology. Based on our group’s experience with a commercially available confocal laser endomicroscope (ZEISS CONVIVO), we critically address the current state of intraoperative CLE in brain tumor surgery, the applicability of classical histological criteria and the strategies required to further improve the diagnostic accuracy of CLE. We finally discuss how a widespread use of CLE in neurosurgery may modify the role of neuropathologists in intraoperative consultation, generating both new opportunities and new challenges.
Keywords: Confocal laser endomicroscopy, Intraoperative consultation, Digital biopsies, Brain tumors, Resection margin, In vivo microscopy
Introduction
Intraoperative consultation in the form of frozen section analysis and/or intraoperative cytology is an integral part of neuropathology. Its relative importance compared to other diagnostic activities is arguably higher in neuropathology than in most other domains of pathology. This is due to a variety of reasons, including (a) the high percentage of brain tumor surgeries among all surgical procedures, (b) the absence of preoperative biopsy in most cases meaning that intraoperative consultation is usually the first opportunity to obtain a tissue-based diagnosis, (c) the large variety of tumor types with often overlapping imaging features and (d) the difficulty to distinguish tumoral tissue macroscopically from non-tumoral tissue. Nevertheless, intraoperative consultation has not benefited from any major improvements for several decades and remains currently afflicted by the drawbacks of established histological and cytological methods. These comprise tissue freezing leading to morphological artifacts, tissue consumption, delays in the transfer of tissue samples from the operating room to the laboratory and difficulties in cell and tissue processing.
In vivo microscopy based on various techniques such as confocal laser endomicroscopy (CLE) or optical coherence tomography has been used for a long time in ophthalmology and has become available for clinical routine for different indications such as gastrointestinal diseases (1), pulmonary mucosal lesions or urological disorders.
Our opinion piece is based on our own experience with a commercially available ZEISS CONVIVO confocal laser endomicroscope in both preclinical and clinical settings. We here first review technical aspects of CLE and then describe the characteristics of the first clinical-grade CLE device available for neurosurgical applications. Finally we discuss potential advantages and limitations of in vivo CLE as well as its impact on diagnostic practice in surgical neuropathology. With this contribution to the scientific literature, we aim to inform potential future users, to stimulate discussion and research on CLE, and to discuss the potential benefit of CLE in neurosurgery and neuropathology.
Limitations of histological and cytological methods in neuropathology and neurosurgery
Traditionally, intraoperative diagnosis in neurosurgery has relied on standard pathological techniques such as frozen sections and intraoperative cytology, which are followed by analysis of formalin-fixed, paraffin-embedded (FFPE) tissue for definite diagnosis. While these latter techniques are still being considered the gold standard, some of their drawbacks are prolonged time for sampling, transferring, processing and interpreting the samples, artifacts and sampling errors, all which limit their applicability for rapid interpretation of tissue samples. In addition, standard pathological techniques involve a multitude of different processes and specialized personnel. In the case of FFPE tissue, they involve tissue fixation, dehydration, rinsing, embedding in hot paraffin, cooling, freezing in preparation for cutting, microtome cutting, transferring to glass slides, and drying. In routine hematoxylin and eosin (H&E) staining, several more steps are being performed: dewaxing, dehydration, hematoxylin staining, differentiation in mild acid, bluing, eosin staining, dehydration, clearing and cover-slipping, with various washing and alcohol incubation steps in between.
All these procedures can take up to one full day for basic staining, and several more days for advanced subsequent investigations such as immunohistochemistry and molecular analyses (2–4). For digital pathology, the final slides need to be scanned, processed, analyzed and archived.
Confocal laser endomicroscopy in neurosurgery
The disadvantages of traditional histologic and cytologic techniques are increasingly being overcome by newer technologies. Confocal laser endomicroscopy (CLE) allows in vivo noninvasive histological imaging using optical sectioning. By placing pinholes in the light path, optical sectioning enables the microscope to selectively image a specific focal depth by mechanically filtering most out-of-focus photons above and below the focal plane. This allows in situ visualization of tissue with higher contrast compared to conventional wide-field microscopy, eliminating out-of-focus background and scattered light. In addition, endomicroscopy allows for three-dimensional image reconstruction and greater variety of imaging parameters. Most importantly, this technology enables real-time histopathological analysis with increased diagnostic yield without the traditional histopathological process of excision, fixation and staining.
Only recently this new technological advancement has been applied to neurosurgery, by using a wide range of fluorescent dyes as contrast enhancers. A single clinical-grade device for CLE, the ZEISS CONVIVO, is hitherto commercially available with clearance by the Food and Drug Administration (FDA) of the United States of America and CE marking in the European Union. Routine clinical experience with the CLE device is currently being investigated in several clinical trials. Preclinical research indicates that the CLE device could improve tumor visualization at the tumor margin and speed up intraoperative diagnosis. Furthermore, the CLE device is designed to be used directly in the operating room and to allow telepathology, thus bringing the neuropathologist even closer to the neurosurgeon.
Confocal laser endomicroscopy beyond neurosurgery
Standard histology and confocal laser endomicroscopy are not interchangeable nor can one method fully replace the other. Tissue samples can be analyzed by standard histology and subsequent elaborated investigations. Both techniques are time-consuming which can become limiting in the case of frozen sections. By contrast, confocal laser microscopy of tissues yields almost instantaneous information but is not amenable to traditional subsequent analyses that require a resected physical sample. It might, however benefit from new analytical methods such as artificial intelligence. Such methods are being increasingly employed in radiology and termed radiomics or radiogenomics. Unlike the situation in neurosurgery and neuropathology, CLE has already been adopted by multiple medical specialties including gastroenterology, pulmonology otorhinolaryngology and urology since early the 2000s. It is therefore worthwhile to analyze how CLE has been integrated in the workflows of these medical specialties (Table 1). In gastroenterology for instance, CLE has proven its clinical utility for the diagnosis of esophageal premalignant lesions and neoplasms such as Barrett’s esophagus with low-grade and high-grade dysplasia (5,6) and esophageal cancer (7). The usefulness of CLE in the detection of esophageal, stomach and colon cancer has moved beyond early proof of concept to large clinical trials (8–14).
Table 1.
Selected clinical in vivo studies reporting interobserver agreement for CLE imaging and consistency of CLE-based diagnosis with histopathology in various precancerous lesions and cancer entities
| Author, year | Entity | Study objective | Type of classification used | Imaging modalities, fluorophore used and administration protocol |
Interobserver agreement κ statistics |
Consistency with histopathology in % of cases |
|---|---|---|---|---|---|---|
| Urology | ||||||
| Chang et al., 2013(20) | normal urothelium, low and high grade bladder tumors | to determine the interobserver agreement and diagnostic accuracy of CLE for bladder cancer | histological feature-based system |
WLC CLE: n.r. |
for cancer diagnosis: CLE-experienced urologists: CLE: κ=0.80 CLE+WLC: κ=0.80 novice CLE urologists: CLE: κ=0.55 CLE+WLC: κ=0.59 |
for cancer diagnosis: CLE-experienced urologists: CLE: 90% CLE+WLC: 90% novice CLE urologists: CLE: 77% CLE+WLC: 80% |
| Liem et al., 2020(21) | low- and high grade urothelial carcinoma | to validate proposed CLE criteria for diagnosis and grading of urothelial carcinoma | histological feature-based system |
WLC CLE: 300 ml of 0.1% FNa intravesically, indwelling for 5 min |
trained CLE investigators: κ=0.68 |
CLE: all cases: 63.3% benign: 29% low grade: 76% high grade: 70% CLE+WLC: all cases: 68.2% benign: 50% low grade: 79% high grade: 67% |
| Breda et al., 2018(22) | upper tract urothelial carcinoma (UTUC) | to describe initial experience with CLE for the real-time characterization of UTUC | histological feature-based system | CLE: 5 ml of FNa, 3-4 min. allowed to react with the tissue | κ=0.64 |
low-grade UTUC: 100% high-grade UTUC: 83% carcinoma in situ: 100% |
| Wu et al., 2019(23) | bladder tumors | to investigate the value of CLE with respect to histologic diagnosis | histological feature-based system |
WLC CLE: 400 ml of 0.1% FNa intravesically, indwelling for 2-3 min. |
n.r. |
CLE+WLC all cases: 81.0% low-grade: 85.7% high-grade: 80.0% |
| Gastroenterology | ||||||
| Kiesslich et al., 2006(24) | Barrett’s esophagus and associated neoplasia | in vivo diagnosis of Barrett’s epithelium and associated neoplasia | histological feature-based system | CLE: 5 ml of 10% FNa after endoscope introduction into esophagus | κ=0.892 |
Barrett’s esophagus: 96.8% Barrett’s-associated neoplastic changes: 97.4% |
| Gaddam et al., 2011(16) | Barrett’s esophagus (BE) | to create and test novel CLE criteria for dysplastic BE and to evaluate dysplasia prediction using these criteria | histological feature-based system | CLE: 2.5 ml of 10% FNa i.v. 1-8 min. before imaging |
all reviewers: κ=0.61 CLE-experienced reviewers: κ=0.66 CLE-inexperienced reviewers: κ=0.57 cases, in which reviewers felt confident of their assessment: κ=0.95 non-confident cases: κ=0.24 cases with high image quality: κ=0.89 cases with low image quality: κ=0.47 |
for diagnosing dysplasia: all reviewers: 82% CLE-experienced reviewers: 84% CLE-inexperienced reviewers: 79% |
| Vennelaganti et al., 2018(25) | colon polyps | to develop CLE criteria for distinguishing hyperplastic polyps from tubular adenomas and evaluate its performance characteristics | histological feature-based system | n.r. (previously recorded CLE sequences) | among all 8 investigators: κ=0.73 |
overall: 84.9% high confidence cases: 91.4% low confidence cases: 65.5% |
| di Pietro et al., 2019(6) | low-grade dysplasia (LG) in Barrett’s esophagus (BE) | to identify endomicroscopic features and develop CLE diagnostic criteria for BE-related LGD | histological feature-based system | CLE: 2.5 ml of 10% FNa i.v. |
CLE-experienced investigators: κ=0.71 CLE-non-experienced investigators: κ=0.60 |
CLE-experienced investigators: 82.1% CLE-non-experienced investigators: 74.5% |
| Otorhinolaryngology | ||||||
| Sievert et al., 2022(26) | laryngeal and hypopharyngeal tumors | to develop a classification system to objectively differentiate between malignant and benign mucosal lesions | scoring-based system | CLE: 2.5 ml of 10% FNa i.v., readministration after 8 minutes |
CLE-expert investigators: κ=0.78 CLE-naïve investigators: κ=0.58 |
CLE experts: 90.8% CLE-naive raters: 86.2% |
| Neurosurgery | ||||||
| Abramov et al., 2021(27) | gliomas, choroid plexus carcinoma, metastases | to assess image characteristics after intraoperative redosing of FNa | quantitative image analysis | CLE: FNa 2-5 mg/kg i.v. 3-180 min. prior to imaging | n.r. |
FNa-redose group: (image acquisition at a mean of 6.4 min. after SF redosing) 1 CLE-experienced neuropathologist: 83% 2 CLE-experienced neurosurgeons: 83% 2 CLE-inexperienced neurosurgeons: 83% 2 CLE-inexperienced non-neurosurgeons: 83% |
| Acerbi et al., 2020(28) | glioblastoma | to study the accuracy of the ZEISS CONVIVO CLE system when used ex vivo in giving an intraoperative diagnosis during surgical removal of glioblastoma | qualitative image interpretation | CLE: FNa 5 mg/kg i.v. at anesthesia induction, images recorded between 84-214 minutes after FNa administration | n.r. |
CLE vs. frozen sections / permanent histology as gold standard obtaining tumor diagnosis: 80% / 80% at tumor core 80% / 67% at tumor margin categorizing morphological patterns: 93% / 87% at tumor core 80% / 67% at tumor margin |
| Belykh et al., 2020(29) | high- and low-grade glioma, meningiomas, brain metastases, choroid plexus carcinoma, craniopharyngioma, schwannoma | to assess the feasibility and diagnostic accuracy of CLE optical biopsies of brain lesions | qualitative image interpretation |
CLE: gliomas and meningiomas: FNa 2 mg/kg i.v., at anesthesia induction metastatic lesions: FNa 5 mg/kg i.v., at anesthesia induction in one case: FNa 40 mg/kg, at induction of anesthesia |
n.r. |
CLE-experienced neuropathologist: 75% CLE-experienced neurosurgeon: 78% CLE-inexperienced neurosurgeon: 71% |
| Abramov et al., 2022(30) | glioblastoma, anaplastic astrocytoma, anaplastic oligodendroglioma, pilocytic astrocytoma, subependymoma, meningioma, pineocytoma, hemangioblastoma, schwannoma, choroid plexus papilloma, perineurioma, mature teratoma, metastases, reactive brain tissue | to evaluate the safety and feasibility of using the first clinical-grade CLE system using fluorescein sodium for intraoperative in vivo imaging of brain tumors | qualitative image interpretation | CLE: FNa 5 mg/kg i.v., ≤ 5 minutes before imaging at surgeon’s request; images recorded between 5-193 minutes after FNa administration | n.r. |
CLE vs. frozen sections: 94% CLE vs. permanent histology: 92%, of which glioma: 93%, and reactive brain tissue: 82% |
n.r.: not reported, CLE: confocal laser endomicroscopy, WLC: white light cystoscopy, FNa: sodium fluorescein
As for all new methods, interpretation criteria for CLE have to be developed, validated and adopted by the medical community. While interpretation guidelines for CLE images are in their infancy in neuropathology, various interpretation criteria and classification systems have been developed and validated in urology and gastroenterology owing to the earlier adoption of CLE in these areas. These diagnostic systems range from simple criteria such as the presence of dark aggregates of cells as the CLE criterion for malignant mucinous lesions of the pancreas (15) to elaborate morphological criteria and diagnostic scores such as the ones published for dysplasia for Barrett’s esophagus (16,17), oral squamous cell carcinomas (18) and bladder cancer (19) and others. These diagnostic systems use features of tissue and cellular architecture found in CLE images to distinguish normal from malignant tissue, or to distinguish between different types of lesions. Scoring methods assign positive and negative scores to specific tissue or cell features. If the score is beyond a predetermined threshold, there is a very high likelihood of malignancy (18).
Similar to all diagnostic procedures requiring human interpretation, CLE is also affected by the pathologist’s subjectivity. In medical disciplines that require interpretation of diagnostic images, such as pathology and radiology, interobserver studies are commonly done to ensure that results are reproducible. Diagnostic methods showing higher levels of agreement between observers are deemed more reliable. While this aspect has not yet been fully investigated for brain tumors, reports on interobserver agreement exist in various tumor entities (Table 1).
Confocal laser endomicroscopy in the context of digital pathology and telepathology
Implementation of CLE will not happen in a void, but in the context of existing workflows and current developments. First, CLE integrates well into the current movement towards digital pathology. A fundamental challenge of digital pathology is digital microscopy, i.e. the adoption of digital images. This made workflows more complex in almost all applications as glass slides cannot be eliminated but remain the source of the ultimate digital image. This contrasts with the situation in digital radiology where workflows were simplified by the elimination of films. From this perspective, CLE as well as other techniques for in vivo microscopy offers the advantage of being intrinsically digital which has a number of positive consequences, including the absence of tissue consumption, the lack of a delay for scanning glass slides and the possibility of teleconsultation.
In the conceptual framework of telepathology (Table 2), CLE is most similar to dynamic telemicroscopy since it is potentially very interactive with the possibility of real-time discussion of findings with the neurosurgeon who holds the CLE probe and needs to identify areas of interest, and with a second person who operates the CLE device.
Table 2.
Paradigms for telepathology and their respective advantages and limitations
|
Telepathology modality |
Description |
Advantages |
Limitations |
|---|---|---|---|
|
Static telemicroscopy |
Microphotographs sent by email, etc. |
Limited resources required Does not require high bandwidth connections |
Requires skilled operator on-site to take representative microphotographs Slow Limited interaction |
|
Dynamic telemicroscopy |
Real-time sharing of microscopic image |
Feasible with standard microscopes equipped with a camera More interactive |
Requires skilled operator on-site More demanding regarding bandwidth |
|
Robotic telemicroscopy |
Remote operation of the microscope |
Very interactive Various focus planes possible Less dependent on skilled operators on site |
High bandwidth required More expensive devices |
|
Whole-slide imaging |
Digital microscopy of previously scanned slides |
Navigation independent of operator on site |
Time-consuming Expensive equipment Single focus plane |
Confocal laser endomicroscopy in the context of intraoperative consultation: strengths and limitations
Traditionally, frozen section and/or cytological preparations are used in variable combination for intraoperative diagnosis in most centers. Both techniques have specific strengths and limitations. While frozen sections provide more information on the tissue architectural level and are more similar to histological slides, cytological preparations provide better nuclear morphological details and are not susceptible to freezing artifacts. Given that intraoperative cytology requires only minimal technical equipment, it is performed on-site in some centers which eliminates transfer to the pathology laboratory and accelerates processing. It requires however the presence of neuropathologists on-site or the presence of equipment for telepathology.
CLE will likely result in faster turnaround times even than on-site cytology while being intrinsically prone to teleconsultation. This may turn out particularly advantageous given the limited availability of neuropathologists in many places. The implementation of CLE in clinical routine will however require both neurosurgeons and neuropathologists to adopt a novel conceptual approach.
Table 3.
Time requirement for intraoperative microscopic diagnosis using CLE and competing techniques
| Frozen section | On-site cytology | CLE | |
| Sampling | rapid | rapid | less rapid (?) |
| Transfer to laboratory | typically minutes or longer | - | - |
| Processing | 5 - 15 minutes | 1 – 2 minutes | - |
| Interpretation | few minutes | few minutes | few minutes |
| Feasible without removing tissue | no | no | yes |
| Time required (neuropathology) | ++ | ++++ | + |
The ultimate clinical utility of CLE may very well differ in terms of specific indications. When neuropathologists are required to assess sample adequacy for diagnosis and ancillary diagnostic techniques, the highest possible diagnostic accuracy probably obtained with frozen section and cytology will arguably outweigh any advantage in turnaround time or non-invasiveness of CLE. Conversely, CLE may turn out as the method of choice for the identification of residual tumor cell infiltration in the periphery of resection cavities, because its speed and non-invasiveness allow for much more extensive sampling than tissue biopsies. CLE thus has the potential to change current paradigms for maximal safe resection of brain tumors.
A hybrid approach combining CLE and tissue biopsies may be envisioned for intraoperative diagnosis. Here, CLE would provide a faster response, while frozen section or cytology would serve as confirmatory techniques.
Table 4.
Expected potential of CLE to supplement or replace frozen sections
| Question | Impact on clinical decision | Specific requirements | Potential for CLE |
| Lesional tissue obtained? | Additional biopsies required: yes/no | Actual tissue and greatest possible accuracy required | Probably limited |
| Diagnosis? |
Surgical strategy Ancillary techniques |
Greatest possible accuracy required Usually no repetitive samples |
To be assessed |
| Margin status? | Extend or stop resection | Ideally, multiple and repetitive sampling |
Significant; potential game changer |
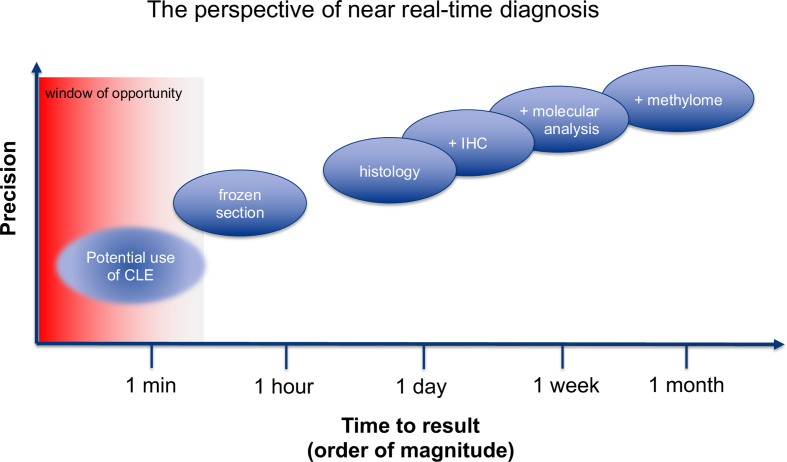
Any novel technique can potentially add something to the existing armamentarium, either by adding precision - as historically seen with gene sequencing or methylome analysis - or by providing information even if limited more rapidly than existing techniques. Current diagnostic practice in neuropathology is characterized by a whole set of different diagnostic modalities. In terms of turnaround times, any technique that is slower than existing ones may be of interest if it adds, either alone or as a complement to other techniques, diagnostic precision. Hematoxylin-eosin-stained slides of FFPE tissue will typically be available within one day, immunohistochemical stainings may require an additional working day. Based on this, patients may be informed about their diagnosis before they leave hospital. Molecular analyses often require around one week which is sufficient for discussion in a multidisciplinary tumor conference before starting adjuvant therapy. Frozen sections and cytology are sufficiently fast for intraoperative consultation, which makes a somewhat lower precision than that of histological slides acceptable. Their turnaround times are however typically too long for extensive repetitive sampling. Therefore, CLE may prove clinically highly useful by allowing repeated analysis or analysis of multiple sites, even if its accuracy were ultimately found to be somewhat lower than that of frozen sections (Figure 1).
Figure 1.
Schematic illustration of turnaround time versus diagnostic precision. An ideal technique would fit in the upper left corner, i.e. offer the greatest possible accuracy with the shortest possible turnaround. Nevertheless, any new technique that is either more precise or faster than available techniques is expected to improve the diagnostic armamentarium. In the case of CLE, the turnaround time in order of one to few minutes allows for multiple repetitive analyses, which are impractical with frozen sections due to the time constraints for their preparation. For a given question, CLE may provide significant benefit by providing fast results even if its precision were lower than that of frozen sections.
General aspects of interpretation
Correlation between confocal laser endomicroscopy and standard histology
In vivo confocal endomicroscopy produces a novel representation of tissue structures that neuropathologists are well accustomed to interpret (Figure 2). As for any new technique, it will be crucial to learn to what extent previously defined morphological criteria can be applied and to identify the differences as compared to traditional techniques. Our experience with CLE images matches H&E slides for different types of lesions. The cellular structures most consistently identifiable in confocal laser endomicroscopy are cell nuclei due to their fainter fluorescence as compared to adjacent tissue. Nuclear size, nuclear size variation as well nuclear spatial distribution are generally well visualized by CLE. On static images, red blood cells are occasionally difficult to distinguish from neural nuclei as they produce a similar type of negative image due to their low fluorescence. We have found their uniform size and round shape useful for their identification.
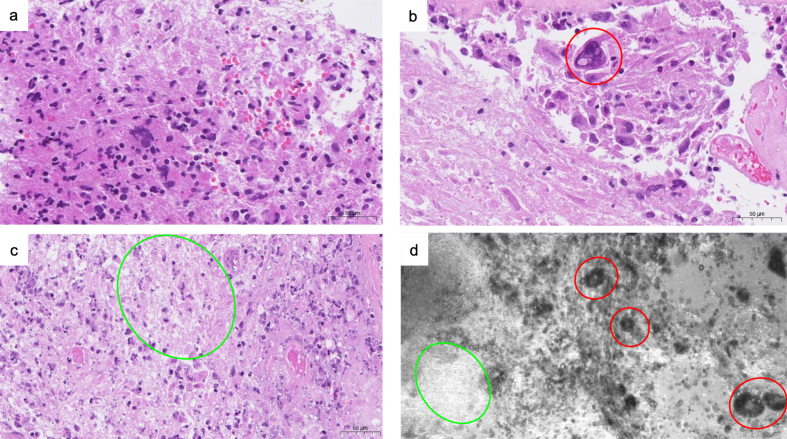
Figure 2.
Example of morphologic patterns in glioblastoma in H&E slides (a, b, c) and in CLE (d), illustrating how similar morphological findings of the same lesions can be visualized with both imaging techniques. Red circles indicate giant cells, green circles indicate necrotic areas.
Correlation of CLE-based morphology and standard histology in clinical routine will likely be an important basis of continuous training for all neuropathologists, allowing them to refine diagnostic criteria. It should be kept in mind, however, that any sole morphological analysis of CNS tumors has significant limitations in the age of a molecular tumor classification. In particular, tumor types defined by molecular alterations cannot be diagnosed with certitude by histology or by any other morphological technique including CLE. CLE may face similar limitations regarding lesions that are considered high-grade solely due to molecular features. Neither of these limitations, however, fundamentally thwart the clinical utility of intraoperative CLE. Three clinical trials are currently investigating CLE in neurosurgery and neuropathology: a 200 patient multicenter trial in Germany (INVIVO, NCT04597801), a 200 patient trial in Berne, Switzerland (CLEBT, NCT04280952) and a trial in Milano, Italy (Besta Institute Review Board, verbal n. 72/2020) focusing on the concordance between CLE and definitive histopathological analysis. The results of these trials will clarify the potential clinical benefit of CLE in neurosurgery and neuropathology and define the role of CLE in the neurosurgical armamentarium.
Development and refinement of diagnostic criteria for CLE
CLE is a promising method which achieves in vivo imaging during neurosurgery at high magnification and in real-time without tissue manipulation. CLE is expected to help to distinguish more easily between healthy and neoplastic tissue, thus addressing a major challenge in brain tumor surgery. By optimizing tumor resection and preventing tumor relapse, CLE is expected to offer a significant advantage to patients. In order to achieve these goals, the neuropathologist and/or the neurosurgeon will require basic knowledge of the cytoarchitecture of different tumor types and subtypes imaged by CLE. In addition, entity-specific interpretation criteria and tumor classification guidelines are needed, similar to the previous situation in urology and gastroenterology.
Here, we would first like to discuss and present the general aspects of interpretation of CLE images, based on the thorough examination of thousands of CLE images and the corresponding histology from different brain tumor samples, and before establishing definite diagnostic criteria for each tumor type and subtype.
In general, our CLE images revealed features similar to already known tissue architecturea and morphology. The CLE images correlated well with the corresponding histology (Figure 2). Unique aspects of individual cells and other surrounding tumor tissue elements were observed while the CLE probe acquired images throughout its focal-depth range. CLE images of brain tumors demonstrated high-contrast background with features of hypercellularity, atypic nuclei, microvascular proliferation, necrosis, and/or abnormal cell clustering and grouping (Figures 3, 6, 7). By contrast, CLE images of normal brain tissue showed a rather quiet, dark and normocellular background (Figure 3e). Erythrocytes were visualized as abundant, round to oval shaped cells that appeared to be much smaller than tumor cells. These features were already sufficient to allow a quick intraoperative diagnosis and to classify the tissue as normal or pathologic, independent of the exact tumor type and subtype, supporting the intraoperative decision making.
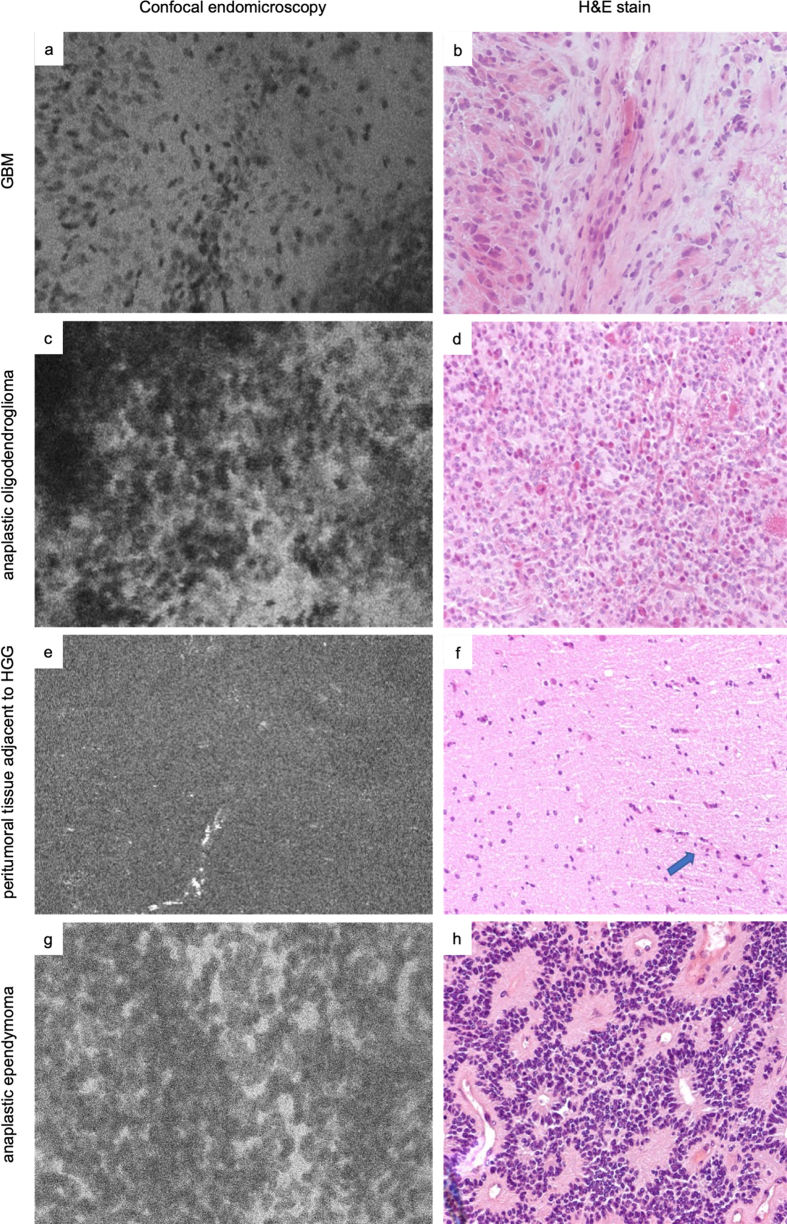
Figure 3.
Examples of morphologic patterns in various tumors on in vivo CLE images recorded in comparison with microscopically matched H&E slides from the same region of interest. Pleomorphic cells and hypercellularity in a case of glioblastoma multiforme (GBM) (a-b), a case of anaplastic oligodendroglioma (c-d), a case of high-grade glioma (HGG) showing a region of normal brain tissue and a highlighted vessel (arrow in H&E slide, bright streak in CLE image) adjacent to the neoplastic tissue (e-f), and a case of anaplastic ependymoma (g-h).
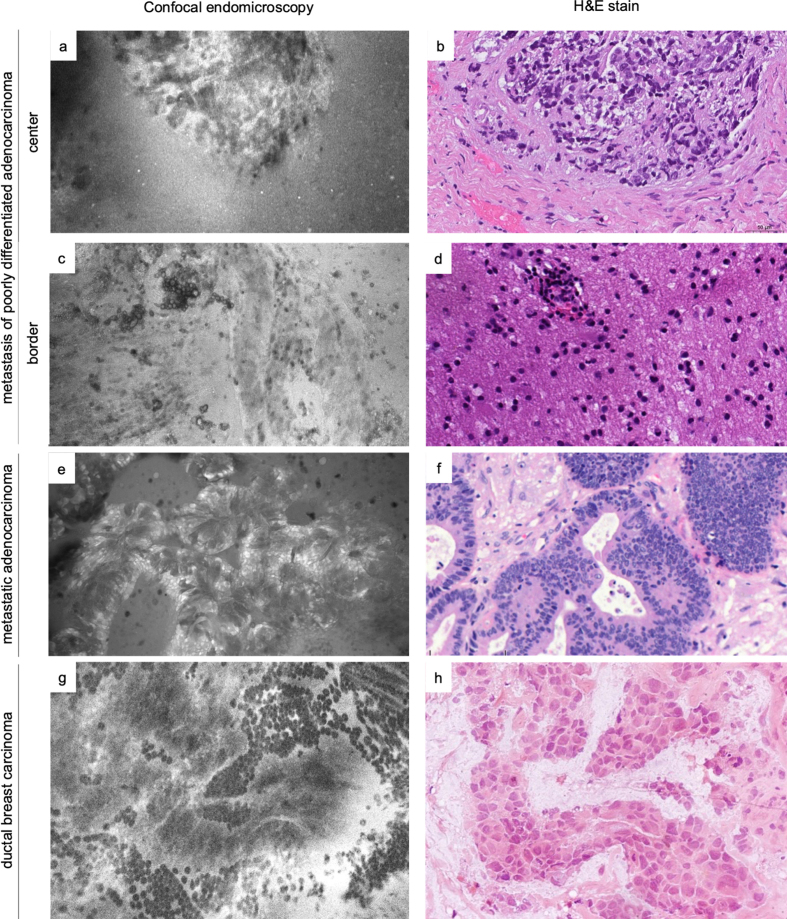
Figure 6.
Comparative examples of metastases in in vivo and ex vivo CLE images and H&E slides. Shown are a metastasis of a poorly differentiated carcinoma (a-b) and reactive changes (gliosis and discrete lymphocytic inflammatory infiltrate) at the border of the same lesion (c-d), metastatic adenocarcinoma (e-f) and metastasis of ductal breast carcinoma (g-h, H&E of frozen section). For illustrative purposes, both in vivo and ex vivo CLE images are included showing that morphology is represented consistently independent of the acquisition modality (a, c, and e: ex vivo; g: in vivo).
Figure 7.
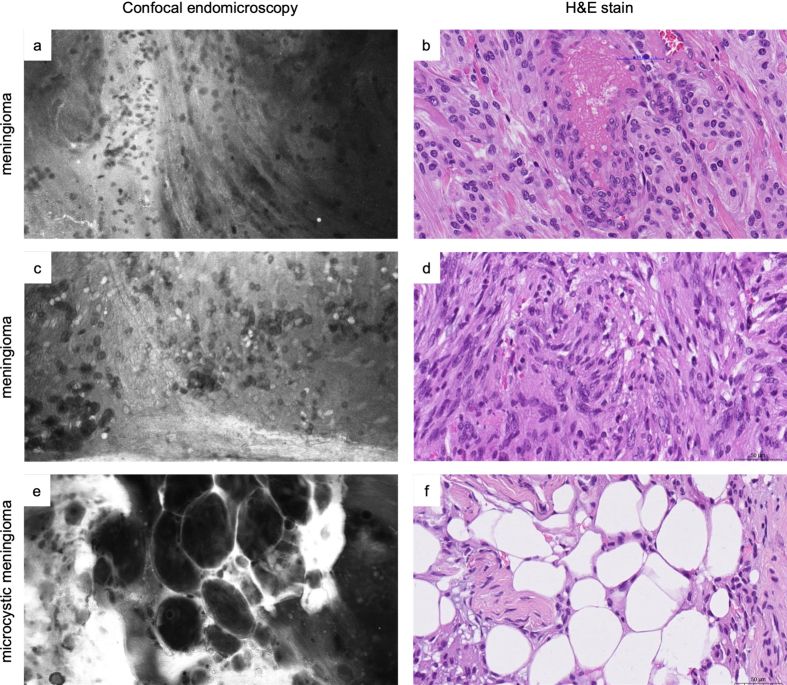
Comparative examples of tumor morphology in ex vivo CLE images and matched H&E slides in two distinct cases of meningioma (a-b and c-d) and microcystic meningioma (e-f). While in vivo imaging is the preferred usage scenario of CLE, ex vivo imaging is useful for training purposes. It enables recording images over a longer period than what is practicable during surgery. It can also help to point out specific tissue characteristics that might otherwise be more time-consuming to achieve intraoperatively.
Possible use cases for CLE
As mentioned previously, CLE images showed the presence or absence of typical histomorphological characteristics of high-grade gliomas (Figures 2 and 3) as observed on routine histological examination such as microvascular proliferation and/or necrosis in addition to hypercellularity and pleomorphism. Distinguishing between a low-grade glioma (CNS WHO grade 1/2) and a high-grade glioma (CNS WHO grade 3/4) is important, because the therapeutic options differ: simple resection in low-grade tumors versus additional radiation and chemotherapy in high-grade tumors. An intraoperative statement regarding the malignant potential of the tumor is often asked, and can help the neurosurgeon decide if further resection should be undertaken or not. However, making a distinction between a low-grade glioma CNS WHO grade 2 and a high-grade glioma CNS WHO grade 3 can be very demanding, since the differentiation between these tumors mostly relies on the presence of mitoses, which are hitherto difficult to identify on CLE images. These limitations should however not affect the integration of molecular data into the tumor grading. Furthermore, CLE is able to access the center of a brain tumor which may be useful for rapid tumor recognition and confirmation. Last but not least, CLE bears the big advantage to evaluate the borders of the tumor, recognizes abnormal histological features, and optimizes surgical resection in marginal regions if necessary.
Due to the diffuse growth pattern of most glial tumors, it is often challenging during surgery to distinguish neoplastic tissue from normal or reactive brain tissue without special or enhanced imaging techniques, often resulting in incomplete tumor resection. Here, virtual biopsies of gliomas at the tumor margins using CLE are of particular interest, because they allow an immediate histological assessment. In such cases, a specific pattern of cellular structures showing delineation of nuclei appearing darker than cytoplasm can often be noticed. Moreover, small clusters of tumor cells or individual tumor cells within brain tissue can sometimes be identified, and a tumor border can often be delineated.
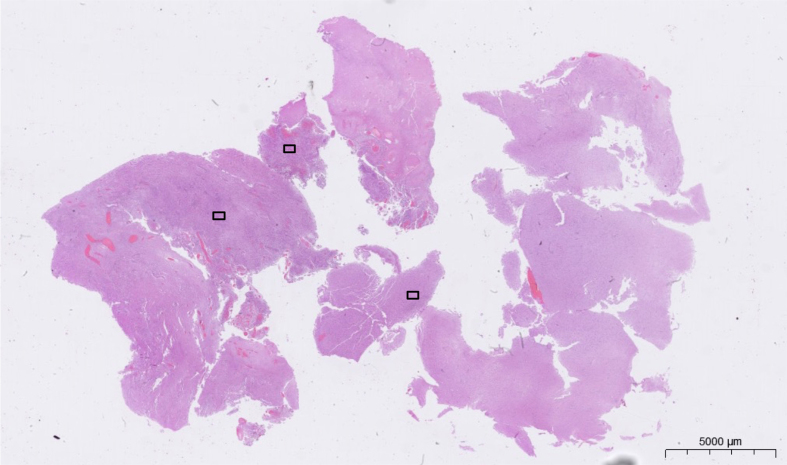
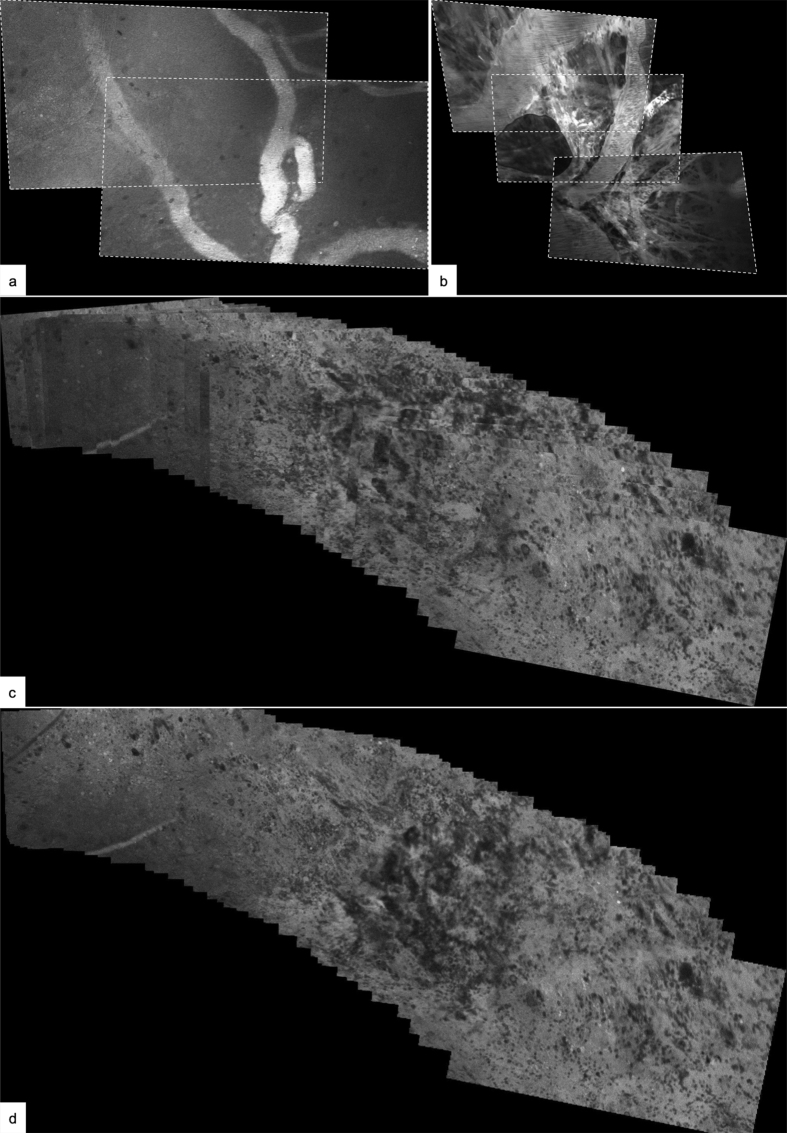
It should be pointed out that the field of view of CLE images is small, approximately 475 μm x 267 μm in the case of the ZEISS CONVIVO (Figure 4). Using CLE to decide whether or not to extend tumor removal can thus be challenging. Thus, when CLE provides an intraoperative preliminary diagnosis based on high magnification images, and a low-power histological image is not available yet, attention must be paid to avoid a potential pitfall for misleading diagnosis, as the entire lesion cannot be overviewed in a single CLE image yet. Consequently, the focus should be on detecting abnormal histoarchitectural features in such cases. Due to the intrinsically digital nature of CLE images, image stitching techniques (Figure 5) can compensate the small field of view of current generation CLE devices. By scanning the tissue surface of the region of interest, CLE can generate a digital map in near real-time. Here, the CLE probe tip constantly acquires images which are digitally stitched together as they are acquired. The resulting digital map then covers a much greater area and allows a better overview of the region of interest than the individual CLE images.
Figure 4.
Representation of the field of view of the ZEISS CONVIVO device superimposed over a low-power histological image of a glioblastoma.
Figure 5.
Experimental image processing (a,b) using stitching techniques. Serial images with overlapping content can be stitched together and result in a greater field of view. Illustrative example of two (a) and three (b) serial images of vasculature with overlapping image content. The images were extracted from an image series recorded over several seconds during which the CLE probe was moved along the tissue surface. Tilting of the probe tip or tissue compression during image series acquisition can result in perspective distortion, which has been corrected for in these examples. Composite of 45 serial images of tumor tissue (c, d): The CLE probe was maneuvered along the tissue surface while serial images were acquired. The images were aligned based on overlapping content (c), which results in a much greater field of view compared to the individual images. Because the images are stacked onto each other, the borders of the individual images are clearly recognizable, as the images on top of the stack overlay the images further below. Further image processing methods, such as blending, can create a seamless tissue map and allow assessment of the tissue in its continuity (d). For clinical purposes it has to be made sure that such methods do not introduce diagnostically relevant artifacts.
Confocal laser endomicroscopy of specific tumor types
In our experience, CLE of metastatic tumor lesions presented mostly high-contrast, clear and good quality images with histomorphological features that were highly diagnostic of the tumor type (Figure 6). A sharp delineation between the neoplastic and healthy brain tissue as well as a grouped growth pattern of tumor cells displaying a high nuclear-to-cytoplasmic ratio, sometimes with a detectable prominent nucleolus, were easily recognizable in CLE images.
Imaging of meningiomas often revealed moderate hypercellular tissue with relatively monomorphic tumor cells and patterns characteristic of a subtype, such as a fibrous background, whorls or microcystic changes (Figure 7). Psammoma bodies were always easily identified when present in the tumor sample. Meningiomas of CNS WHO grade 2 and 3 seemed to have increased cellular atypia than CNS WHO grade 1 tumors, although this distinction was mostly hard to draw.
In the same manner, schwannomas exhibited a fascicular architecture composed of spindled cells with elongated cytoplasmic processes, which were easy to recognize on CLE images. Features such as Antoni A and Antoni B patterns or Verocay bodies were not always detected, even when present in the matched histological samples.
Another important aspect to consider when evaluating CLE images as a neuropathologist, is the quality of the images obtained by the neurosurgeon. In the beginning, lack of experience in handling the CLE probe can cause difficulties in obtaining adequate images for diagnosis. Indeed, the area of interest can be obscured by hand instability or by acquisition of images with blood or movement artifacts.
At the beginning of the learning curve, we found that ex vivo CLE imaging was very useful for training purposes. Such ex vivo images can be easily obtained, allow careful selection of the imaging window and artifact-free depiction of the features of interest. When both the neurosurgeon and the neuropathologist gain experience with the CLE probe, high quality in vivo images are then guaranteed.
Potential impact of CLE on the role of neuropathologists
The future integration of CLE in clinical routine may have a significant impact on the role of neuropathologists in the interdisciplinary team and on neuropathologists’ workload. On one hand, the intrinsic suitability of CLE for telepathology may facilitate distribution of the workload onto a larger team of neuropathologists possibly working at different hospital sites. This may also increase efficiency since the neuropathologists would no longer need to move to the frozen section laboratory or to stay there between subsequent intraoperative consultations. Furthermore, closer interaction due to increased and real-time intraoperative consultation may enhance the visibility of neuropathologists to clinical colleagues and thereby contribute favorably to the role of neuropathologists in the interdisciplinary team. On the other hand, a possible increase in intraoperative consultations may increase the workload of neuropathologists and their limited workforce.
Some neuropathologists may be sceptic about CLE since CLE gives neurosurgeons immediate access to a tool of microscopic analysis. There could be fears that the neurosurgeons may be tempted to interpret CLE images on their own, which ultimately might be seen to render neuropathologists superfluous. We are convinced, however, that such fears would not be warranted and that neuropathologists have any reason to be confident in the importance of their morphological competences. When neurosurgeons became more and more comfortable in interpreting CLE images, this will undoubtedly enhance the quality of the interdisciplinary collaboration and of the entire diagnostic process.
Challenges in the clinical implementation of CLE
Further challenges to be considered for CLE are economic aspects. Clinical implementation of CLE technology may be initially restricted to prosperous countries able to afford the cost of the novel
technology. On a global scale, many people do not have access to adequate neurosurgical care for brain tumors. We deem it easier to implement in vivo CLE in an existing setting of micro-neurosurgery, than to build up infrastructure for frozen section and train staff from scratch in neuropathology. In this scenario, an added benefit would be the easier CLE technology dissemination of knowledge with, e.g. via joint expert teleconsultations from the operating room, as compared to standard slide-based histology.
In many healthcare systems, billing in neuropathology is closely linked to technical analyses of tissues, which means that adequate reimbursement for reading of CLE images may be difficult to obtain. Furthermore, specific training will have to be developed and implemented in order to permit widespread implementation of CLE. Additionally, meticulous quality control procedures assessing diagnostic accuracy and turnaround times will be necessary. Finally, the respective roles, strengths and limitations of frozen section, intraoperative cytology and CLE depending on the clinical context will have to be clarified in order to allow this entire armamentarium of diagnostic modalities to unfold its full potential.
Conclusion and outlook
CLE has a significant potential as an additional tool in intraoperative consultation for CNS lesions. The main strengths of CLE relate to its speed, the possibility to analyze tissue without prior resection, and its intrinsic suitability for telepathology. The commercial availability of a first clinical-grade device will facilitate future widespread application. Data obtained through previous experimental use of CLE will provide a basis for interpretation in clinical use cases. Further refinement of diagnostic criteria and of diagnostic accuracy in routine settings will be paramount.
Furthermore, CLE offers unique perspectives for intraoperative diagnosis at the tissue level, thereby enhancing the role of neuropathologists in the interdisciplinary care team. Ultimately, CLE promises to complement the intraoperative diagnostic armamentarium in neuropathology and neurosurgery and thereby to contribute favorably to the clinical management of CNS tumors.
Conflicts of interest
The Departments of Neurosurgery in Milan and Bern receive fees from Carl Zeiss Meditec AG, Oberkochen, Germany, for lectures at international congresses and support for research using the ZEISS CONVIVO device. Dr. Karl Quint receives fees for coordinating the community of researchers and clinicians working with the ZEISS CONVIVO device. Prof. Ekkehard Hewer and Dr. Maragkou have received honoraria from Carl Zeiss Meditec AG. Carl Zeiss Meditec AG has not played any role in analysis or interpretation of the data and has not been involved in the preparation of the manuscript.
References
- Goetz M. Confocal laser endomicroscopy, Current indications and future perspectives in gastrointestinal diseases. Endoscopia (2012) 24:67–74.
- Sadeghipour A, Babaheidarian P. Making Formalin-Fixed, Paraffin Embedded Blocks. Methods Mol Biol Clifton NJ (2019) 1897:253–268. 10.1007/978-1-4939-8935-5_22 [DOI] [PubMed]
- Sy J, Ang L-C. Microtomy: Cutting Formalin-Fixed, Paraffin-Embedded Sections. Methods Mol Biol Clifton NJ (2019) 1897:269–278. 10.1007/978-1-4939-8935-5_23 [DOI] [PubMed]
- Canene-Adams K. Preparation of formalin-fixed paraffin-embedded tissue for immunohistochemistry. Methods Enzymol (2013) 533:225–233. 10.1016/B978-0-12-420067-8.00015-5 [DOI] [PubMed]
- Pilonis ND, Januszewicz W, di Pietro M. Confocal laser endomicroscopy in gastro-intestinal endoscopy: technical aspects and clinical applications. Transl Gastroenterol Hepatol (2022) 7:7. 10.21037/tgh.2020.04.02 [DOI] [PMC free article] [PubMed]
- di Pietro M, Bertani H, OʼDonovan M, Santos P, Alastal H, Phillips R, Ortiz-Fernández-Sordo J, Iacucci M, Modolell I, Reggiani Bonetti L, et al. Development and Validation of Confocal Endomicroscopy Diagnostic Criteria for Low-Grade Dysplasia in Barrett’s Esophagus. Clin Transl Gastroenterol (2019) 10:e00014. 10.14309/ctg.0000000000000014 [DOI] [PMC free article] [PubMed]
- Xiong Y-Q, Ma S-J, Zhou J-H, Zhong X-S, Chen Q. A meta-analysis of confocal laser endomicroscopy for the detection of neoplasia in patients with Barrett’s esophagus. J Gastroenterol Hepatol (2016) 31:1102–1110. 10.1111/jgh.13267 [DOI] [PubMed]
- Meining A, Bajbouj M, Schmid RM. Confocal fluorescence microscopy for detection of gastric angiodysplasia. Endoscopy (2007) 39:E145–E145. 10.1055/s-2007-966109 [DOI] [PubMed]
- Kiesslich R, Goetz M, Neurath MF. Confocal laser endomicroscopy for gastrointestinal diseases. Gastrointest Endosc Clin N Am (2008) 18:451–466, viii. 10.1016/j.giec.2008.03.002 [DOI] [PubMed]
- Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology (2004) 127:706–713. 10.1053/j.gastro.2004.06.050 [DOI] [PubMed]
- Hsiung P-L, Hsiung P-L, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, Sahbaie P, Crawford JM, Lowe AW, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med (2008) 14:454–458. 10.1038/nm1692 [DOI] [PMC free article] [PubMed]
- Kakeji Y, Yamaguchi S, Yoshida D, Tanoue K, Ueda M, Masunari A, Utsunomiya T, Imamura M, Honda H, Maehara Y, et al. Development and assessment of morphologic criteria for diagnosing gastric cancer using confocal endomicroscopy: an ex vivo and in vivo study. Endoscopy (2006) 38:886–890. 10.1055/s-2006-944735 [DOI] [PubMed]
- Meining A, Wallace MB. Endoscopic Imaging of Angiogenesis In Vivo. Gastroenterology (2008) 134:915–918. 10.1053/j.gastro.2008.02.049 [DOI] [PubMed]
- Wang TD, Friedland S, Sahbaie P, Soetikno R, Hsiung P-L, Liu JTC, Crawford JM, Contag CH. Functional imaging of colonic mucosa with a fibered confocal microscope for real time in vivo pathology. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc (2007) 5:1300–1305. 10.1016/j.cgh.2007.07.013 [DOI] [PMC free article] [PubMed]
- Feng Y, Chang X, Zhao Y, Wu D, Meng Z, Wu X, Guo T, Jiang Q, Zhang S, Wang Q, et al. A new needle-based confocal laser endomicroscopy pattern of malignant pancreatic mucinous cystic lesions (with video). Endosc Ultrasound (2020) 10:200–206. 10.4103/eus.eus_35_20 [DOI] [PMC free article] [PubMed]
- Gaddam S, Mathur SC, Singh M, Arora J, Wani SB, Gupta N, Overhiser A, Rastogi A, Singh V, Desai N, et al. Novel probe-based confocal laser endomicroscopy criteria and interobserver agreement for the detection of dysplasia in Barrett’s esophagus. Am J Gastroenterol (2011) 106:1961–1969. 10.1038/ajg.2011.294 [DOI] [PubMed]
- Tofteland N, Singh M, Gaddam S, Wani SB, Gupta N, Rastogi A, Bansal A, Kanakadandi V, McGregor DH, Ulusarac O, et al. Evaluation of the updated confocal laser endomicroscopy criteria for Barrett’s esophagus among gastrointestinal pathologists. Dis Esophagus Off J Int Soc Dis Esophagus (2014) 27:623–629. 10.1111/dote.12121 [DOI] [PubMed]
- Oetter N, Knipfer C, Rohde M, von Wilmowsky C, Maier A, Brunner K, Adler W, Neukam F-W, Neumann H, Stelzle F. Development and validation of a classification and scoring system for the diagnosis of oral squamous cell carcinomas through confocal laser endomicroscopy. J Transl Med (2016) 14:159. 10.1186/s12967-016-0919-4 [DOI] [PMC free article] [PubMed]
- Wu J, Wang Y-C, Dai B, Ye D-W, Zhu Y-P. Optical biopsy of bladder cancer using confocal laser endomicroscopy. Int Urol Nephrol (2019) 51:1473–1479. 10.1007/s11255-019-02197-z [DOI] [PubMed]
- Chang TC, Liu J-J, Hsiao ST, Pan Y, Mach KE, Leppert JT, McKenney JK, Rouse RV, Liao JC. Interobserver Agreement of Confocal Laser Endomicroscopy for Bladder Cancer. J Endourol (2013) 27:598–603. 10.1089/end.2012.0549 [DOI] [PMC free article] [PubMed]
- Liem EIML, Freund JE, Savci-Heijink CD, de la Rosette JJMCH, Kamphuis GM, Baard J, Liao JC, van Leeuwen TG, de Reijke TM, de Bruin DM. Validation of Confocal Laser Endomicroscopy Features of Bladder Cancer: The Next Step Towards Real-time Histologic Grading. Eur Urol Focus (2020) 6:81–87. 10.1016/j.euf.2018.07.012 [DOI] [PubMed]
- Breda A, Territo A, Guttilla A, Sanguedolce F, Manfredi M, Quaresima L, Gaya JM, Algaba F, Palou J, Villavicencio H. Correlation Between Confocal Laser Endomicroscopy (Cellvizio®) and Histological Grading of Upper Tract Urothelial Carcinoma: A Step Forward for a Better Selection of Patients Suitable for Conservative Management. Eur Urol Focus (2018) 4:954–959. 10.1016/j.euf.2017.05.008 [DOI] [PubMed]
- Wu J, Wang Y-C, Dai B, Ye D-W, Zhu Y-P. Optical biopsy of bladder cancer using confocal laser endomicroscopy. Int Urol Nephrol (2019) 51:1473–1479. 10.1007/s11255-019-02197-z [DOI] [PubMed]
- Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, Hoffman A, Jung M, Nafe B, Galle PR, et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc (2006) 4:979–987. 10.1016/j.cgh.2006.05.010 [DOI] [PubMed]
- Vennelaganti S, Vennalaganti P, Mathur S, Singh S, Jamal M, Kanakadandi V, Rai T, Hall M, Gupta N, Nutalapati V, et al. Validation of Probe-based Confocal Laser Endomicroscopy (pCLE) Criteria for Diagnosing Colon Polyp Histology. J Clin Gastroenterol (2018) 52:812–816. 10.1097/MCG.0000000000000927 [DOI] [PubMed]
- Sievert M, Mantsopoulos K, Mueller SK, Eckstein M, Rupp R, Aubreville M, Stelzle F, Oetter N, Maier A, Iro H, et al. Systematic interpretation of confocal laser endomicroscopy: larynx and pharynx confocal imaging score. Acta Otorhinolaryngol Ital Organo Uff Della Soc Ital Otorinolaringol E Chir Cerv-facc (2022) 42:26–33. 10.14639/0392-100X-N1643 [DOI] [PMC free article] [PubMed]
- Abramov I, Dru AB, Belykh E, Park MT, Bardonova L, Preul MC. Redosing of Fluorescein Sodium Improves Image Interpretation During Intraoperative Ex Vivo Confocal Laser Endomicroscopy of Brain Tumors. Front Oncol (2021) 11:668661. 10.3389/fonc.2021.668661 [DOI] [PMC free article] [PubMed]
- Acerbi F, Pollo B, De Laurentis C, Restelli F, Falco J, Vetrano IG, Broggi M, Schiariti M, Tramacere I, Ferroli P, et al. Ex Vivo Fluorescein-Assisted Confocal Laser Endomicroscopy (CONVIVO® System) in Patients With Glioblastoma: Results From a Prospective Study. Front Oncol (2020) 10:606574. 10.3389/fonc.2020.606574 [DOI] [PMC free article] [PubMed]
- Belykh E, Zhao X, Ngo B, Farhadi DS, Byvaltsev VA, Eschbacher JM, Nakaji P, Preul MC. Intraoperative Confocal Laser Endomicroscopy Ex Vivo Examination of Tissue Microstructure During Fluorescence-Guided Brain Tumor Surgery. Front Oncol (2020) 10:599250. 10.3389/fonc.2020.599250 [DOI] [PMC free article] [PubMed]
- Abramov I, Park MT, Belykh E, Dru AB, Xu Y, Gooldy TC, Scherschinski L, Farber SH, Little AS, Porter RW, et al. Intraoperative confocal laser endomicroscopy: prospective in vivo feasibility study of a clinical-grade system for brain tumors. J Neurosurg (2022) 1:1–11. 10.3171/2022.5.JNS2282 [DOI] [PubMed]