Atypical teratoid/rhabdoid tumors (AT/RT) are aggressively growing malignant embryonal neoplasms of the central nervous system (CNS), which mainly affect young children. Loss of SMARCB1/INI1 (or SMARCA4/BRG1 in rare cases) is recognized as the genetic hallmark of AT/RTs. Furthermore, these tumors can be distinguished into three distinct DNA-methylation based molecular subgroups (i.e. -MYC, -SHH and -TYR) [1],[2],[3]. While most AT/RTs are considered to occur de novo, previous studies have recognized secondary SMARCB1/INI1-deficient rhabdoid tumors arising from other low grade CNS tumors in young patients [4],[5],[6],[7],[8],[9]. Moreover, three AT/RTs, which harbored epigenetic and mutational characteristics of pleomorphic xanthoastrocytoma (PXA), while being entirely void of nuclear SMARCB1/INI1 expression were recently described in older children [10]. We here report the first case of an AT/RT with molecular features of PXA in an older adult.
A 62-year-old woman presented with diffuse headaches since several weeks. Medical examination revealed no clinically significant neurological deficits. An MRI showed a right-sided tumor in temporomesial location with dimensions of 3.8 x 3.7 x 4.1 cm. The tumor displayed contrast-enhancement, central necrosis und perifocal edema (Figure 1a). Additionally, a smaller lesion of the infundibulum was identified. Preoperative lumbar puncture with examination of cerebrospinal fluid revealed extensive presence of tumor cells. The temporomesial tumor was subtotally resected.
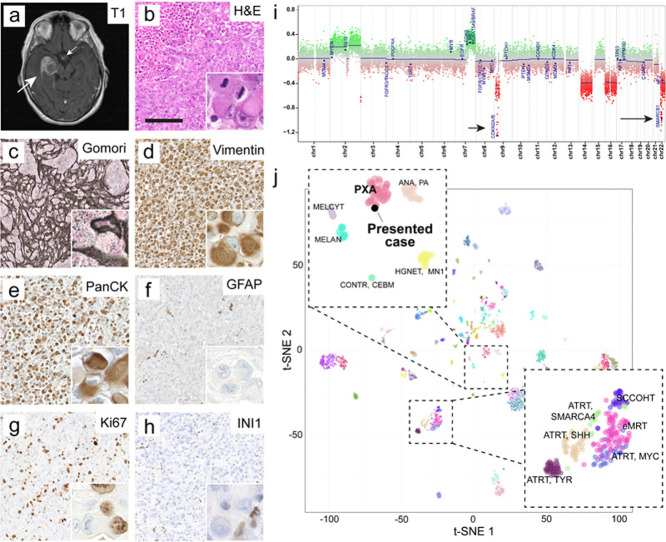
Figure 1. Radiology, histopathology and epigenetic analysis.
a) Representative axial MRI demonstrating the preoperative finding of a large temporomesial tumor (large arrow). Contrast enhancement was found partially within the tumor and additionally in the region of the infundibulum (small arrow). T1-weighted image plus contrast medium.
b) Histomorphology revealed geographical necrosis, prominent rhabdoid morphology and brisk mitotic activity of tumor cells (inset).
c) The tumor showed a prominent network of reticular fibres, visible in the Gomori silver impregnation.
d) Immunostaining for vimentin was vastly strongly positive.
e) Immunostaining for cytokeratins (AE1/3) was vastly strongly positive in the majority of tumor cells.
f) Immunostaining for GFAP was negative in tumor cells while demarcating residual brain tissue.
g) Ki67 proliferative index amounted to about 40%.
h) Tumor cells exhibited loss of nuclear SMARCB1/INI1 staining. Retained nuclear staining was found in blood vessels and inflammatory cells (inset). Scale bar in b – h is 150 µm.
i) Copy number profile of the tumor indicated a homozygous CDKN2A/B (short arrow) and SMARCB1/INI1 (long arrow) deletion.
j) tSNE including a reference set of brain tumors (GSE90496, [11]) showed affiliation of the tumor to the group of PXA.
Clicking the figure will lead you to the full virtual slide (H&E).
Upon histopathological examination, we saw a highly cellular, partially necrotic tumor, which was entirely composed of pleomorphic rhabdoid cells with eosinophilic cytoplasms (Figure 1b). Gomori-staining demonstrated a prominent reticulin network (Figure 1c). Immunohistochemical staining showed positivity for vimentin (Figure 1d) and cytokeratin AE1/3 (PanCK, Figure 1e). GFAP was negative within the tumor cells (Figure 1f). Strong proliferation was demonstrated by brisk mitotic activity and a proliferative index of 40% in the Ki67-staining (Figure 1g). SMARCB1/INI1 nuclear signal was consistently lost in tumor cells (Figure 1h). Further immunohistochemical stainings are displayed in Supplementary Figure 1. DNA was extracted from the tumor and subjected to DNA methylation profiling using the Illumina EPIC BeadChip array. Using the brain tumor methylation classifier (v11b4 and v12.3) [11], the tumor did not match with a defined methylation class (no calibrated score was ≥ 0.3). The copy number profile revealed a homozygous SMARCB1/INI1 and CDKN2A/B deletion, gains in chromosomes 2 and 7q as well as losses of chromosomes 9p, 14q, 16 and 22q (Figure 1i). T-SNE analysis displayed similarity of the case with PXA (Figure 1j). DNA panel sequencing confirmed a BRAF V600E mutation (Table 1). Taking all diagnostic layers together, we saw a malignant rhabdoid tumor, not elsewhere classified, with BRAF V600E mutation, homozygous SMARCB1/INI1 and CDKN2A/B deletion, and a methylation profile with similarity to PXA. In correspondence with the provisional designation of Thomas et al. [10], the case was signed out as AT/RT with molecular features of PXA. Postoperative clinical staging of the patient revealed a smaller lesion of uncertain dignity in the cervical myelon. No further malignancies outside of the CNS were found. Unfortunately, the patient developed postoperative hemi-plegia, infarctions of the basal ganglia and displayed progressive worsening of the clinical status. Palliative chemotherapy was administered with intrathecal application of Methotrexate. The patient died in the 6th postoperative week.
Table 1. NGS DNA panel results.
| Results with frequency < 5 and/or coverage < 50 were excluded): | |||||||||
| Gene | Variant | Amino acid change | Coverage | Allele frequency | Interpretation | ||||
| BRAF | NM_0004333.4: c.1799T>A | NP_004324.2: p.Val600Glu | 4298 | 49.12 % | relevant | ||||
| NF1 | NM_001042492.2:c.480G>T | NP_001035957.1: p.Arg160Ser | 125 | 5.6 % | unclear relevance | ||||
| ATRX | NM_000489.4: c.4345A>G | NP_000480.3: p.Lys1449Glu | 151 | 5.3 % | unclear relevance | ||||
| Regions covered by QIASeq Targeted DNA Panel CDHS-21330Z-424: | |||||||||
| Coding regions with splice sites | |||||||||
| ATRX | EGFR | NF1 | NF2 | PTEN | TP53 | ||||
| Hotspot mutations | |||||||||
| AKT | BRAF | CTNNB1 | FGFR1 | FGFR2 | H3F3A | HIST1H3B | |||
| HIST1H3C | IDH1 | IDH2 | KRAS | PIK3CA | PIK3R1 | TERT (Promoter) | |||
As it is the case for the herein presented tumor, it remains unclear, if AT/RTs with molecular features of PXA correspond to secondary rhabdoid tumors, which have emerged from and entirely outgrown preexisting PXAs. In contrast, such tumors might also occur independently from precursor lesions and therefore represent a distinct entity. Of note, the CNS WHO classification of 2016 has previously recognized high-grade rhabdoid components with loss of SMARCB1/INI1 in anaplastic PXAs and suggested the term “SMARCB1-deficient anaplastic PXA” for such lesions [12]. However, since the tumor presented here was entirely void of SMARCB1/INI1 retained tumor cells and a preexisting lesion had not been described beforehand, the tumor’s origin is left to speculation. It is moreover important to note that the single genetic and epigenetic alterations we found are not pathognomonic for PXAs [13] and the tumor did not match with a defined methylation class of the brain tumor classifier. However, the combination of all molecular findings – including the epigenetic resemblance to PXAs via tSNE analysis – clearly demonstrates similarity with molecular features of PXAs.
A complicating aspect of the current and soon to be updated CNS WHO classification of 2016 is that SMARCB1/INI1-deficient rhabdoid brain tumors tend to be vastly designated as AT/RTs. The herein presented case exemplarily demonstrates that - especially without molecular investigations - this may lead to the subsumption of malignancies with various epigenetic and mutational landscapes as well as different cellular origins. While the preceding summary of the new WHO classification of 2021 [14] has announced the recognition of epigenetic analyses in AT/RTs, it is currently still unclear how SMARCB1/ INI1-deficient brain tumors with rhabdoid morphology and lack of epigenetic features of AT/RTs are to be classified. Future studies should evaluate if predictive and prognostic implications of these tumors call for a more concise classification and terminology.
Data availability
The data that supports the findings of this study is available from the corresponding author upon request. A digital H&E stained slide is deposited here.
Conflict of Interest
The authors have no competing interests to declare.
Acknowledgements
We thank Celina Soltwedel, Carolina Janko, Karin Gehlken, Ulrike Rumpf, Tasja Lempertz, Nicole Bernhardt, Helena Zinn and Ulrich Schüller (Hamburg) for excellent technical support. We thank Annika Wefers for helpful discussions. We thank Martin Hasselblatt and Christian Thomas from the Institute of Neuropathology of the University Hospital Münster for confirming the diagnosis. J.N. was supported by the Deutsche Forschungsgemeinschaft (DFG, Emmy Noether programme). M.D. was supported by the Erich und Gertrud Roggenbuck-Stiftung.
Ethical statement
Study approval was obtained from the local ethics committee of the Hamburg State Chamber of Physicians. The patient and/or guardian gave their informed consent for scientific use of the data.
Author Contributions
M.D. and J.N. conceived the study and drafted the manuscript. All authors acquired and analyzed data and approved the final version of the manuscript.
Supplementary Material
References
- Atypical teratoid/rhabdoid tumors are comprised of three epigenetic subgroups with distinct enhancer landscapes. Johann P, Erkek S, Zapatka M, Kerl K, Buchhalter I, Hovestadt V, Jones D, Sturm D, Hermann C, Segura Wang M, Korshunov A, Rhyzova M, Gröbner S, Brabetz S, Chavez L, Bens S, Gröschel S, Kratochwil F, Wittmann A, Sieber L, Geörg C, Wolf S, Beck K, Oyen F, Capper D, Sluis P, Volckmann R, Koster J, Versteeg R, Deimling A, Milde T, Witt O, Kulozik A, Ebinger M, Shalaby T, Grotzer M, Sumerauer D, Zamecnik J, Mora J, Jabado N, Taylor M, Huang A, Aronica E, Bertoni A, Radlwimmer B, Pietsch T, Schüller U, Schneppenheim R, Northcott P, Korbel J, Siebert R, Frühwald M, Lichter P, Eils R, Gajjar A, Hasselblatt M, Pfister S, Kool M. Cancer Cell. 2016 Mar;29(3) doi: 10.1016/j.ccell.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Atypical teratoid/rhabdoid tumors—current concepts, advances in biology, and potential future therapies. Frühwald M, Biegel J, Bourdeaut F, Roberts C, Chi S. Neuro-Oncology. 2016 Jun;18(6) doi: 10.1093/neuonc/nov264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atypical teratoid rhabdoid tumour: from tumours to therapies. Richardson E, Ho B, Huang A. Journal of Korean Neurosurgical Society. 2018 May 01;61(3) doi: 10.3340/jkns.2018.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atypical teratoid/rhabdoid tumor evolving from an optic pathway ganglioglioma: Case study1. Allen J, Judkins A, Rosenblum M, Biegel J. Neuro-Oncology. 2006 Jan 01;8(1) doi: 10.1215/S1522851705000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atypical teratoid/rhabdoid tumor arising in the setting of a pleomorphic xanthoastrocytoma. Chacko G, Chacko A, Dunham C, Judkins A, Biegel J, Perry A. Journal of Neuro-Oncology. 2007 Jul 23;84(2) doi: 10.1007/s11060-007-9361-z. [DOI] [PubMed] [Google Scholar]
- Atypical teratoid/rhabdoid tumor arising in a ganglioglioma: genetic characterization. Kleinschmidt-DeMasters B, Birks D, Aisner D, Hankinson T, Rosenblum M. American Journal of Surgical Pathology. 2011 Dec;35(12) doi: 10.1097/PAS.0b013e3182382a3f. [DOI] [PubMed] [Google Scholar]
- Atypical teratoid rhabdoid tumor diagnosis after partial resection of dysembryoplastic neuroepithelial tumor: case report and review of the literature. Nadi M, Ahmad T, Huang A, Hawkins C, Bouffet E, Kulkarni A. Pediatric Neurosurgery. 2016;51(4) doi: 10.1159/000443405. [DOI] [PubMed] [Google Scholar]
- Rhabdoid component emerging as a subclonal evolution of paediatric glioneuronal tumours. Bertrand A, Rondenet C, Masliah-Planchon J, Leblond P, Fourchardière A, Pissaloux D, Aït-Raïs K, Lequin D, Jouvet A, Freneaux P, Sevestre H, Ranchere-Vince D, Tauziede-Espariat A, Maurage C, Silva K, Pierron G, Delattre O, Varlet P, Frappaz D, Bourdeaut F. Neuropathology and Applied Neurobiology. 2018 Feb;44(2) doi: 10.1111/nan.12379. [DOI] [PubMed] [Google Scholar]
- Secondary INI1-deficient rhabdoid tumors of the central nervous system: analysis of four cases and literature review. Nobusawa S, Nakata S, Yoshida Y, Yamazaki T, Ueki K, Amano K, Yamamoto J, Miyahara M, Sugai T, Nakazato Y, Hirato J, Yokoo H. Virchows Archiv. 2020 May;476(5) doi: 10.1007/s00428-019-02686-7. [DOI] [PubMed] [Google Scholar]
- Atypical teratoid/rhabdoid tumor (AT/RT) with molecular features of pleomorphic xanthoastrocytoma. Thomas C, Federico A, Sill M, Bens S, Oyen F, Nemes K, Johann P, Hartmann C, Hartmann W, Sumerauer D, Paterno V, Samii A, Kordes U, Siebert R, Frühwald M, Paulus W, Kool M, Hasselblatt M. American Journal of Surgical Pathology. 2021 Sep;45(9) doi: 10.1097/PAS.0000000000001694. [DOI] [PubMed] [Google Scholar]
- DNA methylation-based classification of central nervous system tumours. Capper D, Jones D, Sill M, Hovestadt V, Schrimpf D, Sturm D, Koelsche C, Sahm F, Chavez L, Reuss D, Kratz A, Wefers A, Huang K, Pajtler K, Schweizer L, Stichel D, Olar A, Engel N, Lindenberg K, Harter P, Braczynski A, Plate K, Dohmen H, Garvalov B, Coras R, Hölsken A, Hewer E, Bewerunge-Hudler M, Schick M, Fischer R, Beschorner R, Schittenhelm J, Staszewski O, Wani K, Varlet P, Pages M, Temming P, Lohmann D, Selt F, Witt H, Milde T, Witt O, Aronica E, Giangaspero F, Rushing E, Scheurlen W, Geisenberger C, Rodriguez F, Becker A, Preusser M, Haberler C, Bjerkvig R, Cryan J, Farrell M, Deckert M, Hench J, Frank S, Serrano J, Kannan K, Tsirigos A, Brück W, Hofer S, Brehmer S, Seiz-Rosenhagen M, Hänggi D, Hans V, Rozsnoki S, Hansford J, Kohlhof P, Kristensen B, Lechner M, Lopes B, Mawrin C, Ketter R, Kulozik A, Khatib Z, Heppner F, Koch A, Jouvet A, Keohane C, Mühleisen H, Mueller W, Pohl U, Prinz M, Benner A, Zapatka M, Gottardo N, Driever P, Kramm C, Müller H, Rutkowski S, Hoff K, Frühwald M, Gnekow A, Fleischhack G, Tippelt S, Calaminus G, Monoranu C, Perry A, Jones C, Jacques T, Radlwimmer B, Gessi M, Pietsch T, Schramm J, Schackert G, Westphal M, Reifenberger G, Wesseling P, Weller M, Collins V, Blümcke I, Bendszus M, Debus J, Huang A, Jabado N, Northcott P, Paulus W, Gajjar A, Robinson G, Taylor M, Jaunmuktane Z, Ryzhova M, Platten M, Unterberg A, Wick W, Karajannis M, Mittelbronn M, Acker T, Hartmann C, Aldape K, Schüller U, Buslei R, Lichter P, Kool M, Herold-Mende C, Ellison D, Hasselblatt M, Snuderl M, Brandner S, Korshunov A, Deimling A, Pfister S. Nature. 2018 Mar 22;555(7697) doi: 10.1038/nature26000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Louis D, Perry A, Reifenberger G, Deimling A, Figarella-Branger D, Cavenee W, Ohgaki H, Wiestler O, Kleihues P, Ellison D. Acta Neuropathologica. 2016 Jun;131(6) doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- Biology and grading of pleomorphic xanthoastrocytoma—what have we learned about it? Vaubel R, Zschernack V, Tran Q, Jenkins S, Caron A, Milosevic D, Smadbeck J, Vasmatzis G, Kandels D, Gnekow A, Kramm C, Jenkins R, Kipp B, Rodriguez F, Orr B, Pietsch T, Giannini C. Brain Pathology. 2021 Jan;31(1) doi: 10.1111/bpa.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Louis D, Perry A, Wesseling P, Brat D, Cree I, Figarella-Branger D, Hawkins C, Ng H, Pfister S, Reifenberger G, Soffietti R, Deimling A, Ellison D. Neuro-Oncology. 2021 Aug 02;23(8) doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon request. A digital H&E stained slide is deposited here.