Abstract
Background and Aim
In chronic hepatitis B infection, antiviral therapy significantly reduces the incidence of complications. This study aimed to present real-life 12-month effectiveness and safety data for TAF.
Materials and Methods
This Pythagoras Retrospective Cohort Study included patients from 14 centers in Turkiye. The study presents 12-month results of 480 patients treated with TAF as initial therapy or after switching from another antiviral drug.
Results
The study shows treatment of about 78.1% patients with at least one antiviral agent (90.6% tenofovir disoproxil [TDF]). The rate of undetectable HBV DNA increased in both treatment-experienced and naive patients. In TDF-experienced patients, the rate of alanine transaminase (ALT) normalization increased slightly (1.6%) within 12 months, but the change was not statistically significant (p=0.766). Younger age, low albumin, and high body mass index and cholesterol were identified as risk factors for abnormal ALT after 12 months, but no linear relationship was detected. In TDF-experienced patients, renal and bone function indicators showed significant improvement three months after the transition to TAF and remained stable for 12 months.
Conclusion
Real-life data demonstrated effective virological and biochemical responses with TAF therapy. After switching to TAF treatment, gains in kidney and bone functions were achieved in the early period.
Keywords: Hepatitis B, real life, tenofovir alafenamide
Introduction
Research states that about one-third of the 250 million people infected with Hepatitis B Virus (HBV) have a chronic disease and active viral replication.[1] If untreated, adverse outcomes such as cirrhosis, hepatic decompensation, and liver cancer will develop in 15%-40% of the patients.[1,2] Therefore, Nucleos(t)ide Analogs (NA), which suppress viral replication and slow disease progression, are essential options for treating this infection.[1] However, NA therapy is unlikely to provide a cure, defined as undetectable HBV DNA and loss of Hepatitis B surface antigen (HBsAg).[2,3] Therefore, most patients require lifelong treatment to suppress viral load.[3]
Tenofovir Alafenamide Fumarate (TAF), a phosphonamide prodrug of tenofovir, is hydrolyzed to tenofovir in hepatocytes.[4] Phosphorylation of tenofovir yields the active metabolite, tenofovir-diphosphate (TFV-DP). Formulation of TAF is to deliver the active form found in hepatocytes more efficiently, with less than one-tenth the dose of Tenofovir Disoproxil Fumarate (TDF).[4,5] Thus, lower serum concentrations provide similar efficacy without long-term kidney and bone-related side effects.[1,4] Reports show that long-term use of TDF causes adverse effects such as reduced Bone Mineral Density (BMD) and renal toxicity.[3] It is better for patients with a high potential for bone and renal-related side effects to switch from TDF to TAF. This is also true for those with bone and renal effects resulting after long-term treatment.[6,7] Our objective in this study is to present TAF’s efficacy and safety data at 12 months in Chronic Hepatitis B (HBV) patients in the using real-life data.
Materials and Methods
Study Population
This multicenter retrospective cohort study involved infectious diseases or gastroenterology departments of 14 centers in the Southeast Anatolia region of Turkiye. The study covered infectious diseases in these units. In Turkiye, health insurance covers TAF therapy for specific indications, since February 2019. The study included patients aged 18 years and older who started TAF therapy with the following indications: the presence of proteinuria, history of drug use affecting bone mineral density (BMD), presence of osteoporosis, low phosphorus level (<2.5 mg/dL), chronic steroid use, history of atraumatic bone fracture, glomerular filtration rate (GFR) <60 mL/min/1.73 m2, history of dialysis, and history of renal transplantation. There we no patients with HIV infection in the study. Physicians collected data using a prepared database. Each physician from the chosen centers keyed in patient results between February 2019 and January 2020. This was for patients who started the TAF therapy, and agreed to a follow up until January 2021. Data obtained at 0 (baseline), 3, 6, and 12 months was recorded.
Data Collection
The analyzed data set included patient gender, age, chronic diseases, known duration of HBV infection, family history of Hepatitis B, Body Mass Index (BMI), antiviral treatment experience, interferon history, other drugs used, and the use of drugs other than TDF that affect BMD. The results of HBsAg and HBV DNA assessments of Hepatitis B infection were recorded. Undetectable HBV DNA was defined as below the lowest level detected by real-time reverse transcriptase PCR (<14 IU/mL). Renal function was assessed with serum creatinine; estimated glomerular filtration rate (eGFR) calculated using the Modification of Diet in Renal Disease (MDRD) study equation, and serum phosphate.
Normal serum Alanine Transaminase (ALT) level was defined according to the upper limits of ≤35 U/L in men and ≤25 U/L in women as recommended by the American Association for the Study of Liver Diseases (AASLD).[8] In addition, normal ALT was evaluated according to the normal range of the hospital laboratory kit (<40 U/L). Lipid profiles were assessed based on measurements of total cholesterol (mg/dL), low-density lipoprotein cholesterol (LDLc) (mg/dL), and high-density lipoprotein cholesterol (HDLc) (mg/dL). BMD and femoral and vertebral T-scores assessed at 6-month intervals were used as indicators of bone function. Patients were categorized as NA-naive (CHB, prophylaxis) or NA-experienced. Those classified as NA-experienced were divided into subcategories according to their TDF experience before switching to TAF. Follow-up evaluations were performed during routine outpatient visits as determined by the following physician.
The Ethics Committee approval was obtained from the local Non-Interventional Clinical Studies Ethics Committee (number 12 on 02.10.2019).
All procedures performed in human participant studies followed the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Statistical Analysis
Categorical descriptive data were expressed as frequency and percentage, while continuous variables were expressed as mean and standard deviation or median and maximum/minimum values. Wilcoxon signed-rank test and Friedman’s Analysis Of Variance with Bonferroni Correction were used to compare repeated measures of ALT, Aspartate Transaminase (AST), phosphorus, total cholesterol, LDLc, HDLc, and eGFR during treatment follow-up in independent groups. McNemar’s test was used to compare repeated checks of ALT normalization.
Statistical analyses were performed using SPSS Statistics 25.0 (IBM Corp, Armonk, NY). As the biochemical endpoint in treating HBV infection is achieving ALT normalization,[7] we performed a chi-square or Mann-Whitney U test followed by univariate and multivariate logistic regression analysis of significant variables to determine the risk factors for ALT elevation according to AASLD criteria at 12 months. The results were expressed as odds ratio (OR) and 95% confidence interval (CI). For all analyses, p<0.05 was accepted as statistically significant.
Results
Baseline Characteristics
The median age of the patients was 46.5 (36–59) years, and 327 (68.1%) were men. Of the 480 patients starting TAF therapy, 375 (78.1%) were treatment-experienced, and 105 (21.9%) were treatment-naive. TAF has been initiated in 85 (81.0%) treatment-naive patients for prophylaxis and 20 (19.0%) due to CHB. Most (90.6%) patients with NA treatment experience received TDF before switching to TAF. The three most common reasons for switching to TAF were the use of drugs affecting BMD (42.9%), osteoporosis (22.3%), and low phosphorus (6.0%). The patient’s baseline characteristics are presented in Table 1.
Table 1.
Baseline patient characteristics (n=480)
| n | % | Median | IQR | |
|---|---|---|---|---|
| Gender | ||||
| Male | 327 | 68.1 | ||
| Female | 153 | 31.9 | ||
| Age in years | 46.5 | 36–59 | ||
| Chronic disease | 172 | 35.8 | ||
| Diabetes mellitus | 36 | 7.5 | ||
| Hypertension | 39 | 8.1 | ||
| Chronic heart failure | 4 | 0.8 | ||
| Others | 93 | 19.4 | ||
| Unknown | 18 | 3.8 | ||
| Known duration of HBV infection (years) | 5 | 3–9 | ||
| Familial history of Hepatitis B | 185 | 38.5 | ||
| Previous treatment | ||||
| Naive | 105 | 21.9 | ||
| Tenofovir disoproxil fumarate (total) | 340 | 70.8 | ||
| Entecavir | 15 | 3.1 | ||
| Lamivudine | 8 | 1.6 | ||
| Telbivudine | 5 | 1.0 | ||
| Tenofovir disoproxil fumarate + Lamivudine | 8 | 1.6 | ||
| Lamivudine + Entecavir | 3 | 0.6 | ||
| Entecavir + Telbivudine | 4 | 0.8 | ||
| Unknown | 7 | 1.4 | ||
| Interferon history | 73 | 15.2 | ||
| Additional drug usage | 259 | 54.0 | ||
| Use of non-TDF drug affecting BMD | 299 | 62.3 | ||
| Body mass index (kg/m2) | 26.6 | 23.3–29.3 | ||
| HBV DNA | ||||
| Detectable | 78 | 16.3 | ||
| Undetectable | 352 | 73.3 | ||
| Unknown | 50 | 10.4 | ||
| Alanine transaminase | ||||
| Abnormal | 173 | 36.0 | ||
| Normal | 304 | 63.3 | ||
| Unknown | 3 | 0.6 | ||
| Proteinuria | 40 | 8.3 | ||
| White blood cell count (×109/L) | 6.40 | 5.00–7.70 | ||
| Thrombocyte (×109/L) | 217 | 166–268 | ||
| Alanine transaminase (U/L) | 27 | 20–40 | ||
| Aspartate transaminase (U/L) | 27 | 21–36 | ||
| International normalized ratio | 1.10 | 1.00–1.20 | ||
| Total bilirubin (mg/dL) | 0.85 | 0.50–1.20 | ||
| Creatinine (mg/dL) | 0.9 | 0.72–1.04 | ||
| Phosphorus (mg/dL) | 3.0 | 2.6–3.4 | ||
| Albumin (g/dL) | 3.8 | 3.2–4.3 | ||
| Gamma-glutamyl transferase(U/L) | 33 | 22–43 | ||
| Alpha fetoprotein (U/L) | 2.90 | 1.70–3.31 | ||
| eGFR (mL/min/1.73 m2) | 97 | 85–107 | ||
| Lipid profile (mg/dL) | ||||
| Total cholesterol | 180 | 153–210 | ||
| High-density lipoprotein | 48 | 39–59 | ||
| Low-density lipoprotein | 101 | 88–121 | ||
| Hip T score | -1.5 | -2.0– -0.9 | ||
| Spine T score | -2.0 | -3.0– -1.3 |
HBV: Hepatitis B virus; TDF: Tenofovir disoproxil fumarate; eGFR: Estimated glomerular filtration rate; IQR: Interquartile range.
Virological and Biochemical Effect
The comparison of HBV DNA detection in treatment-naive and experienced patients at the beginning and at 12 months of TAF therapy is presented in the Figure 1. HBV DNA was measured at baseline and 12 months in 259 patients. The proportion of treatment-experienced patients with undetectable HBV DNA increased from 89.6% at baseline to 99.4% at 12 months. HBV DNA was undetectable in all NA-naive CHB patients at 12 months.
Figure 1.
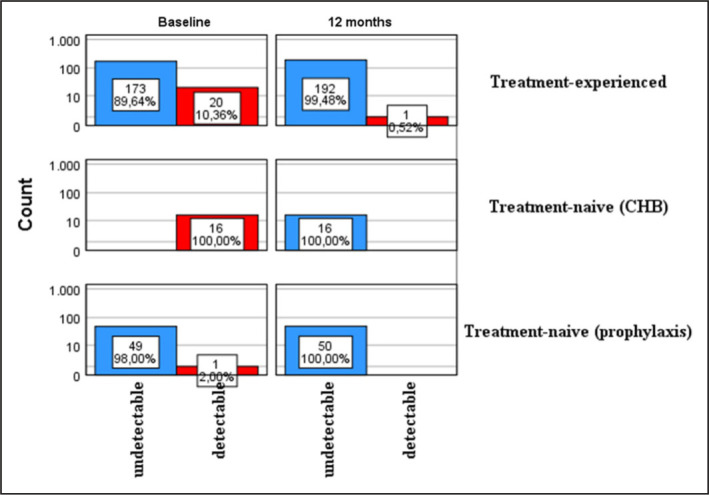
Comparison of rates of HBV DNA detection in treatment-naive and -experienced patients in the first 12 months of TAF therapy.
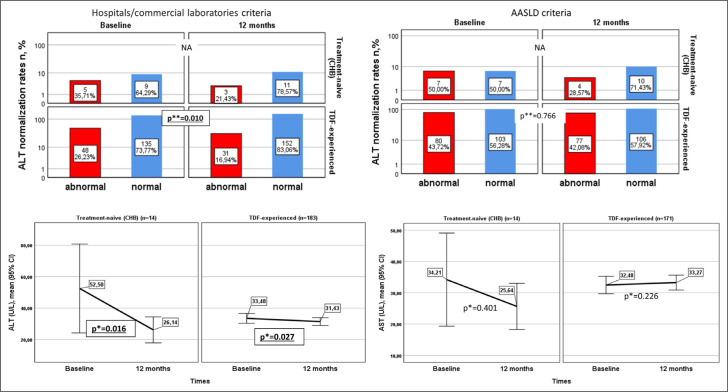
The comparison of ALT normalization rate and mean ALT and AST levels in the 183 TDF-experienced patients and 14 treatment-naive CHB patients whose ALT and AST levels were evaluated at baseline and 12 months is presented in Figure 2. In TDF-experienced patients, the rate of ALT normalization according to AASLD criteria increased from 56.3% to 57.9%. However, this difference was not statistically significant (p=0.766). Based on hospital laboratory criteria (≤40 U/L), this rate increased from 73.8% to 83.1%, and the difference (9.3%) was significant (p=0.001). In treatment-naive CHB patients, the rate of ALT normalization compared to baseline increased according to both criteria. Still, a statistical comparison could not be made because none of the patients had normal ALT at baseline or abnormal ALT at 12 months. Mean ALT values decreased from baseline in TDF-experienced and treatment-naive CHB patients, while there was no statistically significant difference in mean AST values (Fig. 2).
Figure 2.
Comparison of ALT normalization rate and mean ALT and AST values in treatment-naive and TDF-experienced patients in the first 12 months of treatment.
*: Wilcoxon Signed-Rank Test; **: McNemar Test (NA: Not applicable).
The analysis of risk factors associated with ALT elevation according to AASLD criteria at 12 months after switching to TAF in TDF-experienced patients is presented in Table 2. Indicators found to be significant in univariate analysis were included in univariate logistic regression. A statistically significant correlation existed between ALT elevation at 12 months and age, BMI, cholesterol, and albumin. However, none of the parameters showed a linear correlation in multivariate regression analysis (Table 2).
Table 2.
Determinants of high ALT according to AASLD criteria at month 12 after transition from TDF to TAF
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | p* | Odds ratio (95% CI) | p* | |
| Age | 0.97 (0.95–0.99) | 0.021 | 1.01 (0.98 –1.05) | 0.316 |
| BMI (kg/m2) | 1.09 (1.01–1.19) | 0.017 | 1.06 (0.96–1.17) | 0.246 |
| Total cholesterol (mg/dL) | 1.01 (1.00–1.03) | 0.002 | 1.01 (0.99–1.02) | 0.167 |
| Albumin (mg/dL) | 0.56 (0.33–0.95) | 0.034 | 0.54 (0.26–1.34) | 0.105 |
| Total bilirubin (mg/dL) | 1.21 (0.75–1.98) | 0.424 | ||
| INR | 3.63 (0.44–29.87) | 0.230 | ||
| AFP (U/L) | 1.17 (0.94–1.47) | 0.154 | ||
ALT: Alanine transaminase; AASLD: American Association for the Study of Liver Diseases; TDF: Tenofovir Disoproxil Fumarate; TAF: Tenofovir Alafenamide Fumarate; BMI: Body mass index; INR: International normalized ratio; AFP: Alpha fetoprotein; CI: Confidence interval; *: P<0.05.
Renal and Bone Densitometry Results of Patients Switched from TDF to TAF
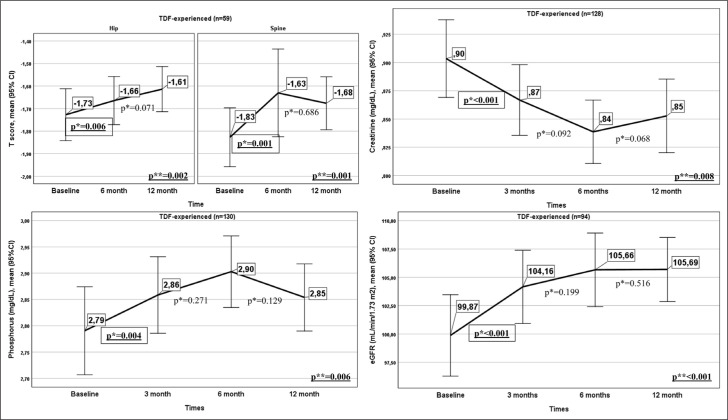
The renal and bone results of patients switched from TDF to TAF are presented in Figure 3. Creatinine, eGFR, and phosphorus data from all four follow-up times within 12 months of switching to TAF were available in 94 patients, 130 patients, and 128 patients, respectively. All three biomarkers had statistically significant changes in the first 12 months (Fig. 3). This was due to substantial increases in eGFR and phosphorus at month 3 compared to baseline and a significant decrease in creatinine at month 3 (p<0.001, p=0.004, and p<0.001, respectively). There were no significant changes in eGFR, phosphorus, or creatinine values between months 3 and 6 or months 6 and 12 (p>0.05).
Figure 3.
Renal and bone outcomes in patients switched from TDF to TAF.
*: Wilcoxon signed rank test; **: Friedman’s F.
In the 59 patients whose BMD was evaluated at baseline, month 6, and month 12 after switching from TDF to TAF, both hip and spine T scores were significantly increased at 6 months compared to baseline (p=0.006 and p=0.001, respectively). However, there was no significant change between months 6 and 12 (p>0.05).
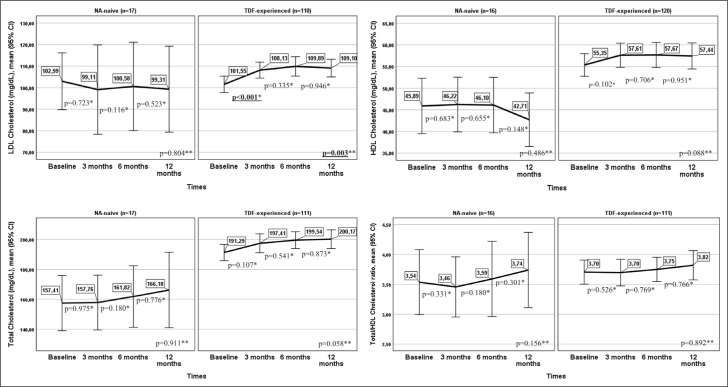
Change in Lipid Profile
The patients’ lipid profiles at baseline and months 3, 6, and 12 of TAF therapy are presented according to treatment history in Figure 4. In the 110 TDF-experienced patients with data from all four follow-up points, LDLc changed significantly from baseline to month 12 of treatment (p=0.003). This difference was associated with increased LDLc values at month 3 compared to baseline (p<0.001). There were no statistically significant changes in LDLc values between months 3 and 6 or months 6 and 12. TC, HDLc, and TC/HDLc ratio showed no significant differences between baseline and 3, 6, and 12 months in TDF-experienced or treatment-naive patients.
Figure 4.
Lipid profiles at baseline and 3, 6, and 12 months according to treatment history.
*: Wilcoxon signed rank test; **: Friedman’s F.
Discussion
In continuation of a previous study evaluating the real-life data of TAF therapy in the first 6 months, this study presents 12-month effectiveness and safety data.[9] The best predictor of HBV infection treatment response is the course of HBV DNA. Achieving undetectable HBV DNA is directly associated with favorable clinical outcomes.[10] In non-inferiority studies of TAF versus TDF, the rate of undetectable HBV DNA at month 12 of TAF therapy was 94% in HBeAg-negative patients and 64% in HBeAg-positive patients.[11,12] In our study, the proportion of treatment-naive (CHB) patients and treatment-experienced patients with undetectable HBV DNA levels at 12 months increased compared to baseline. Over 99% of patients achieved an excellent virological response. The continuation of this effective antiviral therapy may be explained by the efficient extraction of TAF, which has a longer plasma half-life than TDF, in hepatocytes.[13,14]
ALT normalization has been described as an additional endpoint of long-term suppression of viral replication in the treatment of HBV infection.[7] In previously reported real-life data, rates of ALT normalization according to both AASLD and hospital/commercial laboratory criteria increased in patients who switched from TDF to TAF but not significantly. This was attributed to the short follow-up period of 12 months.[15] Although ALT normalization was not significant in our study based on the AASLD criteria when using hospital laboratory criteria, the rate increased significantly, and mean ALT values decreased significantly. This result may be indicative of better results over more extended follow-up periods. In this study, the rate of ALT normalization by AASLD criteria showed a slight increase (56.3% to 57.9%) at 12 months after switching from TDF to TAF, as in previous studies. In a previous study, this was found to be related to the likelihood of ALT elevation in overweight patients.[16] In our study, although they were not found to be significant in the multivariate model, the significant association with low albumin, younger age, high cholesterol, and BMI suggests that there are multiple indicators of ALT normalization.
The mechanism underlying the renal toxicity of TDF is based on the inhibition of mitochondrial DNA polymerase in tubular cells caused by high intracellular tenofovir concentrations.[1,17] Serum creatinine and phosphorus levels have been used to indicate tubular function.[17] Although TDF has a well-tolerated safety profile, serum creatinine elevation above 0.5 mg/dL and serum phosphorus levels below 2 mg/dL were detected in 2.3% and 1.5% of CHB patients who completed TDF treatment for 10 years, respectively.[18] TAF is excreted mainly in the feces (37.1%) and very little via the kidneys (<1%).[13] In addition, TAF reaches intrahepatic active drug concentrations with lower systemic tenofovir, resulting in fewer renal side effects.[19-21] Real-life studies have demonstrated improved renal function indicators in CHB patients who switched from TDF to TAF. Indicators that deteriorated during TDF therapy recovered early after the switch to TAF and showed sustained stabilization.[20] In one study, mean serum phosphorus levels improved significantly up to 3 months after switching from TDF to TAF and were unchanged at 6 months.[21] In another study, the same result was observed for eGFR for up to 6 months, regardless of the duration of TDF use.[15] In our study, mean levels of the renal function indicators phosphorus, creatinine, and eGFR showed significant improvements in the first 3 months and remained stable. This supports evidence that the renal side effects of TDF resolve quickly and that TAF is safe for long-term use.
The adverse effects of TDF on BMD have been associated with phosphorus excretion, increased bone turnover, and direct adverse effects on osteoblast mineralization.[12,21,22] In non-inferiority trials of TAF, a much lower reduction in BMD was observed with TAF than with TDF.[11,12] As in our study, real-life data showed that BMD improved significantly after switching from TDF to TAF.[21] This suggests the reduction in BMD associated with TDF can be reversed quickly. TDF-induced bone loss in the hip has been reported previously and is limited here.[23] However, in our study, we observed increased BMD in both the hip and spine, and although the increase in the hip was not statistically significant, it continued after 6 months. This may be attributed to more significant TDF-associated bone loss in the hip.
In a randomized, double-blind, placebo-controlled study in HIV-infected patients, TC and LDLc values and the percentage of patients with high TC and LDLc levels decreased significantly after 12 weeks of TDF/emtricitabine (FTC) treatment.[24] Another study showed that mean TC and LDLc levels were significantly lower in healthy volunteers who received TDF for 2 weeks compared to placebo.[25] In our study, LDLc significantly increased in TDF-experienced patients in the first 3 months after switching to TAF. Another study with a similar design demonstrated that although treatment-experienced CHB patients exhibited a slight and transient increase in mean TC and LDLc at 6 months, it did not persist at 1 year of treatment.[26]
Similarly, our study observed a significant increase in mean LDLc in the first 3 months in TDF-experienced patients but no significant change at 6 and 12 months. These findings may be associated with losing tenofovir’s lipid-lowering effect with decreased systemic exposure. The lack of a significant change in lipid profile among the treatment-naive patients in our study also supports this inference. In addition, no change in TC/HDLc ratio was reported after the TDF/FTC intervention.[24] We obtained a similar result. This ratio has been reported as the best independent lipid predictor of coronary heart disease (CHD) in the Turkish population.[27,28] This necessitates discussion and investigation of the clinical significance of this change in LDLc regarding CHD risk in prospective studies with larger patient samples.
The strengths of our study are that we examined all aspects of effectiveness and safety data in a large cohort. However, the study has several limitations. One of these is that more than a 1-year follow-up may be needed to evaluate the effectiveness and safety of TAF. Another limitation is the relatively small number of treatment-naive patients and that patients were not evaluated separately according to HBeAg positivity/negativity. In addition, the lack of data for the entire cohort for each determined follow-up point resulted in data loss for comparing repeated measures. In conclusion, TAF is an effective antiviral drug with adequate safety. It is a promising treatment option in the real world, especially in the event of side effects that can occur in TDF-experienced patients.
Footnotes
How to cite this article: Karasahin O, Akdemir Kalkan I, Dal T, Altunisik Toplu S, Harputluoglu M, Mete AO, et al. First year real life experience with tenofovir alafenamide fumarate: The pythagorean cohort. Hepatology Forum 2023; 4(2):61–68.
Ethics Committee Approval
The Dicle University Non-Interventional Clinical Research Ethics Committee granted approval for this study (date: 02.10.2019, number: 12).
Peer-review
Externally peer-reviewed.
Author Contributions
Concept – OK, IAK, TD, SAT, MH, AOM, SK, FS, YY,FF, OKan, SN, DI, FAktar, SKa, NT, SOB, YB, YT, DA, MMO, MKC; Design – OK,IAK, TD, SAT, AOM, SK, FS, YY, FE, SN, FA, SKa, NT, SOB, YB, YT, FAktar, MMO, MKC; Supervision – MKC, YB, YT, FAktar, TD, OKan, DI, MH; Materials – OK, MMO, YD, MA; Data Collection and/or Processing – OK, IAK, SAT,MH, AOM, SK, FS, YY, FE, OKan, SN, DI, FAktar, SKa, NT, SOB, MA, YD; Analysis and/or Interpretation – OK, MMO, YD, MA; Literature Search – OK, IAK, TD,YY, MKC, YB, YT, FA; Writing – OK, IAK, MKC, TD, YY, MMO, MA; Critical Reviews – OK, IAK, MKC, YB, YT, FAktar, TD.
Conflict of Interest
The authors have no conflict of interest to declare.
Financial Disclosure
The authors declared that this study has received no financial support.
References
- 1.Roade L, Riveiro-Barciela M, Esteban R, Buti M. Long-term efficacy and safety of nucleos(t)ides analogues in patients with chronic hepatitis B. Ther Adv Infect Dis. 2021;8:2049936120985954. doi: 10.1177/2049936120985954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang LS, Covert E, Wilson E, Kottilil S. Chronic Hepatitis B infection: a review. JAMA. 2018;319(17):1802–1813. doi: 10.1001/jama.2018.3795. [DOI] [PubMed] [Google Scholar]
- 3.Papatheodoridis GV, Manolakopoulos S, Dusheiko G, Archimandritis AJ. Therapeutic strategies in the management of patients with chronic Hepatitis B virus infection. Lancet Infect Dis. 2008;8(3):167–178. doi: 10.1016/S1473-3099(07)70264-5. [DOI] [PubMed] [Google Scholar]
- 4.Buti M, Riveiro-Barciela M, Esteban R. Tenofovir alafenamide fumarate: a new tenofovir prodrug for the treatment of chronic Hepatitis B infection. J Infect Dis. 2017;216(8):S792–S796. doi: 10.1093/infdis/jix135. [DOI] [PubMed] [Google Scholar]
- 5.Charlton MR, Alam A, Shukla A, Dashtseren B, Lesmana CRA, Duger D, et al. An expert review on the use of tenofovir alafenamide for the treatment of chronic hepatitis B virus infection in Asia. J Gastroenterol. 2020;55(9):811–823. doi: 10.1007/s00535-020-01698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YC, Su YC, Li CY, Hung SK. 13-year nationwide cohort study of chronic kidney disease risk among treatment-naïve patients with chronic hepatitis B in Taiwan. BMC Nephrol. 2015;16:110. doi: 10.1186/s12882-015-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver, Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karasahin O, Akdemir Kalkan I, Dal T, Altunisik Toplu S, Harputoglu M, Mete AO, et al. Real-life data for tenofovir alafenamide fumarate treatment of hepatitis B: The pythagoras cohort. Hepat Mon. 2021;21(2):e104943. [Google Scholar]
- 10.Kau A, Vermehren J, Sarrazin C. Treatment predictors of a sustained virologic response in Hepatitis B and C. J Hepatol. 2008;49(4):634–651. doi: 10.1016/j.jhep.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, et al. GS-US-320-0108 Investigators Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):196–206. doi: 10.1016/S2468-1253(16)30107-8. [DOI] [PubMed] [Google Scholar]
- 12.Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, et al. GS-US-320-0110 Investigators Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):185–195. doi: 10.1016/S2468-1253(16)30024-3. [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration Prescribing information for vemlidy (tenofovir alafenamide) 2020. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208464s008lbl.pdf] (assessed date: 20 April 2021)
- 14.Babusis D, Phan TK, Lee WA, Watkins WJ, Ray AS. Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340. Mol Pharm. 2013;10(2):459–466. doi: 10.1021/mp3002045. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko S, Kurosaki M, Tamaki N, Itakura J, Hayashi T, Kirino S, et al. Tenofovir alafenamide for hepatitis B virus infection including switching therapy from tenofovir disoproxil fumarate. J Gastroenterol Hepatol. 2019;34(11):2004–2010. doi: 10.1111/jgh.14686. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa E, Nomura H, Nakamuta M, Furusyo N, Koyanagi T, Dohmen K, et al. Kyushu University Liver Disease Study (KULDS) Group Tenofovir alafenamide after switching from entecavir or nucleos(t)ide combination therapy for patients with chronic hepatitis B. Liver Int. 2020;40(7):1578–1589. doi: 10.1111/liv.14482. [DOI] [PubMed] [Google Scholar]
- 17.Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, Shlipak MG. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26(7):867–875. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcellin P, Wong DK, Sievert W, Buggisch P, Petersen J, Flisiak R, et al. Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liver Int. 2019;39(10):1868–1875. doi: 10.1111/liv.14155. [DOI] [PubMed] [Google Scholar]
- 19.Lampertico P, Buti M, Fung S, Ahn SH, Chuang WL, Tak WY, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in virologically suppressed patients with chronic hepatitis B: a randomised, double-blind, phase 3, multicentre non-inferiority study. Lancet Gastroenterol Hepatol. 2020;5(5):441–453. doi: 10.1016/S2468-1253(19)30421-2. [DOI] [PubMed] [Google Scholar]
- 20.Farag MS, Fung S, Tam E, Doucette K, Wong A, Ramji A, et al. Effectiveness and renal safety of tenofovir alafenamide fumarate among chronic hepatitis B patients: real-world study. J Viral Hepat. 2021;28(6):942–950. doi: 10.1111/jvh.13500. [DOI] [PubMed] [Google Scholar]
- 21.Farag MS, Fung S, Tam E, Doucette K, Wong A, Ramji A, et al. Effectiveness and Renal Safety of Tenofovir Alafenamide Fumarate among Chronic Hepatitis B Patients: Real-World Study. J Viral Hepat. 2021;28(6):942–950. doi: 10.1111/jvh.13500. [DOI] [PubMed] [Google Scholar]
- 22.Casado JL. Renal and bone toxicity with the use of tenofovir: Understanding at the end. AIDS Rev. 2016;18(2):59–68. [PubMed] [Google Scholar]
- 23.Gill US, Zissimopoulos A, Al-Shamma S, Burke K, McPhail MJ, Barr DA, et al. Assessment of bone mineral density in tenofovir-treated patients with chronic hepatitis B: can the fracture risk assessment tool identify those at greatest risk? J Infect Dis. 2015;211(3):374–382. doi: 10.1093/infdis/jiu471. [DOI] [PubMed] [Google Scholar]
- 24.Santos JR, Saumoy M, Curran A, et al. The lipid-lowering effect of tenofovir/emtricitabine: a randomized, crossover, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61(3):403–408. doi: 10.1093/cid/civ296. [DOI] [PubMed] [Google Scholar]
- 25.Randell PA, Jackson AG, Zhong L, Yale K, Moyle GJ. The effect of tenofovir disoproxil fumarate on whole-body insulin sensitivity, lipids and adipokines in healthy volunteers. Antivir Ther. 2010;15(2):227–233. doi: 10.3851/IMP1518. [DOI] [PubMed] [Google Scholar]
- 26.Sarı ND, Köksal I, Erdem H, Yıldız D, İnce N, Yamazhan T, et al. Digital Hepatology Connect, January 15-16 2021. Changes in lipid profile among HBV patients treated with tenofovir alafenamide: Turkey’s experience of real life setting, AASLD-TASL. [Google Scholar]
- 27.Onat A, Hergenç G, Uzunlar B, Ceyhan K, Uyarel H. Türk toplumunda koroner risk faktörü olarak HDL-kolesterol: öngördürücülüğü, belirleyicileri ve ilişkileri. Türk Kardiyol Dern Arş. 2003;31:9–16. [Turkish] [Google Scholar]
- 28.Onat A, Can G, Yüksel H, Ademoğlu E, Ünaltuna NE, Kaya A. İstanbul: Logos Yayıncılık; 2017. TEKHARF 2017 Tıp dünyasının kronik hastalıklara yaklaşımına öncülük. [Turkish] [Google Scholar]