Abstract
Background and Aim
This study investigated the risk of the development of primary biliary cholangitis (PBC) in individuals who were incidentally identified as having positive antimitochondrial antibodies (AMA)-M2.
Materials and Methods
We retrospectively reviewed extractable nuclear antibody (ENA) panel test results to identify the incidental AMA-M2-positive patients. Patients who filled the diagnostic criteria for PBC were excluded. AMA-M2-positive patients were further evaluated by physical examination, liver biochemistry, liver ultrasonography, and transient elastography (TE) and were also closely followed.
Results
We included 48 (n=45, 93% female) individuals with a median age of 49 (range: 20–69) years. The median follow-up duration was 27 months (range: 9–42) after the detection of AMA-M2. Thirty-three (69%) patients had concomitant autoimmune/inflammatory disorders. Twenty-eight (58%) individuals showed seropositivity for ANA, and 21 had (43%) positive AMA. Fifteen (31%) patients developed typical PBC according to the international PBC diagnostic criteria during the follow-up, and five of them (18%) had significant fibrosis (≥8.2 kPA) by TE at the time of PBC diagnosis.
Conclusion
Two-thirds of the incidental AMA-M2-positive patients developed typical features of PBC after a median 27-month follow-up. Our results suggest that AMA-M2 patients should be closely followed up to detect the late development of PBC
Keywords: Anti-mitochondrial M2 antibody, AMA-M2, primary biliary cholangitis, immunoblot
Introduction
Primary biliary cholangitis (PBC) is an autoimmune, chronic liver disease characterized by the destruction of the intrahepatic biliary ducts. PBC diagnostic criteria include chronic cholestasis, positive serum antimitochondrial antibodies (AMA) or specific antinuclear antibodies (ANA) reactivity, or histologically confirmed chronic non-suppurative, lymphocytic, and granulomatous destruction of the intrahepatic bile ducts.[1]
Serum AMA reactivity is the hallmark feature of PBC.[2] The antimitochondrial antibody targets the 2-oxoacid dehydrogenase complexes, including the pyruvate dehydrogenase complex (PDC-E2), branched-chain 2-oxoacid dehydrogenase complex, and 2-oxoglutaric acid dehydrogenase complex.[3,4] Antimitochondrial M2 antibody (AMA-M2) is the most specific subtype for PBC diagnosis, specifically targeting the PDC-E2 complex.[4]
AMA reactivity is highly specific for PBC diagnosis. More than 95% of all PBC patients are positive for AMA. AMA could be detected in serum years before the diagnosis.[5] Nevertheless, the AMA positivity in the general population is high (1/1000) with respect to the low prevalence of PBC (0,4/1000).[1,3] AMA positivity can rarely be seen in other causes of liver disorders, including autoimmune hepatitis, viral hepatitis (B, C), or non-alcoholic fatty liver disease, and in patients with other immune-mediated disorders such as Sjögren’s syndrome, rheumatoid arthritis (RA), systemic sclerosis, and inflammatory myositis.[4,6,7]
Early retrospective studies from the United Kingdom indicated that AMA-positive healthy individuals might naturally evolve to PBC.[8] Controversially, a prospective cohort study from France reported that nearly half of the AMA-positive non-PBC individuals were unrelated to PBC diagnosis or poor hepatic outcomes. Only one in six patients finally developed into PBC after a median 5-year follow-up.[9] Another study from Austria reported that 10% of AMA-positive individuals developed PBC during 6 years of follow-up.[10] The EASL PBC guideline considers incidental AMA reactivity and recommends annual liver function test assessments of AMA-positive patients with normal biochemical results.[1]
Indirect immunofluorescence testing is the gold standard for AMA detection among different serological methods.[4] A titer of 1/40 or higher is necessary for PBC diagnosis. AMA-M2 detection using immunosorbent assay or western blot techniques would be helpful in the absence or weak seropositivity of AMA-IIF.[11] Several recent studies suggested that AMA-M2 dot blotting is more sensitive and specific for PBC.[12,13]
The majority of studies have evaluated the relevance of AMA positivity in the general population or in patients who had a diagnosis of immune-meditated disorders while little data are available about the outcome of individuals who have seropositivity for AMA-M2. In this study, we investigated the natural evolution of incidental AMA-M2 positivity to PBC diagnosis.
Materials and Methods
Subject Baseline Data
From January 2016 to May 2021, 11459 Extractable Nuclear Antigen Antibodies (ENA) Immunoblot (IB) panel test (Euroimmun, Germany), including AMA-M2, was performed to screen or establish connective tissue disease. One hundred and nineteen (1.03%) of 11459 individuals had positive AMA-M2 antibodies. Twenty-one patients were excluded due to prior PBC diagnosis, two due to underage consenting, and one due to death early after the AMA-M2 testing. The remaining 95 patients were invited to the study, and half agreed to participate. Figure 1 summarizes the flowchart of the enrolled patients.
Figure 1.
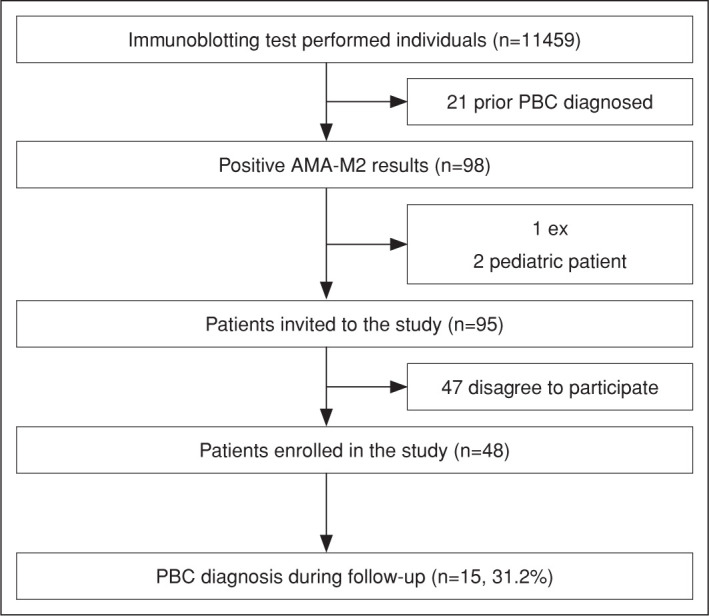
Flow chart of enrolled patients.
PBC: Primary biliary cholangitis; AMA-M2: Antimitochondrial M2 antibody; IFA: Indirect immunofluorescence test.
The patient’s medical history, baseline laboratory results, medical specialty indicating initial ENA panel, and liver imaging results were screened from the hospital’s electronic database. Table 1 summarizes the specialties indicating the AMA-M2 testing. The protocol was approved by Marmara University School of Medicine Research’s Ethics Committee (Approval number 06.2022.885, dated June 03, 2022) and conducted in accordance with the ethical standards of the Declaration of Helsinki.
Table 1.
Specialties indicating the AMA-M2 testing
| Specialties | n | % |
|---|---|---|
| Rheumatology | 31 | 64.5 |
| Internal medicine | 9 | 18.75 |
| Neurology | 5 | 10 |
| Other | 3 | 6.2 |
AMA-M2: Antimitochondrial M2 antibody.
Follow-up
We contacted all PBC non-established individuals who tested positive for AMA-M2 antibodies by phone. We invited them to our hepatology outpatient clinic for a detailed follow-up examination, including symptom assessment, physical examination, laboratory analysis, liver ultrasonography, and liver and spleen stiffness measurements (LSM and SSM) with transient elastography (TE) (FibroScan® 630 Expert; Echosens, Paris, France). Patients who participated in at least two outpatient visits were included in the final analysis.
Laboratory Evaluation
Liver enzymes tested during or before AMA-M2 IB testing were recorded as the baseline results. During the hepatology visits, alkaline phosphatase (ALP), γ-glutamyl transferase, alanine aminotransferase, aspartate aminotransferase, full blood count, ANA, anti-smooth muscle antibody (ASMA), anti-liver-kidney microsomal (LKM) antibody, AMA, IgG, and IgM were obtained. AMA and ANA were detected using the indirect immunofluorescence assay (IIF) method (Euroimmun Inc.). Titers equal to or higher than 1:40 and 1:80 were considered positive for AMA and ANA, respectively.
Statistical Analysis
The distribution of variables was assessed by the Shapiro-Wilk test, and the significance level was set as α=0.05 two-tailed. Descriptive statistics were shown as median (range) in nonnormally distributed variables and as mean in normally distributed variables. Patients’ groups were compared using the Mann Whitney-u test, Chi-square test, and independent sample t-tests.
Results
Patients Characteristics
A total of 48 (96% female) individuals with a median age of 49 years (range: 20–69) were included in the study. The median follow-up duration was 27 months (range: 9–42). Thirty-three patients (68.7%) had concomitant autoimmune/inflammatory disorders, including RA (n=10), Sjögren’s syndrome (n=5), and mixed connective tissue disorders (n=5), which were the most common. Concomitant diagnosed immunological diseases in AMA-M2-positive patients are presented in Table 3. Six patients were associated with metabolic-associated fatty liver disease (MAFLD). Comorbidities were type II diabetes mellitus in five patients, asthma, Cushing’s disease, hypothyroidism, and multiple myeloma in one patient each. Six patients had a family history of cirrhosis, and two had a family history of hepatocellular carcinoma. The baseline clinical and laboratory characteristics of AMA-M2-positive patients are summarized in Table 2.
Table 3.
Antibody positivity and PBC diagnosis rates according to underlying autoimmune/inflammatory disorders
| Immune-mediated inflammatory disease | ANA positivity, n | AMA-IIF positivity, n | PBC diagnosis, n |
|---|---|---|---|
| Rheumatoid arthritis (n=10) | 3 | 2 | 4 |
| Sjögren’s disease (n=5) | 4 | 1 | 0 |
| Mixed connective tissue disorders (n=5) | 3 | 3 | 2 |
| Systemic lupus erythematosus (n=4) | 4 | 0 | 0 |
| Ankylosing spondylitis (n=5) | 3 | 3 | 3 |
| Systemic sclerosis (n=2) | 2 | 1 | 1 |
| Sarcoidosis (n=1) | 0 | 0 | 0 |
| Psoriasis (n=1) | 0 | 0 | 0 |
| Multiple sclerosis (n=2) | 1 | 0 | 0 |
| Familial mediterranean fever (n=1) | 1 | 1 | 0 |
ALP: Alkaline phosphatase; GGT: Gamma glutamine transferase; ANA: Antinuclear antibody; AMA-IIF: Antimitochondrial antibody with indirect immunofluorescence method; PBC: Primary biliary cirrhosis.
Table 2.
Baseline clinical and laboratory characteristics of AMA-M2-positive patients
| Parameter | All patients (n=48) | PBC (n=15) diagnosed individuals | Non-PBC (n=33) individuals | p |
|---|---|---|---|---|
| n/median (range) | ||||
| Age* | 48.5 (20–70) | 56.3 (40–69) | 44.9 (20–70) | 0.005 |
| Sex (male/female) | 2/46 | 0/15 | 2/31 | |
| Follow-up period | 27 (9–42) | 27.6 (13–42) | 26.69 (9–41) | 0.744 |
| Direct bilirubin (mg/dL) | 0.14 (0.06–0.66) | 0.15 (0.8–0.66) | 0.13 (0.06–0.37) | 0.224 |
| Total bilirubin (mg/dL) | 0.29 (0.1–1.3) | 0.39 (0.17–0.85) | 0.28 (0.1–1.30) | 0.476 |
| Albumin (g/L) | 44 (35–50) | 44 (35–47) | 44 (36–50) | 0.973 |
| IgM | 1.5 (0.41–7.13) | 1.87 (0.41–7.13) | 1.41 (0.45–6.68) | 0.032 |
| PBC-specific ANA (+) | 7/48 | 4/15 | 3/33 | 0.270 |
| AMA (+) | 21/48 | 13/15 | 8/33 | 0.000 |
| ALP (IU/L) | 81 (31–503) | 126 (61–503) | 73 (31–110) | 0.000 |
| GGT (IU/L) | 21.3 (6.5–646) | 48.5 (9.6–646) | 17.4 (6.5–127) | 0.005 |
| AST (IU/L) | 20.4 (8.7–124) | 27.3 (17.8–124.9) | 17 (8.7–31) | 0.000 |
| ALT (IU/L) | 16.1 (6.6–53) | 19.5 (6.6–48.8 | 14.7 (6.8–53) | 0.322 |
: Represents the mean value and normal distribution; PBC: Primary biliary cholangitis; ANA: Antinuclear antibody; AMA: Antimitochondrial antibody; ALP: Alkaline phosphatase; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; GGT: Gamma glutamine transferase.
Twenty-eight individuals underwent TE analysis. Among them, 11 individuals were finally diagnosed with PBC. Five of them (17.8%) had significant fibrosis (≥F2 or 8.2 kPA). Two of those five patients were in the PBC non-established group. Twenty-three of the 28 patients had spleen stiffness measurement. 52.2% (n=12) had increased spleen stiffness (≥21 kPA), and two of them had an increased risk of clinically significant portal hypertension (>50 kPA).
Twenty-one patients (43.7%) tested positive for AMA antibodies by indirect immunofluorescence (IIF) during regular follow-ups. Twenty-eight (58.3%) tested positive for ANA, one patient tested positive for anti-LKM, and none tested positive for ASMA.
Patients Diagnosed with PBC
Fifteen patients were diagnosed with PBC according to the international suggested guideline criteria. Six patients showed elevated ALP after 6 months of follow-up and met the PBC diagnostic. Figure 2 represents the cumulative PBC diagnosis rate during follow-up. All PBC-diagnosed patients were female, with a median age of 55 years at the time of diagnosis. Ten patients were ANA-positive, and four showed a nuclear dot pattern. Five patients had negative ANA results, and two had negative AMA-IIF serology. Four patients had RA, and one had Hashimoto’s disease. Three patients had accompanying ankylosing spondylitis (AS), two had mixed connective tissue disorder, and one had systemic sclerosis. Five patients had no other autoimmune/inflammatory disorders, but one had asthma, and one had ACTH-independent Cushing’s disease.
Figure 2.
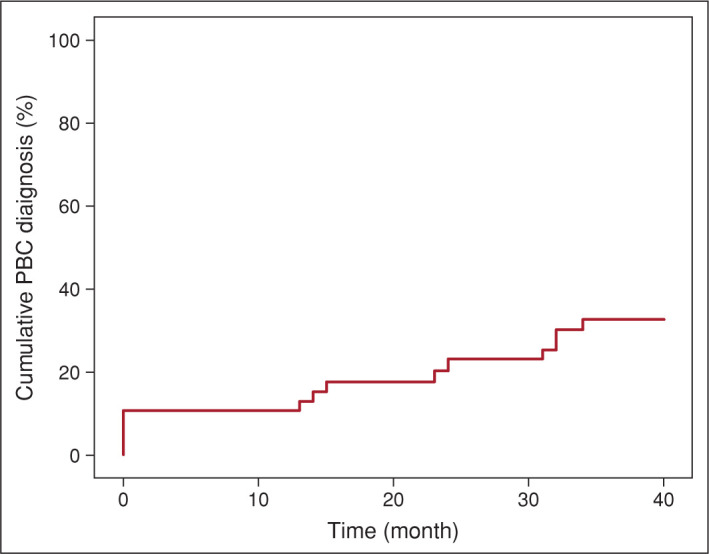
Cumulative PBC diagnosis rate during follow-up.
PBC: Primary biliary cholangitis.
Eleven patients with a definitive diagnosis of PBC underwent TE measurement. Two patients had F4 fibrosis (28.4 kPA and 30.6 kPA), one had F2 fibrosis (9.3 kPA), five had F1 fibrosis (between 6.2 and 8.0 kPA), and the remaining four had no fibrosis (F0, <6 kPA). All but two patients had successful spleen stiffness measurements, and seven of these nine patients had increased spleen stiffness (≥21 kPA), including one patient with clinically significant portal hypertension (>50 kPA).
Symptom analysis revealed fatigue in six patients, pruritus in five, steatorrhea in three, and osteoporosis in four. Two patients had a history of unexplained jaundice before the study inclusion.
AMA-M2-Positive Patients with Non-Established PBC
Twenty-three patients who tested positive for AMA-M2 had no established PBC diagnosis during the follow-up. They did not show ALP elevation or clinical features regarding PBC. Diagnoses of other autoimmune/inflammatory disorders in these groups were RA (n=6), Sjögren’s syndrome (n=5), SLE (n=4), connective tissue disorders (n=3), AS (n=2), psoriasis (n=2), sarcoidosis (n=1), systemic sclerosis (n=1), and multiple sclerosis (n=1). One patient had multiple myeloma, and two patients had MAFLD.
Patients with PBC were significantly younger than those individuals with AMA-M2 positive (median age 45±55 years; p=0.01). Furthermore, they had a lower LSM score compared to PBC-diagnosed individuals (median LSM 4.3 kPA vs 7.3 kPA, p=0.007).
AMA-IIF-Positive Patients
Twenty-one patients showed positive AMA-IIF test results. Five of these showed nuclear dot-patterned ANA positivity. Thirteen patients (61.9%) were diagnosed with PBC. Fifteen AMA-positive individuals had autoimmune/inflammatory disorders, including RA (n=4), connective tissue disorders (n=3), AS (n=3), FMF (n=1), Sjögren’s disease (n=1), systemic sclerosis (n=1), and psoriasis (n=1). They had a median 6.7 kPa LSM score. Nine patients experienced fatigue, seven experienced pruritus, and five experienced steatorrhea. The frequency of seropositivity for ANA and AMA and PBC diagnosis rates are shown in Table 3.
AMA-IIF-Negative Patients
Twenty-seven individuals had negative AMA-IIF serology. Fifteen individuals had ANA titers between 1/80 and 1/320. One patient had a centromeric pattern, and one had nuclear dot-patterned ANA positivity. Twenty patients had coexisting autoimmune/inflammatory disorders, RA (n=6), SLE (n=4), or Sjögren’s disease (n=4). Other extrahepatic autoimmune/inflammatory disorders included sarcoidosis (n=1), systemic sclerosis (n=1), and AS (n=1). Twelve of these patients had fatigue, seven experienced pruritus, and five had osteoporosis. Two AMA-IIF-negative and ANA-positive patients were diagnosed with PBC. One patient had a history of jaundice in the past.
Discussion
PBC is an insidious disease often asymptomatic and incidentally diagnosed with abnormal liver biochemistry results, revealing mild-to-moderate cholestasis.[14] AMA is an important serum marker for PBC diagnosis. AMA reactivity is enough to diagnose PBC without needing a biopsy when combined with elevated ALP levels.[1] There is plenty of evidence about the risk of PBC development in AMA-positive and healthy individuals. Although AMA-M2 is the most specific AMA subtype for PBC diagnosis, it can also be found in 1% of healthy individuals. Little data are available about the clinical relevance of AMA-M2 positivity in healthy individuals.[4]
In our study population, AMA-M2 was positive in about 1% of individuals. The study population mainly included patients diagnosed with or suspected of having autoimmune/inflammatory disorders, and do not convey the general population. Studies conducted on 8126 and 5011 healthy Chinese individuals showed that the frequency of AMA-M2 was 0.23% and 0.16%, respectively.[15,16] Other large population-based studies from China reported AMA-M2 seropositivity in 0.74% of 20970 healthy individuals.[17] AMA-M2 seropositivity rate was reported to be higher in those individuals aged >60 years (1.31% of 6008 individuals).[18] Mitochondrial antibodies can also be detected in patients with non-liver autoimmune disorders or coexisting hepatic and extrahepatic autoimmune disorders.[19,20] In a multinational study including Turkish patients, a large number of autoimmune diseases were reported to be associated with autoimmune liver disease.[21] Most of the patients had concomitant autoimmune/immune-mediated disorders in our population, which may explain the numerically higher AMA-M2 positivity rate.
Our study evaluated the long-term clinical relevance of AMAM2 positivity in non-PBC subjects.[9,10,22] We observed that nearly one-third of the incidental AMA-M2-positive patients were finally diagnosed with PBC in a relatively short follow-up period. Importantly, some of the PBC-diagnosed patients had baseline elevated ALP levels before AMA-M2 IB testing. These patients were not referred to a gastroenterologist by the clinician who requested the AMA-M2 test. A recent cohort study from France reported that 13% of AMA-positive patients were underdiagnosed by physicians despite having elevated ALP levels at the time of initial testing.[9] This shows low awareness of PBC among non-hepatic specialists, which could increase the risk of disease progression due to delays in treatment.
AMA positivity in healthy individuals and its evolution to PBC remain controversial. A very early small cohort study from the United Kingdom (UK) revealed that 16 of 29 AMA-positive, non-PBC individuals developed elevations in ALP during follow-up, and 23 of them fulfilled the PBC diagnostic criteria for a mean 8.7-year follow-up period.[8] This was the highest rate of PBC progression among the AMA-positive individuals. The most important nuance of this study was that all patients had autoimmune disorders, particularly arthritis and thyroid diseases. The rate of PBC development decreased in studies reported in the following years. A prospective cohort study from France reported that nearly half of the AMA-positive non-PBC individuals were unrelated to PBC diagnosis or liver-related poor outcomes. Only 16% of patients eventually developed PBC within 5 years.[9] Another study from Austria reported that 10% (6 out of 158) of AMA-positive individuals developed PBC at 6 years of follow-up.[10] In the most recent study from China, only 4.3% of 139 AMA-positive individuals developed PBC during a 4.6-year median follow-up period.[23]
We did not perform a liver biopsy on any of the individuals. In the very early UK cohort, all patients underwent liver biopsy, and the majority showed histological features compatible with PBC, while in the French cohort, a minority of patients underwent liver biopsy, and none of those patients displayed histological lesions suggestive of PBC.[8,9] In the era of highly sensitive autoantibodies, the additional diagnostic value of liver biopsy in this group is controversial. Besides the diagnostic clues, liver biopsy detects fibrosis in patients with underlying indolent but slowly progressive PBC. Because liver biopsy is an invasive and expensive procedure, we prefer to screen for fibrosis noninvasively by TE.
We reported a higher percentage of PBC development over a relatively short period of time.
To explain this, two unique features of the study design should be emphasized. First, AMA-M2 is more specific than AMA in the diagnosis of PBC. Second, the study population was composed of patients with highly suspicious or diagnosed autoimmune/inflammatory disorders, especially rheumatologic and neurologic autoimmune populations, just like the very early United Kingdom AMA cohort.[8] PBC non-established individuals in this study had a lower median age than the definite PBC-diagnosed patient group. This may be a clue to expect an increase in PBC diagnosis after a longer follow-up period.
Conclusion
One-third of the incidental AMA-M2-positive patients with autoimmune features developed PBC during a 27-month follow-up period. AMA-M2 positivity requires detailed evaluation or close follow-up for PBC diagnosis, specifically in patients with other autoimmune/inflammatory disorders.
Footnotes
How to cite this article: Ergenc I, Gozaydinoglu B, Keklikkiran C, Yilmaz Y. The risk of development of primary biliary cholangitis among incidental antimitochondrial M2 antibody-positive patients. Hepatology Forum 2023; 4(2):69–73.
Ethics Committee Approval
The Marmara University Clinical Research Ethics Committee granted approval for this study (date: 03.06.2022, number: 06.2022.885).
Peer-review
Externally peer-reviewed.
Author Contributions
Concept – IE, BG, YY; Design – IE, BG, YY; Supervision – YY, IE; Materials – CK, BG; Data Collection and/or Processing – BG, CK, IE; Analysis and/or Interpretation – IE, BG, YY; Literature Search – BG, IE; Writing – BG, IE; Critical Reviews – IE, YY.
Conflict of Interest
The authors have no conflict of interest to declare.
Financial Disclosure
The authors declared that this study has received no financial support.
References
- 1.European Association for the Study of the Liver, Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67(1):145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Bassendine MF, Jones DE, Yeaman SJ. Biochemistry and autoimmune response to the 2-oxoacid dehydrogenase complexes in primary biliary cirrhosis. Semin Liver Dis. 1997;17(1):49–60. doi: 10.1055/s-2007-1007182. [DOI] [PubMed] [Google Scholar]
- 3.Shuai Z, Wang J, Badamagunta M, Choi J, Yang G, Zhang W, et al. The fingerprint of antimitochondrial antibodies and the etiology of primary biliary cholangitis. Hepatology. 2017;65(5):1670–1682. doi: 10.1002/hep.29059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colapietro F, Lleo A, Generali E. Antimitochondrial antibodies: from bench to bedside. Clin Rev Allergy Immunol. 2022;63(2):166–177. doi: 10.1007/s12016-021-08904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi S, Cauch-Dudek K, Heathcote EJ, Lindor K, Jorgensen R, Klein R. Antimitochondrial antibody profiles: are they valid prognostic indicators in primary biliary cirrhosis? Am J Gastroenterol. 2002;97(4):999–1002. doi: 10.1111/j.1572-0241.2002.05620.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen CH, Lee CM, Chen CH, Hu TH, Wang JH, Hung CH, et al. Prevalence and clinical relevance of serum autoantibodies in patients with chronic hepatitis C. Chang Gung Med J. 2010;33(3):258–265. [PubMed] [Google Scholar]
- 7.Luo JC, Hwang SJ, Li CP, Lu RH, Chan CY, Wu JC, Chang FY, Lee SD. Clinical significance of serum auto-antibodies in Chinese patients with chronic hepatitis C: negative role of serum viral titre and genotype. J Gastroenterol Hepatol. 1998 May;13(5):475–479. doi: 10.1111/j.1440-1746.1998.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 8.Mitchison HC, Bassendine MF, Hendrick A, Bennett MK, Bird G, Watson AJ, et al. Positive antimitochondrial antibody but normal alkaline phosphatase: is this primary biliary cirrhosis? Hepatology. 1986;6(6):1279–1284. doi: 10.1002/hep.1840060609. [DOI] [PubMed] [Google Scholar]
- 9.Dahlqvist G, Gaouar F, Carrat F, Meurisse S, Chazouillères O, Poupon R, et al. French network of Immunology Laboratories Large-scale characterization study of patients with antimitochondrial antibodies but nonestablished primary biliary cholangitis. Hepatology. 2017;65(1):152–163. doi: 10.1002/hep.28859. [DOI] [PubMed] [Google Scholar]
- 10.Zandanell S, Strasser M, Feldman A, Tevini J, Strebinger G, Niederseer D, et al. Low rate of new-onset primary biliary cholangitis in a cohort of anti-mitochondrial antibody-positive subjects over six years of follow-up. J Intern Med. 2020;287(4):395–404. doi: 10.1111/joim.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386(10003):1565–1575. doi: 10.1016/S0140-6736(15)00154-3. Erratum 2015 Lancet, 386(10003)1536. [DOI] [PubMed] [Google Scholar]
- 12.Bargou I, Mankaï A, Jamaa A, Ben Jazia I, Skandrani K, Sfar H, et al. Detection of M2 antimitochondrial antibodies by dot blot assay is more specific than by enzyme linked immunosorbent assay. Pathol Biol (Paris) 2008;56(1):10–14. doi: 10.1016/j.patbio.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Poyatos E, Morandeira F, Climent J, Mas V, Castellote J, Bas J. Detection of anti-mitochondrial 2-oxoacid dehydrogenase complex subunit’s antibodies for the diagnosis of primary biliary cholangitis. Clin Immunol. 2021:108749. doi: 10.1016/j.clim.2021.108749. [DOI] [PubMed] [Google Scholar]
- 14.Leuschner U. Primary biliary cirrhosis--presentation and diagnosis. Clin Liver Dis. 2003;7(4):741–758. doi: 10.1016/s1089-3261(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 15.Liu HY, Wang LX, Liu YF. [Frequencies of autoantibodies specific for primary biliary cirrhosis in a general adult population group] Zhonghua Gan Zang Bing Za Zhi. 2008 Dec;16(12):922–5. [Chinese] [PubMed] [Google Scholar]
- 16.Jiang XH, Zhong RQ, Fan XY, Hu Y, An F, Sun JW, et al. Characterization of M2 antibodies in asymptomatic Chinese population. World J Gastroenterol. 2003;9(9):2128–2131. doi: 10.3748/wjg.v9.i9.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Wang C, Liu X, Liu J, Wang M, Gao Z, et al. [Evaluation of markers associated with primary biliary cirrhosis in a population of anti-mitochondrial antibody-M2-positive individuals] Zhonghua Gan Zang Bing Za Zhi. 2014;22(10):735–738. doi: 10.3760/cma.j.issn.1007-3418.2014.10.004. [Chinese] [DOI] [PubMed] [Google Scholar]
- 18.Chen B, Wu X, Su C, Zhao L. Screening of anti-M2 mitochondrial antibody in elderly population. Chin J Gen Pract. 2014;6:680–682. [Google Scholar]
- 19.Karakaya F, Oztekin S, Ozturk Y, Kalkan C, Ellik ZM. Elhan AH, et al. Clinical significance of concomitant extrahepatic autoimmune disease in patients with autoimmune liver disease. Hepatol Forum. 2021;2(1):3–6. doi: 10.14744/hf.2020.2020.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demir N, Efe C, Olmez S, Onur A, Yoshida EM. Anti-SLA and AMA-positive celiac disease: A report of two cases and evaluation of autoimmune liver serology. Hepatol Forum. 2020;1(2):68–71. doi: 10.14744/hf.2020.2020.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efe C, Wahlin S, Ozaslan E, Berlot AH, Purnak T, Muratori L, et al. Autoimmune hepatitis/primary biliary cirrhosis overlap syndrome and associated extrahepatic autoimmune diseases. Eur J Gastroenterol Hepatol. 2012;24(5):531–534. doi: 10.1097/MEG.0b013e328350f95b. [DOI] [PubMed] [Google Scholar]
- 22.Metcalf JV, Mitchison HC, Palmer JM, Jones DE, Bassendine MF, James OF. Natural history of early primary biliary cirrhosis. Lancet. 1996;348(9039):1399–1402. doi: 10.1016/S0140-6736(96)04410-8. [DOI] [PubMed] [Google Scholar]
- 23.Duan W, Chen S, Li S, Lv T, Li B, Wang X, et al. The future risk of primary biliary cholangitis (PBC) is low among patients with incidental anti-mitochondrial antibodies but without baseline PBC. Hepatol Commun. 2022;6(11):3112–3119. doi: 10.1002/hep4.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]


