Abstract
Background
Surgery for obstructive sleep apnea (OSA) has changed in concept and technique that transformed from radical excision to functional reconstruction. The aim of this study was to investigate the safety and effectiveness of palatal hybrid surgery in OSA patients.
Methods
Palatal hybrid surgery is a tissue-specific technique (mucosa-preservation, tonsil-excision, fat-ablation, muscle-relocation/suspension) used in treating OSA patients with velopharyngeal obstruction. The study included 46 consecutive adults OSA patients. The palatal hybrid surgery annotates uvulopalatopharyngoplasty in stereoscopic reconstruction of tonsillar fossa (pharyngoplasty), omni-suspension of the soft palate (palatoplasty) and advancement of uvula (uvuloplasty).
Results
No patient experienced airway compromise, voice change or persistent nasal regurgitation following palatal hybrid surgery. One patient existed postoperative tonsillar fossa bleeding received conservative treatment. Postoperative pain in visual analogue scale (VAS) showed average score of 3, 3, 2, 0 at the 1st, 3rd, 7th, 14th day, respectively. Perioperative snoring severity (VAS) (8.7 vs 2.6) and daytime sleepiness (Epworth Sleepiness Scale) (11.3 vs 5.5) all improved significantly (p < 0.001). Posterior air space in retropalatal area increased from 8.4 to 11.1 mm (p < 0.001). Home sleep test showed that apnea–hypopnea index significantly reduced from 41.8 to 18.2 event/h and minimal oxygen saturation increased from 72.4 to 81.5% (p < 0.001). The success rate in individual Friedman stage was 100% (stage I), 63% (stage II) and 58% (stage III) with a total success rate of 63%.
Conclusion
Palatal hybrid surgery using tissue-specific maneuver annotates UPPP in concept and technique. The results show that palatal hybrid surgery is mini-invasive with low morbid and is effective in improving subjective clinic symptoms, objective sleep parameters and success rate of OSA.
Keywords: Palatal hybrid surgery, Uvulopalatopharyngoplasty, Omni-suspension of soft palate, Stereoscopic reconstruction of tonsillar fossa, Push and pull maneuver, Obstructive sleep apnea
At a glance of commentary
Scientific background on the subject
Traditional UPPP uses radical excision of redundant soft palate to enlarge velopharynx and improve sleep-related breathing disorder. UPPP had been criticized for its low success rate in treating obstructive sleep apnea and high complication rate in disturbance of swallowing and phonation.
What this study adds to the field
Palatal hybrid surgery adopts suspension of the muscles, ablation of the fat and excision of the tonsils. These hybrid method offers physiological reconstruction with significant reduction of complication and increase of success rate.
Introduction
Obstructive sleep apnea (OSA) is characterized by repeated obstruction of the upper airway during sleep [1]. The consequences of OSA involve many systems especially in increased risk of stroke and cardiovascular disease [2,3]. Continuous positive airway pressure (CPAP) is the gold standard and 1st line treatment [4], by contrast, surgery is an alternative and salvage treatment and for those patients who are unwilling or poor compliance to CPAP therapy [5].
Among various sleep surgeries, uvulopalatopharyngoplasty (UPPP) is the most commonly used surgery for snoring and OSA [6]. Nevertheless, UPPP was criticized by its low success rate and high complications [7]. Traditional UPPP adhered to the excision of “redundant” soft tissue to enlarge upper airway space [8]. Therefore, general outcomes of excision-based technique were associated with the grade of tonsil and largely attributable to tissue volume reduction from tonsillectomy [9,10]. Further, excessive excision of the pharyngeal muscle for enlargement of airspace incurs complications in swallowing and articulation [11].
Modified UPPP procedures have been implemented for decades to improve its safety and efficacy in OSA patients [[12], [13], [14], [15], [16], [17]]. Among them, lateral pharyngoplasty [18] and Barbed reposition pharyngoplasty [19] had major impacts on concept and technique in the evolution of palatal surgery. Lateral pharyngoplasty addressed on the stabilization of lateral pharyngeal wall and lessened its collapse during sleep [18]. Barbed reposition pharyngoplasty focused on the suspension of the soft palate and stretching of pharyngeal muscle [19]. These two techniques inspired and encouraged many modifications of palatal surgery for OSA [[20], [21], [22], [23]].
From the pathophysiological viewpoint, narrowing of the velopharyngeal space in OSA patients can be attributable to anatomical obstruction from increased tissue volume and physiological collapse from decreased muscle tone during sleep with different proportion in individuals [24] Among the pharyngeal components, tonsil and fat are belong to volume-based obstruction and muscle is part of tone-based collapse [24]. Therefore, an integrated palatal surgery needs to fulfill the improvement in both obstruction and collapse.
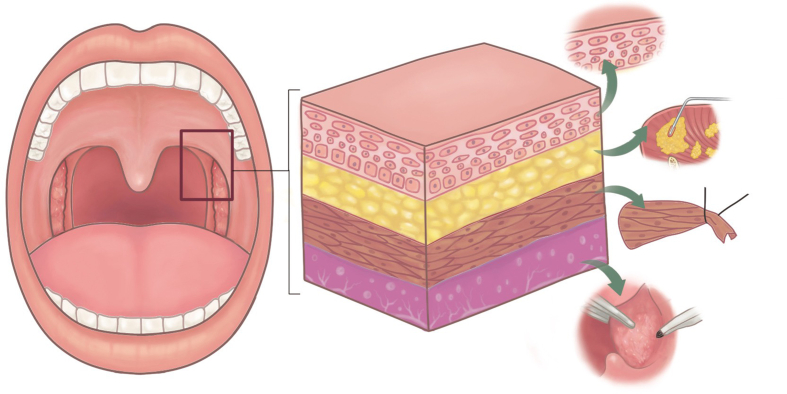
In this proof-of-concept study, we propose a novel technique-palatal hybrid surgery that involves tissue-individualized technique (mucosa-preservation, fat-ablation, tonsil-excision, muscle-relocation/suspension) in palatal surgery for OSA (Fig. 1). The use of tissue-specific hybrid technique in UPPP is based on anatomy (obstruction) and physiology (collapse) to maximize the velopharyngeal space for respiration, moreover, with no damage to normal pharyngeal function in phonation and swallowing. The objective of this study is to investigate the safety and efficacy of palatal hybrid surgery in OSA patients. The results from this study may be helpful in contributing to integrated precision surgery in treating OSA patients.
Fig. 1.
Palatal hybrid surgery is a tissue-specific technique that involves mucosa-preservation, fat-ablation, tonsil-excision, muscle-relocation&suspension.
Method
This study was approved by The Institutional Review Board of Linkou-Chang Gung Memorial Hospital, Taoyuan, Taiwan (202100843B0).
Study population
Forty-six consecutive OSA patients who underwent palatal hybrid surgery for OSA were reviewed in a tertiary referral sleep center at Linkou Chang Gung Memorial Hospital. Eligible candidates for palatal hybrid surgery were diagnosed OSA (apnea/hypopnea index (AHI) > 5 events/h) on preoperative sleep test with clinical symptoms (snoring with/without daytime sleepiness, unrefreshed sleep, choking, gasping, morning headache). All patients were unsatisfied to conservative treatments and unwilling or poor compliance to CPAP therapy. Inclusion criteria included: age between 20 and 65 years, BMI <32 kg/m2, mouth opening space ≥3 finger breadth (4 cm), velopharyngeal obstruction in Muller's maneuver [25], complete questionnaires (visual analogue scale (VAS) of snoring, Epworth sleepiness scale [26] (ESS)) and home sleep test before and 6 months after surgery. Exclusion criteria included significant retrognathia or syndromic patients, Friedman tongue position [27] IV, severe medical disease, previous palate surgery, and high risk for general anesthesia (American Society of Anesthesiologists physical status class > 2).
Home sleep test
Patients with sleep-disordered breathing were routinely received polysomnography in our sleep center before treatment for decades. However, the sleep center have been repeatedly placed on lockdown to prevent spread of COVID-19 in the past two to three years. Further, the subjects needed to show certificate of negative in PCR test for the allowance of in-lab sleep test according to local rule. These largely impeded the process of in-lab sleep test and reduced patients' motivation to receive standard polysomnography. In the epidemic situation, we consequently shifted in-lab sleep test to home sleep test for safety and non-delayed diagnosis of OSA in highly-suspected symptomatic patients. For those patients who were suspected of being co-morbid with insomnia and other sleep disorders or major medical diseases received standard polysomnography.
The portable home sleep test we used in this study was ApneaLink® (ResMed, Sydney, Australia) [28,29], which is powered by two 1.5-V AA batteries and fixed on to the user's chest with the supplied belt. Parameters measured with are pulse oximetry and oronasal flow via nasal cannula to record the flow signal. Once the user presses the start button, ApneaLink® is capable of monitoring breathing patterns and measures apneas or hypopneas as well as flow limitation, snoring sounds, and inspiratory flow. The analog signal is digitalized and scanned on respiratory events, which fulfill the predetermined definitions for hypopneas and apneas. An event log shows and saves the total number of apneas, hypopneas and AHIs per hour recording time. Those parameters are analyzed automatically, but were reviewed and rescored by an experienced sleep specialist.
Surgical procedure
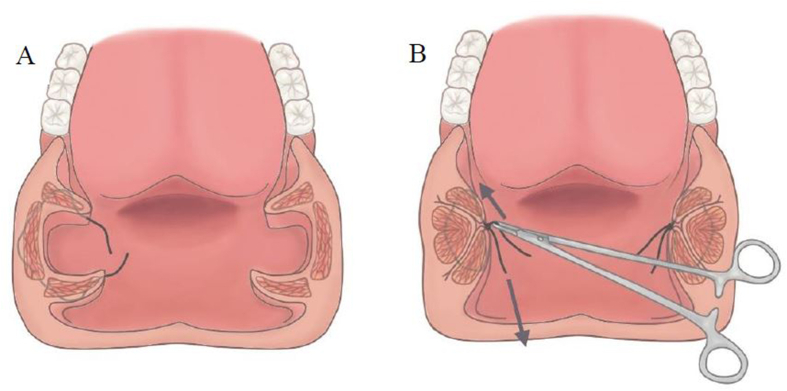
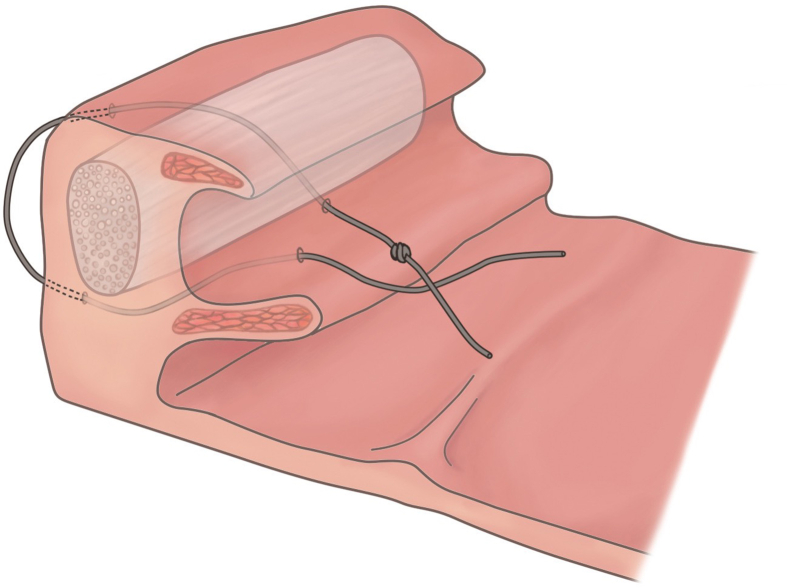
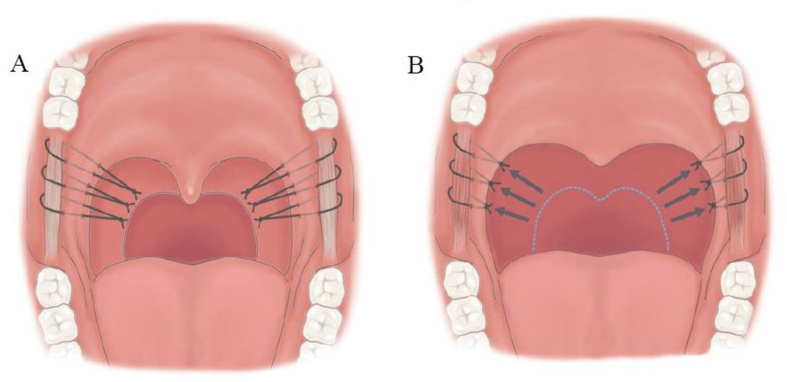
The palatal hybrid surgery was performed under general anesthesia through intra-oral intubation. Head was hyper-extended and an adequately size of mouth gag was used to expose the oropharynx. Procedure of palatal hybrid surgery include: 1. PlasmaBlade-assisted, pillar mucosa-preserved tonsillectomy. A PEAK PlasmaBlade (model: cutting-4, coagulation-2, Medtronic) was used to incise the pillar mucosa along its junction with tonsil (cutting model) and dissect the tonsils from underlying muscle in superior-lateral-inferior-medial sequence (coagulation model). 2. Alation of supratonsillar adipose tissue. An electrocautery wand (cutting model, 20 W) was used to ablate the supratonsillar fat for further expansion of lateral dimension of the velopharynx and facilitating maximal suspension of the soft palate. 3. Stereoscopic reconstruction of tonsillar fossa. (pharyngoplasty) The tonsillar fossa reconstruction started from the inferior pole to middle tonsillar fossa as an upward approach style. Whole muscle layer suture involving the palatopharyngeus, superior pharyngeal constrictor and palatoglossus muscles was performed by a 2-0 Vicryl (Fig. 2A). Seamless closure of the fossa was achieved by push and pull maneuver (keeping the knot at the junction of palatopharyngeus muscle and mucosa of posterior pillar, simultaneously pushing the posterior pillar upward by needle holder and pulling the string downward) (Fig. 2B). The tie was then locked through square knot. The tonsillar fossa reconstruction needed 3–4 stitches according to the tonsil size. The tonsillar fossa was remodeled to a three-dimensional structure after this procedure. 4. Raphe-based omni-suspension of the soft palate. (palatoplasty) The suspension procedure started by a stay suture of the palatopharyngeus muscle using a 1-0 Vicryl, the needle then penetrated the mucosa above the pterygomandibular raphe, pressed down with bundle suture of the raphe, passed through tonsillar fossa, and finally the two strings were tied together to accomplish fixing the palatopharyngeus muscle to the pterygomandibular raphe for suspension of the soft palate (Fig. 3). The suspension started from most superior part of the palatopharyngeus muscle, adjacent to the lateral aspect of the musculus uvula then with interruption of 5 mm inferiorly. Totally, three suspension sutures toward anterior, middle, lateral directions were implemented to expand the velopharyngeal space and diminish its collapse (Fig. 4A and B). Mucosa of anterior and posterior was approximated by 3-0 Vicryl. 5. Advancement of uvula. (uvuloplasty) The uvuloplasty was implemented by horizontal incision of the redundant mucosa at the uvular root and mattress sutures of the raw wound by 3-0 Vicryl. In case of long uvula (>1.5 cm), uvular tip (non-muscular part) was removed.
Fig. 2.
Whole muscle layer suture includes the palatopharyngeus, superior pharyngeal constrictor and palatoglossus muscles. (A) Seamless closure of the tonsillar fossa is achieved by “push and pull” maneuver (simultaneously pushing the posterior pillar upward by needle holder and pulling the string downward). (B).
Fig. 3.
Using the pterygomandibular raphe as an anchor, palatopharyngeus muscle is pulled to the raphe for the suspension of the soft palate.
Fig. 4.
Three suspension sutures toward anterior, middle, lateral directions are implemented to expand the velopharyngeal space and diminish its collapse. (A) The soft palate is raised and the velopharyngeal space is enlarged in both anterior–posterior and lateral dimensions. (B).
Nasal surgery
Nasal surgery was performed simultaneously with palatal hybrid surgery in OSA patients who had nasal obstruction and deviated nasal septum. The procedure of nasal surgery used in this study was mini-invasive septoturbinoplasty (MIST), which involves excision of deviated septum, incision of the nasal septum along the nasal floor for drainage, two to three horizontal trans-septal sutures, and out-fracture of inferior turbinate [24].
Outcome measurement
Change of AHI six months after palatal hybrid surgery in home sleep test was the primary outcome. Other outcomes included changes of minimal oxygen saturation and apnea index, etc; image data of posterior air space in retropalatal space on lateral cephalometry; questionnaire in daytime sleepiness measured by the Epworth Sleepiness Scale [26] (range, 0–24); snoring severity by visual analogue scale (VAS, range, 0–10), and postoperative pain by visual analogue scale (range, 0–10). Classical success (Sher's criteria [7]) was defined as reduction of AHI >50% and postoperative AHI <20.
Statistical analysis
Continuous data are presented as mean and standard deviation (SD) and were compared using the paired Student t test between preoperative and postoperative values. Categorical data are presented as numbers and percentages. Two-tailed p values < 0.05 were considered statistically significant. All statistical analyses were performed using Graph Pad Prism 7.00 for Windows (Graph Pad Software Inc., San Diego, CA, USA) and G∗Power 3.1.9.2 software (Heinrich-Heine University, Dusseldorf, Germany).
Results
Study population
Forty-six OSA patients were enrolled in this study with the general profile of middle age, male, overweighted, severe severity in OSA, small tonsil, high tongue position, and unfavorable Friedman stage (Table 1).
Table 1.
Baseline characteristics.
| Variable (n = 46) | mean (SD) |
|---|---|
| Age (year) | 38.4 (9.3) |
| Male:female | 45:1 |
| BMI (kg/m2) | 26.2 (3.3) |
| MMP I:II:III | 5:9:32 |
| Tonsil size I:II:III | 27:9:10 |
| Stage I:II:III | 3:19:24 |
| PAS (mm) | 8.4 (2.4) |
| AHI (event/h) | 41.8 (22.8) |
| AI (event/h) | 25.8 (24.3) |
| ODI (event/h) | 38.5 (21.2) |
| Snoring index (event/h) | 267.1 (186.6) |
| Mean oxygen saturation (%) | 92.6 (3.5) |
| Lowest oxygen saturation (%) | 72.4 (11.1) |
| <90% saturation (%) | 22.0 (22.1) |
| ESS (0–24) | 11.3 (5.5) |
| VAS snore (0–10) | 8.7 (2.4) |
Continuous variables express as mean (SD).
Abbreviation: SD: standard deviation; BMI: body mass index; MMP: modified Mallampati grade; PAS: posterior air space; AHI: apnea/hypopnea index; AI: apnea index; ODI: oxygen desaturation index; ESS: Epworth sleepiness score; VAS: visual analogue scale.
Recovery
All patients were extubated smoothly after palatal hybrid surgery at operation room and then remained hospitalized in regular ward for two days. No airway compromise was noted. One patient experienced tonsillar wound bleeding at 10th day after surgery that ceased following conservative treatment. Two stitch granulomas over the area of pterygomandibular raphe (L't) were noted and subsided after gargling with Betadine solution. Globus sensation of the throat was common and dramatically improved after removal of retained stitches on low edge of the soft palate at the 2nd postoperative week. Dehiscence of tonsillar fossa was noted in 7 patients and the dehiscence rate was 15% (7/46) in total population and 7.5% (7/92) in total tonsillar fossa. Average postoperative pain score in VAS was mild and showed 3 (1st day), 3 (3rd day), 2 (7th day), 0 (14th day). Additionally, there was no subjective alternation in voice, articulation or taste disturbance experienced one month postoperatively.
Surgical outcomes
Six months after surgery, there was no significant change in BMI. Home sleep test showed significant improvement in AHI (−21.1 event/h), Lowest oxygen saturation (+9.1%) and snoring (−134.0 event/h). Subjective snoring in VAS (70% reduction) and daytime sleepiness in ESS (−5.8) also improved significantly. The posterior air space in retropalatal area at lateral cephalogram increased (+2.7 mm) significantly (Table 2). The classical success rate in individual Friedman stage was 100% (3/3) in stage I, 63% (12/19) in stage II, and 58% (14/24) in stage III with an overall success rate of 63%.
Table 2.
Changes in home sleep test, questionnaire, and cephalometry before and after palatal hybrid surgery.
| Variable | preoperative | postoperative | Change | p |
|---|---|---|---|---|
| BMI (kg/m2) | 26.2 (3.3) | 26.9 (2.9) | +0.7 | 0.06 |
| AHI (event/h) | 41.8 (22.8) | 18.2 (15.3) | −23.6 | <0.0001 |
| supine AHI (event/h) | 45.0 (22) | 23.6 (17.0) | −21.4 | <0.0001 |
| non-supine AHI (event/h) | 18.2 (22.1) | 9.4 (14.2) | −8.8 | 0.0009 |
| AI (event/h) | 25.8 (24.3) | 4.7 (7.5) | −21.1 | <0.0001 |
| ODI (event/h) | 38.5 (21.2) | 18.1 (15.1) | −20.4 | <0.0001 |
| Snoring index (event/h) | 267.1 (186.6) | 133.13 (139.3) | −133.97 | 0.0001 |
| mean SpO2 (%) | 92.6 (3.5) | 94.4 (1.5) | +1.8 | 0.0003 |
| Lowest SpO2 (%) | 72.4 (11.1) | 81.5 (6.7) | +9.1 | <0.0001 |
| <90% SpO2 (%) | 22.0 (22.1) | 7.7 (13.8) | −14.3 | 0.0001 |
| ESS (0–24) | 11.3 (5.5) | 5.5 (2.9) | −5.8 | <0.0001 |
| VAS snore (0–10) | 8.7 (2.4) | 2.6 (1.8) | −6.1 | <0.0001 |
| PAS (mm) | 8.4(2.4) | 11.1(2.7) | +2.7 | <0.0001 |
Continuous variables express as mean (SD).
Abbreviation: SD: standard deviation; BMI: body mass index; MMP: modified Mallampati grade; PAS: posterior air space; AHI: apnea/hypopnea index; AI: apnea index; ODI: oxygen desaturation index; ESS: Epworth sleepiness score; VAS: visual analogue scale.
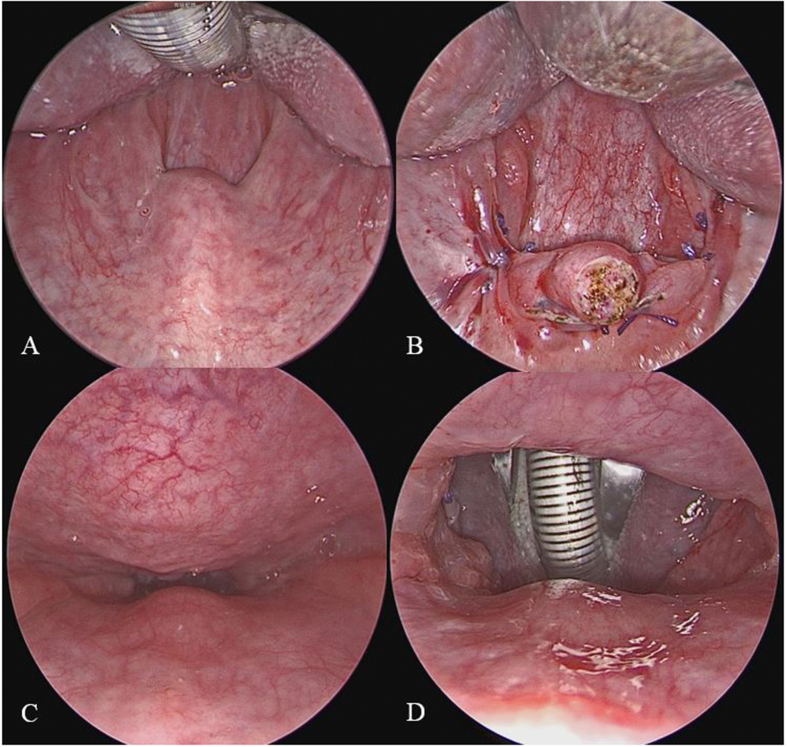
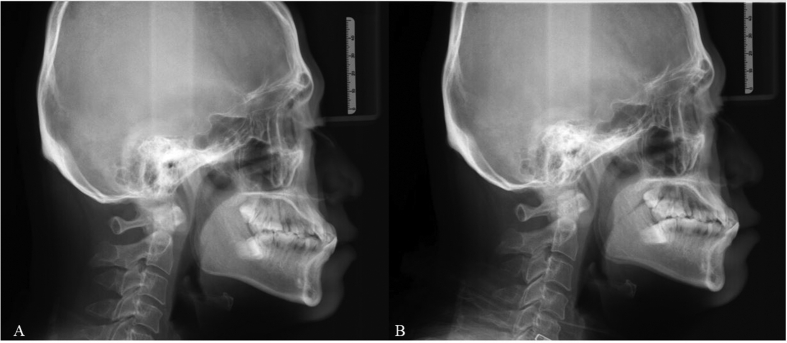
Perioperative changes of oropharyngeal structure (0° rigid endoscope, intraoral) and retropalatal space (70° rigid endoscope, trans-nasal) in one patient are demonstrated in Fig. 5. Perioperative change of posterior air space in retropalatal area at lateral cephalogram is demonstrated in Fig. 6.
Fig. 5.
Perioperative changes of oropharyngeal structure (0° rigid endoscope, intraoral) and retropalatal space (70° rigid endoscope, trans-nasal) demonstrate significant enlargement of the airspace.
Fig. 6.
Posterior air space at retropalatal area in lateral cephalogram increases after palatal hybrid surgery.
Discussion
This study proposes a tissue-specific integrated surgical technique (mucosa-preservation, fat-ablation, tonsil-excision, muscle-suspension) named palatal hybrid surgery to annotate UPPP on stereoscopic reconstruction of tonsillar fossa (pharyngoplasty), raphe-based omni-suspension of soft palate (palatoplasty), and advancement of uvula (uvuloplasty). The results showed minimal morbidities of voice and swallowing in conjunction with significant improvement in subjective symptoms (snoring, daytime sleepiness), objective polysomnographic parameters and airway space. The success rate increased remarkably in Friedman stage III patients.
Annotation of pharyngoplasty
Lateral pharyngeal wall obstruction is one important part contributing to OSA [30]. From the image study, negative pressure in the airway induces lateral pharyngeal wall collapse [31]. The intraoral components of oropharyngeal lateral wall are tonsil, palatoglossus muscle, palatopharyngeus muscle, and superior pharyngeal constrictor muscle. From the viewpoint of anatomic obstruction, tonsillectomy obtains more airspace in large tonsils (grade III, IV) than small tonsils (grade I, II). Consequently, tonsil size is always an outcome predictor of UPPP for OSA [9]. However, this ignores physiological collapse from lateral pharyngeal wall. Lateral pharyngoplasty and relocation pharyngoplasty tried to focus on lateral pharyngeal wall [18,22]. In the study, palatal hybrid surgery performed whole muscle layer suture involving the superior pharyngeal constrictor muscle in closing the tonsillar fossa. The fixation of superior pharyngeal constrictor muscle with palatopharyngeus and palatoglossus muscles constitutes a shield against negative airway pressure and pharyngeal collapse to some extent. In addition, The push& pull maneuver constructs a stereoscopic structure of tonsillar fossa that avoids the posterior displacement of the tongue while direct closure of the palatopharyngeus and palatoglossus muscles after tonsillectomy. This adverse effect can be observed in the decrease of retroglossal space after UPPP in image study especially in patients with big tonsils [32]. PlasmaBlade-assisted tonsillectomy offers low thermal injury to the underlying muscle layer in compassion to cautery-assisted tonsillectomy [33]. Preservation of pillar mucosa contributes to tension-free suture of anterior and posterior pillars. The foregoing are helpful to reduce postoperative dehiscence of tonsillar fossa and related complications.
Annotation of palatoplasty
Soft palate is the commonest site of snore generator and airway obstruction in OSA patients [34]. Classic UPPP excising the “redundant” soft palate for the improvement of snoring and airway obstruction incurred not only complications in swallowing and articulation but also potential “silent apnea” [8]. Barbed reposition pharyngoplasty [19] and suspension palatoplasty [23] addressed on suspension, instead of excision, of the soft palate to avoid velopharyngeal insufficiency. In the study, no patient experienced alternation in voice and nasal regurgitation in the 1st month's follow up. Raphe-based suspension technique provides robust support to expand the velopharyngeal air space against palatal unwinding as time goes by. Further, omni-suspension to individually anterior, middle, and lateral direction offers integrated tension to resist velopharyngeal obstruction from anterior–posterior, lateral, and concentric collapse during sleep. The ablation of supratonsillar adipose tissue further enlarges the velopharyngeal space and facilitates full omni-suspension of the soft palate. Uvuloplasty advances the uvula that reduces turbulence of airflow and vibration of the uvula, and consequently lessens the snoring.
Annotation in safety of UPPP
Three major concerns in the safety of UPPP are postoperative pain, bleeding and velopharyngeal insufficiency. Intolerable pain is most criticized after traditional UPPP. Previous studies showed moderate to severe pain associated with dysphagia after UPPP [[35], [36], [37]]. The average pain scores (VAS) after palatal hybrid surgery are 3, 3, 2 and 0 in the 1st, 3rd, 7th, and 14th day, respectively. The significant reduction of postoperative pain is mainly attributable to the decreased tonsillar fossa dehiscence by stereoscopic reconstruction and the use of suspension instead of excision of pharyngeal muscle. Other factors are also related to the mild post-operative pain in palatal hybrid surgery: use of low thermal injury tool-PlasmaBlade [33], mucosa-preserved tonsillectomy and intravenous injection of Ketorolac [38]. The decreased postoperative pain facilitates normal swallowing and enhanced recovery after surgery. Post-operative tonsillar bleeding is another major concern in safety of UPPP. Previous report revealed 7.8% of tonsillar bleeding after UPPP [39]. In this study, one patient experienced tonsillar fossa bleeding at the 10th day and ceased after conservative treatment. Risk of post-UPPP bleeding is related to systemic co-morbid such as hypertension, coagulation disorders, administration of anti-coagulation medication and local condition such as hypertrophic tonsils (grade III, IV), operative tonsil in infectious status. These factors need to be controlled or excluded to decrease the emergence of post-operative tonsillar bleeding. In palatal hybrid surgery, whole muscle layer suture with push & pull maneuver facilitates sealing off the dead space and seamless closure of the tonsillar fossa that contribute to decreased of tonsillar fossa dehiscence (7.5% in total tonsillar fossa) and diminish the incidence of post-operative tonsillar bleeding. Velopharyngeal insufficiency and voice change after UPPP are annoying to OSA patients in quality of life. Traditional UPPP excised the “redundant” soft palate that jeopardized the integrity of pharyngeal sphincters (levator veli palatinus muscle and uvular muscle) and consequently led to velopharyngeal insufficiency and voice change [40]. Palatal hybrid surgery used suspension technique to expand the velopharyngeal space with no damage to the pharyngeal muscle. Previous study showed the suspension technique not only advances but also lifts soft palate that facilitates closure of the velopharynx during swallowing and phonation of vowel sounds [41]. In the study, no persistent nasal regurgitation or hypernasality observed at the 1st month after surgery. Lump in the throat is common and annoying in UPPP patients [42]. The cause of lumping throat after UPPP is mainly from physical contact between firm scar at palatal edge and dorsal tongue during swallowing or radical uvulectomy leading to dryness and phlegm accumulation. Palatal hybrid surgery preserves mucosa of palatal edge to avoid “hard” contact during swallowing and maintains uvular function in lubrication and swinging off the phlegm that consequently contribute to the diminution of post-UPPP lumping throat.
Annotation in efficacy of UPPP
The principal assessment of UPPP outcome includes subjective symptoms, objective airway space and sleep apnea. Two major clinical complaints in OSA patient are snoring and daytime sleepiness. Systemic review showed UPPP improved snoring and daytime sleepiness in OSA patients [43,44]. The study showed significant improvement in snoring (70% reduction in VAS) and daytime sleepiness (−5.8 in ESS) after palatal hybrid surgery. The results here suggest that palatal hybrid surgery using mini-invasive technique can achieve significant improvement in snoring and daytime sleepiness. Traditional UPPP excised the redundant soft palate and tonsils to enlarge the retropalatal space and the changes of the air space were positively associated with the tonsil size. Using pterygomandibular raphe as the anchor, palatal hybrid surgery suspends soft palate to expand the velopharyngeal space. In lateral cephalometry, the posterior air space at retropalatal area increased from 8.4 mm to 11.1 mm (+32%). Instead of tonsil size, broad posterior pillars (webbing) facilitates omni-suspension and assures a wide retropalatal space after palatal hybrid surgery. Changes of AHI have been used to judge surgical success or failure of OSA for decades. The AHI decreased significantly from 41.8 to 18.2 event/hr after palatal hybrid surgery. According to Sher's criteria, the success rate of UPPP was 41% [7]. The success rate of UPPP in individual Friedman stage I, II and III was 80%, 40% and 8%, respectively [45]. In the study, the success rate in stage I, II and III was 100%, 63% and 58%, respectively. It's noteworthy that palatal hybrid surgery remarkably improved the success rate in unfavorable anatomic structure (Friedman staging III). This could be due to full suspension of the soft palate augmenting the retropalatal space and stereoscopic reconstruction of tonsillar fossa lessening lateral pharyngeal wall collapse and avoiding backward pulling of the tongue. We presume these techniques provide adequate tension to help resist airway collapse from soft palate and lateral pharyngeal wall during sleep. However, the suboptimal success rate and residual AHI also indicate the limitation of sole palatal surgery in treating OSA and suggest a further treatment for persistent airway obstruction found during drug-induced sleep endoscopy in failed patients.
There are several limitations of this study. 1. This is a small sample size study targeting on a overwhelmingly male OSA patients that may not be generalized to the whole OSA patients. 2. The use of home sleep test may under-estimate the severity of OSA. It's possible because ApneaLink® monitors breathing patterns and measures apneas or hypopneas since the user presses the start button without the monitor of EEG. However, previous study compared ApneaLink with polysomnography and showed acceptable reliability [29]. Further, we excluded OSA patients co-morbid with insomnia and other sleep disorders to lessen the potential bias of under-estimate. Under the COVID-19 pandemic, we believe home sleep test will be more commonly used in the follow-up of non-CPAP treatment. 3. Surgical intervention, in this study, is a concurrent nasal/palatal surgery thus the contribution of palatal surgery in the reduction of AHI is unclear. However, our previous study revealed that nasal surgery alone improved subjective snoring and daytime sleepiness but not objective AHI [46]. Thus we consider changes in AHI in this study can represent objective outcome of palatal hybrid surgery to OSA. Further study with control group or stage surgery is warrant to elucidate individual role. 4. The cause of surgical failure either from residual palatal obstruction or persistent hypopharyngeal obstruction is unclear. An extended study is ongoing to identify the obstruction site by drug-induced sleep endoscopy and the cause of obstruction by test of tongue strength/endurance in surgical failure patients.
Conclusion
Palatal hybrid surgery uses the tissue-specific technique instead of excision-based technique to treat anatomical obstruction and physiological collapse in OSA patients. Palatal hybrid surgery annotates UPPP in pharyngoplasty by stereoscopic reconstruction of tonsillar fossa and palatoplasty by omni-suspension of the soft palate. The results show that it is low-morbid in postoperative pain, voice, swallowing and lumping throat with significant improvement in subjective snoring, daytime sleepiness, objective sleep apnea, and success rate particular in unfavorable Friedman stage III.
Conflicts of interest
The authors have declared that no conflicts of interest exists.
Acknowledgments
The author would like to express our gratitude to Mrs. Shin-Jao Lee for the data collection. The authors wish to thank Mr. Nan-Chen Huang for the original illustrations in HY Li. Deep sleep everynight (in Chinese), M news/Walkers Cultural Co., Ltd. Ark Couture Publishing House.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Strollo P.J., Roger R.M. Obstructive sleep apnea. N Engl J Med. 1996;334(2):99–104. doi: 10.1056/NEJM199601113340207. [DOI] [PubMed] [Google Scholar]
- 2.Yaggi H.K., Concato J., Kernan W.N., Lichtman J.H., Brass L.M., Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 3.Bradley T.D., Floras J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan C.E., Issa F.G., Berthon-Jones M., Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 5.Li H.Y., Lee L.A., Tsai M.S., Chen N.H., Chuang L.P., Fang T.J., et al. How to manage continuous positive airway pressure (CPAP) failure- hybrid surgery and integrated treatment. Auris Naris Larynx. 2020;47(3):335–342. doi: 10.1016/j.anl.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Fujita S., Conway W., Zorick F., Roth T. Surgical correction of anatomic azbnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 1981;89(6):923–934. doi: 10.1177/019459988108900609. [DOI] [PubMed] [Google Scholar]
- 7.Sher A.E., Schechtman K.B., Piccirillo J.F. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19(2):156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 8.Simmons F.B., Guilleminault C., Silvestri R. Snoring and some obstructive sleep apnea can be cured by oropharyngeal surgery: palatopharyngoplasty surgery. Arch Otolaryngol. 1983;109(8):503–507. doi: 10.1001/archotol.1983.00800220009003. [DOI] [PubMed] [Google Scholar]
- 9.Tschopp S., Tschopp K. Tonsil size and outcome of uvulopalatopharyngoplasty with tonsillectomy in obstructive sleep apnea. Laryngoscopy. 2019;129(12):449–454. doi: 10.1002/lary.27899. [DOI] [PubMed] [Google Scholar]
- 10.Li H.Y., Wang P.C., Lee L.A., Chen N.H., Fang T.J. Prediction of uvulopalatopharyngoplasty outcome: anatomy-based staging system versus severity-based staging system. Sleep. 2006;29(12):1537–1541. doi: 10.1093/sleep/29.12.1537. [DOI] [PubMed] [Google Scholar]
- 11.Katsantonis G.P., Friedman W.H., Krebs F.J., Walsh J.K. Nasopharyngeal complications following uvulopalatopharyngoplasty. Laryngoscope. 1987;97(3 Pt 1):309–314. [PubMed] [Google Scholar]
- 12.Fairbanks D.N. Uvulopalatopharyngoplasty complications and avoidance strategies. Otolaryngol Head Neck Surg. 1990;102(3):239–245. doi: 10.1177/019459989010200306. [DOI] [PubMed] [Google Scholar]
- 13.Li H.Y., Li K.K., Chen N.H., Wang P.C. Modified uvulopalatopharyngoplasty: the extended uvulopalatal flap. Am J Otolaryngol. 2003;24(5):311–316. doi: 10.1016/s0196-0709(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 14.Li H.Y. In: Sleep-related breathing disorders. Lin H.C., editor. Karger; Adv Otorhinolaryngol; Basel: 2017. Updated palate surgery for obstructive sleep apnea; pp. 74–80. [DOI] [PubMed] [Google Scholar]
- 15.Li H.Y. In: Prevention, screening, and treatments for obstructive sleep apnea: beyond positive airway pressure (PAP) Toh S.T., editor. Elsevier; Sleep Med Clin; New York: 2019. Palatal surgery for obstructive sleep apnea: from ablation to reconstruction; pp. 51–58. [Google Scholar]
- 16.Li H.Y. In: Sleep apnea and snoring. 2nd ed. Friedman M., Jacobowitz O., editors. Elsevier; New York: 2019. Uvulopalatopharyngoplasty: patient selection and effects on airway; pp. 191–197. [Google Scholar]
- 17.Shen S.C., Li H.Y. State-of-the art in reconstructive palatal surgery techniques for obstructive sleep apnea. Cur Sleep Med Rep. 2020;6:67–75. [Google Scholar]
- 18.Cahali M.B. Lateral pharyngoplasty: a new treatment for obstructive sleep apnea hypopnea syndrome. Laryngoscope. 2003;113(11):1961–1968. doi: 10.1097/00005537-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Vicini C., Hendawy E., Campanini A., Eesa M., Bahgat A., Alghamdi S., et al. Barbed reposition pharyngoplasty (BRP) for OSAHS: a feasibility, safety, efficacy and teachability pilot study. “We are on the giant’s shoulders”. Eur Arch Otorhinolaryngol. 2015;272(10):3065–3070. doi: 10.1007/s00405-015-3628-3. [DOI] [PubMed] [Google Scholar]
- 20.Friedman M., Ibrahim H.Z., Vidyasagar R., Pomeranz J., Joseph N.J. Z-palatoplasty (ZPP): a technique for patients without tonsils. Otolaryngol Head Neck Surg. 2004;131(1):89–100. doi: 10.1016/j.otohns.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 21.Pang K.P., Woodson B.T. Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2007;137(1):110–114. doi: 10.1016/j.otohns.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Li H.Y., Lee L.A. Relocation pharyngoplasty for obstructive sleep apnea. Laryngoscope. 2009;119(12):2472–2477. doi: 10.1002/lary.20634. [DOI] [PubMed] [Google Scholar]
- 23.Li H.Y., Lee L.A., Kezirian E.J., Nakayama M. Suspension palatoplasty for obstructive sleep apnea – a preliminary study. Sci Rep. 2018;8(1):4224. doi: 10.1038/s41598-018-22710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H.Y., Lee L.A., Hsin L.J., Fang T.J., Lin W.N., Chen H.C., et al. Intrapharyngeal surgery with integrated treatment for obstructive sleep apnea. Biomed J. 2019;42(2):84–92. doi: 10.1016/j.bj.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amali A., Amirzargar B., Sadeghi M., Saedi B. Muller’s maneuver in patients with obstructive sleep apnea. J Sleep Sci. 2016;1:148–150. [Google Scholar]
- 26.Johns M.W. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103(1):30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 27.Friedman M., Ibrahim H., Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg. 2002;127(1):13–21. doi: 10.1067/mhn.2002.126477. [DOI] [PubMed] [Google Scholar]
- 28.Stehling F., Keull J., Olivier M., Große-Onnebrink J., Mellies U., Stuck B.A. Validation of the screening tool ApneaLink® in comparison to polysomnography for the diagnosis of sleep-disordered breathing in children and adolescents. Sleep Med. 2017;37:13–18. doi: 10.1016/j.sleep.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Chen H., Lowe A.A., Bai Y., Hamilton P., Fleetham J.A., Almeida F.R. Evaluation of a portable recording device (ApneaLinkTM) for case selection of obstructive sleep apnea. Sleep Breath. 2009;13(3):213–219. doi: 10.1007/s11325-008-0232-4. [DOI] [PubMed] [Google Scholar]
- 30.Kezirian E.J., Hohenhorst W., de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011;268(8):1233–1236. doi: 10.1007/s00405-011-1633-8. [DOI] [PubMed] [Google Scholar]
- 31.Schwab R.J., Gupta K.B., Gefter W.B., Metzger L.J., Hoffman E.A., Pack A.I. Upper airway and soft-tissue anatomy in normal subjects and patients with sleep-disordered breathing – significance of the lateral pharyngeal walls. Am J Respir Crit Care. 1995;152(5 Pt 1):1673–1689. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 32.Li H.Y., Li K.K., Chen N.H., Wang C.J., Liao Y.F., Wang P.C. Three-dimensional computed tomography and polysomnography findings following extended uvulopalatal flap surgery for obstructive sleep apnea. Am J Otolaryngol. 2005;26(1):7–11. doi: 10.1016/j.amjoto.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Tsai M.S., Li H.Y. Comparison of post-operative pain between pulsed electron avalanche knife (PEAK) and electrocautery in uvulopalatopharyngoplasty. J Taiwan Otolaryngol Head Neck Surg. 2014;49:170–176. [Google Scholar]
- 34.Lee L.A., Yu J.F., Lo Y.L., Chen Y.S., Wang D.L., Cho C.M., et al. Energy types of snoring sounds in patients with obstructive sleep apnea syndrome: a preliminary observation. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0053481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rombaux P., Hamoir M., Bertrand B., Aubert G., Liistro G., Rodenstein D. Postoperative pain and side effects after uvulopalatopharyngoplasty, laser-assisted uvulopalatoplasty, and radiofrequencytissue volume reduction in primary snoring. Laryngoscope. 2003;113(12):2169–2173. doi: 10.1097/00005537-200312000-00023. [DOI] [PubMed] [Google Scholar]
- 36.Troell R.J., Powell N.B., Riley R.W., Li K.K., Guilleminault C. Comparison of postoperative pain between laser-assisted uvulopalatoplasty, uvulopalatopharyngoplasty, and radiofrequency volumetric tissue reduction of the palate. Otolaryngol Head Neck Surg. 2000;122(3):402–409. doi: 10.1016/S0194-5998(00)70056-8. [DOI] [PubMed] [Google Scholar]
- 37.Nikanne E., Virtaniemi J., Aho M., Kokki H. Ketoprofen for postoperative pain after uvulopalatopharyngoplasty and tonsillectomy: two-week follow-up study. Otolaryngol Head Neck Surg. 2003;129(5):577–581. doi: 10.1016/S0194-59980301579-1. [DOI] [PubMed] [Google Scholar]
- 38.Lee L.A., Wang P.C., Cheng N.H., Fang T.J., Huang H.C., Lo C.C., et al. Alleviation of wound pain after surgeries for obstructive sleep apnea. Laryngoscope. 2007;117(9):1689–1694. doi: 10.1097/MLG.0b013e318093edf3. [DOI] [PubMed] [Google Scholar]
- 39.Kim J.A., Jung H.H. Predictive factors of immediate postoperative complications after uvulopalatopharyngoplasty. Laryngoscope. 2005;115(10):1837–1840. doi: 10.1097/01.mlg.0000173199.57456.2b. [DOI] [PubMed] [Google Scholar]
- 40.Tang J.A., Salapatas A.M., Bonzelaar L.B., Friedman M. Long-term incidence of velopharyngeal insufficiency and other sequelae following uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 2017;156(4):606–610. doi: 10.1177/0194599816688646. [DOI] [PubMed] [Google Scholar]
- 41.Li H.Y., Lee L.A., Fang T.J., Lin W.Y. Evaluation of velopharyngeal function after relocation pharyngoplasty for obstructive sleep apnea. Laryngoscope. 2010;120(5):1069–1073. doi: 10.1002/lary.20850. [DOI] [PubMed] [Google Scholar]
- 42.Röösli C., Schneider S., Häusler R. Long-term results and complications following uvulopalatopharyngoplasty in 116 consecutive patients. Eur Arch Otorhinolaryngol. 2006;263(8):754–758. doi: 10.1007/s00405-006-0051-9. [DOI] [PubMed] [Google Scholar]
- 43.Levin B.C., Becker G.D. Uvulopalatopharyngoplasty for snoring: long-term results. Laryngoscope. 1994;104(9):1150–1152. doi: 10.1288/00005537-199409000-00017. [DOI] [PubMed] [Google Scholar]
- 44.Browaldh N., Bring J., Friberg D. SKUP3 RCT; continuous study: changes in sleepiness and quality of life after modified UPPP. Laryngoscope. 2016;126(6):1481–1491. doi: 10.1002/lary.25642. [DOI] [PubMed] [Google Scholar]
- 45.Friedman M., Vidyasagar R., Bliznikas D., Joseph N. Does severity of obstructive sleep apnea/hypopnea syndrome predict uvulopalatopharyngoplasty outcome? Laryngoscope. 2005;115(12):2109–2113. doi: 10.1097/01.MLG.0000181505.11902.F7. [DOI] [PubMed] [Google Scholar]
- 46.Li H.Y., Wang P.C., Chen Y.P., Lee L.A., Fang T.J., Lin H.C. Critical appraisal and meta-analysis of nasal surgery for obstructive sleep apnea. Am J Rhinol Allergy. 2011;25(1):45–49. doi: 10.2500/ajra.2011.25.3558. [DOI] [PubMed] [Google Scholar]