Abstract
Background:
Lentigo maligna/lentigo maligna melanoma (LM/LMM) can present with subclinical extension that may be difficult to define preoperatively and lead to incomplete excision and potential recurrence. Preliminarily studies have used reflectance confocal microscopy (RCM) to assess LM/LMM margins.
Objective:
To evaluate the correlation of LM/LMM subclinical extension defined by RCM compared with the gold standard histopathology.
Methods:
Prospective study of LM/LMM patients referred for dermatologic surgery. RCM was performed at the clinically defined initial surgical margin followed by margin-controlled staged excision with paraffin-embedded tissue, and histopathology was correlated with RCM results.
Results:
Seventy-two patients were included. Mean age was 66.8 years (standard deviation, 11.1; range, 38–89); 69.4% were men. Seventy of 72 lesions (97.2%) were located on the head and neck with mean largest clinical diameter of 1.3 cm (range, 0.3–5). Diagnostic accuracy for detection of residual melanoma in the tumor debulk (after biopsy) had a sensitivity of 96.7% and a specificity of 66.7% when compared with histopathology. RCM margin assessment revealed an overall agreement with final histopathology of 85.9% (κ = 0.71; P <.001).
Limitations:
No RCM imaging beyond initial planned margins was performed.
Conclusion:
RCM showed moderate to excellent overall agreement between RCM imaging of LM/LMM and histopathology of staged excision margins.
Keywords: lentigo maligna, margins, melanoma, Mohs surgery, reflectance confocal microscopy, staged excision, surgery
Lentigo maligna/lentigo maligna melanoma (LM/LMM) is a melanoma subtype arising on chronic sun-damaged skin. LM/LMM poses diagnostic and surgical challenges because it can present with subclinical extension that may be difficult to define preoperatively, leading to incomplete excision.1,2 This in turn can lead to additional surgery or a
potential increase in local recurrence.1–3 The difficulty of defining margins in LM/LMM arises from the presence of irregular, ill-defined borders and surrounding lentigines amid a background of sun-damaged skin. In addition, LM/LMM occurs more often in the head and neck, a complex area that contains highly functional and cosmetic structures.4,5
Several studies have shown that the recommended 5-mm margins for wide excision in melanoma in situ may not be sufficient to achieve clearance of melanomas of the LM/LMM subtype.3,6–10 Several surgical techniques have been developed to better assess margins and detect subclinical extension. These treatment modalities include mainly Mohs micrographic surgery (ie, using frozen sections) and staged excision procedures (ie, using permanent formalin-fixed paraffin-embedded sections).3,11 Defining the LM/LMM subclinical extension presurgically may improve surgical planning, help anticipate defect size, and boost the shared decision-making process.
Reflectance confocal microscopy (RCM) is a US Food and Drug Administration–approved noninvasive imaging modality that uses a near-infrared (830 nm), low-intensity (30 mW) diode laser and provides quasi-histologic images.12 RCM has shown good reliability for the diagnosis of LM/LMM.13,14 Presurgical RCM is effective at estimating melanoma margins, and RCM findings correlate well with final defect size.15–19 Most of these studies to date, however, have included a limited number of patients,16–19 lacked 1:1 histopathologic correlation,20,21 and/or are based on retrospective analysis.15 The objective of this study was to prospectively evaluate the correlation of subclinical extension defined by RCM compared quadrant-by-quadrant with the gold standard histopathology in a larger cohort of patients with diagnosis of LM/LMM.
METHODS
This was an institutional review board–approved, prospective study conducted between July 2017 and June 2019. All patients signed informed consent before RCM imaging.
Inclusion and exclusion criteria
Consecutive adult patients (≥18 years old) with biopsy-proven stage 0 or IA melanoma according to the American Joint Committee on Cancer, eighth edition, of the LM/LMM subtype presenting for dermatologic surgery evaluation who were willing to participate in the study were included. We excluded patients with melanomas located on anatomic areas where RCM was not able to adequately evaluate lesions (eg, retroauricular fold) or if there were open wounds after biopsy.
Clinical characteristics
Patients had an initial consultation including clinical examination, dermoscopy, and Wood’s lamp evaluation. Demographic characteristics (age, sex) were recorded. All biopsy specimens were evaluated by an expert dermatopathologist (K.J.B.) to confirm the diagnosis and evaluate histopathologic features (eg, Breslow thickness). Tumor location, maximum diameter (cm), and Breslow thickness (mm) were recorded. It was documented if “clinical residual lesion” (ie, evidence of pigmented lesion after the biopsy evaluated with the naked eye) was present. Clinical photos were taken with a digital camera (VEOS DS3; Canfield Inc, Parsippany, NJ).
RCM mapping of LM/LMM
The residual clinical LM/LMM lesion (aided by Wood’s lamp) was circled and the initial surgical margin selected based on histopathology by the dermatologic surgeon.8 RCM examination was performed immediately after the consultation visit with a commercially available, handheld RCM device (Vivascope 3000; Caliber I.D., Rochester, NY). RCM imaging consisted of examination of the residual clinical lesion centrally and examination of surgical margin quadrants at the 12 to 3, × to 6, 6 to 9, and 9 to 12 o’clock positions with the aid of specially designed paper rings, as previously described (Fig 1, A).22,23 Paper ring diameter was selected based on initial planned surgical margins.
Fig 1.
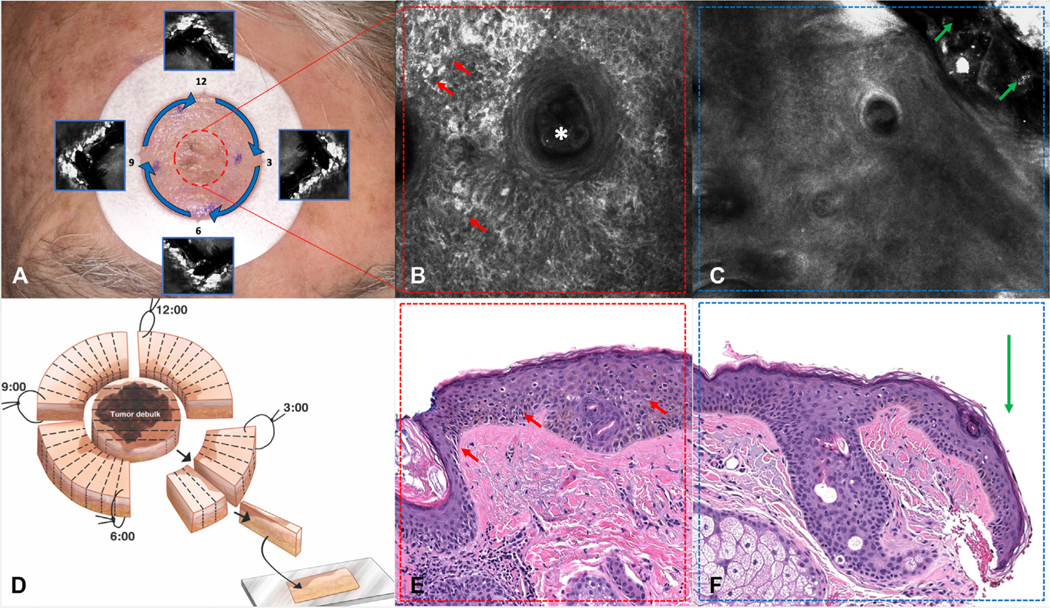
Reflectance confocal microscopy (RCM) mapping of lentigo maligna (LM). A, The adhesive paper ring is positioned 5 mm from the residual LM (center of the lesion; red dashed circle) and margins are imaged at the paper ring (blue arrows). Quadrants are defined by the “notches” (blue squares). B, RCM image of the residual LM showing large, bright, hyper-reflectile cells (red arrows) surrounding a hair follicle (white asterisk) (750 × 750 μm). C, RCM image of the 5-mm margin (blue arrows on A) showing normal skin (green arrows show paper ring edge) (750 × 750 μm). D, Staged excision with radial sections. E, Histopathology showing LM (center of the lesion, red dashed circle in A) (red arrows) (hematoxylin and eosin stain; original magnification: ×40). F, Histopathology of the 12 to × o’clock quadrant showing no evidence of melanoma at the surgical margin (green arrow).
The center of the lesion was examined with RCM to evaluate the characteristics of residual LM/LMM. The aim was to evaluate 100% of the remaining visible lesion/scar (Fig 1, A and B). Then, the initial surgical margin was evaluated inside the paper ring at the level of the dermoepidermal junction. RCM probe was positioned at the 12 o’clock margin and continuously moved clockwise at the 12 to 3, × to 6, 6 to 9, and 9 to 12 o’clock positions using the paper ring notches as references (Fig 1, A and C). Single images, stacks, and videos were taken. RCM LM/LMM criteria used to evaluate the center of the lesion were those previously described by Guitera et al.13 Margins were considered positive if there were at least 1 large round cell (>20 μm) or at least × dendritic or round cells regardless of size, as described by Pellacani et al.17
RCM examination was recorded at bedside in real time and blinded to the final histopathology report. Results were recorded as RCM positive or RCM negative for LM/LMM on the center of the lesion (1 data point) and for each quadrant (4 data points). If 1 quadrant was not possible to evaluate because of the anatomic location, it was recorded as “quadrant not assessable.” All evaluations were performed by the same 2 investigators (C.N.-D. and M.C.) by consensus. A third investigator (S.A. or K.L.) helped to solve disagreements.
Histopathologic analysis and RCM correlation
Staged excision was performed as surgically planned by an expert dermatologic surgeon, as described by Hazan et al,3 using formalin-fixed, paraffin-embedded tissue stained with routine hematoxylin and eosin and immunostains if necessary (Fig 1, D). The center (tumor debulk) of the lesion was processed and serial-sectioned for melanoma prognostic variables (Fig 1, E). The 4 quadrants were processed radially to evaluate initial surgical margins (Fig 1, F).3 Dermatopathology results were recorded by 2 investigators (C.N.-D., S.A.) as melanoma positive or melanoma negative at the center (1 data point) and at each surgical margin quadrant (4 data points) to correlate with RCM findings.
Study outcomes and statistical analysis
The primary outcome was the correlation of RCM with the final histopathologic status both at the center (tumor debulk) of the residual clinical LM/LMM and at each initial surgical margin (quadrants). Secondary outcomes included the sensitivity and specificity for the aforementioned parameters.
Descriptive statistics including means, medians, range, standard deviation (SD), and relative frequencies were used to describe the study participants, the characteristics of the procedures, and the RCM characteristics. Percent overall agreement along with kappa statistics were estimated for RCM and histologic assessments for each lesion quadrant. We interpreted kappa using the guidelines established by Landis and Koch.24 A separate sensitivity analysis was performed by randomly shifting 25% of quadrants counterclockwise to assess the robustness of the study findings. Measures of diagnostic accuracy including sensitivity, specificity, positive predictive value, and negative predictive value along with exact 95% binomial confidence intervals (CIs) for overall RCM classification and individual RCM features were estimated. Alpha level was 5% for all evaluations, and all tests were 2-sided. Analyses were performed using Stata v.14.2 (Stata Corp, College Station, TX).
RESULTS
Eighty-three patients with biopsy-proven LM/LMM were imaged. Eleven patients were subsequently excluded: 5 declined treatment, 2 were treated with off-label imiquimod, 2 with radiotherapy, and 2 with wide excision. Seventy-two were included in the final analysis contributing 354 RCM data points (72 center, 282 margins). Six quadrants in 4 patients were not assessable because of anatomic location (2 located inside the ear concha, 1 on the lower eyelid, 1 on the nasal crease, and 2 retroauricular).
Demographics and tumor characteristics
Mean age was 66.8 years (SD, 11.1; range, 38–89); 69.4% (50/72) were men. Most lesions were located on the head and neck (97.2%; 70/72). Mean size of the lesions was 1.3 cm in largest diameter (SD, 0.9; range, 0.3–5.0). Sixteen of 72 melanomas (22.2%) were invasive with a mean Breslow thickness of 0.5 mm (SD, 0.4; range, 0.2–1.0). Mean initial surgical margin size was 4.6 mm (SD, 1.2; range 3.0–10.0). Details are shown in Table I.
Table I.
Demographic and tumor characteristics of all melanoma patients (N = 72)
| Variable | Value |
|---|---|
|
| |
| Mean age, y (SD) | 66.8 (11.1) |
| Gender, male | 50 (69.4) |
| Location | |
| Cheek | 30 (41.7) |
| Chin | 2 (2.8) |
| Ear | 3 (4.2) |
| Forehead | 4 (5.6) |
| Hand | 1 (1.4) |
| Jawline | 1 (1.4) |
| Nose | |
| Nasal dorsum | 3 (4.2) |
| Nasal sidewall | 4 (5.6) |
| Nasal tip | 6 (8.3) |
| Nasolabial fold | 1 (1.4) |
| Neck | 4 (5.6) |
| Periocular | 4 (5.6) |
| Scalp | 5 (6.9) |
| Shoulder | 1 (1.4) |
| Temple | 3 (4.2) |
| Mean size, cm (SD) | 1.3 (0.9) |
| In situ | 51 (79.7) |
| Clinical residual | 36 (50.0) |
Values are n (%) unless otherwise defined. SD, Standard deviation.
Overall RCM to histopathology correlation
RCM had an overall agreement with the final histopathologic status of 85.8% (κ = 0.71) (Table II). Figs 2 and 3 show RCM mapping of LM cases.
Table II.
Agreement statistics for the overall sample and the 4 lesion quadrants and lesion center
| Location | Agreement (%) | Expected agreement (%) | κ | SE | Z | P |
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | 85.88 | 50.89 | 0.71 | 0.05 | 13.51 | <.001 |
| Tumor debulk | 91.67 | 74.07 | 0.68 | 0.12 | 5.79 | <.001 |
| Quadrant 1 | 85.71 | 54.90 | 0.68 | 0.12 | 5.73 | <.001 |
| Quadrant 2 | 83.10 | 56.60 | 0.61 | 0.11 | 5.33 | <.001 |
| Quadrant 3 | 81.69 | 56.62 | 0.58 | 0.12 | 4.89 | <.001 |
| Quadrant 4 | 87.14 | 56.94 | 0.70 | 0.12 | 6.03 | <.001 |
SE, Standard error.
Fig 2.

Reflectance confocal microscopy (RCM) mapping with lentigo maligna (LM) at 5-mm surgical margins. A, Biopsy-proven LM with ill-defined borders and background of extensive photodamage. The red dashed circle represents the clinically outlined LM, and the blue dashed circle represents the initial surgical margin. B, RCM image of the residual LM (red dashed circle in A) showing bright, large, nucleated dendritic cells arranged in sheet-like structures (red arrows) with folliculotropism (white asterisk) (750 × 750 μm). C, RCM image of 5-mm surgical margin (blue dashed circle in A) showing LM with bright dendritic cells (red arrows) extending to the paper ring margin (green arrows) (750 × 750 μm). Inset shows a videomosaic at the × to 6 o’clock quadrant. D, Histopathology of the tumor debulk showing LM (red arrows) correlating to B (hematoxylin and eosin stain; original magnification: ×40). E, Histopathology of the × to 6 o’clock quadrant showing LM (red arrows) extending to surgical margin (green arrow); correlating to C.
Fig 3.

Reflectance confocal microscopy (RCM) mapping with no clinically evident lentigo maligna (LM) with 5-mm positive surgical margins. A, Biopsy-proven LM with no residual clinical pigmentation surrounded by freckles and lentigines (inset). The red dashed circle represents the biopsy site, and the blue dashed circle represents the initial surgical margin. B, RCM image of the biopsy site (red dashed circle in A) showing residual LM with bright, large, nucleated dendritic cells (red arrows) surrounding hair follicles (white asterisk) (750 × 750 μm). C, RCM image of the 5-mm surgical margin (blue dashed circle in A) showing LM with bright dendritic cells (red arrows) extending to the paper ring margin (green arrows) (750 × 750 μm). D, Histopathology of the tumor debulk showing LM (red arrows) correlating to B (hematoxylin and eosin stain; original magnification: ×40). E, Histopathology of the 6 to 9 o’clock quadrant showing LM (red arrows) extending to the free edge (green arrow) correlating to C. F, Immunohistochemistry highlighting LM cells at the 6 to 9 o’clock quadrant margin (microphthalmia transcription factor stain; original magnification: ×40X).
RCM assessment of residual LM/LMM (tumor debulk).
Histopathology showed residual melanoma on the tumor debulk in 60 of 72 cases (83.3%). RCM evaluation of the central portion of the lesion found that 62 of 72 cases (86.1%) had residual melanoma. RCM had an agreement with the tumor debulk final histopathologic status of 91.7% (κ = 0.68) (Table II). RCM had a sensitivity of 96.7% (95% CI, 88.5%−99.6%), a specificity of 66.7% (95% CI, 34.9%−90.1%), positive predictive value of 93.6% (95% CI, 86.7%−97.0%), and negative predictive value of 80.0% (95% CI, 49.2%−94.3%) for the detection of residual melanoma in the tumor debulk. The number of true positives, true negatives, false positives, and false negatives were 58, 8, 4, and 2, respectively.
Thirty-six of 72 cases (50.0%) did not have any clinical residual pigmentation at the time of RCM examination (secondary to excisional biopsy) and hence presented as a pink scar-like area. Lesions with no clinical residual pigmentation tended to occur in younger patients (63.3 vs 70.4 years; P = .006) and in smaller size LM/LMMs (0.9 vs 1.7 cm; P <.001). There were no differences by gender (P = .8). In this subgroup of patients 25 of 36 patients (69.4%) still had residual melanoma on the debulked tissue. RCM had a sensitivity of 92.0% (95% CI, 73.9%−99.0%), specificity of 72.7% (95% CI, 39.0%−93.9%), positive predictive value of 88.5% (95% CI, 74.4%−95.3%), and negative predictive value of 80.0% (95% CI, 50.2%−94.1%). The number of true positives, true negatives, false positives, and false negatives were 23, 8, 3, and 2, respectively (Fig 3).
RCM assessment of initial surgical margins (quadrants).
Margins were involved with LM/LMM at the delineated ring borders on histopathology in 36 of 72 patients (50.0%). In all, 18.1% (n = 13), 12.5% (n = 9), 6.9% (n = 5), and 12.5% (n = 9) had 1, 2, 3, or all 4 quadrants involved on histopathology. RCM margin assessment revealed an overall agreement with the final histopathology of 85.8% (κ = 0.71; P <.001). Agreement per quadrant ranged between 81.7% and 87.1%, with kappa values ranging from 0.58 to 0.70 (Table II). When a random sample of 25% of the lesion quadrants was shifted to a counterclockwise quadrant to account for minor angular variations, overall agreement remained similar at 83.9% (κ = 0.67; P < .001). Quadrant agreement also remained similar and ranged between 78.9% and 87.1% (Supplemental Table I, available via Mendeley at 10.17632/35nw64ymnh.2).
DISCUSSION
In this study there was moderate to excellent overall agreement (>80%) between RCM and histopathology in LM/LMM patients undergoing staged excision. We evaluated diagnostic accuracy for detection of residual melanoma (after a biopsy) in the tumor debulk and observed a sensitivity of 96.7% and a specificity of 66.7% when compared with the gold standard (histopathology). Similar results were observed for RCM and histopathology for the detection of subclinical extension of LM/LMM at the initial surgical margins (80%−90% agreement; κ = 0.58–0.70). Our study validates the utility of RCM for residual LM/LMM diagnosis and surgical margin evaluation in a large cohort of 72 patients.
Traditionally, LM/LMM margins have been delineated with clinical examination, Wood’s lamp, and dermoscopy with limited accuracy.25 Additionally, scouting biopsies have been used to determine subclinical extension; however, they provide focal and static information.18 RCM is a noninvasive in vivo skin imaging modality that can scan, in real time, LM/LMM before surgery. Studies show RCM provides good correlation with histopathologic findings in patients with LM/LMM for diagnostic purposes.13,20,26 One challenge has been detecting residual melanoma after biopsies. Our study confirms that scarring tissue does not significantly affect the diagnostic role of RCM.17,18,21,23,27,28
Preliminary studies have used RCM to delineate LM/LMM margins.15,17–19,29 Yelamos et al18 included 22 patients and found that surgical margins were a mean of 0.76 mm larger than the RCM estimate. Pellacani et al17 performed a prospective study of 23 patients and found a 92% sensitivity and a 57% specificity for RCM margin evaluation. The study by Pellacani et al also highlights the relevance of RCM in the absence of margin-controlled surgery (staged excision or Mohs micrographic surgery), because they performed RCM-guided traditional wide excision. Guitera et al15 included 29 patients and found that in 59% RCM identified subclinical lesions beyond 5-mm margins; of note, RCM changed management in 73% of their patients. Collectively, RCM studies in Europe,17,21,29 Australia,15 and now the United States18,19 confirm the role of RCM as an adjunctive presurgical tool in LM/LMM management.
RCM mapping may have clinical implications to improve LM/LMM management in multiple clinical scenarios. First, when margin-controlled surgery (eg, Mohs surgery) is not available, RCM may better define surgical margins, ultimately avoiding incomplete excision before reconstruction.1,8,17,30 Second, even if margin-controlled surgeries are available, RCM mapping can translate into a more precise and efficient excision/reconstruction process (ie, anticipating primary closure vs flap vs graft). Similar to traditional wide-field imaging (eg, magnetic resonance imaging, computed tomography), RCM could be used by the physician as a complementary surgical planning tool given its higher cellular detail resolution.31 Finally, careful surgical margin planning in complex and large head and neck LM/LMM can remove the “unknown” factor for the patient. This ultimately may lead to improved patient satisfaction.32
Limitations
Our objective was to evaluate the presence or absence of subclinical extension in a real clinical setting with RCM and compare it with the gold standard. No RCM imaging beyond the initial surgical margin was performed because radial correlation has been demonstrated by Yelamos et al.18 RCM evaluation and surgery were not performed on the same day, theoretically affecting the 1:1 RCM–histopathologic correlation. However, this limitation did not seem to affect results when a subset of cases was rotated counterclockwise (Supplemental Table I). Strategies to overcome this potential limitation include same-day RCM and surgery with coordination between surgeons and confocalists, using “scores” as described by Pellacani et al,17 and the use of transparent dressings to create “templates” that can be conveniently stored and saved for the day of surgery.33 Another limitation is that we used a ring shape to evaluate lesions and some LM/LMM may have irregular borders. The use of a metal-ink pen could be a potential solution.34
The accuracy of RCM LM/LMM mapping may not be considered sufficient for broader clinical practice when compared with histopathology. The specificity for detecting residual LM/LMM in the tumor debulk (central portion) was 66.7%. However, there were only 4 false positives of the 72 cases imaged. Inflammation after biopsy could play a role in these cases. Positive predictive value was 93.3%, which may be a more useful metric in this clinical context. More importantly, for LM/LMM margin assessment we found 81% to 87% agreement between RCM and histopathology. Atypical melanocytes can occur in normal sun-exposed skin, and distinguishing LM/LMM from sun-damage melanocytic hyperplasia can be challenging both on RCM and in histopathologic evaluations and may be seen as RCM false positives.2,35,36 Some melanomas are pauci-cellular and difficult to evaluate with routine hematoxylin and eosin and may explain false-negative quadrants on RCM. Finally, this study was performed in a tertiary cancer center with experience in RCM; RCM mapping is a time-consuming process than can take 20 to 60 minutes. However, we anticipate an increase in use of RCM given the recent acquisition of a category I Current Procedural Terminology code in the United States.37
Conclusion
RCM showed substantial correlation with initial surgical margins when compared with histopathology in patients with LM/LMM. Going forward, LM/LMM RCM mapping may provide important benefits by improving surgical planning, reducing the “unknown” factor, and potentially reducing the number of surgical stages, which ultimately can reduce costs and improve patient satisfaction.
CAPSULE SUMMARY.
Reflectance confocal microscopy margin assessment at the initial surgical margin of lentigo maligna revealed an overall agreement with final histopathology of 85.9%.
Reflectance confocal microscopy mapping of lentigo maligna can improve surgical planning, potentially reducing rate of positive margins, reducing number of surgical stages, reducing costs, and improving patient satisfaction.
Funding sources:
Supported in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748. The funder played no role in any aspect of the study.
Dr Rossi has received grant funding from The Skin Cancer Foundation and the A.Ward Ford Memorial Grant for research related to this work and has served on an advisory board, as a consultant, or given educational presentations for Allergan, Inc, Galderma Inc, Evolus Inc, Elekta, Biofrontera, Quantia, Merz Inc, Dynamed, Skinuvia, Perf-Action, and LAM therapeutics. Dr Nehal has received royalties from publishing companies for books and book chapters.
Abbreviations used:
- CI
confidence interval
- LM/LMM
lentigo maligna/lentigo maligna melanoma
- RCM
reflectance confocal microscopy
- SD
standard deviation
Footnotes
Conflicts of interest:
All other authors have no conflicts of interest to declare.
REFERENCES
- 1.Rzepecki AK, Hwang CD, Etzkorn JR, et al. The “rule of 10s” versus the “rule of 2s”: high complication rates after conventional excision with postoperative margin assessment of specialty site versus trunk and proximal extremity melanomas. J Am Acad Dermatol. 2021;85(2):442–452. [DOI] [PubMed] [Google Scholar]
- 2.Fosko SW, Navarrete-Dechent CP, Nehal KS. Lentigo maligna-challenges, observations, imiquimod, confocal microscopy, and personalized treatment. JAMA Dermatol. 2018;154:879–881. [DOI] [PubMed] [Google Scholar]
- 3.Hazan C, Dusza SW, Delgado R, Busam KJ, Halpern AC, Nehal KS. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142–148. [DOI] [PubMed] [Google Scholar]
- 4.Rigual NR, Popat SR, Jayaprakash V, Jaggernauth W, Wong M. Cutaneous head and neck melanoma: the old and the new. Expert Rev Anticancer Ther. 2008;8:403–412. [DOI] [PubMed] [Google Scholar]
- 5.Hoersch B, Leiter U, Garbe C. Is head and neck melanoma a distinct entity? A clinical registry-based comparative study in 5702 patients with melanoma. Br J Dermatol. 2006;155:771–777. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. Melanoma: (version 2.2018). [Google Scholar]
- 7.Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743–748. [DOI] [PubMed] [Google Scholar]
- 8.Kunishige JH, Doan L, Brodland DG, Zitelli JA. Comparison of surgical margins for lentigo maligna versus melanoma in situ. J Am Acad Dermatol. 2019;81(1):204–212. [DOI] [PubMed] [Google Scholar]
- 9.Karanetz I, Stanley S, Knobel D, et al. Melanoma extirpation with immediate reconstruction: the oncologic safety and cost savings of single-stage treatment. Plast Reconstr Surg. 2016; 138:256–261. [DOI] [PubMed] [Google Scholar]
- 10.Navarrete-Dechent C, Aleissa S, Ariyan C, Busam KJ, Nehal KS. Comment on “Comparison of surgical margins for lentigo maligna versus melanoma in situ.” J Am Acad Dermatol. 2019; 81(4):e115–e116. [DOI] [PubMed] [Google Scholar]
- 11.Kelley LC, Starkus L. Immunohistochemical staining of lentigo maligna during Mohs micrographic surgery using MART-1. J Am Acad Dermatol. 2002;46:78–84. [DOI] [PubMed] [Google Scholar]
- 12.Rajadhyaksha M, Marghoob A, Rossi A, Halpern AC, Nehal KS. Reflectance confocal microscopy of skin in vivo: from bench to bedside. Lasers Surg Med. 2017;49:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080–2091. [DOI] [PubMed] [Google Scholar]
- 14.Wurm E, Pellacani G, Longo C, et al. The value of reflectance confocal microscopy in diagnosis of flat pigmented facial lesions: a prospective study. J Eur Acad Dermatol Venereol. 2017;31:1349–1354. [DOI] [PubMed] [Google Scholar]
- 15.Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149: 692–698. [DOI] [PubMed] [Google Scholar]
- 16.Chen CS, Elias M, Busam K, Rajadhyaksha M, Marghoob AA. Multimodal in vivo optical imaging, including confocal microscopy, facilitates presurgical margin mapping for clinically complex lentigo maligna melanoma. Br J Dermatol. 2005;153:1031–1036. [DOI] [PubMed] [Google Scholar]
- 17.Pellacani G, De Carvalho N, Ciardo S, et al. The smart approach: feasibility of lentigo maligna superficial margin assessment with hand-held reflectance confocal microscopy technology. J Eur Acad Dermatol Venereol. 2018;32(10):1687–1694. [DOI] [PubMed] [Google Scholar]
- 18.Yelamos O, Cordova M, Blank N, et al. Correlation of handheld reflectance confocal microscopy with radial video mosaicing for margin mapping of lentigo maligna and lentigo maligna melanoma. JAMA Dermatol. 2017;153:1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menge TD, Hibler BP, Cordova MA, Nehal KS, Rossi AM. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74:1114–1120. [DOI] [PubMed] [Google Scholar]
- 20.Cinotti E, Fiorani D, Labeille B, et al. The integration of dermoscopy and reflectance confocal microscopy improves the diagnosis of lentigo maligna. J Eur Acad Dermatol Venereol. 2019;33(10):e372–e374. [DOI] [PubMed] [Google Scholar]
- 21.Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40:247–256. [DOI] [PubMed] [Google Scholar]
- 22.Marino ML, Rogers T, Sierra Gil H, Rajadhyaksha M, Cordova MA, Marghoob AA. Improving lesion localization when imaging with handheld reflectance confocal microscope. Skin Res Technol. 2016;22:519–520. [DOI] [PubMed] [Google Scholar]
- 23.Navarrete-Dechent C, Mori S, Cordova M, Nehal KS. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7–e8. [DOI] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 25.Robinson JK. Use of digital epiluminescence microscopy to help define the edge of lentigo maligna. Arch Dermatol. 2004; 140:1095–1100. [DOI] [PubMed] [Google Scholar]
- 26.Cinotti E, Labeille B, Debarbieux S, et al. Dermoscopy vs. reflectance confocal microscopy for the diagnosis of lentigo maligna. J Eur Acad Dermatol Venereol. 2018;32(8):1284–1291. [DOI] [PubMed] [Google Scholar]
- 27.Navarrete-Dechent C, Cordova M, Liopyris K, et al. Reflectance confocal microscopy and dermoscopy aid in evaluating repigmentation within or adjacent to lentigo maligna melanoma surgical scars. J Eur Acad Dermatol. 2020;34(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarrete-Dechent C, Cordova M, Aleissa S, et al. Reflectance confocal microscopy confirms residual basal cell carcinoma on clinically negative biopsy sites before Mohs micrographic surgery a prospective study. J Am Acad Dermatol. 2019;81:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couty E, Tognetti L, Labeille B, et al. In vivo reflectance confocal microscopy combined with the “spaghetti technique” for the identification of surgical margins of lentigo maligna: experience in 70 patients. J Eur Acad Dermatol Venereol. 2018;32:e366–e368. [DOI] [PubMed] [Google Scholar]
- 30.Shin TM, Etzkorn JR, Sobanko JF, et al. Clinical factors associated with subclinical spread of in situ melanoma. J Am Acad Dermatol. 2017;76:707–713. [DOI] [PubMed] [Google Scholar]
- 31.Navarrete-Dechent C, Cordova M, Postow MA, et al. Evaluation of the response of unresectable primary cutaneous melanoma to immunotherapy visualized with reflectance confocal microscopy: a report of 2 cases. JAMA Dermatol. 2019;155:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee EH. Patient expectations and performance measures in dermatologic surgery. Clin Dermatol. 2016;34:111–113. [DOI] [PubMed] [Google Scholar]
- 33.Navarrete-Dechent C, Hibler BP, Cordova F, Cordova M. Lentigo maligna margin template for surgical excision using reflectance confocal microscopy and a transparent adhesive dressing. Dermatol Surg. 2020;46(7):967–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alessia S, Navarrete-Dechent C, Banzhaf CA, et al. Using a metallic ink pen to assist in the demarcation of skin lesions under reflectance confocal microscopy. J Am Acad Dermatol. 2019;81(6):e173–e174. [DOI] [PubMed] [Google Scholar]
- 35.Barlow JO, Maize J Sr, Lang PG. The density and distribution of melanocytes adjacent to melanoma and nonmelanoma skin cancers. Dermatol Surg. 2007;33:199–207. [DOI] [PubMed] [Google Scholar]
- 36.Weyers W, Bonczkowitz M, Weyers I, Bittinger A, Schill WB. Melanoma in situ versus melanocytic hyperplasia in sun-damaged skin. Assessment of the significance of histopathologic criteria for differential diagnosis. Am J Dermatopathol. 1996;18:560–566. [DOI] [PubMed] [Google Scholar]
- 37.American Medical Association. Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. [Google Scholar]


