Abstract
The devastating impact of Tuberculosis (TB) has been a menace to mankind for decades. The World Health Organization (WHO) End TB Strategy aims to reduce TB mortality up to 95% and 90% of overall TB cases worldwide, by 2035. This incessant urge will be achieved with a breakthrough in either a new TB vaccine or novel drugs with higher efficacy. However, the development of novel drugs is a laborious process involving a timeline of almost 20–30 years with huge expenditure; on the other hand, repurposing previously approved drugs is a viable technique for overcoming current bottlenecks in the identification of new anti-TB agents. The present comprehensive review discusses the progress of almost all the repurposed drugs that have been identified to the present day (∼100) and are in the development or clinical testing phase against TB. We have also emphasized the efficacy of repurposed drugs in combination with already available frontline anti-TB medications along with the scope of future investigations. This study would provide the researchers a detailed overview of nearly all identified anti-TB repurposed drugs and may assist them in selecting the lead compounds for further in vivo/clinical research.
1. Introduction
Tuberculosis has been a persistent global threat for centuries and is the leading cause of death worldwide caused by Mycobacterium tuberculosis(M.tb).1 Despite the major attempts to control the disease spread, the rate of infection seems to be declining very slowly. The cumulative reduction achieved from 2015 to 2019 was almost half (9%) as compared to the 20% milestone aimed by the WHO. Approximately 10.0 million people fell ill with TB in 2020, and it took 1.3 million lives in HIV-negative people and 0.21 million among HIV-positive people.1 Although the effective regimens for treatment of drug-sensitive TB with 95% cure are accessible, the lengthy treatments and toxic effects of drugs result in low adherence to TB treatment causing emerging cases of multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB that have further deteriorated the current situation.2 It is no surprise that the current first-line laborious regimen of Rifampicin, Isoniazid, Ethambutol, and Pyrazinamide sees suboptimal compliance,3 further promoting resistance. Moreover, treatment for MDR-TB is lengthier (9–24 months), complex (involving combinations of 5–7 drugs), and poorly tolerated. Patients infected with MDR M.tb have higher mortality than those infected with drug-susceptible M.tb.4 Various newly approved drugs (e.g., Bedaquiline, Delamanid) have shown promising treatment outcomes against MDR and XDR-TB;5,6 however, resistance and failure of these drugs have already been observed in routine clinical practice.7 As a result, research into new anti-TB drugs has become critical.
Over the years various studies and trials have been undertaken with the aim to repurpose the existing drugs for addressing the bottleneck in the development of novel anti-TB agents. According to the National Institute of Health (NIH), drug repurposing is broadly defined as discovering new uses for approved drugs to provide the quickest possible transition from bench to bedside,8 which can further be explained as a process of identification of new therapeutic indications from old/failed/investigational/FDA approved drugs, to the treatment of diseases unrelated to the drug’s initial clinical usage.9 This Review sheds light on almost all worthy drug candidates (years: 2010–2022) that seem to have potential anti-TB activity against drug resistant isolates and discusses their efficacy, mechanism of action, toxic effects, and synergistic effects. The various up-to-date approaches for repurposing existing drugs have also been briefly discussed in the following sections along with the associated challenges and limitations. We have also highlighted the basis adopted by WHO for including various repurposed drugs in the treatment regimen of drug-resistant TB along with their progress in treating the disease.
2. Methodology
Various articles pertaining to (i) repurposing strategies/approaches, (ii) repurposed drugs for TB, (iii) in silico/in vitro evaluations, (iv) murine investigations, and (v) clinical trials were found and collected using PubMed.gov and Web of Science. Except for the information on in silico tools/software and other repurposing approaches, the papers containing information on Tuberculosis drug repurposing were carefully chosen for this study. The ClinicalTrials.gov website was employed to look for the ongoing clinical studies on repurposed anti-TB drugs. The search for published articles was confined to the years January 2010 through March 2022, although not exclusively.
3. Drug Repurposing for Tuberculosis: Strategies and Approaches
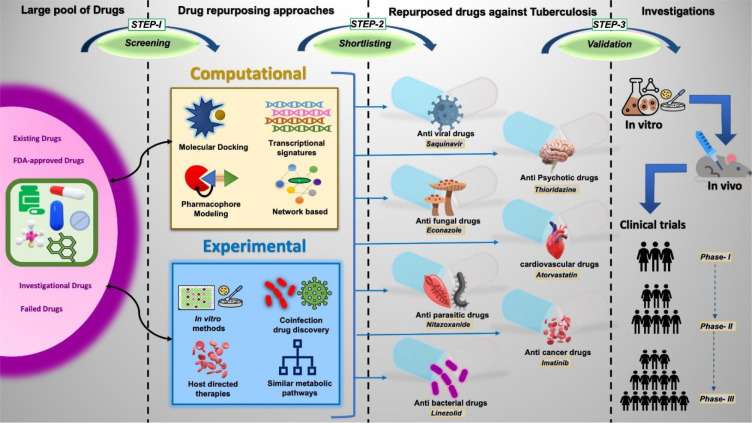
The terms “Drug repurposing” and “Drug repositioning” are often used interchangeably to indicate the new therapeutic usages of already approved and established drugs. Drugs are designed primarily to treat a specific illness or condition, but these may also cross react or interact with nonspecific targets, resulting in secondary biological effects, which certainly opens up a gateway for their utilization against other causes as well. Since the pharmacokinetics and pharmacodynamics studies are already known for such drugs, the tedious process of toxicity profiling, target validation, hit-to-lead optimization, and/or in vivo metabolic studies are rarely required when are repurposed for other diseases.10 This strategy comprehends lower risk of failure, as ∼45% failure rate is associated with safety and toxicity issues in classical drug discovery method with additional benefits of cutting short the time period to around 5–7 years11 and also involves comparatively less investment of resources. Sildenafil, Minoxidil, Thalidomide, Valproic acid, and Methotrexate are some of the well-known examples of repurposed drugs related to various diseases.12,13 To speed up the end TB strategy process this method of drug identification is attracting a plethora of researchers in the field of TB. Figure 1 depicts the steps involved in the drug repurposing process for TB. The process begins with the large pool of drugs (old/failed/investigational/FDA approved). Various computational or experimental repurposing approaches like molecular docking,14 pharmacophore modeling,15 and high throughput drug screening (HTDS)16 are used to screen these drugs for their potential to inhibit M.tb survival. From such screening, the lead bioactive compounds are selected for further validations in preclinical and clinical trial studies to evaluate molecular pathophysiology, contraindications, dosage, and synergistic effects.
Figure 1.
Illustration of the steps involved in the drug repurposing process. Step 1: Screening – identifying potential repurposed drug candidate from a large pool of drugs using appropriate computational or experimental methodologies, Step 2: Shortlisting– selection of potential lead compounds, Step 3: Validation – validating the discovered drug through preclinical and clinical trial investigations.
4. Repurposed Drug Candidates against Tuberculosis
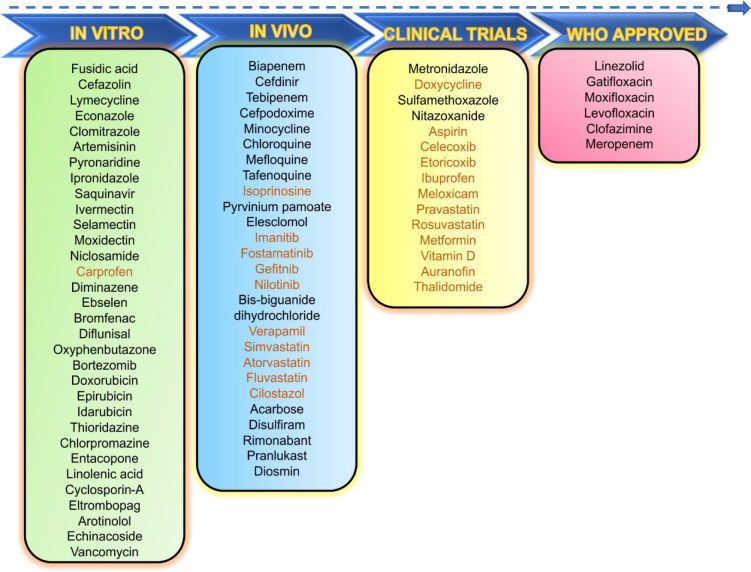
The drugs discussed in this study are categorized into three main categories: (i) anti-infective drugs, (ii) non-anti-infective drugs, and (iii) miscellaneous drugs. Figure 2 highlights the drugs of these categories that have entered clinical trials with high efficiency against TB, as well as those with potential anti-TB activity as demonstrated by various in vitro and in vivo investigations until March 2022.
Figure 2.
Potential repurposed drugs under preclinical or clinical stages of investigations against TB. In vitro (n = 32), in vivo (n = 27), clinical studies (n = 21), WHO approved for TB treatment (n = 6). Drug names in orange represent drugs under investigation as adjunctive agents for TB-treatment.
4.1. Repurposed Anti-Infective Drugs against TB
Repurposing of anti-infective compounds is based on the concept of “spectrum expansion”, in which a chemical having broad-spectrum activity and efficacy against one pathogen (for example, bacterial species) may be tested against another pathogen.17 Since M.tb is a bacterial species, various broad-spectrum antibacterial agents have been reported to be effective against this bacillus. Similarly, the drugs used to treat infections caused by viruses and unicellular prokaryotic and eukaryotic organisms have been widely studied for their antimycobacterial properties. Table 1 and Table 2 list descriptions of nearly all anti-infective repurposed drugs that have been identified with potential anti-TB activity.
Table 1. WHO Approved Antibacterial Repurposed Drugs Included in Treatment Regimen for Drug-Resistant TB.
| S. no. | Drug name and class | Group | Mechanism of action/drug target | Critical concentration | Patient daily dosage | Refs |
|---|---|---|---|---|---|---|
| 1 | Linezolid (Oxazolidinone) | Group-A | 23S rRNA of the 50S subunit | 1 mg/L using LJ medium | 600 mg | (48,49) |
| 2 | Moxifloxacin (Fluoroquinolone) | Group-A | DNA-Gyrase | 1 mg/L using LJ medium | 400 mg | (48,49) |
| 3 | Levofloxacin (Fluoroquinolone) | Group-A | DNA-Gyrase | 2 mg/L using LJ medium | 1 g | (48,49) |
| 4 | Clofazimine (Riminophenazine derivative) | Group-B | Antimicrobial activity is membrane-directed | 1 mg/L using MGIT-DST | 100 mg | (48,49) |
| 5 | Meropenem (β-Lactams) | Group-C | Inhibit cell wall synthesis | NA | 2 g with Clavulanic acid (125 mg) | (48,49) |
Table 2. Repurposed Anti-Infective Drugs against Tuberculosis, Their Mechanisms of Action, MICs, and Ongoing Investigationsa.
| S. no. | Drug name (Drug class) | Mechanism of action/Drug target in M.tb | Minimum inhibitory concentration (MIC) in M.tb | Current status/ongoing studies | Refs |
|---|---|---|---|---|---|
| Antibacterial drugs | |||||
| 1 | Gatifloxacin (Fluoroquinolone) | DNA-Gyrase | 0.5 mg/L | Phase I, II trials completed | NCT0039608449 |
| 2 | Metronidazole (Nitroimidazole) | Break DNA helical structure | 16 μg/mL or higher | Phase II trials completed | NCT00425113 |
| 3 | Doxycycline (Tetracycline) | Inhibit host MMPs and act as HDT | 200 mg/kgb MIC – 0.016 μg/mL | Phase II trials completed | NCT0277499345 |
| 4 | Sulfamethoxazole (Sulpha drugs) | Inhibits synthesis of dihydrofolic acid | 9.5 mg/L | Phase II trials completed | NCT0183298768 |
| 5 | Biapenem (Carbapenem) | Inhibit cell wall synthesis | 2.5–5 μg/mL | in vivo | (69) |
| 6 | Tebipenem (β-Lactams) | Inhibit cell wall synthesis | 1.25–2.5 μg/mL | in vivo | (33) |
| 7 | Minocycline (Tetracycline) | Bind to 30S ribosomal subunit, inhibit protein synthesis | MIC50 < 2 mg/L | in vitro and in vivo | (42) |
| 8 | Cefdinir (Cephalosporin) | Inhibit cell wall synthesis | 2–4 mg/L (H37Ra), 0.5 and 16 mg/L (clinical Isolates) | in vitro and in vivo | (38) |
| 9 | Cefpodoxime (Cephalosporin) | Inhibit cell wall synthesis | >128 μg/mL (without Calvulanate), 32 μg/mL (with Calvulanate) | in vitro and in vivo | (70) |
| 10 | Fusidic acid (Fusidane) | Inhibit translocation | 32–64 mg/L | in silico and in vitro | (71) |
| 11 | Lymecycline (Tetracycline) | TrpD, CoaA | 10–100 μg/mL(H37Rv) | in silico and in vitro | (43) |
| 12 | Cefazolin (Cephalosporin) | Bind penicillin binding proteins, halt peptidoglycan synthesis | 64 mg/L (H37Ra) and, 2–8 mg/L with15 mg/L avibactam | in vitro | (39) |
| 13 | Vancomycin (Glycopeptide) | Inhibit cell wall synthesis | 0.5 mg/L (H37Ra),3 mg/L (H37Rv)12–96 mg/L (MDR isolates) | in vitro | (47) |
| Antifungal Drugs | |||||
| 15 | Econazole (Azole) | Lanosterol 14 α-demethylase | MIC90- 4 μg/mL (M.tb clinical isolates) | in vitro, in vivo | (51) |
| 16 | Clomitrazole (Azole) | Lanosterol 14 α-demethylase | MIC90- 8 μg/mL (M.tb clinical isolates) | in vitro | (51) |
| 17 | Artemisinin | Membrane-associated protein | 75 μg/mL | in vitro | (53) |
| Anti-Protozoal Drugs | |||||
| 18 | Nitazoxanide (Thiazolide) | Disrupt membrane potential, pH homeostasis | 16 μg/mL (H37Rv),12 to 28 μg/mL (M.tb clinical isolates) | Phase II trials completed | NCT0268424072 |
| 19 | Chloroquine (Class 4-aminoquinoline) | Efflux pump inhibitor | NA | in silico, in vivo | (60) |
| 20 | Mefloquine (Quinine) | Interfere with mycolic acids biosynthesis | 4–16 μg/mL (M.tb clinical isolates) | in vitro | (73) |
| 21 | Tafenoquine (Quinine) | NA | 1.25–80 μM (MDR strain) | in vitro | (61) |
| 22 | Pyronaridine (Benzonaphthyridine) | Inhibit DNA synthesis | 5 μg/mL | in silico, in vitro | (58) |
| 23 | Ipronidazole (Nitroimidazole) | NA | 16 μg/mL (clinical isolates) | in vitro | (74) |
| Antiviral Drugs | |||||
| 24 | Isoprinosine | HDT against M.tb | NA | in vivo | (62) |
| 25 | Saquinavir | HDT against M.tb | 5 to 20 μg/mL | in vitro | (63) |
| Anti-Helminthic Drugs | |||||
| 26 | Pyrvinium pamoate (Naphthoic acid) | NA | 1.5 to 4.8 μg/mL (H37Rv, MDR isolates) | in vitro, in vivo | (75) |
| 27 | Ivermectin (Avermectin) | NA | 6 μg/mL (H37Rv) | in vitro | (64) |
| 28 | Selamectin (Avermectin) | NA | 3 μg/mL (H37Rv) | in vitro | (64,66) |
| 29 | Moxidectin (Avermectin) | NA | 3 μg/mL (H37Rv) | in vitro | (64) |
| 30 | Niclosamide | NA | 5 μM (BCG, Beijing) | in silico, in vitro | (76, 77) |
NA: Not available, CT: clinical trials.
Patient daily dosage.
4.1.1. Antibacterial Agents
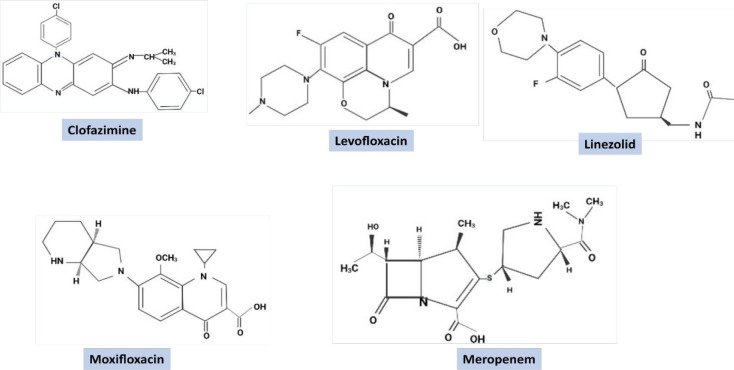
The drugs of this category are the most successful examples of repurposed drugs for TB. Five antibacterial repurposed drugs are already approved by WHO for the treatment of drug-resistant TB. The critical concentration of these drugs along with drug group and patient daily dosage are mentioned in Table 1 and their chemical structures are provided in Figure 3. One of these drugs is Linezolid. It belongs to the Oxazolidinone class with broad-spectrum antimicrobial activity and primarily acts in the early stage of protein synthesis by inhibiting the 50S ribosomal subunit.18 In 2011, the WHO classified Linezolid as a group 5 agent (drugs with unclear efficacy/activity in MDR-TB) due to the lack of sufficient evidence on its safety and efficacy, but in 2018, it was upgraded to group A (highly prioritized drugs for MDR-TB treatment).19 The evidence for linezolid in drug-resistant TB treatment has been evaluated in five meta-analyses; an evidence summary for the 2018 WHO recommendation has already been published.20,21 However, there is still a disagreement about Linezolid use, which is currently being addressed by the ZeNix study (NCT03086486), and its primary results have highlighted a high success rate of 93%, 89%, 91%, and 84% in patients receiving varied concentration of Linezolid (1200, 600 mg/kg) for different durations along with Bedaquiline and Pretomanid (BPaL). The study also reported peripheral neuropathy and myelosuppression as common adverse effects of the drug.22 A similar study also recently reported a 90% success rate (favorable outcome, observed six months after the completion of therapy, in terms of efficacy and safety end points in 98 patients out of 109) of BPaL in 109 XDR and MDR-TB cases with well manageable toxicity cases (NCT02333799).23 A finding has shown that Linezolid in combination with Bedaquiline is effective and safe in the treatment of XDR-TB among third-trimester pregnancy cases.24 The resistance for this drug has been reported due to the mutation at nucleotide position 2061 in the rrl gene that encodes rRNA 23S and T460C mutation in the rplC gene encoding 50S ribosomal protein L3 RplC.25 Although Linezolid is effective against MDR/XDR-TB, the toxicity and associated side effects limit its use only up to DR-TB. Sutezolid and Delpazolid are two Linezolid congeners under investigation in early clinical trials (NCT01225640) with a hope of lower toxicity, safer profile, and effectiveness like Linezolid.26,27 Another antibacterial agent Clofazimine (CZM) was utilized as an anti-leprosy drug, and presently it constitutes a position in group B (compounds which are recommended to use conditionally as anti-TB agents of second choice) of WHO-approved medications for MDR-TB treatment.19 CZM has antimycobacterial effects owing to phospholipase inhibition and anti-inflammatory properties due to effects on potassium transporters.28 In M.tb it works as a prodrug where it is activated by type 2-NADH dehydrogenase and generates reactive oxygen species (ROS).29 In a recent study, MDR-TB patients receiving a shorter treatment regimen containing CFZ drug (100 mg/day) had faster sputum culture conversion and successful outcome rates than those who received 18-month treatment regimen without CFZ, indicating its imperative activity in the case of MDR-TB treatment.30 The preclinical analysis of CFZ against COVID-19 due to its inhibitory activity against numerous coronaviruses and antagonizing replication of SARS-CoV-2 and MERS-CoV itself highlights its repurposing potential for human use.31 An open-label, multicenter, randomized controlled trial involving CFZ has entered phase 3 to compare the efficacy and safety of a study plan of 6 months of Bedaquiline, Delamanid, and Linezolid, with Levofloxacin, and CFZ compared to control strategy of South African Standard of Care for 9 months for treating the Rifampicin resistant TB (NCT04062201). CFZ potential for repurposing in a short-course TB therapy is further strengthened by several studies in various stages (NCT03828201, NCT04311502). The biggest disadvantage of using this drug is its extended half-life (∼70 days in humans) and the substance’s long-term retention in tissues and skin pigmentation.30 Thus, new analogs with a shorter half-life, without toxicities and lesser retention properties, are required to entirely exploit the potential of this drug.
Figure 3.
Chemical structures of five WHO approved repurposed drugs for TB treatment.
Various antibiotics belonging to the class of β-lactams were initially not the preferred choice to test against TB due to their low efficacy. However, to get over the resistance to β-lactam, Clavulanic acid, a potent inhibitor of β-lactamase enzyme, was developed that re-establishes the β-lactam activity.32 β-Lactam antibiotics of the Cephalosporin family like Ceftriaxone, Ceftazidime, and Cefdinir and the Carbapenem family, especially Meropenem, Feropenem, Ertapenem, and Imipenem, are more effective against M.tb than Penicillin derivatives.33,34 These Carbapenems have been classified as group C (noncore drugs) by WHO. Combined administration of Carbapenem–Cilastatin has been found effective against MDR/XDR-TB with excellent patient compliance.35,36 However, further research is needed to identify the possible role and cost-effectiveness of Ertapenem (which can be given intramuscularly) as an alternative for Meropenem and Imipenem–Cilastatin. Increasing resistance among commensal bacteria, the need for intravenous infusion, multiple daily doses, and their high cost are few drawbacks associated with the use of Carbapenems. A comprehensive study by García et al. demonstrated the importance of Cephalosporins for TB therapy wherein it has been used in combination with Rifampicin and Ethambutol and found 4- to 64-times more active against M.tb and exhibiting a synergistic effect with new anti-TB drugs viz. Pretomanid, Bedaquiline, Delamanid, and SQ109.37 Another antibiotic, Cefdinir (semisynthetic Cephalosporin), demonstrated bacterial killing and sterilizing activity against both drug sensitive and resistant M.tb isolates even in the absence of a β-lactamase inhibitor. Due to its oral formulation, ability to penetrate inside clinically pertinent anatomical sites, and good efficacy against MDR-TB cases, Cefdinir is a probable candidate for further exploration as a TB-therapeutics.38 A research group has demonstrated the efficacy of Cefazolin, a member of the Cephalosporin family, in combination of Avibactam against both drug sensitive and MDR-TB clinical strains by using an intracellular hollow fiber model of TB (HFS-TB) and suggested the use of this drug combination as an alternative to treat TB cases in children.39 MIC against the M.tb laboratory strain H37Ra was 64 mg/L for Cefazolin alone, that decreased to 2 mg/L in the presence of 15 mg/L Avibactam. The findings are still needed to validate by clinical trial studies.
The effect of Trimethoprim/Sulfamethoxazole (TMP/SMX) or Co-trimoxazole for TB was observed during their administration in HIV-TB coinfection cases.40 SMX and TMP both are FDA approved antibacterial drugs that act by inhibiting the folic acid biosynthesis pathway. The bactericidal effect of Co-trimoxazole in combination with a first-line anti-TB drug (either Isoniazid or Rifampicin) along with their potential to prevent emergence of drug resistance has been observed under in vitro studies.41 In a trial of an HIV-MDR-TB coinfection case, efficiency of the MDR-TB treatment by TMP/SMX brought in a reduction of time to sputum conversion. A clinical trial to study the effect of Isoniazid and Co-trimoxazole as strategies in reducing infection in HIV-infected children and reducing mortality rate in phase 3 has been completed, but results are still awaited (NCT00330304).
Various antibiotics of the Tetracycline family, viz., Minocycline, Doxycycline, and Lymecycline, are being investigated as potential anti-TB agents. Deshpande et al. demonstrated the bactericidal effect of Minocycline directly on extracellular bacilli and indirectly on intracellular bacilli, via concentration dependent granzyme A-mediated apoptosis. The involvement of Minocycline in inhibiting sonic hedgehog–patched–gli signaling was also reported in the same study.42 In another study Lymecycline (10 μg/mL) alone and in combination of Isoniazid (0.2 μg/mL) and Rifampin (2 μg/mL) substantially inhibited M.tb growth by 93.47%, 99.25%, and 98.35%, respectively, with trpD as a potential drug target.43 TrpD encodes an enzyme probable anthranilate phosphoribosyl transferase, required for the tryptophan amino acid biosynthesis pathway.44 Doxycycline was recently identified as a successful adjunctive host directed therapy (HDT) in controlling TB-associated tissue degradation caused by matrix metalloproteinases (MMPs) in Phase II trial research, and the drug is also advised for further larger investigations.45
Vancomycin which belongs to the class of glycopeptides was initially considered to be ineffective against M.tb.46 However, a recent study has suggested that Vancomycin mediated M.tb killing is better achieved by the inhalational drNg delivery route. The same study has also reported the synergistic effect of d-Cycloserine in reducing Vancomycin concentration that is required for M.tb growth inhibition.47
4.1.2. Antifungal Agents
Econazole, Clotrimazole (Azole-based antifungal drugs), and Artemisinin are few of the antifungal drugs analyzed for their repurposing potential against TB. The existence of a homologous gene of a fungal enzyme (lanosterol 14 α-demethylase) in M.tb, is the basis behind repurposing Econazole and Clotrimazole against M.tb. The azole drugs cause inhibition of this fungal enzyme and have anti-TB effects both in vitro and in vivo, including drug-resistant clinical isolates.17 However, due to their poor oral bioavailability, the use of Azoles as oral drugs has been limited. The challenge is solved by using and evaluating nanoparticles encapsulated with an Econazole-containing regimen that has reached the point of animal testing.50 Recently in vitro activity of Clotrimazole, Econazole, and Nitroimidazole (Metronidazole (MZ) and Ipronidazole (IPZ)) has been analyzed for both latent and active forms of MDR and XDR-TB treatments.51
Artemisinin (a peroxy containing sesquiterpenoid) and Artesunate (derivative of Artemisinin) are a group of drugs extracted from the Chinese medicinal herb plant Artemisia annua, exhibiting antimalarial and antifungal properties.52 The antitubercular property of Artemisinin and its derivatives was first reported in 2016 using different in vitro as well as in vivo tests.53 A study using a DosRST-dependent fluorescent reporter strain of M.tb and screening a 540,000-compound small-molecule library described Artemisinin as a novel inhibitor of the DosRST signaling pathway, directly targeting heme-based DosS and DosT sensor kinases and persistence-linked physiological processes such as triacylglycerol synthesis and antibiotic tolerance of M.tb.16 Further findings suggests that the mechanism behind Artemisinin’s inhibition of DosRST signaling is due to Artemisinin’s oxidation and alkylation of the DosS and DosT heme.54
4.1.3. Anti-Protozoal Agents
Metronidazole, Nitazoxanide, Pyronaridine, Chloroquine, and Tafenoquine are the antiprotozoal agents with potential anti-TB properties as reported by multiple studies. Metronidazole is a Nitroimidazole derivative and a part of the Imidazole family used for treating protozoal and bacterial infections. The rationale behind the use of Nitroimidazole derivatives against TB was provided by the findings of Wayne and Sramek, who tested Metronidazole against TB,55 and found its bactericidal effect against the dormant form of M.tb. However, in a phase-II clinical trial study of MDR-TB patients receiving 500 mg Metronidazole thrice daily were recorded to develop peripheral neuropathies, and this drug was reported to be too toxic for long-term usage.56 Two other de novo drugs of Nitroimidazole class OPC-67683 (Delamanid) and PA-824 (Pretomanid) have been developed by the TB Alliance and approved for TB treatment. These compounds are prodrugs that become active through nitro-reduction by Mycobacteria and release ROS responsible for inhibition of protein and lipid (affecting cell wall) biosynthesis.57
Pyronaridine is a highly efficient antimalarial drug against Plasmodium falciparum and Plasmodium vivax. It has been found to have antitubercular action in vitro, with MIC of 5 μg/mL. Pyronaridine was identified to enhance M.tb susceptibility to Rifampicin in a THP-1 macrophage infection model leading to 16-fold reduction in the MIC. In vitro results revealed that Pyronaridine inhibits M.tb RNA polymerase activity.58
Another antiprotozoal drug, Chloroquine, is an aminoquinoline derivative that has been widely used as an antimalarial drug since 1940.58 Its role in TB as a host cell efflux pump inhibitor59 and phagosome acidification inhibitor has been observed.60 Clearance of anti-TB drugs Isoniazid and Pyrazinamide takes place by efflux pump, breast cancer resistance protein-1 (BCRP-1) present on macrophages.59 Chloroquine acts on BCRP-1, which is overexpressed in M.tb infected macrophages and promotes the M.tb survival by extruding anti-TB drugs into the Extracellular fluid (ECF).59 Recently a study has shown the inhibitory effect of Chloroquine on phagosome acidification, a prime phenomenon in redox physiology of M.tb and generation of heterogeneous population of drug-tolerant macrophages during infection, that resulted in increased sensitivity of drug-tolerant M.tb to treatment with improving lung pathology and reduced relapse time after chemotherapeutic treatment in a BALB/c mouse of TB infection. These results indicated the potential of Chloroquine to enhance the efficacy and reduction in time required for TB treatment.60 Recent research demonstrated for the first time that Tafenoquine is effective against M.tb with lower MICs values compared to other antimalarial medications such as Chloroquine, Mefloquine, and Primaquine. Furthermore, Tafenoquine concentrations ranging from 1.25 to 80 μM had varying effects against sensitive and MDR strains of M.tb, ranging from moderate (reduction of 1.8 log CFU/mL) to strong bactericidal activity (reduction of 4.2 log CFU/ml).61
4.1.4. Antiviral Agents
Isoprinosine and Saquinavir are two antiviral repurposed candidates for TB. Isoprinosine (Inos) or Immunovir is a synthetic purine derivative with immune-modulatory effects that is used in combination with interferon (IFN) therapy in the trachea to treat subacute sclerosing panencephalitis.62 Isoprinosine has been shown to improve host immune responses in vivo by producing pro-inflammatory cytokines and increasing T-cell subset proliferation required for the immune resistance against M.tb.62 However, further studies are required to evaluate its antimycobacterial effect either as direct target or as HDT. Saquinavir, an HIV protease inhibitor, is being investigated as a possible host-directed treatment for M.tb infection, particularly in the context of HIV-M.tb coinfection.63 Recently the repurposing of Saquinavir against M.tb has not only shown its significance in intracellular killing of M.tb in infected macrophages but also enhanced the expression of the antigen presentation machinery of HLA class II type at the cell surface. Additionally, the increased effects in T-lymphocyte priming, proliferation, and IFN-γ secretion have also been reported that suggest the imperative character of Saquinavir as an anti-TB medication.
4.1.5. Anti-Helminthic Agents
Avermectin, Pyrvinium pamoate, and Nitazoxanide are a few of the antihelminthic compounds that have been repurposed for TB. Avermectins, a broad-spectrum class of antihelminthic drugs that includes Ivermectin, Selamectin, and Moxidectin, exhibit potential activity in vitro against M.tb and M. ulcerans with MIC values ranging from 1 to 8 mg/L and 4 to 8 mg/L, respectively.64,65 They were also found effective against MDR and XDR-M.tb clinical isolates as well.65 However, their mode of action in M.tb is still unknown except for Selamectin which in a recent study was shown to interact with M.tb flavo-enzyme DprE1.66 Further studies are required to examine the mode of action of the remaining drugs as well, to completely understand their anti-TB activity. Pyrvinium pamoate has been shown to alter glucose and glycogen utilization pathways of M.tb(67) thus acting as a strong inhibitor of M.tb at a concentration of 1.5 to 4.8 μg/mL against H37Rv and MDR clinical isolate.
Nitazoxanide, initially used as an antidiarrhea drug, has been reported to impede both replicating and nonreplicating forms of M.tb with MIC of 16 μg/mL against H37Rv as well as drug sensitive and resistant clinical isolates.78,79 In 1982, Niclosamide was approved for use in humans to treat tapeworm infection, and recent research has reported its repurposed effect to inhibit the growth of the attenuated M.tb strain (H37Ra) with a MIC of 0.5–1 μM.80 However, possible toxicity to mammalian cells was observed at these dosages, limiting its usefulness as an anti-TB medication.81 In another study using a model of M.tb and HIV coinfection involving human macrophages, Niclosamide (1.25 μM) inhibited the replication of both the pathogens more than half without causing considerable host cell death.76 These contradictory findings require more thorough investigations to reconfirm the debatable nature of Niclosamide as a potential anti-TB agent.
4.2. Repurposed Non-Anti-Infective Agents against TB
Drugs from various pharmacological classes, such as anti-inflammatories, anti-diabetics, anticancer, and so on, that are used to treat various deficiencies and disorders, can also be used to treat pathogen-causing diseases due to their secondary biological effects or may also be used as adjunctive therapies. The medications from each of these pharmacological groups that are being reported to have anti-TB properties are discussed in the following sections and detailed in Table 3.
Table 3. Repurposed Non-Anti-Infective Drugs against Tuberculosis, Their Mechanism of Action, MICs, and Ongoing Investigationsa.
| S. no. | Drug name (Drug class) | Mechanism of action/Drug target in M.tb | Minimum inhibitory concentration (MIC) against M.tb | Current status/ongoing studies | Refs |
|---|---|---|---|---|---|
| NSAIDS (Anti-inflammatory Drugs) | |||||
| 1 | Meloxicam (Benzothiazine) | Cyclooxygenase-2 (COX-2) inhibitor | 7.5 mg for 8 weeksb | Phase III trial, results awaited | NCT02060006 |
| 2 | Aspirin (Acetylsalicylic acid) | Down-regulate the transcription and translation machinery | 3–20 mg/kg (murine model) | Phase II trial, results awaited | NCT0223736585,87 |
| 3 | Etoricoxib (Bipyridines) | Cyclooxygenase-2 (COX-2) inhibitor, used as HDT | NA | Phase I/II trial underway | NCT02503839128 |
| 4 | Ibuprofen (Propionic acid derivative) | InfB (Rv2839c) | 75 mg/L (H37Rv) | Phase II trials underway | NCT0278190984 |
| 5 | Celecoxib (Pyrazoles) | Efflux pump inhibitor | 16 μg/mL (M. smegmatis) ATCC 14468 | Phase I, trial completed | NCT02602509129 |
| 6 | Carprofen (2-Arylpropanoid acid) | Target the respiration process by disrupting membrane potential, inhibit drug efflux mechanism | 40 μg/mL or 146 μM (H37Rv) | in vitro | (90) |
| 7 | Diminazene (Phenylhydrazines) | MycP1 | 150 μM (58% inhibition) | Machine learning, in vitro | (91) |
| 8 | Ebselen (Benzoselenazole) | LdtMt2, cysA2, frdC, and glpD2 | 2.5 μg/mL | in vitro | (93, 94, 130) |
| 9 | Bromfenac (Benzophenone derivative) | Zmp1 and PDF | 100 μM | in vitro | (131) |
| 10 | Diflunisal (Salicylic acid derivative) | Zmp1 and PDF | 50 μM | in vitro | (131) |
| 11 | Oxyphenbutazone (Pyrazolidines) | Oxyphenbutazone converts into reactive species and deplete thiols and flavin nucleotides | 200 μM (H37Rv) | in vitro | (89) |
| Anticancer Drugs | |||||
| 12 | Fostamatinib (Kinase inhibitor) | Transmembrane Serine/Threonine-Protein Kinase B (Rv0014c, Rv0015c) | MIC50–7.98 μM | in silico, in vitro, in vivo | (132,133) |
| 13 | Elesclomol (Bis-thio-hydrazide amide) | Induce oxidative stress and generate ROS | 4 μg/mL | Knowledge-based and in vivo | (99) |
| 14 | Bis-biguanide dihydrochloride | NA | 0.05 μg/mL (clinical isolates) | in vitro, in vivo | (100) |
| 15 | Imatinib (Kinase inhibitor) | Increase myelopoiesis, phagosome maturation and acidification, autophagy in M.tb-infected mice and macrophages | 10 μM (H37Rv) | in vitro and in vivo | (96) |
| 16 | Gefitnib (Kinase inhibitor) | Increase lysosomal biogenesis and modulate cytokine signaling | 10 μM | in vitro and in vivo | (134) |
| 17 | Nilotinib (Kinase inhibitor) | Modulate autophagy in M. bovis infected macrophages, activation of PI3k/Akt/mTOR | 10 μM, 20 μM in M.bovis infected BMDM, RAW264.7, THP-1 cells | in vitro and in vivo | (135) |
| 18 | Bortezomib (Boronic acid derivative) | Caseinolytic proteases (ClpP1P2) | 4.3 μM | in vitro | (98) |
| 19 | Doxorubicin (Anthracycline) | MtbGyrB47 | IC50 range 2.1–4.7 μM | in silico and in vitro | (109,132) |
| 20 | Epirubicin (Anthracycline) | MtbGyrB47 | MIC90 –6.3 μM | in silico, in vitro | (109) |
| 21 | Idarubicin (Anthracycline) | MtbGyrB47 | MIC50 –4.7 μM | in silico, in vitro | (109) |
| 22 | Sorafenib | MtArgJ, FadD32 | MIC90 10 μg/mL | in silico, in vitro | (103,104) |
| Cardiovascular Drugs | |||||
| 23 | Pravastatin (Statins) | Stimulate host immune response. | 40 mg and Rifafour daily for 14 daysb EC50- 7.8 μM (M.tb infected macrophages) | Phase II trial underway | NCT03882177115 |
| 24 | Rosuvastatin (Statins) | Inhibit cellular cholesterol biosynthesis and induces autophagy | NA | Phase II trial in planning | NCT04504851115 |
| 25 | Verapamil (Phenylalkylamines) | Efflux pump, Ca2+ channel inhibitor, interferes with membrane energetics | 512 μM | Knowledge-based and in vivo | (136,137) |
| 26 | Simvastatin (Statins) | Inhibit de novo synthesis of cholesterol, induce autophagy in M.tb infected PBMC’s | 1, 20 μM (M.tb infected PBMC’s) | Knowledge-based and in vivo | (117) |
| 27 | Atorvastatin (Statins) | Inhibit cellular cholesterol biosynthesis, stimulates host immune response | EC75 - 2 μM (BCG infected THP cells) | in vivo studies | (138) |
| 28 | Fluvastatin (Statins) | Inhibit cellular cholesterol biosynthesis, stimulates host immune response | EC50 - 0.032 μM (M.tb infected macrophages) | in vivo | (115) |
| 29 | Cilostazol (Quinolinone derivative) | PDEi | 30 mg/kg dosage administered to mice models | in vivo studies | (139,140) |
| Anti-Psychotic Drugs | |||||
| 30 | Thioridazine (Phenothiazine derivative) | NDH-2, efflux pump inhibitor | 32 and 70 mg/kg (Balb/c Mouse model) | in vivo | (141) |
| 31 | Fluspirilene (Diphenylbutylpiperidine) | modulating the autophagic/lysosomal response | NA | in vitro and in vivo studies | (120) |
| 32 | Pimozide, (Diphenylbutylpiperidine) | modulating the autophagic/lysosomal response | NA | in vitro and in vivo studies | (120) |
| 33 | Chlorpromazine (Phenothiazine) | Inhibitor of efflux pump, K+ transport and | 47 μM (H37Rv) | in vitro studies | (136,142) |
| Anti-Parkinson Disease Drugs | |||||
| 34 | Entacopone (Nitrocatechol) | InhA | 205 μM (H37Rv) | in vitro | (123) |
| 35 | Tolcapone (Nitrocatechol) | InhA | 457 μM (H37Rv) | in vitro | (123) |
| Anti-Diabetic Drugs | |||||
| 36 | Metformin (Biguanide drug) | AMPK modulator, activate autophagy, target Rv0235c | 500–1000 mg orallyb | Phase I trials, in vivo | (124,125) NCT04930744 |
| 37 | Acarbose | murE, ppiB | 0.05–0.1 μg/mL | in vivo studies | (127,143) |
NA – Not available; CT – clinical trials.
Patient daily dosage.
4.2.1. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs are a huge class of drugs with diversity that act by inhibiting cyclooxygenase (COX) to diminish prostaglandin production, thus lessening inflammation, fever, and pain.82 Several studies have supported the use of NSAIDs in adjunctive therapy, making the members of this class easy to repurpose. Ibuprofen, Celecoxib, Aspirin, Etoricoxib, Meloxicam, Oxyphenbutazone, Diflunisal, Bromfenac, Diclofenac, Diminazene, and Ebselen are a few examples of this category. Drugs from this class help in healing the tissues damaged due to extensive drug treatment and host–pathogen interaction.83 Ibuprofen84 and Aspirin85 are the common and most studied NSAID drugs found in most households. Ibuprofen establishes a direct inhibitory impact in whole-cell screening assays84 and protects mice models from TB progression.86 A phase II clinical trial study evaluating the use of Ibuprofen as an adjunctive therapy in XDR-TB patients is already underway (NCT02781909). The synergistic effect of Aspirin with Pyrazinamide has been demonstrated in a murine model,87 but its unpleasant interaction with Isoniazid88 and lack of significant effect in a TB rabbit model make it less promising as an immunomodulator adjunct therapy. However, it is more beneficial in Meningitis-TB, which is characterized by severe inflammation, than in pulmonary TB, due to its anti-inflammatory properties (NCT02237365). Other NSAIDs that have been investigated in clinical studies include Etoricoxib, Meloxicam, and Celecoxib (NCT02503839, NCT02060006) (NCT02602509). On the other hand, Oxyphenbutazone, Diflunisal, Bromofenac, and Diclofenac have been discontinued due to their high toxicity.89 Carprofen has been discovered to have a direct antitubercular specific mode of action, with a MIC of 40 μg/mL. The reported antitubercular effects such as disrupting efflux pumps, biofilms, and membrane potentials of Carprofen are due to its pleiotropic mechanism of action that minimizes the risk and effect of resistance mutations.90 A study described Diminazene as the potential inhibitor of MycP1 (a protease) of M.tb, with a 58.0% inhibition rate at 150 mM concentration.91 In later studies, the anti-TB activities of Diminazene derivatives, synthesized by substituting different electron withdrawing group at benzene ring, were found superior over Diminazene due to the position and nature of electron-withdrawing groups.92 The effect of Ebselen as an anti-TB agent and its synergistic effect with Isoniazid have been found effective against H37Rv and three clinically used drug-resistant strains (JAL2287, BND320, and MYC431).93 In addition, a recent study showed that Ebselen imparts low cytotoxicity against macrophages, indicating that it might be used as an anti-TB.94
4.2.2. Anticancer Drugs
Various anticancer medications due to certain similarities between bacterial pathogens and cancer cells95 are now being investigated as anti-TB agents such as Imatinib,96 Sorafenib (SRB),97 Bortezomib,98 Elesclomol,99 and bis-biguanide dihydrochloride.100 Imatinib, also known as Gleevec, is currently used to treat chronic myelogenous leukemia and has been found to disrupt the M.tb pathogenesis machinery as well. It has been demonstrated to elicit an antipathogen host immune response to eliminate the mycobacterial infection. Imatinib has been proposed as HDT against TB and thus suggested to be beneficial in the case of drug-resistant strains as well.96 Imatinib, upon administration, decreases the bacterial load and number of lesions in infected mice. It acted synergistically when given in combination with other standard anti-TB drugs.101,102 A phase II B clinical trial (NCT03891901) is currently underway to determine the most effective dose of Imatinib that can be used as adjunctive therapy with TB treatment regimens (Rifabutin, Pyrazinamide, Isoniazid, and Ethambutol).
SRB has been studied as a metabolic inhibitor of the arginine biosynthesis pathway in M.tb by targeting its unique arginine biosynthesis enzyme MtArgJ, exclusively present in M.tb, thereby blocking arginine production and impeding M.tb survival.103 SRB in combination of Rifampicin and Isoniazid resulted in increased killing of the M.tb by inhibiting ABCG2 efflux pumps of the bone-marrow mesenchymal stem cells (BM-MSCs).97 A recent study reported fatty acid degradation protein D32 (FadD32) as another target of SRB.104
Another anticancer drug Bortezomib is a proteasome inhibitor that is used for treating multiple myeloma.105 This drug inhibits the growth of tumor cell by affecting (NFkB) signaling pathway of activated B cells and by inhibiting interleukin (IL)-6 signaling.106 Antimycobacterial properties of this drug were seen in target mechanism-based whole cell screen against M. smegmatis and M.tb due to inhibition of caseinolytic protease (ClpP1P2).98 Short half-life following intravenous administration, poor pharmacokinetics, high cost, and significant side effects such as peripheral neuropathy, neutropenia, and cytopenia restrict the use of Bortezomib for TB treatment.107 Later on, many dipeptidyl boronate derivatives of the drug have been synthesized with a specific aim of enhancing inhibition of M.tb protease particularly with low toxicity.107
Elesclomol is a biohydrazide, known for its antiproliferative activity that causes apoptotic cell death by generating oxidative stress.108 In a study testing its in vitro anti-TB activity, MIC99 was found to be lower for clinically isolated MDR or XDR strains as compared to M.tb H37Rv (4 μg/mL). The drug was detected to be effective against M.tb infected bone-marrow-derived macrophages, and the synergistic effect of this drug was observed when used in combination with Rifampicin.99 Moreover, the sensitivity of M.tb to Elesclomol was increased by >65-fold when supplemented with copper, suggesting its potential candidature as a repurposed agent for anti-TB therapy.99
Bis-biguanide dihydrochloride (BBD) was identified as an anti-TB agent in a cell-based high-throughput screening study.100 The antimycobacterial effects of BBD were seen by studying its role in inhibiting both intracellular and extracellular growth of M. smegmatis, slow-growing M. bovis BCG, as well as MDR clinical isolates. The reduction in CFU counts in lung and spleen samples of M.tb infected mice was observed more in BBD treated mice compared to Rifampicin, with no possible side effects, suggesting the advancement of BBD as an alternative new drug for TB treatment.100
In a computational study Epirubicin and Doxorubicin, which are anthracycline antibiotics used as anticancer drugs, were found to be promising inhibitors of M.tb DNA gyrase. These drugs noted to inhibit the gyrase catalytic cycle by binding to ATPase binding pocket located at the N-terminal domain of gyrase B (Mtb GyrB47), thereby affecting the growth of M.tb.109
4.2.3. Drugs against Cardiovascular Diseases
Verapamil, a synthetic derivative of papaverine, is well-known as a calcium channel blocker, and is being utilized to treat heart disorders, hypertension, migraine arrhythmia, and angina.110 To identify target gene mutations connected to drug resistance and to analyze the involvement of efflux pumps in drug resistance levels, researchers used whole-genome sequencing and characterization in the presence and absence of the efflux pump inhibitor Verapamil. Six MDR/XDR and three mono-drug-resistant clinical isolates of M.tb showed a substantial decrease in Rifampicin and Isoniazid MICs after Verapamil administration. The same study also reported the overexpression of various efflux pump genes, viz., Rv876, Rv1145, Rv2936, Rv1146 Rv2333, Rv2459, Rv849, Rv2938, Rv933, Rv1250, and Rv1819 after anti-TB drugs treatment and 4-fold downregulation of at least one gene after Verapamil exposure that highlights the potential role of Verapamil in lowering the expression of efflux pumps in M.tb strains.111
The Statin family of drugs, mainly prescribed to hyperlipidaemic patients for lowering the risk of stroke and cardiovascular disorders, has also been repurposed for TB.112 They act by blocking hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase, a crucial and rate-limiting enzyme in the cholesterol synthesis.113 Higher cholesterol is considered as a predisposing factor for TB as cholesterol acts as a binding molecule for the bacteria to enter the macrophage.114 So, reduction in membrane cholesterol also restricts the entry of bacterium inside macrophages.114 Fluvastatin, Atorvastatin, Lovastatin, Pitavastatin, Simvastatin, Rosuvastatin, and Pravastatin are a few examples of this class.113 Several animal studies have been conducted to investigate the positive impact of statins in the treatment and prevention of TB.115 Among these, Atorvastatin was discovered to be a well-tolerated and safe drug which has entered in clinical studies as a repurposed medication for TB treatment. Two clinical studies on Atorvastatin, the first to depict the effect of Atorvastatin with standard anti-TB drugs in Nigeria (NCT04721795) and the second to study the effect of Atorvastatin in reducing lung inflammation after completion of TB treatment in HIV and Non-HIV TB patients (NCT04147286), has entered in Phase II. The role of Simvastatin, another member of the statin family, in enhancing immune responses and activity of standard anti-TB drugs in macrophages, has been studied. The synergistic effect of the drug Simvastatin with Rifampicin, Isoniazid, or Pyrazinamide because of increased bacillary killing, decreased colony-forming units in lungs of chronically infected BALB/c mice, and reduction in time needed to attain culture-negative lungs has been demonstrated.116 Increased secretion of IL-12 and IL-1b cytokines in untreated peripheral blood mononuclear cells and secretion of IL-10 cytokines in infected peripheral blood mononuclear cells support the role of Simvastatin in enhancing host immune response.117 Statin Adjunctive Therapy for TB (StAT- TB), a phase-II clinical trial study, is currently in the recruitment phase to study the safety, mechanism of action, and dosage of Pravastatin in treatment of TB (NCT03456102). Various other cardiovascular drugs which are found by in silico analysis to inhibit imperative drug targets of M.tb are described in Supplementary Table S1.
4.2.4. Anti-Psychotic Drugs
Among the numerous anti-psychotic medications, those observed as anti-TB repurposing agents include Chlorpromazine, Thioridazine, Nemonapride, Fluspirilene, and Pimozide.118−120 Chlorpromazine and Thioridazine are neuroleptic drugs from the phenothiazine class and have antihistaminic or anti-psychotic properties. Phenothiazines primarily function by inhibiting the activity of an essential enzyme, NADH: menaquinone oxidoreductase, in the energy metabolism pathway.121 Chlorpromazine is the first commercially available phenothiazine having bacterial growth suppression properties122 along with severe side effects for psychoses that resulted in less demand of this drug as an antimicrobial agent. Later, Thioridazine, with less toxicity and more efficacy against drug resistant TB in vitro and in the mouse model, was identified.118 However, the efficacy and safety of Thioridazine against TB is yet to be explored in clinical trials.118
Nemonapride was identified as a likely contender for drug repurposing with the molecular target of Rv3247c in M.tb in a study based on in silico analysis.119 The role of two other anti-psychotic drugs, Fluspirilene and Pimozide of class diphenylbutylpiperidine, in inhibiting intracellular growth of M.tb in both pro- and anti-inflammatory primary human macrophages has been observed.120 These drugs were found effective against MDR M.tb strains and another intracellular bacterium, Salmonella enterica serovar Typhimurium (Stm). Both of these drugs act by regulating autophagy, thus altering the lysosomal response.120
4.2.5. Anti-Parkinson Drugs
Entacapone and Tolcapone drugs have been widely used for treatment of Parkinson’s disease.
These drugs mainly target catechol-O-methyltransferase (COMT), an important human enzyme that is primarily involved in degradation of neurotransmitters.123 Both drugs have been found effective against M.tb at a concentration lower than the toxic concentration to eukaryotic cells.123 It has been predicted that both drugs inhibit M.tb enoyl–acyl carrier protein reductase (InhA)123 that is crucial for mycolic acid biosynthesis. However, further research is still required to completely understand their potential role against M.tb.
By use of an integrated approach based on systems-level information for ligand selection, and genome-wide investigations, another anti-Parkinson drug Droxidopa was identified as a possible candidate for drug repurposing in a study, wherein Rv0098, Rv0390, Rv3588c, Rv2244, Rv2465c, Rv2763c, Rv3247c, Rv1094, Rv3607c, Rv3048c, Rv0321, and Rv2607 were identified as metabolic drug targets.119
4.2.6. Anti-Diabetic Drugs
Metformin, an anti-diabetic drug, is a disruptor of mitochondrial complex-I of the respiratory metabolic pathway and has been considered as a potential combinatorial therapeutic option against TB.124,125 In recent studies, Metformin treatment inhibited M.tb growth and reduced disease pathogenesis by initiating the host-mediated responses via activation of adenosine monophosphate-activated protein kinase (AMPK).124,125 In another study, Metformin exposure to macrophages has been observed causing increased production of antibacterial ROS necessary for reducing bacterial load during infection.125,126 A study titled “Safety and Tolerability of Metformin in People with TB and Human Immunodeficiency Virus (HIV)” is currently in phase II trials (NCT04930744). Another anti-diabetic drug, Acarbose, has recently been proposed as an anti-TB agent, with bacteriostatic action on M. smegmatis growth in vitro. Acarbose also inhibits M.tb biofilm development and substantially decreases (2–4 fold) the dose of Isoniazid and Ethambutol.127
4.3. Miscellaneous Anti-TB Repurposed Agents
The comprehensive details on almost all the miscellaneous repurposed drugs under in vitro, in vivo, or clinical trial investigations are detailed in Table-4, and the most important among them are discussed below. The FDA approved drugs which are being analyzed by in silico approaches to inhibit M.tb protein targets are also mentioned in Supplementary Table S1.
Table 4. Miscellaneous Repurposed Drugs against Tuberculosis, Their Mechanism of Action, MICs, and Ongoing Investigationsa.
| S. no. | Drug name (Class of the drug) | Mechanism of action/Drug target in M.tb | Current use in therapeutics | Minimum inhibitory concentration (MIC) against M.tb | Current status/Ongoing studies | Refs |
|---|---|---|---|---|---|---|
| 1 | Vitamin D (Vitamins) | promotes autophagy in M.tb-infected monocytes | Supplements for calcium and phosphorus | (5000 IU of vitD3 for 2 months)b with standard ATT. | Several CT completed | NCT01580007 |
| NCT02237365146,159 | ||||||
| 2 | Auranofin (Gold salt) | Target TrxB2, and disrupt the redox balance | Rheumatoid arthritis | 3–6 mg/dayb 0.5 μg/mL | Phase II trial completed | NCT02968927149 |
| 3 | Thalidomide | Inhibit TNF-α, used as HDT | Immunomodulatory drug | 3–5 mg/kg/dayb | Various CT completed | (160) |
| 4 | Disulfiram (Thiocarbamate) | Inhibitor of MetA, alter methionine pool and redox status | Alcohol withdrawal drug | 5.3 μM | In vitro and in vivo | (161,162) |
| 5 | Rimonabant (Carbohydrazide) | Target MmpL3 protein, and inhibit cell wall synthesis | Antiobesity drug | 25 μg/mL (H37Rv) | In vitro, In vivo | (151,152) |
| 6 | Pranlukast | Arginine biosynthetic enzyme inhibitor (MtArgJ) | Antiasthma drug | MIC90 = 5 μg/mL (H37Rv) | In vitro, In vivo | (103) |
| 7 | Linolenic acid (Polyunsaturated fatty acid) | NA | Nutritional supplementation | 200 μg/mL (H37Rv) | In vitro | (163) |
| 8 | Diosmin (Phlebotonics) | l,d-transpeptidase enzymes | Hemorrhoids | NA | In vitro and In vivo | (157) |
| 9 | Cyclosporine-A (Immunosuppressants) | Disturb biofilm formation by inhibiting PpiB protein. | Organ transplant | NA | In silico and in vitro | (154,164) |
| 10 | Eltrombopag | Zmp1 and PDF | Thrombocytopenia or aplastic anemia | 6.25 μM (H37Rv), 12.5 μM (Beijing isolates) | in vitro | (131) |
| 11 | Arotinolol | Zmp1 and PDF | Antihypertensive therapy | 3.125 μM (H37Rv), 25 μM (Beijing isolates) | in vitro | (131) |
| 12 | Echinacoside (Phenylethanoid glycoside) | GyrB47 | Parkinson’s, Alzheimer’s, osteoporosis, and hepatitis | MIC90 –12 μM | in silico and in vitro | (109) |
NA: Not available, CT: clinical trials.
Patient daily dosage.
4.3.1. 1α,25-Dihydroxy-Vitamin D
Toll like receptors (TLR) of human macrophages trigger the Vitamin D mediated antimicrobial response in humans. Activation of TLR on human macrophages results in overexpression of both Vitamin D receptor and hydroxylase genes. It is then followed by the activation of beta-defensin 2 and human cathelicidin LL-37, two antimicrobial peptides (AMP) generated by lung epithelial cells, monocytes/macrophages, and neutrophils that can reduce M.tb growth and modulate antimicrobial responses, respectively.144,145 In M.tb infected macrophages/monocytes, active Vitamin D, 1,25(OH)2D3, causes autophagy, which can suppress the infection via an LL-37-dependent mechanism. As a consequence, multiple studies have linked Vitamin D in sufficiency to an increased risk of developing active tuberculosis. In a clinical trial investigation, patients receiving 5000 IU of Vitamin D3 daily along with regular Anti-TB treatment (ATT) had faster rate of sputum culture negatives than those who received placebo. This study showed that Vitamin D3 has favorable benefits as an adjuvant therapy and has the potential to be used as an HDT in the treatment of TB.146 However, some clinical trials have shown it to have no effect on TB, and tuberculous spondylitis was reported in a patient receiving Vitamin-D for 7 weeks (NCT02968927).147
4.3.2. Auranofin
Auranofin is a gold complex originally developed to use as an anti-rheumatic agent.148 The role of Auranofin as thioredoxin reductase (TrxR) inhibitor of M.tb has been reported that results in thiol-redox depletion and blemished defense against oxidative stress.149 It was potentially being investigated as adjunctive HDT in phase II clinical trial (NCT02968927) with patient daily dosage of 3–6 mg/day, and findings reported thrombocytopenia, acute gastroenteritis, disseminated intravascular coagulation, and hypoxaemia as suspected unexpected serious adverse reaction with treatment failure in recipients.147
4.3.3. Rimonabant
Rimonabant is an endocannabinoid receptor antagonist originally used to treat obesity.150 Antibacterial activity of this drug against M.tb H37Rv has been observed.151 Further studies have focused on the effects of Rimonabant derivatives against M.tb H37Rv that have proved as highly potent anti-TB agent with lesser MIC of 0.39 μg/mL, representing a good candidate for further in vivo experimental validation. Mycolate flippase, MmpL3, of M.tb has been found as a direct target of Rimonabant.152
4.3.4. Pranlukast (PRK)
PRK is a FDA permitted molecule and inhibitor of human cysteinyl leukotrienereceptor-1 (hCysLTR1). It has been used in treating chronic bronchial asthma.153 Like SRB, PRK has been found to be a metabolic inhibitor of the arginine biosynthesis pathway in M.tb by targeting arginine biosynthesis enzyme MtArgJ exclusively present in M.tb, thereby blocking arginine production.103 The blocking of this pathway results in reduction of M.tb survival. Studies reflect more potency of PRK than SRB against bacterial survival at both in vitro and in vivo levels. PRK also inhibits the 5-lipoxygenase (5-LO) signaling in M.tb infected macrophages, a pathway that facilitates bacteria to survive inside the host and thus improves the effectiveness of the PRK for the pathogen. The same study showed that PRK works efficiently in a combinatorial approach with the standard anti-TB drugs. In M.tb mice models, the effect of PRK alone and in combination with Rifampicin was seen in terms of reduction in tubercular granulomas and bacterial burden in lung without causing any harm to the host.
4.3.5. Cyclosporin A
Biofilm formation is one of the generic strategies used by various avirulent and virulent bacteria including mycobacteria, to overcome stress encountered during infections that acts as a barrier in drug tolerance and immune surveillance. The matrix of biofilm mainly comprises biopolymers and extracellular components mainly formed by proteins. Therefore, the proteins responsible for the biofilm formation can be used as a drug target, for instance, peptidyl-prolyl isomerase (PpiB) protein. Drugs that disrupt biofilm development and reduce the dose value of anti-TB drugs can be utilized as therapeutic treatments.154 Kumar et al. identified two FDA-approved drugs, Acarbose and Cyclosporine-A, as anti-TB agents in their study. Both of these drugs had a bacteriostatic action on M. smegmatis growth in vitro, whereas gallium nanoparticle (GaNP)g had a bactericidal effect on M. smegmatis growth. Both of these drugs also inhibit the biofilm formation by M.tb. The presence of these drugs during coculturing of M.tb also significantly reduced the (2–4 fold) dosage of Isoniazid and Ethambutol.154 Similarly, other proteins responsible for the biofilm formation can also be studied further to identify novel M.tb drug targets.
4.3.6. Diosmin (DIO)
Enzymes that helps in synthesis of the M.tb cell wall and are absent in eukaryotes can be a better choice to target for the drug development. Recently one study has targeted the l,d-transpeptidase enzyme that is crucial for cell wall synthesis in mycobacteria.155 This enzyme is required for introducing nonclassical type cross-linkage of peptidoglycan between the neighboring meso-DAP residues.156 The drug Diosmin was further recognized as a potential repurposed inhibitor of this enzyme via several in vitro and in vivo analyses. Its substantial synergistic effect with Amoxicillin-Clavulanic acid (AMC) in TB treatment was noted.157 The combination of the aforementioned drugs showed decisive action against M. marinum, although neither drug alone had any impact. After treatment with AMC and DIO, scanning electron microscopy of M. marinum revealed cellular leakage. When infected with M. marinum, the technique of combining AMC and DIO (or DMT) boosted the survival rate of Drosophila melanogaster fly models by up to 60%. The enhanced antimicrobial action of AMC-DIO was also confirmed against M.tb H37Ra and a MDR clinical isolate, but more thorough investigations are still undone.
4.3.7. Fusidic Acid
Researchers have found that the filamentous temperature sensitive mutant Z (FtsZ), a key cytoskeleton cell division protein of bacteria, might be used as a drug target for the development of antibacterial drugs.71 A study using gene ontology-based drug repurposing approach and considering citric acid (inhibitor of M.tb FtsZ) as a reference point has identified four predicted drugs, namely, Fusidic acid (FusA), l-tryptophan, carbamic acid, and 2-(3-guanidinophenyl)-3-mercaptopropanoic acid as potential inhibitors against M.tb FtsZ polymerization. By using different in silico methods such as DFT (Density Function Theory) calculations, molecular docking, and molecular dynamic (MD) simulations, the study revealed FusA as the most potent inhibitor among four even more potent and reactive than the citric acid. The finding that FusA is the best potential MTB-FtsZ polymerization inhibitor was supported by the other post MD analysis parameters including MM/PBSA (molecular mechanics Poisson–Boltzmann surface area) binding free energies, RMSD (root-mean-square deviation), RMSF (root-mean-square fluctuations), RoG (radius of gyration), and hydrogen bond analysis. However, further in-depth preclinical studies are still needed to confirm the anti-TB potential of FusA and other mentioned drugs.
5. Challenges and Limitations
Drug repurposing strategies offer exciting opportunities for developing new therapeutics against Tuberculosis; however, these benefits are often surrounded by huge challenges and limitations. One of these is the lack of public access to valuable data about drugs, e.g., clinical trials. Also, some types of computational data are difficult to handle and integrate. Analyzing such data is laborious and computationally demanding, and it increases the time of research to many folds.158 Even though the pharmacokinetics and pharmacodynamics data of many drugs are available, screening of a compound’s activity and toxicity through biological in vitro or preclinical research is still required for drug-sensitive and drug-resistant isolates.71 In addition, if the current clinical data is obsolete or does not meet the standards of the FDA or European Medicines Agency (EMA) regulatory authorities, new clinical studies may be required according to regulatory rules. The limited number of FDA-approved drugs and their low activity against M.tb has slowed the development of a drug-repurposing strategy for anti-TB drugs. Moreover, some of the repurposed drugs may have better activity in vitro but fail to be reliable under in vivo conditions because of high MICs, toxicities, or contraindications.71 The unique physiology of M.tb potentially imposes a major challenge for the repurposing because the resistance and failure to these drugs may arise quickly.7 Furthermore, the human microbiome could also be highly affected during the lengthy treatment regimen of DR-TB.165 One of the most important limitations of drug repurposing is related to the patent application and intellectual property rights (IPRs). For repositioned drugs the IP protection is limited, and some national legislations impede obtaining a patent for the second therapeutic use of drugs.166 Some IP laws even restrict the repositioned drugs entering the market.167
6. Conclusion
To combat the current state of drug-resistant TB, drug repositioning is an appealing and favored technique in drug development. Numerous computational and experimental approaches for strategically repositioning drugs on a large scale have been advanced over the years. The comprehensive discussion presented in this review sheds light on the anticipated potential of the repurposing technique for identifying important compounds with potential anti-TB action. It also provides deep insights on practically almost all previous and most recent repurposed anti-TB drugs and their synergistic effects with standard anti-TB medications. This large pool of repurposed anti-TB candidates in various investigational and advanced clinical studies implies that drug repurposing is becoming widely acknowledged with a high chance of success. Also, the accomplishment of Linezolid, Moxifloxacin, Levofloxacin, and Clofazimine in MDR-TB treatment shows that drug repurposing for TB treatment is a viable solution to meet the target of global TB eradication. Furthermore, given the rapid advancement of Metformin, Vitamin D3, Statins, and other repurposed adjunctive HDT, it is not unreasonable to expect a combination of HDT and anti-TB drugs to be the definitive treatment for DR-TB in the near future. Nonetheless, the challenges and limitations connected with this technique should not be overlooked and the answers to many key questions regarding repurposed drugs should be addressed through multidisciplinary research at a larger scale before being included in the TB treatment regimen.
Acknowledgments
Dr. Khushbu Sharma acknowledges a Research Associate Fellowship, (Project ID: 2020-6185, File No 45/23/2020/PHA/BMS) from Indian Council of Medical Research, India. Dr. Meetu Agarwal acknowledges, Department of Science and Technology, Ministry of Science and Technology (DST/INSPIRE/04/2019/002743). Dr. Abhinav Grover and Dr. Sonam Grover is grateful to University Grants Commission, India for the faculty Recharge Position. Sonam Grover is grateful to Jamia Hamdard for DST Purse grant and UGC start-up grant (F.4-5/2018(FRP-Start-Up-Grant) (Cycle IV) (BSR).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c05511.
Supplementary Table S1: in silico identified drugs with potential to inhibit M.tb drug targets (PDF)
Author Contributions
# K.S and F.A. contributed equally to the study. K.S, F.A. and S.G., conceptualized the manuscript. K.S and F.A. wrote the Manuscript. T.S., M.A and S.G provided critical inputs and edited the manuscript, A.G and S.G. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Global tuberculosis report 2021; World Health Organization; Geneva, 2021. [Google Scholar]
- Seung K. J.; Keshavjee S.; Rich M. L. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harb Perspect Med. 2015, 5, a017863. 10.1101/cshperspect.a017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sileshi T.; Tadesse E.; Makonnen E.; Aklillu E. The Impact of First-Line Anti-Tubercular Drugs’ Pharmacokinetics on Treatment Outcome: A Systematic Review. Clin Pharmacol 2021, 13, 1–12. 10.2147/CPAA.S289714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung-Delgado K.; Guillen-Bravo S.; Revilla-Montag A.; Bernabe-Ortiz A. Mortality among MDR-TB cases: comparison with drug-susceptible tuberculosis and associated factors. PLoS One 2015, 10, e0119332 10.1371/journal.pone.0119332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan R. Bedaquiline: First FDA-approved tuberculosis drug in 40 years. Int. J. Appl. Basic Med. Res. 2013, 3, 1–2. 10.4103/2229-516X.112228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D. R.; Dalcolmo M.; Tiberi S.; Arbex M. A.; Munoz-Torrico M.; Duarte R.; D’Ambrosio L.; Visca D.; Rendon A.; Gaga M.; Zumla A.; Migliori G. B. New and repurposed drugs to treat multidrug- and extensively drug-resistant tuberculosis. J. Bras Pneumol 2018, 44, 153–160. 10.1590/s1806-37562017000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayanju O.; Limberis J.; Esmail A.; Oelofse S.; Gina P.; Pietersen E.; Fadul M.; Warren R.; Dheda K. Long-term bedaquiline-related treatment outcomes in patients with extensively drug-resistant tuberculosis from South Africa. Eur. Respir. J. 2018, 51, 1800544. 10.1183/13993003.00544-2018. [DOI] [PubMed] [Google Scholar]
- https://ncats.nih.gov/preclinical/repurpose#learn-more.
- Ashburn T. T.; Thor K. B. Drug repositioning: identifying and developing new uses for existing drugs. Nature reviews. Drug discovery 2004, 3, 673–683. 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Maitra A.; Bates S.; Kolvekar T.; Devarajan P. V.; Guzman J. D.; Bhakta S. Repurposing-a ray of hope in tackling extensively drug resistance in tuberculosis. Int. J. Infect Dis 2015, 32, 50–55. 10.1016/j.ijid.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Xue H.; Li J.; Xie H.; Wang Y. Review of Drug Repositioning Approaches and Resources. Int. J. Biol. Sci. 2018, 14, 1232–1244. 10.7150/ijbs.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S.; Verma S. S.; Aggarwal S.; Gupta S. C. Drug repurposing for breast cancer therapy: Old weapon for new battle. Semin Cancer Biol. 2021, 68, 8–20. 10.1016/j.semcancer.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese L.; Fleischer A. B. Thalidomide: current and potential clinical applications. Am. J. Med. 2000, 108, 487–495. 10.1016/S0002-9343(99)00408-8. [DOI] [PubMed] [Google Scholar]
- Tanweer S.; Jamal S.; Mehra S.; Saqib N.; Ahmad F.; Faizan; Grover A.; Grover S. Multifaceted role of drugs: a potential weapon to outsmart Mycobacterium tuberculosis resistance by targeting its essential ThyX. J. Biomol Struct Dyn 2022, 40, 8508. 10.1080/07391102.2021.1913230. [DOI] [PubMed] [Google Scholar]
- Suay-Garcia B.; Falcó A.; Bueso-Bordils J. I.; Anton-Fos G. M.; Pérez-Gracia M. T.; Alemán-López P. A. Tree-Based QSAR Model for Drug Repurposing in the Discovery of New Antibacterial Compounds Against Escherichia coli. Pharmaceuticals (Basel) 2020, 13, 431. 10.3390/ph13120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H.; Colvin C. J.; Johnson B. K.; Kirchhoff P. D.; Wilson M.; Jorgensen-Muga K.; Larsen S. D.; Abramovitch R. B. Inhibitors of Mycobacterium tuberculosis DosRST signaling and persistence. Nat. Chem. Biol. 2017, 13, 218–225. 10.1038/nchembio.2259. [DOI] [PubMed] [Google Scholar]
- Gupta U. D.; Vemuri N.; Gupta P.; Kumar V.; Tanushree P.; Khuller G. K. Efficacy of moxifloxacin & econazole against multidrug resistant (MDR) Mycobacterium tuberculosis in murine model. Indian J. Med. Res. 2015, 142, 323–329. 10.4103/0971-5916.166599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tato M.; de la Pedrosa E. G.; Cantón R.; Gómez-García I.; Fortún J.; Martín-Davila P.; Baquero F.; Gomez-Mampaso E. In vitro activity of linezolid against Mycobacterium tuberculosis complex, including multidrug-resistant Mycobacterium bovis isolates. Int. J. Antimicrob. Agents 2006, 28, 75–78. 10.1016/j.ijantimicag.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Global tuberculosis report 2018; World Health Organization: Geneva, 2018. [Google Scholar]
- Ahmad N.; Ahuja S. D.; Akkerman O. W.; Alffenaar J. C.; Anderson L. F.; Baghaei P.; Bang D.; Barry P. M.; Bastos M. L.; Behera D.; Benedetti A.; Bisson G. P.; Boeree M. J.; Bonnet M.; Brode S. K.; Brust J. C. M.; Cai Y.; Caumes E.; Cegielski J. P.; Centis R.; Chan P. C.; Chan E. D.; Chang K. C.; Charles M.; Cirule A.; Dalcolmo M. P.; D’Ambrosio L.; de Vries G.; Dheda K.; Esmail A.; Flood J.; Fox G. J.; Fréchet-Jachym M.; Fregona G.; Gayoso R.; Gegia M.; Gler M. T.; Gu S.; Guglielmetti L.; Holtz T. H.; Hughes J.; Isaakidis P.; Jarlsberg L.; Kempker R. R.; Keshavjee S.; Khan F. A.; Kipiani M.; Koenig S. P.; Koh W. J.; Kritski A.; Kuksa L.; Kvasnovsky C. L.; Kwak N.; Lan Z.; Lange C.; Laniado-Laborín R.; Lee M.; Leimane V.; Leung C. C.; Leung E. C.; Li P. Z.; Lowenthal P.; Maciel E. L.; Marks S. M.; Mase S.; Mbuagbaw L.; Migliori G. B.; Milanov V.; Miller A. C.; Mitnick C. D.; Modongo C.; Mohr E.; Monedero I.; Nahid P.; Ndjeka N.; O’Donnell M. R.; Padayatchi N.; Palmero D.; Pape J. W.; Podewils L. J.; Reynolds I.; Riekstina V.; Robert J.; Rodriguez M.; Seaworth B.; Seung K. J.; Schnippel K.; Shim T. S.; Singla R.; Smith S. E.; Sotgiu G.; Sukhbaatar G.; Tabarsi P.; Tiberi S.; Trajman A.; Trieu L.; Udwadia Z. F.; van der Werf T. S.; Veziris N.; Viiklepp P.; Vilbrun S. C.; Walsh K.; Westenhouse J.; Yew W. W.; Yim J. J.; Zetola N. M.; Zignol M.; Menzies D. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 2018, 392, 821–834. 10.1016/S0140-6736(18)31644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. C.; Yew W. W.; Tam C. M.; Leung C. C. WHO group 5 drugs and difficult multidrug-resistant tuberculosis: a systematic review with cohort analysis and meta-analysis. Antimicrob. Agents Chemother. 2013, 57, 4097–4104. 10.1128/AAC.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradie F.; Everitt D.; Olugbosi M.; Wills G.; Fabiane S.; Timm J.; Spigelman M.. High rate of successful outcomes treating highly resistant TB in the ZeNix study of pretomanid, bedaquiline and alternative doses and durations of linezolid; IAS 2021, 11th IAS Conference on HIV Science; Berlin, Germany, 2021; pp 18–21.
- Conradie F.; Diacon A. H.; Ngubane N.; Howell P.; Everitt D.; Crook A. M.; Mendel C. M.; Egizi E.; Moreira J.; Timm J.; McHugh T. D.; Wills G. H.; Bateson A.; Hunt R.; Van Niekerk C.; Li M.; Olugbosi M.; Spigelman M. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N Engl J. Med. 2020, 382, 893–902. 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspard M.; Elefant-Amoura E.; Melonio I.; De Montgolfier I.; Veziris N.; Caumes E. Bedaquiline and Linezolid for Extensively Drug-Resistant Tuberculosis in Pregnant Woman. Emerg Infect Dis 2017, 23, 1731–1732. 10.3201/eid2310.161398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckert P.; Hillemann D.; Kohl T. A.; Kalinowski J.; Richter E.; Niemann S.; Feuerriegel S. rplC T460C identified as a dominant mutation in linezolid-resistant Mycobacterium tuberculosis strains. Antimicrob. Agents Chemother. 2012, 56, 2743–2745. 10.1128/AAC.06227-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S.; Dawson R.; Friedrich S. O.; Venter A.; Paige D.; Zhu T.; Silvia A.; Gobey J.; Ellery C.; Zhang Y.; Eisenach K.; Miller P.; Diacon A. H. Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of patients with pulmonary tuberculosis. PLoS One 2014, 9, e94462 10.1371/journal.pone.0094462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Z.; Jing W.; Shi J.; Wen S.; Zhang T.; Huo F.; Shang Y.; Liang Q.; Huang H.; Pang Y. Comparison of In Vitro Activity and MIC Distributions between the Novel Oxazolidinone Delpazolid and Linezolid against Multidrug-Resistant and Extensively Drug-Resistant Mycobacterium tuberculosis in China. Antimicrob. Agents Chemother. 2018, 62, 1. 10.1128/AAC.00165-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholo M. C.; Boshoff H. I.; Steel H. C.; Cockeran R.; Matlola N. M.; Downing K. J.; Mizrahi V.; Anderson R. Effects of clofazimine on potassium uptake by a Trk-deletion mutant of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2006, 57, 79–84. 10.1093/jac/dki409. [DOI] [PubMed] [Google Scholar]
- Yano T.; Kassovska-Bratinova S.; Teh J. S.; Winkler J.; Sullivan K.; Isaacs A.; Schechter N. M.; Rubin H. Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species. J. Biol. Chem. 2011, 286, 10276–10287. 10.1074/jbc.M110.200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y.; Qiu C.; Chen X.; Wang J.; Jing W.; Pan H.; Chen W.; Liu Y.; Li C.; Xi X.; Yin H.; Zeng J.; Zhang X.; Xu T.; Wang Q.; Guo R.; Wang J.; Pang Y.; Chu N. Treatment outcome of a shorter regimen containing clofazimine for multidrug-resistant tuberculosis: a randomized control trial in China. Clin Infect Dis 2020, 71, 1047. 10.1093/cid/ciz915. [DOI] [PubMed] [Google Scholar]
- Yuan S.; Yin X.; Meng X.; Chan J.F.-W.; Ye Z.-W.; Riva L.; Pache L.; Chan C.C.-Y.; Lai P.-M.; Chan C.C.-S.; Poon V.K.-M.; Lee A.C.-Y.; Matsunaga N.; Pu Y.; Yuen C.-K.; Cao J.; Liang R.; Tang K.; Sheng L.; Du Y.; Xu W.; Lau C.-Y.; Sit K.-Y.; Au W.-K.; Wang R.; Zhang Y.-Y.; Tang Y.-D.; Clausen T. M.; Pihl J.; Oh J.; Sze K.-H.; Zhang A. J.; Chu H.; Kok K.-H.; Wang D.; Cai X.-H.; Esko J. D.; Hung I.F.-N.; Li R. A.; Chen H.; Sun H.; Jin D.-Y.; Sun R.; Chanda S. K.; Yuen K.-Y. Clofazimine broadly inhibits coronaviruses including SARS-CoV-2. Nature 2021, 593, 418–423. 10.1038/s41586-021-03431-4. [DOI] [PubMed] [Google Scholar]
- Shahid M.; Sobia F.; Singh A.; Malik A.; Khan H. M.; Jonas D.; Hawkey P. M. Beta-lactams and beta-lactamase-inhibitors in current- or potential-clinical practice: a comprehensive update. Crit Rev. Microbiol 2009, 35, 81–108. 10.1080/10408410902733979. [DOI] [PubMed] [Google Scholar]
- Hugonnet J. E.; Blanchard J. S. Irreversible inhibition of the Mycobacterium tuberculosis beta-lactamase by clavulanate. Biochemistry 2007, 46, 11998–12004. 10.1021/bi701506h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban A. Y.; Bilgin K.; Tasdelen Fisgin N.; Uzun M.; Durupinar B. Effect of Meropenem against multidrug-resistant Mycobacterium tuberculosis. J. Chemother 2008, 20, 395–396. 10.1179/joc.2008.20.3.395. [DOI] [PubMed] [Google Scholar]
- Mirzayev F.; Viney K.; Linh N. N.; Gonzalez-Angulo L.; Gegia M.; Jaramillo E.; Zignol M.; Kasaeva T. World Health Organization recommendations on the treatment of drug-resistant tuberculosis, 2020 update. Eur. Respir. J. 2021, 57, 2003300. 10.1183/13993003.03300-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacon A. H.; van der Merwe L.; Barnard M.; von Groote-Bidlingmaier F.; Lange C.; García-Basteiro A. L.; Sevene E.; Ballell L.; Barros-Aguirre D. β-Lactams against Tuberculosis–New Trick for an Old Dog?. N Engl J. Med. 2016, 375, 393–394. 10.1056/NEJMc1513236. [DOI] [PubMed] [Google Scholar]
- Ramón-García S.; González Del Río R.; Villarejo A. S.; Sweet G. D.; Cunningham F.; Barros D.; Ballell L.; Mendoza-Losana A.; Ferrer-Bazaga S.; Thompson C. J. Repurposing clinically approved cephalosporins for tuberculosis therapy. Sci. Rep 2016, 6, 34293. 10.1038/srep34293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S.; Thomas T.; Howe D.; Malinga L.; Raj P.; Alffenaar J. W.; Gumbo T. Cefdinir and β-Lactamase Inhibitor Independent Efficacy Against Mycobacterium tuberculosis. Front Pharmacol 2021, 12, 677005. 10.3389/fphar.2021.677005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S.; Gumbo T.; Thomas T. Repurposing Cefazolin-Avibactam for the Treatment of Drug Resistant Mycobacterium tuberculosis. Front Pharmacol 2021, 12, 776969. 10.3389/fphar.2021.776969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasse B.; Walker A. S.; Fehr J.; Furrer H.; Hoffmann M.; Battegay M.; Calmy A.; Fellay J.; Di Benedetto C.; Weber R.; Ledergerber B. Co-trimoxazole prophylaxis is associated with reduced risk of incident tuberculosis in participants in the Swiss HIV Cohort Study. Antimicrob. Agents Chemother. 2014, 58, 2363–2368. 10.1128/AAC.01868-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchèze C.; Jacobs W. R. Jr. The combination of sulfamethoxazole, trimethoprim, and isoniazid or rifampin is bactericidal and prevents the emergence of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2012, 56, 5142–5148. 10.1128/AAC.00832-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande D.; Pasipanodya J. G.; Srivastava S.; Martin K. R.; Athale S.; van Zyl J.; Antiabong J.; Koeuth T.; Lee P. S.; Dheda K.; Gumbo T. Minocycline Immunomodulates via Sonic Hedgehog Signaling and Apoptosis and Has Direct Potency Against Drug-Resistant Tuberculosis. Journal of infectious diseases 2019, 219, 975–985. 10.1093/infdis/jiy587. [DOI] [PubMed] [Google Scholar]
- Brindha S. In silico and in vitro screening of FDA-approved drugs for potential repurposing against tuberculosis. BioRxiv 2017, 1. 10.1101/228171. [DOI] [Google Scholar]
- Parish T.; Gordhan B. G.; McAdam R. A.; Duncan K.; Mizrahi V.; Stoker N. G. Production of mutants in amino acid biosynthesis genes of Mycobacterium tuberculosis by homologous recombination. Microbiology (Reading) 1999, 145, 3497–3503. 10.1099/00221287-145-12-3497. [DOI] [PubMed] [Google Scholar]
- Miow Q. H.; Vallejo A. F.; Wang Y.; Hong J. M.; Bai C.; Teo F. S.; Wang A. D.; Loh H. R.; Tan T. Z.; Ding Y.; She H. W.; Gan S. H.; Paton N. I.; Lum J.; Tay A.; Chee C. B.; Tambyah P. A.; Polak M. E.; Wang Y. T.; Singhal A.; Elkington P. T.; Friedland J. S.; Ong C. W. Doxycycline host-directed therapy in human pulmonary tuberculosis. J. Clin Invest 2021, 131, 1. 10.1172/JCI141895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J.; Deng W.; Yang W.; Luo H.; Duan X.; Xie L.; Li P.; Wang R.; Fu T.; Abdalla A. E.; Xie J. Mycobacterium tuberculosis Rv1152 is a Novel GntR Family Transcriptional Regulator Involved in Intrinsic Vancomycin Resistance and is a Potential Vancomycin Adjuvant Target. Sci. Rep 2016, 6, 28002. 10.1038/srep28002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S.; Chapagain M.; van Zyl J.; Deshpande D.; Gumbo T. Potency of vancomycin against Mycobacterium tuberculosis in the hollow fiber system model. J. Glob Antimicrob Resist 2021, 24, 403–410. 10.1016/j.jgar.2021.01.005. [DOI] [PubMed] [Google Scholar]
- WHO consolidated guidelines on drug-resistant tuberculosis treatment; World Health Organization: Geneva, 2019. [PubMed]
- Technical Report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis; World Health Organization: Geneva, 2018.
- Ahmad Z.; Sharma S.; Khuller G. K. Chemotherapeutic evaluation of alginate nanoparticle-encapsulated azole antifungal and antitubercular drugs against murine tuberculosis. Nanomedicine 2007, 3, 239–243. 10.1016/j.nano.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Imperiale B. R.; Cataldi A. A.; Morcillo N. S. In vitro anti-tuberculosis activity of azole drugs against Mycobacterium tuberculosis clinical isolates. Rev. Argent Microbiol 2017, 49, 332–338. 10.1016/j.ram.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Galal A. M.; Ross S. A.; Jacob M.; ElSohly M. A. Antifungal activity of artemisinin derivatives. J. Nat. Prod 2005, 68, 1274–1276. 10.1021/np050074u. [DOI] [PubMed] [Google Scholar]
- Choi W. H. Novel Pharmacological Activity of Artesunate and Artemisinin: Their Potential as Anti-Tubercular Agents. J. Clin Med. 2017, 6, 30. 10.3390/jcm6030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H.; Williams J. T.; Aleiwi B.; Ellsworth E.; Abramovitch R. B. Inhibiting Mycobacterium tuberculosis DosRST Signaling by Targeting Response Regulator DNA Binding and Sensor Kinase Heme. ACS Chem. Biol. 2020, 15, 52–62. 10.1021/acschembio.8b00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G.; Sramek H. A. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1994, 38, 2054–2058. 10.1128/AAC.38.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. W.; Jeon D.; Mountz J. M.; Lee J. D.; Jeong Y. J.; Zia N.; Lee M.; Lee J.; Via L. E.; Lee S.; Eum S. Y.; Lee S. J.; Goldfeder L. C.; Cai Y.; Jin B.; Kim Y.; Oh T.; Chen R. Y.; Dodd L. E.; Gu W.; Dartois V.; Park S. K.; Kim C. T.; Barry C. E. 3rd; Cho S. N. Efficacy and safety of metronidazole for pulmonary multidrug-resistant tuberculosis. Antimicrob. Agents Chemother. 2013, 57, 3903–3909. 10.1128/AAC.00753-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny W. A.; Palmer B. D. The nitroimidazooxazines (PA-824 and analogs): structure-activity relationship and mechanistic studies. Future Med. Chem. 2010, 2, 1295–1304. 10.4155/fmc.10.207. [DOI] [PubMed] [Google Scholar]
- Mori G.; Orena B. S.; Franch C.; Mitchenall L. A.; Godbole A. A.; Rodrigues L.; Aguilar-Pérez C.; Zemanová J.; Huszár S.; Forbak M.; Lane T. R.; Sabbah M.; Deboosere N.; Frita R.; Vandeputte A.; Hoffmann E.; Russo R.; Connell N.; Veilleux C.; Jha R. K.; Kumar P.; Freundlich J. S.; Brodin P.; Aínsa J. A.; Nagaraja V.; Maxwell A.; Mikušová K.; Pasca M. R.; Ekins S. The EU approved antimalarial pyronaridine shows antitubercular activity and synergy with rifampicin, targeting RNA polymerase. Tuberculosis (Edinb) 2018, 112, 98–109. 10.1016/j.tube.2018.08.004. [DOI] [PubMed] [Google Scholar]
- Matt U.; Selchow P.; Dal Molin M.; Strommer S.; Sharif O.; Schilcher K.; Andreoni F.; Stenzinger A.; Zinkernagel A. S.; Zeitlinger M.; Sander P.; Nemeth J. Chloroquine enhances the antimycobacterial activity of isoniazid and pyrazinamide by reversing inflammation-induced macrophage efflux. Int. J. Antimicrob. Agents 2017, 50, 55–62. 10.1016/j.ijantimicag.2017.02.022. [DOI] [PubMed] [Google Scholar]
- Mishra R.; Kohli S.; Malhotra N.; Bandyopadhyay P.; Mehta M.; Munshi M.; Adiga V.; Ahuja V. K.; Shandil R. K.; Rajmani R. S.; Seshasayee A. S. N.; Singh A. Targeting redox heterogeneity to counteract drug tolerance in replicating Mycobacterium tuberculosis. Sci. Transl Med. 2019, 11, 1. 10.1126/scitranslmed.aaw6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrônio M. G. S.; Castelo Branco A.; Abbadi B. L.; Macchi F.; Silveira M. D.; Lock G. A.; Costa T. D.; de Araújo D. M.; Cibulski S.; Bizarro C. V.; Machado P.; Basso L. A.; Rodrigues-Junior V. S. Effects of tafenoquine against active, dormant and resistant Mycobacterium tuberculosis. Tuberculosis (Edinb) 2021, 128, 102089. 10.1016/j.tube.2021.102089. [DOI] [PubMed] [Google Scholar]
- Mishra A. K.; Yabaji S. M.; Dubey R. K. Evaluation of isoprinosine to be repurposed as an adjunct anti-tuberculosis chemotherapy. Med. Hypotheses 2018, 115, 77–80. 10.1016/j.mehy.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Pires D.; Valente S.; Calado M.; Mandal M.; Azevedo-Pereira J. M.; Anes E. Repurposing Saquinavir for Host-Directed Therapy to Control Mycobacterium Tuberculosis Infection. Front Immunol 2021, 12, 647728. 10.3389/fimmu.2021.647728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L. E.; Vilchèze C.; Ng C.; Jacobs W. R. Jr.; Ramón-García S.; Thompson C. J. Anthelmintic avermectins kill Mycobacterium tuberculosis, including multidrug-resistant clinical strains. Antimicrob. Agents Chemother. 2013, 57, 1040–1046. 10.1128/AAC.01696-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omansen T. F.; Porter J. L.; Johnson P. D.; van der Werf T. S.; Stienstra Y.; Stinear T. P. In-vitro activity of avermectins against Mycobacterium ulcerans. PLoS Negl Trop Dis 2015, 9, e0003549 10.1371/journal.pntd.0003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezquerra-Aznárez J. M.; Degiacomi G.; Gašparovič H.; Stelitano G.; Sammartino J. C.; Korduláková J.; Governa P.; Manetti F.; Pasca M. R.; Chiarelli L. R.; Ramón-García S. The Veterinary Anti-Parasitic Selamectin Is a Novel Inhibitor of the Mycobacterium tuberculosis DprE1 Enzyme. Int. J. Mol. Sci. 2022, 23, 771. 10.3390/ijms23020771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U. K. Mechanisms of anthelmintic action. Prog. Drug Res. 1975, 19, 147–157. 10.1007/978-3-0348-7090-0_19. [DOI] [PubMed] [Google Scholar]
- Macingwana L.; Baker B.; Ngwane A. H.; Harper C.; Cotton M. F.; Hesseling A.; Diacon A. H.; van Helden P.; Wiid I. Sulfamethoxazole enhances the antimycobacterial activity of rifampicin. J. Antimicrob. Chemother. 2012, 67, 2908–2911. 10.1093/jac/dks306. [DOI] [PubMed] [Google Scholar]
- Kaushik A.; Ammerman N. C.; Tasneen R.; Story-Roller E.; Dooley K. E.; Dorman S. E.; Nuermberger E. L.; Lamichhane G. In vitro and in vivo activity of biapenem against drug-susceptible and rifampicin-resistant Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2017, 72, 2320–2325. 10.1093/jac/dkx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solapure S.; Dinesh N.; Shandil R.; Ramachandran V.; Sharma S.; Bhattacharjee D.; Ganguly S.; Reddy J.; Ahuja V.; Panduga V.; Parab M.; Vishwas K. G.; Kumar N.; Balganesh M.; Balasubramanian V. In vitro and in vivo efficacy of β-lactams against replicating and slowly growing/nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2013, 57, 2506–2510. 10.1128/AAC.00023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinpelu O.; Lawal M.; Kumalo H.; Mhlongo N. Drug repurposing: Fusidic acid as a potential inhibitor of M. tuberculosis FtsZ polymerization – Insight from DFT calculations, molecular docking and molecular dynamics simulations. Tuberculosis 2020, 121, 101920. 10.1016/j.tube.2020.101920. [DOI] [PubMed] [Google Scholar]
- Bailey M. A.; Na H.; Duthie M. S.; Gillis T. P.; Lahiri R.; Parish T. Nitazoxanide is active against Mycobacterium leprae. PLoS One 2017, 12, e0184107 10.1371/journal.pone.0184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger D.; Vesenbeckh S.; Schönfeld N.; Bettermann G.; Bauer T. T.; Rüssmann H.; Mauch H. Mefloquine as a potential drug against multidrug-resistant tuberculosis. Eur. Respir. J. 2015, 46, 1503–1505. 10.1183/13993003.00321-2015. [DOI] [PubMed] [Google Scholar]
- Imperiale B. R.; Cataldi A. A.; Morcillo N. S. In vitro anti-tuberculosis activity of azole drugs against Mycobacterium tuberculosis clinical isolates. Rev. Argent Microbiol 2017, 49, 332–338. 10.1016/j.ram.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Lougheed K. E. A.; Taylor D. L.; Osborne S. A.; Bryans J. S.; Buxton R. S. New anti-tuberculosis agents amongst known drugs. Tuberculosis (Edinburgh, Scotland) 2009, 89, 364–370. 10.1016/j.tube.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X.; Xu J.; Files M.; Cirillo J. D.; Endsley J. J.; Zhou J.; Endsley M. A. Dual activity of niclosamide to suppress replication of integrated HIV-1 and Mycobacterium tuberculosis (Beijing). Tuberculosis (Edinb) 2019, 116s, S28–s33. 10.1016/j.tube.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleandrova V. V.; Scotti M. T.; Speck-Planche A. Computational Drug Repurposing for Antituberculosis Therapy: Discovery of Multi-Strain Inhibitors. Antibiotics (Basel) 2021, 10, 1005. 10.3390/antibiotics10081005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho L. P.; Lin G.; Jiang X.; Nathan C. Nitazoxanide kills replicating and nonreplicating Mycobacterium tuberculosis and evades resistance. J. Med. Chem. 2009, 52, 5789–5792. 10.1021/jm9010719. [DOI] [PubMed] [Google Scholar]
- Shigyo K.; Ocheretina O.; Merveille Y. M.; Johnson W. D.; Pape J. W.; Nathan C. F.; Fitzgerald D. W. Efficacy of nitazoxanide against clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2013, 57, 2834–2837. 10.1128/AAC.02542-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.; Zhang Y. Antituberculosis activity of certain antifungal and antihelmintic drugs. Tubercle and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease 1999, 79, 319–320. 10.1054/tuld.1999.0212. [DOI] [PubMed] [Google Scholar]
- Kadri H.; Lambourne O. A.; Mehellou Y. Niclosamide, a Drug with Many (Re)purposes. ChemMedChem. 2018, 13, 1088–1091. 10.1002/cmdc.201800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J.; Zumla A. Nonsteroidal antiinflammatory drugs for adjunctive tuberculosis treatment. Journal of infectious diseases 2013, 208, 185–188. 10.1093/infdis/jit153. [DOI] [PubMed] [Google Scholar]
- Canan C. H.; Gokhale N. S.; Carruthers B.; Lafuse W. P.; Schlesinger L. S.; Torrelles J. B.; Turner J. Characterization of lung inflammation and its impact on macrophage function in aging. J. Leukoc Biol. 2014, 96, 473–480. 10.1189/jlb.4A0214-093RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman J. D.; Evangelopoulos D.; Gupta A.; Birchall K.; Mwaigwisya S.; Saxty B.; McHugh T. D.; Gibbons S.; Malkinson J.; Bhakta S. Antitubercular specific activity of ibuprofen and the other 2-arylpropanoic acids using the HT-SPOTi whole-cell phenotypic assay. BMJ. Open 2013, 3, e002672. 10.1136/bmjopen-2013-002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroesen V. M.; Rodríguez-Martínez P.; García E.; Rosales Y.; Díaz J.; Martín-Céspedes M.; Tapia G.; Sarrias M. R.; Cardona P. J.; Vilaplana C. A Beneficial Effect of Low-Dose Aspirin in a Murine Model of Active Tuberculosis. Front Immunol 2018, 9, 798. 10.3389/fimmu.2018.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaplana C.; Marzo E.; Tapia G.; Diaz J.; Garcia V.; Cardona P. J. Ibuprofen therapy resulted in significantly decreased tissue bacillary loads and increased survival in a new murine experimental model of active tuberculosis. Journal of infectious diseases 2013, 208, 199–202. 10.1093/infdis/jit152. [DOI] [PubMed] [Google Scholar]
- Byrne S. T.; Denkin S. M.; Zhang Y. Aspirin and ibuprofen enhance pyrazinamide treatment of murine tuberculosis. J. Antimicrob. Chemother. 2006, 59, 313–316. 10.1093/jac/dkl486. [DOI] [PubMed] [Google Scholar]
- Byrne S. T.; Denkin S. M.; Zhang Y. Aspirin antagonism in isoniazid treatment of tuberculosis in mice. Antimicrob. Agents Chemother. 2007, 51, 794–795. 10.1128/AAC.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B.; Pingle M.; Brickner S. J.; Shah N.; Roberts J.; Rundell M.; Bracken W. C.; Warrier T.; Somersan S.; Venugopal A.; Darby C.; Jiang X.; Warren J. D.; Fernandez J.; Ouerfelli O.; Nuermberger E. L.; Cunningham-Bussel A.; Rath P.; Chidawanyika T.; Deng H.; Realubit R.; Glickman J. F.; Nathan C. F. Nonsteroidal anti-inflammatory drug sensitizes Mycobacterium tuberculosis to endogenous and exogenous antimicrobials. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 16004–16011. 10.1073/pnas.1214188109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A.; Evangelopoulos D.; Chrzastek A.; Martin L. T.; Hanrath A.; Chapman E.; Hailes H. C.; Lipman M.; McHugh T. D.; Waddell S. J.; Bhakta S. Carprofen elicits pleiotropic mechanisms of bactericidal action with the potential to reverse antimicrobial drug resistance in tuberculosis. J. Antimicrob. Chemother. 2020, 75, 3194–3201. 10.1093/jac/dkaa307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza A.; Wagner J. M.; Wei N. N.; Kwiatkowski S.; Zhan C. G.; Watt D. S.; Korotkov K. V. Application of the 4D fingerprint method with a robust scoring function for scaffold-hopping and drug repurposing strategies. J. Chem. Inf Model 2014, 54, 2834–2845. 10.1021/ci5003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappoen D.; Vajs J.; Uythethofken C.; Virag A.; Mathys V.; Kočevar M.; Verschaeve L.; Gazvoda M.; Polanc S.; Huygen K.; Košmrlj J. Anti-mycobacterial activity of 1,3-diaryltriazenes. Eur. J. Med. Chem. 2014, 77, 193–203. 10.1016/j.ejmech.2014.02.065. [DOI] [PubMed] [Google Scholar]
- Padiadpu J.; Baloni P.; Anand K.; Munshi M.; Thakur C.; Mohan A.; Singh A.; Chandra N. Identifying and Tackling Emergent Vulnerability in Drug-Resistant Mycobacteria. ACS Infect Dis 2016, 2, 592–607. 10.1021/acsinfecdis.6b00004. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Lee A. W.; Wu K. K.; Gao P.; Tam K. K.; Rajwani R.; Chaburte G. C.; Ng T. T.; Chan C. T.; Lao H. Y.; Yam W. C.; Kao R. Y.; Siu G. K. H. Screening Repurposed Antiviral Small Molecules as Antimycobacterial Compounds by a Lux-Based phoP Promoter-Reporter Platform. Antibiotics (Basel) 2022, 11, 369. 10.3390/antibiotics11030369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada H.; Martínez-Vázquez M.; López-Jácome E.; González-Pedrajo B.; Andrade Á.; Fernández-Presas A. M.; Tovar-García A.; García-Contreras R. Repurposed anti-cancer drugs: the future for anti-infective therapy?. Expert Rev. Anti Infect Ther 2020, 18, 609–612. 10.1080/14787210.2020.1752665. [DOI] [PubMed] [Google Scholar]
- Napier R. J.; Rafi W.; Cheruvu M.; Powell K. R.; Zaunbrecher M. A.; Bornmann W.; Salgame P.; Shinnick T. M.; Kalman D. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe 2011, 10, 475–485. 10.1016/j.chom.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S.; Angrish N.; Gupta K.; Tyagi A. K.; Khare G. Inhibition of ABCG2 efflux pumps renders the Mycobacterium tuberculosis hiding in mesenchymal stem cells responsive to antibiotic treatment. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases 2021, 87, 104662. 10.1016/j.meegid.2020.104662. [DOI] [PubMed] [Google Scholar]
- Moreira W.; Ngan G. J.; Low J. L.; Poulsen A.; Chia B. C.; Ang M. J.; Yap A.; Fulwood J.; Lakshmanan U.; Lim J.; Khoo A. Y.; Flotow H.; Hill J.; Raju R. M.; Rubin E. J.; Dick T. Target mechanism-based whole-cell screening identifies bortezomib as an inhibitor of caseinolytic protease in mycobacteria. mBio 2015, 6, e00253 10.1128/mBio.00253-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwane A. H.; Petersen R. D.; Baker B.; Wiid I.; Wong H. N.; Haynes R. K. The evaluation of the anti-cancer drug elesclomol that forms a redox-active copper chelate as a potential anti-tubercular drug. IUBMB Life 2019, 71, 532–538. 10.1002/iub.2002. [DOI] [PubMed] [Google Scholar]
- Shen H.; Wang F.; Zeng G.; Shen L.; Cheng H.; Huang D.; Wang R.; Rong L.; Chen Z. W. Bis-biguanide dihydrochloride inhibits intracellular replication of M. tuberculosis and controls infection in mice. Sci. Rep 2016, 6, 32725. 10.1038/srep32725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier R. J.; Norris B. A.; Swimm A.; Giver C. R.; Harris W. A.; Laval J.; Napier B. A.; Patel G.; Crump R.; Peng Z.; Bornmann W.; Pulendran B.; Buller R. M.; Weiss D. S.; Tirouvanziam R.; Waller E. K.; Kalman D. Low doses of imatinib induce myelopoiesis and enhance host anti-microbial immunity. PLoS Pathog 2015, 11, e1004770 10.1371/journal.ppat.1004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A.; Rao N.; Malhotra K. P. Renal tuberculosis in an imatinib-treated chronic myeloid leukemia. J. Bras Nefrol 2020, 42, 366–369. 10.1590/2175-8239-jbn-2019-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A.; Mamidi A. S.; Rajmani R. S.; Ray A.; Roy R.; Surolia A. An allosteric inhibitor of Mycobacterium tuberculosis ArgJ: Implications to a novel combinatorial therapy. EMBO Mol. Med. 2018, 10, 1. 10.15252/emmm.201708038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngidi N. T. P.; Machaba K. E.; Mhlongo N. N. In Silico Drug Repurposing Approach: Investigation of Mycobacterium tuberculosis FadD32 Targeted by FDA-Approved Drugs. Molecules 2022, 27, 668. 10.3390/molecules27030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Q. P.; Zonder J. A. Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system. Curr. Cancer Drug Targets 2014, 14, 517–536. 10.2174/1568009614666140804154511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagoya Y.; Yoshimi A.; Kataoka K.; Nakagawa M.; Kumano K.; Arai S.; Kobayashi H.; Saito T.; Iwakura Y.; Kurokawa M. Positive feedback between NF-κB and TNF-α promotes leukemia-initiating cell capacity. J. Clin Invest 2014, 124, 528–542. 10.1172/JCI68101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira W.; Santhanakrishnan S.; Ngan G. J. Y.; Low C. B.; Sangthongpitag K.; Poulsen A.; Dymock B. W.; Dick T. Towards Selective Mycobacterial ClpP1P2 Inhibitors with Reduced Activity against the Human Proteasome. Antimicrob. Agents Chemother. 2017, 61, 1. 10.1128/AAC.02307-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshner J. R.; He S.; Balasubramanyam V.; Kepros J.; Yang C. Y.; Zhang M.; Du Z.; Barsoum J.; Bertin J. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol. Cancer Ther 2008, 7, 2319–2327. 10.1158/1535-7163.MCT-08-0298. [DOI] [PubMed] [Google Scholar]
- Gl B.; Rajput R.; Gupta M.; Dahiya P.; Thakur J. K.; Bhatnagar R.; Grover A. Structure-based drug repurposing to inhibit the DNA gyrase of Mycobacterium tuberculosis. Biochem. J. 2020, 477, 4167–4190. 10.1042/BCJ20200462. [DOI] [PubMed] [Google Scholar]
- Merison K.; Jacobs H. Diagnosis and Treatment of Childhood Migraine. Curr. Treat Options Neurol 2016, 18, 48. 10.1007/s11940-016-0431-4. [DOI] [PubMed] [Google Scholar]
- Ghajavand H.; Kargarpour Kamakoli M.; Khanipour S.; Pourazar Dizaji S.; Masoumi M.; Rahimi Jamnani F.; Fateh A.; Yaseri M.; Siadat S. D.; Vaziri F. Scrutinizing the drug resistance mechanism of multi- and extensively-drug resistant Mycobacterium tuberculosis: mutations versus efflux pumps. Antimicrob Resist Infect Control 2019, 8, 70. 10.1186/s13756-019-0516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta N. K.; Bruiners N.; Pinn M. L.; Zimmerman M. D.; Prideaux B.; Dartois V.; Gennaro M. L.; Karakousis P. C. Statin adjunctive therapy shortens the duration of TB treatment in mice. J. Antimicrob. Chemother. 2016, 71, 1570–1577. 10.1093/jac/dkw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su V. Y.; Pan S. W.; Yen Y. F.; Feng J. Y.; Su W. J.; Chen Y. M. Statin use and impact on tuberculosis risk. Expert Rev. Anti Infect Ther 2021, 19, 1093–1098. 10.1080/14787210.2021.1892488. [DOI] [PubMed] [Google Scholar]
- Pandey A. K.; Sassetti C. M. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 4376–4380. 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta N. K.; Bruiners N.; Zimmerman M. D.; Tan S.; Dartois V.; Gennaro M. L.; Karakousis P. C. Adjunctive Host-Directed Therapy With Statins Improves Tuberculosis-Related Outcomes in Mice. Journal of infectious diseases 2020, 221, 1079–1087. 10.1093/infdis/jiz517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerry C.; Pinn M. L.; Bruiners N.; Pine R.; Gennaro M. L.; Karakousis P. C. Simvastatin increases the in vivo activity of the first-line tuberculosis regimen. J. Antimicrob. Chemother. 2014, 69, 2453–2457. 10.1093/jac/dku166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-De-Blas P. D. C.; Bobadilla-Del-Valle M.; Sada-Ovalle I.; Estrada-García I.; Torres-González P.; López-Saavedra A.; Guzmán-Beltrán S.; Ponce-de-León A.; Sifuentes-Osornio J. Simvastatin Enhances the Immune Response Against Mycobacterium tuberculosis. Front Microbiol 2019, 10, 2097. 10.3389/fmicb.2019.02097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral L.; Boeree M. J.; Gillespie S. H.; Udwadia Z. F.; van Soolingen D. Thioridazine cures extensively drug-resistant tuberculosis (XDR-TB) and the need for global trials is now!. Int. J. Antimicrob. Agents 2010, 35, 524–526. 10.1016/j.ijantimicag.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Kaur D.; Mathew S.; Nair C. G. S.; Begum A.; Jainanarayan A. K.; Sharma M.; Brahmachari S. K. Structure based drug discovery for designing leads for the non-toxic metabolic targets in multi drug resistant Mycobacterium tuberculosis. J. Transl Med. 2017, 15, 261. 10.1186/s12967-017-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk M. T.; Korbee C. J.; Esselink J. J.; Dos Santos C. C.; van Veen S.; Gordijn I. F.; Vrieling F.; Walburg K. V.; Engele C. G.; Dijkman K.; Wilson L.; Verreck F. A. W.; Ottenhoff T. H. M.; Haks M. C. Repurposing diphenylbutylpiperidine-class antipsychotic drugs for host-directed therapy of Mycobacterium tuberculosis and Salmonella enterica infections. Sci. Rep 2021, 11, 19634. 10.1038/s41598-021-98980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein E. A.; Yano T.; Li L. S.; Avarbock D.; Avarbock A.; Helm D.; McColm A. A.; Duncan K.; Lonsdale J. T.; Rubin H. Inhibitors of type II NADH:menaquinone oxidoreductase represent a class of antitubercular drugs. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 4548–4553. 10.1073/pnas.0500469102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen J. E.; Vergmann B. The antibacterial effect of selected phenothiazines and thioxanthenes on slow-growing mycobacteria. Acta Pathol Microbiol Immunol Scand B 1986, 94, 393–398. 10.1111/j.1699-0463.1986.tb03073.x. [DOI] [PubMed] [Google Scholar]
- Kinnings S. L.; Liu N.; Buchmeier N.; Tonge P. J.; Xie L.; Bourne P. E. Drug discovery using chemical systems biology: repositioning the safe medicine Comtan to treat multi-drug and extensively drug resistant tuberculosis. PLoS Comput. Biol. 2009, 5, e1000423 10.1371/journal.pcbi.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novita B. D.; Ali M.; Pranoto A.; Soediono E. I.; Mertaniasih N. M. Metformin induced autophagy in diabetes mellitus - Tuberculosis co-infection patients: A case study. Indian J. Tuberc 2019, 66, 64–69. 10.1016/j.ijtb.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Padmapriyadarsini C.; Bhavani P. K.; Natrajan M.; Ponnuraja C.; Kumar H.; Gomathy S. N.; Guleria R.; Jawahar S. M.; Singh M.; Balganesh T.; Swaminathan S. Evaluation of metformin in combination with rifampicin containing antituberculosis therapy in patients with new, smear-positive pulmonary tuberculosis (METRIF): study protocol for a randomised clinical trial. BMJ. Open 2019, 9, e024363 10.1136/bmjopen-2018-024363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H.-Y.; Wang N.; Li S.; Hong M.; Wang X.; Feng Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxidative Medicine and Cellular Longevity 2016, 2016, 1–16. 10.1155/2016/2795090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.; Alam A.; Grover S.; Pandey S.; Tripathi D.; Kumari M.; Rani M.; Singh A.; Akhter Y.; Ehtesham N. Z.; Hasnain S. E. Peptidyl-prolyl isomerase-B is involved in Mycobacterium tuberculosis biofilm formation and a generic target for drug repurposing-based intervention. NPJ. Biofilms Microbiomes 2019, 5, 3. 10.1038/s41522-018-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenum S.; Tonby K.; Rueegg C. S.; Rühwald M.; Kristiansen M. P.; Bang P.; Olsen I. C.; Sellæg K.; Røstad K.; Mustafa T.; Taskén K.; Kvale D.; Mortensen R.; Dyrhol-Riise A. M. A Phase I/II randomized trial of H56:IC31 vaccination and adjunctive cyclooxygenase-2-inhibitor treatment in tuberculosis patients. Nat. Commun. 2021, 12, 6774. 10.1038/s41467-021-27029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen R.; Clemmensen H. S.; Woodworth J. S.; Therkelsen M. L.; Mustafa T.; Tonby K.; Jenum S.; Agger E. M.; Dyrhol-Riise A. M.; Andersen P. Cyclooxygenase inhibitors impair CD4 T cell immunity and exacerbate Mycobacterium tuberculosis infection in aerosol-challenged mice. Commun. Biol. 2019, 2, 288. 10.1038/s42003-019-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Munnik M.; Lohans C. T.; Lang P. A.; Langley G. W.; Malla T. R.; Tumber A.; Schofield C. J.; Brem J. Targeting the Mycobacterium tuberculosis transpeptidase Ldt(Mt2) with cysteine-reactive inhibitors including ebselen. Chem. Commun. (Camb) 2019, 55, 10214–10217. 10.1039/C9CC04145A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battah B.; Chemi G.; Butini S.; Campiani G.; Brogi S.; Delogu G.; Gemma S. A Repurposing Approach for Uncovering the Anti-Tubercular Activity of FDA-Approved Drugs with Potential Multi-Targeting Profiles. Molecules 2019, 24, 4373. 10.3390/molecules24234373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues L.; Cravo P.; Viveiros M. Efflux pump inhibitors as a promising adjunct therapy against drug resistant tuberculosis: a new strategy to revisit mycobacterial targets and repurpose old drugs. Expert Rev. Anti Infect Ther 2020, 18, 741–757. 10.1080/14787210.2020.1760845. [DOI] [PubMed] [Google Scholar]
- Kanehiro Y.; Tomioka H.; Pieters J.; Tatano Y.; Kim H.; Iizasa H.; Yoshiyama H. Identification of Novel Mycobacterial Inhibitors Against Mycobacterial Protein Kinase G. Front Microbiol 2018, 9, 1517. 10.3389/fmicb.2018.01517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogi K. M.; Lien K. A.; Johnson J. R.; Krogan N. J.; Stanley S. A. The Tyrosine Kinase Inhibitor Gefitinib Restricts Mycobacterium tuberculosis Growth through Increased Lysosomal Biogenesis and Modulation of Cytokine Signaling. ACS Infect Dis 2017, 3, 564–574. 10.1021/acsinfecdis.7b00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain T.; Zhao D.; Shah S. Z. A.; Sabir N.; Wang J.; Liao Y.; Song Y.; Dong H.; Hussain Mangi M.; Ni J.; Yang L.; Zhou X. Nilotinib: A Tyrosine Kinase Inhibitor Mediates Resistance to Intracellular Mycobacterium Via Regulating Autophagy. Cells 2019, 8, 506. 10.3390/cells8050506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues L.; Ainsa J. A.; Amaral L.; Viveiros M. Inhibition of drug efflux in mycobacteria with phenothiazines and other putative efflux inhibitors. Recent Pat Antiinfect Drug Discov 2011, 6, 118–127. 10.2174/157489111796064579. [DOI] [PubMed] [Google Scholar]
- Gupta S.; Cohen K. A.; Winglee K.; Maiga M.; Diarra B.; Bishai W. R. Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 574–576. 10.1128/AAC.01462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato L. S.; Rosa P. S.; Ferreira J. d. S.; Neumann A. d. S.; da Silva M. G.; do Nascimento D. C.; Soares C. T.; Pedrini S. C. B.; Oliveira D. S. L. d.; Monteiro C. P.; Pereira G. M. B.; Ribeiro-Alves M.; Hacker M. A.; Moraes M. O.; Pessolani M. C. V.; Duarte R. S.; Lara F. A. Statins increase rifampin mycobactericidal effect. Antimicrob. Agents Chemother. 2014, 58, 5766–5774. 10.1128/AAC.01826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiga M.; Agarwal N.; Ammerman N. C.; Gupta R.; Guo H.; Maiga M. C.; Lun S.; Bishai W. R. Successful shortening of tuberculosis treatment using adjuvant host-directed therapy with FDA-approved phosphodiesterase inhibitors in the mouse model. PLoS One 2012, 7, e30749 10.1371/journal.pone.0030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leukes V.; Walzl G.; du Plessis N. Myeloid-Derived Suppressor Cells as Target of Phosphodiesterase-5 Inhibitors in Host-Directed Therapeutics for Tuberculosis. Front Immunol 2020, 11, 451. 10.3389/fimmu.2020.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soolingen D.; Hernandez-Pando R.; Orozco H.; Aguilar D.; Magis-Escurra C.; Amaral L.; van Ingen J.; Boeree M. J. The Antipsychotic Thioridazine Shows Promising Therapeutic Activity in a Mouse Model of Multidrug-Resistant Tuberculosis. PLoS One 2010, 5, e12640 10.1371/journal.pone.0012640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveiros M.; Martins M.; Couto I.; Rodrigues L.; Machado D.; Portugal I.; Amaral L. Molecular tools for rapid identification and novel effective therapy against MDRTB/XDRTB infections. Expert Rev. Anti Infect Ther 2010, 8, 465–480. 10.1586/eri.10.20. [DOI] [PubMed] [Google Scholar]
- Brindha S.; Sundaramurthi J. C.; Velmurugan D.; Vincent S.; Gnanadoss J. J. Docking-based virtual screening of known drugs against murE of Mycobacterium tuberculosis towards repurposing for TB. Bioinformation 2016, 12, 368. 10.6026/97320630012368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. T.; Stenger S.; Tang D. H.; Modlin R. L. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol 2007, 179, 2060–2063. 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- Liu P. T.; Stenger S.; Li H.; Wenzel L.; Tan B. H.; Krutzik S. R.; Ochoa M. T.; Schauber J.; Wu K.; Meinken C.; Kamen D. L.; Wagner M.; Bals R.; Steinmeyer A.; Zügel U.; Gallo R. L.; Eisenberg D.; Hewison M.; Hollis B. W.; Adams J. S.; Bloom B. R.; Modlin R. L. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Mily A.; Rekha R. S.; Kamal S. M.; Arifuzzaman A. S.; Rahim Z.; Khan L.; Haq M. A.; Zaman K.; Bergman P.; Brighenti S.; Gudmundsson G. H.; Agerberth B.; Raqib R. Significant Effects of Oral Phenylbutyrate and Vitamin D3 Adjunctive Therapy in Pulmonary Tuberculosis: A Randomized Controlled Trial. PLoS One 2015, 10, e0138340 10.1371/journal.pone.0138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S.; Ginindza S.; Beattie T.; Arjun N.; Likoti M.; Edward V. A.; Rassool M.; Ahmed K.; Fielding K.; Ahidjo B. A.; Vangu M. D. T.; Churchyard G. Adjunctive host-directed therapies for pulmonary tuberculosis: a prospective, open-label, phase 2, randomised controlled trial. Lancet Respir Med. 2021, 9, 897–908. 10.1016/S2213-2600(20)30448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman K.; Finer R.; Donovan S.; Elder R. C.; Doi J.; Ratliff D.; Ng K. Intestinal uptake and metabolism of auranofin, a new oral gold-based antiarthritis drug. Science 1984, 225, 430–432. 10.1126/science.6429854. [DOI] [PubMed] [Google Scholar]
- Harbut M. B.; Vilchèze C.; Luo X.; Hensler M. E.; Guo H.; Yang B.; Chatterjee A. K.; Nizet V.; Jacobs W. R. Jr.; Schultz P. G.; Wang F. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 4453–4458. 10.1073/pnas.1504022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong T. M.; Heymsfield S. B. Cannabinoid-1 receptor inverse agonists: current understanding of mechanism of action and unanswered questions. Int. J. Obes (Lond) 2009, 33, 947–955. 10.1038/ijo.2009.132. [DOI] [PubMed] [Google Scholar]
- Ramesh R.; Shingare R. D.; Kumar V.; Anand A.; B S.; Veeraraghavan S.; Viswanadha S.; Ummanni R.; Gokhale R.; Srinivasa Reddy D. Repurposing of a drug scaffold: Identification of novel sila analogues of rimonabant as potent antitubercular agents. Eur. J. Med. Chem. 2016, 122, 723–730. 10.1016/j.ejmech.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Li J.; Yang X.; Wu L.; Zhang J.; Yang Y.; Zhao Y.; Zhang L.; Yang X.; Yang X.; Cheng X.; Liu Z.; Jiang B.; Jiang H.; Guddat L. W.; Yang H.; Rao Z. Crystal Structures of Membrane Transporter MmpL3, an Anti-TB Drug Target. Cell 2019, 176, 636–648. 10.1016/j.cell.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Barnes N. C.; Pujet J. C. Pranlukast, a novel leukotriene receptor antagonist: results of the first European, placebo controlled, multicentre clinical study in asthma. Thorax 1997, 52, 523–527. 10.1136/thx.52.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N.; Jena P. K.; Pradhan S. K. Arabinosyltransferase C enzyme of Mycobacterium tuberculosis, a potential drug target: An insight from molecular docking study. Heliyon 2020, 6, e02693 10.1016/j.heliyon.2019.e02693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troudt J.; Creissen E.; Izzo L.; Bielefeldt-Ohmann H.; Casonato S.; Manganelli R.; Izzo A. A. Mycobacterium tuberculosis sigE mutant ST28 used as a vaccine induces protective immunity in the guinea pig model. Tuberculosis (Edinb) 2017, 106, 99–105. 10.1016/j.tube.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietzerbin J.; Das B. C.; Petit J. F.; Lederer E.; Leyh-Bouille M.; Ghuysen J. M. Occurrence of D-alanyl-(D)-meso-diaminopimelic acid and meso-diaminopimelyl-meso-diaminopimelic acid interpeptide linkages in the peptidoglycan of Mycobacteria. Biochemistry 1974, 13, 3471–3476. 10.1021/bi00714a008. [DOI] [PubMed] [Google Scholar]
- Pushkaran A. C.; Vinod V.; Vanuopadath M.; Nair S. S.; Nair S. V.; Vasudevan A. K.; Biswas R.; Mohan C. G. Combination of Repurposed Drug Diosmin with Amoxicillin-Clavulanic acid Causes Synergistic Inhibition of Mycobacterial Growth. Sci. Rep 2019, 9, 6800. 10.1038/s41598-019-43201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M. D.; Holzinger E. R.; Li R.; Pendergrass S. A.; Kim D. Methods of integrating data to uncover genotype-phenotype interactions. Nat. Rev. Genet 2015, 16, 85–97. 10.1038/nrg3868. [DOI] [PubMed] [Google Scholar]
- Rode A. K. O.; Kongsbak M.; Hansen M. M.; Lopez D. V.; Levring T. B.; Woetmann A.; Ødum N.; Bonefeld C. M.; Geisler C. Vitamin D Counteracts Mycobacterium tuberculosis-Induced Cathelicidin Downregulation in Dendritic Cells and Allows Th1 Differentiation and IFNγ Secretion. Front Immunol 2017, 8, 656. 10.3389/fimmu.2017.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Toorn R.; Solomons R. S.; Seddon J. A.; Schoeman J. F. Thalidomide Use for Complicated Central Nervous System Tuberculosis in Children: Insights From an Observational Cohort. Clin Infect Dis 2021, 72, e136 10.1093/cid/ciaa1826. [DOI] [PubMed] [Google Scholar]
- Horita Y.; Takii T.; Yagi T.; Ogawa K.; Fujiwara N.; Inagaki E.; Kremer L.; Sato Y.; Kuroishi R.; Lee Y.; Makino T.; Mizukami H.; Hasegawa T.; Yamamoto R.; Onozaki K. Antitubercular activity of disulfiram, an antialcoholism drug, against multidrug- and extensively drug-resistant Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 2012, 56, 4140–4145. 10.1128/AAC.06445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary D.; Marzuki M.; Lee A.; Bouzeyen R.; Singh A.; Gosain T.; Kidwai S.; Grady C.; Tsotetsi K.; Chawla K.; Shihui F.; Lum J.; Gupta S.; Agarwal N.; Tsenova L.; Kumar Y.; Lee B.; Kumar P.; Thakur K. G.; Singh R.; Singha A. Disulfiram Inhibits M. tuberculosis Growth by Altering Methionine Pool, Redo Status and Host-Immune Response. BioRxiv 2021, 277368. 10.1101/2020.09.01.277368. [DOI] [Google Scholar]
- Choi W. H. Evaluation of anti-tubercular activity of linolenic acid and conjugated-linoleic acid as effective inhibitors against Mycobacterium tuberculosis. Asian Pac J. Trop Med. 2016, 9, 125–129. 10.1016/j.apjtm.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Mitra D.; Mukherjee S.; Das A. K. Cyclosporin A binding to Mycobacterium tuberculosis peptidyl-prolyl cis-trans isomerase A–investigation by CD, FTIR and fluorescence spectroscopy. FEBS Lett. 2006, 580, 6846–6860. 10.1016/j.febslet.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Maier L.; Pruteanu M.; Kuhn M.; Zeller G.; Telzerow A.; Anderson E. E.; Brochado A. R.; Fernandez K. C.; Dose H.; Mori H.; Patil K. R.; Bork P.; Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talevi A.; Bellera C. L. Challenges and opportunities with drug repurposing: finding strategies to find alternative uses of therapeutics. Expert Opin Drug Discov 2020, 15, 397–401. 10.1080/17460441.2020.1704729. [DOI] [PubMed] [Google Scholar]
- Li Y. Y.; Jones S. J. Drug repositioning for personalized medicine. Genome Med. 2012, 4, 27. 10.1186/gm326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.