Abstract

H8–BINOL, a partially reduced form of BINOL, is widely employed in a broad array of organocatalyzed asymmetric methodologies. Over the last 25 years, asymmetric organocatalysis has witnessed an incredible improvement, and an advancement still continues to get a single enantio-enriched product. The broad-spectrum applications of H8–BINOL organocatalyst in C–C bond formation, C-heteroatom bond construction, name reactions, pericyclic reactions, and one pot and multicomponent reaction are attracting the attention of researchers. A diversified unique H8–BINOL-based catalyst has been synthesized and screened for catalytic activity. In this Review we frame out the H8–BINOL catalyzed novel discoveries from the last two decades.
1. Introduction
H8–BINOL constitutes newly designed catalysts to control stereochemistry of products. Owing to the economic and scientific significance of asymmetric methodology, enomorous amounts of organocatalyst were screened.1 Among the pool of organocatalysts, H8–BINOL-based organocatalysts are interesting to control the stereochemistry of the product by their unique orientation (Figure 1) in the transition state.2 The sterically controlled transition state guides the researcher to design new catalysts to use in varied asymmetric chemical transformations.3
Figure 1.
H4–BINOL and H8–BINOL.
H8–BINOL-based catalysts have evidently attracted the attention of the scientific community because of their remarkable chemical properties (Bronsted & Lewis acid, base, and dual activity) and wide range of pharmaceutical privileges. H8–BINOL with diversified structural modifications have found applications in several fields such as asymmetric C–H activation, asymmetric C–C & C-heteroatom bond formation, rearrangement reactions, cycloaddition reactions, photoredox catalysis reactions, asymmetric hydrogenations, addition reactions, elimination reactions, and substituition reactions, as well as in well-known name reactions4 summarized in Figure 2.
Figure 2.
Unique applications of H8–BINOL.
Biomolecules and enzymes in biological systems are frequently enantioselective to their binding ligands. All through designing bioactive scaffolds to interact with these targets, stereoselectivity is an important part. Attention is given while exploring structure since chirality is increasingly important. Binding affinity for a chiral drugs can bind targets with unique selectivity. A market survey depicts that about 80% of drugs (4–9) are chiral5 (Figure 3).
Figure 3.
Potent blockbuster chiral drugs.
Axially chiral H8–BINOL catalysts are more flexible than BINOL catalysts. Due to the flexibility in structure they can mold themselves in such a way in the transition state to install excellent enantioselectivity. Chiral BINOL catalyst (13) reduced to H8–BINOL (2) by platinum/palladium catalyst.6 Chiral H8–BINOL (2-3), H8–BINOL phosphoric acid (10), and phosphoramides (11-12) have been widely applied in the past couple of years. Representative catalysts (10–12) with acidic functional groups are shown in (Figure 4).
Figure 4.
H8 BINOL cataysts.
2. Synthesis of H8–BINOL Molecules
H8–BINOL is a reduced form of BINOL organocatalyst possessing the more excellent key topographies of the completely aromatic complements of precursor BINOL. It is distinguished from BINOL by different solubilities, acidities, and racemization outlines as well as different geometries and bite angles. The critical widespread scenerios of this scaffold make it of exact importance to asymmetric organocatalysis. Mostly, the high degree of tunability, conformational rigidity, and C2 symmetry of H8–BINOL 2, make it a comparatively more versatile catalyst. In accumulation of phosphate groups, other types of functionalities can be affixed to the H8–BINOL scaffold to tune its Bronsted acidity like its counterpart precursor fully aromatic BINOL. H8–BINOL-based N-triflyl phosphoramides and disulfonimides have settled as more powerful alternatives to BINOL-CPAs (Figure 4).
Chiral H8–BINOL with metals like titanium, calcium, and aluminum and other metals forms complexes to employ as a chiral Lewis acid which are uniquely used for enantioselective synthesis (Figure 4). H8–BINOL catalysts are synthesized from BINOL 13 by reduction. Preparation is accomplished by multistep synthesis of BINOL and its reduction. The first step comprises alkylation of the hydroxy group of BINOL followed by selective bromination or iodination using n-BuLi. The second step is the Suzuki coupling reaction of dihalogentated BINOL with various substituted aromatic boronic acids. Finally, 2,2′ substituited BINOL is subjected to deprotection converting OMe to hydroxy group by using tribromoboron to get a compound with free hydroxy groups. These hydroxy groups at 2,2' are used for installation of phosphoric acid. After installation of acidic functionalities the BINOL molecule is subjected to partial hydrogenation using platinum oxide at room temperature7 (Scheme 1).
Scheme 1. Reagents and Conditions: (i) MeOH, acetone, reflux, 6 h; (ii) I2, THF, −78 °C, 3 min; (iii) Pd(PPh3)4 (5 mol %), TMEDA, ArB(OH)2 (3 equiv), THF, K2CO3 (1 M), reflux, 3 h; (iv) BBr3, DCM, 0 °C; (v) POCl3, Pyridine, 80 °C, H2O, HCl, 6 M, 100 °C; (vi) H2, Pd/C (7 mol %), EtOH, 70 °C, 1 h.
3. Applications of H8–BINOL Molecule
The development in synthesis of a varied scaffold with innumerable functionalities of enantiopure and thermally stable (R)- and (S)-H8–BINOL catalysts 2–3 and 10–12 initiates a key milestone in the field of asymmetric organocatalysis. Principally, a great assortment of chiral binaphthyl-based chiral phosphorus acid compounds 10–12, H8–BINOL installed metal complexes having Lewis acid nature, have been given significant weight owing to their adaptability as ligands in enantioselective metal catalyzed reactions, addition reactions, and substitution reactions. The application of these organocatalysts for the preparation of optically active products with the anticipated enantiopurity has been widely explored. This Review highlights that in addition to phosphate, other sorts of functionalities can be affixed to the BINOL scaffold to augment the Bronsted acidity of the catalyst. For example, BINOL-based N-triflyl phosphoramides and disulfonimides, dual functionalities as Lewis acid & base, and metal complexes with Lewis acidity have recently been developed as more powerful alternatives to CPAs (Figure 4). The utmost applicable concepts and books concerning wide-ranging synthetic reactions for binaphthyl-based organocatalysts having acidic and basic functionalities have been published and added in this review. Numerous natural building blocks have been extensively used for the development of chiral ligand libraries, though intrinsic margins were frequently executed by the natural presence of a single enantiomer in the last few decades. Still, the nascent synthetic availability of H8–BINOL in both (R) and (S) enantiomerically pure forms has guided the scientific community towards a massive development in synthesis of new materials and chiral ligands as organocatalyst (Figure 3). Currently, BINOL and more preferably the partially hydrogenated H8–BINOL (Figure 4) are considered typical and unique ligands, systematically used in a range of asymmetric catalytic reactions. The most selective ones are carbon–carbon and carbon–heteroatom bond formation reactions, asymmetric α-allylation and alkylation, hydrogenation, and name reactions including Mannich and Strecker, cycloaddition like Diels–Alder, Aldol, Friedel–Crafts, Robinsons annulation, Michael addition and olefin methathesis, asymmetric C–H activation reactions as well as in heteroatom-transfer reactions, such as epoxidation and Baeyer–Villiger, among rearrangement reactions. The imperious need to develop active and selective systems with the prospect of catalyst recycling and, by the fact that there are no reviews covering this subject, exhaustive examples of the preparation of functionalized ligands is engrossing.8
Hu and his group (Scheme 2) invented a reaction catalyzed with iridium metal and H8–BINOL ligand (21a–21b) for enantioselective hydrogenation of sterically hindered N-arylimines 19a and 19b. Phosphoramidite ligands (21a–21b) have been developed for utilization in enantioselective hydrogenation. The electron donating and withdrawing groups of the phenyl ring of of the substituent exhibited a partial impact on the enantioselectivity. Methyl 2-(2,6-dimethylphenylamino)propanoate 20d can be used as an intermediate for the preparation of the chiral herbicide (1S)-Metolachlor. Their process optimization depicts that KI is a key additive in the presence of dichloromethane as the solvent. The catalytic system employed by Hu and his group gave high enantioselectivities up to 99%. The reduced products are 20a–20c from the hydrogenation of a variety of sterically hindered N-arylimines.9
Scheme 2. Reagents and Conditions: (i) Ir[CODCl2]2 (0.5 mol %), catalyst 21a–b (1.1 mol %), KI (5 mol %), H2 (20 bar), CH2Cl2, rt, 24 h.
Antilla and his group (Scheme 3; Figure 5) brilliantly developed a protocol utilizing an organocatalyst, a 4-tert-butyl phenyl substituted (R)-H8-BINOL calcium phosphate 25, for enantioselective amination of 3-aryl-2-benzofuranones 22 with dibenzylazo dicarboxylate 23. The appeal of the green protocol is the formation of a C–N bond α to the carbonyl group. The product of this reaction, chiral 3-amino-2-benzofuranone, is an intermediate for medicinally important products like (−) fumimycin (antibacterial), sorbicillactone A (antileukemic), and hopeahainol A (acetylcholinesterase inhibitor). The process optimization study reported trifluoromethylbenzene as a best solvent along with molecular sieves of 4 Å. The reaction has broad substrate scope, requires short reaction times (30 min), and requires a relatively low catalyst loading (1–10 mol %). This conversion is superficial via intermediate 26 having a high degree of atom economy, which gave products 24a–24c with good yields and high enantioselectivities (up to 99%). This reaction has excellent ee and a broad substrate scope with mild reaction conditions.10
Scheme 3. Reagents and Conditions: (i) Catalyst 25 (1 mol %), trifluoromethylbenzene, 4 Å molecular sieves, 30 min, rt.
Figure 5.
Asymmetric aryl addition reactions for the synthesis of chiral alcohol 28 were reported by Gau and his group (Scheme 4). Addition of triaryl aluminum in THF solvent to aldehydes 27 catalyzed by the Ti(IV) catalyst of (R)-H8–BINOL 29 produced 28 with varied substituents. In this protocol, substituted arylaluminum reagents were used to afford chiral alcohols 28a–28c in excellent enantioselectivities (up to 97%). The catalytic system was applied to the most diversified aldehydes with electron donating and withdrawing groups. Moreover, diarylmethanols 28 in both R- and S-configurations can be obtained. This protocol is superior to the organozinc compound which is used for the synthesis of diaryl alcohol, propargyl alcohol, and allyl alcohols. These alcohols are very useful intermediates for natural products (macrolide antibiotics). Notably, the catalytic system is extremely efficient with respect to times when the suppression of the background reaction is not required.11
Scheme 4. Reagents and Conditions: (i) Al(Ar)3, THF (1.2 equiv), H8–BINOL catalyst 29 (10 mol %), Ti(O-i-Pr)4 (1.25 equiv), THF/hexane, 0 °C, 10 min.
Da et al. successfully developed (Scheme 5; Figure 6) a highly enantioselective catalytic arylation of substituited aldehyde 31 consuming aryl bromide 30 as the starting aryl source. The catalyst (S)-H8–BINOL–Ti(Oi-Pr)233 complex was used under mild reaction conditions in one pot.12 TMEDA works as a supportive additive to stop racemization. The transmetalation strategy is used to transfer aryllithiums to less reactive arylmetals like titanium is less costly compared to use of aryl Grignard and zinc reagents. The solvent and additive screening gives the best result with THF/hexane as a solvent and TMEDA as an additive. The mechanistic pathway of the reaction demonstrates that an intermediate 33a is formed which gives products 32a–32c with 99% yield and 96% ee.
Scheme 5. Reagents and Conditions: (i) n-BuLi/-78 °C, AlCl3/0 °C–rt, TMEDA, Ti(OiPr)4, catalyst (S)-H8–BINOL 33.
Figure 6.
An efficient rhodium-catalyzed enantioselective arylation by reacting arylboroxines (ArBO)3 with isatin-derived N-Boc/trityl ketimine 34 has been developed by Xu and his group (Scheme 6). Essential enantioselectivity is accomplished by engaging the H8–BINOL-derivative phosphite-olefin 36 as a chiral organocatalyst. The screening of various BINOL and H8–BINOL catalysts reveals that H8–BINOL is the most versatile catalyst. Base and solvent study examined with TEA, DBU, DABCO, and DIPEA showed the use of 3 equiv of DIPEA and dioxane giving the adduct in 82% yield and 96% ee. This method allowed access to a broad variety of valuable tetrasubstituted 3-amino-2-oxindole derivatives 35 in excellent yield (up to 98%) with excellent enantioselectivities.13
Scheme 6. Reagents and Conditions: (i)[Rh(COE)2Cl]2, H8–BINOL catalyst 36, (ArBO)3.
α-Diazoesters are basic nucleophiles for enantioselective C–C bond formation in strategies with the retention of diazo functionality. Singh and his group described (Scheme 7; Figure 7) enantioselective syntheses of phthalides 40 by using a H8–BINOL 40a-based chiral Bronsted acid as an organocatalyst. The reaction has been accomplished via tandem Mannich lactamization and aldol-lactonization reactions, respectively, via intermediate 41a. The optimization study showed that H8–BINOL is a better catalyst than BINOL, with dichloethane as solvent at 0 °C. Total synthesis of (S)-PD 172938 (dopamine D4 ligand) has been established by using this protocol.14a A variety of enantioenriched phthalides 40a–40c (up to 85% ee) containing α-diazoesters were afforded in excellent yields.14b
Scheme 7. Reagents and Conditions: (i) catalyst 41 (5 mol %), DCE, 4 Å molecular sieves, −20 °C.
Figure 7.
Hu and his group grasped (Scheme 8) Ir-catalyzed hydrogenation of 3-alkyl-2-arylquinolines 42 by using chiral phosphine–phosphoramidite ligand 44 with a (Sa)-3,3′-dimethyl H8-naphthyl moiety. The hydrogenation by organocatalyst 44 proceeded in cis-diastereoselectivity and gave the hydrogenation products in >20:1 dr. The substitution pattern on phenyl rings R1 and R2 slightly affected the enantioselectivty and yield. The hydrogenation protocol exhibited broad functional group tolerance, furnishing a wide range of optically active 2,3-disubstituted tetrahydroquinolines 43a–43d in up to 96% ee and with continuous cis-diastereoselectivity.15
Scheme 8. Reagents and Conditions: (i) [Ir(COD)]Cl]2 (0.5 mol %), L = 44 (1.1 mol %), H2 (10 bar), 1,4 dioxane, 80 °C, 24 h.
Xu and his co-worker invented (Scheme 9; Figure 8) an Rh-catalyzed asymmetric arylation of 1,2,5-thiadiazolidine 1,1-dioxide 46 type cyclic ketimines utilizing chiral phosphite-based hybrid olefin ligand 48 and aromatic boronic acid. The eclectic diversity of boronic acids with different electronic and steric demands was effectively reacted with a sequence of cyclic ketimines. Sterically hindered arylboronic acids with ortho substituents on the phenyl rings as well as more inspiring heteroarylboronic acids were well abided. The optimization study showed that the reaction performed with 1.0 equiv of K3PO4 (2.5 M) in toluene gives better results. The reactions generate quaternary carbon containing gem-diaryl-substituted sulfahydantoins 46a–46d and 4-ethoxy-2,3-dihydro-1,2,5-thiadiazole 1,1-dioxides 47 with excellent enantioselectivities.16 The mechanism of reaction favors the formation of less hindered Re-face intermediate 50.
Scheme 9. Reagents and Conditions: (i) [Rh(COE)2Cl]2, Ar2B(OH)2; (ii) LAH, catalyst 48.
Figure 8.
Cascade reaction of 4-aminoindoles 52 with β,γ-unsaturated α-keto esters 51 via C-7 Friedel–Crafts alkylation/N-hemiacetalization catalyzed by H8–BINOL catalyst 54 has been developed by Antilla and his group (Scheme 10; Figure 9). Using a chiral magnesium H8–BINOL-derived bis(phosphate) complex as organocatalyst, the functionalized 1,7-annulated indole scaffolds 53a–53c are obtained in high yields (up to 98%) and excellent enantioselectivities (up to 99%) and diastereoselectivities (up to >20:1) under mild reaction conditions.17 The solvent screening and metal salt with H8–BINOL complex showed that diethyl ether and Mg complexes are the best solvent and complex, respectively, which results in high enantioselectivity and yield. The N-methylated indole substrate was also verified, but it could not provide the C-7-functionalized product, thus indicating that 1-H on the indole substrates is the key to the process. The proposed mechanism of the reaction shows that intermediate 55 was formed and gets converted into product 50 with required enantioselectivity.
Scheme 10. Reagents and Conditions: (i) catalyst 54 (10 mol %), 4 Å molecular sieves, Et2O, rt.
Figure 9.
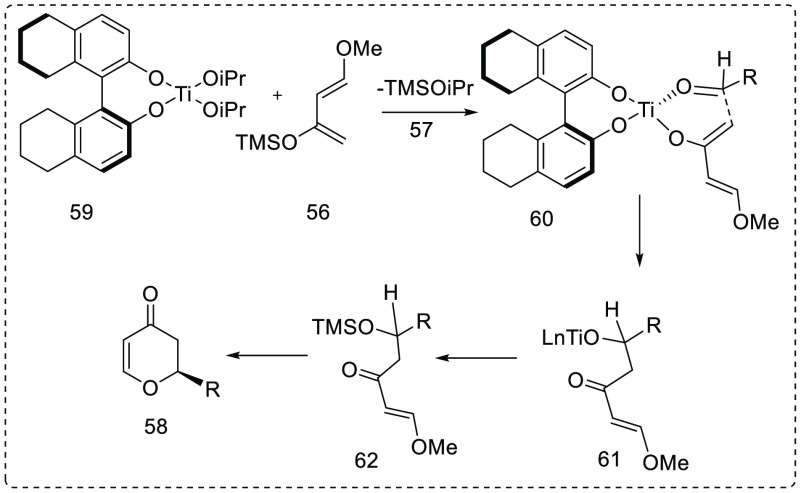
Jiang and his group investigated (Scheme 11; Figure 10) the hetero-Diels–Alder reaction between substituted aldehyde 58 and Danishefsky’s diene 56 using readily accessible Ti(IV)-H8–BINOL (TiHBOL) complex 60 leading to products 2,3-dihydro-2-phenyl-4-pyranone 58 with very high enantioselectivity (up to 99% ee) and yield (92%).18 Screening of alkaloids such as Ti(OiPr)4, Al(OiPr)3, Zr(OEt)4, Sm(OiPr)3, Yb(OiPr)3, and Ce(OiPr)3 reported Ti(OiPr)4 improved yield of reaction. Ratio of Ti(OiPr)4 to ligand is 1:1.5 gave high enantioselectivity. All reactions were carried out in toluene using 20 mol % of Ti–H8–BINOL catalyst at 0 °C temperature.
Scheme 11. Reagents and Conditions: (i) catalyst 59 (20 mol %), TFA, toluene, 0 °C.
Figure 10.
Zhou and his group prepared (Scheme 12; Figure 11) a palladium catalyst supported by a new, (R)-H8–BINOL-derived monophosphine 66. Arylation of 1-naphthyl triflate 63 by efficient coupling of an O-trimethylsilyl ketene acetal 64 produces tertiary centers with high ee. The usefulness of the reaction was demonstrated in a gram-scale synthesis of (S)-Naproxen in 92% enantiomeric excess.19 The mechanism of reaction follows the formation of 62 which reacts with LiOAc to give 68. Intermediate 68 reacts with precursor 64 and forms complex 67 which finally gets converted into product 65.
Scheme 12. Reagents and Conditions: (i) 2% PDMe2(TMEDA), 2.4% catalyst 66, LiOAc; (ii) TFA.
Figure 11.
Gong and his group described (Scheme 13) a protocol employing chiral H8–BINOL catalyst 73 as a Bronsted acid. Direct asymmetric Mannich reaction of cyclic ketones 69 with aldimines yielded a product formed by condensation of 70 and 71. The Mannich reaction gave chiral β-amino carbonyls 72a–72c in high yields with excellent enantioselectivities up to 98% and high diastereomeric ratios up to 98/2 diastereomeric ratio.20 The stereochemical results influenced by electronic properties of the substituent on benzaldehyde precursor 71. Electron donating groups decreases the enantioselectivity. An aliphatic aldehyde follows Mannich reaction with 84/16 dr and 75% ee. Tetrahydropyranone (X = CH2) and N-Boc-protected piperidin-4-ones (X = N-Boc) 69 are both highly reactive toward the imine generated in situ from para-nitrobenzaldehyde 70 and phenylamine 71, leading to the formation of anti-Mannich products with 90% yield and 91% ee, respectively.
Scheme 13. Reagents and Conditions: (i) catalyst 73 (0.5–5 mol %), PhCH3, 0 °C.
Rueping et al. and his group (Scheme 14) reported synthesis of aminohydrazones 76 via enantioselective Bronsted acid 77 catalyzed addition of methyleneaminopyrrolidine 74 to N-Boc imines 75. The matching aminohydrazones 76a–76c have been synthesized in good yields up to 90% ee.21N-Boc-protected aldimine and the pyrrolidine-derived hydrazine give better yield than N-benzyloxycarbonyl- or N-benzoyl-protected aldimines. The process optimization study reported chloroform to be a better solvent at 0 °C using 10 mol % catalyst. The process optimization study screened N-protection of imine with CO2tBu, CO2tCH2Ph, COPh, CH2Ph, 4F-PhCH2, and 4Br-PhCH2 showed that CO2tBu protected imine gives better selectivity. Solvent screening reported that trifluorobenzne is supportive for the reaction.
Scheme 14. Reagents and Conditions: (i) catalyst 77, CHCl3, 0 °C.
Gau and his group (Scheme 15) synthesized diaryl methanols 81a–81b and triarylmethanols 82a–82b in excellent yield with high enantioselectivity. These products formed by reacting AlArEt2(THF) 80 to aldehydes 78 or ketone 79. The titanium(IV) complex of (R)-H8–BINOL catalyst 83 is used for this transformation.22 A screening study of the reaction depicted that the steric hindrance of the substrates had an effect on the reactivity of phenyl transfer to ketones. The phenyl addition to hindered ketones required more times to give products in advanced yields.
Scheme 15. Reagents and Conditions: (i) catalyst 83 (10 mol %), 1.5 equiv Ti(OiPr)4, toluene, 0 °C; (ii) cat. 83 (10 mol %), Ti(OiPr)4 (3.5 equiv), toluene, 0 °C.
Gong and his group (Scheme 16; Figure 12) discovered that the first organocatalytic asymmetric Biginelli reaction using chiral phosphoric acid, derived from H8–BINOL 88, afforded the reaction in high yields of chiral 3,4-dihydropyrimidin-2-(1H)-ones 87a–87c up to 97% ee. Varied substrates, including aldehydes 84, β-keto eters 86, and urea derivatives 85 could be used in this protocol.23 Validation of the reaction protocol improved yield and enantioselectivity slightly by increasing temperature and reaction time in the presence of optimized dichloromethane as a solvent. H8–BINOL catalyst with various substituents at the 3,3′ positions showed that subtituents other than naphthyl worked well.
Scheme 16. Reagents and Conditions: (i) catalyst 88 (10 mol %), CH2Cl2, 250 °C.
Figure 12.
Han and his group (Scheme 17; Figure 13) developed a chiral ligand 92-based protocol for enantioselective synthesis of chiral indolines 91a–91c. The present invention describes a chiral anion-mediated asymmetric Heck/Tsuji–Trost reaction of aryl iodides 89 and 1,3-dienes 99 to afford higher yields and enantioselectivities.24 Chiral anion-mediated chemistry employing chiral phosphate anions as the sole chiral source to generate enantioselective C–C bond via Heck-type reaction was established in this protocol. The precursor for this reaction modified by replacing the aryl diazonium salt with aryl halide enhanced the synthetic utility of the present protocol.
Scheme 17. Reagents and Conditions: (i) Pd(PPh3)4, catalyst 92, Ag2CO3.
Figure 13.
The steric hindrance of substituents at the 3,3′-positions of the binaphthyl backbone in R-BINOL and SPINOL improves yield. The protocol improved further by using H8–BINOL-based phosphoric acids. Switching silver carbonate to other metal carbonates, such as K2CO3, Na2CO3, and PbCO3, did not improve enantioselectivity. An optimization study reported that addition of a small amount of DMSO to the system significantly improve the outcome of the reaction up to 81%.
Zhou and his group (Scheme 18) have successfully developed a methodology for synthesis of pyridyl containing diaryl methanol 95 using a titanium(IV) catalytic system of (R)-H8–BINOLate 96 up to 98% ee from easily available precusors pyridyl aluminum reagents 94 and aldehydes 93.25 Various arylating agents like arylboronic acids, arylzinc, arylaluminum, aryltitanium, aryl lithium, and aryl Grignard reagents were employed for asymmetric protocols. However they gave excellent reports with Ti(OiPr)4. Diverse chiral ligands were tested for asymmetric addition reactions across the carbon oxygen double bond. Comparative study presented that the modified (S)-BINOL derivatives afforded the addition reactions with lower enantioselectivity than (R)-H8–BINOL. In the present scheme (R)-H8–BINOL is the most efficient to afford in 91% yield with excellent enantioselectivity of 90%. The dihedral angle in the transition state of the reaction of H8–BINOL catalyst compared with BINOL is lower. This is the reason that asymmetric addition at aldehydes by a nucleophile gives excellent results. Lowering the temperature up to −10 °C did not recover the enantioselectivity of the product. Aromatic aldehydes with electron withdrawing or electron donating substituents at the para- or meta- position afforded the corresponding alcohols in high yields.
Scheme 18. Reagents and Conditions: (i) 10 mol % catalyst 96, Ti(iOP)4, toluene, 0 °C, 12 h.
Chan and his group described (Scheme 19) the 1,4-addition of dialkylzinc 98 to 2-cyclopentenone, 2-cyclohexenone, and 2-cycloheptenone 97 to yield 3-methyl and ethyl substituted cyclonone 99a–99d with 99% ee by using chiral aryl diphosphite ligand 100 derived from H8-binaphthol.26 Deploying (CuOTf)2·C6H6 as the metal source, 100 as the ligand, and diethyl ether as the solvent delivered the desired product up to 98% yield and 97% ee. An optimized protocol provides a key step en route to anticarcinogenic clavularin B. The ring size also affects the yield of the reaction. Smaller rings gives lower yield and enantioselectivity.
Scheme 19. Reagents and Conditions: (i) (CuOTf)2·C6H6 (1 mol %), catalyst 100 (2 mol %), R2Zn, Et2O, −30 °C.
Krische and his group developed a protocol (Scheme 20) for the chiral-anion-dependent inversion of diastereo- and enantioselectivity in butadiene 102 hydrohydroxy alkylation with alcohol 101 to form products of carbonyl syn-crotylation 103. The observed diastereo- and enantioselectivity is opposite (anti-crotylation) to that observed using H8–BINOL-derived phosphate counterions 104 in combination with DPPF or even (S)-SEGPHOS.27 The importance of this protocol is that for the first time the X-ray crystal structure of a ruthenium complex modified by a chiral phosphate counterion is interpreted.
Scheme 20. Reagents and Conditions: (i) RuH2(CO)(PPh3)3 (5 mol %), (S) SEGPHOS (5 mol %), catalyst 104 (10 mol %).
Chan and his group have developed a protocol (Scheme 21) for asymmetric hydrogenation of enamides 105 leading to α-arylethylamine derivatives 106a–106d with excellent enantioselectivities 99.0% ee using chiral bisaminophosphine ligand 107 and Rh.28 It was noted that the enantioselectivity of the catalyst (R)-4, 107 was sensitive to the solvent used. Higher ee values of 106 were achieved in protic and/or polar solvents. The hydrogen pressure had very little effect on the enantioselectivity. For example, in the hydrogenation of 105 using Rh-(R)-4 catalyst in THF, the following results were obtained: PH2 = 14.5 psi, ee = 92.1%; PH2 = 100 psi, ee = 90.7%; PH2 = 1000 psi, ee = 89.5%. Substitution at the meta- or para-position of the phenyl ring of the parent enamide 105 did not give much influence on the enantioselectivity for the products.
Scheme 21. Reagents and Conditions: (i) [Rh(R)-4(COD)]BF4, catalyst 107.
Shi and his group developed (Scheme 22) a catalytic asymmetric allylic and homoallylic C–H diamination of terminal olefins 108 using di-tert-butyldiaziridinone 109 and H8–BINOL-derived phosphorus amidite ligand 111, giving diamination products 110a–110c in good yields with high regio-, diastereo-, and enantioselectivities.29 The ee was determined by chiral GC (Chiraldex B-DM column) unless otherwise stated. The ee was determined by chiral HPLC (Chiralpak AD column) after the removal of t-butyl groups of 110. The (R,R) configuration was determined by comparing the optical rotation with the reported one. The ee was determined by chiral GC (Chiraldex B-DM column) after the removal of t-butyl groups. The ratio was determined by achiral GC (VA-5MS column). The (2R,3S,4R) configuration was determined by the X-ray structure of diamination product after the removal of the TMS group.
Scheme 22. Reagents and Conditions: (i) 5–10 mol %, Pd2(dba)3, catalyst 111 (1:4.4), 65 °C, 6 h.
Gustafson and his group discovered (Scheme 23; Figure 14) new atropisomer selective synthesis of a diarylamine 114 via phosphoric acid catalyzed 114 halogenation of N-aryl quinoids 112 yielding products 113a–113c in upwards of 95:5 er and 90% yield.30 Atropisomerism, or axial chirality, is ubiquitous throughout modern FDA-approved drugs like binimetinib, bosutinib, and a VEGFR inhibitor from Wyeth on behalf of a potentially atropisomeric N-aryl quinoid. Evaluation study of reaction parameters such as temperature 24 °C, time (6–12 h), solvent (toluene + hexane, 1:1), catalyst (5 mol %) loading, and bromination reagent (0.06 mol). The reaction scope proved to be tolerant of diverse aryl substitutions of the 2,4-positions of the aniline. Electron-rich aryl substitutions yield ers above 95:5 and electron-poor aryl substitutions yield ers around 90:10. The present halogenation protocol was acquiescent to chlorination in the presence of a Lewis basic catalyst and NCS (91% yield with 87:13 er) and iodination using NIS (93% yield 80:20 er), although with somewhat attenuated selectivities.
Scheme 23. Reagents and Conditions: (i) catalyst 114, halogen source, NXS, NXP (X = Br).
Figure 14.
Zhang and his co-worker established a protocol (Scheme 24) of enantioselective addition reaction of pyrrole 117/indoles 116 to 3-vinylindoles 115 by applying chiral imidodiphosphoric acid 120 to afford triarylmethane 118 and 119 with a quaternary chiral center. A series of optically active 1,1,1-triarylethmanes bearing quaternary stereocenters 118a–118b and 119a–119b were synthesized in excellent yields up to 99% and enantioselectivities up to 98%.31 The optimization study found that the substrates with electron donating groups have an inverse pattern in comparison to the substrates with halogen or electron withdrawing groups when they contribute in the reaction.
Scheme 24. Reagents and Conditions: (i) catalyst 120, CHCl3, −20 °C, (ii) Cl–CH2CH2Cl, 0–20 °C, 5 Å molecular sieves.
Gong and his group performed (Scheme 25; Figure 15) enantioselective allylic C–H alkylation reaction of allylarenes 122 with pyrazol-5-ones 121 by the cooperative catalyst of a complex of a palladium and chiral H8–BINOL 126a/b to afford chiral N-heterocycles 124 and 125 in high yields and enantioselectivity (up to 96% ee).32 The palladium-catalyzed C–H activation for C–C bond formation needed an oxidant, and the ligands are expected to survive in the oxidized conditions during the catalytic cycle. Most of the phosphine ligands can be very easily oxidized to five-valence phosphorus species, which are unable to accelerate palladium catalysis. This process has limitations for using chiral ligands. Few of the BINOL-derived phosphoramidite ligands are appropriate with quinone-type oxidants (2,5 DMBQ) in the palladium-catalyzed asymmetric allylic C–H oxidation. Introduction of even more electronically poor 3,5-bis(trifluoromethyl)phenyl groups or 4-nitrophenyl substituents at 3,3′-positions of the binaphthyl backbone increases stereoselectivity. Modification of the amine moiety in the phosphoramidite ligands found that the assimilation of a cyclic piperidine moiety significantly enhanced the catalytic efficiency and enantioselectivity. The chiral H8–BINOL phosphoric acid cocatalyst with H8–BINOL phoshoramide showed a considerable effect on the reaction. The scope of the protocol was tolerant to a broad spectrum of allylbenzene derivatives 122 and 123, installed with either an electronically rich or deficient substituent in different substitution patterns, capable of offering excellent enantioselectivity ranging from 87% to 96% ee. Pyrazol-5-one 121 is tolerable to the presence of an electron donating and withdrawing group on the benzyl ring at the C-4 position. The desired products attained excellent yield and enantioselectivity up to 93%.
Scheme 25. Reagents and Conditions: (i) and (ii) catalyst 126a and b (15 mol %), Pd(dba)2 (7.5 mol %), 2,5-dimethyl benzoquinone (1.2 equiv).
Figure 15.
An enantioselective gold(I)-complex-H8–BINOL 131 catalyzed intramolecular [4 + 2]-cycloaddition of allenes 127 and dienes 128 is reported by Toste and his group (Scheme 26). The reactions allow for the asymmetric synthesis of trans hexahydroindenes 129 and pyrrolidine 130 products using C3-symmetric phosphite gold(I) and ortho aryl phosphoramidite gold(I) complexes as catalysts, respectively.33 Chiral catalyst optimization study of bidentate phosphines toward an enantioselective [4 + 2]-cycloaddition failed to produce any of the desired cycloadduct. This study demonstrated that, despite the linear geometry of gold(I) complexes, chiral monodentate phosphorus-based ligands can be employed in asymmetric gold(I) catalysis.
Scheme 26. Reagents and Conditions: (i) L6AuCl cat. 131 (0.5 mol %), AgBF4 (5 mol %); (ii) L6AuCl catalyst 131 (0.5 mol %), AgSbF6 (5 mol %).
Allylic alcohol and amine are synthesized by alkenyl boronates and alkenyl metals which is an important protocol for SP3 and SP2 carbon–carbon single bond formation. Guo and his group described a novel protocol (Scheme 27; Figure 16) for the synthesis of structurally diverse allylic indole alcohols 135 empolying arylglyoxals 133 with 3-vinylindoles 134 in the presence chiral calcium phosphate 136. The catalyst 136 was prepared by reaction of Ca(OH)2 with H8–B-INOL in dichloromethane solvent.34 Phenylglyoxals 133 with a para-substituted phenyl ring with an electron withdrawing or electron donating group did not affect the reaction protocol. The steric effect of ortho-substituents of phenyl could affect the stereoselectivity of the products.
Scheme 27. Reagents and Conditions: (i) catalyst 136 (10 mol %), DCE, 3 Å molecular sieves, 30 °C.
Figure 16.
Catalyst optimization study reported that the absence of phosphoric acid increased the difficulty to control the stereoselectivity. By referencing Hosomi’s work, the author found that CaCl2 was a suitable catalyst for the aldol reaction of silyl enolates and arylgloxals. They envisioned that the chiral alkali or alkaline earth phosphates would be good catalysts for reaction. Chiral phosphoric acid catalyst and its metal complexes with Li, Na, Mg, K, Ca, and Ba were screened, but calcium complex was found to be more efficient in terms of enantioselectivity and yield.
Gong and his group described (Scheme 28) chiral Bronsted acid 141 catalyzed asymmetric aza-hetero-Diels–Alder reaction between aromatic aldimines 138 and α–β unsaturated cyclohexanone 137. Series of chiral phosphoric acid catalysts were prepared from (R)-BINOL and H8–BINOL as a starting material. Both types of catalyst execute asymmetric addition of cyclohexanone 137 with N-PMP-benzaldimine 138 to afford corresponding bridged compounds 139a–139c with expected enantioselectivity.35 The solvent optimization study reported that nonpolar aprotic solvents toluene and m-xylene led to higher yields than their polar counterparts like halogenated solvents. The phosphoric acid 141-catalyzed direct aza Diels–Alder reaction was extended to a series of benzaldimines (138), with an electron donating or electron withdrawing substituent afforded endoisomer 139 in 70–82% yield and with up to 87% enantioselectivities.
Scheme 28. Reagents and Conditions: (i) catalyst 141 (5 mol %), PhCH3, 20 °C.
Larrosa and his group (Scheme 29) synthesized planar-chiral compounds 144a–144c by a catalytic asymmetric direct C–H arylation of (6-arene)-chromium complexes 142 and aromatic iodides 143. The required enantioselectivity developed by using hemilabile ligand 145 H8–BINAP.36 H8–BINAP with various substituents at the 2,2′ position screened like alcohol, ester, and amide reports low enantioselectivity. H8–BINAP with oxidized phosphine at the 2,2′ position produces excellent yield. The methodology was screened with respect to the fluoroarene chromium complex bearing electron donating and withdrawing groups reported excellent results without any group. Mechanistic studies suggested that the reaction proceeds through a Pd/Ag bimetallic double catalytic cycle where the C–H activation is carried out by Ag.
Scheme 29. Reagents and Conditions: Pd(dba)2 (5 mol %), organocatalyst 145 (5.5 mol %), K2CO3 (2.0 equiv), Ag2CO3 (0.65 equiv), Cy2CHCOOH (0.5 equiv).
Guan and his lab have stated a protocol (Scheme 30; Figure 17) for the synthesis of 2-substituted propanamides 149a–149c. The reaction was subjected to pressure to 50 atmospheric of CO in an autoclave. The glass vassel filled with the first palladium iodide (10 mol %), chiral H8–BINOL phospharamide 150 (11 mol %), followed by addition of styrene and various N-substituted anilines was subjected to CO pressure in an autoclave. The reaction followed the asymmetric Markovnikov rule for hydroaminocarbonylation of alkenes 146 with anilines 147 and carbon monoxide 148 for construction of a diverse array of 2-substituted propanamides 149a–149c with high yields (99%) and enantioselectivities (84%).37
Scheme 30. Reagents and conditions: (i) PdI2, catalyst 150, THF, rt.
Figure 17.
Varied catalysts optimized for enantioselectivity showed that a monodentate chiral phosphine ligand showed the best result in terms of reactivity, regioselectivity, and enantioselectivity. Catalysts were further screened with basic phosphoramidite ligand–ligand scaffolds with a range of electronic variation at the 3,3′-positions of the H8–BINOL framework were prepared and screened. Encouragingly, the yield and ee of ligand 150 achieved good results. 4-iBuOPh and phenoxazinyl (POA) substituents at phosphorus atoms afforded excellent results. The optimal reaction conditions for anilines 147 with electron donating substituents, such as alkyl and methoxy, afforded the desired products in high yield (62–98%) and high ee (91–98%).
The sterically hindered anilines like bulky 2,6-dimethylaniline and 2,6-diisopropylaniline were compatible with the optimized reaction conditions. Halogen substituted anilines and strong electron withdrawing groups were tolerated in the reaction to deliver the desired products in high yields and ee’s. The synthetic utility of this Pd-catalyzed asymmetric Markovnikov hydroaminocarbonylation screened for synthesis of intermediates of ibuprofen, naproxen, flurbiprofen, and ketoprofen and were worked out effectively.
Li and his group reported (Scheme 31; Figure 18) a palladium-catalyzed asymmetric [4 + 4] cycloaddition of γ-methylidene-δ-valerolactones 151 with substituited anthranils 152 using H8–BINOL organocatalyst 154 producing functionalized tetrahydrobenzo[b]-azocine derivatives 153a–153c with yield up to 92%. The product obtained with 20:1 distereoselectivity and 99% enantiosectivity.38 The electron donating group on anthanils increases yield, and withdrawing groups reduce the yield of the reaction. The catalyst screened for varied substituents on the nitrogen atom of the P–N bond of catalyst 154 showed that diaryl methane group with methyl substituent on aromatic ring afforeded high enantioselectivity.
Scheme 31. Reagents and conditions: (i) Pd2(dba)3·CHCl3 (2.5 mol %), catalyst 154 (11%), Et3B (20 mol %), xylene/PhCl (1:1), 10 °C, 4 Å molecular sieves.
Figure 18.
Anthranils with various functional groups, such as halides (F, Cl, Br), methyl ester, trifluoromethyl group, nitrile, silyl ether, and benzoate, were compatible, affording the [4 + 4] cycloaddition products n in good to excellent yields, diastereoselectivities, and enantioselectivities (up to 92% yield, 12:1 dr, 99% ee).
Peng and his group (Scheme 32; Figure 19) developed chemoselective catalytic transfer hydrogenation of fluorinated alkynyl ketimines 155a achieved by employing chiral phosphoric acid 157 as catalyst with benzothiazoline 155b as hydride source, providing the corresponding chiral fluorinated propargylamine 156a–156c products.39
Scheme 32. Reagents and Conditions: (i) H8–BINOL catalyst 157 (10 mol %), CH2Cl2, 40 °C, 48 h.
Figure 19.
Under optimal conditions the scope of various substituted fluorinated alkynyl ketimines (156a), N-arylsubstituted substrates with electron donating or electron withdrawing substituent on the aromatic ring, slickly converted to the corresponding products in high yields and excellent enantioslectivities.
Antilla and his group reported (Scheme 33; Figure 20) an enantioselective method for desymmetrization of meso-epoxides 158 using aromatic thiols and aromatic selenium hydrides 160 using metal H8–BINOL phosphates 161 resulting in β-hydroxyl sulfides and selenides 160a–160d obtained in excellent yield with enantioselectivity.40 Historically, a wide range of azide, amine, oxygen, and carbon-centered nucleophiles have been employed for the desymmetrization of meso-epoxides, but the use of thiols remains relatively limited. A recent report of Shibasaki and Sun for the epoxide ring opening using thiol is restricted to particular substrates. Antila and his group used Li–H8–BINOL phosphate could activate epoxides toward nucleophilic addition, generating chiral 1,2-functionalized thiols. Aromatic thiols with electron withdrawing, donating groups, bulky thiols, heteroatom-containing functional groups produces β-hydroxy sulfides in excellent yield and asymmetric induction. Epoxides were screened by using fused six-membered rings, cycloheptene oxide, and cyclopentene oxide cis- and trans-1,4-cyclohexadiene dioxide as a substrate for this reaction, thus obtaining the β-hydroxyl sulfide in excellent yield but only moderate ee. For acyclic epoxide, Li–H8–BINOL phosphate did not yield the desymmetrization product.
Scheme 33. Reagents and Conditions: (i) Li, catalyst 161 (10–20 mol %), p-xylene, 4 Å molecular sieves, 48 h, rt.
Figure 20.
Liu and his group settled an asymmetric version of the Morita-Baylis-Hillman reaction using H8–BINAM organocatalyst. Catalyst H8–BINAM thiourea catalyst 167 was synthesized using H8–BINAM and isothiocyanate (Scheme 34). Asymmetric Morita-Baylis-Hillman reaction adduct was synthesized using 2-cyclohexen-1-one or 2-cyclopenten-1-one 165 with substituted aromatic aldehydes 164. The organocatalyst H8–BINAM was used in combination with 1,4-diazabicyclo[2.2.2]octane (DABCO). The adduct of Morita-Baylis-Hillman reactions 166a–166c obtained up to 79% yield and up to 88% enantiomeric excess.41
Scheme 34. Reagents and Conditions: (i) catalyst 167 (20 mol %), DABCO (20 mol %), toluene, rt, 43–99%.
Masson and his group described (Scheme 35) a chiral phosphoric acid 72 catalyzed electrophilic amination of enecarbamates 168 with dibenzylazodicarboxylate 169 and oxygenated, aromatic ring, heterocycle, or thiol containing nucleophiles 170 affording a wide range of 1,2-disubstituted 1,2-diamines 171a–171c with excellent enantiselectivity.42
Scheme 35. Reagents and Conditions: (i) catalyst 172 (10 mol %), NuH promoter; (ii) Raney Ni, R2O.
Zhang and his group (Scheme 36) developed an aza-Friedel–Crafts reaction between pyrrole 173 and enamides 174 (N-acetyl)/175 (N-tosyl). The H8–BINOL phosphoramide catalyst 178a was used for reaction of pyrrole 173 with enamide 174 employing catalyst 178a to give product 176. Similarly, catalyst 178b was used for the reaction between pyrrole 173 with N-tosyl enamide 175 to afford product 177. The products aryl-(2-pyrrolyl)methanamine 176a–176b and 177a–177b mentioned in the reaction were obtained in excellent yield.43 Optimization of various solvents showed that 96% ee was observed when the solvent was 1,4-dioxane with less chemoselectivity. The yield was improved intensely by adding 5 Å molecular sieves. α-Phenyl-substituted enamides 174 with electron withdrawing or donating groups at the ortho, meta, and para positions of the phenyl rings gave rise to the quaternary carbon centered products 177a–b in high yields with excellent enantioselectivities.
Scheme 36. Reagents and Conditions: (i) 0.2 mol % catalyst 178a; (ii) 0.3 mol % cat. 178b.
Harada and his group developed a protocol (Scheme 37) using 3-aryl H8–BINOL 182 grafted on the surface of silica gel using a hydrosilane derivative as a precursor, and the ensuing silica-supported ligand was employed in the enantioselective alkylation and arylation of aromatic aldehydes 179 in the presence of Ti(OiPr)4. The reactions via Et2Zn, Et3B, and ArMgX 180 reagents corresponding chiral alcohols 181a–181d with excellent ee.44
Scheme 37. Reagents and Conditions: (i) catalyst 182 (6 mol %) reused 14 times.
The heterogeneous titanium catalyst derived from the silica-supported H8–BINOL ligand exhibited high activity and enantioselectivity at 6 mol % loading not only in the reaction using Et2Zn but also using Et3B and aryl Grignard reagents with the fact that it could be reused up to 14 times without appreciable deterioration of the activity.
Chiral counterion directed organocatalysis reaction was established by Gong and his group. The protocol described reaction (Scheme 38; Figure 21) of enamides 183 with indolyl alcohols 184 using H8–BINOL phosphoric acid 172. H8–BINOL reacted with indolyl alcohol 186 to give a carbocation as an electrophilic center. Nucleophile enamide 183 attacks 186 selectively from one side to afford β-aryl 3-(3 indolyl)propanones 185a–185c in high yields (up to 96%) and enantioselectivity (up to 96% ee).45 A survey of solvents revealed that dichloromethane initiates reaction with excellent yield. The N-protecting group of enamide has a great influence on the enantioselectivity, hence replacement of Ac (acetyl) with benzoyl as the protecting group improves the yield. The scope of the reaction explored for the aryl substituent of (1H-indol-3-yl)(aryl)methanols (184) depicted that electron-rich and electron-deficient aryl groups were tolerable.
Scheme 38. Reagents and Conditions: (i) cat. (10 mol %) 172, CH2Cl2, −30 °C.
Figure 21.
Walsh and co-worker reported (Scheme 39) catalytic asymmetric addition of allyl group to ketones and generated α-allylic alcohols. Methallylation of ketones 188 achieved by employing catalytic system of titanium tetraisopropoxide and H8–BINOL. Various ketones 188 and tetramethallylstannane 189 reacted enantioselectively to afford tertiary homoallylic alcohols 190a–190d in excellent yields (up to 99% and enantioselectivity up to 90%). Ozonolysis of the resulting products provides access to chiral α-hydroxy ketones, which are not readily prepared from direct asymmetric aldol reaction of acetone with ketones.46 The catalyst screening study for various substituent at 3,3′ and 6,6′ positions of (R)-BINOL phosphoric acid produced low enantioselectivity. Further comparative study with these BINOL phosphoric acids with H8–BINOL chiral phosphoric showed that H8–BINOL produced better results. To maximize enantioselectivity, a variety of solvents were examined in the protocol with H8–BINOL, and it was reported that acetonitrile and propionitrile produce highest yield.
Scheme 39. Reagents and Conditions: (i) catalyst 191 (30 mol %), Ti(O-iPr)4 (30 mol %), isopropanol (20 equiv), CH2Cl2, rt.
Blanchet and co-worker developed (Scheme 40; Figure 22) an enantioselective aldol reaction between ethyl 2-oxoacetate 193 and alicyc ketones 192 to afford chiral β-hydroxylketones 194a–194d. The protocol was developed by using Bronsted acid catalyst-H8–BINOL-derived phosphoric acid 195. Various catalysts like pTSA, BINOL, and H8–BINOL were screened, and it was found that H8–BINOL gives better stereoselectivity. The protocol tolerates diverse nucleophilic ketones 192 present in alicyclic rings, aliphatic groups, fused alicyclic-aromatic rings, and α,β-unsaturated ketones. The electrophilic precursor aldehydes 193 like benzyl glyoxalate, i-propyl glyoxalate, and ethyl glyoxalate were screened and their tolerability reported. This method affords an efficient synthesis of various β-hydroxy ketones, most of which could not be synthesized using enamine organocatalysis as possible by present protocol.47
Scheme 40. Reagents and Conditions: (i) catalyst 195 (5 mol %), toluene, rt–0 °C.
Figure 22.
To optimize the yield and selectivity of the aldol product of various nonprotic solvents tested (toluene, xylene, THF, Et2O, Bu2O, CH2Cl2, CH3CN), toluene was identified to afford the highest diastereo- and enantioselectivity at room temperature. Interestingly, reactions could be performed at 50 °C with little erosion of enantioselectivity. Decreasing the reaction temperature had a beneficial impact on the overall selectivity. An optimal amount of 5 mol % catalyst was determined, while 1.5 mol % led to slightly decreased selectivity and 10 mol % to no improvement. Carrying out the reaction in nearly solventless conditions, using only toluene provided by the commercial solution of the glyoxylate, led to a significant improvement of both reaction rates and selectivities (dr syn/anti 70:30 and 93:7 er for the syn isomer). Investigated nucleophile partners (192) such as pentan-2-one, tetrahydrothiopyran-4-one, tetrahydropyran-4-one, and cyclopentanone gave slightly lower selectivities than cyclohexanone under optimized reaction conditions.
Lin Pu and co-workers reported (Scheme 41; Figure 23) the addition of diphenylzinc 196 to aromatic aliphatic aldehydes 197 catalyzed by organocatalyst 199 to yield chiral alchols 198a–198d up to 89% ee.48 Chiral catalyst (S)-4 199 used to catalyze the reaction of diphenylzinc with various aliphatic and aromatic aldehydes by applying the optimum conditions exhibited high enantioselectivity for all the substrates. The solvent screened showed that THF is the best solvent in the presence of 10 mol % catalyst.
Scheme 41. Reagents and Conditions: (i) 10 mol % catalyst 199, THF, rt.
Figure 23.
Ding and his group (Scheme 42) successfully applied a combinatorial coordination chemistry strategy combined with high-throughput screening techniques to engineer practical enantioselective catalysts for asymmetric hetero-Diels–Alder reaction. Reaction of varied substituted aldehydes 200 with Danishefsky’s diene 201 in the presence of H8–BINOL catalytic system 203 produced heterodienone 202 with excellent yield and enantioselectivity.49 The diol ligands employed for catalyst preparation include commercially available or easily prepared tartaric acid derivatives, BINOL, 6,6′-Br2–BINOL, H4–BINOL, H8–BINOL, 3,3′-Br2–BINOL, 3,3′-Br2–H8–BINOL, 3,3′-Ph2–BINOL, 3,3′-Ph2-H8–BINOL, and 3,3′-(SPh)2-H8–BINOL were examined, and it was concluded that 203 is an excellent catalyst. The solvent effect was examined on the reaction in both catalyst preparation and the reaction process, and it was found that the catalyst prepared in toluene is superior to that obtained in diethyl ether. The most important report was that solvent-free conditions gave quantitative yield and much higher enantioselectivity (up to 99%). Screening of diversified substituted aromatic and heterocyclic aldehyde 200 showed up to 98% ee and >99% yield.
Scheme 42. Reagents and Conditions: (i) 0.1–0.005 mol % Ti(OiPr)4: catalyst 203 (1:1), solvent free, rt; (ii) CF3COOH.
Harada and his group developed (Scheme 43) a methodology for the synthesis of chiral secondary alcohols 207 employing organocatalyst. The protocol mentioned is enantioselective alkylation of aldehydes 205 using functionalized alkylboron reagents 206. This reagent is prepared from terminal olefin precursors 204 by hydroboration reaction. Akly bornane 206 undergo enantioselective addition to aldehydes in the presence of a catalytic amount (5 mol %) of 3-(3,5-diphenylphenyl)-H8–BINOL 208. Reaction produced corresponding functionalized alcohols 207a–207d in 99% ee in the presence of excess titanium tetraisopropoxide.50
Scheme 43. Reagents and Conditions: (i) catalyst 208 (5 mol %), Ti(OiPr)4 (3 equiv), THF, reflux.
Gong and his group performed a novel asymmetric multicomponent reaction (Scheme 44). The protocol settled under inert atmosphere by adding initially Pd(OAc)2 phosphoramide ligand 213 and dialkylmalonate 210 in a Schlenk tube. Under vacuum 1,3-butadiene 209 and aryl iodides 211 in MTBE were added. This reaction proceeded via a palladium-catalyzed cascade arylation and asymmetric allylic alkylation reaction, providing an efficient strategy for the enantioselective 1,2 difunctionalized 212a–212c product.51 Reaction optimization study using solvents like MTBE, THF, DCM, MeCN, and DMF showed that the highest enantioselectivity of 87% ee was observed in MTBE. A variety of arylbutadienes 209 and aryl iodides 211, possessing same aryl groups, were first examined in the presence of 5 mol % of Pd(OAc)2 and 10 mol % of catalyst in MTBE at 80 °C. It is necessary to mention that the structures of two different regiomers are generated. The presence of either electron-releasing or -deficient substituent at the para-position was nicely tolerated and highly regioselectively generated the target products in high yields and enantioselectivity. The substitution pattern had considerable effect on both regio- and stereoselectivities.
Scheme 44. Reagents and Conditions: (i) Pd(OAc)2 (5 mol %), catalyst 213 (10 mol %), MTBE, 80 °C, 72 h.
Baik and his group invented an asymmetric protocol using (Scheme 45) copper bromide and H8–BINOL catalytic system for enantiotopic selective allylation of gem-diborylalkanes 215 with allyl bromides 214. The combination of copper(I) bromide and H8–BINOL derived phosphoramidite ligand 217 worked out to be the most effective catalytic system to provide various enantio-enriched homoallylic boronate esters 216a–216d. The product 216 contained a boron-substituted stereogenic center that is solely derived from gem-diborylalkanes 215, in good yields with high enantiomeric ratios under mild conditions.52 The effect of an amino group of (R)-BINOL-derived phosphoramidite ligands found them to have negative effects; on the other hand, (R)-H8–BINOL-based phosphoramidite as a ligand afforded 216 in good yield with 95:5 er. Spirobiindanediol and TADDOL-derived phosphoramidite ligand displayed lower efficiencies. Allyl bromides 214 bearing methyl, hexyl, cyclohexyl, and phenyl ring bearing electronically neutral, donating, or withdrawing substituents, naphthyl- and heteroaryl-containing as a substituent at the C2-position were produced in good yields and er.
Scheme 45. Reagents and Conditions: (i) 0.1 equiv catalyst 217, 5 mol % CuBr, 2 equiv LiOtBu, THF, rt, 30 h.
Schaus and co-worker performed (Scheme 46) the Morita-Baylis-Hillman reaction using organocatalyst. The reaction started with the precursor by the addition of cyclohexanone 219 to aldehydes 218 in the presence of catalyst 221 and produced chiral alcohols 220 with maximum yield and enantioselectivity.53 The catalyst optimization and recruitment study revealed that patially reduced H8–BINOL with substituent at 3,3′ influences the yield of the reaction. Screening of trialkylphosphines such as PMe3 or P(n-Bu)3 in the reaction afforded a yield similar to that of PEt3 but lower enantioselectivities (50% and 64% ee, respectively). The variety of aromatic and aliphatic aldehydes screened with a diverse substituent pattern leads to optimum yield and enantioselectivity.
Scheme 46. Reagents and Conditions: (i) 2–20 mol % catalyst 221, P(OEt)3.
Toste and his group (Scheme 47) utilized phosphoramidite ligand 224 with AuI catalyst in the development of intramolecular cycloadditions of allenenes 223 to give 3,4-disubstituted pyrrolidines 223 as major products.54 Comparative study of BINOL with diverse 3,3′ substituent, VANOL backbone, VAPOL backbone derived catalyst, and H8–BINOL 3,3′ substituted catalyst showed that H8–BINOL-based catalyst favors reaction. Allenes 222 with various electron donating and withdrawing groups cyclizes in the presence of MeOH, H2O, aromatic rings, and allyl alcohol EtOH to produce corresponding products. Stereochemical observation noted in this protocol that both cis and trans pathways are competitive, but in the presence of a nucleophile (MeOH), the trans pathway is preferred. On the other hand, in the absence of a viable nucleophile, the cis pathway is operative. These findings also suggest that a more polar solvent, such as MeNO2, could further increase the preference for the trans pathway. In addition, we speculate that the interaction between the carbocation and gold may serve to direct the attack of the nucleophile from the opposite side trans attack.
Scheme 47. Reagents and Conditions: (i) catalyst 224 AuCl (5 mol %), 5% AgBF4, MeOH.
Yoshikai and his group (Scheme 48) developed a protocol for enantioselective installation of an alkyl group at the C2 position of indole 225 whose nitrogen is protected by the Boc protecting group. Styrene 226 was used as the alkyl source. Cobalt–chiral phosphoramidite catalyst generated from Co(acac)4 and 228 was used to induce the required enantioselectivity. The reaction affords 1,1-diarylethane 227 products in moderate to good yields with good enantioselectivities under mild conditions.55 The chiral catalyst screening during optimization of chiral ligands like BINOL-phosphoramidite, (S)-Monophos (MOP), (−) TADDOL-phosphoramidite, (S)-BINAP, and H8–BINOL-phosphoramidite depicted that H8–BINOL-phosphoramidite results high yield and enantioselectivity. Diversified N-protected indoles 225 with protection at nitrogen such as benzyl, phenyl, Cbz, Tosyl (Ts), Boc, and −CONEt2 were screened, and results were excellent with -Boc protection. The reactions of indole with a substituent on the 5- or 6-position and 4-methoxystyrene were achieved with enantioselectivities higher than 83% ee. The 7-ethylindole substituent resulted in reduced reactivity and enantioselectivity. Use of CoBr2 instead of Co(acac)3 was essential to achieve a reasonable conversion; otherwise, the reaction was rather sluggish.
Scheme 48. Reagents and Conditions: (i) catalyst Co(acac)3, L-228, Me3SiCH2MgCl, THF, rt, 12 h, 88%.
Styrenes 226 bearing a para- or meta-substituent on the aromatic ring afforded the corresponding hydroarylation products in yields of 48–88% ee. High ee values (85–86%) were achieved in the reactions of 4-methoxystyrene and 4-trimethylsilylstyrene.
Zhang and his group developed (Scheme 49) organocatalytic H8–BINOL 232 catalyzed Pictet-Spengler reaction of biphenyl-2-amines 229 and aromatic aldehydes 230 to yield 6-aryl-5,6-dihydrophenanthridines 231 with high yields (up to 98%) and enantioselectivities (up to 99% ee).56 Solvents screened with the best catalyst 232 enantioselectivity could be improved to 79–89% ee by using o/m-xylene as solvent, but the yield decreased slightly. The reaction was performed in chloroform resulting in 97% yield and 93% ee obtained in only 18 h. The catalyst loading from 2% to 10% 5 Å molecular sieves in chloroform at 0 °C. Aromatic aldehydes with different substituents like electron withdrawing 4-NO2, 4-CN, and 4-CF3 groups afforded products with excellent yields and enantioselectivities (95–98% yields, 94–99% ee). The scope of biphenyl-2-amines was subsequently investigated with electron withdrawing substituents on the 4-position of the aniline moiety providing the corresponding 5,6-dihydrophenanthridines 232 in 79–88% yields and 82–99% ee.
Scheme 49. Reagents and Conditions: (i) 5 mol % catalyst 232, CHCl3, 5 Å molecular sieves.
Harada and his group (Scheme 50) described a method for preparing enantio-enriched secondary alcohols 235 by starting from aryl bromides 231 and aldehydes. Aryl bromides were first treated with BuLi, and the resulting aryllithium reagents 234 were mixed with titanium tetraisopropoxide and magnesium bromide in the presence of 3-(3,5-diphenylphenyl)-H8–BINOL 236 and titanium tetraisopropoxide, producing the corresponding chiral alcohols 235a–235c in high enantioselectivities and in high yields.57
Scheme 50. Reagents and Conditions: (i) catalyst 236 (2 mol %), Ti(OiPr)4, MgBr2, CH2Cl2.
An enatioselective protocol for the synthesis of β,β-disubstituted allylic alcohols 239 has been developed by Harada and his group (Scheme 51). The reaction was started with readily available terminal alkynes 238a and aldehydes 237. Trisubstituted (E)-vinylaluminum reagent 238 was generated regio- and stereoselectively by the Zr-catalyzed carboalumination of the alkynes 238 with Me3Al. Intermediate 238 was used in consequent enantioselective addition reactions with aldehydes 237 using a DPP-H8–BINOL-derived titanium catalyst 240 at a catalytic amount.58
Scheme 51. Reagents and Conditions: (i) Me3Al, Cp2ZrCl2; (ii) 5 mol % catalyst 240, Ti(iOPr)4, THF, 0 °C.
To replace the undesirable racemic pathway, reaction parameters, such as the amount of Me3Al (2.4 equiv) for the carbometalation step, temperature (25 °C), solvent (THF), and concentrations of catalyst, were optimized.
Harada and his group developed a protocol (Scheme 52) for the asymmetric alkylation of aldehydes 241 by triethyl borane 242 and chiral catalyst 3-(3,5-diphenylphenyl)-H8–BINOL-derived titanium(IV) 244 in the presence of an excess amount of titanium tetraisopropoxide. The reaction proceeds with a low catalyst, exhibiting high enantioselectivity in the chiral secondary alcohol 243 product.59
Scheme 52. Reagents and Conditions: (i) 2 mol % catalyst 244, Ti(OiPr)4 (3 equiv), THF, 66 °C.
Harada et al. developed a general one pot method (Scheme 53) for the highly enantioselective synthesis of α-substituted allylic alcohols 247a–247c starting from readily available terminal alkynes 246 and aldehydes 245 and proceeding via vinylaluminum reagents. Ni(dppp)Cl2 (3 mol %) was treated with terminal alkyne (3 equiv) and Me2AlH (3 equiv) and stirred, and subsequently H8–BINOL substituted aldehydes and titanium isopropoxide were added at 0 °C to produce substituted allylic alcohols. High enantioselectivities were achieved at a low catalyst loading (5 mol %) in the subsequent addition reaction. The study showed the applicability of the DPP-H8–BINOL derived titanium catalyst 248 for the enantioselective carbonyladdition of organoaluminum reagents 246 and demonstrates the versatility of this catalytic system.60
Scheme 53. Reagents and Conditions: (i) 5 mol % catalyst 248, Ti(OiPr)4 (3 equiv), THF, 0 °C.
Functionalized alkylzinc halides 250 can be employed in the enantioselective addition to aldehydes 249 by using a titanium(IV) catalyst derived from a H8–BINOL 252 in the presence of [Ti(OiPr)4] and MgBr2 (Scheme 54). A range of functionalities, including olefin, chlorine atoms, protected alcohols, amides, and cyano groups, are tolerated in the present reaction, providing the corresponding functionalized alcohols 251 in high yields and enantioselectivities.61
Scheme 54. Reagents and Conditions: (i) [Ti(OiPr)4] (7.2 equiv) and catalyst 252, MgBr2 (1.2 equiv), CH2Cl2, 0 °C, 3 h.
Harada and his co-worker performed arylation of the carbonyl group of diversified aldehydes 254 enantioselectively with organotitanium 255 and H8–BINOL catalyst. Heteroaryllithium reagents 253 were treated with ClTi(OiPr)3 to produce organotitanium precursor 255. Titanium complexes formed with H8–BINOL 257 (Scheme 55) exhibit excellent catalytic activity for nucleophilic addition of heteroaryl group across the carbon oxygen bond of aldehyde 254. The protocol produced diaryl-, aryl-, heteroaryl-, and diheteroarylmethanol derivatives 256 in high enantioselectivity at low catalyst loading (0.2–2 mol %).62
Scheme 55. Reagents and Conditions: (i) 0.2–2.0 mol % catalyst 257, CH2Cl2, 0 °C.
Harada and his group (Scheme 56) investigated that Grignard reagent 259 can be used in asymmetric arylation of aldehydes 258 for the synthesis of chiral alcohols 260 in the presence of the chiral titanium catalyst derived from H8–BINOL 261 and titanium tetraisopropoxide.63 The scope of the protocol was expanded to electron withdrawing and donating group phenyl rings of the Grignard reagent and aldehydes with aryl and alkyl groups.
Scheme 56. Reagents and Conditions: (i) 2 mol % catalyst 261, Ti(OiPr)4 (1 equiv), CH2Cl2, 0 °C, 3 h.
Da and his group (Scheme 57) have successfully employed H8–BINOL organocatalyst 265 for catalytic asymmetric arylation of aldehydes 262 by using a variety of aryl and heteroaryl Grignard reagent 263 with enantioselectivities as high as 99% of chiral alcohols 264 products.64 Different ArMgBr reagents, with electron donating or electron withdrawing para-substituents, gave excellent enantioselectivities and yields, up to 99%. The aromatic and heteroaromatic aldehydes with electron donating and electron withdrawing substituents were found to undergo arylation with PhMgBr with excellent enantioselectivities at 94% yield and up to 99% ee.
Scheme 57. Reagents and Conditions: (i) 15% catalyst (S) H8–BINOL 265, Ti(OiPr)4, THF, rt, 90% yield.
Da and his group (Scheme 58) developed a protocol which involved a catalytic asymmetric addition of aryllithium nucleophiles 267 (generated from aryl bromide and n-BuLi) to α-bromoenals or α-methylenals 266 to afford product α-methyl or bromo-substituted allylic alcohols 268. (S)-H8–BINOL catalyst 269 was used along with titanium isopropoxide under inert reaction condition.65 A series of solvents, ether, toluene, acetonitrile, hexane, and THF, were investigated so as to improve enantiomeric excess. THF/hexane resulted in a slightly higher ee than THF; both of these achieved higher enantioselectivity than the other four solvents. Hence, THF/hexane was the ideal solvent. PhLi was produced in situ from PhBr and n-BuLi; the ratio of PhBr to n-BuLi could influence on the enantioselectivity.
Scheme 58. Reagents and Conditions: (i) cat. 269, AlCl3, TMEDA, Ti(OiPr)4, THF/hexane, 40 °C, 3 h.
Enantioselectivity was highest at 1:1.2 of PhBr/n-BuLi. The protocol was also applicable to electron donating and withdrawing substituent on precursor cinnamaldehyde 267 with excellent enantioselectivity.
Zhang and his group reported an asymmetric version of the Pictet-Spengler reaction for synthesis of chiral 5,6-dihydroindolo[1,2-a]quinoxalines 272 (Scheme 59). His group synthesized H8–BINOL-imidodiphosphoric acid catalyst 273 and applied for the Pictet-Spengler-type reaction involving indolyl anilines 270 and ketones 271. They afforded products 272a–272c with excellent selectivity.66
Scheme 59. Reagents and Conditions: (i) 2 mol % catalyst 273, 5 Å molecular sieves anisol or 1,4 dioxane.
The enantioselectivity was increased when the reaction condition was optimized in terms of temperature (−20 °C), solvent (anisole), and organocatalyst (2 mol %) using molecular sieves. The synthetic scheme showed tolerance to a variety of substituent patterns on precursor molecules. Substrate 270 with a methyl group on a 3-position of the indole moiety gave rise to 273 in high yield with excellent enantioselectivity (up to 92% yield and up to 95% ee). Replacing the substituent ethyl group on pyruvate 271 to phenyl group afforded DHIQ 273 with 96% yield and 94% ee. Substrate 270 bearing electron donating, electron withdrawing, or aryl groups on the 5-position of the indole part reacted with either ethyl pyruvate/phenyl pyruvate 271 vigorously produced the chiral DHIQs 272 with high yields and enantioselectivities.
Cao and his team (Scheme 60; Figure 24) invented a protocol by using vinyl-substituted oxyallyl carbonates 274 as a new C,O-dipole catalyzed by Pd2(dba)3-H8–BINOL 279 for enantioselective (3 + 2) cycloaddition. The corresponding oxyallyl-Pd species 280 was weakly nucleophilic to react with activated carbonyl compounds 275 and 276, affording multisubstituted and enantio-enriched oxazolidinones 277 and 1,3-dioxolanes 278 with a high degree of chemo- and stereoselectivity. The synthetic transformations of the oxazolidinone product were carried out to build enantio-enriched α-chiral aminoketone and epoxy derivatives.67
Scheme 60. Reagents and Conditions: (i) Pd2(dba)3, ligand catalyst 279, THF.
Figure 24.
Lin and his group (Scheme 61; Figure 25) applied a [3 + 3] addition protocol via a novel asymmetric Friedel–Crafts alkylation/trans-amidation tandem reaction using N-arylpyrazoline 281 and 2-oxaindole 282 for the enantio- and diasteroselective synthesis of pyrazolo[3,4-b]pyridin-6-ones 283 employing synergistic chiral phosphoric acid 284 and MgSO4 catalysis.68 Investigation of various additives such as MgSO4, Na2SO4, and molecular sieves (5 Å, 4 Å, and 3 Å molecular sieves), but only MgSO4 is suitable for this conversion. Solvent screening also reports a mixed result about dichloroethane, 1,4-dioxane, and carbon tetrachloride.69
Scheme 61. Reagents and Conditions: (i) CPA catalyst 284, MgSO4, 1,4-dioxane: CCl4 (1:1), 40 °C.
Figure 25.
Hui and his group developed (Scheme 62) a photochemical carbene 288 insertion reaction in 1,4-dicarbonyl compound 287 for the construction of enantio-enriched under visible light and H8–BINOL catalyst 290-based Bronsted catalyst.70 The carbene species with transition metal can participate in a variety of chemical transformations. However, transition metal catalysts are difficult to recover and expensive and replaced by visible light (blue LED) in this protocol. During process optimization study, additives such as Na2SO4, MgSO4, and 4 Å molecular sieves were screened. MgSO4 was a beneficial additive and dichloromethane is a supportive solvent.
Scheme 62. Reagents and Conditions: (i) 5 mol % catalyst 290, MgSO4, DCM, N2, −25 °C, 24 h.
The Cu(CH3CN)BF4 reacted with chiral ligand 294 to produce a metal complex which catalyzes asymmetric 1,3-dipolar cycloaddition (Scheme 63) of azomethine ylides 292 and electron deficient alkenes 291. The reaction produced various enantio-enriched pyrrolidine derivatives 293a–293c. The protocol has been established by Wang and his co-workers.71 Triethyl amine as a base and toluene as a solvent were identified as the best choices in terms of reactivity and enantioselectivity. Enantio-enriched pyrrolidine derivatives are very useful synthetic building blocks for natural alkaloids and pharmaceuticals.
Scheme 63. Reagents and Conditions: (i) Cu(CH3CN)4BF4 and L-294 (5 mol %), Et3N (20 mol %), PhMe, 10 °C.
4. Summary
The present review covers the most competent asymmetric enantioselective protocols catalyzed by H8–BINOL, an axially chiral catalyst used as a Bronsted acid as well as Lewis acid in C–C and C-heteroatom bond formation. These catalysts have also made entry into asymmetric C–H activation reactions since the early 2000s. Recently, the trend to develop enantioselective and diastereoselective protocols underwent scale up in industries for chiral drugs, agrochemicals, natural products, and other chiral medicinally important molecules using organocatalyst with unique features. The present review highlighted the challenges, problems, limitations, and future prospects of organocatalyst with the hope that by referring to this review new work with innovative protocols will be done in the next decade by scientists.
Acknowledgments
Author is thankful to Professor Vimal K. Jain, Director, Centre of Excellence in Basic Sciences, Mumbai, India-400047.
Biography

Dr. Rajpratap Kshatriya completed his Master of Science in Chemical Sciences from S. P. Pune University, India with first rank in Organic Chemistry Specialization. He worked in Pharmaceutical Process Development R & D of IPCA Laboratory, Mumbai, RPG Life Sciences Mumbai & other pharmaceutical Research and development organizations. He completed his PhD course from S. P. Pune University in the area of Synthetic Natural Products. After submission of PhD thesis he started to work with Prof. Arvind Lali (Institute of Chemical Technology, Matunga Mumbai, previously known as UDCT) as a Post Doc RA on a project “HPLC Method development and validation for natural products using analytical techniques”. In 2016 he moved to University of Kwazulu Natal South Africa for postdoctoral research at Department of Pharmacy, School of Pharmaceutical Sciences. He worked with Prof. Tricia Naicker & Thavender Govender at Catalysis and Peptide Research Unit (CPRU) in Department of Pharmacy in the area of medicinal chemistry and organocatalysis. In 2017 he moved to Institute of Chemical Technology (previously known as UDCT), Mumbai, India at department of Fine Chemical Technology (previously Dyestuff Technology), in the group of Dr. Satyajit Saha. He worked on CSIR fellowship as CSIR-Post Doc in the area of asymmetric organocatalysis using R-BINOL catalyst. In 2019 he moved to Uka Tarsadia University as Research Professor. In 2022 he joined with Prof. Vimal K. Jain as Post Doc RA at School of Chemical Sciences, UM-DAE Centre for Excellence in Basic Sciences, University of Mumbai, Kalina, Santacruz (E), Mumbai, India. He worked there in the area of Organometallic Chemistry. He worked on methodology development of selenium, sulphur and phosphrous-based organic chemistry and asymmetric organometallic chemistry. Presently he is working in Anant Pharmaceutical Pvt. Ltd. as a Senior Research Scientist (Group Leader). He is working with Dr. Ajit Godbole in the area of impurity development, novel chiral methodology development & Contract research work.
The author declares no competing financial interest.
References
- a Zheng C.; You S.-L. Recent Development of Direct Asymmetric Functionalization of Inert C–H Bonds. RSC Adv. 2014, 4, 6173–6214. 10.1039/c3ra46996d. [DOI] [Google Scholar]; b Liu Y.; Li W.; Zhang J. Chiral ligands designed in China. Natl. Sci. Rev. 2017, 4 (3), 326–358. 10.1093/nsr/nwx064. [DOI] [Google Scholar]; c Terada M. Binaphthol-derived phosphoric acid as a ver-satile catalyst for enantioselective carbon–carbon bond forming reactions. Chem. Commun. 2008, 4097–4112. 10.1039/b807577h. [DOI] [PubMed] [Google Scholar]; d Kampen D.; Reisinger C. M.; List B.. Asymmetric Organocatalysis, 1st ed., List B., Ed.; Springer Berlin: Heidelberg, 2009; pp 1–37. [Google Scholar]; e Parmar D.; Sugiono E.; Rueping M.. Asymmetric Bronsted Acid Catalysis, 1st ed., Rueping M., Ed.; Wiley-VCH Germany, 2016; pp 1–240. [Google Scholar]; f Dalko P. I.Enantioselective Organocatalysis: Reactions and Experimental Procedures, 1st ed., Dalko P. I., Ed.; Wiley-VCH Germany, 2007; pp 1–17. [Google Scholar]; g Ikariya T.; Shibasaki M.. Bifunctional Molecular Catalysis, 1st ed., Shibasaki M., Ed.; Springer Berlin, Heidelberg, 2011; pp 1–210. [Google Scholar]; h Yue Q.; Liu B.; Liao G.; Shi B.-F. Binaphthyl Scaffold: A Class of Versatile Structure in Asymmetric C–H Function-alization. ACS Catal. 2022, 12 (15), 9359–9396. 10.1021/acscatal.2c02193. [DOI] [Google Scholar]; i Noyori R. Asymmetric Catalysis: Science and Opportuni-ties (Nobel Lecture). Angew. Chem., Int. Ed. 2002, 41, 2008–2022. . [DOI] [PubMed] [Google Scholar]; j Mikami K., Lautens M., Eds. New Frontiers in Asymmet-ric Catalysis; Wiley: Hoboken, 2007. [Google Scholar]; k Sandoval C. A.; Noyori R.. An Overview of Recent Developments in Metal-Catalyzed Asymmetric Transformations. In Organic Chemistry–Breakthroughs and Perspectives, Ding K.-L.; Dai L.-X.; Eds.; Wiley-VCH: Weinheim, Germany, 2012; Chapter 9, pp 335–363. [Google Scholar]; l Wang P.-S; Chen D.-F; Gong L.-Z.. Asymmetric Organocatalysis Combined with Metal Catalysis, 1st ed., Gong L.-Z., Ed.; Springer: Berlin, 2020; pp 185. [Google Scholar]
- a Heumann L. V.; Keck G. E. A New Method for the Synthesis of H4-BINOL. J. Org. Chem. 2008, 73, 4725–4727. 10.1021/jo8004556. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Pellissier H. Recent developments in enantioselective titanium-catalyzed transformations. Coord. Chem. Rev. 2022, 463, 214537. 10.1016/j.ccr.2022.214537. [DOI] [Google Scholar]; c Wang J.; Zheng S.; Rajkumar S. Chiral phosphoric acid-catalyzed stereodivergent synthesis of trisubstituted allenes and computational mechanistic studies. Nat. Commun. 2020, 11, 5527. 10.1038/s41467-020-19294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Soai K.; Niwa S. Enantioselective addition of organozinc reagents to aldehydes. Chem. Rev. 1992, 92 (5), 833–856. 10.1021/cr00013a004. [DOI] [Google Scholar]
- a Abreu A. R.; Pereira M. M.; Bayón J. C. Synthesis of new bis-BINOL-2,2′-ethers and bis-H8-BINOL-2,2′-ethers evaluation of their Titanium complexes in the asymmetric ethylation of benzaldehyde. Tetrahedron 2010, 66 (3), 743–749. 10.1016/j.tet.2009.11.047. [DOI] [Google Scholar]; b Doyle A. G.; Jacobsen E. N. Small-Molecule H-Bond Donors in Asymmetric Catalysis. Chem. Rev. 2007, 107 (12), 5713–5743. 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]; c Simón L.; Goodman J. M. Mechanism of BINOL–Phosphoric Acid-Catalyzed Strecker Reaction of Benzyl Imines. J. Am. Chem. Soc. 2009, 131 (11), 4070–4077. 10.1021/ja808715j. [DOI] [PubMed] [Google Scholar]; d Simón L.; Goodman J. M. Theoretical Study of the Mechanism of Hantzsch Ester Hydrogenation of Imines Catalyzed by Chiral BINOL-Phosphoric. Acids J. Am. Chem. Soc. 2008, 130 (27), 8741–8747. 10.1021/ja800793t. [DOI] [PubMed] [Google Scholar]; e Simón L.; Goodman J. M. A Model for the Enantioselectivity of Imine Reactions Catalyzed by BINOL–Phosphoric Acid Catalysts. J. Org. Chem. 2011, 76 (6), 1775–1788. 10.1021/jo102410r. [DOI] [PubMed] [Google Scholar]; f Bhaskararao B.; Sunoj R. B. Origin of Stereodiver-gence in Cooperative Asymmetric Catalysis with Simulta-neous Involvement of Two Chiral Catalysts. J. Am. Chem. Soc. 2015, 137 (50), 15712–15722. 10.1021/jacs.5b05902. [DOI] [PubMed] [Google Scholar]; g Bihani M.; Zhao J. C.-G. Advances in Asymmetric Diastereodivergent Catalysis. Adv. Synth. Catal. 2017, 359, 534. 10.1002/adsc.201601188. [DOI] [Google Scholar]; h Beletskaya I. P.; Nájera C.; Yus M. Stereodivergent Catalysis. Chem. Rev. 2018, 118, 5080–5200. 10.1021/acs.chemrev.7b00561. [DOI] [PubMed] [Google Scholar]; i Martínez S.; Veth L.; Lainer B.; Dydio P. Challenges and Opportunities in Multicataly-sis. ACS Catal. 2021, 11, 3891–3915. 10.1021/acscatal.0c05725. [DOI] [Google Scholar]; j Zhou Q.-L.Privileged Chiral Ligands and Catalysts, 1st ed., Zhou Q.-L., Ed.; Wiley-VCH Weinheim, 2011. [Google Scholar]; k Shao Z.; Zhang H. Com-bining transition metal catalysis and organocatalysis: A broad new concept for catalysis. Chem. Soc. Rev. 2009, 38, 2745–2755. 10.1039/b901258n. [DOI] [PubMed] [Google Scholar]; l Sunoj R. B. Transition State Models for Understanding the Origin of Chiral Induction in Asymmetric Catalysis. Acc. Chem. Res. 2016, 49 (5), 1019–1028. 10.1021/acs.accounts.6b00053. [DOI] [PubMed] [Google Scholar]
- a Parmar D.; Sugiono E.; Raja S.; Rueping M. Complete Field Guide to Asymmetric BINOL-Phosphate Derived Bro̷nsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Bro̷nsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev. 2014, 114, 9047–9153. 10.1021/cr5001496. [DOI] [PubMed] [Google Scholar]; b Tay J.-H.; Arguelles A. J.; Nagorny P. Direct Interconversion of BINOL and H8-BINOL-Based Chiral Bro̷nsted Acids Using Single-Step Red/Ox Manipulations. Org. Lett. 2015, 17, 3774. 10.1021/acs.orglett.5b01754. [DOI] [PubMed] [Google Scholar]; c Parmar D.; Sugiono E.; Raja S.; Rueping M. Addition and Correction to Complete Field Guide to Asymmetric BINOL-Phosphate Derived Bro̷nsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Bro̷nsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev. 2017, 117, 10608–10620. 10.1021/acs.chemrev.7b00197. [DOI] [PubMed] [Google Scholar]; d Akiyama T. Stronger Bro̷nsted Acids. Chem. Rev. 2007, 107, 5744. 10.1021/cr068374j. [DOI] [PubMed] [Google Scholar]; e Doyle A. G.; Jacobsen E. N. Small-Molecule H-Bond Donors in Asymmetric Catalysis. Chem. Rev. 2007, 107, 5713. 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]; f Pereira M. M.; Calvete M. J. F.; Carrilho R. M. B; Abreu A. R. Synthesis of binaphthyl based phosphine and phosphite ligands. Chem. Soc. Rev. 2013, 42, 6990–7027. 10.1039/c3cs60116a. [DOI] [PubMed] [Google Scholar]
- a Yin Q.; Shi Y.; Wang J.; Zhang X. Direct catalytic asymmetric synthesis of α-chiral primary amines. Chem. Soc. Rev. 2020, 49 (17), 6141. 10.1039/C9CS00921C. [DOI] [PubMed] [Google Scholar]; b Scott K. A.; Ropek N.; Melillo B.; Schreiber S. L.; Cravatt B. F.; Vinogradova E. V. Stereochemical diversity as a source of discovery in chemical biology. Curr. Res. Chem. Biol. 2022, 2, 100028. 10.1016/j.crchbi.2022.100028. [DOI] [Google Scholar]; c Cabré A.; Verdaguer X.; Riera A. Recent Advances in the Enantioselective Synthesis of Chiral Amines via Transition Metal-Catalyzed Asymmetric Hydrogenation. Chem. Rev. 2022, 122, 269–339. 10.1021/acs.chemrev.1c00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Fan Y.-S.; Jiang Y.-J.; An D.; Sha D.; Antilla J. C.; Zhang S. H8-BINOL Chiral Imidodiphosphoric Acids Catalyzed Enantioselective Synthesis of Dihydroindolo–/–pyrrolo[1,2-a]quinoxalines. Org. Lett. 2014, 16, 6112. 10.1021/ol502965p. [DOI] [PubMed] [Google Scholar]; b Watanabe S.; Nakaya N.; Akai J.; Kanaori K.; Harada T. Silica-Supported Catalyst for Enantioselective Arylation of Aldehydes under Batch and Continuous-Flow Conditions. Org. Lett. 2018, 20 (9), 2737–2740. 10.1021/acs.orglett.8b00945. [DOI] [PubMed] [Google Scholar]
- a Tay J.-H.; Arguelles A. J.; Nagorny P. Direct Interconversion of BINOL and H8-BINOL-Based Chiral Bro̷nsted Acids Using Single-Step Red/Ox Manipulations. Org. Lett. 2015, 17, 3774–3777. 10.1021/acs.orglett.5b01754. [DOI] [PubMed] [Google Scholar]; b Korostylev A.; Tararov V. I.; Fischer C.; Monsees A.; Borner A. Convenient and Efficient Reduction of 1,1‘-Binaphthyls to H8–1,1‘-Binaphthyl Derivatives with Pd and Ru Catalysts on Solid Support. J. Org. Chem. 2004, 69, 3220–3221. 10.1021/jo0497609. [DOI] [PubMed] [Google Scholar]; c Cram D. J.; Helgeson R. C.; Peacock S. C.; Kaplan L. J.; Domeier L. A.; Moreau P.; Koga K.; Mayer J. M.; Chao Y. Host-guest complexation. 8. Macrocyclic polyethers shaped by two rigid substituted dinaphthyl or ditetralyl units. J. Org. Chem. 1978, 43, 1930–1946. 10.1021/jo00404a019. [DOI] [Google Scholar]; d McDougal N. T.; Schaus S. E. Asymmetric Morita–Baylis–Hillman Reactions Catalyzed by Chiral Bro̷nsted Acids. J. Am. Chem. Soc. 2003, 125, 12094–12095. 10.1021/ja037705w. [DOI] [PubMed] [Google Scholar]; e Bartoszek M.; Beller M.; Deutsch J.; Klawonn M.; Köckritz A.; Nemati N.; Pews-Davtyan A. A convenient protocol for the synthesis of axially chiral Bronsted acids. Tetrahedron 2008, 64, 1316–1322. 10.1016/j.tet.2007.11.067. [DOI] [Google Scholar]; f Bartoszek M.; Beller M.; Deutsch J.; Klawonn M.; Kockritz A.; Nemati N.; Pews-Davtyan A. A convenient protocol for the synthesis of axially chiral Bro̷nsted acids. Tetrahedron 2008, 64 (7), 1316–1322. 10.1016/j.tet.2007.11.067. [DOI] [Google Scholar]
- a Doyle A. G.; Jacobsen E. N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 2007, 107, 5713. 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]; b Yamamoto H.; Boxer M. Super Bro̷nsted Acid Catalysis in Organic Synthesis. Chimia 2007, 61, 279. 10.2533/chimia.2007.279. [DOI] [Google Scholar]; c Terada M. Chiral phosphoric acids as versatile catalysts for enantioselective transformations. Synthesis 2010, 2010, 1929. 10.1055/s-0029-1218801. [DOI] [Google Scholar]
- Hou C. J.; Wang Y. H.; Zheng Z.; Xu J.; Hu X. P. Chiral Phosphine–Phosphoramidite Ligands for Highly Efficient Ir-Catalyzed Asymmetric Hydrogenation of Sterically Hindered N-arylimines. Org. Lett. 2012, 14, 3554–3557. 10.1021/ol301618r. [DOI] [PubMed] [Google Scholar]
- Liu R.; Krishnamurthy S.; Wu Z.; Tummalapalli K. S. S.; Antilla J. C. Chiral Calcium Phosphate Catalyzed Enantioselective Amination of 3-Aryl-2-benzofuranones. Org. Lett. 2020, 22 (20), 8101–8105. 10.1021/acs.orglett.0c03059. [DOI] [PubMed] [Google Scholar]
- Wu K.-H.; Gau H.-M. Remarkably Efficient Enantioselective Titanium(IV)–(R)-H8-BINOLate Catalyst for Arylations to Aldehydes by Triaryl(tetrahydrofuran)aluminum Reagents. J. Am. Chem. Soc. 2006, 128, 14808–14809. 10.1021/ja062080y. [DOI] [PubMed] [Google Scholar]
- Yang Y.-X.; Liu Y.; Zhang L.; Jia Y.-E.; Wang P.; Zhuo F.-F.; An X.-T.; Chao-Shan Da C.-S. Aryl bromides as inexpensive starting materials in the catalytic enantioselective arylation of aryl aldehydes: The additive TMEDA enhances the enantioselectivity. J. Org. Chem. 2014, 79 (21), 10696–10702. 10.1021/jo502070r. [DOI] [PubMed] [Google Scholar]
- Yu Y.-N.; Qi W.-Y.; Wu C.-Y.; Xu M.-H. Rhodium-Catalyzed Enantioselective Addition of Arylboroxines to Isatin-Derived N-Boc Ketimines Using Chiral Phosphite–Olefin Ligands: Asymmetric Synthesis of 3-Aryl-3-amino-2-oxindoles. Org. Lett. 2019, 21 (18), 7493–7497. 10.1021/acs.orglett.9b02787. [DOI] [PubMed] [Google Scholar]
- a Belliotti T. R.; Brink W. A.; Kesten S. R.; Rubin J. R.; Wustrow D. J.; Zoski K. T.; Whetzel S. Z.; Corbin A. E.; Pugsley T. A.; Heffner T. G.; Wise L. D. Bioorg. Med. Chem. Lett. 1998, 8, 1499. 10.1016/S0960-894X(98)00252-2. [DOI] [PubMed] [Google Scholar]; b Ray S. K.; Sadhu M. M.; Biswas R. G.; Unhale R. A.; Singh A. K. A General Catalytic Route to Enantioenriched Isoindolinones and Phthalides: Application in the Synthesis of (S)-PD 172938. Org. Lett. 2019, 21 (2), 417–422. 10.1021/acs.orglett.8b03597. [DOI] [PubMed] [Google Scholar]
- Hu X.-H.; Hu X.-P. Highly Diastereo-and Enantioselective Ir-Catalyzed Hydrogenation of 2, 3-Disubstituted Quinolines with Structurally Fine-Tuned Phosphine–Phosphoramidite Ligands. Org. Lett. 2019, 21 (24), 10003–10006. 10.1021/acs.orglett.9b03925. [DOI] [PubMed] [Google Scholar]
- Li Y.; Yu Y.-N.; Xu M.-H. Simple Open-Chain Phosphite-Olefin as Ligand for Rh-Catalyzed Asymmetric Arylation of Cyclic Ketimines: Enantioselective Access to gem-Diaryl α-Amino Acid Derivatives. ACS Catal. 2016, 6 (2), 661–665. 10.1021/acscatal.5b02403. [DOI] [Google Scholar]
- He H.; Cao Y.; Xu J.; Antilla J. C. Catalytic Asymmetric C-7 Friedel–Crafts Alkylation/N-Hemiacetalization of 4-Aminoindoles. Org. Lett. 2021, 23 (8), 3010–3014. 10.1021/acs.orglett.1c00699. [DOI] [PubMed] [Google Scholar]
- Wang B.; Feng X.; Huang Y.; Liu H.; Cui X.; Jiang Y. A Highly Enantioselective Hetero-Diels–Alder Reaction of Aldehydes with Danishefsky’s Diene Catalyzed by Chiral Titanium(IV)5,5‘,6,6‘,7,7‘,8,8‘-Octahydro-1,1‘-bi-2-naphthol Complexes. J. Org. Chem. 2002, 67 (7), 2175–2182. 10.1021/jo016240u. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Liu Z.; Zhou J. An Enantioselective, Intermolecular α-Arylation of Ester Enolates To Form Tertiary Stereocenters. J. Am. Chem. Soc. 2011, 133 (40), 15882–15885. 10.1021/ja2066829. [DOI] [PubMed] [Google Scholar]
- Guo Q.-X.; Liu H.; Guo C.; Luo S.-W.; Gu Y.; Gong L.-Z. Chiral Bro̷nsted Acid-Catalyzed Direct Asymmetric Mannich Reaction. J. Am. Chem. Soc. 2007, 129 (13), 3790–3791. 10.1021/ja068236b. [DOI] [PubMed] [Google Scholar]
- Rueping M.; Sugiono E.; Theissmann T.; Kuenkel A.; Köckritz A.; Pews-Davtyan A.; Nemati N.; Beller M. An Enantioselective Chiral Bro̷nsted Acid Catalyzed Imino–Azaenamine Reaction. Org. Lett. 2007, 9 (6), 1065–1068. 10.1021/ol063112p. [DOI] [PubMed] [Google Scholar]
- Zhou S.; Wu K.-H.; Chen C.-A.; Gau H.-M. Highly Enantioselective Arylation of Aldehydes and Ketones Using AlArEt2(THF) as Aryl Sources. J. Org. Chem. 2009, 74 (9), 3500–3505. 10.1021/jo900348p. [DOI] [PubMed] [Google Scholar]
- Chen X.-H.; Xu X.-Y.; Liu H.; Cun L.-F.; Gong L.-Z. Highly enantioselective organocatalytic Biginelli reaction. J. Am. Chem. Soc. 2006, 128, 14802–14803. 10.1021/ja065267y. [DOI] [PubMed] [Google Scholar]
- Xu J.-C.; Yin Y.-Z.; Han Z.-Y. Asymmetric Counteranion Directed Catalytic Heck/Tsuji–Trost Annulation of Aryl Iodides and 1, 3-Dienes. Org. Lett. 2021, 23 (10), 3834–3838. 10.1021/acs.orglett.1c00910. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Tu B.; Ge M.; Li Y.; Chen L.; Wang W.; Zhou S. Asymmetric Addition of Pyridyl Aluminum Reagents to Aldehydes Catalyzed by a Titanium(IV) Catalytic System of (R)-H8-BINOLate. J. Org. Chem. 2015, 80 (16), 8307–8313. 10.1021/acs.joc.5b01410. [DOI] [PubMed] [Google Scholar]
- Liang; Au-Yeung T. T.-L.; Chan A. S. C. Highly Enantioselective 1,4-Addition of Diorganozinc Reagents to Cyclic Enones Using Chiral Diphosphite Ligands Derived from H8-Binaphthol. Org. Lett. 2002, 4 (22), 3799–3801. 10.1021/ol026581+. [DOI] [PubMed] [Google Scholar]
- McInturff E. L.; Yamaguchi E.; Krische M. J. Chiral-anion-dependent inversion of diastereo-and enantioselectivity in carbonyl crotylation via ruthenium-catalyzed butadiene hydrohydroxyalkylation. J. Am. Chem. Soc. 2012, 134 (51), 20628–20631. 10.1021/ja311208a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.-Y.; Pai C.-C.; Chan A. S. C. Asymmetric Synthesis of Chiral Amine Derivatives through Enantioselective Hydrogenation with a Highly Effective Rhodium Catalyst Containing a Chiral Bisaminophosphine Ligand. J. Am. Chem. Soc. 1998, 120 (23), 5808–5809. 10.1021/ja974045k. [DOI] [Google Scholar]
- Du H.; Zhao B.; Shi Y. Catalytic asymmetric allylic and homoallylic diamination of terminal olefins via formal C– H activation. J. Am. Chem. Soc. 2008, 130 (27), 8590–8591. 10.1021/ja8027394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya S. D.; Toenjes S. T.; Yamamoto N.; Maddox S. M.; Gustafson J. L. Catalytic Atroposelective Synthesis of N-Aryl Quinoid Compounds. J. Am. Chem. Soc. 2020, 142 (5), 2198–2203. 10.1021/jacs.9b12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X.-K.; Zhang H.; Gao J.-G.; Sun D.-Y.; Qin X.-S.; Jiang G.-F.; Zhang G.-L.; Zhang S. Asymmetric Synthesis of 1,1,1-Triarylethanes by Chiral Imidodiphosphoric Acid Catalyzed Nucleophilic Addition of Pyrrole and Indoles to 3-Vinylindoles. J. Org. Chem. 2019, 84 (19), 12562–12572. 10.1021/acs.joc.9b02024. [DOI] [PubMed] [Google Scholar]
- Lin H.-C.; Wang P.-S.; Tao Z.-L.; Chen Y.-G.; Han Z.-Y.; Gong L.-Z. Highly Enantioselective Allylic C–H Alkylation of Terminal Olefins with Pyrazol-5-ones Enabled by Cooperative Catalysis of Palladium Complex and Bro̷nsted Acid. J. Am. Chem. Soc. 2016, 138 (43), 14354–14361. 10.1021/jacs.6b08236. [DOI] [PubMed] [Google Scholar]
- Gonzalez A. Z.; Toste F. D. Gold(I)-Catalyzed Enantioselective [4 + 2]-Cycloaddition of Allene-dienes. Org. Lett. 2010, 12 (1), 200–203. 10.1021/ol902622b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-Y.; Yuan W.-Q.; Tang S.; Huang Y.-W.; Xue J.-H.; Fu L.-N.; Guo Q.-X. Chiral Calcium Phosphate Catalyzed Asymmetric Alkenylation Reaction of Arylglyoxals with 3-Vinylindoles. Org. Lett. 2017, 19 (5), 1120–1123. 10.1021/acs.orglett.7b00143. [DOI] [PubMed] [Google Scholar]
- Liu H.; Cun L.-F.; Mi A.-Q.; Jiang Y.-Z.; Gong L.-Z. Enantioselective Direct Aza Hetero-Diels– Alder Reaction Catalyzed by Chiral Bro̷nsted Acids. Org. Lett. 2006, 8 (26), 6023–6026. 10.1021/ol062499t. [DOI] [PubMed] [Google Scholar]
- Batuecas M.; Luo J.; Gergelitsová I.; Krämer K.; Whitaker D.; Vitorica-Yrezabal I.; Larrosa I. Catalytic Asymmetric C–H Arylation of (η6-Arene)Chromium Complexes: Facile Access to Planar-Chiral Phosphines. ACS Catal. 2019, 9 (6), 5268–5278. 10.1021/acscatal.9b00918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y.-H.; Yang H.-Y.; Chen M.; Wu F.; Xu X.-X.; Guan Z.-H. Asymmetric Markovnikov hydroaminocarbonylation of alkenes enabled by palladium-monodentate phosphoramidite catalysis. J. Am. Chem. Soc. 2021, 143 (1), 85–91. 10.1021/jacs.0c11249. [DOI] [PubMed] [Google Scholar]
- Gao C.; Wang X.; Liu J.; Li X. Highly Diastereo- and Enantioselective Synthesis of Tetrahydrobenzo[b]azocines via Palladium-Catalyzed [4 + 4] Cycloaddition. ACS Catal. 2021, 11 (5), 2684–2690. 10.1021/acscatal.0c05515. [DOI] [Google Scholar]
- Chen M.-W.; Yang Q.; Deng Z.; Zhou Y.; Ding Q.; Peng Y. Organocatalytic Asymmetric Reduction of Fluorinated Alkynyl Ketimines. J. Org. Chem. 2018, 83 (15), 8688–8694. 10.1021/acs.joc.8b00873. [DOI] [PubMed] [Google Scholar]
- Ingle G.; Mormino M. G.; Antilla J. C. Lithium BINOL Phosphate Catalyzed Desymmetrization of meso-Epoxides with Aromatic Thiols. Org. Lett. 2014, 16 (21), 5548–5551. 10.1021/ol502527q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N. M.; Liu X.-G. Asymmetric Morita-Baylis-Hillman reaction of arylaldehydes with 2-cyclohexen-1-one catalyzed by chiral bis (thio) urea and DABCO. Org. Lett. 2008, 10 (6), 1043–1046. 10.1021/ol7028806. [DOI] [PubMed] [Google Scholar]
- Dumoulin A.; Bernadat G.; Masson G. Enantioselective Three-Component Amination of Enecarbamates Enables the Synthesis of Structurally Complex Small Molecules. J. Org. Chem. 2017, 82 (3), 1775–1789. 10.1021/acs.joc.6b03093. [DOI] [PubMed] [Google Scholar]
- Wu K.; Zhuo M. H.; Sha D.; Fan Y. S.; An D.; Jiang Y. J.; Zhang S. Q. H8-BINOL chiral imidodiphosphoric acid catalyzedhighly enantioselective aza-Friedel–Craftsreactions of pyrroles and enamides/imines. Chem. Commun. 2015, 51, 8054. 10.1039/C5CC00685F. [DOI] [PubMed] [Google Scholar]
- Akai J.; Watanabe S.; Michikawa K.; Harada T. Application of a Heterogeneous Chiral Titanium Catalyst Derived from Silica-Supported 3-Aryl H8-BINOL to Enantioselective Alkylation and Arylation of Aldehydes. Org. Lett. 2017, 19 (13), 3632–3635. 10.1021/acs.orglett.7b01625. [DOI] [PubMed] [Google Scholar]
- Guo Q.-X.; Peng Y.-G.; Zhang J.-W.; Song L.; Feng Z.; Gong L.-Z. Highly Enantioselective Alkylation Reaction of Enamides by Bro̷nsted-Acid Catalysis. Org. Lett. 2009, 11 (20), 4620–4623. 10.1021/ol901892s. [DOI] [PubMed] [Google Scholar]
- Kim J. G.; Camp E. H.; Walsh P. J. Catalytic Asymmetric Methallylation of Ketones with an (H8-BINOLate)Ti-Based Catalyst. Org. Lett. 2006, 8 (20), 4413–4416. 10.1021/ol061417y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pousse G.; Cavelier F. L.; Humphreys L.; Rouden J.; Blanchet J. Bro̷nsted acid catalyzed asymmetric aldol reaction: a complementary approach to enamine catalysis. Org. Lett. 2010, 12 (16), 3582–3585. 10.1021/ol101176j. [DOI] [PubMed] [Google Scholar]
- Qin Y.-C.; Pu L. Highly Enantioselective Addition of Diphenylzinc to Aliphatic and Aromatic Aldehydes Catalyzed by a Readily Available H8-Binol Derivative. Angew. Chem., Int. Ed. 2006, 45, 273–277. 10.1002/anie.200503206. [DOI] [PubMed] [Google Scholar]
- Long J.; Hu J.; Shen X.; Ji B.; Ding K. Discovery of exceptionally efficient catalysts for solvent-free enantioselective hetero-Diels– Alder reaction. J. Am. Chem. Soc. 2002, 124 (1), 10–11. 10.1021/ja0172518. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Kawasaki H.; Harada T. Enantioselective Alkylation of Aldehydes Using Functionalized Alkylboron Reagents Catalyzed by a Chiral Titanium Complex. Org. Lett. 2013, 15 (16), 4198–4201. 10.1021/ol4019248. [DOI] [PubMed] [Google Scholar]
- Wu X.; Lin H.-C.; Li M.-L.; Li L.-L.; Han Z.-Y.; Gong L.-Z. Enantioselective 1, 2-difunctionalization of dienes enabled by chiral palladium complex-catalyzed cascade arylation/allylic alkylation reaction. J. Am. Chem. Soc. 2015, 137 (42), 13476–13479. 10.1021/jacs.5b08734. [DOI] [PubMed] [Google Scholar]
- Kim M.; Park B.; Shin M.; Kim S.; Kim J.; Baik M.-H.; Cho S. H. Copper-Catalyzed Enantiotopic-Group-Selective Allylation of gem-Diborylalkanes. J. Am. Chem. Soc. 2021, 143 (2), 1069–1077. 10.1021/jacs.0c11750. [DOI] [PubMed] [Google Scholar]
- McDougal N. T.; Schaus S. E. Asymmetric Morita– Baylis– Hillman reactions catalyzed by chiral Bro̷nsted acids. J. Am. Chem. Soc. 2003, 125 (40), 12094–12095. 10.1021/ja037705w. [DOI] [PubMed] [Google Scholar]
- González A. Z.; Benitez D.; Tkatchouk E.; Goddard W. A.; Toste F. D. Phosphoramidite gold (I)-catalyzed diastereo-and enantioselective synthesis of 3, 4-substituted pyrrolidines. J. Am. Chem. Soc. 2011, 133 (14), 5500–5507. 10.1021/ja200084a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.-S.; Yoshikai N. Cobalt-Catalyzed Enantioselective Directed C–H Alkylation of Indole with Styrenes. Org. Lett. 2015, 17 (1), 22–25. 10.1021/ol503119z. [DOI] [PubMed] [Google Scholar]
- Wang C.; An D.; Guan X.; Fan Y.; Liu G.; Zhang G.; Zhang S. Organocatalytic Enantioselective Synthesis of 6-Aryl-5,6-dihydrophenanthridines by a Modified Pictet–Spengler Reaction of Biphenyl-2-amines and Aromatic Aldehydes. Eur. J. Org. Chem. 2017, 2017, 1865–1869. 10.1002/ejoc.201700185. [DOI] [Google Scholar]
- Nakagawa Y.; Muramatsu Y.; Harada T. Catalytic Enantioselective Synthesis of Diarylmethanols from Aryl Bromides and Aldehydes by Using Organolithium Reagents. Eur. J. Org. Chem. 2010, 2010, 6535–6538. 10.1002/ejoc.201001254. [DOI] [Google Scholar]
- Morimoto H.; Harada T. Catalytic Enantioselective Synthesis of Trisubstituted Secondary Allylic Alcohols Starting from Terminal Alkynes and Aldehydes. Eur. J. Org. Chem. 2015, 2015, 7378–7383. 10.1002/ejoc.201500910. [DOI] [Google Scholar]
- Ukon T.; Harada T. Catalytic Asymmetric Alkylation of Aldehydes by Using Trialkylboranes. Eur. J. Org. Chem. 2008, 2008, 4405–4407. 10.1002/ejoc.200800561. [DOI] [Google Scholar]
- Kumar R.; Kawasaki H.; Harada T. Catalytic Enantioselective Synthesis of α-Substituted Secondary Allylic Alcohols from Terminal Alkynes and Aldehydes via Vinylaluminum Reagents. Chem.—Eur. J. 2013, 19, 17707–17710. 10.1002/chem.201303619. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y.; Kanehira S.; Hayashi Y.; Harada T. Catalytic Enantioselective Alkylation of Aldehydes by Using Organozinc Halide Reagents. Chem.—Eur. J. 2013, 19, 3311–3314. 10.1002/chem.201204346. [DOI] [PubMed] [Google Scholar]
- Uenishi A.; Nakagawa Y.; Osumi H.; Harada T. Practical Enantioselective Arylation and Heteroarylation of Aldehydes with In Situ Prepared Organotitanium Reagents Catalyzed by 3-Aryl-H8-BINOL-Derived Titanium Complexes. Chem.—Eur. J. 2013, 19, 4896–4905. 10.1002/chem.201203946. [DOI] [PubMed] [Google Scholar]
- Muramatsu Y.; Harada T. Catalytic asymmetric aryl transfer reactions to aldehydes with Grignard reagents as the aryl source. Chem.—Eur. J. 2008, 14, 10560–10563. 10.1002/chem.200801612. [DOI] [PubMed] [Google Scholar]
- Fan X.-Y.; Yang Y.-X.; Zhuo F.-F.; Yu S.-L.; Li X.; Guo Q.-P.; Du Z.-X.; Da C.-S. AlCl3 and BDMAEE: A Pair of Potent Reactive Regulators of Aryl Grignard Reagents and Highly Catalytic Asymmetric Arylation of Aldehydes. Chem.—Eur. J. 2010, 16, 7988–7991. 10.1002/chem.201000974. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Sun L.; Li Y.-Y.; Liu Y.; Yang Y.-X.; Yuan R.; Wang P.; Da C.-S. Catalytic Asymmetric Arylation of Enals to Enantioenriched Linear Trisubstituted Allylic Secondary Alcohols by using Aryl Lithiums Generated In Situ from Aryl Bromides. ChemCatChem. 2013, 5, 3516–3519. 10.1002/cctc.201300655. [DOI] [Google Scholar]
- Fan Y.-S.; Jiang Y.-J.; An D.; Sha D.; Antilla J. C.; Zhang S. H8-BINOL Chiral Imidodiphosphoric Acids Catalyzed Enantioselective Synthesis of Dihydroindolo–/–pyrrolo[1,2-a]quinoxalines. Org. Lett. 2014, 16 (23), 6112–6115. 10.1021/ol502965p. [DOI] [PubMed] [Google Scholar]
- Pan X.; Yu L.; Wang S.; Wu R.; Ou C.; Xu M.; Chen B.; Gao Y.; Ni H.-L.; Hu P.; Wang B.-Q.; Cao P. Pd-Catalyzed Enantioselective (3+ 2)-Cycloaddition of Vinyl-Substituted Oxyallyl Carbonates with Isocyanates and Ketones. Org. Lett. 2022, 24 (11), 2099–2103. 10.1021/acs.orglett.2c00290. [DOI] [PubMed] [Google Scholar]
- Woldegiorgis A. G.; Han Z.; Lin X. Asymmetric [3 + 3] Annulation to Construct Trifluoromethylated Pyrazolo[3,4-b]pyridin-6-ones via Chiral Phosphoric Acid and MgSO4 Synergistic Catalysis. Org. Lett. 2022, 24 (22), 4058–4063. 10.1021/acs.orglett.2c01513. [DOI] [PubMed] [Google Scholar]
- Xu J.-C.; Yin Y.-Z.; Han Z.-Y. Asymmetric Counteranion Directed Catalytic Heck/Tsuji–Trost Annulation of Aryl Iodides and 1, 3-Dienes. Org. Lett. 2021, 23 (10), 3834–3838. 10.1021/acs.orglett.1c00910. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Wang Z.; Wang Z.; Chu Y.; Wang S.; Hui X.-P. Visible-Light-Mediated Formal Carbene Insertion Reaction: Enantioselective Synthesis of 1,4-Dicarbonyl Compounds Containing All-Carbon Quaternary Stereocenter. ACS Catal. 2022, 12 (9), 5510–5516. 10.1021/acscatal.2c00064. [DOI] [Google Scholar]
- Chang X.; Yang Y.; Shen C.; Xue K.; Wang Z.-F.; Cong H.; Tao H.-Y.; Chung L. W.; Wang C.-J. β-Substituted Alkenyl Heteroarenes as Dipolarophiles in the Cu(I)-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides Empowered by a Dual Activation Strategy: Stereoselectivity and Mechanistic Insight. J. Am. Chem. Soc. 2021, 143 (9), 3519–3535. 10.1021/jacs.0c12911. [DOI] [PubMed] [Google Scholar]