Abstract
Acute kidney injury (AKI) is a common consequence of sepsis with a mortality rate of up to 40%. The pathogenesis of septic AKI is complex and involves several mechanisms leading to exacerbated inflammatory response associated with renal injury. A large body of evidence suggests that inflammation is tightly linked to AKI through bidirectional interaction between renal and immune cells. Preclinical data from our and other laboratories have identified in complement system activation a crucial mediator of AKI. Partial recovery following AKI could lead to long-term consequences that predispose to chronic dysfunction and may also accelerate the progression of preexisting chronic kidney disease. Recent findings have revealed striking morphological and functional changes in renal parenchymal cells induced by mitochondrial dysfunction, cell cycle arrest via the activation of signaling pathways involved in aging process, microvascular rarefaction, and early fibrosis. Although major advances have been made in our understanding of the pathophysiology of AKI, there are no available preventive and therapeutic strategies in this field. The identification of ideal clinical biomarkers for AKI enables prompt and effective therapeutic strategy that could prevent the progression of renal injury and promote repair process. Therefore, the use of novel biomarkers associated with clinical and functional criteria could provide early interventions and better outcome. Several new drugs for AKI are currently being investigated; however, the complexity of this disease might explain the failure of pharmacological intervention targeting just one of the many systems involved. The hypothesis that blood purification could improve the outcome of septic AKI has attracted much attention. New relevant findings on the role of polymethylmethacrylate-based continuous veno-venous hemofiltration in septic AKI have been reported. Herein, we provide a comprehensive literature review on advances in the pathophysiology of septic AKI and potential therapeutic approaches in this field.
Keywords: Sepsis, Acute kidney injury, Molecular mechanisms, Complement system, Dysregulated immune response, Polymethylmethacrylate membrane hemofilter
Introduction
Sepsis-induced acute kidney injury (SI-AKI) is a common clinical complication of the critically ill patients and is associated with unacceptable high risk for mortality, and its impact extends into long-term outcomes, predisposing to the development of chronic kidney disease (CKD). SI-AKI has a complex and unique pathophysiology principally characterized by an exacerbated immune response associated with systemic endothelial dysfunction and alterations of renal resident cells that may promote progression of kidney disease. The host immune response is enhanced by several mediators including damage- and pathogen-associated molecular patterns (DAMPs/PAMPs) that bind the pattern recognition receptors (PRR), such as toll-like receptors (TLR), expressed on the surface of immune cells [1, 2, 3], inducing their subsequent activation. Additionally, renal resident cells are able to directly interact with these factors, through TLR-2 and TLR-4, and actively participate in amplifying renal damage [1].
A crucial mediator of innate immune response involved in sepsis is the complement system. The kidney is particularly susceptible to complement cascade, activated both by the pathogen itself and by damaged tissue. Additionally, the end product of complement activation, the C5b-9 complex, is associated to the development of multiple organ failure (MOF) as well as other complement fragments with AKI severity after cardiac surgery [4]. Several preclinical and clinical studies have underlined the involvement of complement factors in the pathogenesis of AKI [5]. Unresolved recovery of renal function is associated with a great risk of physiological and structural changes that lead to the progression of chronic renal failure [6]. A broad range of potential pathophysiological mechanisms have been proposed to be involved in AKI-to-CKD transition, including hypoxia and microvascular rarefaction, persistence of chronic inflammation and cell cycle arrest, development of interstitial fibrosis, cell and tissue senescence, and mitochondrial dysfunction [5, 7]. Although considerable advances have been made in our understanding of the pathophysiology of AKI and AKI-to-CKD process, there are no effective and standardized therapeutic strategies in this field.
The introduction of highly sensitive and diagnostic-specific biomarkers might enable the prompt detection and effective treatment of SI-AKI. This approach is critical for the development of new therapies that could take place in the earliest stages, before kidney damage occurs.
Several molecules have been tested in preclinical and clinical studies, to recover mitochondrial dysfunction, inflammation, and oxidative stress. Most of these interventions have been proven ineffective since sepsis is a complex disease that involves several mediators. Clinical studies showed that the use of adsorptive membrane hemofilter reduced systemic inflammation, improving blood pressure and urine output in critically ill patients [8]. In line with these findings, our group reported relevant results on the role of polymethylmethacrylate (PMMA)-based continuous veno-venous hemofiltration (CVVH) in an animal model of SI-AKI. PMMA treatment induced significant modulation of hemodynamic parameters, with preservation of renal function and avoidance of structural changes in the renal parenchyma of endotoxemic animals. This review deeply analyzes the potential mechanisms involved in the pathogenesis of septic AKI and the new advance in diagnosis and therapeutic strategies.
Pathophysiology of SI-AKI
As it is now largely recognized, SI-AKI pathophysiology is complex and multifactorial. Over and above intrarenal hemodynamic changes, a key role has emerged for several elements such as inflammation, vascular dysfunction, bioenergetics, and tubular cell adaptation to injury [1, 9, 10]. Consistently, Gomez et al. [11] proposed a “unified theory” of SI-AKI, consolidating the various mechanisms into a coherent framework of synergic interactions.
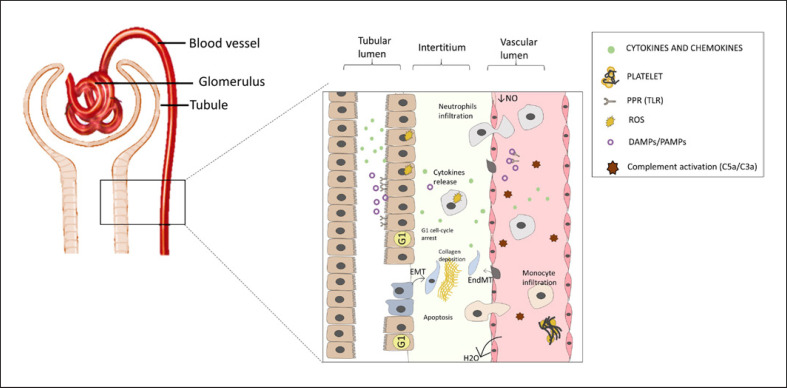
Hyperinflammation is a pivotal hallmark in the pathophysiology of SI-AKI, characterized by a humoral and a cellular mediator which exacerbate the renal injury. However, as the new sepsis definition implies, hyperinflammation leads to neutrophil and macrophage/lymphocyte infiltration followed by MOF and poor outcomes [12]. Mounting evidence has shown that in AKI-induced sepsis, inflammatory mediators including DAMPs/PAMPs are released in the intravascular compartment. DAMP/PAMP interaction with tubular injured cells via PRRs enhances the inflammatory damage by stimulating the production of cytokines (i.e., TNF-α, IL-6, and IL-1β), chemokines (i.e., MCP-1), and adhesion molecules (i.e., VCAMs and ICAMs) (Fig. 1) [13]. Notably, among the PRR family, TLRs are the most extensively studied, following DAMP/PAMP interaction, leading to the recruitment of innate immune system cells [14]. On the other hand, DAMP/PAMP interaction with tubular epithelial cells (TECs) stimulates the production of reactive oxygen species (ROS) resulting in exacerbating oxidative stress, mitochondrial injury, and apoptosis [15]. Thus, the pro-inflammatory renal microenvironment milieu induced by sepsis plays a central role in causing tubular dysfunction. In this scenario, renal resident cells actively participate in amplifying this “cytokine storm” and perpetuating the renal damage [16].
Fig. 1.
Pathophysiology of SI-AKI. SI-AKI is recognized by a complex mechanism characterized by the interplay between resident renal cells and immune system. Hyperinflammation is a physiological stimulus triggered by sepsis injury and is characterized by a humoral and a cellular mediator which exacerbate the renal injury. Hyperinflammation leads to neutrophil and macrophage/lymphocyte infiltration followed by organ failure. PAMPs/DAMPs interaction with tubular injured cells via PRRs enhances the inflammatory damage by stimulating the production of cytokines and adhesion molecules. On the other hand, DAMP/PAMP interaction with proximal TECs results in ROS production, apoptosis, and cell cycle arrest.
The most relevant morphological change observed in the proximal and distal tubule is cellular shedding, characterized by cellular desquamation with increased permeability, resulting in the leakage of glomerular filtrate from the tubular lumen to the interstitium [17, 18]. According to these findings, in a recent study, a swine model of sepsis showed substantial histological changes compared to healthy animals. The observed differences included morphological changes such as vacuolization, epithelial flattening, and necrosis associated with the glomerular capillary rarefaction with monocyte infiltration [19].
Besides the pro-inflammatory microenvironment, the vascular rarefaction after SI-AKI leads to hemodynamic derangement and tissue hypoxia. Regardless of hemodynamic circulation, several clinical studies have observed a wide heterogeneity in blood flow distribution within tissues [20]. The same microcirculatory derangement has been described in different models of SI-AKI [21].
In this regard, we should consider two important pathological processes. First, local hypoxia generated in hyperperfused areas worsens the inflammation and triggers an adaptive metabolic downregulation of the TECs. Of note, hypoxia per se exerts a central role in the pathogenesis of both vascular dysfunction and renal injury [22]. Second, the production of the pro-inflammatory cytokines by endothelial cells increases the expression of adhesion molecules, resulting in leukocyte adhesion, micro-thrombi formation, and endothelial damage exacerbation. In addition, endothelial decline is associated with nitric oxide (NO) reduction. Consequently, the loss of NO-mediated vasodilation further cooperates to worsen a preexisting hyperperfused environment [23, 24]. Finally, the patchy pattern of NO distribution in renal parenchyma involves a heterogeneous distribution of renal blood flow caused by microcirculatory dysfunction in SI-AKI [18, 25].
Alongside hypoxia, an increasing number of studies suggest that oxidative stress is an important hallmark of sepsis-induced tubular injury, as it seems spatially associated with renal areas of sluggish flow [26]. Interestingly, in vitro experiments revealed increased production of ROS in TECs and podocytes treated with bacteria-derived toxins or plasma from septic patients [27, 28]. Nevertheless, postmortem studies on septic patients have shown the heterogeneous distribution of tubular cellular injury with apical vacuolization but without extensive apoptosis or necrosis [22]. Accordingly, recent experimental studies provide essential insights into the central interplay between SI-AKI and apoptosis. The paucity of TECs apoptosis may be explained by the metabolic adaptations to a harmful renal environment favoring cell survival to the detriment of organ function.
In this scenario, mitochondrial dysfunction together with the oxidative outburst orchestrates the metabolic adaptation of TEC resulting in energy optimization, reprogramming metabolism, and counteraction of proapoptotic triggers [25, 29, 30]. Therefore, inflammation implies an optimization and rearrangement of energy consumption, supporting vital functions. Interestingly, the downregulation of tubular transporters (i.e., ion channel and solute carriers) associated with apoptosis inhibition suggests an adaptive mechanism for survival [31]. Although it is still unclear how metabolic reprogramming occurs, experimental studies in septic AKI indicate that the energy requirement may induce a switch from aerobic glycolysis to anaerobic glycolysis [32].
Hence, in SI-AKI physiopathology, the interplay of inflammation and microvascular dysfunction characterizes and amplifies renal injury. In addition, mitochondrial dysfunction may orchestrate a complete metabolic rearrangement, favoring cell survival processes (such as mitophagy, autophagy, and cell cycle arrest), with significant reduction in kidney function (i.e., tubular absorption and secretion of solutes).
Role of Immune Response and Complement System in SI-AKI
Recent evidence has observed that hyperinflammation in the renal environment after SI-AKI is associated with elevated innate and adaptive immune responses [33]. Additionally, a broad range of mediators such as cytokines and chemokines together with complement system have been identified as pivotal factors in sepsis-related tissue injury. Notably, the complement system has been demonstrated to have a significant function since its activation affects organ damage resulting in poor prognosis for septic patients [34].
In this point of view, Singbartl et al. [33] proposed a bidirectional interplay between the immune system and SI-AKI. Following the early host-microbial interactions, a widespread activation of the innate immune system coordinates a defensive response followed by macrophage/lymphocyte infiltration engaged by pro-inflammatory mediators released by injured cells.
First, both DAMPs and PAMPs participate in the development of hyperinflammation since they activate macrophage via TLR [35]. The binding between DAMPs/PAMPs and TLRs in both immune and non-immune cells triggers the assembly of inflammasome which mediates the maturation and secretion of pro-inflammatory factors such as pro-IL-18 and pro-IL-1β [36]. As previously underlined, a wave of inflammatory cells including monocyte/macrophages and T- and B-cells infiltrate the renal interstitium. Many findings showed the importance of the TLRs in the development of sepsis, as the expression of TLR-2 and TLR-4 in monocytes of sepsis patients was upregulated when compared with healthy individuals [37].
Furthermore, a mechanistic role of lymphocytes in the pathogenesis of SI-AKI has been described. Especially an inflammatory subset of CD4+ T cells, the Th17, stimulates neutrophil infiltration by IL-17 production. Remarkably, hyperactivated CD4+Th17 cells were associated with poor outcomes in patients with septic shock [38]. IL-17 knockout mice exhibit a reduced neutrophil infiltration correlated with a reduced TECs apoptosis in SI-AKI. Additionally, IL-17 expression was associated with renal fibrosis in AKI-to-CKD transition [39].
Besides immune cell activation, cytokines and complement systems mediate a tight crosstalk between inflammatory and renal resident cells [40, 41, 42]. Cytokines such as are IL-6, IL-17, IL-8, and TNF-α have a wide range of action in endothelial dysfunction, immune cell activation, and exert an antiapoptotic and profibrotic activity [33].
Together with any other immune mediator, the complement system plays a crucial function in SI-AKI [34]. Complement involves numerous factors which exert a broad number of physiological functions. Mainly, the complement system exerts a first-line defense against bacterial infection and mediates the cross-link between innate and adaptive immunity. Recent clinical and experimental findings suggest that complement activation is associated with MOF and detrimental outcomes during septic shock [43]. Additionally, anaphylatoxins C3a and C5a are mainly involved in vascular permeability, kidney fibrosis, and leukocyte extravasation [44, 45]. Similarly, anaphylatoxins C3a triggers a local inflammation and chemotaxis by binding with receptors on peripheral blood mononuclear cells (i.e., C3aR, C5aR1, and C5aR2) [5]. By this observation, severe septic patients showed elevated levels of C5a strongly associated with MOF and reduced survival rates [46].
Notably, a further player recently detected in the complement activation is PTX3 [47]. Following SI-AKI, PTX3 protein stimulates the classical pathway activation via C1q, resulting in the worsening of injury [48]. In our recent study in a swine model of LPS-induced AKI, PTX3 and C5b-9 deposits significantly increased in peritubular and glomerular capillaries after 24 h of LPS infusion [19]. Besides, increased activation of complement pathways was observed. These findings corroborate the essential role of PTX3 in complement activation and severity of sepsis disease. Finally, further evidence indicates a deep association between serum PTX3 levels and injury severity in several inflammatory and cardiovascular diseases [47].
Additionally, a tight association was found between C5b-9 and MOF development in SI-AKI [49]. C5b-9 is believed to play an important role in the pathogenesis of various kidney diseases by causing cellular injury together with tissue fibrosis and inflammation [50]. Animal models of SI-AKI show a significant activation of C5b-9, especially in the tubulointerstitial compartment. Several in vitro experiments have suggested that C5b-9 exerts a profibrotic activity associated with the progression of renal injury. In addition, human glomerular epithelial cells and TECs treated with C5b-9 significantly increased collagen synthesis and cytokine production [51, 52]. Similar results were obtained in endothelial cells [53]. Collectively, these findings support the crucial role of the immune response and complement in the pathophysiology of SI-AKI and suggest a critical role of inflammation in the AKI-to-CKD transition.
AKI-to-CKD Transition
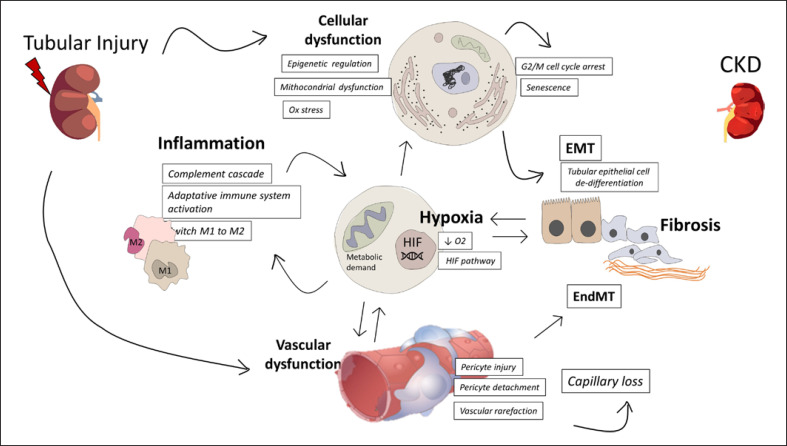
An adaptive response to AKI may lead to the complete recovery of the damage with the total repair of pathological changes [54]. On the other hand, depending on the severity and frequency of the lesion, a maladaptive repair may affect the renal tissue leading to the so-called “AKI-to-CKD transition” which exacerbates the risk of developing CKD and end-stage renal disease. Regardless of the causes of AKI, CKD progression ensue a well-defined pathway leading to the detrimental effect of renal fibrosis and chronic damage [55]. So far, several pathophysiological occurrences and actors have been investigated in the maladaptive response to AKI (Fig. 2).
Fig. 2.
Pathophysiological mechanism involved in the AKI-to-CKD transition. Several mechanisms contribute to maladaptive repair to AKI leading to CKD progression. Main mechanism includes inflammation, hypoxia, vascular rarefaction, and cellular dysfunction which ultimately lead to kidney fibrosis. HIF, hypoxia-inducible factor; EMT, epithelial/mesenchymal transition; EndMT, endothelial/mesenchymal transition.
Tubulointerstitial Fibrosis
Regardless of the different origins of the acute damage, tubulointerstitial fibrosis is one of the main driving forces of AKI-to-CKD progression. Tubulointerstitial fibrosis occurs as a consequence of extracellular matrix (ECM) deposition via mesenchymal cells (fibroblasts or pericytes) or both tubular and endothelial cells by tubular epithelial/mesenchymal transition (EMT) or endothelial/mesenchymal transition, respectively. Of note, EMT arises when TECs reach a mesenchymal phenotype and lose the ability to re-differentiate since they arrest the cell cycle in the G2/M phase [56]. Interestingly, a causal association between cell cycle arrest and fibrosis was supposed since, following AKI, the expression of both epithelial and mesenchymal markers (i.e., N-cadherin, vimentin, and αSMA) in tubular cells promote a partial EMT associated with a senescence-related secretory phenotype [57]. The consequent profibrotic factors production such as TGF-β1, PDGF-β, and CTGF/CCN2 together with stress-induced factors expression (i.e., JNK and MCP-1) enhances an interstitial profibrotic milieu [58]. Other profibrotic growth factors produced by injured TEC are listed in Table 1. Additionally, the production of PAMPs and DAMPs triggers a systemic inflammatory response marked by both humoral (cytokines and chemokines) and cellular (dendritic cells, macrophages, NK, and neutrophils) components boosting the renal inflammation [59]. At once, the Wnt/β-catenin signaling pathway seems to play a pivotal role in this process by supporting both the inflammatory response and ECM deposition [60, 61].
Table 1.
Profibrotic growth factors involved in AKI-to-CKD transition
| Factors | Repored effect | Study | Authors |
|---|---|---|---|
| NGAL and KIM-1 | Profibrotic and pro-inflammatory | In vivo | Ko et al. 2010 [66] |
| SerpinA3 | Profibrotic and pro-inflammatory | In vivo | Navarro et al. 2019 [67] |
| CSF-1 | M2 macrophage activation and polarization | In vivo | Wang et al. 2015 [68] |
| CTGF | Profibrotic, fibroblast proliferation, and cytokine production | In vivo and in vitro | Geng et al. 2012 [69] |
| Notch pathway | Profibrotic and pro-inflammatory | In vivo and in vitro | Kobayashi et al. 2008 [70] |
| WNT/β catenin pathway | Fibroblast to myofibroblast differentiation and regulation of Klotho | In vivo and in vitro | Maarouf et al. 2016 [71] |
| Kuang et al. 2021 [72] | |||
| Xiao et al. 2016 [73] | |||
| VEGF | Macrophage recruitment; VEGF promoter gene hypermethylation at HIF-1α binding site promote fibrosis | In vivo | Leonard et al. 2008 [74] |
| HIF | HIF-1α promotes fibrogenesis via EMT and profibrotic gene activation | In vivo and in vitro | Rosenberger et al. 2002 [75] |
| Higgins et al. 2007 [76] | |||
| YAP | YAP activation promote renal fibrosis via KLF4 and MCP-1 | In vivo | Xu et al. 2021 [77] |
| Zheng et al. 2021 [78] | |||
| NFAT | Increased expression of NFAT2 contributes to renal fibrosis | In vivo and in vitro | Xie et al. 2021 [79] |
| SIK1 | Profibrotic effect via EMT process | In vivo and in vitro | Hu et al. 2021 [80] |
| Ang II | Profibrotic, vascular rarefaction, and stimulation of cytokine production | In vitro and in vivo | Chou et al. 2018 [81] |
| Snail 1 | Induction of partial EMT | In vivo and in vitro | Grande et al. 2015 [82] |
Increasing studies corroborate the role of capillary rarefaction in the development of renal fibrosis and chronic failure after AKI [62]. First, AKI-induced vascular injury leads to long-term implication since renal vascular endothelial cells exhibit a poor regenerative capacity. Besides, experimental evidence in an animal model of endotoxemia-induced AKI suggests that endothelial/mesenchymal transition is one of the most important mechanisms in augmenting capillary rarefaction and chronic interstitial fibrosis [63, 64, 65]. In addition, the detachment of the pericytes from the capillaries represents another mechanism involved in AKI-induced CKD. Evidence demonstrated that pericyte migration was followed by pericyte-to-myofibroblast trans-differentiation during renal ischemia/reperfusion injury and endotoxemia, resulting in the loss of endothelial integrity and vascular rarefaction on one side and advancing collagen deposition on the other [59, 66, 67, 68]. Hence, the interplay between vascular dysfunction, inflammation, and ECM neogenesis are the three mainly detrimental outcomes causing the AKI-to-CKD breakthrough.
Hypoxia and Mithocondrial Dysfunction
Hypoxia is one of the most pivotal triggers for the maladaptive repair of acute damage. The counteracting player of hypoxia is the hypoxia-inducible factor (HIF) activated during the hypoxic state which upregulates plentiful target of downstream genes controlling hematopoiesis, angiogenesis, and metabolism [69]. Furthermore, plenty of evidence highlighted an HIF-dependent regulation addressed for several pro-fibrogenic genes including Col-I, PAI-1, ET-1, CTGF, MMP-2, and TIMP1 [70]. Additionally, HIF activation can stimulate both the proliferation and recruitment of inflammatory cells to the injury site [69]. Consistent with these findings, the genetic ablation of HIF-1α in TECs improved the development of long-term tubulointerstitial fibrosis and inflammation in a mouse model of AKI [71].
Mitochondrial dysfunction affects cellular function, leading to the loss of kidney function during acute injury. In this scenario, an abnormal mitophagy is associated with a failure of renal recovery after AKI by increasing the susceptibility to extended injury in tubular cells [7]. Several studies on ischemia/reperfusion and sepsis models showed a weakened renal repair due to the persistent disruption of mitochondrial homeostasis resulting in severe tubular damage [72, 73]. Key elements in mitophagy, including the PINK1-PARK2 pathway and BNIP3-mediated mitophagy pathway, seem to exert a protective role in preserving the renal tubular integrity and the normal renal function following injury [74]. Interestingly, the PINK1-PARK2 downregulation in a model of LPS-induced sepsis, correlated with TGF-β1 activation, mitochondrial ROS production, and inhibition of mitophagy [75].
Cellular Senescence
Hypoxia, mitochondrial dysfunction, and epigenetic change affect cell cycle arrest and cellular senescence[76]. Cellular senescence is involved in the detrimental consequences of long-term AKI damage and, consequently, in accelerating the maladaptive repair linking AKI to CKD. One of the well-known hallmarks of renal senescence is the downregulation of the antiaging molecule Klotho since the renal environment plays a central role in its regulation and homeostasis [77]. Cellular and animal models show an increase in downregulation of Klotho expression following AKI, outlining a thigh regulation by a few numbers of factors including HIF. For instance, inflammatory components together with the complement mediators such as C1 and C5a can reduce Klotho expression [60, 78]. Finally, it has been suggested that several triggers, including the Notch signaling pathway, play an effective function in activating others pro-senescent molecules such as p21 [79].
Several epigenetic mechanisms orchestrate structural and functional changes in AKI, leading to extension of the tubular and vascular injury. During an acute lesion, the so-called “hypoxia memory” mediates important epigenetic changes in renal cellular chromatin thanks to histone acetylation and histone methylation processes [80, 81, 82, 83, 84]. Several endogenous mediators such as complement factors can affect the histone modification by various mechanisms. For instance, the complement C5a factor induces fibroblast-like phenotype and ECM deposition, via epigenetic modification in TECs [60].
Renal Replacement Therapy in SI-AKI
In course of sepsis, current therapeutic strategies are based on hemodynamic stability, support therapy, and early appropriate antibiotic administration to counteract infection. However, the use of inappropriate antibiotics is associated to overall increased risk of mortality [85] whereas every hour of delay in the administration increases mortality by 8% [86]. Moreover, delayed and/or inappropriate antibiotic administration seems responsible for the developing of multidrug resistance gram-negative sepsis [87]. Indeed, gram-negative sepsis (Enterobacteriaceae, Pseudomonas aeruginosa, and Klebsiella pneumonia) has the highest incidence of multidrug resistance sepsis [88]. The most common drugs associated with AKI are aminoglycosides, vancomycin, radiocontrast media, cisplatin, amphotericin B, foscarnet, and osmotically active agents [89]. Thus, they should be used with caution to avoid renal damage, according to the KDIGO AKI guidelines.
Another important consideration is that there is no specific treatment to prevent or recover renal injury in septic patients, and the support by renal replacement therapy (RRT) becomes necessary when renal function is compromised [90]. In most critically ill patients, the indication for RRT is unquestionable, and the procedure should be initiated without delay. Indeed, the 2012 KDIGO AKI guidelines [91] suggested early initiating RRT in patients with urgent indications such as severe acidosis, severe hyperkalemia, and acute lung edema.
However, the initiation of RRT might find several obstacles related to unstable hemodynamic phase of septic patients. In addition, delays in RRT could compromise acid-base, electrolyte, and fluid balance, causing more severe complications of AKI. Since 2012, several studies have attempted to provide an answer to this issue. Recent randomized clinical trials have evaluated the optimal timing to start RRT in critically ill AKI patients. A meta-analysis, mostly derived from observational studies, suggested a reduction in 28-day mortality in favor of earlier starts [92]. In contrast, both in the Artificial Kidney Initiation in Kidney Injury (AKIKI) multicenter trial [93] and Dialysis Early versus Late in the Intensive Care Unit (IDEAL-ICU) study [94], the benefit of an early RRT start was not provided. Therefore, these controversial findings contribute to lack of a strong recommendation for the use of early RRT in the KDIGO guidelines. In addition, the Surviving Sepsis Campaign Guidelines contain weak recommendations for the choice of intermittent hemodialysis or continuous RRT (CRRT) [95].
In the late 1970s, CRRT was introduced in the intensive care unit [96] in order to manage critically ill patients needing renal support therapy due to AKI and sepsis [97]. CRRT includes four basic techniques as follows: CVVH, continuous veno-venous hemodialysis, and continuous veno-venous hemodiafiltration. CRRT is being increasingly performed in ICU because it offers certain practical advantages such as the cardiovascular tolerance, the control of electrolyte, and acid-base homeostasis [98]. Bellomo et al. [99] suggested that CRRT should be the therapy of choice for critically ill patients requiring RRT, especially for those with hemodynamic instability. CRRT has been criticized for its lack of specificity because it removes both useful molecules and inflammatory mediators [100]. However, the absence of specificity could be seen as an advantage considering the complexity of the septic process.
High-volume hemofiltration (HVHF) is defined as a CRRT with a convective dose >35 mL/kg per h. The benefits of HVHF were investigated in sepsis-like syndromes such as resuscitated cardiac arrest patients [101] and patients with severe acute pancreatitis [102]. A multicenter study (Hemofiltration Study: High Volume in Intensive Care [IVOIRE]) investigated the 28-, 60-, or 90-day mortality after HVHF (70 mL/kg per h) or standard hemofiltration (35 mL/kg per h) for 96 h. No differences were found in terms of survival rate, renal function, hemodynamics, or organ failure between two treatment modalities. However, the results were underpowered since only 30% of the estimated sampling size was effectively recruited [103]. In accordance, another clinical study that compared the HVHF treatment with two different convective doses (85 mL/kg and 50 mL/kg per h) failed to demonstrate improved survival and renal outcome [104].
Some clinical studies demonstrated that HVHF induced an improvement in hemodynamics and organ perfusion and a decrease in circulating inflammatory cytokines. However, these improvements did not ameliorate survival and clinical outcome [95]. Otherwise, recent clinical trial with septic children and children with septic AKI assessed the efficacy of HVHF (convective dose of 50–100 mL/kg/h) in decreasing the plasma concentration of inflammatory mediators and improving hemodynamics and survival rate [105]. The discordance between these findings is probably due to the use of conventional hemofilters in HVHF [106], such as membranes without adsorbing properties [107]. Combining HVHF and adsorptive membranes may optimize the technique, providing a significant clinical outcome and mortality benefit in septic patients.
Experimental Therapies for SI-AKI: Blood Purification Approaches
The complexity of septic disease characterized by a dysfunctional immune response with a cytokine storm, poor clinical outcomes, and low survival rates led to optimize available extracorporeal blood purification techniques [96, 106]. Systemic immune imbalance is the common denominator between renal failure and sepsis. Indeed, early renal dysfunction has been well documented in experimental septic models with systemic inflammation [19, 63, 67, 68, 108] and also in critically ill patients, according to SOFA scores [8, 109]. Ronco et al. [110] proposed the “cytokine peak hypothesis,” affirming that the early removal of both pro- and anti-inflammatory mediators from the bloodstream may effectively prevent the toxic tolerance, reducing local and systemic injuries. In addition, the removal of cytokines might influence the level of local inflammatory mediators, preserving organ function [110, 111, 112]. The hypothesis that blood purification improves the outcome of SI-AKI remains to be established.
In the last decades, new adsorptive membranes have been developed to offer both renal support and amelioration of hemodynamic stability. These membranes have the advantage to enhance the clearance of middle-to-high molecular weight mediators. Polymyxin B is a resin membrane with demonstrated capacity to bind endotoxin, decreasing circulating LPS levels in septic patients [113]. Multiple clinical trials have been conducted to determine the efficacy of PMX-HP and have shown conflicting results [114]. A multicenter pilot trial enrolled 36 surgical patients with intra-abdominal sepsis and demonstrated that the treatment with 2-h PMX-HP improved left ventricular function and decreased RRT requirement [114]. Accordingly, another clinical trial, Early Use of Polymyxin B Hemoperfusion in Abdominal Sepsis (EUPHAS), reported significant improvements in terms of hemodynamic stability, renal function, and 28-day survival in patients with severe intra-abdominal sepsis, treated with PMX-HP [115]. A multicenter trial, Safety and Efficacy of Polymyxin B Hemoperfusion for Septic Shock (EUPHRATES) analyzed the effects of 2 PMX-HP sessions versus hemoperfusion plus standard therapy in 450 patients with septic shock. The authors did not find significant improvement in survival rate and renal function [116]; however, a post hoc analysis of the trial focusing on patients with endotoxin activity assay between 0.6 and 0.9 reported a significant improvement in mortality as well as in hemodynamic and respiratory endpoints [117]. Interestingly, Srisawat et al. [118] demonstrated for the first time that PMX-HP improved mHLA-DR expression in severe sepsis patients, providing beneficial effects in immune response.
CytoSorb membrane is used as hemoperfusion cartridge to absorb and remove high cytokines. Several studies showed the capacity of this membrane to remove cytokines, complement mediators, PAMPs, and DAMPs [119, 120, 121]. However, these studies did not report improvements in terms of hemodynamics and renal function. CytoSorb has been used to reduce inflammatory response in course of severe pancreatitis or cardio-pulmonary bypass [122]. The first results of the clinical trial, NCT02312024, related to the use of CytoSorb adsorber in real-life critically ill patients, reported significantly decrease of IL-6 levels and no declines in SOFA scores [123].
Oxiris is AN69-based membrane designed specifically for cytokine and LPS removal, through its surface treated with polyethyleneimine and heparin [95]; it is capable of removing inflammatory mediators. Moreover, a randomized double-blind crossover study of septic shock-related acute renal failure showed beneficial hemodynamic effects and reduced levels of LPS and pro-inflammatory mediators compared to standard hemofilter [124]. However, evidence supporting its favorable outcomes on renal function has to be demonstrated.
PMMA Membrane Hemofilter: Recent Advances
Another membrane with adsorptive properties is the PMMA characterized by larger and longer pores and an overall high-specific surface area dedicated, almost exclusively, to trap substances with high molecular weights [95]. PMMA is principally composed of two methylmethacrylate polymer elements that, when mixed together, generated a synthetic polymeric membrane [95]. This filter was developed for dialysis treatment in chronic field; moreover, PMMA showed excellent capacity to remove β2-MG by adsorption, decreasing toxic effects of this pathogenic molecule, involved in dialysis-related amyloidosis [125]. In the last forty years, the potential of this adsorptive membrane has been well recognized. Indeed, several clinical studies revealed significant effects in critically ill patients that cannot be explained as those of RRT alone [95]. In particular, there was a rapid improvement in several clinical symptoms and also clinical parameters such as recovery of urine output and mean arterial pressure [109, 126, 127]. Of course, these results could be related to the capacity of PMMA to remove pathogenic mediators.
Matsuda et al. [126] investigated the effectiveness of PMMA-CHDF treatment in 43 consecutive septic shock patients with AKI, comparing it to a standard hemofilter made of polyacrylonitrile. Following 24 h of treatment, the authors found an increase of urine output and amelioration of hemodynamic stability compared with standard hemofilter. Accordingly, Sakamoto et al. [127] showed a great efficacy of PMMA for removal of several cytokines in septic patients. Then, these clinical findings showed that PMMA membrane hemofilter in CHDF modality reduced systemic inflammation and improved hemodynamic stability and renal function in critically ill patients [8].
Different publications show in vitro data on the effect of PMMA high adsorptive properties in removing IL-6, IL-8, IL1-β, TNF-α, and HMGB-1 [95, 128]. Recently, a new version of PMMA membrane hemofilter for continuous RRT obtained the CE mark and is available for the clinical use in Europe. This membrane, HEMOFEEL CH-1.8 W, is a non-ionic PMMA membrane with an effective surface area of 1.8 m2, an internal hollow fiber diameter of 240 μm, and a wall thickness of 30 μm. A declared cutoff of 38 kD with an ultrafiltration rate of 30 mL/h/mm Hg, measured in standard experimental setting condition with a Qb = 200 mL/min.
Considering the principal clinical signs for AKI, such as the significant increase in serum creatinine, decrease in urine output, and the sepsis-induced hypotension, our group recently demonstrated the effectiveness of PMMA to recover renal function and hemodynamic status compared to polysulphone (PS) treatment in a swine model of LPS-induced AKI [19]. Moreover, histological analysis and, in particular, Masson trichrome staining underlined the impact of PMMA in reducing renal damage and early fibrosis with respect to PS [19]. Interestingly, at systemic level, we found that PMMA and not PS reduced LBP, serum complement activation, and significantly reduced circulating sCD40 and sCD40L [19].
LBP is an acute-phase protein, synthesized by hepatocytes that enhances and amplifies cellular response to endotoxin, and it is crucial in the development of early renal fibrosis [63, 67, 129, 130, 131]. Interestingly, PMMA significantly removed LBP from blood circulation, suggesting its efficacy in preventing renal fibrosis and the subsequent progression to CKD. In addition, it was able to modulate the sCD40L/sCD40 axis, through the removal of both mediators [19]. Then, the ability of PMMA is to remove those mediators that are present in large number, preserving homeostatic balance, assuring better immune competence in septic patients, and avoiding immunodepression phase and secondary infections.
Several studies reported that the gene expression profiles of circulating leukocytes correlate with renal diseases [132, 133] and might be a potentially useful tool for discovery-oriented studies of the pathogenesis of sepsis and severe infection [134, 135]. Indeed, such studies are based on the assumption that molecular profiling of circulating blood cells might reflect physiological and pathological events occurring in different tissues of the body [135]. Interestingly, we analyzed gene expression profile of circulating leukocytes, and we provided molecular explanation of the PMMA effectiveness by modulation of PBMC transcriptome [19]. In particular, our analysis demonstrated an increased expression of several genes involved in inflammatory response and complement system activation with significant downregulation in PMMA-treated peripheral blood mononuclear cells that was also associated to better renal recovery [19].
In addition, hemadsorption with this new membrane-modulated local and systemic complement activation was contributing to the balance between pro- and anti-inflammatory processes. Therefore, the use of the new PMMA membrane hemofilter might prevent an exacerbated inflammatory response on one hand and the paralysis of cell-mediated immunity on the other, resulting in early recovery of renal function. Considering these findings, we believe that PMMA-CVVH treatment might represent a promising therapeutic strategy to modulate cytokine storm and to assure immune competence with a significant impact on short- and long-term outcomes for patients with systemic inflammatory syndrome.
Conclusion
The early application of blood purification therapies to remove circulating inflammatory mediators and bacterial toxins might improve immune homeostasis, preventing the subsequent molecular mechanisms involved in SI-AKI and CKD progression. Despite initially promising results in preclinical studies, the application of these novel techniques in clinical studies did not provide sustainable survival benefits. Large-scale randomized controlled trials could measure the effectiveness of this intervention in septic-AKI field. Then, the hypothesis that blood purification improves the outcome of septic AKI remains to be established. Therefore, technological advancements in blood purification approaches and well-designed, prospective randomized controlled trials are the way to obtain concrete evidence in terms of clinical outcome.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was supported by University of Bari “Aldo Moro” and the Italian Ministry of Health (Giovani Ricercatori 2011-2012, GR-2011-02351027, granted to Giuseppe Castellano; Fondo Sociale Europeo, Azione I.2 “Attrazione e Mobilità Internazionale dei Ricercatori” – AIM-1810057 – activity 2 granted to Alessandra Stasi).
Author Contributions
Alessandra Stasi contributed to conceptualization, design, writing, and editing of the work. Rossana Franzin and Gianvito Caggiano contributed to the conceptualization, design, and drafting of the work; Gianvito Caggiano conceived figure and table; Rosa Losapio, Marco Fiorentino, and Carlo Alfieri contributed to the draft; Loreto Gesualdo and Giovanni Stallone critically revised the manuscript; Giuseppe Castellano contributed substantially to the work reported by critical revisions and draft editing. All authors have read and agreed to the published version of the manuscript.
verified
Funding Statement
This study was supported by University of Bari “Aldo Moro” and the Italian Ministry of Health (Giovani Ricercatori 2011-2012, GR-2011-02351027, granted to Giuseppe Castellano; Fondo Sociale Europeo, Azione I.2 “Attrazione e Mobilità Internazionale dei Ricercatori” – AIM-1810057 – activity 2 granted to Alessandra Stasi).
References
- 1.Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96((5)):1083–1099. doi: 10.1016/j.kint.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilgili B, Haliloğlu M, Cinel İ. Sepsis and acute kidney injury. Turk J Anaesthesiol Reanim. 2014;42((6)):294–301. doi: 10.5152/TJAR.2014.83436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rimmelé T, Payen D, Cantaluppi V, Marshall J, Gomez H, Gomez A, et al. Immune cell phenotype and function in sepsis. Shock. 2016;45((3)):282–291. doi: 10.1097/SHK.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCullough JW, Renner B, Thurman JM. The role of the complement system in acute kidney injury. Semin Nephrol. 2013 Nov;33((6)):543–556. doi: 10.1016/j.semnephrol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franzin R, Stasi A, Fiorentino M, Stallone G, Cantaluppi V, Gesualdo L, et al. Inflammaging and complement system a link between acute kidney injury and chronic graft damage. Front Immunol. 2020;11:734. doi: 10.3389/fimmu.2020.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franzin R, Stasi A, Fiorentino M, Simone S, Oberbauer R, Castellano G, et al. Renal Delivery of Pharmacologic Agents During Machine Perfusion to Prevent Ischaemia-Reperfusion Injury From Murine Model to Clinical Trials. Front Immunol. 2021;12:673562. doi: 10.3389/fimmu.2021.673562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He L, Wei Q, Liu J, Yi M, Liu Y, Liu H, et al. AKI on CKD heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017 Nov;92((5)):1071–1083. doi: 10.1016/j.kint.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumura Y, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Watanabe E, et al. Treatment of septic shock with continuous HDF using 2 PMMA hemofilters for enhanced intensity. Int J Artif Organs. 2012;35((1)):3–14. doi: 10.5301/ijao.5000044. [DOI] [PubMed] [Google Scholar]
- 9.Chvojka J, Sýkora R, Karvunidis T, Raděj J, Kroužecký A, Novák I. New developments in septic acute kidney injury. Physiol Res. 2010;59((6)):859–869. doi: 10.33549/physiolres.931936. [DOI] [PubMed] [Google Scholar]
- 10.Stasi A, Franzin R, Fiorentino M, Squiccimarro E, Castellano G, Gesualdo L. Multifaced Roles of HDL in Sepsis and SARS-CoV-2 Infection Renal Implications. Int J Mol Sci. 2021;22((11)):5980. doi: 10.3390/ijms22115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez H, Ince C, de Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014 Jan;41((1)):3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) J Am Med Assoc. 2016 Feb;315((8)):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderberg SB, Luther T, Frithiof R. Physiological aspects of Toll-like receptor 4 activation in sepsis-induced acute kidney injury. Acta Physiol. 2017 Mar;219((3)):573–588. doi: 10.1111/apha.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramnath RD, Ng SW, Guglielmotti A, Bhatia M. Role of MCP-1 in endotoxemia and sepsis. Int Immunopharmacol. 2008 Jun;8((6)):810–818. doi: 10.1016/j.intimp.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Quoilin C, Mouithys-Mickalad A, Lécart S, Fontaine-Aupart M-P, Hoebeke M. Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim Biophys Acta. 2014;1837((10)):1790–1800. doi: 10.1016/j.bbabio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Cantaluppi V, Quercia AD, Dellepiane S, Ferrario S, Camussi G, Biancone L. Interaction between systemic inflammation and renal tubular epithelial cells. Nephrol Dial Transpl. 2014 Nov;29((11)):2004–2011. doi: 10.1093/ndt/gfu046. [DOI] [PubMed] [Google Scholar]
- 17.Bellomo R, Wan L, Langenberg C, May C. Septic acute kidney injury new concepts. Nephron Exp Nephrol. 2008 Sep;109((4)):e95–e100. doi: 10.1159/000142933. [DOI] [PubMed] [Google Scholar]
- 18.Garofalo AM, Lorente-Ros M, Goncalvez G, Carriedo D, Ballén-Barragán A, Villar-Fernández A, et al. Histopathological changes of organ dysfunction in sepsis. Intensive Care Med Exp. 2019 Jul;7((Suppl 1)):45. doi: 10.1186/s40635-019-0236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stasi A, Franzin R, Divella C, Sallustio F, Curci C, Picerno A, et al. PMMA-based continuous hemofiltration modulated complement activation and renal dysfunction in LPS-induced acute kidney injury. Front Immunol. 2021 Apr;12:605212. doi: 10.3389/fimmu.2021.605212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Post EH, Kellum JA, Bellomo R, Vincent JL. Renal perfusion in sepsis from macro- to microcirculation. Kidney Int. 2017 Jan;91((1)):45–60. doi: 10.1016/j.kint.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 21.Legrand M, Bezemer R, Kandil A, Demirci C, Payen D, Ince C. The role of renal hypoperfusion in development of renal microcirculatory dysfunction in endotoxemic rats. Intensive Care Med. 2011 Sep;37((9)):1534–1542. doi: 10.1007/s00134-011-2267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerolle N, Nochy D, Guérot E, Bruneval P, Fagon JY, Diehl JL, et al. Histopathology of septic shock induced acute kidney injury apoptosis and leukocytic infiltration. Intensive Care Med. 2010 Mar;36((3)):471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 23.Lowry JL, Brovkovych V, Zhang Y, Skidgel RA. Endothelial nitric-oxide synthase activation generates an inducible nitric-oxide synthase-like output of nitric oxide in inflamed endothelium. J Biol Chem. 2013 Feb;288((6)):4174–4193. doi: 10.1074/jbc.M112.436022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trzeciak S, Cinel I, Phillip Dellinger R, Shapiro NI, Arnold RC, Parrillo JE, et al. Resuscitating the microcirculation in sepsis the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med. 2008 May;15((5)):399–413. doi: 10.1111/j.1553-2712.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013 Mar;187((5)):509–517. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang CC, Ma MC, Chien CT, Wu MS, Sun WK, Chen CF. Hypoxic preconditioning attenuates lipopolysaccharide-induced oxidative stress in rat kidneys. J Physiol. 2007 Jul;582((Pt 1)):407–419. doi: 10.1113/jphysiol.2006.122747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariano F, Cantaluppi V, Stella M, Romanazzi GM, Assenzio B, Cairo M, et al. Circulating plasma factors induce tubular and glomerular alterations in septic burns patients. Crit Care. 2008 Mar;12((2)):R42. doi: 10.1186/cc6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen M, Jahnukainen T, Bao W, Daré E, Ceccatelli S, Celsi G. Uropathogenic Escherichia coli toxins induce caspase-independent apoptosis in renal proximal tubular cells via ERK signaling. Am J Nephrol. 2003;23((3)):140–151. doi: 10.1159/000069853. [DOI] [PubMed] [Google Scholar]
- 29.Gómez H, Kellum JA, Ronco C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol. 2017 Mar;13((3)):143–151. doi: 10.1038/nrneph.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Nourbakhsh N, Pham H, Tham R, Zuckerman JE, Singh P. Evolution of altered tubular metabolism and mitochondrial function in sepsisassociated acute kidney injury. Am J Physiol Ren Physiol. 2020 Aug;319((2)):F229–F244. doi: 10.1152/ajprenal.00390.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Good DW, George T, Watts BA. Lipopolysaccharide directly alters renal tubule transport through distinct TLR4-dependent pathways in basolateral and apical membranes. Am J Physiol Ren Physiol. 2009;297((4)):866–874. doi: 10.1152/ajprenal.00335.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W, et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun. 2014 Jul;5:4436. doi: 10.1038/ncomms5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singbartl K, Formeck CL, Kellum JA. Kidney-immune system crosstalk in AKI. Semin Nephrol. 2019 Jan;39((1)):96–106. doi: 10.1016/j.semnephrol.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Markiewski MM, Deangelis RA, Lambris JD. Complexity of complement activation in sepsis. J Cell Mol Med. 2008;12((6A)):2245–2254. doi: 10.1111/j.1582-4934.2008.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar V. Inflammasomes pandora's box for sepsis. J Inflamm Res. 2018;11:477–502. doi: 10.2147/JIR.S178084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong L, Medford ARL, Hunter KJ, Uppington KM, Millar AB. Differential expression of Toll-like receptor (TLR)-2 and TLR-4 on monocytes in human sepsis. Clin Exp Immunol. 2004 May;136((2)):312–319. doi: 10.1111/j.1365-2249.2004.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maravitsa P, Adamopoulou M, Pistiki A, Netea MG, Louis K, Giamarellos-Bourboulis EJ. Systemic over-release of interleukin-17 in acute kidney injury after septic shock clinical and experimental evidence. Immunol Lett. 2016 Oct;178:68–76. doi: 10.1016/j.imlet.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Luo C-J, Luo F, Zhang L, Xu Y, Cai G-Y, Fu B, et al. Knockout of interleukin-17A protects against sepsis-associated acute kidney injury. Ann Intensive Care. 2016;6((1)):56. doi: 10.1186/s13613-016-0157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zager RA, Johnson AC, Lund S, Hanson S. Acute renal failure determinants and characteristics of the injury-induced hyperinflammatory response. Am J Physiol Renal Physiol. 2006;291((3)):F546–F456. doi: 10.1152/ajprenal.00072.2006. [DOI] [PubMed] [Google Scholar]
- 41.Andres-Hernando A, Dursun B, Altmann C, Ahuja N, He Z, Bhargava R, et al. Cytokine production increases and cytokine clearance decreases in mice with bilateral nephrectomy. Nephrol Dial Transpl. 2012;27((12)):4339–4347. doi: 10.1093/ndt/gfs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medica D, Franzin R, Stasi A, Castellano G, Migliori M, Panichi V, et al. Extracellular Vesicles Derived from Endothelial Progenitor Cells Protect Human Glomerular Endothelial Cells and Podocytes from Complement- and Cytokine-Mediated Injury. Cells. 2021;10((7)):1675. doi: 10.3390/cells10071675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charchaflieh J, Wei J, Labaze G, Hou YJ, Babarsh B, Stutz H, et al. The role of complement system in septic shock. Clin Dev Immunol. 2012;2012:407324. doi: 10.1155/2012/407324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng Q, Li K, Sacks SH, Zhou W. The role of anaphylatoxins C3a and C5a in regulating innate and adaptive immune responses. Inflamm Allergy Drug Targets. 2009;8((3)):236–246. doi: 10.2174/187152809788681038. [DOI] [PubMed] [Google Scholar]
- 45.Curci C, Castellano G, Stasi A, Divella C, Loverre A, Gigante M, et al. Endothelial-to-mesenchymal transition and renal fibrosis in ischaemia/reperfusion injury are mediated by complement anaphylatoxins and Akt pathway. Nephrol Dial Transpl. 2014 Apr;29((4)):799–808. doi: 10.1093/ndt/gft516. [DOI] [PubMed] [Google Scholar]
- 46.Riedemann NC, Guo R-F, Ward PA. A key role of C5a/C5aR activation for the development of sepsis. J Leukoc Biol. 2003 Dec;74((6)):966–970. doi: 10.1189/jlb.0403137. [DOI] [PubMed] [Google Scholar]
- 47.Porte R, Davoudian S, Asgari F, Parente R, Mantovani A, Garlanda C, et al. The long pentraxin PTX3 as a humoral innate immunity functional player and biomarker of infections and sepsis. Front Immunol. 2019;10:794. doi: 10.3389/fimmu.2019.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma YJ, Garred P, Stover CM, Ma YJ, Garred P. Pentraxins in complement activation and regulation. Front Immunol. 2018;9:3046. doi: 10.3389/fimmu.2018.03046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmad FM, A Al-Binni M, Bani Hani A, Abu Abeeleh M, Abu-Humaidan AHA. Complement terminal pathway activation is associated with organ failure in sepsis patients. J Inflamm Res. 2022 Jan;15:153–162. doi: 10.2147/JIR.S344282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koopman JJE, van Essen MF, Rennke HG, de Vries APJ, van Kooten C. Deposition of the membrane attack complex in healthy and diseased human kidneys. Front Immunol. 2021;11:599974. doi: 10.3389/fimmu.2020.599974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torbohm I, Schonermark M, Wingen AM, Berger B, Rother K, Hansch GM. C5b-8 and C5b-9 modulate the collagen release of human glomerular epithelial cells. Kidney Int. 1990 Apr;37((4)):1098–1104. doi: 10.1038/ki.1990.91. [DOI] [PubMed] [Google Scholar]
- 52.Abe K, Li K, Sacks SH, Sheerin NS. The membrane attack complex up regulates collagen gene expression in renal tubular epithelial cells. Clin Exp Immunol. 2004 Apr;136((1)):60–66. doi: 10.1111/j.1365-2249.2004.02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benzaquen LR, Nicholson-Weller A, Halperin JA. Terminal complement proteins C5b-9 release basic fibroblast growth factor and platelet-derived growth factor from endothelial cells. J Exp Med. 1994 Mar;179((3)):985–992. doi: 10.1084/jem.179.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, et al. Progression after AKI understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol. 2016 Mar;27((3)):687–697. doi: 10.1681/ASN.2015030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takaori K, Yanagita M. Insights into the mechanisms of the acute kidney injury-to-chronic kidney disease continuum. Nephron. 2016;134((3)):172–176. doi: 10.1159/000448081. [DOI] [PubMed] [Google Scholar]
- 56.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16((5)):535–543. doi: 10.1038/nm.2144. 1p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fiorentino M, Grandaliano G, Gesualdo L, Castellano G. Acute kidney injury to chronic kidney disease transition. Contrib Nephrol. 2018;193:45–54. doi: 10.1159/000484962. [DOI] [PubMed] [Google Scholar]
- 58.Yang L. How acute kidney injury contributes to renal fibrosis. Adv Exp Med Biol. 2019;1165:117–142. doi: 10.1007/978-981-13-8871-2_7. [DOI] [PubMed] [Google Scholar]
- 59.Guzzi F, Cirillo L, Roperto RM, Romagnani P, Lazzeri E. Molecular mechanisms of the acute kidney injury to chronic kidney disease transition an updated view. Int J Mol Sci. 2019;20((19)):4941. doi: 10.3390/ijms20194941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castellano G, Franzin R, Sallustio F, Stasi A, Banelli B, Romani M, et al. Complement component C5a induces aberrant epigenetic modifications in renal tubular epithelial cells accelerating senescence by Wnt4/βcatenin signaling after ischemia/reperfusion injury. Aging. 2019 Jul;11((13)):4382–4406. doi: 10.18632/aging.102059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huffstater T, Merryman WD, Gewin LS. Wnt/β-Catenin in acute kidney injury and progression to chronic kidney disease. Semin Nephrol. 2020 Mar;40((2)):126–137. doi: 10.1016/j.semnephrol.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Basile DP. The endothelial cell in ischemic acute kidney injury implications for acute and chronic function. Kidney Int. 2007 Jul;72((2)):151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 63.Castellano G, Stasi A, Intini A, Gigante M, di Palma AM, Divella C, et al. Endothelial dysfunction and renal fibrosis in endotoxemia-induced oliguric kidney injury possible role of LPS-binding protein. Crit Care. 2014 Oct;18((5)):520. doi: 10.1186/s13054-014-0520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, et al. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Ren Physiol. 2011 Mar;300((3)):F721–F733. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xavier S, Vasko R, Matsumoto K, Zullo JA, Chen R, Maizel J, et al. Curtailing endothelial TGF-β signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J Am Soc Nephrol. 2015 Apr;26((4)):817–829. doi: 10.1681/ASN.2013101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castellano G, Franzin R, Stasi A, Divella C, Sallustio F, Pontrelli P, et al. Complement activation during ischemia/reperfusion injury induces pericyte-to-myofibroblast transdifferentiation regulating peritubular capillary lumen reduction through pERK signaling. Front Immunol. 2018 May;9:1002. doi: 10.3389/fimmu.2018.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castellano G, Stasi A, Franzin R, Sallustio F, Divella C, Spinelli A, et al. LPS-binding protein modulates acute renal fibrosis by inducing pericyte-to-myofibroblast trans-differentiation through TLR-4 signaling. Int J Mol Sci. 2019 Jul;20((15)):3682. doi: 10.3390/ijms20153682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sallustio F, Stasi A, Curci C, Divella C, Picerno A, Franzin R, et al. Renal progenitor cells revert LPS-induced endothelial-to-mesenchymal transition by secreting CXCL6 and BPIFA2 antiseptic peptides. FASEB J. 2019;33((10)):10753–66. doi: 10.1096/fj.201900351R. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Ren Physiol. 2014 Dec;307((11)):F1187–F1195. doi: 10.1152/ajprenal.00425.2014. [DOI] [PubMed] [Google Scholar]
- 70.Liu J, Kumar S, Dolzhenko E, Alvarado GF, Guo J, Lu C, et al. Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight. 2017 Sep;2((18)):e94716. doi: 10.1172/jci.insight.94716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shu S, Wang Y, Zheng M, Liu Z, Cai J, Tang C, et al. Hypoxia and hypoxia-inducible factors in kidney injury and repair. Cells. 2019 Feb;8((3)):207. doi: 10.3390/cells8030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van der Slikke EC, Star BS, van Meurs M, Henning RH, Moser J, Bouma HR. Sepsis is associated with mitochondrial DNA damage and a reduced mitochondrial mass in the kidney of patients with sepsis-AKI. Crit Care. 2021 Jan;25((1)):36. doi: 10.1186/s13054-020-03424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Ren Physiol. 2012 Apr;302((7)):F853–F864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li S, Lin Q, Shao X, Zhu X, Wu J, Wu B, et al. Drp1-regulated PARK2-dependent mitophagy protects against renal fibrosis in unilateral ureteral obstruction. Free Radic Biol Med. 2020 May;152:632–649. doi: 10.1016/j.freeradbiomed.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Bhatia D, Chung K-P, Nakahira K, Patino E, Rice MC, Torres LK, et al. Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis. JCI Insight. 2019 Dec;4((23)):e132826. doi: 10.1172/jci.insight.132826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Franzin R, Stasi A, Ranieri E, Netti GS, Cantaluppi V, Gesualdo L, et al. Targeting Premature Renal Aging from Molecular Mechanisms of Cellular Senescence to Senolytic Trials. Front Pharmacol. 2021;12:630419. doi: 10.3389/fphar.2021.630419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kurzhagen JT, Dellepiane S, Cantaluppi V, Rabb H. AKI an increasingly recognized risk factor for CKD development and progression. J Nephrol. 2020 Dec;33((6)):1171–1187. doi: 10.1007/s40620-020-00793-2. [DOI] [PubMed] [Google Scholar]
- 78.Castellano G, Intini A, Stasi A, Divella C, Gigante M, Pontrelli P, et al. Complement modulation of anti-aging factor klotho in ischemia/reperfusion injury and delayed graft function. Am J Transpl. 2016;16((1)):325–333. doi: 10.1111/ajt.13415. [DOI] [PubMed] [Google Scholar]
- 79.Kobayashi T, Terada Y, Kuwana H, Tanaka H, Okado T, Kuwahara M, et al. Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney Int. 2008 Jun;73((11)):1240–1250. doi: 10.1038/ki.2008.74. [DOI] [PubMed] [Google Scholar]
- 80.Li Z, Li N. Epigenetic modification drives acute kidney injury-to-chronic kidney disease progression. Nephron. 2021;145((6)):737–747. doi: 10.1159/000517073. [DOI] [PubMed] [Google Scholar]
- 81.Irifuku T, Doi S, Sasaki K, Doi T, Nakashima A, Ueno T, et al. Inhibition of H3K9 histone methyltransferase G9a attenuates renal fibrosis and retains klotho expression. Kidney Int. 2016 Jan;89((1)):147–157. doi: 10.1038/ki.2015.291. [DOI] [PubMed] [Google Scholar]
- 82.Sasaki K, Doi S, Nakashima A, Irifuku T, Yamada K, Kokoroishi K, et al. Inhibition of SET domain-containing lysine methyltransferase 7/9 ameliorates renal fibrosis. J Am Soc Nephrol. 2016 Jan;27((1)):203–215. doi: 10.1681/ASN.2014090850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nangaku M, Hirakawa Y, Mimura I, Inagi R, Tanaka T. Epigenetic changes in the acute kidney injury-to-chronic kidney disease transition. Nephron. 2017;137((4)):256–259. doi: 10.1159/000476078. [DOI] [PubMed] [Google Scholar]
- 84.Ogbadu J, Singh G, Aggarwal D. Factors affecting the transition of acute kidney injury to chronic kidney disease potential mechanisms and future perspectives. Eur J Pharmacol. 2019 Dec;865:172711. doi: 10.1016/j.ejphar.2019.172711. [DOI] [PubMed] [Google Scholar]
- 85.Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003 Nov;115((7)):529–535. doi: 10.1016/j.amjmed.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 86.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34((6)):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 87.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant enterobacteriaceae epidemiology and prevention. Clin Infect Dis. 2011 Jul;53((1)):60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 88.Busani S, Roat E, Serafini G, Mantovani E, Biagioni E, Girardis M. The role of adjunctive therapies in septic shock by gram negative MDR/XDR infections. Can J Infect Dis Med Microbiol. 2017;2017:2808203. doi: 10.1155/2017/2808203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gameiro J, Fonseca JA, Outerelo C, Lopes JA. Acute kidney injury from diagnosis to prevention and treatment strategies. J Clin Med. 2020 Jun;9((6)):1704. doi: 10.3390/jcm9061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seabra VF, Balk EM, Liangos O, Sosa MA, Cendoroglo M, Jaber BL. Timing of renal replacement therapy initiation in acute renal failure a meta-analysis. Am J Kidney Dis. 2008 Aug;52((2)):272–284. doi: 10.1053/j.ajkd.2008.02.371. [DOI] [PubMed] [Google Scholar]
- 91.Kidney Disease Improving Global Outcomes KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012 Mar;2:1–138. [Google Scholar]
- 92.Pasin L, Boraso S, Tiberio I. Early initiation of renal replacement therapy in critically ill patients a meta-analysis of randomized clinical trials. BMC Anesthesiol. 2019 May;19((1)):62. doi: 10.1186/s12871-019-0733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016 Jul;375((2)):122–133. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 94.Barbar SD, Clere-Jehl R, Bourredjem A, Hernu R, Montini F, Bruyère R, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018 Oct;379((15)):1431–1442. doi: 10.1056/NEJMoa1803213. [DOI] [PubMed] [Google Scholar]
- 95.Hattori N, Oda S. Cytokine-adsorbing hemofilter old but new modality for septic acute kidney injury. Ren Replace Ther. 2016;2((1)):41. [Google Scholar]
- 96.Rimmelé T, Kellum JA. Clinical review blood purification for sepsis. Crit Care. 2011;15((1)):205. doi: 10.1186/cc9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Metnitz PGH, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002 Sep;30((9)):2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 98.Uchino S, Bellomo R, Ronco C. Intermittent versus continuous renal replacement therapy in the ICU impact on electrolyte and acid-base balance. Intensive Care Med. 2001 Jun;27((6)):1037–1043. doi: 10.1007/s001340100953. [DOI] [PubMed] [Google Scholar]
- 99.RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009 Oct;361((17)):1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 100.Ratanarat R, Permpikul C, Ronco C. Renal replacement therapy in acute renal failure which index is best for dialysis dose quantification? Int J Artif Organs. 2007 Mar;30((3)):235–243. doi: 10.1177/039139880703000309. [DOI] [PubMed] [Google Scholar]
- 101.Laurent I, Adrie C, Vinsonneau C, Cariou A, Chiche J-D, Ohanessian A, et al. High-volume hemofiltration after out-of-hospital cardiac arrest a randomized study. J Am Coll Cardiol. 2005 Aug;46((3)):432–437. doi: 10.1016/j.jacc.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 102.Jiang HL, Xue WJ, Li DQ, Yin AP, Xin X, Li CM, et al. Influence of continuous veno-venous hemofiltration on the course of acute pancreatitis. World J Gastroenterol. 2005 Aug;11((31)):4815–4821. doi: 10.3748/wjg.v11.i31.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Honoré PM, Jacobs R, Boer W, Joannes-Boyau O, de Regt J, de Waele E, et al. New insights regarding rationale therapeutic target and dose of hemofiltration and hybrid therapies in septic acute kidney injury. Blood Purif. 2012;33((1–3)):44–51. doi: 10.1159/000333837. [DOI] [PubMed] [Google Scholar]
- 104.Zhang P, Yang Y, Lv R, Zhang Y, Xie W, Chen J. Effect of the intensity of continuous renal replacement therapy in patients with sepsis and acute kidney injury a single-center randomized clinical trial. Nephrol Dial Transpl. 2012 Mar;27((3)):967–973. doi: 10.1093/ndt/gfr486. [DOI] [PubMed] [Google Scholar]
- 105.Li WB, Yin LY, Zhang XQ. Evaluation of safety and efficacy of different continuous blood Purification methods in treating infantile sepsis. J Biol Regul Homeost Agents. 2018;32((3)):663–667. [PubMed] [Google Scholar]
- 106.Atan R, Crosbie D, Bellomo R. Techniques of extracorporeal cytokine removal a systematic review of the literature on animal experimental studies. Int J Artif Organs. 2013 Mar;36((3)):149–158. doi: 10.5301/ijao.5000128. [DOI] [PubMed] [Google Scholar]
- 107.Yumoto M, Nishida O, Moriyama K, Shimomura Y, Nakamura T, Kuriyama N, et al. In vitro evaluation of high mobility group box 1 protein removal with various membranes for continuous hemofiltration. Ther Apher Dial. 2011 Aug;15((4)):385–393. doi: 10.1111/j.1744-9987.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 108.Clark E, Molnar AO, Joannes-Boyau O, Honoré PM, Sikora L, Bagshaw SM. High-volume hemofiltration for septic acute kidney injury a systematic review and meta-analysis. Crit Care. 2014 Jan;18((1)):R7. doi: 10.1186/cc13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakada TA, Oda S, Matsuda KI, Sadahiro T, Nakamura M, Abe R, et al. Continuous hemodiafiltration with PMMA hemofilter in the treatment of patients with septic shock. Mol Med. 2008;14((5–6)):257–263. doi: 10.2119/2007-00108.Nakada. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ronco C, Tetta C, Mariano F, Wratten ML, Bonello M, Bordoni V, et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis the peak concentration hypothesis. Artif Organs. 2003;27((9)):792–801. doi: 10.1046/j.1525-1594.2003.07289.x. [DOI] [PubMed] [Google Scholar]
- 111.Oshihara W, Fujieda H, Ueno Y. A new poly(methyl methacrylate) membrane dialyzer, NF, with adsorptive and antithrombotic properties. Contrib Nephrol. 2017;189:230–236. doi: 10.1159/000450806. [DOI] [PubMed] [Google Scholar]
- 112.Honoré PM, Matson JR. Extracorporeal removal for sepsis acting at the tissue level: the beginning of a new era for this treatment modality in septic shock. Crit Care Med. 2004 Mar;32((3)):896–897. doi: 10.1097/01.ccm.0000115262.31804.46. [DOI] [PubMed] [Google Scholar]
- 113.Sato T, Shoji H, Koga N. Endotoxin adsorption by polymyxin B immobilized fiber column in patients with systemic inflammatory response syndrome the Japanese experience. Ther Apher Dial. 2003 Apr;7((2)):252–258. doi: 10.1046/j.1526-0968.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- 114.Vincent J-L, Laterre P-F, Cohen J, Burchardi H, Bruining H, Lerma FA, et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock. 2005 May;23((5)):400–405. doi: 10.1097/01.shk.0000159930.87737.8a. [DOI] [PubMed] [Google Scholar]
- 115.Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock the EUPHAS randomized controlled trial. JAMA. 2009 Jun;301((23)):2445–2452. doi: 10.1001/jama.2009.856. [DOI] [PubMed] [Google Scholar]
- 116.Dellinger RP, Bagshaw SM, Antonelli M, Foster DM, Klein DJ, Marshall JC, et al. Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level the EUPHRATES randomized clinical trial. JAMA. 2018 Oct;320((14)):1455–1463. doi: 10.1001/jama.2018.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klein DJ, Foster D, Walker PM, Bagshaw SM, Mekonnen H, Antonelli M. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia a post hoc analysis of the EUPHRATES trial. Intensive Care Med. 2018 Dec;44((12)):2205–2212. doi: 10.1007/s00134-018-5463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Srisawat N, Tungsanga S, Lumlertgul N, Komaenthammasophon C, Peerapornratana S, Thamrongsat N, et al. The effect of polymyxin B hemoperfusion on modulation of human leukocyte antigen DR in severe sepsis patients. Crit Care. 2018 Oct;22((1)):279. doi: 10.1186/s13054-018-2077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schädler D, Pausch C, Heise D, Meier-Hellmann A, Brederlau J, Weiler N, et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients a randomized controlled trial. PLoS One. 2017;12((10)):e0187015. doi: 10.1371/journal.pone.0187015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Friesecke S, Stecher S-S, Gross S, Felix SB, Nierhaus A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock a prospective single-center study. J Artif Organs. 2017 Sep;20((3)):252–259. doi: 10.1007/s10047-017-0967-4. [DOI] [PubMed] [Google Scholar]
- 121.Zuccari S, Damiani E, Domizi R, Scorcella C, D'Arezzo M, Carsetti A, et al. Changes in cytokines haemodynamics and microcirculation in patients with sepsis/septic shock undergoing continuous renal replacement therapy and blood purification with CytoSorb. Blood Purif. 2020;49((1–2)):107–113. doi: 10.1159/000502540. [DOI] [PubMed] [Google Scholar]
- 122.Morris C, Gray L, Giovannelli M. Early report the use of CytosorbTM haemabsorption column as an adjunct in managing severe sepsis: initial experiences, review and recommendations. J Intensive Care Soc. 2015 Aug;16((3)):257–264. doi: 10.1177/1751143715574855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Friesecke S, Träger K, Schittek GA, Molnar Z, Bach F, Kogelmann K, et al. International registry on the use of the CytoSorb® adsorber in ICU patients study protocol and preliminary results. Med Klin Intensivmed Notfmed. 2019 Nov;114((8)):699–707. doi: 10.1007/s00063-017-0342-5. [DOI] [PubMed] [Google Scholar]
- 124.Shum HP, Chan KC, Kwan MC, Yan WW. Application of endotoxin and cytokine adsorption haemofilter in septic acute kidney injury due to Gram-negative bacterial infection. Hong Kong Med J. 2013 Dec;19((6)):491–497. doi: 10.12809/hkmj133910. [DOI] [PubMed] [Google Scholar]
- 125.Ishikawa I, Chikazawa Y, Sato K, Nakagawa M, Imamura H, Hayama S, et al. Proteomic analysis of serum outflow dialysate and adsorbed protein onto dialysis membranes (polysulfone and pmma) during hemodialysis treatment using SELDI-TOF-MS. Am J Nephrol. 2006;26((4)):372–380. doi: 10.1159/000094779. [DOI] [PubMed] [Google Scholar]
- 126.Matsuda K, Moriguchi T, Harii N, Yanagisawa M, Harada D, Sugawara H. Comparison of efficacy between continuous hemodiafiltration with a PMMA high-performance membrane dialyzer and a PAN membrane hemofilter in the treatment of septic shock patients with acute renal failure. Contrib Nephrol. 2011;173:182–190. doi: 10.1159/000329058. [DOI] [PubMed] [Google Scholar]
- 127.Sakamoto Y, Mashiko K, Obata T, Matsumoto H, Hara Y, Kutsukata N, et al. Effectiveness of continuous hemodiafiltration using a polymethylmethacrylate membrane hemofilter after polymyxin B-immobilized fiber column therapy of septic shock. ASAIO J. 2008;54((1)):129–132. doi: 10.1097/MAT.0b013e31815d2f01. [DOI] [PubMed] [Google Scholar]
- 128.Monard C, Rimmelé T, Ronco C. Extracorporeal blood purification therapies for sepsis. Blood Purif. 2019;47((Suppl 3)):1–14. doi: 10.1159/000499520. [DOI] [PubMed] [Google Scholar]
- 129.Dunzendorfer S, Lee H-K, Soldau K, Tobias PS. Toll-like receptor 4 functions intracellularly in human coronary artery endothelial cells roles of LBP and sCD14 in mediating LPS responses. FASEB J. 2004 Jul;18((10)):1117–1119. doi: 10.1096/fj.03-1263fje. [DOI] [PubMed] [Google Scholar]
- 130.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011 Oct;121((10)):4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stasi A, Intini A, Divella C, Franzin R, Montemurno E, Grandaliano G, et al. Emerging role of Lipopolysaccharide binding protein in sepsis-induced acute kidney injury. Nephrol Dial Transpl. 2017 Jul;32((1)):24–31. doi: 10.1093/ndt/gfw250. [DOI] [PubMed] [Google Scholar]
- 132.Preston GA, Waga I, Alcorta DA, Sasai H, Munger WE, Sullivan P, et al. Gene expression profiles of circulating leukocytes correlate with renal disease activity in IgA nephropathy. Kidney Int. 2004;65((2)):420–430. doi: 10.1111/j.1523-1755.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 133.Alcorta D, Preston G, Munger W, Sullivan P, Yang JJ, Waga I, et al. Microarray studies of gene expression in circulating leukocytes in kidney diseases. Exp Nephrol. 2002;10((2)):139–149. doi: 10.1159/000049909. [DOI] [PubMed] [Google Scholar]
- 134.Fannin RD, Auman JT, Bruno ME, Sieber SO, Ward SM, Tucker CJ, et al. Differential gene expression profiling in whole blood during acute systemic inflammation in lipopolysaccharide-treated rats. Physiol Genomics. 2005;21((1)):92–104. doi: 10.1152/physiolgenomics.00190.2004. [DOI] [PubMed] [Google Scholar]
- 135.Mohr S, Liew CC. The peripheral-blood transcriptome new insights into disease and risk assessment. Trends Mol Med. 2007;13((10)):422–432. doi: 10.1016/j.molmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 136.Ko GJ, Grigoryev DN, Linfert D, Jang HR, Watkins T, Cheadle C, et al. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Ren Physiol. 2010 Jun;298((6)):F1472–F1483. doi: 10.1152/ajprenal.00619.2009. [DOI] [PubMed] [Google Scholar]
- 137.Sánchez-Navarro A, Mejía-Vilet JM, Pérez-Villalva R, Carrillo-Pérez DL, Marquina-Castillo B, Gamba G, et al. SerpinA3 in the early recognition of acute kidney injury to chronic kidney disease (CKD) transition in the rat and its potentiality in the recognition of patients with CKD. Sci Rep. 2019;9((1)):10350. doi: 10.1038/s41598-019-46601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y, Chang J, Yao B, Niu A, Kelly E, Breeggemann MC, et al. Proximal tubule-derived colony stimulating factor-1 mediates polarization of renal macrophages and dendritic cells and recovery in acute kidney injury. Kidney Int. 2015 Dec;88((6)):1274–1282. doi: 10.1038/ki.2015.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Geng H, Lan R, Singha PK, Gilchrist A, Weinreb PH, Violette SM, et al. Lysophosphatidic acid increases proximal tubule cell secretion of profibrotic cytokines PDGF-B and CTGF through LPA2- and Gαq-mediated Rho and αvβ6 integrin-dependent activation of TGF-β. Am J Pathol. 2012 Oct;181((4)):1236–1249. doi: 10.1016/j.ajpath.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J, et al. Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J Am Soc Nephrol. 2016 Mar;27((3)):781–790. doi: 10.1681/ASN.2014121188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kuang Q, Wu S, Xue N, Wang X, Ding X, Fang Y. Selective wnt/β-catenin pathway activation concomitant with sustained overexpression of miR-21 is responsible for aristolochic acid-induced AKI-to-CKD transition. Front Pharmacol. 2021;12:667282. doi: 10.3389/fphar.2021.667282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, et al. Sustained activation of wnt/β-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol. 2016;27((6)):1727–1740. doi: 10.1681/ASN.2015040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Ren Physiol. 2008 Dec;295((6)):F1648–F1657. doi: 10.1152/ajprenal.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, et al. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002 Jul;13((7)):1721–1732. doi: 10.1097/01.asn.0000017223.49823.2a. [DOI] [PubMed] [Google Scholar]
- 145.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007 Nov;117((12)):3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu D, Chen P-P, Zheng P-Q, Yin F, Cheng Q, Zhou Z-L, et al. KLF4 initiates sustained YAP activation to promote renal fibrosis in mice after ischemia-reperfusion kidney injury. Acta Pharmacol Sin. 2021 Mar;42((3)):436–450. doi: 10.1038/s41401-020-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zheng Z, Li C, Shao G, Li J, Xu K, Zhao Z, et al. Hippo-YAP/MCP-1 mediated tubular maladaptive repair promote inflammation in renal failed recovery after ischemic AKI. Cell Death Dis. 2021 Aug;12((8)):754. doi: 10.1038/s41419-021-04041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xie ZY, Dong W, Zhang L, Wang MJ, Xiao ZM, Zhang YH, et al. NFAT inhibitor 11R-VIVIT ameliorates mouse renal fibrosis after ischemia-reperfusion-induced acute kidney injury. Acta Pharmacol Sin. 2022 Aug;43((8)):2081–2093. doi: 10.1038/s41401-021-00833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hu J, Qiao J, Yu Q, Liu B, Zhen J, Liu Y, et al. Role of SIK1 in the transition of acute kidney injury into chronic kidney disease. J Transl Med. 2021 Dec;19((1)):69. doi: 10.1186/s12967-021-02717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chou YH, Chu TS, Lin SL. Role of renin-angiotensin system in acute kidney injury-chronic kidney disease transition. Nephrology. 2018 Oct;23((Suppl 4)):121–125. doi: 10.1111/nep.13467. [DOI] [PubMed] [Google Scholar]
- 151.Grande MT, Sánchez-Laorden B, López-Blau C, de Frutos CA, Boutet A, Arévalo M, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015 Sep;21((9)):989–997. doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]