Abstract
Programmed cell death activated by herpes simplex virus 1 mutants can be caspase dependent or independent depending on the nature of the infected cell. The recently discovered mitochondrial apoptosis-inducing factor (AIF) on activation is translocated to the nucleus and induces programmed cell death that is caspase independent. To assess the role of AIF and also to assay apoptosis-related events in primary human embryonic lung (HEL) fibroblasts, cells were mock infected or infected with wild-type virus previously shown not to induce apoptosis in continuous lines of primate cells or with the d120 mutant lacking infected cell protein no. 4 (ICP4) and were shown to induce apoptosis in all cell lines tested. Cells exposed to dexamethasone or osmotic shock induced by sorbitol were the positive controls. The results were as follows: (i) AIF was translocated to the nucleus in all infected cell cultures and in cells treated with dexamethasone or sorbitol, but cells infected with the wild type-virus showed no evidence of undergoing programmed death. (ii) Cytochrome c was released from mitochondria of cells infected with the d120 mutant or exposed to dexamethasone or sorbitol but not from mitochondria in cells treated with sorbitol and infected with the wild-type virus. (iii) Poly(ADP-ribose) polymerase was cleaved in mock-infected cells exposed to sorbitol or dexamethasone and in cells infected with the d120 mutant but not in either untreated cells infected with wild-type virus or cells exposed to sorbitol and then infected with wild-type virus. In contrast to HEp-2 cells, neither d120 infection nor treatment with dexamethasone or sorbitol caused fragmentation of DNA in HEL fibroblasts. Electron microscopic examination showed chromatin condensation and vacuolization in a fraction of cells infected with d120 but not in wild-type virus-infected cells or cells treated with dexamethasone or sorbitol. We conclude that AIF is translocated to the nucleus in infected cells but apoptosis does not ensue in wild-type-infected cells. HEL fibroblasts infected with the d120 virus exhibit symptoms of classical apoptosis, such as cytochrome c release and cleavage of poly(ADP-ribose) polymerase observed also in cells undergoing caspase 3-dependent programmed cell death in which AIF is either not involved or not a contributory factor.
Earlier publications from this and other laboratories have shown that herpes simplex virus (HSV) mutants in functions expressed early in infection induce apoptosis and that the basic mechanisms responsible for the apoptosis depend on the type of infected cell (1, 2, 7–9). For example, mutant tsB7 retains viral DNA in capsids at nuclear pores in cells infected and maintained at nonpermissive temperatures. At the nonpermissive temperature, this virus induced apoptosis in Vero cells but not in SK-N-SH cells (7). Another mutant, d120, lacks the α4 genes (5). This virus expresses predominantly α genes and induces apoptosis in virtually all cell lines tested (8, 9, 14). Curiously, the apoptosis induced by SK-N-SH cells is caspase 3 independent, as evidenced by failure to cleave specific substrates and resistance to caspase inhibitors, and is not accompanied by cleavage of poly(ADP-ribose) polymerase (PARP), whereas in HEp-2 cells infected with the same mutant, apoptosis was caspase 3 dependent (8, 9). The lack of evidence of caspase involvement led us to explore the possibility that HSV-1 activates the mitochondrial apoptosis-inducing factor (AIF) described several years ago.
Briefly, AIF is a ubiquitous flavin adenine dinucleotide flavoprotein of 613 amino acid residues (human). The mature form has a predicted mass of 57 kDa (15, 17). On activation, AIF is translocated from the mitochondrial intermembrane space to the nucleus, where it proteolytically activates a nuclear endonuclease (18, 19). It causes chromatin condensation and cleavage of DNA into relatively large fragments (3, 4, 15, 17). AIF is activated by dexamethasone but is not blocked by specific inhibitors (e.g., Z-VAD.fmk) of Ca2+, serine or cysteine proteases known to be involved in programmed cell death (4, 17).
To study the role of AIF in HSV-1-infected cells, we cloned a DNA fragment encoding the carboxyl-terminal 292 amino acids of AIF from uninfected cells based on the published sequence and made a polyclonal antibody to the protein. To enable us to study the role of AIF in the course of infection, we carried out all of the studies described in this report in human embryonic lung (HEL) fibroblasts. The latter cells were also of interest because all preceding studies were done with cells derived from malignant tumors in which mutations to longevity may have altered the natural apoptotic cascade. We report that in HEL fibroblasts, AIF is translocated in cells infected with both the wild type and a d120 mutant, and since the wild-type virus does not induce apoptosis, it follows that HSV blocks AIF-induced apoptosis. We also report that in HEL fibroblasts infected with the d120 mutant or treated with dexamethasone or sorbitol, cytochrome c was released from mitochondria and PARP was cleaved but cellular DNA was not fragmented. Wild-type virus blocked cleavage of PARP but not the release of cytochrome c from mitochondria in cells treated with sorbitol. These results indicate that HSV can induce changes associated with programmed cell death in primary human cells characterized by a limited life span.
Relevant to this report are also observations that HSV blocks apoptosis induced by exogenous agents (7–9, 11, 12, 13, 16).
MATERIALS AND METHODS
Cells and viruses.
HEL fibroblasts were obtained from Aviron (Mountain View, Calif.). HSV-1(F) is the prototype HSV-1 strain used in this laboratory (6). The HSV-1(KOS)d120 mutant, a gift of N. DeLuca, carries a deletion in both copies of the α4 gene and was grown in a Vero cell line (E5) expressing α4 (5).
Plasmid.
An EcoRI-SalI fragment encoding the carboxyl-terminal 292 amino acids of AIF was amplified by PCR from the Human Lymphocyte Matchmaker cDNA Library (Clontech, Palo Alto, Calif.) with primers CGAATTCAGGCTCGAGCCTTGGGCACAG and GCGTCGACTCAGTCTTCATGAATGTTGAATAG. The amplified PCR fragment was inserted into EcoRI and SalI sites of pGEX-4T-2, resulting in plasmid (pRB5413).
Antibodies.
The polyclonal serum to AIF was generated as follows. Escherichia coli BL21 was transformed with (pRB5413). The fusion protein encoded by the plasmid was purified from a large-scale culture as recommended by the manufacturer (Pharmacia). Two rabbits were injected at Josman Laboratories (Napa, Calif.) subcutaneously with 1 mg of fusion protein each time at 14-day intervals. The serum used in the studies reported here was collected 1 week after the fourth immunization. Monoclonal antibodies to cytochrome c clone 7H8.2C12 were purchased from PharMingen, San Diego, Calif. Monoclonal antibodies to PARP were purchased from Santa Cruz Biotechnology, Santa Cruz, Calif.
Induction of apoptosis.
Osmotic shock was induced by exposing HEL fibroblasts to sorbitol. Cells were mock infected or infected with 10 PFU of HSV-1(F) or HSV-1(KOS) d120 mutant per cell and then exposed to 1 M sorbitol for 1 h, following by 6 h of incubation at 37°C in Dulbecco's modified Eagle's medium (DMEM) containing 1% newborn calf serum. As positive controls for programmed cell death, HEL fibroblasts were exposed to 500 mM dexamethasone (Sigma, St. Louis, Mo.) for 18 h as described elsewhere (10).
Immunoblot assays.
Protein concentrations of whole-cell lysates were determined with the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.). Infected or uninfected cell lysates (50 mg of protein per lane) were electrophoretically separated in 7.5% denaturing polyacrylamide gel. Proteins were then electrically transferred to a nitrocellulose sheet (Bio-Rad), blocked for 2 h in 5% milk (in phosphate-buffered saline [PBS]) at room temperature, and then reacted with a rabbit polyclonal antibody specific for PARP (Santa Cruz Biotechnology). The antibody was diluted 1:300. The protein bands were visualized by an enhanced chemiluminescent detection (ECL) system (Pierce, Rockford, Ill.) according to the instructions of the manufacturer.
Subcellular fractionation.
Replicate cultures of HEL fibroblasts (2 × 106 cells per culture) were either mock infected or were infected with 10 PFU of HSV-1(F) or the HSV-1 d120 mutant per cell. At the indicated times after infection, they were collected and rinsed twice with 5 ml of PBS. The cell pellet was gently resuspended in 200 μl of ice-cold lysis buffer (20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 0.1% NP-40, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 250 mM sucrose, 0.1 mM TLCK [Na-p-tosyl-l-lysine-chloromethyl-ketone], 0.1 mM TPCK [tolyl-sulfonyl-phenylalanyl-chloromethyl-ketone], 0.5 mM phenylmethylsulfonyl fluoride). After 5 min on ice, the nuclei were pelleted by centrifugation for 10 min at 750 × g and resuspended in the lysis buffer. The supernatant fluids were centrifuged again at 10,000 × g for 20 min. The cytosolic fraction (supernatant fluid) was transferred to new tubes, and the pellets that represented the mitochondrial fraction were resuspended in lysis buffer.
Localization of AIF and cytochrome c.
The protein concentrations in the mitochondrial, nuclear, and cytosolic fractions were determined by the Bio-Rad protein assay. Equivalent amounts of these three fractions were electrophoretically separated in 12% denaturing polyacrylamide gel. Proteins were then electrically transferred to a nitrocellulose sheet, blocked for 2 h in 5% milk (in PBS) at room temperature, and then reacted for 16 h at 4°C with the primary antibody diluted in PBS. Polyclonal antibody specific for AIF was diluted 1:5,000, whereas monoclonal antibody against cytochrome c was diluted 1:500. The protein bands were visualized by an ECL system.
DNA fragmentation assay.
Infected or treated cells were collected, washed in PBS, lysed in a solution containing 10 mM Tris-HCl, pH 8.0, 10 mM EDTA, and 0.5% Triton X-100, and digested with 0.1 mg of RNase A/ml at 37°C for 1 h, and then cells were centrifuged at 12,000 rpm for 25 min in an Eppendorf microcentrifuge to pellet chromosomal DNA. The supernatant fluids were digested with 1 mg of proteinase K/ml at 50°C for 2 h in the presence of 1% sodium dodecyl sulfate, extracted with phenol and chloroform, precipitated in cold ethanol, and subjected to electrophoresis on 1.5% agarose gels containing 0.5 μg of ethidium bromide per ml. DNA fragments were visualized by UV light transillumination. Photographs were taken with the aid of a computer-assisted image processor (Eagle Eye II; Stratagene).
RESULTS
AIF is translocated from mitochondria to the nucleus in cells infected with wild-type or mutant viruses.
Two series of experiments were done to test whether AIF is translocated in the nucleus of infected cells.
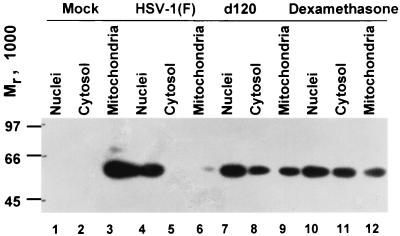
In the first series of experiments, replicate cultures of HEL fibroblasts containing 2 × 106 cells each were mock infected or infected with 10 PFU of HSV-1(F) or d120 virus per cell. The cells were harvested at 24 h after infection and fractionated as described in Materials and Methods. As a positive control, one set of mock-infected cultures was treated with dexamethasone (500 μM) for 18 h. The results shown in Fig. 1 were as follows. (i) In mock-infected cells, AIF was associated with the mitochondrial fraction (Fig. 1, lanes 1 to 3). (ii) Both the wild-type virus HSV-1(F) and the d120 mutant caused the translocation of AIF from the mitochondria to the nuclei of infected cells (Fig. 1, lanes 3 to 8). In this experiment, the translocation was more pronounced in wild-type virus-infected cells than in cells infected with the d120 mutant.
FIG. 1.
Immunoblot showing the distribution of AIF in untreated mock-infected cells or cells treated with dexamethasone or in cells infected with HSV-1(F) and d120. The untreated mock-infected cells and infected cells were harvested 24 h after exposure to virus. The dexamethasone-treated cells were harvested 18 h after exposure to the drug.
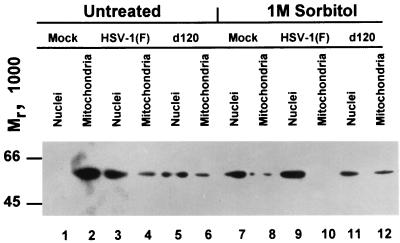
In the second series of experiments, replicate cultures of HEL fibroblasts were mock infected or infected with 10 PFU of HSV-1(F) or d120 mutant per cell. A replica set of cultures was treated with 1 M sorbitol, followed by 5 h of incubation and then infection as described above. All cultures were harvested at 18 h after infection, solubilized, fractionated, and processed as described in Materials and Methods. The results shown in Fig. 2 essentially paralleled those seen in Fig. 1. The key finding of this experiment was that sorbitol was very effective in inducing translocation of AIF from the mitochondria to the nucleus (lanes 7 to 12).
FIG. 2.
Immunoblot showing the distribution of AIF in mock-infected cells or infected cells either untreated or treated with sorbitol. HEL fibroblasts were harvested at 18 h after mock infection or infection (10 PFU/cell) with HSV-1(F) or d120. After infection, the cells were exposed to sorbitol as described in Materials and Methods.
Earlier studies from this and other laboratories have shown that HSV does block programmed cell death induced by sorbitol. The results presented here show that wild-type virus induces rather than blocks translocation of AIF to the nucleus—a requirement for AIF-induced programmed cell death.
Release of cytochrome c from HEL fibroblasts infected with HSV.
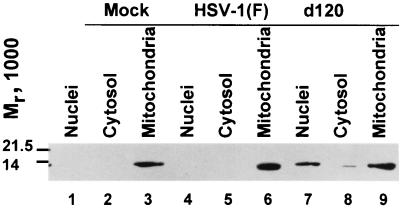
The translocation of AIF from mitochondria of HEL fibroblasts infected with wild-type and mutant viruses prompted us to examine the status of cytochrome c. Earlier studies from this laboratory have shown that cytochrome c is released from the mitochondria of cells infected with the d120 mutant but not from cells infected with wild-type virus. Two series of experiments were done. In the first, replicate cultures of HEL fibroblasts were mock infected or exposed to 10 PFU of wild-type virus per cell. The cells were harvested at 12 h after infection, fractionated, and processed as described in Materials and Methods. The results (Fig. 3) were that cytochrome c was associated with mitochondria in mock-infected cells (lanes 1 to 3) and cells infected with the wild-type virus (lanes 4 to 6). In cells infected with the d120 mutant, cytochrome c was associated with the cytosolic and nuclear fractions. The presence of cytochrome c in the nucleus was surprising and not reproducible in other experiments; most likely, it represented contamination from another compartment.
FIG. 3.
Immunoblot showing the distribution of cytochrome c in mock-infected and infected cells. Replicate cultures containing 2 × 106 HEL fibroblasts were harvested 12 h after being mock infected or infection with HSV-1(F) or d120 mutant virus (10 PFU/cell) and were processed as described in Materials and Methods.
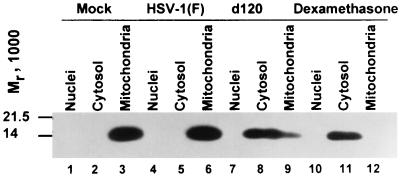
In the second series of experiments, the cells were infected as described above but were harvested at 24 h after infection. Included in this experiment was a positive control consisting of dexamethasone-treated mock-infected cells. As in the preceding section, the dexamethasone concentration was 500 μM and cells were harvested at 18 h after treatment. The results of this experiment (Fig. 4) indicate that cytochrome c was released from mitochondria into the cytosol in cells infected with the d120 mutant and in mock-infected cells treated with dexamethasone. In essence, the behavior of d120 mutant HEL fibroblasts was similar to that of HEp-2 cells infected with the same mutant.
FIG. 4.
Immunoblot showing the distribution of cytochrome c in untreated and dexamethasone-treated mock-infected cells and in cells infected with HSV-1(F) or the d120 mutant. The cells were harvested 24 h after mock infection (untreated cells) or infection. The HEL fibroblasts treated with dexamethasone were harvested 18 h after exposure to the drug. The procedures were as described in the legend to Fig. 1 and in Materials and Methods.
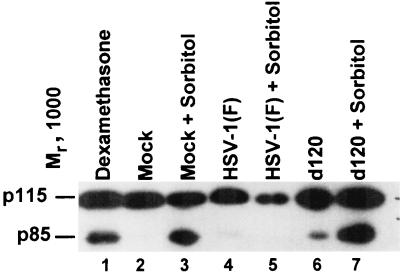
PARP is cleaved on HEL fibroblasts infected with the d120 mutant with or without simultaneous treatment with sorbitol but not in cells infected with the wild-type virus.
Earlier studies have shown that the d120 mutant triggers in HEp-2 cells the cleavage of PARP in caspase 3-dependent pathways of programmed cell death and that HSV-1 does not block PARP cleavage in cells infected and exposed to osmotic shock. To determine whether PARP is cleaved in HEL fibroblasts, replicate cultures were mock infected or exposed to 10 PFU of HSV-1(F) or the d120 mutant. Infected untreated cells were harvested at 18 h after infection. Another set of replicate cultures was exposed to 1 M sorbitol for 1 h and then incubated for 5 h and finally either mock infected or infected and maintained for 18 h. A replicate culture was exposed to dexamethasone (500 μM) for 18 h and then harvested. The cells were processed as described in Materials and Methods. The results (Fig. 5) were as follows: PARP was cleaved in cells infected with the d120 virus and in both dexamethasone- and sorbitol-treated cells. In these studies, PARP was not cleaved in cells infected with HSV-1(F) or in cells treated with sorbitol and then infected with this virus. This observation is in contrast to the results of studies done with HEp-2 cells in which HSV-1(F) did not block the cleavage of PARP following osmotic shock induced by exposure to sorbitol.
FIG. 5.
Photograph of cell lysates electrophoretically separated in denaturing gels and reacted with antibody to PARP. Cells were harvested at 18 h after mock infection or infection (10 PFU/cell) with HSV-1(F) or d120. For sorbitol treatment, cells were mock infected or infected with HSV-1(F) or d120 and then exposed to sorbitol for 1 h and collected at 5 h after treatment. The dexamethasone-treated cells were harvested 18 h after exposure to the drug.
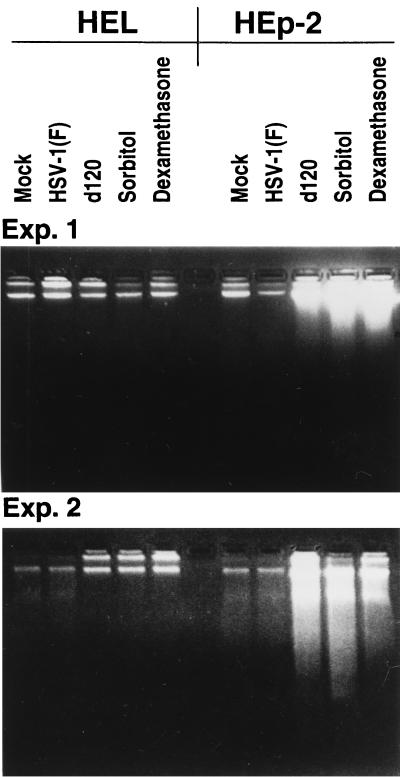
DNA is not fragmented in HEL fibroblasts infected with d120 or treated with dexamethasone or sorbitol.
In light of the finding described above, it was of interest to determine whether d120, sorbitol, or dexamethasone caused fragmentation of cellular DNA or other morphologic changes characteristic of apoptotic cells. In the first series of experiments, replicate cultures of HEL fibroblast or HEp-2 cells were exposed to 10 PFU of HSV-1(F) or d120 per cell or treated with dexamethasone for 18 h or with 1 M sorbitol for 1 h followed by 5 h of incubation in medium without the drug or infected with wild-type virus and maintained for an additional 18 h at 37°C. The harvested cells were tested for the presence of fragmented DNA as described in Materials and Methods. The results (Fig. 6) of two experiments showed no evidence of DNA fragmentation in HEL fibroblasts either infected or treated with the dexamethasone or sorbitol. In contrast, DNA fragmentation was readily observed in HEp-2 cells treated with sorbitol or dexamethasone or infected with the d120 mutant.
FIG. 6.
Photograph of two agarose gels containing electrophoretically separated DNA fractions from HEL fibroblasts or HEp-2 cells that were either mock infected or infected with HSV-1(F) or d120 mutant. In each experiment, replicate mock-infected cells were exposed to sorbitol (1 M) or dexamethasone (500 μM). Infected cells and mock-infected cells either untreated or treated with dexamethasone were harvested 18 h after infection or exposure to the drug. The cultures exposed to sorbitol for 1 h were then incubated for an additional 5 h in DMEM supplemented with 1% calf serum. The cells were processed as described in Materials and Methods.
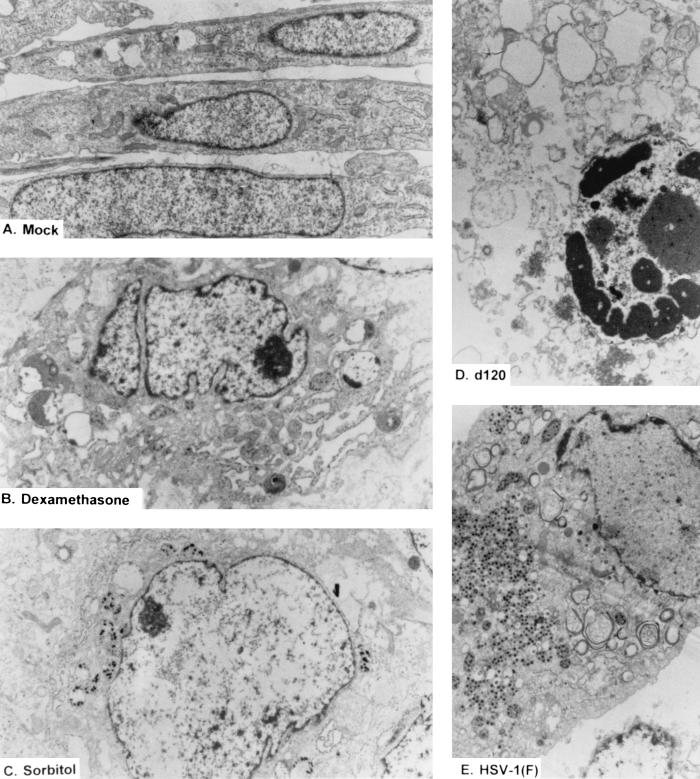
In the second series of experiments, cultures treated in exactly the same fashion as described above were fixed, sectioned, and examined with the aid of an electron microscope for morphologic manifestation of infection and treatment. Electron microphotographs depicting the most commonly observed cell types are shown in Fig. 7. Mock infected cells sectioned along the long axis showed the typical morphology of healthy uninfected cells. Approximately 30 to 40% of the cells infected with the d120 mutant showed condensation of chromatin and vacuolization. The remainder resembled the uninfected cells treated with either sorbitol or dexamethasone. In the latter, small dense punctate structures replaced the uniformly staining nucleoplasm, giving the nuclei an empty appearance.
FIG. 7.
Electron micrographs of thin sections of replicate cultures of mock-infected HEL fibroblasts either untreated (A) or treated with dexamethasone (B) or sorbitol (C) or infected with wild-type HSV-1(F) (10 PFU/cell [E]) or d120 (10 PFU/cell [D]). The infected cells were harvested at 18 h after infection. Osmotic shock was induced in cells exposed to 1 M sorbitol for 1 h. The cells were then incubated in DMEM supplemented with 1% calf serum for 5 h before being harvested. As a positive control for programmed cell death, HEL fibroblasts were exposed to 500 μM dexamethasone for 18 h. The cells were fixed with glutaraldehyde, sectioned, and examined in a Siemens 102 electron microscope. The sections were photographed at ×6,000 (A), ×6,000 (B), ×5,000 (C), ×8,000 (D), or ×5,000 (E).
We conclude that the HEL fibroblasts appear to be more resistant to fragmentation of DNA and morphologic changes characteristic of apoptosis than HEp-2 cells, as described in this and earlier studies on virus-induced programmed cell death.
DISCUSSION
The impetus to carry out the studies described in this report stemmed from two observations. First, HSV mutants can cause programmed cell death that is insensitive to specific caspase inhibitors or in which caspase activation could not be demonstrated on the basis of cleavage of synthetic or natural substrates (8). Although caspase-independent programmed cell death has been described extensively in the literature, the pathways are not fully understood. AIF, a relatively ubiquitous and probably primitive activator of programmed cell death (3, 17), was of potential interest.
The second observation, which formed the basis of these studies, is that the cell lines used in earlier studies are derived from human tumors (HEp-2, HeLa, SK-N-SH) (7–9) or are immortal (Vero). These cell lines are suitable for a large number of problems related to viral gene functions. However, since in these cell lines the immortality reflects a regulatory defect impinging on programmed cell death, it seemed appropriate to determine whether HSV induces apoptosis in primary human embryonic fibroblasts characterized by a limited life span.
The key results and their significance may be summarized as follows: (i) All of the studies done to date indicate that cells infected with certain mutants in early functions exhibit a stress response to infection. The earliest indication of a stress response leading to apoptosis is release of cytochrome c from mitochondria, followed by activation of procaspases and ultimately cleavage of key proteins. The wild-type HSV-1 blocks cytochrome c release in all cell lines tested to date. Furthermore, at the other extreme, the wild-type virus does not induce the classical chromatin condensation and cleavage of DNA that are the hallmarks of apoptosis. Nevertheless, wild-type virus does induce the translocation of AIF from the mitochondria to the nucleus. In an earlier publication from this laboratory, we argued that since mutants arrested in early functions induce cytochrome c release, wild-type virus must in some fashion block cytochrome c release (8, 9). In the case of AIF, since wild-type virus does not block its translocation, the failure of AIF to induce apoptosis indicates that its function is blocked at another point in the course of the viral replicative cycle.
(ii) The mutant d120 induces in HEL fibroblasts symptoms characteristic of cell death, such as release of cytochrome c, cleavage of PARP caused by activated caspases, and condensation of chromatin in approximately 30 to 40% of infected cells. Were this not the case, and were HEL fibroblasts showing other symptoms of programmed cell death unrelated to caspases, a detailed study on the contribution of AIF would be appropriate. In HEL fibroblasts, the contribution of AIF is overshadowed by the activation of the caspase cascade and, at this moment at least, is not amenable to solution.
(iii) The response to d120 infection of HEL is similar but not identical to that of infected HEp-2 cells. The sole difference is that the HEL fibroblasts are more resistant to fragmentation of DNA than HEp-2 cells, as shown in Fig. 6. The implications of this finding are threefold. First, the results give credence and significance to the earlier studies done on immortal cell lines derived from human tumors, in that virus mutants can induce proapoptotic manifestations in euploid cells. Second, the observation that caspase inhibitors blocked apoptosis in HEp-2 cells infected with the d120 mutant has the dual effect of underlying the role of caspases in the evolution of the apoptotic cascade while at the same time excluding the contribution of caspase-independent programmed cell death induced by AIF. Lastly, the resistance of HEL fibroblasts to DNA degradation underscores the earlier observation that the manifestation of programmed cell death is cell type dependent (7–9).
ACKNOWLEDGMENTS
These studies were aided by Public Health Service grants from the National Cancer Institute (CA47451, CA71933, and CA78766).
REFERENCES
- 1.Auber M, Blaho J A. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J Virol. 1999;73:2803–2813. doi: 10.1128/jvi.73.4.2803-2813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auber M, O'Toole J, Blaho J A. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J Virol. 1999;73:10359–10370. doi: 10.1128/jvi.73.12.10359-10370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardi P, Scorrano L, Colonna R, Petroniil V, Lisa F. Mitochondria and cell death. Eur J Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 4.Daugas E, Susin S, Zamazami N, Marzol I, Ferri K, Irinopoulou T, Larochette N, Prevost M, Leber B, Andrews D, Penninger J, Kroemer G. Mitochondrial-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000;14:729–739. [PubMed] [Google Scholar]
- 5.DeLuca N A, McCarth A, Schaffer P A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;58:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ejercito P M, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 7.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvan V, Brandimarti R, Roizman B. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J Virol. 1999;73:3219–3226. doi: 10.1128/jvi.73.4.3219-3226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galvan V, Brandimarti R, Munger J, Roizman B. Bcl-2 blocks a caspase-dependent pathway of apoptosis activated by herpes simplex virus 1 infection in HEp-2 cells. J Virol. 2000;74:1931–1938. doi: 10.1128/jvi.74.4.1931-1938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorman A M, Hirt U A, Orrenius S, Ceccatelli S. Dexamethasome pre-treatment interferes with apoptotic death in glioma cell. Neuroscience. 2000;96:417–425. doi: 10.1016/s0306-4522(99)00565-5. [DOI] [PubMed] [Google Scholar]
- 11.Jerome K R, Fox R, Chen Z, Sears A E, Lee H-E, Corey L. Herpes simplex virus inhibits apoptosis through the action of two genes, US5 and US3. J Virol. 1999;73:8950–8957. doi: 10.1128/jvi.73.11.8950-8957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerome K R, Tait J F, Koelle D M, Corey L. Herpes simplex virus type 1 renders infected cells resistant to cytotoxic T-lymphocyte-induced apoptosis. J Virol. 1998;72:436–441. doi: 10.1128/jvi.72.1.436-441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koyama A H, Miwa Y. Suppression of apoptotic DNA fragmentation in herpes simplex virus type 1-infected cells. J Virol. 1997;71:2567–2571. doi: 10.1128/jvi.71.3.2567-2571.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leopardi R, Roizman B. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc Natl Acad Sci USA. 1996;93:9583–9587. doi: 10.1073/pnas.93.18.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenzo H, Susin S, Penninger J, Kroemer G. Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ. 1999;6:516–524. doi: 10.1038/sj.cdd.4400527. [DOI] [PubMed] [Google Scholar]
- 16.Sieg S, Yildirim Z, Smith D, Kayagaki N, Yagita H, Huang Y, Kaplan D. Herpes simplex virus type 2 inhibition of Fas ligand expression. J Virol. 1996;70:8747–8751. doi: 10.1128/jvi.70.12.8747-8751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Susin S, Lorenzo M, Zamazami N, Marzol I, Snow B, Brothers G, Mangon J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett D, Aebersold R, Siderovski D, Penninger J, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing-factor (AIF) Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 18.Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsujimoto Y, Shimizu S. Bcl-2 family: life-or-death switch. FEBS Lett. 2000;466:6–10. doi: 10.1016/s0014-5793(99)01761-5. [DOI] [PubMed] [Google Scholar]