Abstract
Muscle-directed gene therapy with adeno-associated viral (AAV) vectors is undergoing clinical development for treating neuromuscular disorders and for systemic delivery of therapeutic proteins. Although these approaches show considerable therapeutic benefits, they are also prone to induce potent immune responses against vector or transgene products owing to the immunogenic nature of the intramuscular delivery route, or the high doses required for systemic delivery to muscle. Major immunological concerns include antibody formation against viral capsid, complement activation, and cytotoxic T cell responses against capsid or transgene products. They can negate therapy and even lead to life-threatening immunotoxicities. Herein we review clinical observations and provide an outlook for how the field addresses these problems through a combination of vector engineering and immune modulation.
Keywords: adeno-associated virus, skeletal muscle, immune response, CD8 T cell, antibody
INTRODUCTION
Gene therapy drugs based on adeno-associated viral (AAV) vectors have received regulatory approval for treatments of several genetic diseases by in vivo gene transfer, including Leber's congenital amaurosis, spinal muscular atrophy (SMA), and hemophilia A and B.1,2 However, immune responses against vector or therapeutic transgene products continue to complicate AAV gene therapies, despite this vector's low innate immunogenicity compared with many other delivery systems.3,4 Natural infection with AAV creates pre-existing humoral and T cell immunity in the human population. Multiple factors such as vector dose and design, target organ, and route of administration determine the risk of B and T cell activation after vector administration.
Skeletal muscle is an attractive target tissue for in vivo gene transfer owing to ease of access, long life span of muscle fibers, and the ability to secrete proteins into circulation. In fact, the first target in clinical gene therapy for hemophilia B was skeletal muscle, and the first approved AAV gene therapy product, Glybera, to treat lipoprotein lipase (LPL) deficiency, was also administered intramuscularly.5 Moreover, skeletal muscles (especially diaphragm), cardiac, and other muscles are critical targets for correcting muscular dystrophies, certain lysosomal storage diseases, and other neuromuscular disorders by gene therapy including gene editing.
IMPACT OF ANTIBODIES AGAINST CAPSID ON EFFICACY OF INTRAMUSCULAR AAV ADMINISTRATION
One of the major hurdles to AAV-mediated gene augmentation is the presence of pre-existing neutralizing antibodies (NAbs) against the viral capsid.6 About 80% of the human population develops such antibodies against various serotypes (starting during childhood), with prevalence varying substantially depending on capsid and geography.7–9 One would expect high titers of pre-existing NAbs to preclude patients from receiving gene therapy with the respective serotype/capsid sequence. However, the impact of NAbs on muscle gene transfer is less clear as conflicting results have been reported from different clinical studies.
For example, a clinical trial in hemophilia B patients showed that intramuscular administration of AAV2 encoding human factor 9 gene led to successful transduction and transgene expression despite the presence of pre-existing NAbs (titers ranging from 1:10 to 1:1,000).10 Similarly, in clinical trials with intramuscular injection of alipogene tiparvovec (Glybera), more than half of patients (15/26) had pre-existing antibodies to AAV1, yet most of these patients achieved LPL expression.11
However, in one of the earliest phase I study in α1-antitrypsin (AAT)-deficient patients, only 1 patient out of 12 had transient and subtherapeutic levels of AAT in serum.12 This was attributed to the presence of pre-existing antibodies against AAV2 capsid in almost all of the patients. In a follow-up clinical study, nine AAT-deficient patients were treated with intramuscular injections of AAV1-AAT over a range of doses.13 Of note, four patients had previously been injected with the AAV2-AAT vector. Three of these patients received the same vector dose of AAV1-AAT as AAV2-AAT, whereas a fourth patient was given a higher dose.
In the intermediate group, two patients (previously injected with AAV2-AAT) achieved subtherapeutic levels of AAT in plasma that lasted up to 90 days in one patient and up to a year in the other. In clinical trials for different forms of limb-girdle muscular dystrophy (LGMD) using AAV1 vector, patients with pre-existing AAV1 NAbs nonetheless showed transgene expression in muscles fibers.14–16 Subsequent treatment-emergent/increase of NAb titers did not affect transgene expression.12,13,17
Although not entirely conclusive, a trend emerges that pre-existing humoral immunity against AAV capsid is less of an obstacle for gene transfer by direct intramuscular injection. In contrast, NAbs that form after gene transfer are likely to prevent readministration. Therefore, immune suppression protocols are being explored to prevent NAb formation upon initial gene transfer. For instance, B cell depletion using anti-CD20 combined with mTOR inhibition with sirolimus (rapamycin) to target B and T cells is being tested in patients with Pompe disease.18,19 Switching capsid sequence for readministration may be helpful within limitations.
For example, in a preclinical study with nonhuman primates, Greig et al. showed that readministration using heterologous serotype is possible by intramuscular administration when NAb titers are below a certain level at the time of second vector administration.20 In contrast, pre-existing humoral immunity is more likely to negatively impact AAV transduction after intravenous administration of AAV vector, thereby complicating systemic vector delivery to muscle.18
CAPSID-SPECIFIC CELLULAR RESPONSES UPON INTRAMUSCULAR AAV ADMINISTRATION
The first indication of a cytotoxic T cell response to AAV gene therapy was observed in a clinical study of AAV2-mediated liver gene transfer of human coagulation factor IX in hemophilia B patients.21 In this study, a patient in the high-dose cohort lost factor IX expression after initially achieving therapeutic levels. This was accompanied by a transient and self-resolving increase in transaminases. Further studies implemented a capsid-specific CD8+ T cell response, which was not observed in preclinical studies.22 Immune suppressive regimens (prophylactic or on demand), primarily based on steroid drugs, are now being employed and, to some extent, are successful in controlling/preventing the elicitation of cellular responses.23 CD8+ T cell responses against capsid were also observed in muscle gene transfer.12–17
Although inflammatory responses and T cell infiltrates were observed in muscle biopsies of patients treated with AAV1-AAT, these did not severely affect transgene expression long term and eventually diminished concomitant with the recruitment of FoxP3+ regulatory T cells (Treg).13,17,24 Interestingly, Mueller et al. showed the persistence of AAV capsids at the site of injection for up to 12 months after vector administration.24 The presence of AAV capsids long after vector injection may mimic a state of chronic viral infection. Cellular infiltrates were observed to express PD-1 and PD-L1, perhaps representing an exhausted phenotype.
However, the potential for reactivation of such T cells is unclear. Apoptosis of infiltrating mononuclear cells at the site of vector administration was also observed in clinical trials for LGMD.14,15 It should be pointed out, however, that patients were given immunosuppressive drugs before vector administration in these studies.
In LPL gene transfer, one of the patients had elevated levels of creatine kinase in plasma 1 month after vector administration, which corresponded with a decline in transgene expression.25 High number of AAV capsid-specific T cells as estimated by enzyme-linked immunosorbent spot (ELISpot) assay on the peripheral blood mononuclear cells of this patient suggested the rejection of AAV transduced muscle fibers by T cells.25 Furthermore, this study demonstrated a clear difference in the kinetics of activation of AAV capsid-specific CD8+ T cells at low- and high-dose cohorts wherein at low vector dose, activation of CD8+ T cells occurred at around 3 months and at high dose activation occurred around 1 month postvector administration.
TRANSGENE-SPECIFIC ADAPTIVE IMMUNE RESPONSES IN INTRAMUSCULAR AAV ADMINISTRATION
Although not as frequent, transgene-specific cellular immune responses are also a potential concern to the success of AAV gene therapy. This is particularly the case in treating Duchenne muscular dystrophy (DMD), where progressive degeneration and wasting of muscle fibers establish a proinflammatory environment in the muscle architecture. In a phase I trial with AAV2.5 vector encoding a truncated but functional version of the dystrophin protein, Mendell et al. found that two of the three patients in the high dose (1 × 1011 vector genomes [vg]/kg) cohort developed revertant dystrophin-specific T cell responses, whereas the third patient had a T cell response against minidystrophin.26
One patient in the low-dose (2 × 1010 vg/kg) cohort also had pre-existing cellular responses against revertant dystrophin that seemed to accelerate the development of T cell response after gene transfer in this patient. Interestingly, despite the presence of vg in all these patients, muscle biopsies from only two patients (one in each cohort) had minidystrophin expression. Of these two patients, one (in the high-dose cohort) developed a delayed (day 60 postinjection) T cell response.
T cell responses against dystrophin before gene transfer may reflect the sporadic expression of dystrophin epitopes in revertant fibers. Surveys of larger cohorts of DMD patients indeed found such pre-existing T cell responses. These studies also indicated that the probability of developing dystrophin-specific T cell response increases with the age of DMD patients and early treatment with glucocorticoids could have an immune modulatory effect.27,28
However, in a clinical trial of Becker muscular dystrophy (BMD), patients in both dose cohorts (3 × 1011 vg/kg per leg, i.e., 6 × 1011 vg/kg; and 6 × 1011 vg/kg per leg, i.e., 1.2 × 1012 vg/kg) developed transgene (follistatin)-specific T cell responses.29 Immunosuppression was employed with only limited success, indicating that superior immune modulatory regimens should be developed.
Perhaps not surprisingly, the underlying mutation of the defective gene is a major determinant of antigen-specific immune responses against the transgene product because it will govern whether neoepitopes are being presented after gene transfer. Hence, gene deletions, inversions, frameshift mutations, and early stop codons (nonsense mutations) are more likely to predispose to immune responses than missense mutations. Unexpectedly, however, rare cases of CD8+ T cell responses against AAT in patients with AAT deficiency resulting from a missense mutation revealed the potential for polymorphic sequence differences between endogenous and transgene to be another source of neoepitopes.30
The possibility of antibody formation that could clear a secreted transgene product and thereby prevent therapy was illustrated in a clinical trial that aimed to use AAV gene transfer to skeletal muscle for systemic delivery of an antibody against HIV.31 Even when using humanized backbone sequences, such “anti-idiotypic antibodies” may form against the antigen-specific variable part of the immunoglobulin. To prevent antibody formation against factor IX in muscle-directed gene therapy for hemophilia B, only patients with F9 missense mutations were included.10 This requirement was dropped in hepatic gene transfer, which is more likely to result in immune tolerance due to the immune regulatory pathways that are active in the liver.3,21,32
IMMUNE RESPONSES UPON SYSTEMIC DELIVERY OF AAV VECTORS TO MUSCLE
Treatment of DMD and other neuromuscular disorders is only effective if the vector is delivered to multiple muscle groups, which requires systemic administration of large vector doses through a blood vessel. For instance, multiple clinical trials attempt to treat DMD using this approach.33 This route not only exposes the vector to pre-existing NAbs but also creates a source of immunotoxicities. At vector doses ≥1014 vg/kg, complement activation has been observed in multiple patients, which was often associated with a decline in platelet counts and thrombotic microangiopathy (TMA), causing kidney damage (presumably from activation of endothelial cells) and hemolysis.34
Hence, investigators started adapting monoclonal antibody therapy against complement components (e.g., anti-C5, eculizumab) as a mitigation strategy to overcome these events, which appear largely antibody dependent. Thus, the classical pathway of complement activation likely plays a critical role, although direct binding of AAV capsid to components of the complement system, such as iC3b, may further increase the response.34,35 Complement activation, TMA, and liver toxicity have also been observed in systemic AAV9 gene therapy for SMA.36–39
Primary and memory antibody responses may contribute to complement activation by AAV–antibody complexes, which is unlikely serotype-specific. More work is needed to define the potential role of capsid structure in vivo, given that AAV capsids may differ in their kinetics of clearance and biodistribution.40
Dystrophin-specific CD8+ T cell activation is another major problem in systemic gene therapy for DMD. In systemic AAV microdystrophin trials, it was found that a region that is present in microdystrophin but absent in DMD patients (due to deletion mutations) might have induced T cell responses.26,34 Therefore, patients with such mutations are currently excluded from clinical trials. This decision was made in part because of several incidents of myocarditis in DMD patients after AAV gene transfer, suspecting a T cell response to be the cause of this cardiac pathology.41,42
A recent study in nonhuman primates receiving AAV9 vector (designed to treat the lysosomal storage disorder Pompe disease, a disease that also severely affects muscle function) demonstrated the potential for CD8+ T cell responses against a transgene product expressed in the heart to result in myocarditis.43
These high-dose systemic deliveries also pose a risk for severe liver toxicities as the liver functions as a sink for vector. Accumulation of high vector loads in the liver may trigger the targeting of hepatocytes by capsid-specific CD8+ T cells or be associated with other toxicities through yet undefined mechanisms. Importantly, both liver toxicities and TMA (and other pathologies resulting from complement activation) can be fatal for the patient.36,37,39,44,45 More detailed information on the incidence and outcome of these toxicities is not yet available in peer-reviewed publications but is expected to become available in the future.
CONCLUSIONS AND OUTLOOK
Muscle-directed gene transfer is an important path for treating various neuromuscular disorders and is also attractive for systemic protein delivery. However, a tendency to induce B and T cell responses against capsid and transgene products and immunotoxicities associated with high-dose systemic delivery to muscle pose serious hurdles. Novel capsids that are effective at lower doses and are detargeted from the liver will hopefully help avoid the latter complications.46,47 Although monoclonal antibody treatments or other drugs targeting complement components are being adopted as an adjunct therapy, the efficacy of such modulators is yet to be discerned.
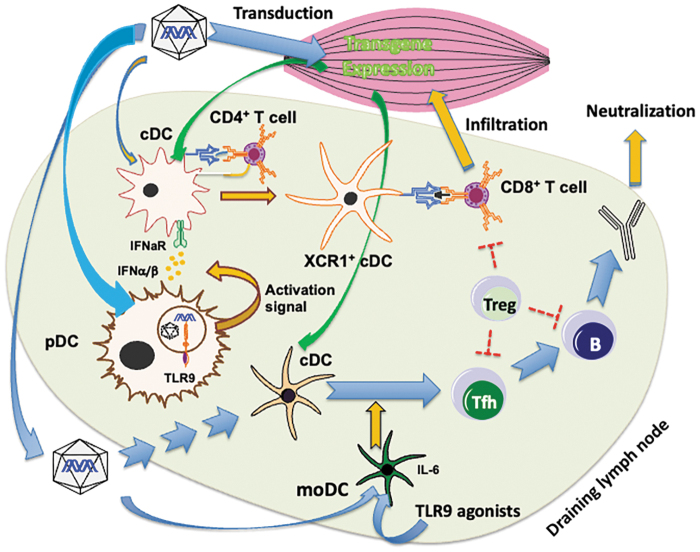
More mechanistic preclinical studies are needed to guide the clinical development of vectors with reduced risk of CD8+ T cell activation and to generate more effective transient immune suppression protocols. For example, we know that innate immune signals are requisite for T cell activation and may be derived from the activation of pattern recognition receptors. Sensing of AAV genomes by the endosomal DNA receptor TLR9 promotes CD8+ T cell responses through induction of interferon type I (Fig. 1),48–51 which already prompted vector developers to eliminate CpG motifs (which in their unmethylated form, such as in viral DNA, are potent agonists for TLR9) from transgene cassettes.3,4,52–56
Figure 1.
Known immune response mechanisms in AAV muscle gene transfer. The immune response starts locally in the draining lymph nodes of transduced muscle. TLR9 signaling in pDCs upon sensing vector genomes induces IFNα/β expression, which conditions cross-presenting cDCs. Combined with costimulatory signals from CD4+ T helper cells, this leads to the priming of CD8+ T cells against AAV capsid or transgene products. These may infiltrate transduced muscle and target muscle fibers that display peptides derived from capsid or transgene product through MHC I. Transport of the capsid or transgene product to dendritic cells in the T–B cell border may lead to activation of Tfh cells, which promote B cell activation and germinal center formation, leading to antibody formation, memory B cells, and plasma cells. moDCs activated by AAV or exogenous DNA enhance Tfh activation, thereby increasing antibody production. Other innate and cytokine signaling pathways driving B and T cell responses likely exist. Treg can limit B and T cell responses. Preventing TLR9 signaling (e.g., through CpG depletion of vector genomes), eliminating transgene expression in dendritic cells (e.g., by incorporation of miRNA target sequences into transcripts of the transgene), and blockade of cytokine signaling or costimulation represent some of the current and emerging approaches to prevent immune responses. AAV, adeno-associated viral; cDCs, conventional dendritic cells; miRNA, microRNA; moDCs, monocyte-derived/inflammatory dendritic cells; pDCs, plasmacytoid dendritic cells; Tfh, T follicular helper; Treg, regulatory T cells.
Activation of monocyte-derived dendritic cells by AAV or exogenous DNA enhances antibody formation by increasing activation of T follicular helper cells, for example, by induction of IL-6 (Fig. 1).57,58 Further basic research should aim to identify other targetable innate and cytokine signaling pathways that may be derived from the vector (pathogen-associated molecular patterns) or tissue damage (damage-associated molecular patterns) and promote B and/or T cell responses. Administration of steroid drugs is a conventional treatment for DMD and is widely used in clinical AAV gene therapy to prevent inflammatory and, more specifically, CD8+ T cell responses. Regimens better tailored to AAV gene transfer, based on mechanistic considerations and large animal studies, can be developed.
Although AAV vectors are generally inefficient in transducing professional antigen-presenting cells (APCs such as dendritic cells), recent studies suggest that transgene expression in APCs occurs at sufficient levels to contribute to CD8+ T cell activation59–62 and that the use of muscle cell-specific promoters is insufficient to prevent this, especially at high vector doses.63–66 Inclusion of microRNA target sites that result in the degradation of the transgene messenger RNA in APCs has been shown to be effective in murine models.60 Transient in vivo inactivation of immunoglobulins or blockade of neonatal Fc receptors represent potential avenues to overcome pre-existing NAbs.60,67–69
Going forward, the field continues to face challenges posed by the immune system. These are likely to extend to AAV delivery of bacterial nucleases in gene editing approaches.70,71 Nonetheless, several highly promising avenues are being developed to manage the immunological hurdle. These include the development of novel vectors with improved efficacy and reduced accumulation in the liver combined with the incorporation of features that reduce innate immune signaling and antigen presentation into vector design, and the development of more tailored immune suppression regimens that are informed by mechanistic studies. Immune optimization of muscle gene therapy will increase safety, and extend gene therapy to more patients, even if they have unfavorable mutations.
AUTHOR DISCLOSURE
R.W.H. is serving on the scientific advisory board of the Regeneron Pharmaceuticals–Intellia Therapeutics collaboration on gene editing for hemophilia B, has served on a Biomarin roundtable on clinical gene therapy for hemophilia A, and has received funding from Spark Therapeutics for preclinical gene therapy studies. D.D. is a member of the scientific advisory board for Solid Biosciences and an equity holder of Solid Biosciences. D.D. is a member of the scientific advisory board for Sardocor Corp. D.D. is an inventor of several issued and filed patents on microdystrophin gene therapy and recombinant AAV vectors. The other author has no competing financial interests.
FUNDING INFORMATION
The research on AAV immune response in the Herzog laboratory is currently supported by NIH grants R01HL131093, R01AI051390, and P01HL160472. The research on DMD gene therapy in the Duan laboratory is currently supported by NIH grants R01NS90634 and R01AR70517.
REFERENCES
- 1. Mendell JR, Al-Zaidy SA, Rodino-Klapac LR, et al. . Current clinical applications of in vivo gene therapy with AAVs. Mol Ther 2021;29(2):464–488; doi: 10.1016/j.ymthe.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pierce GF, Herzog RW. Two gene therapies for hemophilia available: Now what? Mol Ther 2023;31(4):919–920; doi: 10.1016/j.ymthe.2023.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shirley JL, de Jong YP, Terhorst C, et al. . Immune responses to viral gene therapy vectors. Mol Ther 2020;28(3):709–722; doi: 10.1016/j.ymthe.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verdera HC, Kuranda K, Mingozzi F. AAV vector immunogenicity in humans: A long journey to successful gene transfer. Mol Ther 2020;28(3):723–746; doi: 10.1016/j.ymthe.2019.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buning H. Gene therapy enters the pharma market: The short story of a long journey. EMBO Mol Med 2013;5(1):1–3; doi: 10.1002/emmm.201202291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schulz M, Levy DI, Petropoulos CJ, et al. . Binding and neutralizing anti-AAV antibodies: Detection and implications for rAAV-mediated gene therapy. Mol Ther 2023;31(3):616–630; doi: 10.1016/j.ymthe.2023.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erles K, Sebokova P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol 1999;59(3):406–411; doi: [DOI] [PubMed] [Google Scholar]
- 8. Calcedo R, Vandenberghe LH, Gao G, et al. . Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009;199(3):381–390; doi: 10.1086/595830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boutin S, Monteilhet V, Veron P, et al. . Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum Gene Ther 2010;21(6):704–712; doi: 10.1089/hum.2009.182 [DOI] [PubMed] [Google Scholar]
- 10. Manno CS, Chew AJ, Hutchison S, et al. . AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 2003;101(8):2963–2972; doi: 10.1182/blood-2002-10-3296 [DOI] [PubMed] [Google Scholar]
- 11. Ferreira V, Petry H, Salmon F. Immune responses to AAV-vectors, the Glybera example from bench to bedside. Front Immunol 2014;5:82; doi: 10.3389/fimmu.2014.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brantly ML, Spencer LT, Humphries M, et al. . Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum Gene Ther 2006;17(12):1177–1186; doi: 10.1089/hum.2006.17.1177 [DOI] [PubMed] [Google Scholar]
- 13. Brantly ML, Chulay JD, Wang L, et al. . Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A 2009;106(38):16363–16368; doi: 10.1073/pnas.0904514106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mendell JR, Rodino-Klapac LR, Rosales XQ, et al. . Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol 2010;68(5):629–638; doi: 10.1002/ana.22251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, et al. . Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol 2009;66(3):290–297; doi: 10.1002/ana.21732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herson S, Hentati F, Rigolet A, et al. . A phase I trial of adeno-associated virus serotype 1-gamma-sarcoglycan gene therapy for limb girdle muscular dystrophy type 2C. Brain 2012;135(Pt 2):483–492; doi: 10.1093/brain/awr342 [DOI] [PubMed] [Google Scholar]
- 17. Flotte TR, Trapnell BC, Humphries M, et al. . Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: Interim results. Hum Gene Ther 2011;22(10):1239–1247; doi: 10.1089/hum.2011.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corti M, Cleaver B, Clement N, et al. . Evaluation of readministration of a recombinant adeno-associated virus vector expressing acid alpha-glucosidase in Pompe disease: Preclinical to clinical planning. Hum Gene Ther Clin Dev 2015;26(3):185–193; doi: 10.1089/humc.2015.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corti M, Elder M, Falk D, et al. . B-cell depletion is protective against anti-AAV capsid immune response: A human subject case study. Mol Ther Methods Clin Dev 2014;1:14033; doi: 10.1038/mtm.2014.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greig JA, Calcedo R, Grant RL, et al. . Intramuscular administration of AAV overcomes pre-existing neutralizing antibodies in rhesus macaques. Vaccine 2016;34(50):6323–6329; doi: 10.1016/j.vaccine.2016.10.053 [DOI] [PubMed] [Google Scholar]
- 21. Manno CS, Pierce GF, Arruda VR, et al. . Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med 2006;12(3):342–347; doi: 10.1038/nm1358 [DOI] [PubMed] [Google Scholar]
- 22. Mingozzi F, Maus MV, Hui DJ, et al. . CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med 2007;13(4):419–422; doi: 10.1038/nm1549 [DOI] [PubMed] [Google Scholar]
- 23. Nathwani AC, Reiss UM, Tuddenham EG, et al. . Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371(21):1994–2004; doi: 10.1056/NEJMoa1407309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mueller C, Chulay JD, Trapnell BC, et al. . Human Treg responses allow sustained recombinant adeno-associated virus-mediated transgene expression. J Clin Invest 2013;123(12):5310–5318; doi: 10.1172/JCI70314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mingozzi F, Meulenberg JJ, Hui DJ, et al. . AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood 2009;114(10):2077–2086; doi: 10.1182/blood-2008-07-167510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mendell JR, Campbell K, Rodino-Klapac L, et al. . Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med 2010;363(15):1429–1437; doi: 10.1056/NEJMoa1000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anthony K, Ala P, Catapano F, et al. . T cell responses to dystrophin in a natural history study of Duchenne muscular dystrophy. Hum Gene Ther 2023; doi: 10.1089/hum.2022.166 [DOI] [PubMed] [Google Scholar]
- 28. Flanigan KM, Campbell K, Viollet L, et al. . Anti-dystrophin T cell responses in Duchenne muscular dystrophy: Prevalence and a glucocorticoid treatment effect. Hum Gene Ther 2013;24(9):797–806; doi: 10.1089/hum.2013.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mendell JR, Sahenk Z, Malik V, et al. . A phase 1/2a follistatin gene therapy trial for Becker muscular dystrophy. Mol Ther 2015;23(1):192–201; doi: 10.1038/mt.2014.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calcedo R, Somanathan S, Qin Q, et al. . Class I-restricted T-cell responses to a polymorphic peptide in a gene therapy clinical trial for alpha-1-antitrypsin deficiency. Proc Natl Acad Sci U S A 2017;114(7):1655–1659; doi: 10.1073/pnas.1617726114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Priddy FH, Lewis DJM, Gelderblom HC, et al. . Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: A phase 1 randomised controlled trial. Lancet HIV 2019;6(4):e230–e239; doi: 10.1016/S2352-3018(19)30003-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herzog RW. Complexity of immune responses to AAV transgene products—Example of factor IX. Cell Immunol 2019;342:103658; doi: 10.1016/j.cellimm.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. US National Library of Medicine. Clinical Trials. Available from: https://clinicaltrials.gov/ct2/results?cond=DMD&term=AAV&cntry=&state=&city=&dist= [Last accessed: May 9, 2023].
- 34. Lek A, Atas E, Hesterlee SE, et al. . Meeting report: 2022 Muscular Dystrophy Association summit on ‘safety and challenges in gene transfer therapy.’ J Neuromuscul Dis 2023;10(3):327–336; doi: 10.3233/JND-221639 [DOI] [PubMed] [Google Scholar]
- 35. Smith CJ, Ross N, Kamal A, et al. . Pre-existing humoral immunity and complement pathway contribute to immunogenicity of adeno-associated virus (AAV) vector in human blood. Front Immunol 2022;13:999021; doi: 10.3389/fimmu.2022.999021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Byrne BJ, Elder M, Leon-Astudillo C, et al. . Secondary hemophagocytic lymphohistiocytosis following Zolgensma therapy: An evolving story on the innate response to systemic gene therapy. Mol Ther 2022;30(12):3503–3504; doi: 10.1016/j.ymthe.2022.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chand D, Mohr F, McMillan H, et al. . Hepatotoxicity following administration of onasemnogene abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. J Hepatol 2021;74(3):560–566; doi: 10.1016/j.jhep.2020.11.001 [DOI] [PubMed] [Google Scholar]
- 38. Galletta F, Cucinotta U, Marseglia L, et al. . Hemophagocytic lymphohistiocytosis following gene replacement therapy in a child with type 1 spinal muscular atrophy. J Clin Pharm Ther 2022;47(9):1478–1481; doi: 10.1111/jcpt.13733 [DOI] [PubMed] [Google Scholar]
- 39. Guillou J, de Pellegars A, Porcheret F, et al. . Fatal thrombotic microangiopathy case following adeno-associated viral SMN gene therapy. Blood Adv 2022;6(14):4266–4270; doi: 10.1182/bloodadvances.2021006419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Logan GJ, Mietzsch M, Khandekar N, et al. . Structural and functional characterization of capsid binding by anti-AAV9 monoclonal antibodies from infants after SMA gene therapy. Mol Ther 2023; doi: 10.1016/j.ymthe.2023.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pfizer DMD Gene Therapy Team. Letter to the Duchenne Muscular Dystrophy Community, Sent Through Parent Project Muscular Dystrophy (PPMD). September 28, 2021. Available from: http://join.parentprojectmd.org/site/DocServer/A_Message_from_Pfizer_on_our_DMD_Clinical_Program_-_Sept.pdf [Last accessed: April 27, 2023].
- 42. Sarepta Therapeutics, Inc. Sarepta Therapeutics' Investigational Gene Therapy SRP-9001 for Duchenne Muscular Dystrophy Demonstrates Significant Functional Improvements Across Multiple Studies. June 7, 2022. Available from: https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-investigational-gene-therapy-srp-9001 [Last accessed: April 27, 2023].
- 43. Hordeaux J, Ramezani A, Tuske S, et al. . Immune transgene-dependent myocarditis in macaques after systemic administration of adeno-associated virus expressing human acid alpha-glucosidase. Front Immunol 2023;14:1094279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Philippidis A. Fourth boy dies in clinical trial of Astellas' AT132. Hum Gene Ther 2021;32(19–20):1008–1010; doi: 10.1089/hum.2021.29182.bfs [DOI] [PubMed] [Google Scholar]
- 45. Shieh PB, Bonnemann CG, Muller-Felber W, et al. . Re: “moving forward after two deaths in a gene therapy trial of myotubular myopathy” by Wilson and Flotte. Hum Gene Ther 2020;31(15–16):787; doi: 10.1089/hum.2020.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tabebordbar M, Lagerborg KA, Stanton A, et al. . Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 2021;184(19):4919..e22–4938.e22; doi: 10.1016/j.cell.2021.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weinmann J, Weis S, Sippel J, et al. . Identification of a myotropic AAV by massively parallel in vivo evaluation of barcoded capsid variants. Nat Commun 2020;11(1):5432; doi: 10.1038/s41467-020-19230-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martino AT, Suzuki M, Markusic DM, et al. . The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood 2011;117(24):6459–6468; doi: 10.1182/blood-2010-10-314518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rogers GL, Shirley JL, Zolotukhin I, et al. . Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8(+) T cells. Blood 2017;129(24):3184–3195; doi: 10.1182/blood-2016-11-751040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rogers GL, Suzuki M, Zolotukhin I, et al. . Unique roles of TLR9- and MyD88-dependent and -independent pathways in adaptive immune responses to AAV-mediated gene transfer. J Innate Immun 2015;7(3):302–314; doi: 10.1159/000369273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu J, Huang X, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest 2009;119(8):2388–2398; doi: 10.1172/JCI37607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bertolini TB, Shirley JL, Zolotukhin I, et al. . Effect of CpG depletion of vector genome on CD8(+) T cell responses in AAV gene therapy. Front Immunol 2021;12:672449; doi: 10.3389/fimmu.2021.672449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Faust SM, Bell P, Cutler BJ, et al. . CpG-depleted adeno-associated virus vectors evade immune detection. J Clin Invest 2013;123(7):2994–3001; doi: 10.1172/JCI68205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Konkle BA, Walsh CE, Escobar MA, et al. . BAX 335 hemophilia B gene therapy clinical trial results: Potential impact of CpG sequences on gene expression. Blood 2021;137(6):763–774; doi: 10.1182/blood.2019004625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pan X, Yue Y, Boftsi M, et al. . Rational engineering of a functional CpG-free ITR for AAV gene therapy. Gene Ther 2022;29(6):333–345; doi: 10.1038/s41434-021-00296-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shirley JL, Keeler GD, Sherman A, et al. . Type I IFN sensing by cDCs and CD4(+) T cell help are both requisite for cross-priming of AAV capsid-specific CD8(+) T cells. Mol Ther 2020;28(3):758–770; doi: 10.1016/j.ymthe.2019.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Butterfield JSS, Biswas M, Shirley JL, et al. . TLR9-activating CpG-B ODN but not TLR7 agonists triggers antibody formation to factor IX in muscle gene transfer. Hum Gene Ther Methods 2019;30(3):81–92; doi: 10.1089/hgtb.2019.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kuranda K, Jean-Alphonse P, Leborgne C, et al. . Exposure to wild-type AAV drives distinct capsid immunity profiles in humans. J Clin Invest 2018;128(12):5267–5279; doi: 10.1172/JCI122372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lu Y, Song S. Distinct immune responses to transgene products from rAAV1 and rAAV8 vectors. Proc Natl Acad Sci U S A 2009;106(40):17158–17162; doi: 10.1073/pnas.0909520106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Muhuri M, Zhan W, Maeda Y, et al. . Novel combinatorial microRNA-binding sites in AAV vectors synergistically diminish antigen presentation and transgene immunity for efficient and stable transduction. Front Immunol 2021;12:674242; doi: 10.3389/fimmu.2021.674242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Veron P, Allo V, Riviere C, et al. . Major subsets of human dendritic cells are efficiently transduced by self-complementary adeno-associated virus vectors 1 and 2. J Virol 2007;81(10):5385–5394; doi: 10.1128/JVI.02516-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xiao Y, Muhuri M, Li S, et al. . Circumventing cellular immunity by miR142-mediated regulation sufficiently supports rAAV-delivered OVA expression without activating humoral immunity. JCI Insight 2019;5(13):e99052; doi: 10.1172/jci.insight.99052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Boisgerault F, Gross DA, Ferrand M, et al. . Prolonged gene expression in muscle is achieved without active immune tolerance using microrRNA 142.3p-regulated rAAV gene transfer. Hum Gene Ther 2013;24(4):393–405; doi: 10.1089/hum.2012.208 [DOI] [PubMed] [Google Scholar]
- 64. Wang B, Li J, Fu FH, et al. . Construction and analysis of compact muscle-specific promoters for AAV vectors. Gene Ther 2008;15(22):1489–1499; doi: 10.1038/gt.2008.104 [DOI] [PubMed] [Google Scholar]
- 65. Wang L, Dobrzynski E, Schlachterman A, et al. . Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood 2005;105(11):4226–4234; doi: 10.1182/blood-2004-03-0848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu YL, Mingozzi F, Rodriguez-Colon SM, et al. . Therapeutic levels of factor IX expression using a muscle-specific promoter and adeno-associated virus serotype 1 vector. Hum Gene Ther 2004;15(8):783–792; doi: 10.1089/1043034041648453 [DOI] [PubMed] [Google Scholar]
- 67. Herzog RW, Biswas M. Neutralizing the neutralizers in AAV gene therapy. Mol Ther 2020;28(8):1741–1742; doi: 10.1016/j.ymthe.2020.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Horiuchi M, Hinderer CJ, Shankle HN, et al. . Neonatal Fc receptor inhibition enables adeno-associated virus gene therapy despite pre-existing humoral immunity. Hum Gene Ther 2023; doi: 10.1089/hum.2022.216 [DOI] [PubMed] [Google Scholar]
- 69. Leborgne C, Barbon E, Alexander JM, et al. . IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat Med 2020;26(7):1096–1101; doi: 10.1038/s41591-020-0911-7 [DOI] [PubMed] [Google Scholar]
- 70. Hakim CH, Kumar SRP, Perez-Lopez D, et al. . Assessment of the gene therapy immune response in the canine muscular dystrophy model. Methods Mol Biol 2023;2587:353–375; doi: 10.1007/978-1-0716-2772-3_18 [DOI] [PubMed] [Google Scholar]
- 71. Hakim CH, Kumar SRP, Perez-Lopez DO, et al. . Cas9-specific immune responses compromise local and systemic AAV CRISPR therapy in multiple dystrophic canine models. Nat Commun 2021;12(1):6769; doi: 10.1038/s41467-021-26830-7 [DOI] [PMC free article] [PubMed] [Google Scholar]