Abstract
Adeno-associated virus (AAV)-based gene therapies are emerging strategies in Duchenne muscular dystrophy (DMD) treatment. Exposure to wild-type AAV can lead to development of neutralizing antibodies (NAbs) and blocking of AAV transduction, thereby limiting the delivery of AAV vector-based gene therapy. Therefore, it is imperative to check for the presence of AAV NAbs in a patient who is a candidate for gene therapy. We prospectively enrolled 101 genetically confirmed males with DMD (median age 11 years, 48% ambulatory and 59% on steroids) and performed AAV neutralization assays against AAV2, AAV8, AAV9, and AAVrh74 serotypes. The serotype analysis showed that AAV9 (36%) and AAVrh74 (32%) seroprevalence was lower compared with AAV2 (56%) and AAV8 (47%). Interestingly, age was not correlated with NAb titer for any of the capsids. NAb responses were observed at a higher frequency in African American participants and at a lower frequency in Caucasian participants for all four serotypes. Further analysis showed no significant differences in NAb titers regardless of serotype and whether participants were taking steroids or not. Finally, we observed higher AAV8, AAV9, and AAVrh74 seroprevalence and significantly higher AAV2 and AAV8 NAb titers in participants who were ambulatory compared with nonambulatory participants. Overall, these data identify AAV9 and AAVrh74 as the two serotypes with lower pre-existing NAb titers in this study's cohort of 101 males with DMD, possibly showing their utility in future gene therapy applications in treatment of this cohort of patients with DMD.
Keywords: Duchenne muscular dystrophy, adeno-associated virus, AAV9, AAVrh74, neutralizing antibody, seroprevalence
INTRODUCTION
Duchenne muscular dystrophy (DMD) is an X-linked, chronic, progressive muscular disease caused by a mutation in the dystrophin (DMD) gene.1 To rescue affected muscles, gene replacement trials using a microdystrophin construct packed in an adeno-associated virus (AAV) vector are ongoing in adolescents and children. Although promising, there are several considerations before systemic gene delivery can be established as therapy.2,3 Most notable of these considerations was the presence of pre-existing neutralizing antibodies (NAbs) to AAV serotypes, which will negatively impact the efficacy of the in vivo gene therapy uptake.4–7
Identifying participants for AAV gene therapy trials can be challenging because of high rates of pre-existing NAbs against AAV.8–11 Most notably, numerous studies have shown pre-existing NAbs against AAV2 to be relatively high in the cohorts screened, ranging from 47% to 96%. Another serotype choice for AAV gene therapy to treat DMD is AAV9, a capsid under investigation in three clinical trials (NCT Nos. 03368742, 04240314, and 03362502).
Although AAV9 has not been evaluated as extensively as AAV2 for pre-existing NAbs, one study showed that 33.5% of a cohort of 62 participants were positive for AAV9 NAbs.11 Other serotypes are also under investigation in clinical trials, such as AAVrh74 to also treat DMD (NCT No. 02376816) and AAV8 to treat X-linked myotubular myopathy (NCT No. 03199469).
While the AAV-based gene therapies are being developed for muscular dystrophy, it may be prudent to screen males with DMD for NAbs to these AAV serotypes of interest that are currently used in clinical trials. On review of the literature, we found limited studies on the seroprevalence of NAbs for AAV in DMD patients.12,13 In this study, we set out to determine the seroprevalence rates of AAV serotypes 2, 8, 9, and rh74 in a large cohort of male DMD patients (n = 101) from Atlanta, GA.
MATERIALS AND METHODS
Study cohort
In this prospective study, we consecutively recruited genetically confirmed males with DMD followed in the muscular dystrophy clinic at Children's Healthcare of Atlanta (Atlanta, GA). The study duration was from June 2016 to May 2021. Study participants' data on age, genetic mutation, ethnic background, steroid use, and ambulatory status were recorded. The study was approved by the local Institutional Review Board.
Written informed consent and age-appropriate assent were obtained from the parents or legal guardians and patients. Spanish-only speaking patients received certified IRB consent and assent forms translated in Spanish.
AAV reporter vectors
AAV2 and AAV9 vectors encoding firefly luciferase under the control of a CMV promoter were obtained from the University of North Carolina Viral Vector Core. The gene encoding the AAVrh74 capsid was synthesized by GenScript and cloned into an AAV rep/cap DNA expression plasmid, which also expressed AAV2 replicase. AAV8 and AAVrh74 were produced and quantified, as previously described, using AAV8 and AAVX POROS CaptureSelect columns (Thermo Fisher Scientific) and quantified by quantitative PCR.6 Titered vectors were stored at −80°C until use.
AAV neutralization assay
The study procedure included a one-time collection of 5 mL of blood for the AAV neutralization assay during the standard of care blood draw for 25 hydroxy vitamin D levels. Patient serum was separated and stored at −80°C. Deidentified and coded study samples were transferred to the Emory National Primate Center Vaccine Biomarker Laboratory, Emory University, for AAV neutralization assays.
Heat-inactivated serum samples were diluted in complete media (Dulbecco's modified Eagle's medium [DMEM] supplemented with 10% fetal bovine serum [FBS], Pen/Strep, and l-glutamine; VWR) at ratios of 1:5, 1:20, 1:80, and 1:240 and plated in duplicate. Previously known seropositive and seronegative samples were included with each assay. Serum dilutions were combined with 50 μL of ∼1010–1011 AAV viral genomes/mL encoding firefly luciferase and incubated at 37°C in 5% CO2 for 1 h. These dilutions were used to produce >1,000 relative luciferase units upon transduction.
During incubation, confluent monolayers of HeLa or HEK293T cells (ATCC) seeded at 25,000 cells/well (AAV multiplicity of infection (MOI) range 20,000–200,000) were infected with viral particles of adenovirus-5 (ATCC) to increase luciferase expression of AAV2 and AAVrh74-transduced cells.
After incubation, the antibody–virus mixtures were aliquoted onto the adenovirus-5 HeLa cell monolayers and incubated at 37°C in 5% CO2. After 24–48 h, plates were removed from the incubator and washed once with phosphate-buffered saline (PBS) before addition of the firefly luciferase substrate (Bright-Glo assay kit; Bio-Rad). Plates were read on a BioTek luminometer within 15 min of substrate addition. Inhibition was determined by normalizing relative light units values against wells without serum and reported as a percentage.
Neutralizing titers were reported as the estimated dilution at which 50% of AAV transduction has been inhibited (IC50), using the nonlinear regression analysis in GraphPad Prism, v9.4.0, software. Samples with IC50 titers >1:5 were considered seropositive based on previous recommendations14 and a neutralizing titer that has been previously shown to block transduction of the liver.15 Low, moderate, and high positive NAb titers were defined as dilution of <1:20, 1:20–1:240, and >1:240, respectively.
Five study subjects had repeat blood draws for AAV neutralization titers during the study period.
AAV enzyme-linked immunosorbent assay
Binding antibodies (BAbs) against AAV2, AAV8, AAV9, and AAVrh74 were determined as previously described.6 Briefly, 96 half-well enzyme-linked immunosorbent assay (ELISA) plates were coated with 8 × 109 vg/mL of the respective AAV capsid and incubated overnight at 4°C. Plates were washed twice with PBS +0.005% Tween-20 (PBS-T) and blocked with 2% bovine serum albumin (BSA) in PBS for 1 h at 37°C.
Heat-inactivated serum samples were diluted 1:240 in 2% BSA in PBS and plated in duplicate on the ELISA plates. Plates were incubated for 1 h at 37°C and then washed five times with PBS-T. An HRP-conjugated goat anti-human IgG secondary antibody (Jackson ImmunoResearch) diluted 1:5,000 in 2% BSA in PBS was added to each plate. Plates were then incubated for 1 h at 37°C and washed 10 times with PBS-T.
TMB solution (Thermo Fisher) was added to each plate and plates were incubated at room temperature until color development, typically 1–2 min. Reactions were stopped using the TMB stop solution (SeraCare) and plates were read at an optical density (OD) of 450 nm on a plate reader (BioTek). Data are reported as average 450 nm OD (OD450) values at 1:240 dilution.
Statistical analysis
Descriptive statistics were used for data analysis, as determined by GraphPad Prism, v9.4.0, and statistical significance was defined as p-value <0.05.
RESULTS
Cohort demographics
One hundred one males with DMD and median age of 11 years (range 2–20 years) were enrolled. Cohort demographics are provided in Table 1. All study participants, except for one, had a confirmed pathogenic mutation in the dystrophin gene (n = 73 for out-of-frame deletion; n = 21 for point mutation; n = 6 for duplication; and n = 1 for unknown). Ethnic backgrounds of study participants were as follows: Caucasian 47.5% (48/101), Hispanic 31.7% (32/101), African American 16.8% (17/101), and Southeast Asian Indian 4% (4/101).
Table 1.
Study cohort with positive and negative antiadeno-associated virus neutralizing antibody titers
| Category | Analyzed for AAV2 and AAV9 NAbs (n = 101) | Analyzed for AAV8 and AAVrh74 NAbs (n = 92) |
|---|---|---|
| Age | ||
| <5 Years | 7% (n = 7) | 7% (n = 6) |
| 5–12 Years | 52% (n = 53) | 51% (n = 47) |
| >12 Years | 41% (n = 41) | 42% (n = 39) |
| Ethnic background | ||
| Caucasian | 48% (n = 49) | 47% (n = 43) |
| Hispanic | 31% (n = 31) | 33% (n = 30) |
| African American | 17% (n = 17) | 16% (n = 15) |
| Southeast Asian Indian | 4% (n = 4) | 4% (n = 4) |
| Steroid use | ||
| Deflazacort | 42% (n = 43) | 43% (n = 39) |
| Prednisone | 17% (n = 17) | 17% (n = 16) |
| Steroid-naive | 41% (n = 41) | 40% (n = 37) |
| Ambulatory status | ||
| Ambulatory | 48% (n = 48) | 47% (n = 43) |
| Age range, years (median) | 5–17 (8) | 5–17 (8) |
| Nonambulatory | 45% (n = 46) | 47% (n = 43) |
| Age range, years (median) | 8–20 (15) | 8–20 (15) |
| Toddler/infant | 7% (n = 7) | 6% (n = 6) |
AAV, adeno-associated virus; NAbs, neutralizing antibodies.
At the time of study enrollment, 59.4% (60/101) were receiving oral steroids (n = 43, deflazacort; and n = 17, prednisone) and 47.5% (48/101) were ambulatory. Due to sampling limitations, all 101 samples were assayed for NAbs against AAV2 and AAV9, while a 92-sample subset of the collected samples was assayed for NAbs against AAV8 and AAVrh74. The same 92 samples were assayed for BAbs against all four AAV serotypes.
Seroprevalence of AAV NAbs within the cohort
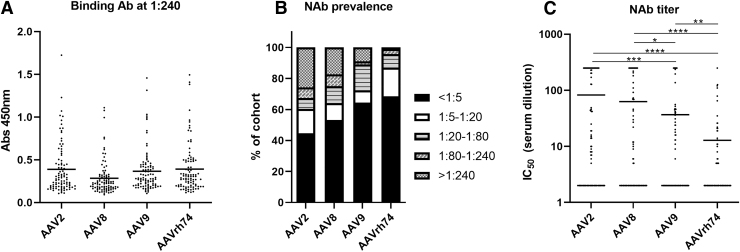
For this study, we assessed NAb prevalence against AAV2, AAV8, AAV9, and AAVrh74, with the latter three of particular interest because of the ongoing clinical trials using these capsids. Inclusion of AAV2 served as a historical comparison. When testing for BAbs by ELISA at the highest dilution used for the neutralization assay (1:240), we observed BAbs to some extent for nearly all samples (Fig. 1A).
Figure 1.
AAV seroprevalence in study participants. (A) Overall seroprevalence rates for AAV2, AAV8, AAV9, and AAVrh74 NAbs in the study cohort. Rates are grouped by participant samples with IC50 titers within the listed titer ranges. (B) Comparison of AAV2, AAV8, AAV9, and AAVrh74 IC50 NAb titers for each participant. Serum samples were premixed with indicated AAV vector encoding firefly luciferase. Sample and vector mixtures were then added to cells and incubated for 24–48 h. Firefly luciferase expression was determined, and neutralization values were normalized to cells transduced only with the vector. IC50 titers were calculated using a nonlinear regression analysis. Black bar indicates mean. (C) Ninety-two participant samples were screened for pre-existing binding antibodies against the indicated AAV serotype by ELISA. Serum samples were diluted 1:240 and added to plates coated with the indicated serotype. Binding antibodies were determined with an anti-human IgG secondary antibody. Black bar indicates mean. Note: no significant differences in binding antibodies were determined based on serotype comparisons; *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001 based on unpaired Student's t-test. AAV, adeno-associated virus; ELISA, enzyme-linked immunosorbent assay; NAbs, neutralizing antibodies.
While we did not observe any significant differences in OD450 values, the average OD450 value of BAbs against AAV8 was the lowest. We next assessed NAb titers, and within our cohort, 63.4% (n = 64/101) of participants were AAV seropositive against at least one of the serotypes assessed. Of note, 23 of the 92 participants who we tested against all 4 AAV serotypes had NAb titers against all four serotypes.
We observed that 44% of participants were seronegative for AAV2, 53% were seronegative for AAV8, 64% were seronegative for AAV9, and 68% were seronegative for AAVrh74 (Fig. 1B). The largest number of participants who were seropositive for AAV2 and AAV8 had NAb titers >1:240. In contrast, the largest number of AAV9-seropositive participants had NAb titers ranging from 1:80 to 1:240, while the largest number of AAVrh74-seropositive participants had NAb titers ranging from 1:5 to 1:20.
While we used a 1:5 cutoff for identifying a participant as seronegative in this study, the cutoff value may not be what is used for an actual clinical trial. Thus, we reasoned that determining IC50 values for each participant may be beneficial (Fig. 1C). We observed a significantly lower NAb titer for AAVrh74 compared with the other three AAV capsids tested (p < 0.0001 for AAV2 and AAV8 and p < 0.01 for AAV9).
Additionally, NAb titers for AAV9 were significantly lower than for AAV2 (p < 0.001) and AAV8 (p = 0.0374). Finally, no correlation between age and NAb titer was observed for any of the four AAV serotypes (Fig. 2).
Figure 2.
Age does not correlate with NAb titers for the study cohort. Correlation plots based on age and NAb titers determined in Fig. 1 for the indicated AAV serotype. No significant correlation was observed for any serotype based on the Pearson r correlation analysis.
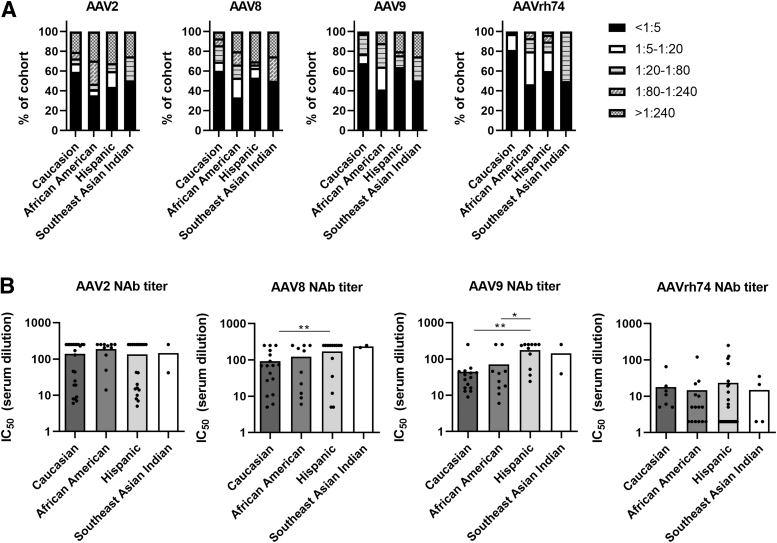
Higher rate of AAV NAbs in minority groups
We next divided our cohort based on ethnicity. Seropositivity for AAV2 NAbs was highest in African Americans (65%), followed by Hispanics (56%), Southeast Asian Indians (50%), and Caucasians (41%) (Fig. 3A). For AAV8, seropositivity was highest in African Americans (67%), followed by Southeast Asian Indians (50%), Hispanics (47%), and Caucasions (40%).
Figure 3.
AAV seroprevalence in study participants based on ethnicity. (A) Overall seroprevalence rates for AAV2, AAV8, AAV9, and AAVrh74 NAbs in the study cohort grouped based on ethnicity. Refer to Table 1 for ethnic background information. (B) Average AAV2, AAV8, AAV9, and AAVrh74 NAb titers based on each indicated ethnicity. Note that only participant samples with NAb titers >1:5 were used for the analysis; *p < 0.05 and **p < 0.01 based on unpaired Student's t-test. All other comparisons are not significant.
AAV9 seropositivity was similar to that observed for AAV8. The highest rates were for African Americans (59%), then Southeast Asian Indians (50%), followed by Hispanics (36%) and Caucasians (32%). This trend was also observed for AAVrh74 seropositivity, with the rate for African Americans at 53%, Southeast Asian Indians at 50%, Hispanics at 40%, and Caucasians at 19%.
For participants seropositive for AAV2 NAbs, we observed no significant differences in average AAV2 NAb titers, with all four group averages ranging from 1:134 to 1:188 (Fig. 3B). In contrast, we did observe significantly higher NAb titers against AAV8 and AAV9 in Hispanics compared with Caucasians (p = 0.0329 and p < 0.001, respectively). In Hispanics, NAb titers against AAV9 were also significantly higher compared with African Americans (p = 0.0211).
No significant differences were seen between ethnic groups for AAVrh74 NAb titers. Average NAb titers against AAV8 ranged from 1:92 to 1:235 for the four groups, while they ranged from 1:44 to 1:175 against AAV9 and from 1:14 to 1:23 against AAVrh74.
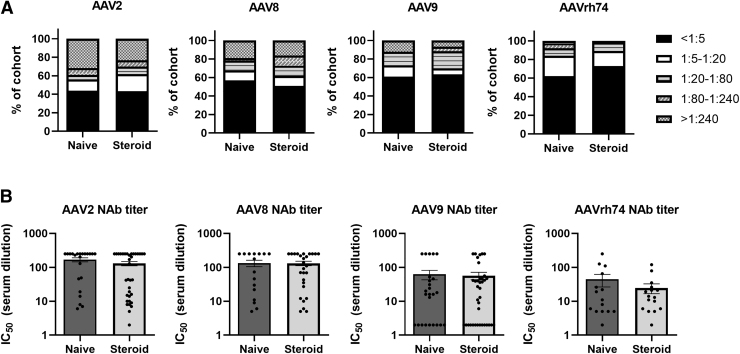
Ambulatory status, not steroid use, correlated with higher AAV2 NAb titers
Within our cohort, we identified 54% of the participants who were receiving oral steroids when samples were taken for analysis. A nearly identical rate of AAV2 seropositivity was observed in individuals not receiving steroids (57% vs. 56%, respectively) (Fig. 4A).
Figure 4.
AAV seroprevalence in study participants based on steroid use. (A) Overall seroprevalence rates for AAV2, AAV8, AAV9, and AAVrh74 NAbs in the study cohort grouped based on steroid use: naïve, n = 41, and steroids, n = 58, for AAV2 and AAV9 analysis; and naïve, n = 37, and steroids, n = 55, for AAV8 and AAVrh74 analysis. (B) Average NAb titers for indicated AAV serotypes for naïve and steroid using groups. Note that only participant samples with NAb titers >1:5 were used for the analysis. No group comparisons were significantly different based on unpaired Student's t-test.
Those receiving steroids had a higher rate of AAV8 seropositivity compared with those not receiving steroid therapy (49% vs. 43%, respectively). However, there was a slightly lower rate of AAV9 seropositivity in participants receiving steroids compared with those who were not (37% vs. 39%, respectively). A similar trend was also observed for AAVrh74 seropositivity, with the group receiving steroids being 27% positive for AAVrh74 NAbs compared with 38% positive in the group not receiving steroid treatment.
We observed no significant difference in NAb titers against any of the serotypes (Fig. 4B). Average NAb titer ranges were 1:130–1:146 for AAV2, 1:131–1:133 for AAV8, 1:57–1:62 for AAV9, and 1:24–1:44 for AAVrh74. We did observe a nonsignificant trend of lower NAb titers in the steroid-treated group for all four serotypes.
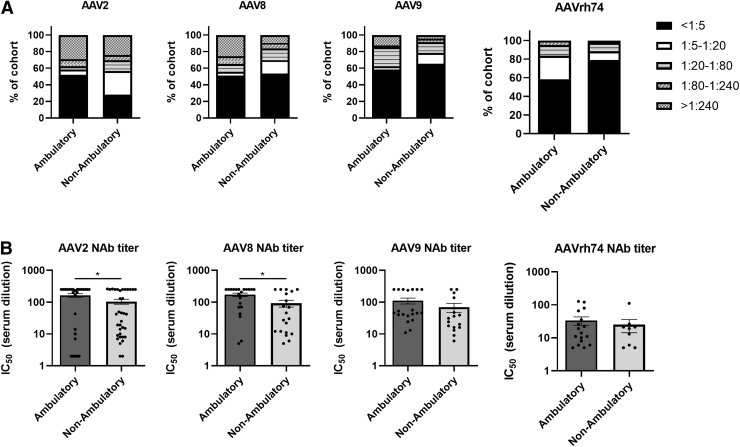
Our final subgroup analysis was based on ambulatory status, of which 59% of participants were ambulatory at the time of collecting samples for the study. A lower rate of AAV2-seropositive participants was observed in the ambulatory subgroup (48%) compared with the nonambulatory group (72%) (Fig. 5A). NAb rates were nearly equal for AAV8, 49% seropositive in the ambulatory subgroup compared with 47% in the nonambulatory subgroup.
Figure 5.
AAV seroprevalence in study participants based on ambulatory status. (A) Overall seroprevalence rates for AAV2, AAV8, AAV9, and AAVrh74 NAbs in the study cohort grouped based on ambulatory status: ambulatory, n = 48, and nonambulatory, n = 46, for AAV2 and AAV9 analysis. Ambulatory, n = 43, and nonambulatory, n = 43, for AAV8 and AAVrh74 analysis. (B) Average NAb titers for indicated serotypes for ambulatory and nonambulatory participants. Note that only participant samples with NAb titers >1:5 were used for the analysis; *p < 0.05 based on unpaired Student's t-test. All other comparisons are not significant.
The trend for AAV9 was reversed compared with AAV2, with 42% of the ambulatory subgroup participants being AAV9 seropositive and the nonambulatory group having a 35% rate of AAV9-seropositive participants. Finally, we observed that only 9% of participants in the nonambulatory subgroup were AAVrh74 seropositive, while 42% were seropositive in the ambulatory group.
For participants who were positive for NAbs, we observed significantly higher average NAb titers in the ambulatory group for AAV2 (1:162) and AAV8 (1:171) compared with the nonambulatory group (1:104 for AAV2 and 1:92 for AAV8; Fig. 5B). However, we did not observe significant differences in AAV9 or AAVrh74 NAb titers. The average AAV9 NAb titers were 1:110 and 1:69 and average AAVrh74 NAb titers were 1:33 and 1:25 for the ambulatory and nonambulatory subgroups, respectively.
DISCUSSION
In this study, we examined serum samples for the AAV seroprevalence rate in a large cohort of males with DMD, with the goal of screening, preparing, and informing DMD patients about upcoming AAV-based gene therapies. Inherited neuromuscular disorders are at the forefront of in vivo gene therapies.2 Onasemnogene abeparvovec (ZOLGENSMA) is one such example that is FDA approved for spinal muscular atrophy (SMA). This gene therapy treatment and several others in development employ AAV9 vectors.
Studies of healthy human donors and SMA gene therapy candidates have shown that prior exposure to the wild-type AAV can lead to the presence of pre-existing serum NAb titers.4 AAV microdystrophin is currently being developed for males with DMD; however, there are limited published studies on the AAV seroprevalence in this population.12,13 Early identification of DMD patients who are seronegative against a specific AAV serotype may increase the number of patients who can receive AAV gene therapy.
While early identification may be a difficult task, there are other methods under evaluation to overcome pre-existing NAbs against AAV. These include engineering AAV capsids that escape NAb responses,16–18 utilizing traditional plasmapheresis to remove immunoglobulins,19,20 and treating hosts with IgG-degrading endopeptidases such as IdeZ and IdeS.21,22
Our study showed a 54% AAV seroprevalence rate against AAV2 or AAV9 in a cohort of males with DMD (n = 101) in Atlanta. Leborgne et al. examined 130 French males with DMD and found a similar AAV seroprevalence rate.12 They observed a slightly lower frequency of AAV2-seropositive participants (about 44%), but did not test for AAV9. They also showed 30–80% seroprevalence against AAV1, AAV2, and AAV8 in healthy subjects; however, our study lacked a control arm to determine the AAV seroprevalence in age-matched healthy males in the Atlanta metro area.
Additionally, they had a 6-year follow-up and seroconversion rate of only 11% (n = 2/19). This finding underscores the concept that patients who are identified as seronegative should be notified and advised to limit contact with others to avoid seroconversion before AAV therapy is administered.
To date, this is the first study to compare AAV8, AAV9, and AAVrh74 NAb prevalence in a DMD cohort in the United States. The AAV seroprevalence analysis showed a lower rate for AAV9 and AAVrh74 NAb titers compared with AAV2 and AAV8 NAb titers in the study population. The lower seroprevalence and low to moderate NAb titers for AAV9 and AAVrh74 provide confirming evidence that these two capsids could be suitable for AAV gene therapy in DMD patients.
Three clinical trials are already evaluating AAV9 vectors (NCT No. 04240314, NCT No. 03368742, and NCT No. 03362502) to treat DMD and another trial is evaluating AAVrh74 (NCT No. 02376816). While our study shows a higher rate of AAVrh74-negative participants, this has not been observed for other studies. It was previously shown that participants in another screening study had higher prevalence of seropositivity for AAVrh74 compared with AAV9.23 It is worth noting that those findings were based on BAbs, where in our study, we did not observe significant differences based on BAb analysis.10
The results here do highlight the ongoing debate in the gene therapy field about the best assay for screening AAV gene therapy participants. Some trials have used total BAbs based on ELISA, while others used the neutralization assay. Recently, a study showed that BAbs may enhance AAV transduction in vitro and in vivo.5 Based on this, we reasoned that the neutralization assay may be more inclusive for finding future cohorts of participants for AAV gene therapy and it was the rationale for using the assay for this study.
As more AAV gene therapy clinical trials are conducted, we need to continue discussions on a standardized screening assay and what the antibody titer cutoffs should be for future trials. Recently, Dai et al. screened pig samples for NAbs against AAV1, AAV2, AAV5, AAV6, AAV8, and AAV9.24 The authors thoroughly evaluated the reporter vectors on different cell lines at different MOIs to achieve an efficient signal in the assay.
However, what remains to be understood is how different MOIs of AAV influence the NAb response, which could ultimately impact the seroprevalence results. One possibility to avoid any confounding factors would be to use the same cell line and MOI, regardless of the AAV vector that is used, as demonstrated in a previous study examining AAV6, AAV8, and AAV9 NAb prevalence in dogs.25
While overall rates of seropositivity are helpful, another understudied factor to consider when choosing AAV capsids for gene therapy is the age at which humans are exposed and seroconvert to various AAV serotypes. Our study included a majority of seropositive males in the school-going age of 5–12 years, possibly suggesting that natural exposure to AAV occurs once a child is surrounded by more people.
Although we did not observe any correlation between age and AAV NAb titers, identifying participants in AAV gene therapy trials at the earliest possible age would be ideal. A study by Zygmunt et al. showed that healthy young adults (<18 years) had measurable NAb titers to different serotypes, indicating that exposure to AAV can happen early in life.13 Additionally, identifying other factors may be helpful in determining the route of AAV exposure. This would include studying correlations between AAV seropositive status and socioeconomic status.
Further studies with a deeper analysis of participants' background data would benefit our understanding of AAV exposure. Additionally, a previous study observed higher seroprevalence of NAbs to AAV1, AAV3b, and AAV9 among healthy adult Black and Hispanic blood donors compared with their Caucasian counterparts.10
A recent study also found that seroprevalence was also higher in healthy Black and Hispanic donors for AAV1, AAV3B, AAV5, AAV9, AAVrh74, AAVDJ, and Spark100.10 Similarly, in our study on males with DMD, participants belonging to an ethnic minority background (African American and Hispanic) were more likely to be seropositive compared with Caucasian DMD participants. We urge that these data should be considered when developing broad AAV gene therapies meant for most people regardless of ethnicity.
Additionally, future studies screening larger cohorts of people from ethnic minority backgrounds for AAV seropositive status will need to be conducted. Other study limitations included lack of longitudinal follow-up and repeat AAV testing for all participants, inability to determine the age of seroconversion, and lack of a control arm of healthy individuals. These data would help inform the AAV field about how many people could benefit from any AAV gene therapy and possibly identify regions that are more seronegative than others.
In conclusion, our study shows AAV seroprevalence in a DMD cohort of 101 males in Atlanta, GA, for NAbs against AAV2, AAV8, AAV9, and AAVrh74. AAV9 and AAVrh74 seroprevalence was lower compared with AAV2 and AAV8.
Even though the ethnicity analysis showed African American participants with higher seroprevalence of AAV NAbs and Hispanic participants with higher NAb titers for AAV8 and AAV9, future studies will benefit from evaluation of other factors for determining causes of AAV exposure and development of AAV NAbs.
While future multicenter studies estimating AAV seroprevalence are needed, these data establish a relatively large DMD cohort that could benefit from AAV gene therapy.
ETHICAL PUBLICATION STATEMENT
The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The authors would like to thank Kerry Dibernado, LPN, CCRP, and research coordinators at Children's Healthcare of Atlanta, for study consent, sample collection, and sample processing.
AUTHORs' CONTRIBUTIONS
S.V. conceived the study. S.V., S.N.N., R.R., S.R.U., H.C.P., and T.H.V. organized and supervised the study. S.V., R.R., D.L., and T.H.V. collected and prepared samples. A.A.K., I.L., N.S.C., M.K., D.L., and M.R.G. conducted experiments. S.V., S.N.N., T.H.V., and M.R.G. analyzed the data. S.V., S.N.N., and M.R.G. wrote the article with input and approval from all coauthors.
AUTHOR DISCLOSURE
M.R.G. is a cofounder and consultant for Emmune, Inc., and an inventor on patents with potential royalties licensed to Emmune, Inc. All other authors have no conflicts of interest to disclose.
FUNDING INFORMATION
This work was funded by the Children's Healthcare of Atlanta Foundation Philanthropy Funds, Neuromuscular Program, number 95317-001-48-01, and by NIH grants R00AI138860 and R01AI167724 to M.R.G. and U42OD011023 to the Emory National Primate Research Center.
REFERENCES
- 1. Flanigan KM. Duchenne and Becker muscular dystrophies. Neurol Clin 2014;32:671–688. [DOI] [PubMed] [Google Scholar]
- 2. Mendell JR, Al-Zaidy SA, Rodino-Klapac LR, et al. . Current clinical applications of in vivo gene therapy with AAVs. Mol Ther 2021;29:464–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Byrne BJ, Corti M, Muntoni F. Considerations for systemic use of gene therapy. Mol Ther 2021;29:422–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kruzik A, Fetahagic D, Hartlieb B, et al. . Prevalence of anti-adeno-associated virus immune responses in international cohorts of healthy donors. Mol Ther Methods Clin Dev 2019;14:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fitzpatrick Z, Leborgne C, Barbon E, et al. . Influence of pre-existing anti-capsid neutralizing and binding antibodies on AAV vector transduction. Mol Ther Methods Clin Dev 2018;9:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gardner MR, Mendes DE, Muniz CP, et al. . High concordance of ELISA and neutralization assays allows for the detection of antibodies to individual AAV serotypes. Mol Ther Methods Clin Dev 2022;24:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuchs SP, Martinez-Navio JM, Rakasz EG, et al. . Liver-directed but not muscle-directed AAV-antibody gene transfer limits humoral immune responses in Rhesus monkeys. Mol Ther Methods Clin Dev 2020;16:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li C, Narkbunnam N, Samulski RJ, et al. . Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther 2012;19:288–294. [DOI] [PubMed] [Google Scholar]
- 9. Calcedo R, Vandenberghe LH, Gao G, et al. . Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009;199:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khatri A, Shelke R, Guan S, et al. . Higher seroprevalence of anti-adeno-associated viral vector neutralizing antibodies among racial minorities in the United States. Hum Gene Ther 2022;33(7–8):442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boutin S, Monteilhet V, Veron P, et al. . Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010;21:704–712. [DOI] [PubMed] [Google Scholar]
- 12. Leborgne C, Latournerie V, Boutin S, et al. . Prevalence and long-term monitoring of humoral immunity against adeno-associated virus in Duchenne Muscular Dystrophy patients. Cell Immunol 2019;342:103780. [DOI] [PubMed] [Google Scholar]
- 13. Zygmunt DA, Crowe KE, Flanigan KM, et al. . Comparison of serum rAAV serotype-specific antibodies in patients with Duchenne muscular dystrophy, Becker muscular dystrophy, inclusion body myositis, or GNE myopathy. Hum Gene Ther 2016;28:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorovits B, Fiscella M, Havert M, et al. . Recommendations for the development of cell-based anti-viral vector neutralizing antibody assays. AAPS J 2020;22:24. [DOI] [PubMed] [Google Scholar]
- 15. Manno CS, Pierce GF, Arruda VR, et al. . Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347. [DOI] [PubMed] [Google Scholar]
- 16. Tse LV, Klinc KA, Madigan VJ, et al. . Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc Natl Acad Sci U S A 2017;114:E4812–E4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Selot R, Arumugam S, Mary B, et al. . Optimized AAV rh.10 vectors that partially evade neutralizing antibodies during hepatic gene transfer. Front Pharmacol 2017;8:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lochrie Michael A, Tatsuno Gwen P, Christie B, et al. . Mutations on the external surfaces of adeno-associated virus type 2 capsids that affect transduction and neutralization. J Virol 2006;80:821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bertin B, Veron P, Leborgne C, et al. . Capsid-specific removal of circulating antibodies to adeno-associated virus vectors. Sci Rep 2020;10:864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orlowski A, Katz MG, Gubara SM, et al. . Successful transduction with AAV vectors after selective depletion of anti-AAV antibodies by immunoadsorption. Mol Ther Methods Clin Dev 2020;16:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leborgne C, Barbon E, Alexander JM, et al. . IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat Med 2020;26:1096–1101. [DOI] [PubMed] [Google Scholar]
- 22. Elmore ZC, Oh DK, Simon KE, et al. . Rescuing AAV gene transfer from neutralizing antibodies with an IgG-degrading enzyme. JCI Insight 2020;5:e139881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fu H, Meadows AS, Pineda RJ, et al. . Differential prevalence of antibodies against adeno-associated virus in healthy children and patients with mucopolysaccharidosis III: perspective for AAV-mediated gene therapy. Hum Gene Ther Clin Dev 2017;28:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dai Y, Kavita U, Lampen MH, et al. . Prevalence of pre-existing neutralizing antibodies against adeno-associated virus serotypes 1, 2, 5, 6, 8, and 9 in sera of different pig strains. Hum Gene Ther 2021;33:451–459. [DOI] [PubMed] [Google Scholar]
- 25. Shin J-H, Yue Y, Smith B, et al. . Humoral immunity to AAV-6, 8, and 9 in normal and dystrophic dogs. Hum Gene Ther 2011;23:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]