Abstract
The order Mycocaliciales (Ascomycota) comprises fungal species with diverse, often highly specialized substrate ecologies. Particularly within the genus Chaenothecopsis, many species exclusively occur on fresh and solidified resins or other exudates of vascular plants. In New Zealand, the only previously known species growing on plant exudate is Chaenothecopsisschefflerae, found on several endemic angiosperms in the family Araliaceae. Here we describe three new species; Chaenothecopsismatai Rikkinen, Beimforde, Tuovila & A.R. Schmidt, C.nodosa Beimforde, Tuovila, Rikkinen & A.R. Schmidt, and C.novae-zelandiae Rikkinen, Beimforde, Tuovila & A.R. Schmidt, all growing on exudates of endemic New Zealand conifers of the Podocarpaceae family, particularly on Prumnopitystaxifolia. Phylogenetic analyses based on ribosomal DNA regions (ITS and LSU) grouped them into a distinct, monophyletic clade. This, as well as the restricted host range, suggests that all three taxa are endemic to New Zealand. Copious insect frass between the ascomata contain ascospores or show an early stage of ascomata development, indicating that the fungi are spread by insects. The three new species represent the first evidence of Chaenothecopsis from any Podocarpaceae species and the first from any gymnosperm exudates in New Zealand.
Keywords: Chaenothecopsis , Mycocaliciales, New Zealand, Phyllocladus , plant exudate, Podocarpaceae, Prumnopitys , resinicolous fungi
Introduction
The order Mycocaliciales Tibell & Wedin represents an isolated lineage of non-lichenized ascomycetes with sessile or pin-like ascomata (Tibell and Wedin 2000). Species of this lineage are currently assigned to two families and five genera of which Chaenothecopsis Vain. represents the largest genus. However, generic delimitations within the Mycocaliciales are in need of revision, since molecular studies show that the currently established genera are not monophyletic (e.g. Tibell and Vinuesa 2005; Tuovila 2013).
The substrate ecology of mycocalicoid species currently assigned to Chaenothecopsis is particularly diverse. There are many highly specialized species that have adapted to utilize specific substrates of certain tree species (Tibell 1987; Tuovila 2013) or to live in association with lichens or green algae (Titov 2006). Within Chaenothecopsis a number of species occur exclusively on fresh and recently solidified exudates of diverse gymnosperms and angiosperms, with most of them exhibiting a high level of host specificity (e.g. Tibell and Titov 1995; Tuovila et al. 2013). Most resinicolous Chaenothecopsis species are known from terpenoid conifer resins of temperate boreal forests of the Northern Hemisphere including species of Abies Mill., Larix Mill., Picea A.Dietr., Pinus L. and Tsuga Carrière (e.g. Titov and Tibell 1993; Tibell and Titov 1995; Rikkinen 1999, 2003; Tuovila et al. 2011b). Only two species have so far been reported from conifers of warm temperate forests in Asia (Cunninghamia R.Br.; Tuovila et al. 2013) and an araucarian conifer from New Caledonia (Agathis Salisb.; Rikkinen et al. 2014). Additional Chaenothecopsis species, all belonging to a distinct, monophyletic group, grow on angiosperm exudates of host trees in the Sapindales Juss. ex Bercht. & J. Presl., including Anacardiaceae R.Br. (Khaya A.Juss. and Rhus L.; Tuovila et al. 2011a) and Simaroubaceae DC. (Ailanthus Desf.; Tuovila et al. 2014), as well as the Apiales Nakai (Kalopanax Miq. (Tuovila et al. 2014), Pseudopanax K.Koch (Beimforde et al. 2017), and Schefflera J.R.Forst. & G.Forst. (Samuels and Buchanan 1983)). Of the mycocalicioid fungi so far known from New Zealand, most species of Chaenothecopsis are believed to be more or less cosmopolitan and live as saprophytes on the lignum of local conifers or angiosperms (Tibell 1987). Only one New Zealand species, Chaenothecopsisschefflerae (Samuels & D.E. Buchanan) Tibell, is known from plant exudates so far. It occurs exclusively on angiosperm exudates produced by different species of endemic Araliaceae Juss. (Schefflera, Pseudopanax; Samuels and Buchanan 1983; Beimforde et al. 2017).
Several fossils in Paleogene amber demonstrate that the ascoma morphology and resinicolous ecology of conifer-associated taxa have remained unchanged for tens of millions of years (Rikkinen and Poinar 2000; Tuovila et al. 2013; Rikkinen et al. 2018; Rikkinen and Schmidt 2018), but the evolutionary origin of the resinicolous ecology within the Mycocaliciales is still unclear. Molecular phylogenetic analyses indicate that the resinicolous ecology on conifer resin predates fungi occupying angiosperm exudate. Chaenothecopsis species from angiosperm exudates are grouped in a well-supported monophyletic group, suggesting a single origin of this ecological mode, whereas species on conifer resin are scattered throughout the genus, suggesting a longer evolutionary history (e.g. Rikkinen et al. 2014; Tuovila et al. 2014; Beimforde et al. 2017).
Here we describe three new Chaenothecopsis species that grow mainly on exudates of Prumnopitystaxifolia (Banks & Sol. ex D. Don) de Laub. (Podocarpaceae Endl.), an endemic New Zealand gymnosperm also known as black pine or Mataī. The morphology of each species is examined using light and scanning electron microscopy (SEM) and their phylogenetic relationships are elucidated based on ribosomal DNA data of the internal transcribed spacer region (ITS) and the large ribosomal subunit (nucLSU). The new species are described as Chaenothecopsismatai, C.nodosa and C.novae-zelandiae. They represent the first Chaenothecopsis species from any species of the conifer family Podocarpaceae and the first report of Chaenothecopsis species associated with gymnosperm exudate from New Zealand.
Methods
Biological material
Chaenothecopsis specimens were collected from Prumnopitystaxifolia (Podocarpaceae) growing in different localities in the North and South Islands of New Zealand (Fig. 1, Suppl. material 1). Specimens were also collected on exudates of Phyllocladustrichomanoides D. Don (Podocarpaceae) from the North Island. Type specimens are deposited in the New Zealand Fungarium (PDD), Landcare Research in Auckland (Suppl. material 1).
Figure 1.
Typical habitats of Chaenothecopsis species from Podocarpaceae in northern New Zealand A collecting specimens of Chaenothecopsisnovae-zelandiae (PDD 110742) from a trunk of Prumnopitystaxifolia along Te Whaiti Road B (detail of A): Prumnopitystaxifolia with old, partly charred lesions CPrumnopitystaxifolia hosting Chaenothecopsismatai (PDD 110746) along Ruatahuna Road D colonized exudate of PrumnopitystaxifoliaE (detail of D): exudate colonized by Chaenothecopsismatai (PDD 110746). Scale bars: 4 cm (D); 2 cm (E).
Light microscopy and scanning electron microscopy
Morphological features (Figs 2–10) of the fungal specimens were studied and imaged using a Carl Zeiss StereoDiscovery V8 dissection microscope, a Leica DMLS microscope and a Carl Zeiss AxioScope A1 compound microscope equipped with Canon EOS 5D digital cameras. Ascomatal details were studied under 40- to 100-fold magnification, sometimes with an additional 1.6-fold magnification. Spores and inner ascomatal structures were analyzed and imaged on a microscope slide in water using Differential Interference Contrast (DIC) illumination. Some diagnostic structures, such as paraphyses and stipe hyphae, were observed by utilizing potassium hydroxide (KOH).
Figure 2.
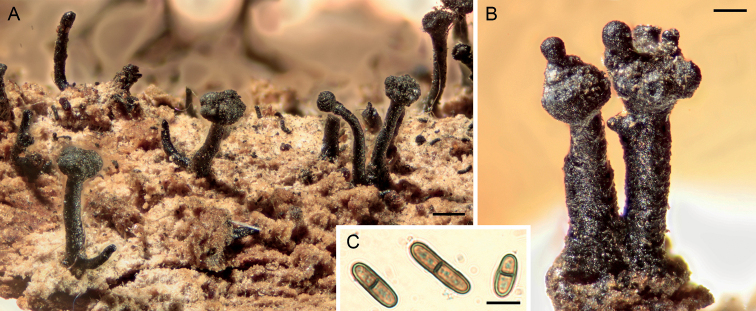
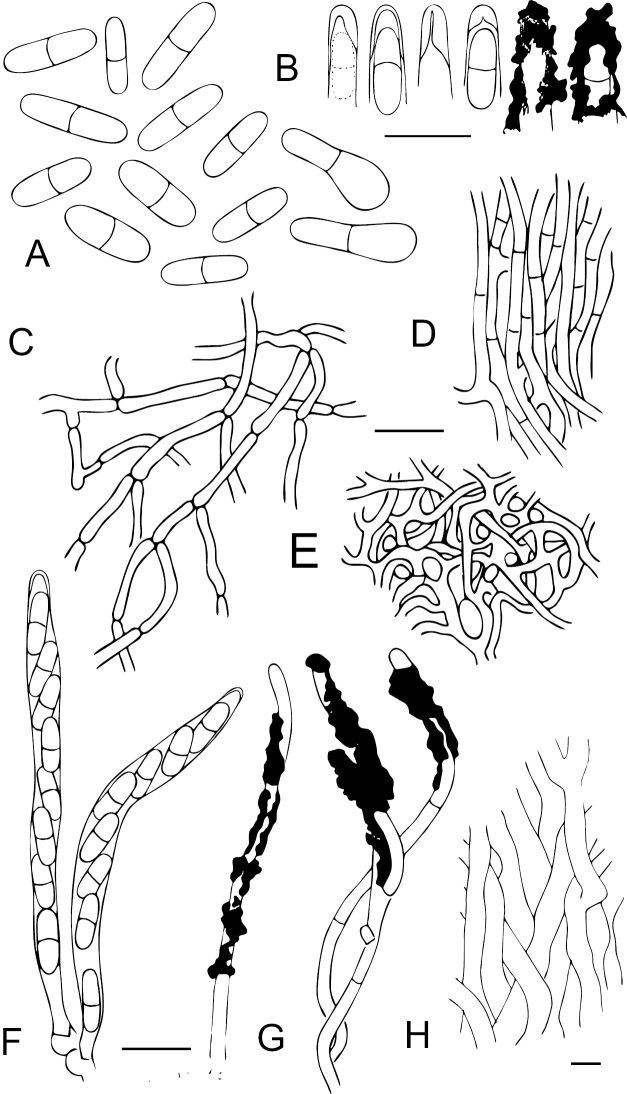
Light micrographs of Chaenothecopsisnovae-zelandiae sp. nov. (PDD 110744). A apothecia on hardened exudate of PrumnopitystaxifoliaB apothecia with proliferating capitula C ascospores. Scale bars: 200 µm (A); 100 µm (B); 5 µm (C).
Figure 10.
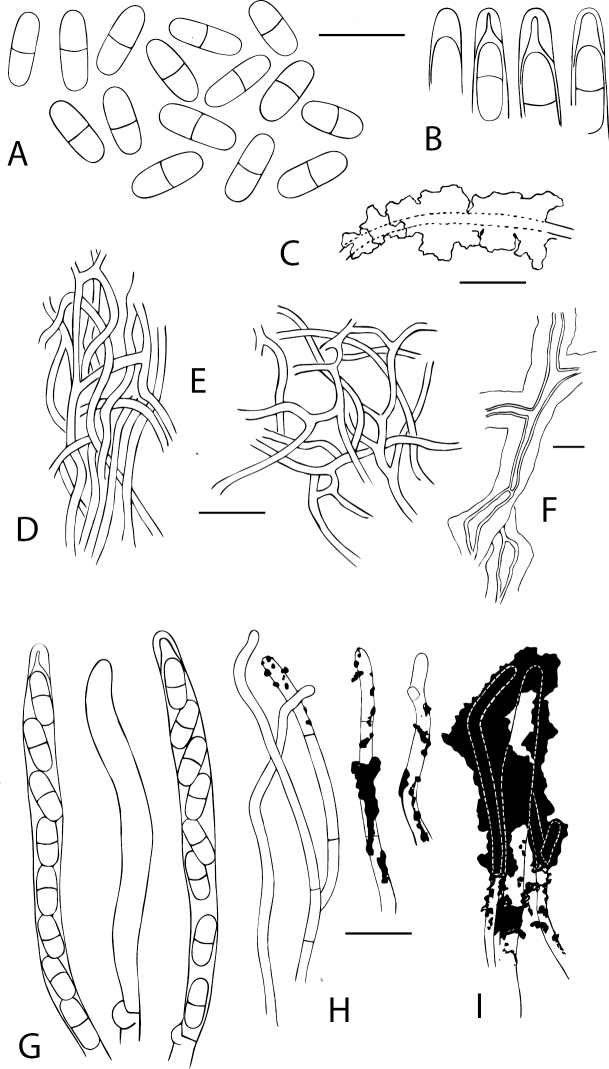
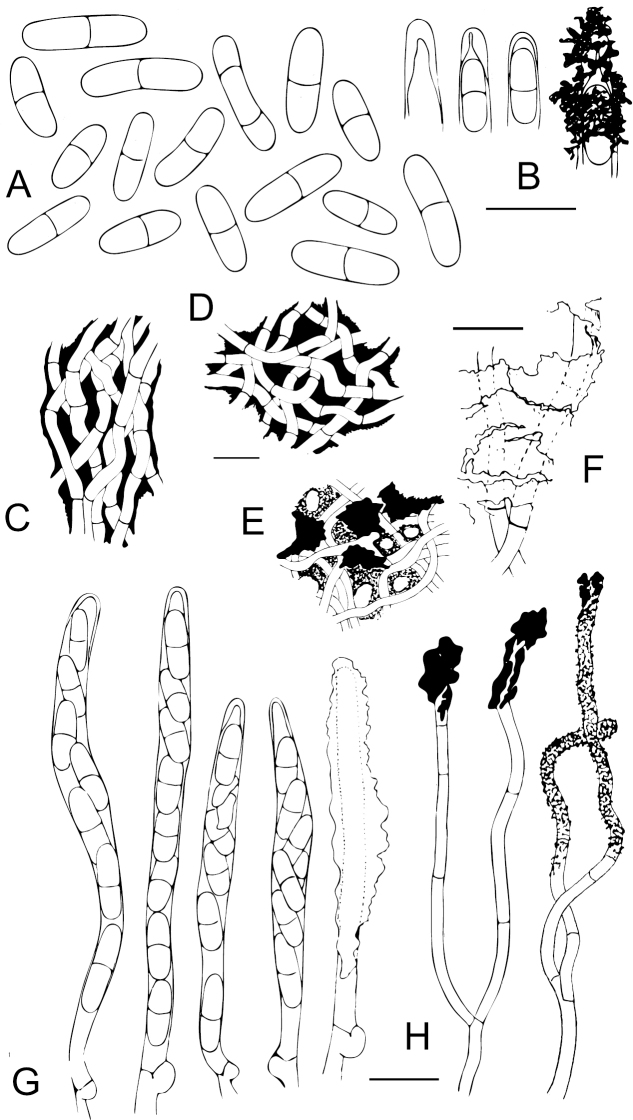
Anatomical details of Chaenothecopsisnodosa sp. nov. A ascospores B ascus tips C hypha of epithecium covered with amorphous material D excipulum structure E stipe hyphae F structure of the hyphae at the base of the stipe G asci with croziers H paraphyses I tips of paraphyses covered with amorphous material. Scale bars: 10 µm.
Light-microscopical images of ascomata on Prumnopitys Phil. exudates were obtained from 40–60 focal planes by using incident and transmitted light simultaneously. Individual images of focal planes were digitally stacked using the software package HeliconFocus 7.0 (Helicon Soft Limited, Kharkiv, Ukraine).
For scanning electron microscopy (Figs 3, 6, 9, 11), air dried specimens of each species were removed from the substrate, placed on a carbon-covered SEM-mount, sputtered by gold/palladium and examined under a Carl Zeiss LEO 1530 Gemini field emission scanning-electron microscope.
Figure 3.
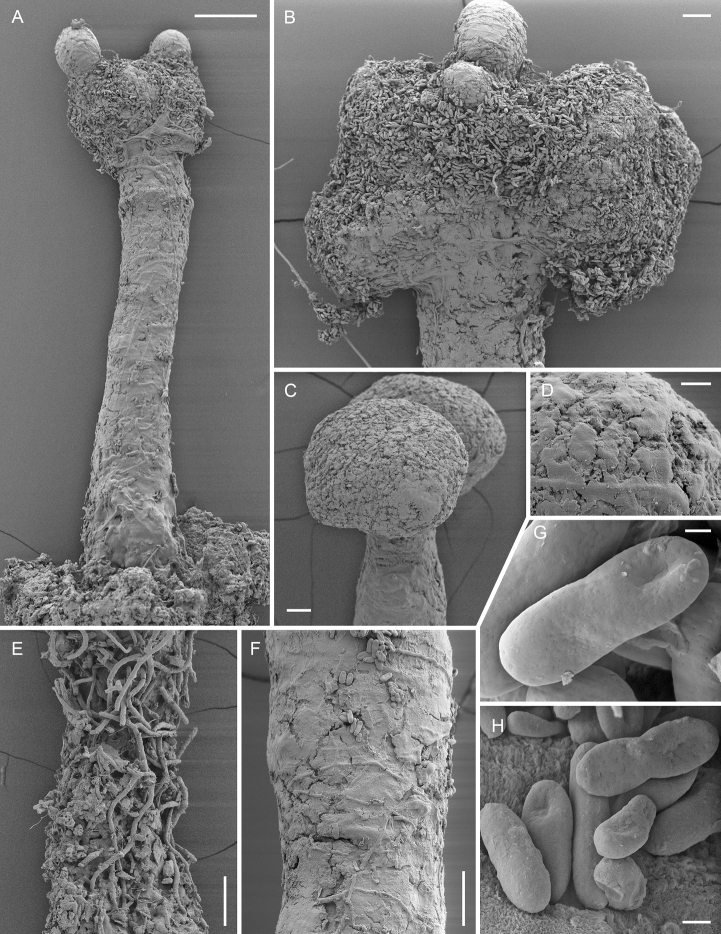
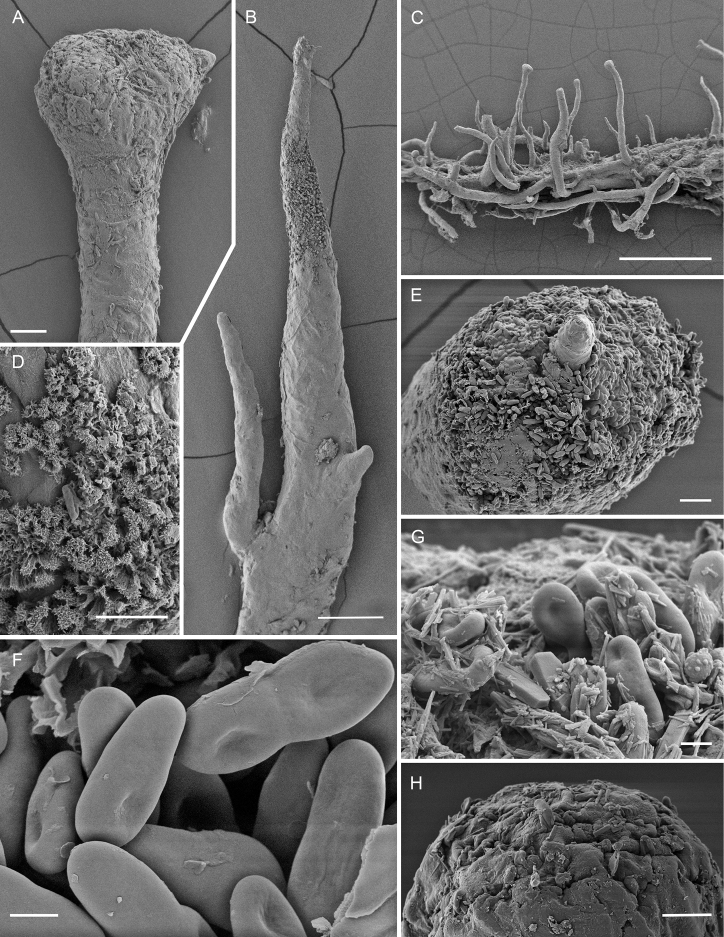
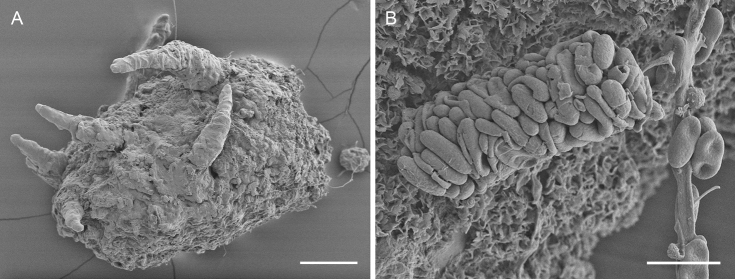
Scanning electron micrographs of Chaenothecopsisnovae-zelandiae sp. nov. (PDD 110744/CBNZ073B) A proliferating apothecium B mature capitulum with ascospores and amorphous material C semi-mature capitulum D (detail of C): epithecium of semi-mature capitulum E orientation of hyphae at the base of deteriorating ascoma F stipe surface G ascospore H ascospores. Scale bars: 100 µm (A); 30 µm (B, C, E, F); 10 µm (D); 2 µm (H); 1 µm (G).
Figure 6.
Scanning electron micrographs of Chaenothecopsismatai sp. nov. (PDD 110749) A semi-mature capitulum B upper part of apothecium C pseudostroma-like growth of apothecia D structure of pruina on stipe surface E proliferating growth of capitulum F ascospores G (detail of E): ascospores and crystals on capitulum surface H mature capitulum. Scale bars: 1 mm (C); 100 µm (B); 30 µm (A); 20 µm (E); 10 µm (D, H); 2 µm (F, G).
Figure 9.
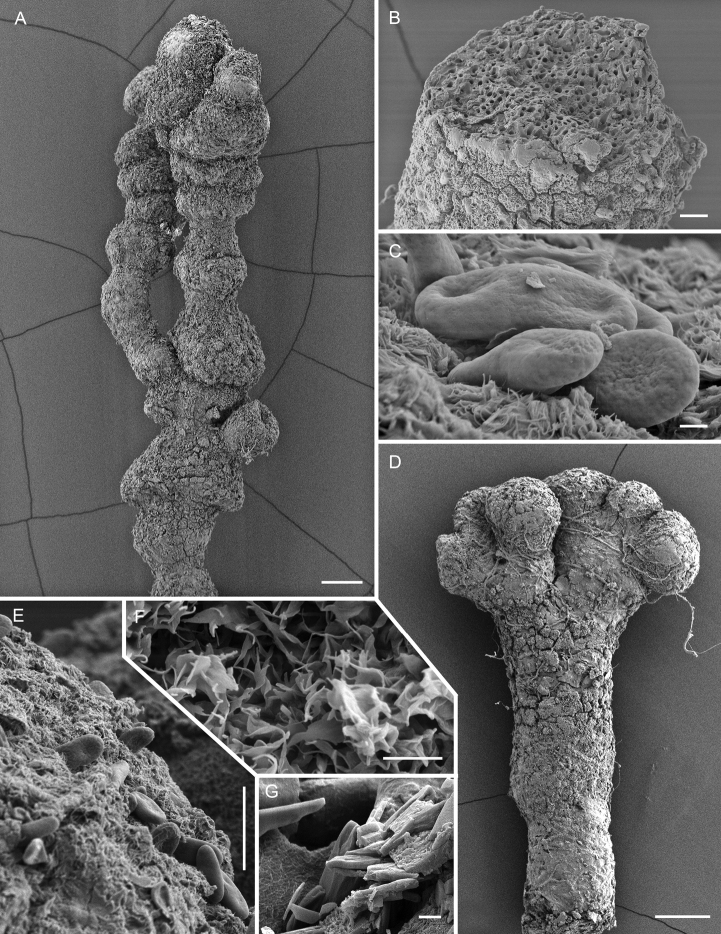
Scanning electron micrographs of Chaenothecopsisnodosa sp. nov. (PDD 110745) A branched ascoma with numerous tightly stacked capitula B cross section of stipe C ascospore ornamentation D compound capitula E–G details of capitulum surface E ascospores on capitulum surface F amorphous material on capitulum surface G crystals on capitulum surface. Scale bars: 100 µm (A, D); 10 µm (B, E); 1 µm (C, F, G).
Figure 11.
Insect fecal pellets associated with Chaenothecopsismatai (A) and Chaenothecopsisnodosa (B) A fecal pellet showing initial ascomata development B insect fecal pellets consisting predominantly of ascospores. Scale bars: 100 µm (A); 10 µm (B).
Spore isolation and cultivation
Cultures were obtained by transferring single ascocarps from the substrate to cavity glass slides containing a drop of sterile 0.9% sodium chloride. All adhering substrate particles were removed and a single mature ascocarp was transferred to a fresh cavity glass slide containing a drop of sterile 0.9% sodium chloride and gently crushed with a sterile scalpel to liberate the spores. Spores were further diluted in 200–300µl sterile 0.9% sodium chloride and transferred to solid potato dextrose media (PDA, Carl Roth, Germany: 4 g/l potato infusion, 20 g/l glucose, 15 g/l agar, pH = 5.6 ± 0.2) using pipettes and filter tips. Inoculates were investigated under a Carl Zeiss StereoDiscovery V8 dissection microscope, initially every 2 days, until germination started. Cultures were subsequently stored in the dark and checked every week in order to detect possible contamination at an early stage. After 5–6 months, cultures were identified using molecular analysis of internal transcribed spacer region (ITS).
DNA extraction, PCR amplification and sequencing
DNA was extracted from all collected representative specimens of Chaenothecopsis. Between 5–10 ascomata of each specimen were crushed with a fine glass mortar and pestle (Carl Roth, Karlsruhe, Germany) prior to DNA-extraction. DNA was subsequently extracted using the DNA Micro Kit from Quiagen (Hilden, Germany) following the manufacturer’s protocol, but modifying the incubation time to at least 24 hours. Samples were held in micro-glass mortars closed with parafilm during the whole incubation time.
The large subunit of nuclear ribosomal RNA (LSU) was amplified using primers pairs LR0R and LR3 (Vilgalys and Hester 1990; Rehner and Samuels 1994), as well as LR5 and LR7 (Vilgalys and Hester 1990). The internal transcribed spacer region (ITS) of the ribosomal DNA was amplified using the primers ITS5 (White et al. 1990) or ITS1F (Gardes and Bruns 1993) and ITS4 (White et al. 1990). Polymerase chain reaction (PCR) was conducted using Taq DNA polymerase (Promega, Madison, WI) by following the manufacturer’s recommendations and PCR conditions with the following steps: (1) hot start with 95 °C for 2 min; (2) 35 cycles of 45 s (ITS) to 60 s (LSU) at 95 °C, 60 s at 52–55 °C and 45 s (ITS) to 60 s (LSU) at 72 °C and (3) 10 min of final elongation at 72 °C. Subsequently, the ITS and LSU rDNA products were purified using PCRapace (Invitek, Berlin, Germany) and sequenced in both directions with a MegaBACE 1000 automated sequencing machine and DYEnamic ET Primer DNA sequencing reagent (Amersham Biosciences, Little Chalfont, UK). Sequences were assembled and edited using Bioedit 5.0.9 (Hall 1999).
Taxon sampling and phylogenetic analysis
While many different Chaenothecopsis species have been reported from New Zealand (Tibell 1987), sequences of only a few, including Chaenothecopsisdebilis (Sm.) Tibell, C.haematopus Tibell and C.schefflerae (Samuels & D.E. Buchanan) Tibell, are available at present in Genbank. Most other sequences were obtained from specimens collected in Europe, primarily Sweden. Some Genbank sequences originating from cultures appeared inconsistent with the sequences from corresponding type material and were excluded from our analyses.
ITS and nucLSU from New Zealand specimens were sequenced in forward and backward direction and sequences were assembled using Bioedit 5.0.9 (Hall 1999). ITS and LSU data sets were aligned separately using MAFFT version 6 (Katoh and Toh 2008) and subsequently combined in Bioedit 5.0.9 (Hall 1999). For phylogenetic analyses only unambiguously alignable DNA regions were selected manually, using the mask function in Bioedit 5.0.9 (Hall 1999). The resulting data set comprises 401 basepairs (bp) of the ribosomal ITS region and 779 bp of the ribosomal LSU region.
The best fitting substitution model for each gene was chosen separately from seven substitution schemes included in the software package jModeltest 2.1.1 (Darriba et al. 2012), and models were selected according to the Bayesian information criterion (Schwarz 1978). The Bayesian information criterion supported the TIM2ef+I+G model as the best fit for the ITS region and the TrN+I+G model for the LSU gene. Both genes were combined in a single data matrix using Bioedit 5.0.9 (Hall 1999) and Bayesian analyses were carried out using Markov chain Monte Carlo in MrBayes 3.2.7 (Ronquist and Huelsenbeck 2003) on the CIPRES Science Gateway v. 3.3 (Miller et al. 2010) without using BEAGLE high-performance library (https://github.com/beagle-dev/beagle-lib).
Four chains were conducted simultaneously for 10 million generations each, sampling parameters every 1000th generation. Average standard deviations of split frequency < 0.01 were interpreted as indicative of independent Markov chain Monte Carlo convergence. A burn-in sample of 2500 trees was discarded for the run and the remaining trees were used to estimate branch lengths and posterior probabilities. Convergence and sufficient chain mixing (effective sample sizes > 200) were controlled using Tracer 1.7.2 (Rambaut and Drummond 2009). GenBank accession numbers of all fungal specimens used for phylogenetic reconstruction are provided in Table 1. The combined data matrix, settings for the Bayesian analyses, and resulting phylogenetic tree (Fig. 12) were deposited in TreeBASE, direct access: http://purl.org/phylo/treebase/phylows/study/TB2:S29864.
Table 1.
GenBank accessions for the fungal ITS and LSU sequences used in this study for phylogenetic analysis (Fig. 12).
| Species name | Voucher | GenBank accessions ITS/LSU | References |
|---|---|---|---|
| Brunneocarposbanksiae Giraldo & Crous | CPC 29841 | NR_147648/NG_066277 | Crous et al. (2016) |
| Caliciopsisindica J. Pratibha & Bhat | GUFCC 4947 | GQ259981/GQ259980 | Pratibha et al. (2011) |
| Chaenothecopsis sp. 1 | Tuovila 09-052 | X119110/JX119119 | Tuovila et al. (2013) |
| Chaenothecopsis sp. 2 | 08-004 (TUR) | KC590480/KC590485 | Tuovila (2014) |
| Chaenothecopsisconsociata (Nádv.) A.F.W. Schmidt | Tibell 22472 (UPS) | AY795851/DQ008999 | Tibell and Vinuesa (2005) |
| Chaenothecopsisdebilis (Sm.) Tibell | Tibell 16643 (UPS) | AY795852/ AY795991 | Tibell and Vinuesa (2005) |
| Chaenothecopsisdiabolica Rikkinen & Tuovila | H:Tuovila 06-035 | JX119109/JX119114 | Tuovila (2013) |
| Chaenothecopsisdolichocephala Titov | Tibell 19281 | AY795854/AY795993 | Tibell and Vinuesa (2005) |
| Chaenothecopsisfennica (Laurila) Tibell | Tibell 16024 (UPS) | AY795857/AY795995 | Tibell and Vinuesa (2005) |
| Chaenothecopsisgolubkovae Tibell & Titov | Titov 6707 (UPS) | AY795859/AY795996 | Tibell and Vinuesa (2005) |
| Chaenothecopsishaematopus Tibell | 16625 (UPS) | AY795861/AY795997 | Tibell and Vinuesa (2005) |
| Chaenothecopsiskhayensis Rikkinen & Tuovila | JR 04G058 | JX122785/HQ172895 | Tuovila et al. (2011a) |
| Chaenothecopsismontana Rikkinen | H:Tuovila 07-086 | JX119105/JX119114 | Tuovila et al. (2013) |
| Chaenothecopsisneocaledonica Rikkinen, Tuovila & A.R. Schmidt | Rikkinen 010179 | KF815196/KF815197 | Rikkinen et al. (2014) |
| Chaenothecopsisnigripunctata Rikkinen | H:Tuovila 06-013 | JX119103/JX119112 | Tuovila et al. (2013) |
| Chaenothecopsismatai Rikkinen, Beimforde, Tuovila & A.R. Schmidt | PDD 110746 | OQ308931/OQ308874 | This study |
| PDD 110749 | OQ308932/OQ308875 | This study | |
| Chaenothecopsisnodosa Beimforde, Tuovila, Rikkinen & A.R. Schmidt | PDD 110743 | OQ308933/OQ308876 | This study |
| PDD 110745 | OQ308934/OQ308877 | This study | |
| Chaenothecopsisnovae-zelandiae Rikkinen, Beimforde, Tuovila & A.R. Schmidt | PDD 110742 | OQ308935/OQ308878 | This study |
| PDD 110744 | OQ308936/OQ308879 | This study | |
| Chaenothecopsispallida Rikkinen & Tuovila | H:JR 010652 | JX122779/JX122781 | Tuovila et al. (2013) |
| Chaenothecopsispusilla (A. Massal.) A.F.W. Schmidt | Tibell 16580 (UPS) | -/ DQ009000.1 | Tibell and Vinuesa (2005) |
| Chaenothecopsispusiola (Ach.) Vain. | H:Tuovila 09-047 | JX119106/JX119115 | Tuovila et al. (2013) |
| Chaenothecopsisquintralis Messuti, Amico, Lorenzo & Vidal-Russ. | BCRU:05233 | -/JQ267741 | Messuti et al. (2012) |
| Chaenothecopsisresinophila Rikkinen & Tuovila | H:JR000424 | JX122780/JX122782 | Tuovila et al. (2013) |
| Chaenothecopsisschefflerae (Samuels & D.E. Buchanan) Tibell | Rikkinen 13183 | KY499965/ KY499967 | Beimforde et al. (2017) |
| Chaenothecopsissitchensis Rikkinen | H:Tuovila 06-033 | JX119102/JX119111 | Tuovila et al. (2013) |
| Chaenothecopsissubparoica (Nyl.) Tibell | Tretiach (hb. Tretiach) | AY795869/- | Tibell and Vinuesa (2005) |
| Chaenothecopsistsugae | H:JR07005B | JX119104/JX119113 | Tuovila et al. (2013) |
| Chaenothecopsisviridireagens Rikkinen | Tibell 22803 (UPS) | AY795872/ DQ013257 | Tibell and Vinuesa (2005) |
| Fusichalaraminuta Hol.-Jech. | CBS 709.88 | KX537754/ KX537758 | Réblová et al. (2017) |
| Mycocaliciumalbonigrum (Nyl.) Tibell | Tibell 19038 | AF223966/ AY796001 | Tibell and Vinuesa (2005) |
| Mycocaliciumsubtile (Pers.) Szatala | JR6450 | OQ308930/OQ308873 | This study |
| Mycocalicium sp. | Tuovila 09-131 (TUR) | KC590482/KC590487 | Tuovila et al. (2014) |
| Sphinctrinaleucopoda Nyl. | Kalb 33829 (hb. Kalb) | AY795875/AY796006 | Tibell and Vinuesa (2005) |
| Sphinctrinaturbinata (Pers.) De Not. | Tibell 23093 (UPS) | AY795877/DQ009001 | Tibell and Vinuesa (2005) |
| Tibell 22478 (UPS) | AY795876/- | Geiser et al. (2006) | |
| AFTOL-ID 1721 | -/ EF413632 | Geiser et al. (2006) | |
| Stenocybepullatula (Ach.) Stein | Tibell 17117 (UPS) | AY795878/AY796008 | Tibell and Vinuesa (2005) |
| Phaeocaliciumpopulneum (Brond. & Duby) A.F.W. Schmidt | Tibell 19286 (UPS) | AY795874/AY796009 | Tibell and Vinuesa (2005) |
| Phaeocaliciumpraecedens (Nyl.) A.F.W. Schmidt | Tuovila 09-240 (TUR) | KC590481/KC590486 | Tuovila et al. (2014) |
| Pyrgillusjavanicus (Mont. & Bosch) Nyl. | AFTOL-ID 342 | DQ826741/DQ823103 | James et al. (2006) |
| Pyrenulaminutispora Aptroot & M. Cáceres | ABL AA11877 | KT820119/- | Gueidan et al. (2016) |
| Pyrenulanitida (Weigel) Ach. | F 5929 | JQ927458/ DQ329023 | del Prado et al. (2006); Weerakoon et al. (2016) |
| Rhopalophoraclavispora (W. Gams) Réblová | CBS 129.74 | KX537751/ MH872573 | Réblová et al. (2017) |
| CBS 281.75 | KX537752/ KX537756 | Réblová et al. (2017) | |
| Verrucariainverecundula Pykälä & Myllys | FILIC650-13 | MK138796/- | Pykälä et al. (2019) |
Figure 12.
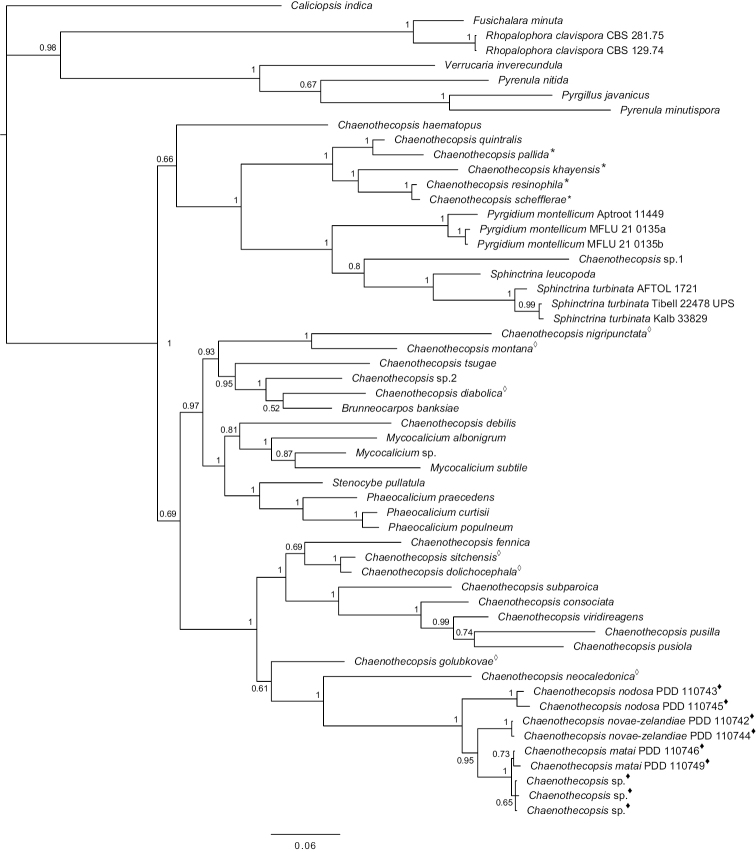
Phylogenetic relationships of mycocalicioid fungi (Mycocaliciales, Ascomycota). Bayesian tree based on partial sequences of the ribosomal internal transcribed spacer region (ITS) and the large ribosomal subunit (LSU). Numbers at branches indicate Bayesian posterior probabilities. The asterisks mark species from angiosperm exudate, white diamonds mark species from conifer resin, black diamonds mark species from podocarpous exudates.
Results
Taxonomy
. Chaenothecopsis novae-zelandiae
Rikkinen, Beimforde, Tuovila & A.R. Schmidt sp. nov.
213FA4AB-516F-5AAF-98AB-B238F92C2C29
MB846458
Figure 4.
Anatomical details of Chaenothecopsisnovae-zelandiae sp. nov. A ascospores B ascus tips C excipulum D stipe hyphae E epithecium with amorphous material and pores F hyphae of excipulum with amorphous material G asci with croziers H paraphyses. Scale bars: 10 µm.
Type.
New Zealand, South Island, State Highway 6 close to Makarora, Otago, ca. 44°13.787'S, 169°13.9708'E, on exudate of Prumnopitystaxifolia, 5 February 2017, holotype: PDD110744, New Zealand Fungarium (PDD), Landcare Research in Auckland, GenBank accession OQ308936/OQ308879.
Diagnosis.
Chaenothecopsisnovae-zelandiae differs from other Chaenothecopsis species by forming mostly solitary ascomata on podocarpous plant exudates, and by having inner ascomatal structures firmly connected by amorphous material and finely ornamented spores, which can be slightly constricted at the septum.
Etymology.
The specific epithet refers to New Zealand where the species was first discovered.
Description.
Apothecia growing on the exudate of Prumnopitystaxifolia, 0.6–1.6 mm tall, growing individually or grouped in small clusters, often branched or proliferating from the capitulum. Stipe glossy black, straight, 80–180 µm wide, sometimes slightly flexuous or curved, frequently branched at the base or, more rarely, in the upper parts. Stipe hyphae mostly covered with a layer of hard pigment partly dissolving in KOH, 6–8 µm wide, with walls two layered, the outer wall brown, 2–4 µm wide and cell walls fused, the inner wall pale to hyaline, c. 0.5–1.5 µm wide, with the hyphae intertwined (textura intricata prismatica), swelling in KOH and the yellowish brown pigment leaking into the medium; hyphae in inner part of the stipe hyaline, slightly intertwined, 3–4.6 µm, swelling in KOH. Capitulum black, in young apothecia hemispherical to sometimes almost spherical, sometimes lobed or multi-headed, 200–400 µm wide. Excipulum hyphae brownish to slightly green, 5–7 µm wide, periclinally arranged or slightly intertwined (textura prismatica), swelling in KOH, with some brown pigment leaking into the medium; wall 2–2.5 µm. Epithecium light green to emerald green, appearing as a crustose layer, usually with crystals, composed of hyphae extending from the excipulum; hyphae attached to the hymenium by the amorphous material; containing various amounts of orange to ruby-red pigment in most ascomata, usually occurring as crystals on the outer walls of hyphae, and sometimes also inside their lumina. Hypothecium light green to hyaline, with the hyphae swelling in KOH. Hymenium light brown to greenish to almost hyaline, swelling in KOH, full of amorphous material strongly congealing the asci and paraphyses together. Paraphyses hyaline, filiform, 1.5–2 µm wide (n = 10), branched, as long or slightly longer than the asci, variously covered with amorphous material, septate at 10–15 µm intervals. Asci cylindrical, 55–60 × 6.1 µm (n = 5), with the apex variously thickened, often penetrated by a short canal; mature asci usually without a thickening, variously covered with light green to hyaline, amorphous material, formed with croziers. Ascospores uniseriate, sometimes partly biseriate, obliquely to periclinally oriented in asci, 1-septate, light brown, cylindrical to slightly ellipsoid, sometimes phaseoliform, smooth, or with a very fine ornamentation, (7.7–) 8–13 (–15.4) × (2.8–) 3–3.9 (–4.5) µm (n = 70) [mean 10.3 × 3.4 µm, Q = (2.1–) 2.4–3.8 (–5.0), mean Q = 3.1]; septa as thick as the spore wall, sometimes constricted.
Ecology and distribution.
Chaenothecopsisnovae-zelandiae has been found only at two locations in temperate broad-leaved rainforests of New Zealand on semi-hardened exudate and exudate-soaked bark on the main trunk of Prumnopitystaxifolia, sometimes growing mixed with Chaenothecopsismatai.
Specimens examined.
Specimens PDD110744 (Figs 2, 3A, B, F–H) and PDD 110742 (Figs 1A, B, 3C, D, E) on exudate of Prumnopitystaxifolia. The specimens are deposited in the New Zealand Fungarium (PDD), Landcare Research in Auckland, with a duplicate specimen (PDD 110742/JR13033) in Helsinki (H). The collection data and GenBank accession numbers are given in Suppl. material 1.
. Chaenothecopsis matai
Rikkinen, Beimforde, Tuovila & A.R. Schmidt sp. nov.
90F17D37-7293-5097-BF4F-0D9275607EF9
MB846459
Figure 5.
Light micrographs of Chaenothecopsismatai sp. nov. (PDD 110749) A branched and intertwined stipes, some developing capitula B ascomata with red pruina C young capitulum with ascospores D semi-mature capitulum E ascospores. Scale bars: 500 µm (A); 100 µm (B, C); 10 µm (D); 2 µm (E).
Figure 7.
Anatomical details of Chaenothecopsismatai sp. nov. A ascospores B ascus tips C stipe hyphae D excipulum structure E epithecium structure F asci with corziers G paraphyses H inner stipe hyphae. Scale bars: 10 µm.
Type.
New Zealand, South Island, Croydon Bush, Dolamore Park, Southland, ca. 46°3.6657'S, 168°49.9135'E, on exudate of Prumnopitystaxifolia. 17 February 2017, Beimforde PDD110749, holotype; New Zealand Fungarium (PDD), Landcare Research in Auckland, GenBank accession OQ308932/OQ308875.
Diagnosis.
Chaenothecopsismatai differs from other Chaenothecopsis species by forming extensive mat-like pseudostromata on podocarpous plant exudates with long, often multi-branched, partially translucent stipes, predominantly slender capitula and smooth septate spores that are often constricted at the septum.
Etymology.
The specific epithet refers to the Maori name of Prumnopitystaxifolia, the exudate-producing tree on which the species was first discovered.
Description.
Apothecia growing on the exudate of Prumnopitystaxifolia, arising from a dense mycelium mat which hardens in dry conditions and swells under humid conditions, forming a loose intertwined network with apices either remaining sterile or developing capitula, sometimes growing individually. Stipe glossy, crustose near stipe apices and pruinose parts, black to brownish, often with a hyaline base and/or apex, 90–240 μm wide, usually 2–7 mm long, or sometimes more than 1 cm long, flexuous or curved, multiple-branched, mostly uniformly thickened, tapering towards the apices, often with an orange to red pruina below the capitula. Stipe hyphae 2–8 µm wide, with walls two-layered, the outer wall brown and the cell walls fused, the inner walls hyaline, c. 0.5–1 µm wide, with the hyphae intertwined (textura prismatica-intricata), swelling in KOH; hyphae in the inner part of stipe hyaline to greenish, 2–6 µm wide, swelling in KOH. Capitulum black, 110–220 µm wide, 100–200 high, lentiform to cupulate, sometimes narrower than or as wide as the stipe. Excipulum hyphae brown to emerald green, 4–7 µm wide, intertwined (textura prismatica-intricata), with outer cell walls fused, swelling in KOH and some brown pigment leaking into the medium. Epithecium brownish to emerald green to hyaline, appearing as crusty layer, usually with crystals, composed of the hyphae of the excipulum and paraphyses forming a variously thickened layer. Containing various amounts of orange to ruby-red pigments in most ascomata, usually occurring as crystals on the outer walls of hyphae, and sometimes also inside their lumina. Hypothecium light brown to greenish hyaline, with the hyphae swelling in KOH. Hymenium brownish to emerald to hyaline, with the hyphae swelling in KOH, orange to red pigments present, full of amorphous material strongly congealing asci and paraphyes together. Paraphyses hyaline, filiform, 1.5–2 µm wide (n = 10), branched, usually slightly longer than the asci, variously covered with amorphous material, septate at 9–19 µm intervals. Asci cylindrical, 47–77 µm high, 5–7 µm wide (n = 8), with the apex variously thickened, often penetrated by a poorly developed canal; mature asci usually without a thickening, formed with croziers, tightly embedded in the hymenium, with light brown-green to hyaline amorphous material making individual asci difficult to observe. Ascospores, smooth, uniseriate, periclinally (to slightly obliquely) oriented in asci, 1-septate, brown, cylindrical to slightly ellipsoid, (7.3–) 8–12.5 (–14) × (2.8–) 3–4.5 (–4.7) µm (n = 60), [mean 10.3 × 3.4 µm, Q = (2–) 3–4.3 (–4.5), mean Q = 3.2]; septa as thick as spore wall, sometimes constricted.
Ecology and distribution.
Chaenothecopsismatai has been found at several locations in temperate broad-leaved rain forests of New Zealand on semi-hardened exudate and exudate-soaked wood and bark on the main trunk of Prumnopitystaxifolia, sometimes growing mixed with Chaenothecopsisnovae-zelandiae. Some specimens of a morphologically-similar Chaentohecopsis species have also been collected from exudate of Phyllocladustrichomanoides (Podocarpaceae), but their detailed analysis awaits more material.
Specimens examined.
PDD110746 (Fig. 1D–E), PDD110747, PDD110748, PDD110749 (Figs 5, 6) on exudate of Prumnopitystaxifolia. The specimens are deposited in the New Zealand Fungarium (PDD), Landcare Research, Auckland, with a duplicate of specimen JR13032 in Helsinki (H). The collection data and GenBank accession numbers are given in Suppl. material 1.
. Chaenothecopsis nodosa
Beimforde, Tuovila, Rikkinen & A.R. Schmidt sp. nov.
8018A732-9725-5BC3-87EA-748B8F380C76
MB846460
Figure 8.
Light micrographs of Chaenothecopsisnodosa sp. nov. (PDD 110745) A branched ascoma with catenulate capitulum B development of this ascoma has involved at least 11 separate stages of capitulum proliferation C detail of compound capitulum D ascospores. Scale bars: 100 µm (A, B, D); 10 µm (C).
Type.
New Zealand, North Island, close to Kakaho Camp site, central North Island, ca. 38°34.0224'S, 175°43.0525'E, on exudate of Prumnopitystaxifolia, 5 April 2015, Beimforde PDD 110745, holotype; New Zealand Fungarium (PDD), Landcare Research in Auckland, GenBank accession OQ308934/OQ308877.
Diagnosis.
Chaenothecopsisnodosa differs from other Chaenothecopsis species by producing capitula in a catenulate stack, consecutively on top of each other, typically covered with a white pruina.
Etymology.
The specific epithet refers to the appearance of catenulate groups of sphaeric capitula stacked on top of each other
Description.
Apothecia growing on the exudate of Prumnopitystaxifolia, 1.0–3.1 mm tall, growing individually and proliferating from the capitulum, often several from a single capitulum or from the stipe, eventually forming catenulate stacks of several capitula on top of each other. Stipe dark brown to black, straight to slightly curved, 100–190 μm wide, becoming crustose with age, often with a white pruina at upper stipe regions, and sometimes with an additional red pruina below. Stipe hyphae 3–8 µm wide, with walls two layered, the outer wall dark brown, 1.5–3.5 µm and with cell walls fused in most parts, the inner wall c. 0.5–1 µm, with the hyphae intertwined (textura prismatica-intricata), swelling in KOH; hyphae in inner parts yellowish to light brown, 2–5 µm wide, swelling in KOH. Capitulum black, lenticular to almost spherical or ellipsoid, 150–420 μm wide, 250–220µm high; typically a white pruina is macroscopically visible on the capitula. Excipulum hyphae light brown to hyaline in younger ascomata, brown in older ascomata, 2–6 µm wide, intertwined (textura prismatica-intricata), swelling in KOH; often covered with a crusty layer of amorphous material and crystals. Epithecium light green to moss green, appearing as a crusty layer, variously (up to 20 µm) thickened, usually with crystals, composed of hyphae extending from the excipulum; hyphae attached to the hymenium by the amorphous material. Hymenium light brown to olive green, with the hyphae swelling in KOH, full of amorphous material strongly congealing the asci and paraphyses together. Paraphyses hyaline, filiform, 1.5–2.5 μm wide (n = 20), sometimes branched, as long as or slightly longer than asci, variously covered with amorphous material, septate at 10–25 μm intervals, with the apices intertwined and agglutinated with the hyphae of the epithecium. Asci cylindrical, 60–77 × 4.9–7.7 μm (n = 8), with the apex variously thickened, penetrated by a minute canal visible only in young asci; mature asci usually without a thickening, variously covered with light green to hyaline, amorphous material, formed with croziers; asci in older capitula disintegrated. Ascospores uniseriate, obliquely to periclinally oriented in the asci, 1-septate, brown, cylindrical to slightly ellipsoid, ornamented, (6.7–) 8.5–9.2 (–10.8) × (3.1–) 3.4–3.9 (–4.6) μm (n = 60) [mean 9.5 × 3.8 μm, Q = (2.8–) 3.5–4.6 (–5.4), mean Q = 3.8]; septa as thick as spore wall.
Ecology and distribution.
Chaenothecopsisnodosa has to date been found only in temperate broad-leaved rainforests of New Zealand on semi-hardened exudate and exudate-soaked exposed wood and bark on the main trunk of Prumnopitystaxifolia.
Specimens examined.
Specimens PDD 110743 and PDD 110745 (Figs 8, 9) on exudate of Prumnopitystaxifolia. The specimens are deposited in the New Zealand Fungarium (PDD), Landcare Research, Auckland. The collection data and GenBank accession numbers are given in Suppl. material 1.
Discussion
Taxonomy and systematics
The new species described here represent the first Chaenothecopsis species from exudates of New Zealand gymnosperms. Only Chaenothecopsisschefflerae had previously been found on New Zealand plant exudates, but this species is restricted to angiosperm exudates of endemic Araliaceae (Beimforde et al. 2017).
All three new species occur on the same substrate, i.e., exudate of Prumnopitystaxifolia and each has a distinctive macroscopic appearance. Chaenothecopsisnodosa tends to produce many capitula in a catenulate stack, consecutively on top of each other (Figs 8A, B, D, 9A) and typically produces a white prunia (Fig. 8A, D). In contrast, C.matai and C.novae-zelandiae produce a reddish pruina (Fig. 5B, C). Ascomata of C.novae-zelandiae have comparatively short stipes and tend to grow individually or in smaller groups (Fig. 2A), whereas C.matai usually produces extensive mat-like pseudostromata on its substrate (Figs 5A, 6C).
Chaenothecopsismatai may form very long, multiply-branched and interwoven stipes, often with hyaline parts at the base or apex (Fig. 5B). This species grows in areas of the host trees where exudate accumulates in a humid environment, e.g., in crevices of trunks or branches, or between forking trunks at the base of trees. In such places, C.matai sometimes forms dense mycelial mats which are soaked with the water-soluble Prumnopitys exudate and from which apothecia and sterile stalks arise, forming a pseudostroma-like network. A pseudostroma-like growth habit has also been observed in Chaenothecopsiscaespitosa (W. Phillips) D. Hawksw., described by Hawksworth (1980). However, in contrast to C.matai, apothecia of C.caespitosa grow in tuft-like structures. Nor does C.caespitosa produce the long, abundantly branched stipes observed in C.matai. In addition, the former species has only been collected from rotting polypores on Taxus branches in Great Britain. A pseudostroma-like growth habit is also known from Mycocaliciumsequoia Bonar (Bonar 1971), a mycocalicioid species growing on exudates of Sequoia Endl. and Sequoiadendron J.Buchholz. However, in contrast to C.matai, M.sequioae has a bright yellow pruina on the capitulum surface and tends to produce very compact stroma-like mycelia in which the stalked ascomata are almost completely embedded.
Chaenothecopsisnodosa is morphologically conspicuous and readily distinguishable from C.matai, C.novae-zelandiae and other resinicolous Chaenothecopsis species with proliferating ascomata, such as C.diabolica Rikkinen & Tuovila (Tuovila et al. 2011b), C.dolichocephala Titov (Tibell and Titov 1995), and C.proliferatus Rikkinen, A. R. Schmidt & Tuovila (Tuovila et al. 2013) on the basis of its catenulate, very tightly stacked capitula. Proliferating ascomata are produced by several resinicolous Chaenothecopsis species from different clades, and are also evident from fossil specimens from Paleogene Baltic and Bitterfeld amber (Tuovila et al. 2013; Rikkinen et al. 2018). One can assume that these types of ascomata can effectively rejuvenate if partially overrun by fresh exudate and thus represent a morphological adaptation to life on plant exudates (Tuovila et al. 2013).
In Mycocaliciales, the assignment of species to particular genera, and the delimitation of species is sometimes challenging when using morphological characters only (Schmidt 1970; Tibell 1984, 1987; Titov 2006; Tuovila 2013). For this reason, besides careful examination of microscopical diagnostic characters (for details see Tuovila and Huhtinen 2020), we used additional information from phylogenetically informative gene regions, the internal transcribed spacer region (ITS) and the large ribosomal subunit (LSU), for species identification and taxonomic assignment. Our phylogenetic tree (Fig. 12) accentuates unresolved issues of generic delimitation within Mycocaliciales (e.g. Tibell and Vinuesa 2005; Tuovila 2013) since species assigned to genera such as Mycocalicium Vain., Phaeocalicium A.F.W. Schmidt and Chaenothecopsis appear not to be monophyletic. The recently erected genus Brunneocarpos Giraldo & Crous (Crous et al. 2016) is nested within Chaenothecopsis, with C.diabolica constituting the sister taxon of Brunneocarposbanksiae Giraldo & Crous.
Our phylogenetic analysis (Fig. 12) places all three new Chaenothecopsis species in a monophyletic clade. The three species also share many morphological features. Additional specimens collected from Phyllocladustrichomanoides are most similar to C.matai, differing only by few base pairs in the ITS region. However, due to the very limited sample material from Phyllocladus Rich. exudates, we were currently not able to study possible differences between C.matai specimens collected from Prumnopitys and Phyllocladus exudates in detail.
Chaenothecopsisneocaledonica Rikkinen, A.R.Schmidt & Tuovila is the sister taxon to the New Zealand clade in our phylogenetic tree (Fig. 12). C.neocaledonica grows on resinous plant exudates of Agathisovata (C.Moore ex Vieill.) Warb. (Araucariaceae Henkel & W.Hochst.), an endemic New Caledonian conifer (Rikkinen et al. 2014). This sister taxon relationship is conceivable due to their geographical proximity. Morphologically, all three New Zealand species differ from C.neocaledonica (and from other resinicolous species with one-septate spores) in the presence of peculiar amorphous material covering the asci and paraphyses, sometimes in a very thick layer (Figs 4B, F, H, 7B, G, 10C, H, I). This material also glues the whole hymenium tightly together and makes asci and paraphyses difficult to observe. In addition, the spores of the New Zealand species are on average narrower than those of C.neocaledonica, and at least some in each studied ascoma were phaseoliforme (resembling kidney-beans) or slightly constricted (C.matai and C.novae-zelandiae) at the septum, in contrast to the strictly cylindrical-fusoid spores of C.neocaledonica.
Endemism and spore dispersal
Most previously known Chaenothecopsis species from temperate forest systems of New Zealand are considered to be cosmopolitan and not strictly host specific. According to Tibell (1987), C.debilis, C.nana Tibell, C.nivea (F. Wilson) Tibell, C.pusilla (A. Massal.) A.F.W. Schmidt and C.savonica (Räsänen) Tibell occur on hard lignum and/or bark of various New Zealand gymnosperms or angiosperms. Other species, such as C.haematopus, C.lignicola (Nádv.) A.F.W. Schmidt, C.nigra Tibell and C.nigropedata Tibell, may also be associated with lichens or algae.
Previously only two Chaenothecopsis species, C.brevipes Tibell and C.schefflerae, were thought to be endemic to New Zealand (Tibell 1987). C.brevipes is a lichenicolous species, characterized by its short stalk and strict association with lichens of the genus Arthonia Ach. (Arthoniaceae). However, this species seems to be more widespread than previously assumed. In New Zealand C.brevipes occurs on Arthoniaplatygraphella Nyl. (Tibell 1987) but was later also noted on other Arthonia species e.g., in Russia (Titov and Tibell 1993), North America and Canada (Selva 2010). C.schefflerae is a species which appears to be endemic to New Zealand as it only occurs on exudates of endemic Araliaceae. This species was initially known only from exudates of Scheffleradigitata (Araliaceae) but was later also found on exudates of Pseudopanax (Beimforde et al. 2017). In any case, C.schefflerae is not closely related to the species described here, as it belongs to a well-supported monophyletic group that includes all other known Chaenothecopsis species from angiosperm exudates.
Chaenothecopsisnovae-zelandiae, C.matai and C.nodosa were predominantly found on exudates of Prumnopitystaxifolia. However, as mentioned above, we also found very limited material of a similar Chaenothecopsis species growing on exudates of Phyllocladustrichomanoides. Thus, it is possible that the new species may also occur on exudates of other Phyllocladus species and possibly even on Prumnopitysferruginea, all of which are also endemic to New Zealand. Although a broader host range is thus possible, we expect that the three new Chaenothecopsis species described here all belong to New Zealand’s endemic mycobiota, both due to their specialized substrates and the fact that they group into a distinct monophyletic lineage in our phylogenetic analyses (Fig. 12).
The exudate outpourings of Prumnopitystaxifolia are sometimes densely covered by numerous Chaenothecopsis ascomata providing shelter to diverse arthropods. Some of our collected specimens, particularly those with numerous ascomata were abundantly littered with insect fecal pellets between or at the base of the ascomata. Scanning electron micrographs revealed spores on the outer surfaces of many fecal pellets, and some smaller fecal pellets consist almost entirely of Chaenothecopsis spores (Fig. 11B), suggesting that associated insects feed on the ascomata and defecate undigested ascospores. This notion is substantiated by our findings of fecal pellets with associated early stages of ascomata development (Fig. 11A). We detected a range of insects and insect remnants between the densely arranged ascomata in several samples, for example lepidopteran cocoons, mites, coleopterans such as a rove beetle (Staphylinidae Latreille) and possibly wood boring beetles as well as insect exuviae, pupae and larvae. These findings, together with the spores and initial ascomata development in the fecal pellets, indicate that the densely growing ascomata provide shelter and food source for diverse insects and that ascospores of the fungi are ingested, but probably not digested by insects. It is thus likely that insects are involved in the spore dispersal of the species described herein, as spores may be consumed by the insects and spread with their excrements or get attached to the insects’ surface when they crawl over the apothecia. It might well be that the spore-dispersing insects are also associated with the host trees and thus guarantee that the spores reach the substrates that are essential for the fungal species to survive.
Ecology on plant exudates and evolution
Some fungi have developed defenses against the toxic components of plant exudates (e.g. Rautio et al. 2012; Adams et al. 2013) but it is uncertain whether this unusual, inherently toxic substrate is preferred to evade competition or whether exudates provide a nutrient source for the fungi. The dependence of some mycocalicioid fungi and other resinicolous ascomycetes on conifer resins and other plant exudates, and the fact that their hyphae grow randomly into this substrate (Beimforde et al. 2020) suggests a nutrient uptake from the exudates. Theoretically, resin and other plant exudates represent oxidizable organic matter, but it has not yet been proven empirically whether fungi are able to metabolize compounds of plant exudates.
Our culture experiments demonstrate that all three species described here grow in vitro on a carbohydrate-based medium (PDA). Still, we cannot exclude that phenolic and/or terpenoid substances of the Prumnopitys exudate may also be degraded by the species. The composition of plant exudate differs greatly between individual plant lineages. The exudates of angiosperms that serve as hosts for some Chaenothecopsis species (Khaya and Rhus (Anacardiaceae), Ailanthus (Simaroubaceae), Kalopanax, Pseudopanax and Schefflera (Araliaceae)) consist of complex hydrophilic, non-polymerized polysaccharides (Langenheim 2003), representing a conceivable nutrient source. In contrast, conifer host trees produce resinous exudates that consist of a mixture of hydrophobic, phenolic and terpenoid components that are toxic for most microorganisms (Bednarek and Osbourn 2009; Sipponen and Laitinen 2011; Rautio et al. 2012) because they damage cell wall structures (Rautio et al. 2011). Nevertheless, terpenoid/phenolic conifer exudates may contain hybrid subgroups such as guaiac gums, guaiac resins, and kino resins (Lambert et al. 2021), which might be degradable by fungi. The composition of Prumnopitys exudate has not yet been studied in detail, but it appears to differ from other conifer exudates (Lambert et al. 2007). According to our observations, the exudate of Prumnopitystaxifolia differs from resins or exudates of most other conifer hosts in being water-soluble, in its dark tint and the strong phenolic fragrance of fresh outpourings. This means that, as recently shown for some Araucaria species (Seyfullah et al. 2022), distinct types of exudate (gum, resin, and gum resin) may co-occur in Prumnopitys.
Our phylogenetic analysis indicates that the three species from Podocarpaceae exudate descend from a common ancestor. Likewise, all known Chaenothecopsis species from various angiosperm exudates also originate from a common ancestor. In contrast, resinicolous species from terpenoid conifer resins have multiple origins and occur in several lineages within the Mycocaliciales, suggesting a longer and more complex evolutionary history. The age of the resinicolous ecology within Mycocaliciales remains uncertain since relationships between individual monophyletic clades have not yet been fully resolved. In any case, resinicolous Chaenothecopsis species from various ambers prove that this ecological mode on conifer resin has existed within the genus for at least 35 million years (Rikkinen and Poinar 2000; Tuovila et al. 2013; Rikkinen et al. 2018; Rikkinen and Schmidt 2018). Recent estimates of divergence times of the Ascomycota place the separation of Mycocaliales and Eurotiomycetes in the Carboniferous (Prieto and Wedin 2013; Beimforde et al. 2014) and the origin of the Mycocaliciales crown group in the late Jurassic, when diverse conifer lineages were present (Lubna et al. 2021). It is possible that Mycocaliciales could have colonized conifers at an early stage of conifer evolution in the Permian, and it might well be that the resinicolous ecology evolved at a very early stage within Mycocaliciales. The oldest New Zealand pollen and macrofossil records of Prumnopitys and Phyllocladus are from Paleocene and Eocene deposits (Lee et al. 2016) and thus fungi on their exudates could have existed since then. Based on the isolated phylogenetic position of this clade from Podocarpaceae exudates, it could well be that this lineage diverged from other Chaenothecopsis clades in the Paleocene or even earlier.
Supplementary Material
Acknowledgements
We thank Daphne Lee (Dunedin) for linguistic assistance, providing help with field work and information about palaeobotanical evidence in New Zealand, Adrienne Stanton (Landcare Research, Auckland) for providing voucher numbers and curating our specimens in the New Zealand Fungarium PDD – Plant Disease Division, Liz Girvan (Dunedin) and Dorothea Hause-Reitner (Göttingen) for assisting in scanning electron microscopy. We also thank the anonymous reviewer for his detailed review of the manuscript. This study was supported by funds provided by the German Research Foundation (project 429296833) as well as by the Academy of Finland (project 343113).
Citation
Beimforde C, Schmidt AR, Tuovila H, Kaulfuss U, Germer J, Lee WG, Rikkinen J (2023) Chaenothecopsis (Mycocaliciales, Ascomycota) from exudates of endemic New Zealand Podocarpaceae. MycoKeys 95: 101–129. https://doi.org/10.3897/mycokeys.95.97601
Supplementary materials
Sampled specimens’ information for the three new Chaenothecopsis species from Podocarpaceae of New Zealand
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Christina Beimforde, Alexander R. Schmidt, Hanna Tuovila, Uwe Kaulfuss, Juliane Germer, William G. Lee, Jouko Rikkinen
Data type
table (word document)
Explanation note
Species name, collection/voucher number, collection date/sites, fungal hosts and locations.
References
- Adams AS, Aylward FO, Adams SM, Erbilgin N, Aukema BH, Currie CR, Suen G, Raffa KF. (2013) Mountain pine beetles colonizing historical and naive host trees are associated with a bacterial community highly enriched in genes contributing to terpene metabolism. Applied and Environmental Microbiology 79(11): 3468–3475. 10.1128/AEM.00068-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek P, Osbourn A. (2009) Plant-microbe interactions: Chemical diversity in plant defense. Science 324(5928): 746–748. 10.1126/science.1171661 [DOI] [PubMed] [Google Scholar]
- Beimforde C, Feldberg K, Nylinder S, Rikkinen J, Tuovila H, Dörfelt H, Gube M, Jackson DJ, Reitner J, Seyfullah LJ, Schmidt AR. (2014) Estimating the Phanerozoic history of the Ascomycota lineages: Combining fossil and molecular data. Molecular Phylogenetics and Evolution 78: 386–398. 10.1016/j.ympev.2014.04.024 [DOI] [PubMed] [Google Scholar]
- Beimforde C, Tuovila H, Schmidt AR, Lee WG, Gube M, Rikkinen J. (2017) Chaenothecopsisschefflerae (Ascomycota: Mycocaliciales): a widespread fungus on semi-hardened exudates of endemic New Zealand Araliaceae. New Zealand Journal of Botany 55(4): 387–406. 10.1080/0028825X.2017.1360368 [DOI] [Google Scholar]
- Beimforde C, Schmidt AR, Rikkinen J, Mitchell JK. (2020) Sareomycetes cl. nov.: A new proposal for placement of the resinicolous genus Sarea (Ascomycota, Pezizomycotina). Fungal Systematics and Evolution 6(1): 25–37. 10.3114/fuse.2020.06.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonar L. (1971) A new Mycocalicium on scarred Sequoia in California. Madroño 21: 62–69. [Google Scholar]
- Crous PW, Wingfield MJ, Richardson DM, Leroux JJ, Strasberg D, Edwards J, Roets F, Hubka V, Taylor PWJ, Heykoop M, Martín MP, Moreno G, Sutton DA, Wiederhold NP, Barnes CW, Carlavilla JR, Gené J, Giraldo A, Guarnaccia V, Guarro J, Hernández-Restrepo M, Kolaik M, Manjón JL, Pascoe IG, Popov ES, Sandoval-Denis M, Woudenberg JHC, Acharya K, Alexandrova AV, Alvarado P, Barbosa RN, Baseia IG, Blanchette RA, Boekhout T, Burgess TI, Cano-Lira JF, Moková A, Dimitrov RA, Dyakov MY, Dueñas M, Dutta AK, Esteve-Raventós F, Fedosova AG, Fournier J, Gamboa P, Gouliamova DE, Grebenc T, Groenewald M, Hanse B, Hardy GESJ, Held BW, Jurjevi Kaewgrajang T, Latha KPD, Lombard L, Luangsa-ard JJ, Lysková P, Mallátová N, Manimohan P, Miller AN, Mirabolfathy M, Morozova OV, Obodai M, Oliveira NT, Ordóñez ME, Otto EC, Paloi S, Peterson SW, Phosri C, Roux J, Salazar WA, Sánchez A, Sarria GA, Shin HD, Silva BDB, Silva GA, Smith MT, Souza-Motta CM, Stchigel AM, Stoilova-Disheva MM, Sulzbacher MA, Telleria MT, Toapanta C, Traba JM, Valenzuela-Lopez N, Watling R, Groenewald JZ. (2016) Fungal planet description sheets: 400–468. Persoonia 36(1): 450–451. 10.3767/003158516X692185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. (2012) jModelTest 2: More models, new heuristics and parallel computing. Nature Methods 9(8): e772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed]
- del Prado R, Schmitt I, Kautz S, Palice Z, Lücking R, Lumbsch HT. (2006) Molecular data place Trypetheliaceae in Dothideomycetes. Mycological Research 110(5): 511–520. 10.1016/j.mycres.2005.08.013 [DOI] [PubMed] [Google Scholar]
- Gardes M, Bruns TD. (1993) ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2(2): 113–118. 10.1111/j.1365-294X.1993.tb00005.x [DOI] [PubMed] [Google Scholar]
- Geiser DM, Gueidan C, Miadlikowska J, Lutzoni F, Kauff F, Hofstetter V, Fraker E, Schoch CL, Tibell L, Untereiner WA, Aptroot A. (2006) Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia 98(6): 1053–1064. 10.1080/15572536.2006.11832633 [DOI] [PubMed] [Google Scholar]
- Gueidan C, Aptroot A, da Silva Cáceres ME, Binh NQ. (2016) Molecular phylogeny of the tropical lichen family Pyrenulaceae: Contribution from dried herbarium specimens and FTA card samples. Mycological Progress 15(1): 1–7. 10.1007/s11557-015-1154-8 [DOI] [Google Scholar]
- Hall T. (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hawksworth DL. (1980) Two little-known members of the Mycocaliciaceae on polypores (Chaenothecopsiscaespitosa, Phaeocaliciumpolyporaeum, Calicium, Mycocalicium). Transactions of the British Mycological Society 74: 650–651. 10.1016/S0007-1536(80)80073-8 [DOI] [Google Scholar]
- James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, Cox CJ, Celio G, Gueidan C, Fraker E, Miadlikowska J, Lumbsch HT, Rauhut A, Reeb V, Arnold AE, Amtoft A, Stajich JE, Hosaka K, Sung GH, Johnson D, O’Rourke B, Crockett M, Binder M, Curtis JM, Slot JC, Wang Z, Wilson AW, Schüssler A, Longcore JE, O’Donnell K, Mozley-Standridge S, Porter D, Letcher PM, Powell MJ, Taylor JW, White MM, Griffith GW, Davies DR, Humber RA, Morton JB, Sugiyama J, Rossman AY, Rogers JD, Pfister DH, Hewitt D, Hansen K, Hambleton S, Shoemaker RA, Kohlmeyer J, Volkmann-Kohlmeyer B, Spotts RA, Serdani M, Crous PW, Hughes KW, Matsuura K, Langer E, Langer G, Untereiner WA, Lücking R, Büdel B, Geiser DM, Aptroot A, Diederich P, Schmitt I, Schultz M, Yahr R, Hibbett DS, Lutzoni F, McLaughlin DJ, Spatafora JW, Vilgalys R. (2006) Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443(7113): 818–822. 10.1038/nature05110 [DOI] [PubMed] [Google Scholar]
- Katoh K, Toh H. (2008) Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics 9(4): 286–298. 10.1093/bib/bbn013 [DOI] [PubMed] [Google Scholar]
- Lambert JB, Kozminski MA, Santiago-Blay JA. (2007) Distinctions among conifer exudates by proton magnetic resonance spectroscopy. Journal of Natural Products 70(8): 1283–1294. 10.1021/np0701982 [DOI] [PubMed] [Google Scholar]
- Lambert JB, Santiago-Blay JA, Wu Y, Contreras TA, Johnson CL, Bisulca CM. (2021) Characterization of phenolic plant exudates by nuclear magnetic resonance spectroscopy. Journal of Natural Products 84(9): 2511–2524. 10.1021/acs.jnatprod.1c00522 [DOI] [PubMed] [Google Scholar]
- Langenheim JH. (2003) Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany. Timber Press, Portland, Cambridge.
- Lee DE, Lee WG, Jordan GJ, Barreda VD. (2016) The Cenozoic history of New Zealand temperate rainforests: Comparisons with southern Australia and South America. New Zealand Journal of Botany 54(2): 100–127. 10.1080/0028825X.2016.1144623 [DOI] [Google Scholar]
- Lubna L, Asaf S, Khan AL, Jan R, Khan A, Khan A, Kim K-M, Lee I-J. (2021) The dynamic history of gymnosperm plastomes: Insights from structural characterization, comparative analysis, phylogenomics, and time divergence. The Plant Genome 14(3): e20130. 10.1002/tpg2.20130 [DOI] [PubMed]
- Messuti MI, Vidal-Russell R, Amico GC, Lorenzo LE. (2012) Chaenothecopsisquintralis, a new species of calicioid fungus. Mycologia 104(5): 1222–1228. 10.3852/12-006 [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. 2010 gateway computing environments workshop (GCE), New Orleans, 8 pp. 10.1109/GCE.2010.5676129 [DOI] [Google Scholar]
- Pratibha J, Amandeep K, Shenoy BD, Bhat DJ. (2010) Caliciopsisindica sp. nov. from India. Mycosphere. Journal of Fungal Biology 1: 65–72. [Google Scholar]
- Prieto M, Wedin M. (2013) Dating the diversification of the major lineages of ascomycota (Fungi). PLoS ONE 8(6): e65576. 10.1371/journal.pone.0065576 [DOI] [PMC free article] [PubMed]
- Pykälä J, Launis A, Myllys L. (2019) Taxonomy of the Verrucariakalenskyi – V.xyloxena species complex in Finland. Nova Hedwigia 109(3–4): 489–511. 10.1127/nova_hedwigia/2019/0553 [DOI] [Google Scholar]
- Rambaut A, Drummond AJ. (2009) Tracer. MCMC Trace analysis tool version v1.7.2. https://github.com/beast-dev/tracer/releases/tag/v1.7.2
- Rautio M, Sipponen A, Lohi J, Lounatmaa K, Koukila-Kähkölä P, Laitinen K. (2012) In vitro fungistatic effects of natural coniferous resin from Norway spruce (Piceaabies). European Journal of Clinical Microbiology & Infectious Diseases 31(8): 1783–1789. 10.1007/s10096-011-1502-9 [DOI] [PubMed] [Google Scholar]
- Réblová M, Untereiner WA, Stepanek V, Gams W. (2017) Disentangling PhialophorasectionCatenulatae: Disposition of taxa with pigmented conidiophores and recognition of a new subclass, Sclerococcomycetidae (Eurotiomycetes). Mycological Progress 16(1): 27–46. 10.1007/s11557-016-1248-y [DOI] [Google Scholar]
- Rehner SA, Samuels GJ. (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98(6): 625–634. 10.1016/S0953-7562(09)80409-7 [DOI] [Google Scholar]
- Rikkinen J. (1999) Two new species of resinicolous Chaenothecopsis (Mycocaliciaceae) from Western North America. The Bryologist 102(3): e366. 10.2307/3244223 [DOI] [PubMed]
- Rikkinen J. (2003) Chaenothecopsisnigripunctata, a remarkable new species of resinicolous Mycocaliciaceae from western North America. Mycologia 95(1): 98–103. 10.1080/15572536.2004.11833136 [DOI] [PubMed] [Google Scholar]
- Rikkinen J, Poinar G. (2000) A new species of resinicolous Chaenothecopsis (Mycocaliciaceae, Ascomycota) from 20 million year old Bitterfeld amber, with remarks on the biology of resinicolous fungi. Mycological Research 104(1): 7–15. 10.1017/S0953756299001884 [DOI] [Google Scholar]
- Rikkinen J, Schmidt AR. (2018) Morphological convergence in forest microfungi provides a proxy for Paleogene forest structure. In: Krings M, Harper CJ, Cúneo NR, Rothwell GW. (Eds) Transformative Paleobotany.Academic Press, London, 527–549. 10.1016/B978-0-12-813012-4.00022-X [DOI]
- Rikkinen J, Tuovila H, Beimforde B, Seyfullah L, Perrichot V, Schmidt AR. (2014) Chaenothecopsisneocaledonica sp. nov.: The first resinicolous mycocalicioid fungus from an araucarian conifer. Phytotaxa 173(1): 49–60. 10.11646/phytotaxa.173.1.4 [DOI] [Google Scholar]
- Rikkinen J, Meinke K, Grabenhorst H, Gröhn C, Kobbert M, Wunderlich J, Schmidt AR. (2018) Calicioid lichens and fungi in amber: Tracing extant lineages back to the Paleogene. Geobios 51(5): 469–479. 10.1016/j.geobios.2018.08.009 [DOI] [Google Scholar]
- Ronquist F, Huelsenbeck JP. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12): 1572–1574. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Samuels GJ, Buchanan DE. (1983) Ascomycetes of New Zealand 5. Mycocaliciumschefflerae sp. nov., its ascal ultrastructure and Phialophora anamorph GARY J. SAMUELS. New Zealand Journal of Botany 21(2): 163–169. 10.1080/0028825X.1983.10428540 [DOI] [Google Scholar]
- Schmidt A. (1970) Anatomisch-taxonoische Untersuchungen an europäischen Arten der Flechtenfamilie Caliciaceae. Mitteilungen aus dem Staatsinstitut für Allgemeine Botanik Hamburg 13: 111–166. [Google Scholar]
- Schwarz G. (1978) Estimating the dimension of a model. Annals of Statistics 6(2): 461–464. 10.1214/aos/1176344136 [DOI] [Google Scholar]
- Selva SB. (2010) New and interesting calicioid lichens and fungi from eastern North America. The Bryologist 113(2): 272–276. 10.1639/0007-2745-113.2.272 [DOI] [Google Scholar]
- Seyfullah LJ, Roberts EA, Jardine PE, Rikkinen J, Schmidt AR. (2022) Uncovering the natural variability of araucariacean exudates from ex situ and in situ tree populations in New Caledonia using FTIR spectroscopy. PeerJ Analytical Chemistry 4: e17. 10.7717/peerj-achem.17 [DOI]
- Sipponen A, Laitinen K. (2011) Antimicrobial properties of natural coniferous rosin in the European Pharmacopoeia challenge test. APMIS: Journal of Pathology, Microbiology and Immunology 119: 720–724. 10.1111/j.1600-0463.2011.02791.x [DOI] [PubMed] [Google Scholar]
- Tibell L. (1984) A reappraisal of the taxonomy of Caliciales. Beiheft zur Nova Hedwigia 79: 597–713. [Google Scholar]
- Tibell L. (1987) Australasian Caliciales. Symbolae Botanicae Upsalienses 27: 1–276. [Google Scholar]
- Tibell L, Titov A. (1995) Species of Chaenothecopsis and Mycocalicium (Caliciales) on exudate. The Bryologist 98(4): e550. 10.2307/3243587 [DOI]
- Tibell L, Vinuesa M. (2005) Chaenothecopsis in a molecular phylogeny based on nuclear rDNA ITS and LSU sequences. Taxon 54(2): 427–442. 10.2307/25065370 [DOI] [Google Scholar]
- Tibell L, Wedin M. (2000) Mycocaliciales, a new order for nonlichenized calicioid fungi. Mycologia 92(3): 577–581. 10.1080/00275514.2000.12061195 [DOI] [Google Scholar]
- Titov A. (2006) Mikokalizievye griby (porjadok Mycocaliciales) Golarktiki [Mycocalicioid fungi (the order Mycocaliciales) of the Holarctic]. KMK Scientific Press, Moskva.
- Titov A, Tibell L. (1993) Chaenothecopsis in the Russian Far East. Nordic Journal of Botany 13(3): 313–329. 10.1111/j.1756-1051.1993.tb00055.x [DOI] [Google Scholar]
- Tuovila H. (2013) Sticky business: Diversity and evolution of Mycocaliciales (Ascomycota) on plant exudates. Publications from the Department of Botany. University of Helsinki 44: 1–142. [Google Scholar]
- Tuovila H, Huhtinen S. (2020) New methods for mycocalicioid fungi. Lichenologist 52(6): 403–413. 10.1017/S0024282920000481 [DOI] [Google Scholar]
- Tuovila H, Cobbinah JR, Rikkinen J. (2011a) Chaenothecopsiskhayensis, a new resinicolous calicioid fungus on African mahogany. Mycologia 103(3): 610–615. 10.3852/10-194 [DOI] [PubMed] [Google Scholar]
- Tuovila H, Larsson P, Rikkinen J. (2011b) Three resinicolous North American species of Mycocaliciales in Europe with a re-evaluation of Chaenothecopsisoregana Rikkinen. Karstenia 51(2): 37–49. 10.29203/ka.2011.447 [DOI] [Google Scholar]
- Tuovila H, Schmidt AR, Beimforde C, Dörfelt H, Grabenhorst H, Rikkinen J. (2013) Stuck in time – a new Chaenothecopsis species with proliferating ascomata from Cunninghamiaresin and its fossil ancestors in European amber. Fungal Diversity 58(1): 199–213. 10.1007/s13225-012-0210-9 [DOI] [Google Scholar]
- Tuovila H, Davey ML, Yan L, Huhtinen S, Rikkinen J. (2014) New resinicolous Chaenothecopsis species from China. Mycologia 106(5): 989–1003. 10.3852/13-178 [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172(8): 4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakoon G, Wolseley PA, Arachchige O, Eugenia da Silva Cáceres M, Jayalal U, Aptroot A. (2016) Eight new lichen species and 88 new records from Sri Lanka. The Bryologist 119(2): 131–142. 10.1639/0007-2745-119.2.131 [DOI] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR Protocols: a Guide to Methods and Applications.Academic Press, New York, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sampled specimens’ information for the three new Chaenothecopsis species from Podocarpaceae of New Zealand
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Christina Beimforde, Alexander R. Schmidt, Hanna Tuovila, Uwe Kaulfuss, Juliane Germer, William G. Lee, Jouko Rikkinen
Data type
table (word document)
Explanation note
Species name, collection/voucher number, collection date/sites, fungal hosts and locations.