SUMMARY
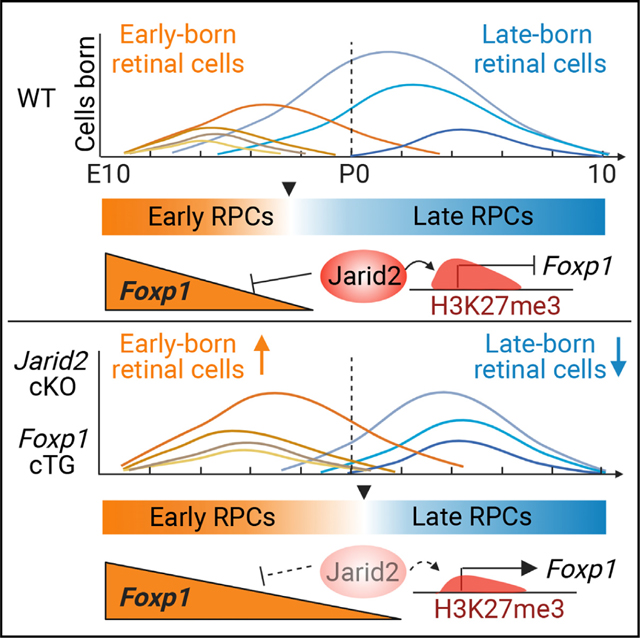
Transitions in competence underlie the ability of CNS progenitors to generate a diversity of neurons and glia. Retinal progenitor cells in mouse generate early-born cell types embryonically and late-born cell types largely postnatally. We find that the transition from early to late progenitor competence is regulated by Jarid2. Loss of Jarid2 results in extended production of early cell types and extended expression of early progenitor genes. Jarid2 can regulate histone modifications, and we find reduction of repressive mark H3K27me3 on a subset of early progenitor genes with loss of Jarid2, most notably Foxp1. We show that Foxp1 regulates the competence to generate early-born retinal cell types, promotes early and represses late progenitor gene expression, and is required for extending early retinal cell production after loss of Jarid2. We conclude that Jarid2 facilitates progression of retinal progenitor temporal identity by repressing Foxp1, which is a primary regulator of early temporal patterning.
In brief
Temporal patterning of neural progenitors is essential to generate neuronal diversity. Zhang et al. find that Jarid2 facilitates retinal progenitor temporal identity transition via H3K27me3 deposition and repression of early genes, most notably Foxp1. They find that Foxp1 is an effector of Jarid2 in regulating timing of retinal neurogenesis.
Graphical Abstract
INTRODUCTION
Temporal patterning of progenitors is critical for generating a diversity of cell types during development of the nervous system, driving the differentiation of neurons and glia in specific proportions in a defined sequence.1,2 Changes in neural progenitor competence govern the ability of progenitors to differentiate into diverse neuronal and glial cell types.3,4 A major challenge is determining how regulation of temporal competence in the vertebrate central nervous system (CNS) is coordinated by transcriptional programs and changes in chromatin accessibility.1,2,5–7
This problem is well defined in the developing retina, since lineage analysis has shown that multipotent retinal progenitor cells (RPCs) progress through temporally regulated competence states during which they generate distinct cell types in a defined sequence that is conserved across species.5,8 In the mouse, early-born cell types (retinal ganglion cells [RGCs], cone photoreceptors, horizontal cells, some amacrine cells) are generated embryonically, while late-born cell types (most rod photoreceptors, remaining amacrine cells, bipolar cells, Müller glia) are generated postnatally, with precise regulation of cell fate specification.7 Cell-intrinsic processes primarily regulate the timing of cell genesis, with late progenitors restricted from generating earlier cell types.8–10 Recent single-cell analysis of mouse and human retinal development has revealed a distinct shift in gene expression and chromatin accessibility from early- to late-stage RPCs, consistent with a change in competence to generate early- versus late-born retinal cell types.11–13 Multiple temporal identity factors and regulators have been shown to regulate RPC competence and timing of retinal cell production, including those with similarity to temporal identity factors first identified in Drosophila such as Ikaros family (Ikzf1 and Ikzf4) and Casz1, as well as other transcription factors such as FoxN4, Pou2f1/Pou2f2, Lhx2, and NFI factors, along with microRNA regulation through Dicer.11,13–25 However, whether additional factors contribute to, and the molecular mechanisms regulating, progressive changes in RPC competence are still unclear.
Histone modifications contribute to changes in the ability of CNS progenitors to generate diverse cell types,3,6,17,26,27 and also play a role in retinal development.28 A key repressive histone modification, methylation of H3 at lysine 27 (H3K27me3) is dynamically regulated on progenitor and differentiation genes during mouse and human retinal development.29 H3K27 methylation is catalyzed by polycomb repressive complex 2 (PRC2), which is composed of obligate core components and an array of accessory proteins.30 PRC2 plays important developmental roles in multiple embryonic lineages including the developing nervous system.27,31,32 In retinal progenitors, loss of core components, Ezh2 or Eed, results in widespread derepression of genes not normally expressed in retinal lineages, accelerated differentiation of late-born cell types, as well as premature cell-cycle exit resulting in progenitor depletion.33–35 These findings suggest that PRC2-mediated gene repression maintains retinal lineage integrity and regulates progenitor differentiation.
Jarid2 (Jumonji, AT-rich interactive domain 2) is best characterized as a component of PRC2.2, one of two alternate subcomplexes that methylate H3K27,36–40 and Jarid2 can also associate with complexes that methylate H3K9.41,42 Jarid2 is required for embryonic development, including brain development, as well as transition between pluripotent and differentiated states of embryonic stem cells (ESCs), although its in vivo function, particularly in CNS development, is not fully defined.39,43–46 In humans, the JARID2 gene is associated with autism spectrum disorder, schizophrenia, and neurodevelopmental delay.47–51 Since Jarid2 has been implicated in CNS development, and plays a role in regulating histone modification and gene repression, we sought to further investigate its function.
Here, we show that Jarid2 is important for temporal patterning of progenitors, facilitating the progression from early to late RPCs. Conditional loss of Jarid2 resulted in a delayed transition from early to late RPC gene expression, and extended production of early-born retinal cell types. We also observed selective loss of H3K27me3 and derepression for a subset of genes expressed in early RPCs, most notably the transcription factor Foxp1. Through gain- and loss-of-function analysis, we show that Foxp1 promotes the production of early retinal cell types, and functions as a key effector in extending early retinal cell production after loss of Jarid2. We conclude that Jarid2 promotes repression of Foxp1 to facilitate the progression from early to late RPC competence.
RESULTS
Loss of Jarid2 affects retinal neurogenesis
We first sought to examine the expression of Jarid2, so we performed in situ hybridization on sections of E14.5 and P0 mouse retina and found that Jarid2 is expressed in retinal progenitors in the neuroblastic layer and differentiating cells in the ganglion cell layer (GCL) (Figures S1A–S1D). To address its function, we conditionally inactivated Jarid2 in retinal progenitors prior to onset of neurogenesis using either Six3-Cre (Six3-Cre Jarid2 cKO)52 or Rax-CreERT2, a tamoxifen-inducible Cre (Rax-Cre Jarid2 cKO)53 (Figure S1E). Tamoxifen interferes with birth, so Rax-Cre Jarid2 cKO pups were collected embryonically, while Six3-Cre Jarid2 cKO was used for postnatal analysis. We confirmed reduction of full-length Jarid2 mRNA from Six3-Cre Jarid2 cKO retina at P0 by RT-PCR (Figure S1F), and by sequencing the knockout product confirmed loss of exon 3, which results in a frameshift and premature stop. We also confirmed reduced protein expression by western blot (Figure S1G).
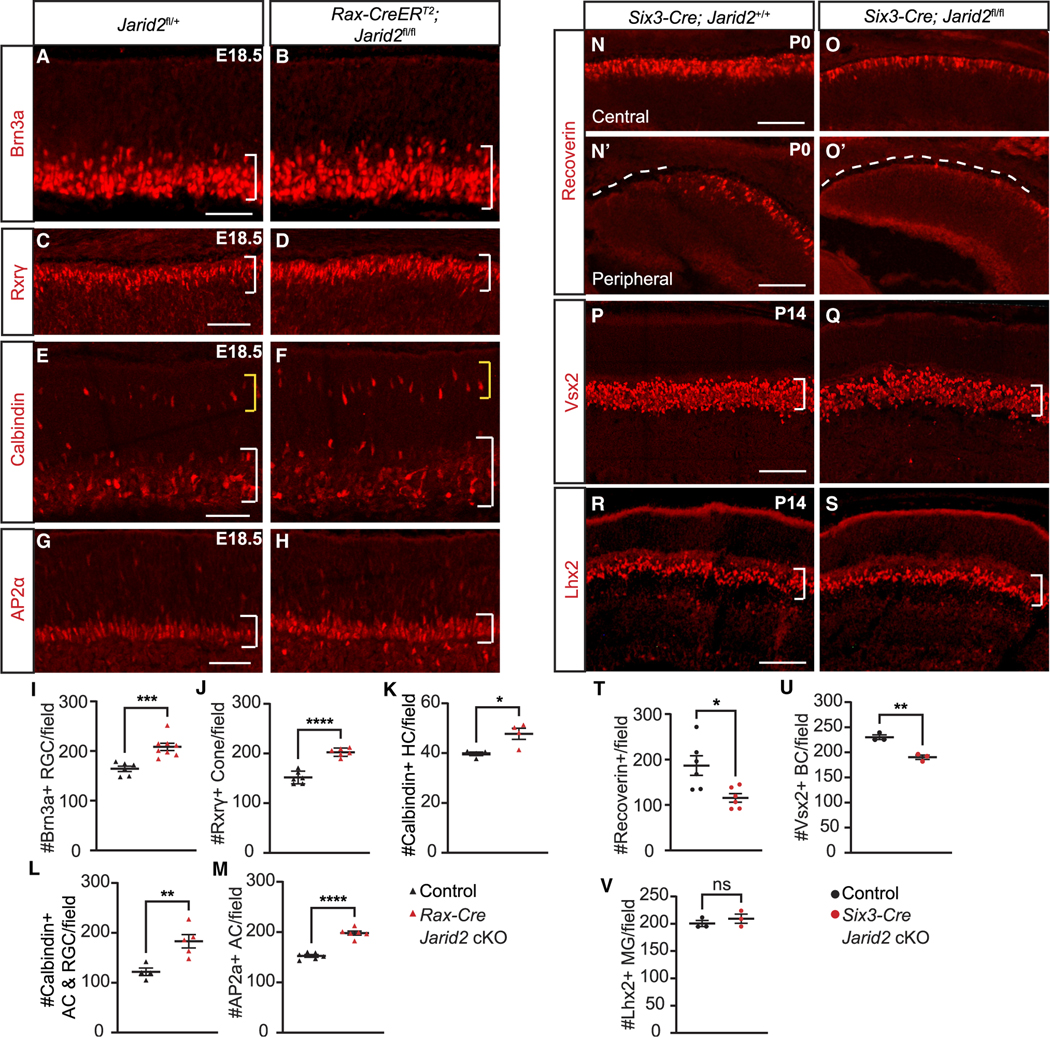
During mouse retina development, RGCs, horizontal cells, cone photoreceptors, and some amacrine cells are born during the embryonic period, so we asked whether the generation of these early-born cell populations is impacted by loss of Jarid2. We generated Rax-Cre Jarid2 cKO by administering tamoxifen at onset of retinal neurogenesis at E10.5,53 and collected embryos at E18.5 after early cell genesis is largely complete. In Rax-Cre Jarid2 cKO retina we observed increased Brn3a+ RGCs in the GCL (Figures 1A, 1B, and 1I). There was also an increase in the number of Rxrγ+ cone photoreceptors (Figures 1C, 1D, and 1J) and increased Calbindin+ horizontal cells in outer retina (Figures 1E, 1F, and 1K). Calbindin also labels a subset of amacrine cells and RGCs, and we found increased Calbindin+ cells in the inner retina (Figures 1E, 1F, and 1L). Consistent with this, there were increased AP2α+ amacrine cells (Figures 1G, 1H, and 1M). We confirmed increased Brn3+ RGCs in Six3-Cre Jarid2 cKO at P0 (Figures S2A, S2B, and S2G). In Six3-Cre Jarid2 cKO there were also increased Calbindin+ amacrine cells (Figures S2C, S2D, and S2H) and increased Rxrγ+ cone photoreceptors (Figures S2E, S2F, and S2I). We found no difference in pH3-labeled mitotic cells at E18.5 in Rax-Cre Jarid2 cKO retina (p = 0.18, Table S6), suggesting that proliferation did not contribute to the shift in early cell number. We did observe an increase in cleaved caspase-3-labeled apoptotic cells at P0 in Six3-Cre Jarid2 cKO retina (p < 0.05, Table S6), but still found that the increased early-born cells persisted, with increased Brn3a+ RGCs, AP2α+ amacrine cells, and Rxrγ+ cone photoreceptors evident in the Six3-Cre Jarid2 cKO retina at P14 (Figures S2S–S2AA). Thus, loss of Jarid2 results in an overall increase in early-born retinal cell populations.
Figure 1. Loss of Jarid2 affects retinal neurogenesis.
Rax-Cre Jarid2 cKO retina at E18.5 shows (A, B, and I) increased Brn3a-labeled RGCs within the GCL (bracket), (C, D, and J) increased Rxrγ-labeled cone photoreceptors (bracket), (E and F) increased calbindin-labeled horizontal cells (yellow bracket, K) and amacrine cells (white bracket, L), and (G, H, and M) increased AP2α-labeled amacrine cells (bracket). At P0, Six3-Cre Jarid2 cKO shows (N, O, and T) reduced Recoverin-labeled photoreceptors in the central retina (N′ and O′) and delayed differentiation in the peripheral retina (dotted line). At P14 (P, Q, and U), Vsx2-labeled bipolar cells were reduced, (R, S and V) but Lhx2-labeled Müller glia were unchanged. Graphs represent mean ± SEM. n = 6 retinas in (J, M, and T) and control in (I), n = 8 for Jarid2 cKO in (I), n = 4 in (K), and control in (L), n = 5 for Jarid2 cKO in (L), and n = 3 in (U and V). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by Student’s t test. Scale bars, 100 μm. See also Figure S2 and Table S6 for statistical details.
One possible explanation for this phenotype is increased production of these cells during their normal developmental window. To address this, we performed analysis of Six3-Cre Jarid2 cKO at E16.5 when early cell genesis is still ongoing. At that time point, we detected no change in the number of Brn3+ RGCs (Figures S2J, S2K, and S2P), Otx2+ photoreceptor precursors (Figures S2L, S2M, and S2Q), or Rxrγ+ cone photoreceptors (Figures S2N, S2O, and S2R). Thus, we conclude that there is not an increased production of these cell populations during the initial period of early cell genesis.
We hypothesized that the increased early-born retinal cells may potentially affect the generation of rod photoreceptors, bipolar cells, and Müller glia, which are largely born after birth. We found a reduced number of photoreceptors labeled for Recoverin in the central retina at P0 in Six3-Cre Jarid2 cKO (Figures 1N, 1O, and 1T). Retinal neuron differentiation follows a central to peripheral gradient, and at the periphery we found that the leading edge of Recoverin labeling was more central in the cKO, suggesting delayed photoreceptor differentiation (Figures 1N′ and 1O′). At P14 the number of Vsx2+ bipolar cells was reduced in Six3-Cre Jarid2 cKO (Figures 1P, 1Q, and 1U), but there was no apparent change in Lhx2+ Müller glia (Figures 1R, 1S, and 1V). Together, we conclude that, with loss of Jarid2, there is enhanced production of early-born retinal neurons during the late embryonic period, and consequently reduced production of later born retinal neurons, suggesting a delayed shift from early to late retinal cell generation.
Loss of Jarid2 results in extended production of early-born retinal neurons
To directly assess whether there is increased production of early retinal cell types during the late embryonic period, we labeled mitotic cells by administering EdU at E16.5 and E17.5, followed by EdU detection and immunostaining for differentiated cell markers at E18.5 (Figure S3A). Compared with controls, Rax-Cre Jarid2 KO retinas showed a significant increase in EdU+Brn3a+ RGCs (Figures S3B, S3C, and S3H). Similarly, we detected an increase in EdU+AP2α+ amacrine cells (Figures S3D, S3E, and S3I) and in EdU+Rxrγ+ cone photoreceptors (Figures S3F, S3G, and S3J). These findings indicate that, with loss of Jarid2, there is increased production of multiple early retinal cell types during the late embryonic period, from E16.5 to birth.
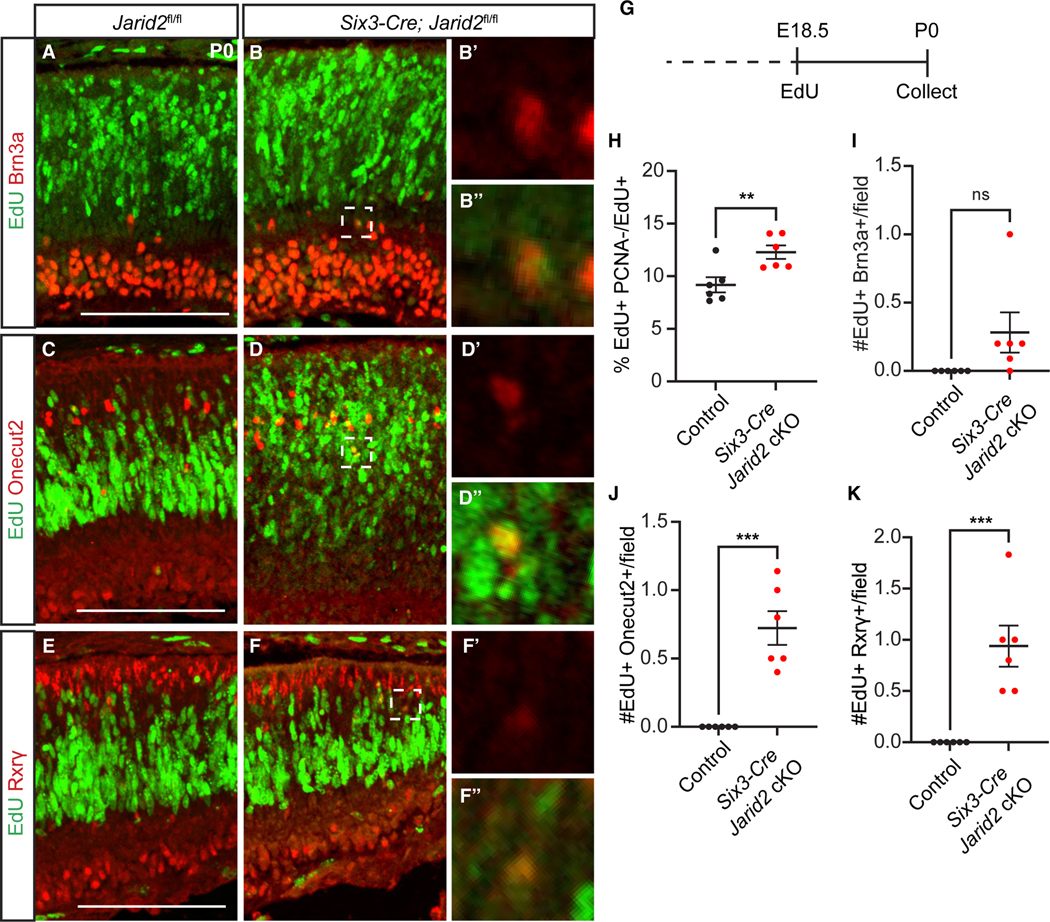
Inactivation of Jarid2 may lead to an increased production of early-born cell types toward the end of their normal window of production, or may extend the window. To distinguish between these possibilities, we administered EdU to control and Six3-Cre Jarid2 cKO animals at E18.5, after all early retinal cell types are normally generated,54 then performed analysis at P0 (Figure 2G). We first asked whether there is a change in cell-cycle exit by combining EdU detection with immunostaining for mitotic marker PCNA. We found that the percent EdU+PCNA−/EdU+ cells was increased, indicating that loss of Jarid2 promotes enhanced cell-cycle exit of progenitors (Figure 2H). When we immunostained for markers of early retinal cell types, control retinas showed no EdU+Brn3a+ RGCs, consistent with RGC genesis being complete. In contrast, EdU+Brn3a+ RGCs were evident in Six3-Cre Jarid2 cKO retinas, although this did not reach significance due to low number and variability (p = 0.08; Figures 2A, 2B, and 2I). Similarly, control retinas showed no EdU+Oc2+ horizontal cells nor EdU+ Rxrγ+ cone photoreceptors, while these were significantly increased in Six3-Cre Jarid2 cKO retinas (Figures 2C–2F, 2J, and 2K). Thus, loss of Jarid2 promotes RPC differentiation with extended production of multiple early-born cell types beyond their normal developmental window.
Figure 2. Loss of Jarid2 promotes extended production of early-born retinal neurons.
Animals were administered EdU at E18.5 and analyzed at P0 (G). Six3-Cre Jarid2 cKO but not control retinas showed EdU-labeled (A, B, and I) Brn3a+ RGCs, (C, D, and J; p = 0.08) Oc2+ horizontal cells, and (E, F, and K) Rxrγ+ cone photoreceptors. (H) Cell-cycle exit (EdU+PCNA−/EdU+) of RPCs was increased in Six3-Cre Jarid2 cKO retina. Zoomed-in images were obtained from a single imaging plane. Graphs represent mean ± SEM, n = 6. **p < 0.01, ***p < 0.00 by Student’s t test. Scale bars, 100 μm. See also Figure S3 and Table S6 for statistical details.
Loss of Jarid2 results in increased early-stage RPC gene expression
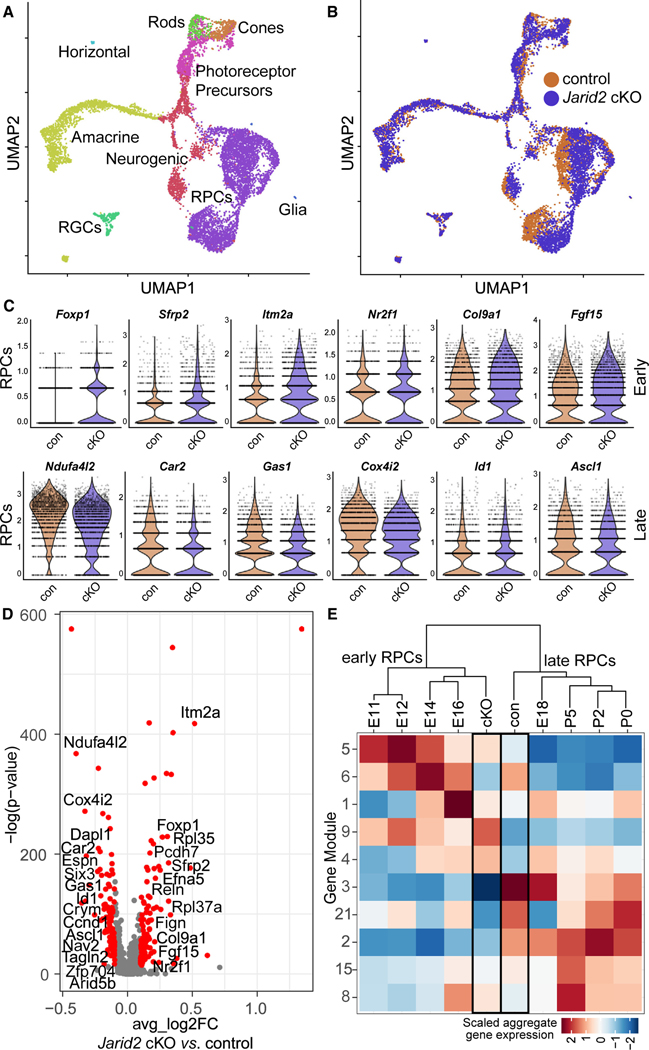
Prior single-cell RNA sequencing (scRNA-seq) analysis of gene expression across the course of retinal neurogenesis (E11–P14) showed a shift from early to late RPC gene expression signatures between E16.5 and E18.5.11 We hypothesized that this transition may be delayed with loss of Jarid2. To determine whether RPC gene expression is altered, we performed scRNA-seq on cells isolated from whole retinas of Rax-Cre Jarid2 cKO and littermate controls at E18.5, and performed the experiment twice to obtain two biological replicates for each sample. We saw a clear overlap between the two batches and genotypes (Figures S4C and S4D), and we identified retinal cell types using established markers (Figures 3A, S4A, and S4B).11 We performed dimensional reduction using spliced transcript counts to narrow the window of gene expression to only mature transcripts. Interestingly, we observed that Jarid2 cKO and control cells were distinguishable in the primary RPC population, arguing that gene expression in this cell population is changed with loss of Jarid2 (Figure 3B).
Figure 3. Increased early RPC gene expression in Rax-Cre Jarid2 cKO RPCs.
(A) UMAP dimensional reduction separates single cells from E18.5 retinas into distinct retinal cell types.
(B) Dimensional reduction based on mature transcripts highlights the difference between Jarid2 cKO and control RPCs.
(C) Violin plots of SCTransform-normalized mature transcript counts in Jarid2 control and cKO RPCs. These selected early and late RPC genes are significantly differentially expressed in the two populations by Wilcoxon rank-sum test (default for Seurat FindMarkers function).
(D) Differential expression analysis comparing total gene expression from Jarid2 cKO and control RPCs. Volcano plot of all expressed genes with significantly changed genes in red. p-adj < 0.05, average log2 fold change > 0.1. Genes are plotted such that those with increased expression in the Jarid2 cKO are on the right.
(E) Module analysis across retinal RPCs. Heatmap represents scaled aggregate gene expression for RPCs from Jarid2 cKO, Jarid2 control, and E11–P5 RPCs (raw data from Clark et al.11). See also Figure S4 and Tables S1 and S2.
To understand the molecular basis for extended generation of early retinal cell types, we focused on primary RPCs and found 125 upregulated and 105 downregulated genes in Jarid2 cKO RPCs compared with control RPCs (Figure 3D, Table S1). We sought to compare these to genes previously found to be enriched in early versus late RPCs, so we reanalyzed published scRNA-seq data to visualize expression of select RPC genes from E11 to P511 (Figure S5). We found that Jarid2 cKO primary RPCs showed significantly increased expression of multiple early-stage RPC genes, including Foxp1, Sfrp2, Itm2a, Nr2f1, Col9a1, and Fgf15, while multiple late-stage RPC genes, including Ndufa4l2, Car2, Gas1, Cox4l2, Isl1, and Ascl1, were decreased (Figures 3C and 3D).
To investigate the possibility of a delayed shift to late-stage RPCs in the absence of Jarid2, we directly compared our findings to gene expression data from E11 to P5 RPCs.11 Using Monocle,55 we identified gene sets, or modules, from this dataset that vary across developing RPCs (Table S2). We compared aggregated module gene expression of RPCs at each time point to that of Jarid2 cKO and control RPCs (Figure 3E). This analysis found that Jarid2 cKO RPCs from E18.5 retinas showed greater similarity in module gene expression to early RPCs (E16.5 and earlier), while Jarid2 control RPCs were more similar to late RPCs (E18.5 and later). Altogether, these findings suggest that loss of Jarid2 results in a delay in the transition from early to late-stage RPCs.
Jarid2 regulates H3K27me3 deposition on genes expressed in early RPCs
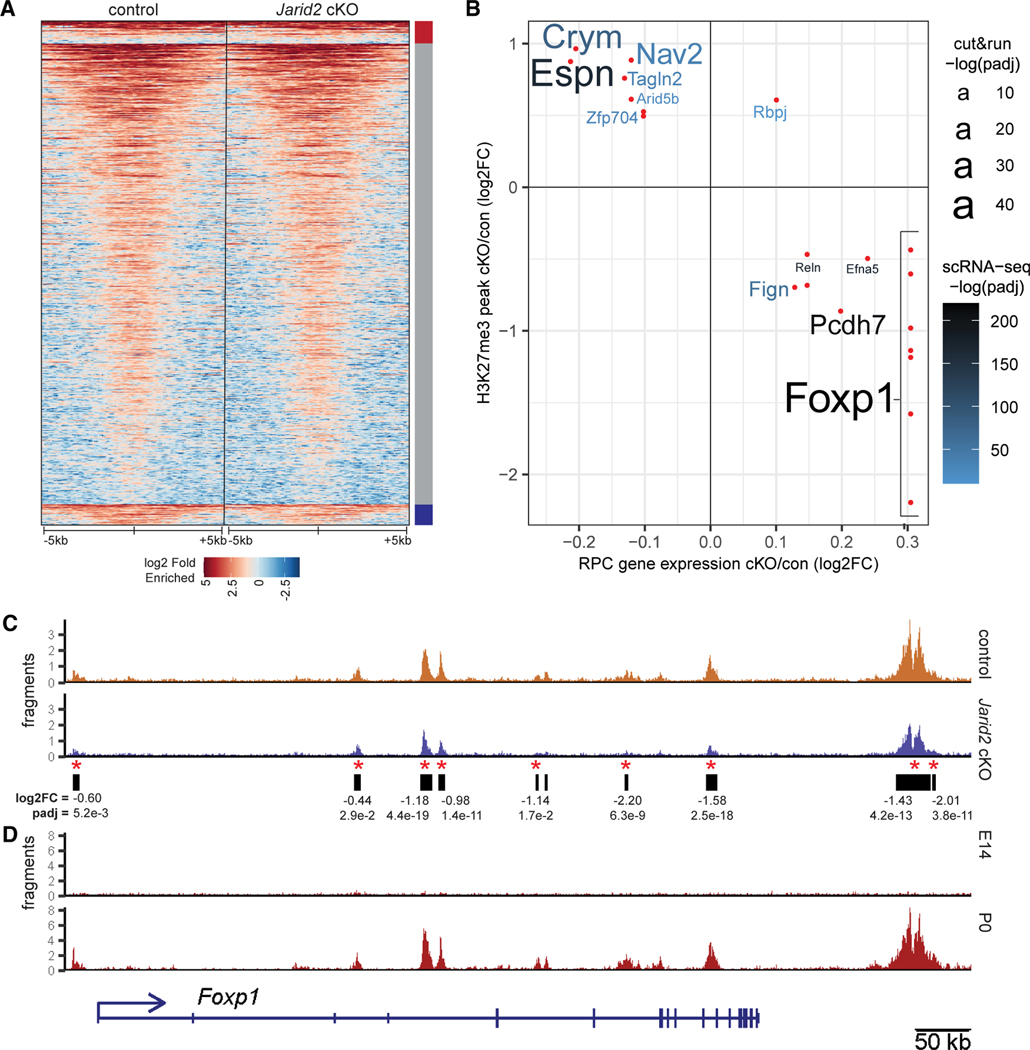
Since Jarid2 can regulate PRC2 activity and recruitment to target genes,36,56 we hypothesized that the observed transcriptional differences in the Jarid2 cKO could be attributed to changes in H3K27me3-mediated gene repression. To test this, we performed CUT&RUN chromatin profiling to define the distribution of H3K27me3 modification in control and Rax-Cre Jarid2 cKO retinas at E18.5. Altogether, we generated 8 samples each from Jarid2 cKO and control animals, in two separate batches. We identified 19,034 H3K27me3-enriched peaks in the control and Jarid2 cKO samples, with 840 significantly increased and 744 significantly decreased in Jarid2 cKO retinas (Figure 4A; Table S3). To reveal cohorts of genes that may be important for the change in temporal patterning of RPCs in Jarid2 cKO, we identified genes associated with differential H3K27me3 peaks that are also differentially expressed in Rax-Cre Jarid2 cKO RPCs (Figure 4B). Since Jarid2 is reported to promote transcriptional repression, we focused on those that lose H3K27me3 repression and have higher RNA expression in the Jarid2 cKO RPCs. Among these, the early RPC gene Foxp1 was notable due its increased expression in Jarid2 cKO RPCs combined with decreased H3K27me3, with at least seven significantly decreased peaks in or near the Foxp1 locus (Figures 4B and 4C).
Figure 4. Jarid2 regulates H3K27me3 deposition at select genes.
(A) Heatmap of H3K27me3 enrichment compared with IgG, 5 kb to either side of the center of merged peaks called from E18.5 Jarid2 control and cKO CUT&RUN experiments. Peaks are grouped according to significant increase (red bar), decrease (blue bar), or no change (gray bar) in Jarid2 cKO. n = 8.
(B) Intersection of gene annotation for differentially regulated H3K27me3 peaks in whole retina and differentially expressed in Jarid2 cKO RPCs. Those peaks with reduced H3K27me3 and increased associated gene expression in Jarid2 cKO RPCs are in the bottom right quadrant. Relative confidence, −log(q value), is indicated by font size for CUT&RUN, or font color for scRNA-seq.
(C) Jarid2 control and cKO H3K27me3 CUT&RUN reads over the Foxp1 gene locus and called peaks (black bars). Red asterisk: significantly reduced peaks (q ≤ 0.05).
(D) H3K27me3 CUT&RUN tracks for wild-type whole retina at E14.5 and P0. See also Figure S6 and Table S3.
To identify the changes in H3K27me3 distribution during early versus late retinal development, we performed CUT&RUN analysis on E14.5 retina during peak early cell production, and on P0 retina, when early cell genesis is complete and late cell production has begun. We observed sparse H3K27me3 labeling of the Foxp1 locus at E14.5, with a substantial increase by P0 (Figure 4D). An inspection of prior H3K27me3 ChIP data across retinal development29 showed that similar regions within the Foxp1 locus progressively acquire H3K27me3 during normal retinal development, from E17.5 to P3 (Figures S6A and S6B). These data suggest that Jarid2 facilitates Foxp1 repression via H3K27me3 deposition during the transition from early to late retinal cell production. Similarly, several previously identified Jarid2 target genes (Pcdh7, Fign, Efna5, and Reln) show reduced H3K27me3 and increased expression in Jarid2 cKO RPCs (Figure 4B). These genes are also expressed in early RPCs (Figure S5) and acquire H3K27me3 from early (E14.5) to late (P0) stages (Figure S6),45,56 although the effect on Foxp1 was the most robust. Altogether, the intersection of H3K27me3 profiling combined with scRNA-seq suggests that Jarid2 promotes the repression of a subset of genes expressed in early RPCs during the transition from early to late RPC states.
Foxp1 regulates the generation of early-born retinal cell types
To better understand genes that may mediate Jarid2 function in RPC competence transitions, we focused our attention on Foxp1, since it encodes a transcription factor, and is thus most likely to mediate changes in temporal competence of progenitors. In addition, Foxp1 has been shown to regulate the window for early neurogenesis during cortical development.57 In the developing retina, Foxp1 was reported as an early RPC gene and shown to decline in expression by E17.5 (Figure S5).11,13,58 To better define Foxp1 temporal expression in the developing mouse retina, we used antibody detection and found that it is expressed in RPCs in the neuroblastic layer at E12.5 and E14.5, with declining expression at E16.5, and virtually no detectable expression in RPCs at P0 (Figure 5A). We saw strong expression of Foxp1 in a subset of RGCs as reported previously58,59 (Figure 5A). Thus, Foxp1 is downregulated in RPCs during the transition from early to late retinal neurogenesis, consistent with the increased H3K27me3-mediated repression we detected at the Foxp1 locus (Figure 4D).
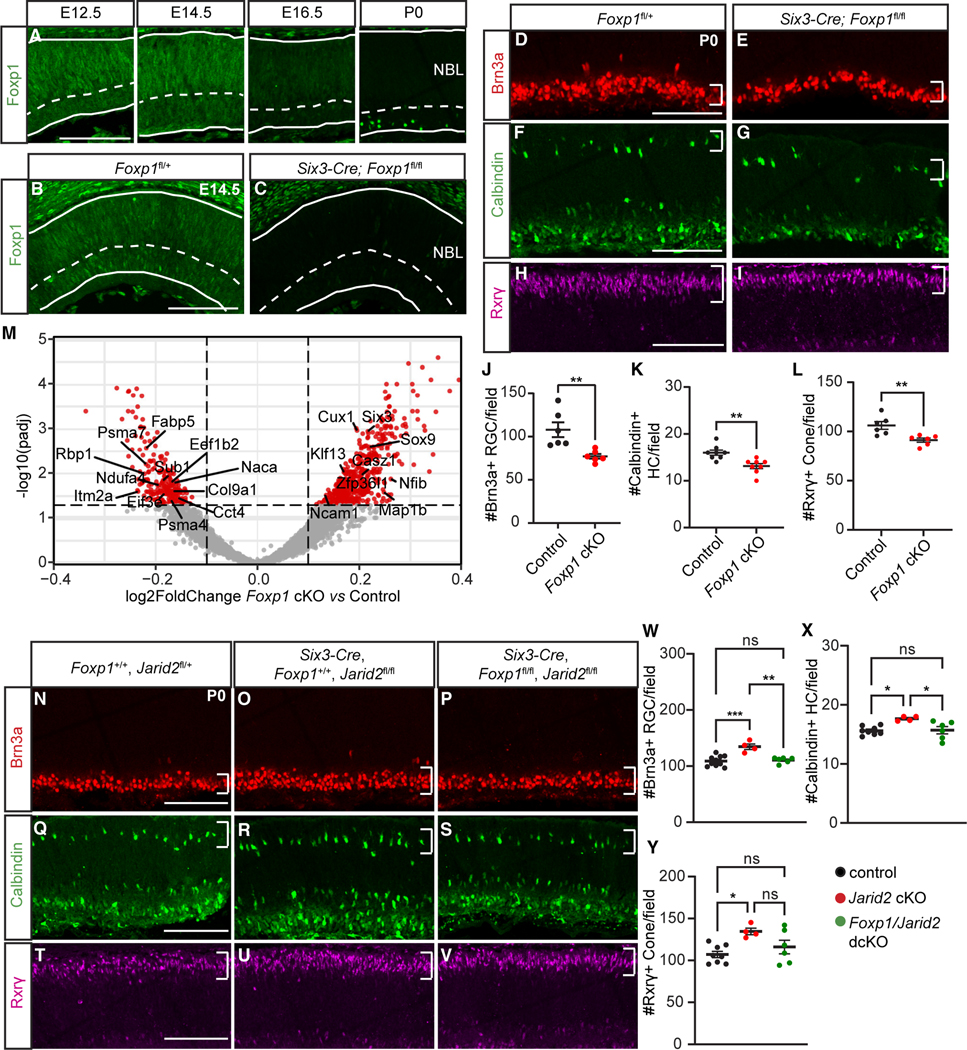
Figure 5. Foxp1 regulates generation of early-born retinal cell types.
(A) Foxp1 expression in wild-type retinas at E12.5, E14.5, E16.5, and P0.
(B and C) Foxp1 expression in the central retina of E14.5 Foxp1fl/+ (control) and Six3-Cre; Foxp1fl/fl (Foxp1 cKO) littermates.
(D–L) Foxp1 cKO retina showed (D, E, and J) reduced Brn3a-labeled RGCs, (F, G, and K) reduced Calbindin-labeled horizontal cells, and (H, I, and L) reduced Rxrγ-labeled cone photoreceptors within central dorsal retinas at P0. Regions of interest marked by white brackets.
(M) Cropped volcano plot of Foxp1 cKO E16.5 bulk RNA-seq results. Red dots represent significantly differentially expressed genes. p-adj < 0.05.
(N–V) Representative images of early-born retinal cell types in (N, Q, and T), control animals in (O, R, and U), and Six3-Cre Jarid2 cKO in (P, S, and V), or Foxp1:Jarid2 double cKO littermates at P0 with (W and Y) associated quantification.
(N–P and W) Brn3a-labeled cells, (Q–S and X) Calbindin-labeled horizontal cells, (T–V and Y) Rxrγ-labeled cone photoreceptors. Region of interest marked by white brackets. NBL, neuroblastic layer. Graphs represent mean ± SEM. n = 6 in (J and L) and Foxp1:Jarid2 double cKO in (W–Y), n = 8 in (K), and control in (X and Y), n = 10 in control in (W), n = 4 in Jarid2 cKO in (W–Y). *p < 0.05, **p < 0.01. ns, not significant. Scale bars, 100 μm. See also Tables S4 and S6 for statistical details.
To determine the function of Foxp1 in early RPCs, we conditionally abolished Foxp1 expression by crossing Six3-Cre mice with Foxp1-floxed mice (Foxp1 cKO),60 and used littermates lacking Cre as controls. The efficiency of Foxp1 cKO in the retina was confirmed by Foxp1 immunostaining at E14.5 (Figures 5B and 5C). To test if the generation of early-born cell types was affected in Foxp1 cKO, we performed immunostaining at P0 for early-born retinal cell type markers. All cell types were present, as reported previously for Dkk3-Cre-mediated Foxp1 cKO,58 so we quantified to detect potential shifts in their proportions. We observed a thinning of the GCL and a significant reduction in Brn3a+ RGCs (Figures 5D, 5E, and 5J). We also found a significant reduction in Calbindin+ horizontal cells in the outer retina (Figures 5F, 5G, and 5K), and a significant reduction in Rxrγ+ cone photoreceptors (Figures 5H, 5I, and 5L). Together this suggests that Foxp1 promotes early-born cell generation. To determine whether the loss of Foxp1 alters the transcriptional profile of RPCs, we performed bulk RNA-seq on E16.5 Foxp1 cKO and littermate control retinas. Several early RPC genes, including Itm2a, Ndufa4, and Col9a1, were downregulated, while a group of late RPC genes, such as Casz1, Nfib, and Sox9, were upregulated, indicating premature expression of late RPC genes in Foxp1 cKO (Figure 5M; Table S4).
We have shown that Jarid2 cKO results in elevated Foxp1 expression at the late embryonic stage, and that loss of Foxp1 reduces early-born cell generation, which is the opposite of the Jarid2 cKO phenotype. Based on these observations, we hypothesized that loss of Jarid2-mediated repression of Foxp1 could be a major factor in the delayed transition to late progenitor competence and extended production of early cell types. To test this, we generated Foxp1:Jarid2 double cKO animals using Six3-Cre. We confirmed that Six3-Cre Jarid2 cKO mice showed increased Brn3a+ RGCs at P0 (Figures 5N, 5O, and 5W). In contrast, Foxp1:Jarid2 double cKO mice showed no significant change in Brn3a+ RGCs compared with littermate controls lacking Cre (Figures 5N, 5P, and 5W). Similarly, the increase in Calbindin+ horizontal cells and Rxrγ+ cone photoreceptors seen in Jarid2 cKO retinas were also reversed in Foxp1:Jarid2 double cKO mice (Figures 5Q–5V, 5X, and 5Y). Thus, elevated Foxp1 is required for increased early-born cell production after loss of Jarid2.
Ectopic Foxp1 expression in RPCs affects retinal cell genesis
Since Foxp1 expression normally declines and is absent in RPCs by P0, we asked whether sustained Foxp1 expression is sufficient for the generation of early-born neurons. We conditionally elevated Foxp1 in RPCs by crossing Six3-Cre mice with conditional Foxp1 transgenic mice expressing Foxp1 under the CAG promoter (Foxp1 cTG).61 We confirmed by immunostaining that Foxp1 expression was increased in retinal progenitors of Six3-Cre Foxp1tg/+ mice, with mosaic levels of expression in radial columns (Figures 6A, 6B, S7A, and S7B). We then assessed effects on the generation of early cell types and found that by P0, overexpression of Foxp1 led to an increase in Brn3a+ RGCs in the GCL similar to the Jarid2 cKO phenotype (Figures 6C, 6D, and 6K). We also observed a significant increase in other early-born cell types, including Onecut2+ horizontal cells (Figures 6E, 6F, and 6L), and Rxrγ+ cone photoreceptors (Figures 6G, 6H, and 6M). We observed more Brn3a+ RGCs in regions with higher Foxp1 levels in Six3-Cre Foxp1tg/+ mice (Figures S7C–S7E), suggesting that RGC generation may be Foxp1 dosage dependent. To test this, we analyzed Foxp1 cTG mice with two copies of the Foxp1 transgene and found that Brn3a+ RGCs were dramatically increased in (Figures 6I and 6J).
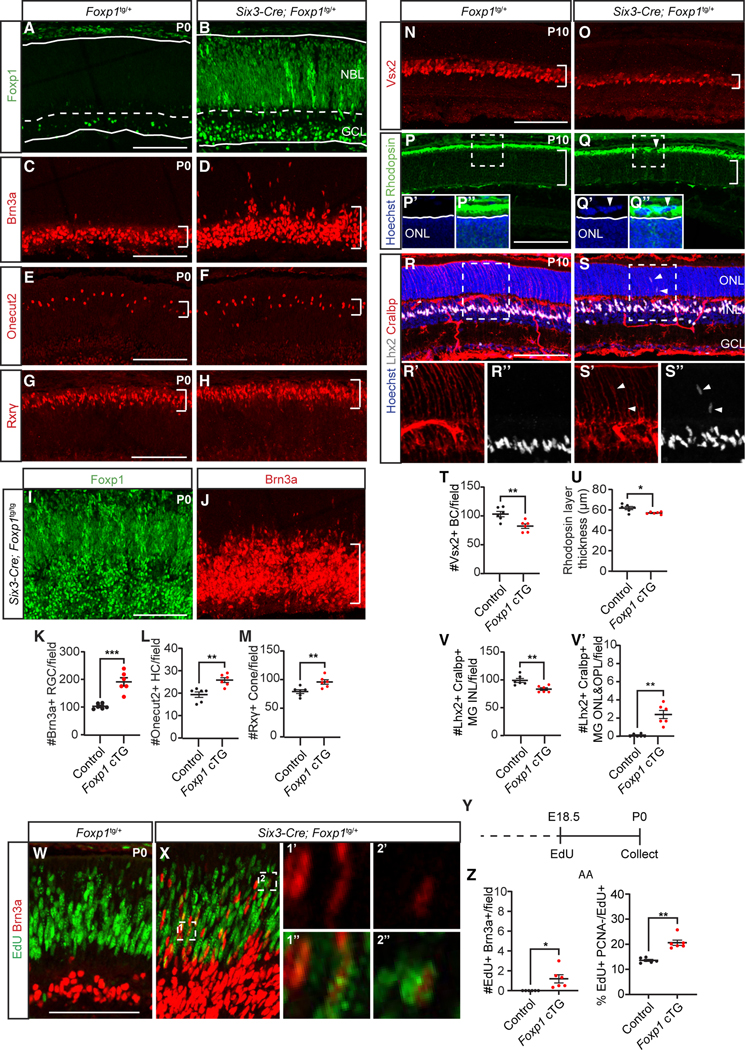
Figure 6. Sustaining Foxp1 in RPCs increases in early-born and decreases in late-born retinal cells.
(A and B) Foxp1 expression in P0 Foxp1tg/+ (control) and Six3-Cre; Foxp1tg/+ (Foxp1 cTG) littermates.
(C–H and K–M) Foxp1 cTG showed increased (C, D, and K) Brn3a-labeled RGCs (E, F, and L) Onecut2-labeled horizontal cells, and (G, H, and M) Rxrγ-labeled cone photoreceptors at P0.
(I and J) Foxp1 expression and Brn3a-labeled RGCs in P0 Six3-Cre; Foxp1tg/tg retina. Foxp1 cTG at P10 showed (N, O, and T) decreased Vsx2-labeled bipolar cells and (P, Q, and U) reduced thickness of Rhodospin-labeled rod photoreceptor layer. Arrowheads in (P) and (Q) indicate abnormal structure in the subretinal region.
(R, S, and V) Foxp1 cTG had reduced Lhx2 and Cralbp-colabeled Müller glia in the INL and (V′) increased Müller glia in the ONL and OPL. Arrowheads in (S, S’, and S′′) indicate mislocated Müller glia in the ONL and OPL.
(Y) Foxp1 cTG administered EdU at E18.5 and analyzed at P0 (W–Z) showed increased EdU+Brn3a+ RGCs. Zoomed-in images were from a single imaging plane. (AA) Foxp1 cTG retina showed increased cell-cycle exit of progenitors. NBL, neuroblastic layer; GCL, ganglion cell layer; ONL, outer nuclear layer; INL, inner nuclear layer. Graphs represent mean ± SEM, n = 6. *p < 0.05, **p < 0.01, ***p < 0.001, by Student’s t test. Scale bars, 100 μm. See also Figure S7 and Table S6 for statistical details.
We then asked whether there was a corresponding effect on late-born cell generation by examining Foxp1 cTG retina at P10. We found a reduction in late-born retinal cells including Vsx2+ bipolar cells (Figures 6N, 6O, and 6T) and Rhodopsin+ rod photoreceptors (Figures 6P, 6Q, and 6U). This is consistent with a prior study showing increased cones and reduced rods following Foxp1 overexpression.58 We also observed disruption of the outer limiting membrane with abnormal Rhodopsin+ cells in the subretinal space (Figures 6P and 6Q) in Foxp1 cTG, although the severity of such aberrant rosette-like structures was variable across sections and animals (Figures S7F–S7H). We found that Lhx2+ and Cralbp+ Müller glia were reduced in the inner nuclear layer (Figures 6R, 6S, and 6V), and some were mislocated to the outer plexiform layer and outer nuclear layer (Figure 6V′). Thus, late-born cell generation is reduced and Müller glia development impacted by misexpression of Foxp1.
To determine if the increased generation of early-born retinal cells was due to an extended window of production, we administered EdU to Foxp1 cTG mice at E18.5 and performed immunostaining for Brn3a+ RGCs at P0. Control retinas showed no Brn3a+ RGCs colabeled with EdU, in contrast to Foxp1 cTG retinas (Figures 6W–6Z), indicating an extended window of RGC genesis. As in Jarid2 cKO, this extended window was coupled with enhanced exit of RPCs, as we found that the percentage of EdU+PCNA−/EdU+ cells was increased (Figure 6AA). Taken together these results suggest that Foxp1 promotes the generation of early-born retinal cells at the expense of late-born cell generation by extending the window of early cell genesis.
Sustaining Foxp1 in RPCs maintains early RPC transcription pattern
To assess if Foxp1 overexpression in RPCs changes the gene expression pattern of RPCs, we performed scRNA-seq on P0 Foxp1 cTG and control littermate retinas (Figure 7A). We observed a dramatic difference in the UMAP embedding of RPCs between Foxp1 cTG and control retinas (Figure 7B). Focusing our analysis on RPCs, we identified 575 differentially expressed genes in the Foxp1 cTG retinas. We noted upregulation of early RPC genes, including Sfrp2, Col9a1, Fgf15, and Itm2a, and downregulation of late RPC genes Ndufa4l2, Car2, Casz1, and Nfib, suggesting that an early RPC transcriptional pattern is artificially maintained in Foxp1 cTG RPCs (Figures 7C and 7D).
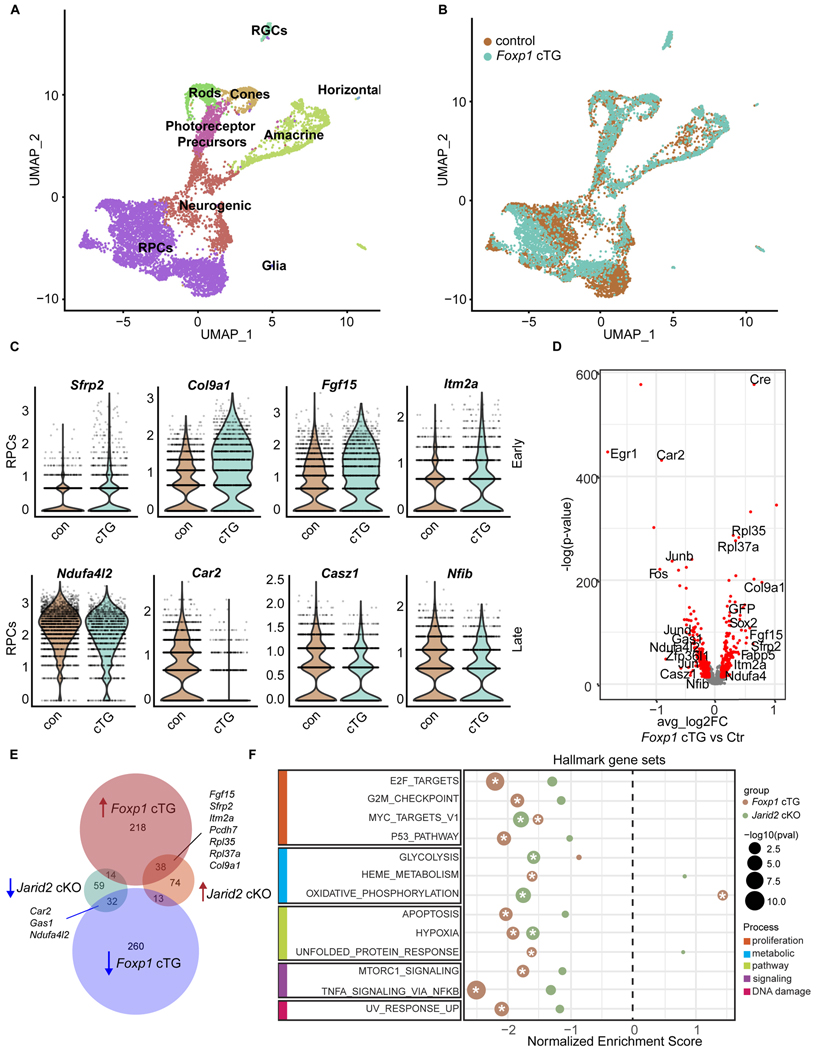
Figure 7. Elevated Foxp1 expression results in increased early RPC gene expression.
(A and B) UMAP dimensional reduction of scRNA-seq data from P0 Foxp1 cTG and littermate control, colored by annotated cell types (A) and colored by genotype (B).
(C) Violin plots of normalized transcript counts of selected early and late RPC genes differentially expressed in Foxp1 control and Foxp1 cTG RPCs.
(D) Volcano plot of all expressed genes, with genes significantly differentially expressed in Foxp1 cTG in red. p-adj < 0.01, average log2 fold change >0.1. Genes are plotted such that those with increased expression in the Foxp1 cTG are on the right.
(E) Venn diagram comparing differentially expressed genes common to Jarid2 cKO and Foxp1 cTG RPCs. RPC marker genes are shown.
(F) GSEA of MSigDB mouse hallmark gene sets. A positive enrichment score indicates enrichment in Jarid2 cKO (green) and Foxp1 cTG (brown) RPCs, while a negative score represents enrichment in the control. Significantly enriched genes sets (p-adj < 0.05) are marked with an asterisk. Gene sets are grouped by shared biological process. See also Table S5.
As the gene expression pattern in Foxp1 cTG RPCs is reminiscent of that in Jarid2 cKO RPCs we compared the genes that were significantly differentially expressed in these two datasets. We found that, of the 230 genes differentially expressed in Jarid2 cKO and 575 in Foxp1 cTG RPCs, 96 were differentially expressed in both conditions. Among these, 96 genes are upregulated early RPC genes (Fgf15, Sfrp2, Itm2a, Pcdh7, Ppl35, Rpl27a, and Col9a1) and downregulated late RPC genes (Car2, Gas1, and Ndufa4l2) (Figure 7E). The large overlap between these two datasets suggests that Foxp1 and Jarid2 may regulate similar biological processes.
We took an unbiased approach to define the biological processes altered in Jarid2 cKO and Foxp1 cTG RPCs. Significantly altered MSigDB mouse hallmark gene sets were identified from each dataset through preranked gene set enrichment analysis (Figure 7F).62 We found that two gene sets were significantly depleted in both Jarid2 cKO and Foxp1 cTG RPCs: Myc targets V1 and hypoxia. Several additional gene sets were significantly reduced in Foxp1 cTG, with Jarid2 cKO data trending in the same direction: E2F targets, G2M checkpoint, P53 pathway, apoptosis, mTORC1 signaling, TNF-α signaling via NF-κB, and UV response. The only significantly discordant result was enrichment of oxidative phosphorylation in Foxp1 cTG RPCs and depletion in Jarid2 CKO RPCs. Thus, we find that Jarid2 and Foxp1 similarly regulate gene signatures of proliferation, biological pathways, cell signaling, and DNA damage.
DISCUSSION
Here, we report that temporal progression of CNS progenitors in developing retina is regulated by Jarid2-mediated repression of a subset of genes expressed in early progenitors, most notably Foxp1, which we show functions as a key effector of Jarid2-dependent regulation of temporal patterning. We thus define an important in vivo role for Jarid2 in facilitating a transition in neural progenitor competence.
H3K27me3 levels are highly dynamic across retinal development,29 and we found that loss of Jarid2 results in a selective decrease in H3K27me3 and derepression of a subset of genes active in early RPCs. This derepression was detected at E18.5, after the transition to late RPCs. Prior evidence suggests that Jarid2 may play a role in targeting active genes for repression. For example, Jarid2/PRC2.2 is required for de novo silencing of genes active in ESCs, during differentiation to neural progenitor cells in vitro.39 In addition, Jarid2, together with Aebp2, can partially overcome inhibition of PRC2 methyltransferase activity caused by activation marks such as H3K4me3 and H3K36me3.38 Jarid2 has also been shown to facilitate H3K9 methylation to promote silencing of the Notch1 locus by SETDB1 during heart development41 and to silence CyclinD1 via G9a/GLP,42 so it is possible that additional Jarid2-mediated histone modifications contribute to silencing of early RPC genes during retinal development, potentially in concert with H3K27me3-mediated repression.63 Overall, our findings show that Jarid2 promotes selective repression of subsets of active genes and a shift in gene expression during temporal pattering of progenitors to promote the change in production from early to late retinal cell types.
We identified Foxp1 as a critical target of Jarid2-mediated polycomb repression, since it was upregulated in Jarid2 mutant RPCs at E18.5 and showed significant reduction in H3K27me3 across the locus. Foxp1 is notable, since its expression is highest at embryonic stages of retina development,58 and scRNA-seq analysis has identified it as a gene enriched in early RPCs.11 Foxp1 encodes a transcription factor of the forkhead box (FOX) family that is expressed in developing CNS, with mutations linked to neurodevelopmental disorders.64 During normal retinal development, we observed little H3K27me3 at the Foxp1 locus at E14.5, when the gene is actively expressed in early RPCs, but saw acquisition of H3K27me3 by birth when the gene is silenced in late RPCs. This is consistent with prior ChIP-seq analysis showing progressive increase in H3K27me3 at the Foxp1 locus from E17.5 to P3,29 coincident with the progressive reduction of Foxp1 expression in RPCs.11,58 It is also possible that changes in the cell composition of the retina contributes to changes in H3K27me3 since the CUT&RUN analysis was performed on total retina. But, together with scRNA-seq analysis, our findings suggest that silencing of Foxp1 expression in RPCs is facilitated by Jarid2-mediated repression, although other regulatory and transcriptional mechanisms likely contribute.13,29
We further establish Foxp1 as a key competence factor for early retinal cell genesis. We first showed that Foxp1 plays a role in early retinal cell specification, since RGCs, cones, and horizontal cells were reduced in Foxp1 cKO retina. A prior study did not report a visible change in retinal neurogenesis with Dkk3-Cre-mediated conditional knockout of Foxp1, potentially due to differences in Cre drivers used or stage of analysis resulting in a more subtle phenotype. They did report that overexpression of Foxp1 resulted in increased cones and reduced rods, consistent with our findings.58 Other related forkhead family transcription factors (Foxp2,4) are also expressed in the developing retina, raising the possibility of compensation by these other factors, although Foxp1 is the most highly expressed during early development.58 We found that elevated expression of Foxp1, using an inducible transgene, phenocopied the loss of Jarid2, resulting in enhanced production of multiple early cell types and reduced generation of late cell types such as bipolar cells and rod photoreceptors. For both loss of Jarid2 and upregulation of Foxp1, this was due to extended production of early cell types. This parallels the role of Foxp1 during cortex development, where its expression in apical radial glia during early cortical neurogenesis acts to gate the window for production of early-born deep layer neurons.57 We found a dose-dependent effect of Foxp1, with two copies of the transgene promoting even higher levels of RGC differentiation, consistent with Foxp1 levels being highest early when RGCs are being produced then gradually declining. Notably, we found that loss of Jarid2 or elevation of Foxp1 expression resulted in enhanced cell-cycle exit of progenitors, which is in contrast to the effects of Foxp1 in promoting self-renewal and maintenance of apical radial glia during cortex development, suggesting potential context-dependent functions.57 Despite divergent effects on progenitor cell proliferation, our findings suggest a conserved role for Foxp1 in CNS progenitors in regulating the period of early neurogenesis.
RNA-seq analysis at E16.5, during the period of early cell generation, revealed that loss of Foxp1 resulted in upregulation of multiple genes normally expressed in late RPCs or Müller glia, including Sox9 and Nfib.11 Conversely, scRNA-seq analysis of Foxp1 cTG retina at P0 showed effects on RPC gene expression, with upregulation of multiple early RPC genes and downregulation of late RPC genes, including known late temporal identity genes Casz1 and Nfib.11,16,17 This raises the possibility that Foxp1 promotes extended production of early retinal cell types by repressing genes in RPCs important for late temporal identity. This would be consistent with a repressor-decay timer mechanism for temporal progression whereby a temporal identity factor initiates expression when its repressor decays to a sufficiently low level, as in Drosophila,65 with decline in Foxp1 facilitated by Jarid2-mediated repression. Interestingly, our findings support predictions from scATAC-seq analysis revealing potential cross-regulatory gene networks involving Foxp1 and Nfi.13 Altogether, our observations support the conclusion that expression of Foxp1 promotes early competence during retinal neurogenesis.
We provide substantial evidence that Foxp1 functions as a primary effector in extending the early competence window after loss of Jarid2. Foxp1 is upregulated in Jarid2 cKO retina, and we find that loss of Jarid2 or increased Foxp1 expression can extend the window for early cell production, have similar effects on temporal patterning of RPC gene expression, and similarly regulate gene signatures of proliferation, biological pathways, cell signaling, and DNA damage. Importantly, we show that Foxp1 acts epistatically to Jarid2, as loss of Foxp1 reverses the enhanced production of early cell types observed with loss of Jarid2 alone. In lymphoma, Foxp1 is targeted and suppressed by Ezh2 gain of function, suggesting that Foxp1 may be regulated by PRC2 in other contexts.66 Foxp1:Jarid2 double cKO mice did not show reduced early cell types as seen in Foxp1 cKO mice alone, suggesting that additional factors may contribute to early cell generation. Several other genes also show increased expression and reduced H3K27me3 in the Jarid2 cKO retina, including previously identified Jarid2 target genes,45,56,67 but they are less highly expressed in RPCs (Figure S5) and are unlikely to drive transcriptional changes.
We found that, while Foxp1 cTG showed reduced Müller glia number and mislocalization, Jarid2 cKO did not affect Müller glia development. This is potentially due to more modest levels of Foxp1 upregulation with loss of Jarid2 than with transgenic Foxp1 misexpression. Consistent with effects on Müller glia development, in Foxp1 cTG retina we observed disruption of the outer limiting membrane with mislocated rod cells in the subretinal space, which is similar to the retinal phenotype reported for the Dicer conditional knockout.23 Overall, we conclude that Foxp1 is critical for extending the early competence window after loss of Jarid2, providing additional mechanistic insight into the interplay between epigenetic regulation, in this case related to histone modifications, and transcriptional regulation of temporal patterning in CNS progenitors. More generally, Jarid2-mediated repression may contribute to competence restriction, whereby late progenitors are restricted from generating earlier cell types.8–10
In Drosophila CNS, polycomb-dependent regulation restricts an early competence window for neuroblasts as they transition from motoneuron to interneuron production.68 PRC2 also plays a role in timing of mammalian cortical neurogenesis and glial differentiation.27,69 In retina, the late temporal factor Casz1 associates with nucleosome remodeling and deacetylase complex, and Jarid2 was also found in this Casz1 interactome suggesting potentially more direct interaction between regulators of temporal identity.17 Based on our findings, it will be interesting to explore how Jarid2 contributes to these diverse roles. While multiple mechanisms likely converge to modulate regulation of temporal patterning in neural progenitors, we propose that dynamic regulation of H3K27me3 by Jarid2 plays a role in restricting progenitor cell competence.
Limitations of the study
During the course of development,7 or as a consequence of genetic manipulation such as Jarid2 cKO or Foxp1 cTG, there are changes in the proportion of different retinal cell populations. While our scRNA-seq analysis revealed gene expression changes within RPCs, we were only able to perform the CUT&RUN analysis for H3K27me3 on bulk retina, so changes in histone modification could be in part due to changes in cell composition. In addition, while loss of Jarid2 resulted in derepression of Foxp1, we were not able to show Jarid2 binding to the Foxp1 locus, despite testing several antibodies, so it remains possible that the effect on Foxp1 repression is indirect.
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Monica Vetter (monica.vetter@neuro.utah.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All raw and processed bulk RNA-seq, single cell RNA-seq, and CUT&RUN data generated for this study have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. Additionally, this paper analyzes existing, publicly available data and the accession numbers for these datasets are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| Goat anti-Brn3 (1:50) | Santa Cruz Biotechnology | Cat#sc6026; RRID: AB_673441 |
| Mouse anti-Brn3a (1:200) | Millipore | Cat#MAB1585; RRID: AB_94166 |
| Mouse anti-Otx2 (1:200) | Santa Cruz Biotechnology | Cat#sc-514195 |
| Rabbit anti-calbindin D-28K (1:2000) | Millipore | Cat#PC253L; RRID: AB_213554 |
| Mouse anti-Rxrγ (1:250) | Santa Cruz Biotechnology | Cat#sc-365252; RRID: AB_10850062 |
| Rabbit anti-recoverin (1:2000) | Millipore | Cat#AB5585; RRID: AB_2253622 |
| Goat anti-Vsx2/Chx10 (1:500) | gift from Dr. Edward M. Levine | N/A |
| Sheep anti-Vsx2/Chx10 (1:500) | Exalpha Biologicals | Cat#X1180P; RRID: AB_2314191 |
| Mouse anti-Rhodopsin (1:200) | Thermo Fisher Scientific | Cat#MA5–11741; RRID: AB_10980983 |
| Rabbit anti-Lhx2 (1:500) | Abcam | Cat# ab184337, RRID: AB_2916270 |
| Mouse anti-Cralbp (1:500) | Novus | Cat# NB100–74392, RRID: AB_2253681 |
| Mouse anti AP-2alpha (1:500) | Santa Cruz Biotechnology | Cat#sc-12726, RRID: AB_667767 |
| Mouse anti-PCNA (1:2000) | Sigma-Aldrich | Cat#P8825, RRID: AB_477413 |
| Rabbit anti-pH3 (Ser10) (1:250) | Millipore | Cat#06–570; RRID: AB_310177 |
| Rabbit anti-Foxp1 (1:2000) | Abcam | Cat#ab16645; RRID: AB_732428 |
| Rabbit anti-H3K27me3 | Millipore | Cat#07–449; RRID: AB_310624 |
| Sheep anti-Onecut2 (1:100) | R&D Systems | Cat#AF6294; RRID: AB_10640365 |
| Donkey anti-rabbit 488 (1:400) | Thermo Fisher Scientific | Cat#A-21206, RRID: AB_2535792 |
| Donkey anti-rabbit 555 (1:400) | Thermo Fisher Scientific | Cat#A31572; RRID: AB_162543 |
| Donkey anti-mouse 488 (1:400) | Thermo Fisher Scientific | Cat#A-21202, RRID: AB_141607 |
| Donkey anti-mouse 555 (1:400) | Thermo Fisher Scientific | Cat#A-31570, RRID: AB_2536180 |
| Donkey anti-sheep 555 (1:400) | Thermo Fisher Scientific | Cat#A-21436, RRID: AB_2535857 |
| Donkey anti-goat 488 (1:400) | Thermo Fisher Scientific | Cat#A-11055, RRID: AB_2534102 |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| DNAse I | Sigma-Aldrich | Cat#D4513 |
| Liberase | Sigma-Aldrich | Cat#5401119001 |
| RBC lysis buffer | eBioscience | Cat#00–4333-57 |
| Tamoxifen | Sigma-Aldrich | Cat#T5648 |
|
| ||
| Critical commercial assays | ||
|
| ||
| Click-iT plus EdU Imaging Kits | Thermo Fisher Scientific | Cat#C10637 |
| RNeasy Plus Mini Kit | Qiagen | Cat#74134 |
| CUT&RUN Assay Kit | Cell Signaling | Cat#86652 |
| Spin column Kit for Cut&Run | Cell Signaling | Cat#14209 |
| RNA ScreenTape Assay | Agilent Technologies | Cat#5067–5576, 5067–5577, 5067–5578 |
| D1000 ScreenTape Assay | Agilent Technologies | Cat#5067–5582, 5067–5583 |
| Kapa Library Quant Kit | Kapa Biosystems | Cat#KK4824 |
| NEBNext Ultra II Directional RNA Library Prep Kit for Illumina | New England Biolabs | Cat#E7760 |
| NEBNext rRNA Depletion Kit v2 | New England Biolabs | Cat#E7400 |
| NEBNext UItra II DNA Library Prep Kit for Illumina | New England Biolabs | Cat#E7645 |
| Chromium Single Cell 3′ Library Prep Kit v3 | 10X Genomics | Cat#PN-1000092 |
| Chromium Next GEM Single Cell 3′ Library Prep Kit v3.1 |
10X Genomics | Cat#PN-1000128 |
| NovaSeq XP workflow v1 and v1.5 | Illumina | Cat#20021664, 20043131 |
| NovaSeq S2 and S4 Reagent Kits | Illumina | Cat#20012860, 20028312 |
|
| ||
| Deposited data | ||
|
| ||
| All raw and analyzed data generated for this study | This paper | GEO: GSE202741 |
| Jarid2 cKO scRNA-seq | This paper | GEO: GSE202734 |
| H3K27me3 CUT&RUN from Jarid2 cKO | This paper | GEO: GSE222955 |
| H3K27me3 CUT&RUN from E14.5 and P0 retina | This paper | GEO: GSE202728 |
| Foxp1 cKO RNA-seq | This paper | GEO: GSE202725 |
| Foxp1 cTG scRNA-seq | This paper | GEO: GSE222956 |
| Single Cell-seq of whole retina E11.5 to P5 | Clark et al.11 | GEO: GSE118614 |
| H3K27me3 ChIP-seq from E17.5, P0, and P3 retina | Aldiri et al.29 | GEO: GSE87037 |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| C57BL/6N-Jarid2tm1a(KOMP)Wtsi/Mmucd mice | KOMP Repository | RRID: MMRRC_048262-UCD |
| B6.Cg-Tg(ACTFLPe)9205Dym/J mice | Jackson Laboratory | RRID: IMSR_JAX:005703 |
| Tg(Six3-Cre)69Frty mice | Dr. Yasuhide Furuta (Furuta et al.52) | RRID: IMSR_JAX:019755 |
| Raxtm1.1(cre/ERT2)Sbls/J mice | Dr. Seth Blackshaw (Pak et al.53) | RRID: IMSR_JAX:025521 |
| Foxp1flox/flox mice | Jackson Laboratory (Feng et al.60) | RRID: IMSR_JAX:017699 |
| Foxp1a transgenic mice | Dr. Hui Hu (Wang et al.61) | MGI:5607369 |
|
| ||
| Software and algorithms | ||
|
| ||
| BBTools v38.34 | Bushnell et al.70 | RRID:SCR_016968 |
| cutadapt v1.16 and v2.8 | Martin71 | RRID:SCR_011841 |
| STAR v2.7.0f and v2.7.6a | Dobin et al.72 | RRID:SCR_004463 |
| featureCounts v1.5.1 | Liao et al.73 | RRID:SCR_012919 |
| DESeq2 v1.38.1 | Love et al.74 | RRID:SCR_015687 |
| CellRanger v3.1.0 | 10X Genomics | RRID:SCR_023221 |
| velocyto v0.17.17 | La Manno et al.75 | RRID:SCR_018167 |
| Seurat v4.0.3 | Stuart et al.87 | RRID:SCR_007322 |
| DoubletFinder v2.0.3 | McGinnis et al.76 | RRID:SCR_018771 |
| Monocle3 v1.0.0 | Qiu et al.55 | RRID:SCR_018685 |
| Novoalign v4.02.02 | Novocraft | RRID:SCR_014818 |
| Samtools v1.10 | Danecek et al.77 | RRID:SCR_002105 |
| Macs2 v2.2.7.1 | Zhang et al.78 | RRID:SCR_013291 |
| Fgsea v1.18.0 | Korotkevich et al.79 | RRID:SCR_020938 |
| Gage v2.42.0 | Luo et al.80 | RRID:SCR_017067 |
| GraphPad Prism v9 | GraphPad | RRID:SCR_002798 |
| NIS-Elements v18 | Nikon | RRID:SCR_014329 |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse strains
The heterozygous Jarid2 knockout first (KO-first) mouse line carrying a Jarid2 KO-first allele was generated in the University of Utah Transgenic and Gene Targeting Core using cryo-archived embryos of Jarid2tm1a(KOMP)Wtsi from KOMP Repository (RRID: MMRRC:048262-UCD). The Jarid2 floxed mice were generated by crossing Jarid2 KO-first mice with B6.Cg-Tg(ACTFLPe) 9205Dym/J mice (RRID: IMSR_JAX:005703) which express flippase (Flp) recombinase to delete the FRT flanked trapping cassette (see Figure S1).
In order to generate retinal Jarid2 conditional knockout (cKO), Jarid2 floxed mice were crossed with Six3-Cre (RRID: IMSR_JAX:019755)52 or Rax-CreERT2 (RRID: IMSR_JAX:025521).53 For Rax-CreERT2 animals, Cre activity was induced by intraperitoneal injection of 75 μg Tamoxifen per gram of body weight to pregnant females at E10.5. Tamoxifen was dissolved in corn oil (Sigma-Aldrich, C8267) at a concentration of 10 mg/ml. Foxp1 floxed (RRID: IMSR_JAX:017699) and Foxp1a transgenic mice (MGI:5607369) were crossed with Six3-Cre mice52 to generateSix3-Cre; Foxp1fl/fl and Six3-Cre; Foxp1tg/+ mice, respectively. To generate Foxp1:Jarid2 double conditional knockout mice, Foxp1fl/fl mice were crossed with Six3-Cre; Jarid2fl/fl.
The morning a plug was detected was considered embryonic day 0.5. All animals were treated within the guidelines of the University of Utah Institutional Animal Care and Use Committee (IACUC) and all experiments were IACUC approved. Both male and female animals were used unless otherwise stated.
METHOD DETAILS
Western blot
Freshly dissected retinal tissue at P0 was homogenized in lysis buffer with HaltProtease Inhibitor Cocktail (ThermoFisher 87786). Protein concentration was determined using BCA (Millipore Sigma B9643–1L). Protein was separated on Bio-Rad Mini-protean TGX Gels 4–20% (Bio-Rad 456–1093) and transferred using Bio-Rad Trans-Blot Turbo Transfer Pack (Bio-Rad 1704156). Membranes were then incubated with the primary antibodies followed by HRP conjugated secondary antibodies, and detection with Clarity Western ECL Substrate (Bio Rad 1705060).
Tissue processing
For embryonic and P0 retinal cryostat sectioning, whole heads were collected. For P10/P14 retinal cryostat sections, eyeballs were enucleated from euthanized mice and the dorsal eye marked by a cauterizer. Tissue was pre-fixed in 4% PFA for 15 minutes and a small incision was made on the cornea. Tissue was further fixed in 4% PFA for 30 minutes, washed 3 times for 20 minutes in PBS, then submerged in 10% and 20% sucrose in PBS at 4°C. Tissue was embedded in OCT compound (Tissue-Tek, Cat #27050) and stored at −80°C. Coronal cryostat sections were made at 16 μm thickness and later processed for RNA in situ hybridization and immunostaining with antibodies listed in the table as previously described.35
EdU incorporation
Pregnant females were given intraperitoneal injection of EdU (2μl 10mM EdU/gram of body weight, Invitrogen #C10637) at specific time points as indicated in the figure legends. EdU staining was performed on cryostat section using Click-iT plus EdU Imaging Kits (Invitrogen 10637 and 10640) prior to immunostaining with antibodies.
Confocal microscopy
Confocal images were acquired on an inverted Nikon A1R Confocal Microscope. Images were acquired at 20X objective with a 3X digital zoom to obtain a 0.2 μm pixel resolution. Stacks through the Z plane were taken at 0.8 μm step distance through at total of about 16 μm, covering the entire thickness of the retina. Image acquisition settings were consistent across ages and genotypes.
RT-PCR
Total RNA was isolated from freshly dissected P0 retina using RNeasy Plus Mini Kit. cDNA was synthesized using SuperScript III first-strand synthesis superMix (Invitrogen 11,752–050) followed by PCR reaction using forward (AGTTGACTCTTCTGCTCGCAC) and reverse (CTCGACGGCCCTTCTTCAAA) primers (Figure S1C). After agarose gel electrophoresis, expected bands were excised and purified before DNA sequencing.
Bulk RNA sequencing
For bulk RNA-seq analysis of Foxp1 cKO, total RNA from E16.5 retinas was isolated using RNeasy Plus Mini Kit. Three biological replicates of each: Six3-Cre; Foxp1fl/fl animals and Foxp1fl/fl controls, all from the same litter, were used for RNA sequencing.
Library generation, sequencing, and alignment were performed by the Huntsman Cancer Institute High-Throughput Genomics and Bioinformatics Analysis Shared Resource. Total RNA concentration and quality were measured by RNA ScreenTape Assay (Agilent Technologies, 5067–5576, 5067–5577). All samples had a RIN value of 9.9 or better. The NEBNext Ultra II Directional RNA Library Prep Kit for Illumina with NEBNext rRNA Depletion Kit v2 (E7400) was used to generate libraries which were qualified by D1000 ScreenTape Assay (Agilent Technologies, 5067–5582, 5067–5583) and quantified with a Kapa Library Quant Kit (Kapa Biosystems, KK4824). Samples were sequenced on an Illumina NovaSeq 6000 instrument using the NovaSeq XP workflow (20043131). A 150 × 150 cycle paired end sequence run was performed using a NovaSeq 6000 S4 reagent Kit v1.5 (20028312).
To clean up the sequencing data for analysis, overlapping reads were grouped by BBTools v38.34 clumpify script,70 specified to remove duplicate reads and optical duplicates at a max distance of 12000. Illumina adapters were trimmed with cutadapt v2.871 using a minimum read length of 20 and minimum overlap between read and adaptor of 6, then aligned to mouse Ensembl genome annotation release 102 with STAR v2.7.6a in two pass mode.72 Reads were assigned to the target that had the largest overlap and uniquely aligned, reverse stranded, reads were counted with featureCounts v1.5.1.73 Genes with fewer than 5 counts in every sample were eliminated and differentially expressed genes were identified using DESeq2 v1.32.0.74 In addition to the variable of interest: genotype, the additional variable: sex, was included in the DESeq2 design formula to mitigate its effect on the results.
Single cell library preparation and sequencing
For single cell analysis of Jarid2 cKO, cells were obtained from two E18.5 tamoxifen-treated female littermates with the genotypes Jaridfl/fl; Rax-CreERT2 (cKO) and Jaridfl/fl (control). For single cell analysis in Foxp1 cTG, P0 retinas from female littermates with the genotypes Foxp1tg/+; Six3-Cre (Foxp1 cTG) and Foxp1tg/+ (control) were used. For each sample, two freshly dissected retinas from one individual animal were pooled and dissociated in PBS, 50 mM HEPES, 0.05 mg/ml DNase I and 0.025 mg/ml Liberase for 35 min with intermediate trituration. Cells were passed through a 40 μm nylon cell strainer, washed with washing buffer (1X PBS, 2% BSA, 0.1% sodium azide, 0.05% EDTA), and red blood cells were lysed in RBC lysis buffer. Cell counts were determined using a Countess. 1× 106 cells was resuspended in 1mL DPBS and provided to the High Throughput Genomics Core for library prep. This protocol was repeated on different days to provide cells for Jarid2 batch I, Jarid2 batch II and Foxp1 cTG.
Single cell libraries were generated with Single Cell 3′ Gene Expression Library Prep v3 reagents for batch I and Next GEM Single Cell 3′ Gene Expression Library prep v3.1 with UDI for batch II, according to manufacturer’s protocol (10X Genomics PN-1000092 and PN-1000128). Sequencing libraries were applied to a NovaSeq flow cell using XP chemistry workflow v1 and v1.5 for batch I and batch II samples respectively (Illumina 20,021,021664 and 20043131). A 150 × 150 cycle paired end sequence run was performed using an Illumina NovaSeq 6000 instrument and NovaSeq S2 reagent Kit for batch I or S4 reagent Kit v1.5 for batch II (Illumina 20012860 and 20028312). Batch I libraries were sequenced in two separate runs to a read depth of > 600 million reads per sample. Batch II libraries were sequenced to > 200 million reads per sample in a single run. Single cell library for Foxp1 cTG was prepared using the same protocol as Jarid2 batch II.
Analysis of single cell RNA-seq data
Single cell RNA-seq data was processed with the CellRanger software from 10X genomics. Briefly, sequencing reads from the Jarid2 experiment were aligned to the mm10 reference genome provided by 10X genomics (version 2020-A from Ensembl 98) while reads from Foxp1 experiment were aligned to a reference including the mm10 genome, GFP, and Cre. Feature-barcode matrices were generated using cellranger count v3.1.0 with expected-cells set to 5000. Spliced and unspliced transcripts counts were also generated from Jarid2 batch II single cell samples with velocyto v0.17.17.75 Total transcripts were used for all analyses unless specified otherwise.
Filtered feature matrices from CellRanger were further filtered with the aid of Seurat v4.0.3. High quality cells were identified by a mitochondrial percentage <7.5 and a high number of genes/features. The feature cut-off was determined independently for each sample by the local minimum cell density between 200 and 2000 features (con I = 1490, cKO I = 1845, con II = 1367, cKO II = 1481. Foxp1 con = 1000, Foxp1 cTG = 1050). Each sample was run, individually, through Seurat’s SCTransform pipeline, regressing out mitochondrial percentage, to generate unsupervised cell clusters. For Jarid2 samples DoubletFinder v2.0.3 identified likely doublets assuming 0.8% doublets in 1000 cells, adjusted for homotypic doublets.76 Doublets were excluded from downstream analysis.
Jarid2 batch I and batch II were combined into a single dataset using the Seurat’s integration anchor pipeline with principal components set to 30 and cluster resolution to 2.81 Foxp1 cTG single cell samples were combined with the merge function. Clusters were manually annotated using expression of known cell markers: Vsx2, Lhx2, and Pax6 for RPCs, Neurog2 and Olig2 for neurogenic cells, Crx for photoreceptor precursors, Thrb for cones, Nrl for rods, Rbpms for retinal ganglion cells, Tfap2a for amacrine cells, and Lhx1 for horizontal cells. Differential expression analysis was performed with Seurat FindMarkers, using logfc.threshold of 0.1. For Foxp1 cTG, markers were further narrowed by q-value <0.01. Using Jarid2 batch II samples only, the SCTranform pipeline was run on spliced transcripts, regressing out cell cycle scores, to obtain a UMAP representing mature transcriptional state. Cell type annotation for the spliced UMAP was carried over from the integrated dataset.
Gene set enrichment analysis was run on RPC gene expression from the Jarid2 cKO dataset and Foxp1 cTG dataset sperately using gene sets obtained from the Molecular Signatures Database.82 Specifically, we used the mouse-ortholog hallmark gene sets v2022.1. All detectable genes were ranked by average log2 fold change between Jarid2 control and cKO or between Foxp1 control and cTG, and analyzed with both fgsea v1.18.079 and gage v2.42.080 using default settings. We only marked gene sets as significant if they were significantly changed, in the same direction, by both methods. Q-values listed are from fgsea results.
Gene module analysis
To identify gene modules that vary across development, we utilized single cell sequencing data from whole retina across from E11 to P511 (GEO: GSE118614). Retinal progenitor cells, as defined in that study were analyzed with Seurat’s SCTransform function followed by dimensional reduction, with cell cycle differences and mitochondrial percentage regressed out of the model. Highly variable features, identified from the retinal progenitor cells, were grouped into 22 modules with Monocle functions.55 Normalized counts were summed for the genes in each module at each age, and scaled across the modules. Only those modules that varied across development, with a standard deviation range >1.25, were selected to display. We also eliminated module 22 for being driven by red blood cell transcripts. Separately, gene expression was aggregated from control and Jarid2 cKO RPCs across these same modules. These data were combined, and values were scaled across each age or condition to generate a heatmap and dendrogram. Hierarchical clustering was performed with hclust using method ward.D2.
CUT&RUN sample and library preparation
The H3K27me3 CUT&RUN was performed in two separate batches. In batch I, two biological replicates were used for control (Jarid2fl/fl) and conditional knockout (Rax-CreERT2; Jarid2fl/fl), one male and one female of each genotype, all from a single litter. Batch II consisted of all male littermates: three biological replicates, each with two technical duplicates, for a total of 12 samples. Single cell suspensions were made using the same method described for single cell sequencing. CUT&RUN83 was performed using a CUT&RUN Assay Kit according to manufacturer’s protocol. 300K single cells were used for each reaction. 2 μl rabbit anti-H3K27me3 antibody was used for H3K27me3 Immunoprecipitation. This antibody has been validated for specificity by ENCODE.84,85 5 μl Rabbit I (DA1E) mAb IgG XP Isotype Control provided by the kit was used as a negative control. DNA fragments were purified on spin columns, eluted with 50 μl buffer (Cell Signaling 14209) and provided to University of Utah High Throughput Genomics Core for library generation.
DNA quality was confirmed by PCR, libraries were created with the NEBNext UItra II DNA Library Prep Kit (New England Biolabs E7645S), and 150 bp paired-end sequencing was performed on a NovaSeq 6000 using the XP chemistry workflow (20021664) and S4 reagent Kit v1.5 (20028312).
CUT&RUN data analysis
All CUT&RUN samples were aligned to mouse genome build mm10 with Novoalign v4.02.02 using standard Illumina TruSeq adapters. Samtools fixmate v1.1077 removed unmapped reads and added mate scores, then bam files were sorted and indexed. Unique Molecular Indexes (UMIs) were sequenced to identify PCR duplicates, and the UMIs were handled by scripts written by the Huntsman Cancer Institute Bioinformatics Core. PCR and optical duplicates were removed with optical distance threshold at 2500. H3K27me3 broad peak calling was done with a Macs2-based pipeline,78 MultiRepMacsChIPSeq v17.7, developed by the University of Utah Bioinformatics Shared Resource (github.com/HuntsmanCancerInstitute/MultiRepMacsChIPSeq). Briefly, multirep_macs2_pipeline.pl was run with the following parameters: q-value threshold for peak calling set to 2, minimum peak size of 600, maximum gap size of 300, minimum mapping quality of 10, q-value cutoff of 0.1, maximum link size of 1500 and mappable genome size 2500000000,. The output of this pipeline includes fragment tracks, log2 fold enrichment of H3K27me3 relative to IgG, identification of called peaks, annotation with Ensembl release 102, and counts of fragments in each peak. Difference calling between control and Jarid2 cKO was done with DESeq2 v1.38.1, controlling for batch effect.
Wild type E14.5 and P0 CUT&RUN samples were prepared, sequenced and analyzed as described above, with the following specific changes: P0 H3K27me3 and P0 IgG samples were run in technical triplicate, E14.5 H3K27me3 was run in duplicate, and E14.5 IgG was a single sample. Only female pups were used to perform CUT&RUN for H3K27me3, and IgG. Samples were purified on DNA spin column (cell signaling #14209) and eluted with 35 μL elution buffer. 5 μL was used for PCR to validate sample quality and the rest used for library preparation. PCR showed specific bands with positive control primers for H3K27me3. MultiRepMacsChIPSeq was run with expected fragment size of 200 to obtain fragment and log2 fold enrichment tracks of H3K27me3 relative to IgG at E14.5 and P0.
For developmental time-course comparison, we obtained fastq files from29 (GEO: GSE87037). Specifically, input samples and ChIP-seq targeting H3K27me3 in whole murine retina at E17.5, P0 and P3 were used. Alignment to mm10 was performed as described above except that, as these are single-read samples, a mate score was not added. Broad H3K27me3 peaks were called for each replicate with the MultiRepMacsChIPSeq pipeline run as above with specific changes: no optical distance set and duplication rate set to 5%. Peaks from individual samples were merged into a single bed file for counting, and differential peak enrichment was done with DESeq2.
QUANTIFICATION AND STATISTICAL ANALYSIS
Confocal images shown are representative of at least 3 animals/6 retinas. For quantification, the number of retinal cells was quantified manually and blindly by averaging total positive cells within the central retinal region (~300 μm length) from 4–5 sections per retina using Nikon Elements software. For EdU birthdating analysis, the number of cells colocalizing EdU with different retinal markers was counted within the central retinal region (~1000 μm length) and averaged per retina. For cell-cycle exit analysis, EdU was injected 24 h before retina collection. Total EdU+ cells and EdU+PCNA-cells were counted within the central retinal region (~300 μm length) and the cell-cycle exit index was calculated as EdU+PCNA−/total EdU+.86 The index was then averaged from 3–4 sections per retina.
Statistical analysis was performed on all image data using GraphPad Prism software and the details (p values, sample sizes and statistical methods) can be found in Table S6. The sample size (n) is the number of experimental units which represents individual eyes. In each experiment, the sample size was determined to meet the criteria that statistical power would be more than 80% with the use of a two-tailed unpaired t test at the significance level (p value) of 0.05. All graph data are presented as mean ± standard error of the mean (SEM). In all graphs, dots represent individual retina. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. ns = not significant.
Supplementary Material
Highlights.
Loss of Jarid2 delays retinal progenitor early- to late-identity transition
Jarid2 mediates H3K27me3 deposition and repression of early genes including Foxp1
Foxp1 regulates the timing of early retinal cell production
Foxp1 is an effector of Jarid2-mediated temporal patterning
ACKNOWLEDGMENTS
This research was supported by the National Eye Institute award R01EY012274 (to M.L.V.). We thank Madelyn Lee for technical assistance. We thank Richard Dorsky, Issam Al Diri, Sarah Anderson, Mariana S. Silveira, Viviane Valenca, and Brad Cairns for providing comments on the manuscript. We thank Seth Blackshaw for providing Rax-CreERT2 mice and Hui Hu for providing Foxp1a transgenic mice. We thank the University of Utah Office of Comparative Medicine for animal husbandry. Research reported in this publication utilized the High-Throughput Genomics and Bioinformatic Analysis Shared Resource at Huntsman Cancer Institute in the University of Utah and was supported by the National Cancer Institute of the National Institutes of Health under award no. P30CA042014. The computational resources used were partially funded by the NIH Shared Instrumentation grant 1S10OD021644-01A1. Sanger DNA sequencing was performed at the DNA Sequencing Core Facility, University of Utah. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
DECLARATION OF INTERESTS
J.Z., J.M.B., F.C., and M.L.V. are co-inventors on a provisional patent (US Provisional Patent Serial No. 63/439,941) that incorporates technology described in this article.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.112237.
REFERENCES
- 1.Holguera I, and Desplan C. (2018). Neuronal specification in space and time. Science 362, 176–180. 10.1126/science.aas9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi AM, Fernandes VM, and Desplan C. (2017). Timing temporal transitions during brain development. Curr. Opin. Neurobiol 42, 84–92. 10.1016/j.conb.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam MA, and Harwell CC (2020). Epigenetic regulation of cortical neurogenesis; orchestrating fate switches at the right time and place. Curr. Opin. Neurobiol 63, 146–153. 10.1016/j.conb.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnefont J, and Vanderhaeghen P. (2021). Neuronal fate acquisition and specification: time for a change. Curr. Opin. Neurobiol 66, 195–204. 10.1016/j.conb.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cepko C. (2014). Intrinsically different retinal progenitor cells produce specific types of progeny. Nat. Rev. Neurosci 15, 615–627. 10.1038/nrn3767. [DOI] [PubMed] [Google Scholar]
- 6.Ray A, Zhu H, Ding A, and Li X. (2022). Transcriptional and epigenetic regulation of temporal patterning in neural progenitors. Dev. Biol 481, 116–128. 10.1016/j.ydbio.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petridou E, and Godinho L. (2022). Cellular and molecular determinants of retinal cell fate. Annu. Rev. Vis. Sci 8, 79–99. 10.1146/annurev-vision-100820-103154. [DOI] [PubMed] [Google Scholar]
- 8.Mattar P, and Cayouette M. (2015). Mechanisms of temporal identity regulation in mouse retinal progenitor cells. Neurogenesis (Austin) 2, e1125409. 10.1080/23262133.2015.1125409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belliveau MJ, Young TL, and Cepko CL (2000). Late retinal progenitor cells show intrinsic limitations in the production of cell types and the kinetics of opsin synthesis. J. Neurosci 20, 2247–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cayouette M, Barres BA, and Raff M. (2003). Importance of intrinsic mechanisms in cell fate decisions in the developing rat retina. Neuron 40, 897–904. 10.1016/s0896-6273(03)00756-6. [DOI] [PubMed] [Google Scholar]
- 11.Clark BS, Stein-O’Brien GL, Shiau F, Cannon GH, Davis-Marcisak E, Sherman T, Santiago CP, Hoang TV, Rajaii F, James-Esposito RE, et al. (2019). Single-cell RNA-seq analysis of retinal development identifies NFI factors as regulating mitotic exit and late-born cell specification. Neuron 102, 1111–1126.e5. 10.1016/j.neuron.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Shiau F, Yi W, Lu S, Wu Q, Pearson JD, Kallman A, Zhong S, Hoang T, Zuo Z, et al. (2020). Single-cell analysis of human retina identifies evolutionarily conserved and species-specific mechanisms controlling development. Dev. Cell 53, 473–491.e9. 10.1016/j.devcel.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyu P, Hoang T, Santiago CP, Thomas ED, Timms AE, Appel H, Gimmen M, Le N, Jiang L, Kim DW, et al. (2021). Gene regulatory networks controlling temporal patterning, neurogenesis, and cell-fate specification in mammalian retina. Cell Rep. 37, 109994. 10.1016/j.celrep.2021.109994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott J, Jolicoeur C, Ramamurthy V, and Cayouette M. (2008). Ikaros confers early temporal competence to mouse retinal progenitor cells. Neuron 60, 26–39. 10.1016/j.neuron.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Liu X, Li S, Huang X, Qian H, Jin K, and Xiang M. (2020). Foxn4 is a temporal identity factor conferring mid/late-early retinal competence and involved in retinal synaptogenesis. Proc. Natl. Acad. Sci. USA 117, 5016–5027. 10.1073/pnas.1918628117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattar P, Ericson J, Blackshaw S, and Cayouette M. (2015). A conserved regulatory logic controls temporal identity in mouse neural progenitors. Neuron 85, 497–504. 10.1016/j.neuron.2014.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattar P, Jolicoeur C, Dang T, Shah S, Clark BS, and Cayouette M. (2021). A Casz1-NuRD complex regulates temporal identity transitions in neural progenitors. Sci. Rep 11, 3858. 10.1038/s41598-021-83395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javed A, Mattar P, Lu S, Kruczek K, Kloc M, Gonzalez-Cordero A, Bremner R, Ali RR, and Cayouette M. (2020). Pou2f1 and Pou2f2 Cooperate to Control the Timing of Cone Photoreceptor Production in the Developing Mouse Retina. Development 147. 10.1242/dev.188730. [DOI] [PubMed] [Google Scholar]
- 19.Javed A, Santos-França PL, Mattar P, Cui A, Kassem F, and Cayouette M. (2023). Ikaros family proteins redundantly regulate temporal patterning in the developing mouse retina. Development 150, dev200436. 10.1242/dev.200436. [DOI] [PubMed] [Google Scholar]
- 20.La Torre A, Georgi S, and Reh TA (2013). Conserved microRNA pathway regulates developmental timing of retinal neurogenesis. Proc. Natl. Acad. Sci. USA 110, E2362–E2370. 10.1073/pnas.1301837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decembrini S, Andreazzoli M, Barsacchi G, and Cremisi F. (2008). Dicer inactivation causes heterochronic retinogenesis in Xenopus laevis. Int. J. Dev. Biol 52, 1099–1103. 10.1387/ijdb.082646sd. [DOI] [PubMed] [Google Scholar]
- 22.Decembrini S, Bressan D, Vignali R, Pitto L, Mariotti S, Rainaldi G, Wang X, Evangelista M, Barsacchi G, and Cremisi F. (2009). MicroRNAs couple cell fate and developmental timing in retina. Proc. Natl. Acad. Sci. USA 106, 21179–21184. 10.1073/pnas.0909167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgi SA, and Reh TA (2010). Dicer is required for the transition from early to late progenitor state in the developing mouse retina. J. Neurosci 30, 4048–4061. 10.1523/JNEUROSCI.4982-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon PJ, Yun S, Clark AM, Monuki ES, Murtaugh LC, and Levine EM (2013). Lhx2 balances progenitor maintenance with neurogenic output and promotes competence state progression in the developing retina. J. Neurosci 33, 12197–12207. 10.1523/JNEUROSCI.1494-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zibetti C, Liu S, Wan J, Qian J, and Blackshaw S. (2019). Epigenomic profiling of retinal progenitors reveals LHX2 is required for developmental regulation of open chromatin. Commun. Biol 2, 142. 10.1038/s42003-019-0375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon KJ, Vissers C, Ming GL, and Song H. (2018). Epigenetics and epitranscriptomics in temporal patterning of cortical neural progenitor competence. J. Cell Biol 217, 1901–1914. 10.1083/jcb.201802117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira JD, Sansom SN, Smith J, Dobenecker MW, Tarakhovsky A, and Livesey FJ (2010). Ezh2, the histone methyltransferase of PRC2, regulates the balance between self-renewal and differentiation in the cerebral cortex. Proc. Natl. Acad. Sci. USA 107, 15957–15962. 10.1073/pnas.1002530107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raeisossadati R, Ferrari MFR, Kihara AH, AlDiri I, and Gross JM (2021). Epigenetic regulation of retinal development. Epigenet. Chromatin 14, 11. 10.1186/s13072-021-00384-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldiri I, Xu B, Wang L, Chen X, Hiler D, Griffiths L, Valentine M, Shirinifard A, Thiagarajan S, Sablauer A, et al. (2017). The dynamic epigenetic landscape of the retina during development, reprogramming, and tumorigenesis. Neuron 94, 550–568.e10. 10.1016/j.neuron.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margueron R, and Reinberg D. (2011). The Polycomb complex PRC2 and its mark in life. Nature 469, 343–349. 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu PP, Xu YJ, Teng ZQ, and Liu CM (2018). Polycomb repressive complex 2: emerging roles in the central nervous system. Neuroscientist 24, 208–220. 10.1177/1073858417747839. [DOI] [PubMed] [Google Scholar]
- 32.Jambhekar A, Dhall A, and Shi Y. (2019). Roles and regulation of histone methylation in animal development. Nat. Rev. Mol. Cell Biol 20, 625–641. 10.1038/s41580-019-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujimura N, Kuzelova A, Ebert A, Strnad H, Lachova J, Machon O, Busslinger M, and Kozmik Z. (2018). Polycomb repression complex 2 is required for the maintenance of retinal progenitor cells and balanced retinal differentiation. Dev. Biol 433, 47–60. 10.1016/j.ydbio.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Iida A, Iwagawa T, Kuribayashi H, Satoh S, Mochizuki Y, Baba Y, Nakauchi H, Furukawa T, Koseki H, Murakami A, and Watanabe S. (2014). Histone demethylase Jmjd3 is required for the development of subsets of retinal bipolar cells. Proc. Natl. Acad. Sci. USA 111, 3751–3756. 10.1073/pnas.1311480111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Taylor RJ, La Torre A, Wilken MS, Cox KE, Reh TA, and Vetter ML (2015). Ezh2 maintains retinal progenitor proliferation, transcriptional integrity, and the timing of late differentiation. Dev. Biol 403, 128–138. 10.1016/j.ydbio.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Mierlo G, Veenstra GJC, Vermeulen M, and Marks H. (2019). The complexity of PRC2 subcomplexes. Trends Cell Biol. 29, 660–671. 10.1016/j.tcb.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Højfeldt JW, Hedehus L, Laugesen A, Tatar T, Wiehle L, and Helin K. (2019). Non-core subunits of the PRC2 complex are collectively required for its target-site specificity. Mol. Cell 76, 423–436.e3. 10.1016/j.molcel.2019.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasinath V, Beck C, Sauer P, Poepsel S, Kosmatka J, Faini M, Toso D, Aebersold R, and Nogales E. (2021). JARID2 and AEBP2 regulate PRC2 in the presence of H2AK119ub1 and other histone modifications. Science 371, eabc3393. 10.1126/science.abc3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petracovici A, and Bonasio R. (2021). Distinct PRC2 subunits regulate maintenance and establishment of Polycomb repression during differentiation. Mol. Cell 81, 2625–2639.e5. 10.1016/j.molcel.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youmans DT, Gooding AR, Dowell RD, and Cech TR (2021). Competition between PRC2.1 and 2.2 subcomplexes regulates PRC2 chromatin occupancy in human stem cells. Mol. Cell 81, 488–501.e9. 10.1016/j.molcel.2020.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mysliwiec MR, Carlson CD, Tietjen J, Hung H, Ansari AZ, and Lee Y. (2012). Jarid2 (Jumonji, AT rich interactive domain 2) regulates NOTCH1 expression via histone modification in the developing heart. J. Biol. Chem 287, 1235–1241. 10.1074/jbc.M111.315945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shirato H, Ogawa S, Nakajima K, Inagawa M, Kojima M, Tachibana M, Shinkai Y, and Takeuchi T. (2009). A jumonji (Jarid2) protein complex represses cyclin D1 expression by methylation of histone H3-K9. J. Biol. Chem 284, 733–739. 10.1074/jbc.M804994200. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi T, Yamazaki Y, Katoh-Fukui Y, Tsuchiya R, Kondo S, Motoyama J, and Higashinakagawa T. (1995). Gene trap capture of a novel mouse gene, jumonji, required for neural tube formation. Genes Dev. 9, 1211–1222. [DOI] [PubMed] [Google Scholar]
- 44.Landeira D, and Fisher AG (2011). Inactive yet indispensable: the tale of Jarid2. Trends Cell Biol. 21, 74–80. 10.1016/j.tcb.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasini D, Cloos PAC, Walfridsson J, Olsson L, Bukowski JP, Johansen JV, Bak M, Tommerup N, Rappsilber J, and Helin K. (2010). JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature 464, 306–310. 10.1038/nature08788. [DOI] [PubMed] [Google Scholar]
- 46.Jung J, Mysliwiec MR, and Lee Y. (2005). Roles of JUMONJI in mouse embryonic development. Dev. Dynam 232, 21–32, an official publication of the American Association of Anatomists. 10.1002/dvdy.20204. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Chen G, Norton N, Liu W, Zhu H, Zhou P, Luan M, Yang S, Chen X, Carroll L, et al. (2009). Whole genome association study in a homogenous population in Shandong peninsula of China reveals JARID2 as a susceptibility gene for schizophrenia. J. Biomed. Biotechnol 2009, 536918. 10.1155/2009/536918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mo W, Liu J, Zhang Z, Yu H, Yang A, Qu F, Hu P, Liu Z, and Hu F. (2018). A study of single nucleotide polymorphisms in CD157, AIM2 and JARID2 genes in Han Chinese children with autism spectrum disorder. Nord. J. Psychiatr 72, 179–183. 10.1080/08039488.2017.1410570. [DOI] [PubMed] [Google Scholar]
- 49.Pedrosa E, Ye K, Nolan KA, Morrell L, Okun JM, Persky AD, Saito T, and Lachman HM (2007). Positive association of schizophrenia to JARID2 gene. Am. J. Med. Genet. B Neuropsychiatr. Genet 144B, 45–51. 10.1002/ajmg.b.30386. [DOI] [PubMed] [Google Scholar]
- 50.Ramos PS, Sajuthi S, Langefeld CD, and Walker SJ (2012). Immune function genes CD99L2, JARID2 and TPO show association with autism spectrum disorder. Mol. Autism 3, 4. 10.1186/2040-2392-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verberne EA, Goh S, England J, van Ginkel M, Rafael-Croes L, Maas S, Polstra A, Zarate YA, Bosanko KA, Pechter KB, et al. (2021). JARID2 haploinsufficiency is associated with a clinically distinct neurodevelopmental syndrome. Genet. Med 23, 374–383. 10.1038/s41436-020-00992-z. [DOI] [PubMed] [Google Scholar]
- 52.Furuta Y, Lagutin O, Hogan BL, and Oliver GC (2000). Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis 26, 130–132. [PubMed] [Google Scholar]
- 53.Pak T, Yoo S, Miranda-Angulo AL, Wang H, and Blackshaw S. (2014). Rax-CreERT2 knock-in mice: a tool for selective and conditional gene deletion in progenitor cells and radial glia of the retina and hypothalamus. PLoS One 9, e90381. 10.1371/journal.pone.0090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young RW (1985). Cell differentiation in the retina of the mouse. Anat. Rec 212, 199–205. 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 55.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, and Trapnell C. (2017). Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982. 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng JC, Valouev A, Swigut T, Zhang J, Zhao Y, Sidow A, and Wysocka J. (2009). Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell 139, 1290–1302. 10.1016/j.cell.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pearson CA, Moore DM, Tucker HO, Dekker JD, Hu H, Miquela-jáuregui A, and Novitch BG (2020). Foxp1 regulates neural stem cell self-renewal and bias toward deep layer cortical fates. Cell Rep. 30, 1964–1981.e3. 10.1016/j.celrep.2020.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki-Kerr H, Baba Y, Tsuhako A, Koso H, Dekker JD, Tucker HO, Kuribayashi H, and Watanabe S. (2017). Forkhead box protein P1 is dispensable for retina but essential for lens development. Invest. Ophthalmol. Vis. Sci 58, 1916–1929. 10.1167/iovs.1620085. [DOI] [PubMed] [Google Scholar]
- 59.Rousso DL, Qiao M, Kagan RD, Yamagata M, Palmiter RD, and Sanes JR (2016). Two pairs of on and off retinal ganglion cells are defined by intersectional patterns of transcription factor expression. Cell Rep. 15, 1930–1944. 10.1016/j.celrep.2016.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng X, Ippolito GC, Tian L, Wiehagen K, Oh S, Sambandam A, Willen J, Bunte RM, Maika SD, Harriss JV, et al. (2010). Foxp1 is an essential transcriptional regulator for the generation of quiescent naive T cells during thymocyte development. Blood 115, 510–518. 10.1182/blood-2009-07-232694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Geng J, Wen X, Bi E, Kossenkov AV, Wolf AI, Tas J, Choi YS, Takata H, Day TJ, et al. (2014). The transcription factor Foxp1 is a critical negative regulator of the differentiation of follicular helper T cells. Nat. Immunol 15, 667–675. 10.1038/ni.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, and Mesirov JP (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550. 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fong KW, Zhao JC, Lu X, Kim J, Piunti A, Shilatifard A, and Yu J. (2022). PALI1 promotes tumor growth through competitive recruitment of PRC2 to G9A-target chromatin for dual epigenetic silencing. Mol. Cell 82, 4611–4626.e7. 10.1016/j.molcel.2022.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Co M, Anderson AG, and Konopka G. (2020). FOXP transcription factors in vertebrate brain development, function, and disorders. Wiley Interdiscip Rev Dev Biol 9, e375. 10.1002/wdev.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Averbukh I, Lai SL, Doe CQ, and Barkai N. (2018). A repressor-decay timer for robust temporal patterning in embryonic Drosophila neuroblast lineages. Elife 7, e38631. 10.7554/eLife.38631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gan L, Yang Y, Li Q, Feng Y, Liu T, and Guo W. (2018). Epigenetic regulation of cancer progression by EZH2: from biological insights to therapeutic potential. Biomark. Res 6, 10. 10.1186/s40364018-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao L, Li J, Ma Y, Wang J, Pan W, Gao K, Zhang Z, Lu T, Ruan Y, Yue W, et al. (2015). Ezh2 is involved in radial neuronal migration through regulating Reelin expression in cerebral cortex. Sci. Rep 5, 15484. 10.1038/srep15484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Touma JJ, Weckerle FF, and Cleary MD (2012). Drosophila Polycomb complexes restrict neuroblast competence to generate motoneurons. Development 139, 657–666. 10.1242/dev.071589. [DOI] [PubMed] [Google Scholar]
- 69.Hirabayashi Y, Suzki N, Tsuboi M, Endo TA, Toyoda T, Shinga J, Koseki H, Vidal M, and Gotoh Y. (2009). Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron 63, 600–613. 10.1016/j.neuron.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 70.Bushnell B, Rood J, and Singer E. (2017). BBMerge - accurate paired shotgun read merging via overlap. PLoS One 12, e0185056. 10.1371/journal.pone.0185056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. j 17, 10–12. [Google Scholar]
- 72.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liao Y, Smyth GK, and Shi W. (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 74.Love MI, Huber W, and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.La Manno G, Soldatov R, Zeisel A, Braun E, Hochgerner H, Petukhov V, Lidschreiber K, Kastriti ME, Lönnerberg P, Furlan A, et al. (2018). RNA velocity of single cells. Nature 560, 494–498. 10.1038/s41586-018-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGinnis CS, Murrow LM, and Gartner ZJ (2019). DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest Neighbors. Cell Syst. 8, 329–337.e4. 10.1016/j.cels.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM, and Li H. (2021). Twelve years of SAMtools and BCFtools. GigaScience 10, giab008. 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, and Liu XS (2008). Model-based analysis of ChIP-seq (MACS). Genome Biol. 9, R137. 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Korotkevich G, Sukhov V, Budin N, Shpak B, Artyomov MN, and Sergushichev A. (2021). Fast gene set enrichment analysis. Preprint at bioRxiv. 10.1101/060012. [DOI] [Google Scholar]
- 80.Luo W, Friedman MS, Shedden K, Hankenson KD, and Woolf PJ (2009). GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinf. 10, 161. 10.1186/1471-2105-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, and Satija R. (2019). Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21. 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, and Tamayo P. (2015). The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425. 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skene PJ, and Henikoff S. (2017). An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. Elife 6, e21856. 10.7554/eLife.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davis CA, Hitz BC, Sloan CA, Chan ET, Davidson JM, Gabdank I, Hilton JA, Jain K, Baymuradov UK, Narayanan AK, et al. (2018). The Encyclopedia of DNA elements (ENCODE): data portal update. Nucleic Acids Res. 46, D794–D801. 10.1093/nar/gkx1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sloan CA, Chan ET, Davidson JM, Malladi VS, Strattan JS, Hitz BC, Gabdank I, Narayanan AK, Ho M, Lee BT, et al. (2016). ENCODE data at the ENCODE portal. Nucleic Acids Res. 44, D726–D732. 10.1093/nar/gkv1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Farhy C, Elgart M, Shapira Z, Oron-Karni V, Yaron O, Menuchin Y, Rechavi G, and Ashery-Padan R. (2013). Pax6 is required for normal cell-cycle exit and the differentiation kinetics of retinal progenitor cells. PLoS One 8, e76489. 10.1371/journal.pone.0076489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw and processed bulk RNA-seq, single cell RNA-seq, and CUT&RUN data generated for this study have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. Additionally, this paper analyzes existing, publicly available data and the accession numbers for these datasets are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| Goat anti-Brn3 (1:50) | Santa Cruz Biotechnology | Cat#sc6026; RRID: AB_673441 |
| Mouse anti-Brn3a (1:200) | Millipore | Cat#MAB1585; RRID: AB_94166 |
| Mouse anti-Otx2 (1:200) | Santa Cruz Biotechnology | Cat#sc-514195 |
| Rabbit anti-calbindin D-28K (1:2000) | Millipore | Cat#PC253L; RRID: AB_213554 |
| Mouse anti-Rxrγ (1:250) | Santa Cruz Biotechnology | Cat#sc-365252; RRID: AB_10850062 |
| Rabbit anti-recoverin (1:2000) | Millipore | Cat#AB5585; RRID: AB_2253622 |
| Goat anti-Vsx2/Chx10 (1:500) | gift from Dr. Edward M. Levine | N/A |
| Sheep anti-Vsx2/Chx10 (1:500) | Exalpha Biologicals | Cat#X1180P; RRID: AB_2314191 |
| Mouse anti-Rhodopsin (1:200) | Thermo Fisher Scientific | Cat#MA5–11741; RRID: AB_10980983 |
| Rabbit anti-Lhx2 (1:500) | Abcam | Cat# ab184337, RRID: AB_2916270 |
| Mouse anti-Cralbp (1:500) | Novus | Cat# NB100–74392, RRID: AB_2253681 |
| Mouse anti AP-2alpha (1:500) | Santa Cruz Biotechnology | Cat#sc-12726, RRID: AB_667767 |
| Mouse anti-PCNA (1:2000) | Sigma-Aldrich | Cat#P8825, RRID: AB_477413 |
| Rabbit anti-pH3 (Ser10) (1:250) | Millipore | Cat#06–570; RRID: AB_310177 |
| Rabbit anti-Foxp1 (1:2000) | Abcam | Cat#ab16645; RRID: AB_732428 |
| Rabbit anti-H3K27me3 | Millipore | Cat#07–449; RRID: AB_310624 |
| Sheep anti-Onecut2 (1:100) | R&D Systems | Cat#AF6294; RRID: AB_10640365 |
| Donkey anti-rabbit 488 (1:400) | Thermo Fisher Scientific | Cat#A-21206, RRID: AB_2535792 |
| Donkey anti-rabbit 555 (1:400) | Thermo Fisher Scientific | Cat#A31572; RRID: AB_162543 |
| Donkey anti-mouse 488 (1:400) | Thermo Fisher Scientific | Cat#A-21202, RRID: AB_141607 |
| Donkey anti-mouse 555 (1:400) | Thermo Fisher Scientific | Cat#A-31570, RRID: AB_2536180 |
| Donkey anti-sheep 555 (1:400) | Thermo Fisher Scientific | Cat#A-21436, RRID: AB_2535857 |
| Donkey anti-goat 488 (1:400) | Thermo Fisher Scientific | Cat#A-11055, RRID: AB_2534102 |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| DNAse I | Sigma-Aldrich | Cat#D4513 |
| Liberase | Sigma-Aldrich | Cat#5401119001 |
| RBC lysis buffer | eBioscience | Cat#00–4333-57 |
| Tamoxifen | Sigma-Aldrich | Cat#T5648 |
|
| ||
| Critical commercial assays | ||
|
| ||
| Click-iT plus EdU Imaging Kits | Thermo Fisher Scientific | Cat#C10637 |
| RNeasy Plus Mini Kit | Qiagen | Cat#74134 |
| CUT&RUN Assay Kit | Cell Signaling | Cat#86652 |
| Spin column Kit for Cut&Run | Cell Signaling | Cat#14209 |
| RNA ScreenTape Assay | Agilent Technologies | Cat#5067–5576, 5067–5577, 5067–5578 |
| D1000 ScreenTape Assay | Agilent Technologies | Cat#5067–5582, 5067–5583 |
| Kapa Library Quant Kit | Kapa Biosystems | Cat#KK4824 |
| NEBNext Ultra II Directional RNA Library Prep Kit for Illumina | New England Biolabs | Cat#E7760 |
| NEBNext rRNA Depletion Kit v2 | New England Biolabs | Cat#E7400 |
| NEBNext UItra II DNA Library Prep Kit for Illumina | New England Biolabs | Cat#E7645 |
| Chromium Single Cell 3′ Library Prep Kit v3 | 10X Genomics | Cat#PN-1000092 |
| Chromium Next GEM Single Cell 3′ Library Prep Kit v3.1 |
10X Genomics | Cat#PN-1000128 |
| NovaSeq XP workflow v1 and v1.5 | Illumina | Cat#20021664, 20043131 |
| NovaSeq S2 and S4 Reagent Kits | Illumina | Cat#20012860, 20028312 |
|
| ||
| Deposited data | ||
|
| ||
| All raw and analyzed data generated for this study | This paper | GEO: GSE202741 |
| Jarid2 cKO scRNA-seq | This paper | GEO: GSE202734 |
| H3K27me3 CUT&RUN from Jarid2 cKO | This paper | GEO: GSE222955 |
| H3K27me3 CUT&RUN from E14.5 and P0 retina | This paper | GEO: GSE202728 |
| Foxp1 cKO RNA-seq | This paper | GEO: GSE202725 |
| Foxp1 cTG scRNA-seq | This paper | GEO: GSE222956 |
| Single Cell-seq of whole retina E11.5 to P5 | Clark et al.11 | GEO: GSE118614 |
| H3K27me3 ChIP-seq from E17.5, P0, and P3 retina | Aldiri et al.29 | GEO: GSE87037 |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| C57BL/6N-Jarid2tm1a(KOMP)Wtsi/Mmucd mice | KOMP Repository | RRID: MMRRC_048262-UCD |
| B6.Cg-Tg(ACTFLPe)9205Dym/J mice | Jackson Laboratory | RRID: IMSR_JAX:005703 |
| Tg(Six3-Cre)69Frty mice | Dr. Yasuhide Furuta (Furuta et al.52) | RRID: IMSR_JAX:019755 |
| Raxtm1.1(cre/ERT2)Sbls/J mice | Dr. Seth Blackshaw (Pak et al.53) | RRID: IMSR_JAX:025521 |
| Foxp1flox/flox mice | Jackson Laboratory (Feng et al.60) | RRID: IMSR_JAX:017699 |
| Foxp1a transgenic mice | Dr. Hui Hu (Wang et al.61) | MGI:5607369 |
|
| ||
| Software and algorithms | ||
|
| ||
| BBTools v38.34 | Bushnell et al.70 | RRID:SCR_016968 |
| cutadapt v1.16 and v2.8 | Martin71 | RRID:SCR_011841 |
| STAR v2.7.0f and v2.7.6a | Dobin et al.72 | RRID:SCR_004463 |
| featureCounts v1.5.1 | Liao et al.73 | RRID:SCR_012919 |
| DESeq2 v1.38.1 | Love et al.74 | RRID:SCR_015687 |
| CellRanger v3.1.0 | 10X Genomics | RRID:SCR_023221 |
| velocyto v0.17.17 | La Manno et al.75 | RRID:SCR_018167 |
| Seurat v4.0.3 | Stuart et al.87 | RRID:SCR_007322 |
| DoubletFinder v2.0.3 | McGinnis et al.76 | RRID:SCR_018771 |
| Monocle3 v1.0.0 | Qiu et al.55 | RRID:SCR_018685 |
| Novoalign v4.02.02 | Novocraft | RRID:SCR_014818 |
| Samtools v1.10 | Danecek et al.77 | RRID:SCR_002105 |
| Macs2 v2.2.7.1 | Zhang et al.78 | RRID:SCR_013291 |
| Fgsea v1.18.0 | Korotkevich et al.79 | RRID:SCR_020938 |
| Gage v2.42.0 | Luo et al.80 | RRID:SCR_017067 |
| GraphPad Prism v9 | GraphPad | RRID:SCR_002798 |
| NIS-Elements v18 | Nikon | RRID:SCR_014329 |