Abstract
Background
Systematic reviews and meta-analyses of randomized clinical trials (RCTs) have reported the benefits of ketogenic diets (KD) in various participants such as patients with epilepsy and adults with overweight or obesity. Nevertheless, there has been little synthesis of the strength and quality of this evidence in aggregate.
Methods
To grade the evidence from published meta-analyses of RCTs that assessed the association of KD, ketogenic low-carbohydrate high-fat diet (K-LCHF), and very low-calorie KD (VLCKD) with health outcomes, PubMed, EMBASE, Epistemonikos, and Cochrane database of systematic reviews were searched up to February 15, 2023. Meta-analyses of RCTs of KD were included. Meta-analyses were re-performed using a random-effects model. The quality of evidence per association provided in meta-analyses was rated by the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) criteria as high, moderate, low, and very low.
Results
We included 17 meta-analyses comprising 68 RCTs (median [interquartile range, IQR] sample size of 42 [20–104] participants and follow-up period of 13 [8–36] weeks) and 115 unique associations. There were 51 statistically significant associations (44%) of which four associations were supported by high-quality evidence (reduced triglyceride (n = 2), seizure frequency (n = 1) and increased low-density lipoprotein cholesterol (LDL-C) (n = 1)) and four associations supported by moderate-quality evidence (decrease in body weight, respiratory exchange ratio (RER), hemoglobin A1c, and increased total cholesterol). The remaining associations were supported by very low (26 associations) to low (17 associations) quality evidence. In overweight or obese adults, VLCKD was significantly associated with improvement in anthropometric and cardiometabolic outcomes without worsening muscle mass, LDL-C, and total cholesterol. K-LCHF was associated with reduced body weight and body fat percentage, but also reduced muscle mass in healthy participants.
Conclusions
This umbrella review found beneficial associations of KD supported by moderate to high-quality evidence on seizure and several cardiometabolic parameters. However, KD was associated with a clinically meaningful increase in LDL-C. Clinical trials with long-term follow-up are warranted to investigate whether the short-term effects of KD will translate to beneficial effects on clinical outcomes such as cardiovascular events and mortality.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-023-02874-y.
Keywords: Ketegenic diet, Umbrella review, Systematic review, Meta-analysis, Weight loss, Seizure
Background
Ketogenic diets (KD) have received substantial attention from the public primarily due to their ability to produce rapid weight loss in the short run [1, 2]. The KD eating pattern severely restricts carbohydrate intake to less than 50 g/day while increasing protein and fat intake [3–6]. Carbohydrate deprivation leads to an increase in circulating ketone bodies by breaking down fatty acids and ketogenic amino acids. Ketones are an alternative energy source from carbohydrates that alter physiological adaptations. These adaptions have been shown to produce weight loss with beneficial health effects by improving glycemic and lipid profiles [7, 8]. KD has also been recommended as a nonpharmacological treatment for medication-refractory epilepsy in children and adults [8, 9]. Evidence suggests that KD has reduced seizure frequency in patients with medication-refractory epilepsy, and even allowing some patients to reach complete and sustained remission.11 However, the exact anticonvulsive mechanism of KD remains unclear [10, 11].
Several systematic reviews and meta-analyses of randomized clinical trials (RCTs) have reported on the use of KD in patients with obesity or type 2 diabetes mellitus (T2DM) to control weight and improve cardiometabolic parameters [1, 12–15], in patients with refractory epilepsy to reduce seizure frequency [16], and in athletes to control weight and improve performance [17]. To date, there has been little synthesis of the strength and quality of this evidence in aggregate. This umbrella review therefore aims to systematically identify relevant meta-analyses of RCTs of KD, summarize their findings, and assess the strength of evidence of the effects of KD on health outcomes.
Methods
The protocol of this study was registered with PROSPERO (CRD42022334717). We reported following the 2020 Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (Additional file 1) [18]. Difference from the original review protocol is described with rationale in Additional file 2: Table S1.
Search strategy and eligibility criteria
We searched PubMed, EMBASE, Epistemonikos, and the Cochrane database of systematic reviews (CDSR) from the database inception to February 15, 2023 (Additional file 2: Table S2). No language restriction was applied. Study selection was independently performed in EndNote by two reviewers (C.P. and PS). After removing duplicates, the identified articles' titles and abstracts were screened for relevance. Full-text articles of the potentially eligible articles were retrieved and selected against the eligibility criteria. Any discrepancies were resolved by discussion with the third reviewer (SKV).
We included studies that met the following eligibility criteria: systematic reviews and meta-analyses of RCTs investigating the effects of any type of KD on any health outcomes in participants with or without any medical conditions compared with any comparators. When more than 1 meta-analysis was available for the same research question, we selected the meta-analysis with the largest data set [19–21]. Articles without full-text and meta-analyses that provided insufficient or inadequate data for quantitative synthesis were excluded.
Data extraction and quality assessment
Two reviewers (CP and PS) independently performed data extraction and quality assessment (Additional file 2: Method S1). Discrepancies were resolved with consensus by discussing with the third reviewer (SKV). We used AMSTAR- 2 -A Measurement Tool to Assess Systematic Reviews- to grade the quality of meta-analyses as high, moderate, low, or critically low by assessing the following elements, research question, a priori protocol, search, study selection, data extraction, quality assessment, data analysis, interpretation, heterogeneity, publication bias, source of funding, conflict of interest [22].
Data synthesis
For each association, we extracted effect sizes (mean difference [MD], the standardized mean difference [SMD], and risk ratio [RR]) of individual studies included in each meta-analysis and performed the meta-analyses to calculate the pooled effect sizes and 95% CIs using a random-effects model under DerSimonian and Laird [23], or the Hartung-Knapp- Sidik-Jonkman approach for meta-analyses with less than five studies [24]. p < 0.05 was considered statistically significant in 2-sided tests. Heterogeneity was evaluated using the I2 statistic. The evidence for small-study effects was assessed by the Egger regression asymmetry test [25]. Statistical analyses were conducted using Stata version 16.0 (StataCorp). We presented effect sizes of statistically significant associations with the known or estimated minimally clinically important difference (MCID) thresholds for health outcomes [14, 26–30].
We assessed the quality of evidence per association by applying the GRADE criteria (Grading of Recommendations, Assessment, Development, and Evaluations) in five domains, including (1) risk of bias in the individual studies, (2) inconsistency, (3) indirectness, (4) imprecision, and (5) publication bias [31]. We graded the strength of evidence (high, moderate, low, and very low) using GRADEpro version 3.6.1 (McMaster University).
Sensitivity analyses
Sensitivity analyses were performed by excluding small-size studies (< 25th percentile) [32] and excluding primary studies having a high risk of bias rated by the Cochrane’s risk of bias 2 tool (RoB 2) for RCTs from the identified associations [19–21, 33].
Results
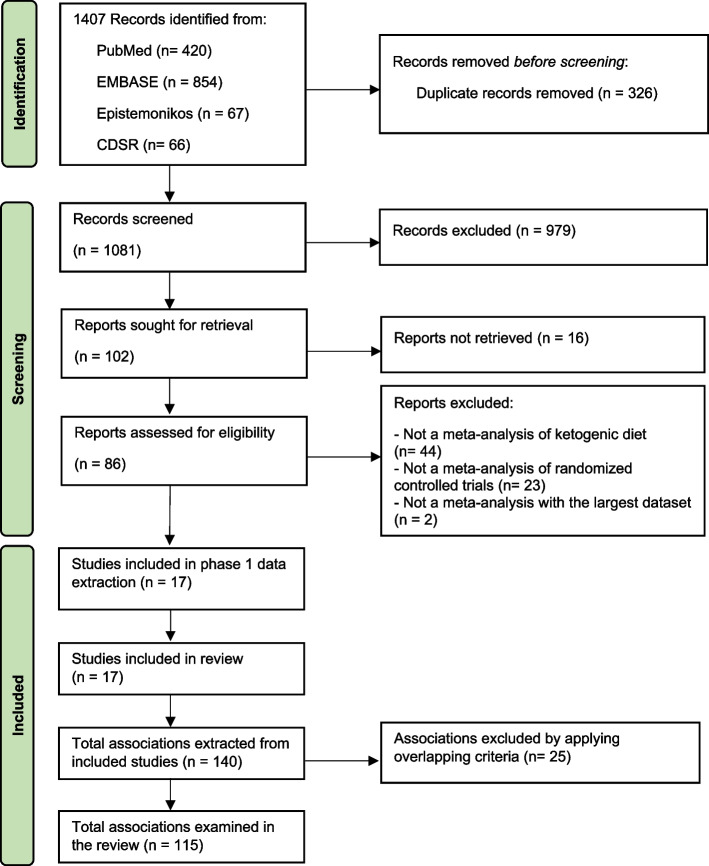
Seventeen meta-analyses were included (Fig. 1 and Additional file 2: Table S3) [1, 2, 15–17, 34–45]. These meta-analyses comprised 68 unique RCTs with a median (interquartile range, IQR) sample size per RCT of 42 (20–104) participants and a median (IQR) follow-up period of 13 (8–36) weeks. The quality of meta-analyses assessed using AMSTAR-2 found that none were rated as high confidence, 2 (12%) as moderate confidence, 2 (12%) as low confidence, and 13 (76.0%) as critically low confidence (Table 1 and Additional file 2: Table S4).
Fig. 1.
Study selection flow of meta-analyses. Abbreviation: CDSR, Cochrane database of systematic review
Table 1.
Characteristics of meta-analyses of randomized clinical trials studying ketogenic diet
| Source | Population | Type of KD | Comparator | Duration of diet | No. of included studies | Total participants | Outcomes | AMSTAR-2 rating |
|---|---|---|---|---|---|---|---|---|
| Alarim et al. 2020 [36] | T2DM adults with overweight or obesity | KD | LCD or RD | 4–8 months | 8 | 653 | Body weight, BMI, FPG, HbA1c, LDL-C, HDL-C, TC, TG | Critically low |
| Amini et al. 2021 [37] | Individuals > 18 years old | KD or K-LCHF | LCD, LFD, HCD, or RD | 2 weeks to 24 months | 18 | 1835 | Body weight, BMI, muscle mass, waist circumference, fat mass, body fat percentage, visceral adipose tissue, | Moderate |
| Ashtary-Larky et al. 2022 [38] | Individuals > 16 years old | KD or K-LCHF | RD or KD | 3 weeks to 3 months | 13 | 244 | BMI, muscle mass, fat mass, body fat percentage | Critically low |
| Bueno et al. 2013 [1] | Adults with obesity | KD | LFD or RD | 3–6 months | 13 | 1415 | Body weight, HDL-C, LDL-C, TG, FPG, HbA1c, fasting insulin, SBP, DBP, CRP | Moderate |
| Cao et al. 2021 [39] | Athletes | K-LCHF | HCD | 4–6 weeks | 10 | 139 | VO2 max, maximal heart rate during exercise, respiratory exchange ratio | Critically low |
| Castellana et al. 2020 [15] | T2DM adults with overweight or obesity | VLCKD | LCD | 1–2 months | 13 | 801 | Body weight | Low |
| Choi et al. 2020 [2] | Adults with overweight or obesity, some with T2DM | KD, K-LCHF, or VLCKD | LCD, LFD, HCD, or RD | 120 min to 24 months | 14 | 734 | Body weight, BMI, waist circumference, HDL-C, LDL-C, TC, TG, FPG, HbA1c, fasting insulin, HOMA-IR, SBP, DBP, CRP, creatinine, C-peptide | Critically low |
| Lee et al. 2021 [40] | Adults with overweight or obesity | KD | RD | 1–6 months | 7 | 255 | Body weight, BMI, fat mass, muscle mass, waist circumference, HDL-C, LDL-C, TC, TG, FPG, VO2 peak | Critically low |
| Lee et al. 2021 [17] | Athletes | KD | RD | 2–24 weeks | 8 | 158 | Body fat percentage, muscle mass, HDL-C, TC, TG, FPG, fasting insulin, heart rate, VO2 max, respiratory exchange ratio, | Critically low |
| Lopez-Espinosa et al. 2021 [41] | Adults with obesity | KD, K-LCHF, or VLCKD | LCD, LFD, or HCD | 24–96 weeks | 10 | 943 | BMI, HDL-C, LDL-C, TC, TG | Critically low |
| Muscogiuri et al. 2021 [42] | Adults with overweight or obesity | VLCKD | LCD | 2–4 weeks | 15 | 835 | Body weight, BMI, fat mass, muscle mass, waist circumference, FPG, HbA1C, HOMA-IR, total cholesterol, triglyceride, HDL-C, LDL-C | Critically low |
| Rafiullah et al. 2022 [35] | T2DM adults | KD | RD | 1–32 weeks | 10 | 800 | Body weight, LDL-C, TG, HbA1c | Critically low |
| Sainsbury et al. 2018 [34] | T2DM adults with overweight or obesity | KD | LCD or LFD | 12–24 weeks | 25 | 2784 | HbA1c | Low |
| Smith et al. 2020[43] | Adults with obesity, some with dyslipidemia | KD | LDF or RD | 12–96 weeks | 25 | 3340 | Muscle mass | Critically low |
| Sourbron et al. 2020 [16] | Children and adolescents (age 1–18 years) with refractory epilepsy | KD or MAD | RD | 3–16 months | 7 | 539 | Seizure frequency reduction | Critically low |
| Vargas-Molina et al. 2022 | Athletes or resistance trained adults | KD | RD | 8–12 weeks | 5 | 111 | Body weight, muscle mass | Critically low |
| Yang et al. 2021 [44] | Adults with cancers | KD or K-LCHF | RD | 6–24 weeks | 6 | 325 | Body weight, HDL-C, LDL-C, TC, TG, FPG, insulin, adverse effects | Critically low |
Abbreviations: AMSTAR-2 A Measurement Tool to Assess Systematic Reviews, BMI body mass index, CRP C-reactive protein, DBP diastolic blood pressure, FPG fasting plasma glucose, HbA1c hemoglobin A1c, HCD high carbohydrate diet, HDL-C high-density lipoprotein cholesterol, HOMA-IR homeostatic model of insulin resistance, K-LCHF ketogenic low-carbohydrate high-fat diet, KD ketogenic diet, LCD low-calorie diet, LDL-C low-density lipoprotein cholesterol, LFD low-fat diet, MAD modified Atkins diet, RD regular diet, SBP systolic blood pressure, T2DM type 2 diabetic mellitus, TC total cholesterol, TG triglyceride, VLCKD very low-calorie ketogenic diet, VO2 max maximum oxygen consumption, VO2 peak peak oxygen consumption
Types of KD identified in this umbrella review were categorized as (1) KD, which limits carbohydrate intake to < 50 g/day or < 10% of the total energy intake (TEI) [35], (2) ketogenic low-carbohydrate, high-fat diet (K-LCHF), which limits carbohydrate intake to < 50 g/day or < 10% of TEI with high amount of fat intake (60–80% of TEI) [38, 46], (3) very low-calorie KD (VLCKD), which limits carbohydrate intake to < 30–50 g/day or 13–25% of TEI with TEI < 700–800 kcal/day, and (4) modified Atkins diet (MAD), which generally limits carbohydrate intake to < 10 g/day while encouraging high-fat foods [15, 47]. Meta-analyses of long-chain triglyceride KD, medium-chain triglyceride KD, and low glycemic index treatment were not identified.
Description and summary of associations
We identified 115 unique associations of KD with health outcomes (Additional file 2: Table S5). The median (IQR) number of studies per association was 3 [4–6], and the median (IQR) sample size was 244 (127–430) participants. Outcomes were associated with KD types, including 40 (35%) KD, 18 (16%) K-LCHF, 13 (11%) VLCKD, 25 (22%) KD or K-LCHF, 5 (4%) KD or VLCKD, 1 (1%) KD or MAD, and 13 (11%) KD, K-LCHF, or VLCKD.
The associations involved 40 (35%) anthropometric measures (i.e., body weight, body mass index [BMI] [calculated as weight in kilograms divided by height in meters squared], waist circumference, muscle mass, fat mass, body fat percentage, and visceral adipose tissue), 37 (32%) lipid profile outcomes (i.e., triglyceride, total cholesterol, high-density lipoprotein cholesterol [HDL-C], and low-density lipoprotein cholesterol [LDL-C]), 22 (19%) glycemic profile outcomes (i.e., hemoglobin A1c [HbA1c], fasting plasma glucose, fasting insulin, and homeostatic model assessment of insulin resistance [HOMA-IR]), 6 (5%) exercise performance (i.e., maximal heart rate, respiratory exchange ratio [RER], maximal oxygen consumption (VO2 max), 5 (4%) blood pressure outcomes (i.e., systolic blood pressure [SBP], diastolic blood pressure [DBP], and heart rate), 1 (1%) outcome associated with seizure frequency reduction ≥ 50% from baseline, and 3 other outcomes (i.e., serum creatinine, C-peptide, and C-reactive protein). In addition, there is 1 association (1%) of adverse events.
Participants in the identified associations included 68 (59%) associations in adults with overweight or obesity with or without T2DM or dyslipidemia, 15 (13%) athletes or resistance-trained adults, 12 (10%) adults with T2DM, 11 (10%) healthy participants ≥ 16 years old, 8 (7%) cancer patients, and 1 (1%) in children and adolescents with epilepsy.
Using GRADE, 115 associations were supported by very low strength of evidence (n = 66, 57%), with the remaining being low (n = 36, 31%), moderate (n = 9, 8%), and high quality of evidence (n = 4, 3%) (Additional file 2: Table S5). Almost half, or 44% (51 associations), were statistically significant based on a random-effects model, of which 51% (26 associations) were supported by a very low level of evidence, followed by low (17 associations [33%]), moderate (4 associations [8%]), and high (4 associations [8%]) levels of evidence. Overall beneficial outcomes associated with KD were BMI [37, 42], body weight [1, 2, 35–37, 41], waist circumference [37, 42], fat mass [37, 42], body fat percentage [38, 40], visceral adipose tissue [37], triglyceride [1, 2, 36, 42], HDL-C [1, 2, 42], HbA1c [2, 34, 35], HOMA-IR [2, 42], DBP [1], seizure frequency reduction ≥ 50% from baseline [16], and respiratory exchange ratio [17, 39]. Adverse outcomes associated with KD were reduced muscle mass [37, 38], and increased LDL-C [2, 35], and total cholesterol [2, 17]. In terms of safety, one association showed no significant increase in adverse events (e.g., constipation, abdominal pain, and nausea) with KD [44].
Eight out of 13 associations supported by moderate to high-quality evidence were statistically significant (Table 2). There were 4 statistically significant associations supported by high-quality evidence, including the following: (1) KD or MAD for 3–16 months was associated with a higher proportion of children and adolescents with refractory epilepsy achieving seizure frequency reduction ≥ 50% from baseline compared with regular diet (RR, 5.11; 95% CI, 3.18 to 8.21) [16], (2) KD for 3 months was associated with reduced triglyceride in adults with T2DM compared with regular diet (MD, -18.36 mg/dL; 95% CI, -24.24 to -12.49, MCID threshold 7.96 mg/dL) [14, 35], (3) KD for 12 months was associated with reduced triglyceride in adults with T2DM compared with regular diet (MD, -24.10 mg/dL; 95% CI, -33.93 to -14.27, MCID threshold 7.96 mg/dL) [14, 35], and (4) KD for 12 months was associated with increased LDL-C in adults with T2DM compared with regular diet (MD, 6.35 mg/dL; 95% CI, 2.02 to 10.69, MCID threshold 3.87 mg/dL) [14, 35]. In addition, there were 4 statistically significant associations supported by moderate-quality evidence: (1) KD for 3 months was associated with reduced HbA1c in adults with T2DM compared with regular diet (MD, -0.61%; 95% CI, -0.82 to -0.40, MCID threshold 0.5%) [14, 35], (2) VLCKD for 4–6 weeks was associated with reduced body weight in T2DM adults with overweight or obesity compared with a low-fat diet or regular diet (MD, -9.33 kg; 95% CI, -15.45 to -3.22, MCID threshold 4.40 kg) [14, 15], (3) K-LCHF for 4–6 weeks was associated with reduced respiratory exchange ratio in athletes compared with a high-carbohydrate diet (SMD, -2.66; 95% CI, -3.77 to -1.54) [39], and (4) K-LCHF for 11–24 weeks was associated with increased total cholesterol in athletes compared with regular diet (MD, 1.32 mg/dL; 95% CI, 0.64 to 1.99) [14, 17].
Table 2.
Summary of significant associations of ketogenic diet with health outcomes supported by moderate to high quality of evidence
| Source | Outcome | Population | Intervention | Duration of KD | Comparator | No. of studies (sample size) | Metric | Random effect size (95% CI) | P value | I2, % | GRADE rating | Clinical importance (MCID threshold) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sourbron et al. 2020 [16] | Seizure frequency reduction ≥ 50% from baseline | Children or adolescents (age 1–18 years) with refractory epilepsy | KD or MAD | 3–16 months | RD | 5 (n = 374) | RR | 5.11 (3.18 to 8.21) | < .001 | 0 | High | N/A |
| Rafiullah et al. 2022 [35] | Triglyceride, mg/dL | Adults with T2DM | KD | 3 months | RD | 4 (n = 283) | MD | -18.36 (-24.24 to -12.49) | < .001 | 0 | High | Yes (MCID 7.96 mg/dL) |
| Rafiullah et al. 2022 [35] | Triglyceride, mg/dL | Adults with T2DM | KD | 12 months | RD | 5 (n = 445) | MD | -24.10 (-33.93 to -14.27) | < .001 | 0 | High | Yes (MCID 7.96 mg/dL) |
| Rafiullah et al. 2022 [35] | LDL-C, mg/dL | Adults with T2DM | KD | 12 months | RD | 4 (n = 389) | MD | 6.35 (2.02 to 10.69) | .004 | 0 | High | Yes (MCID 3.87 mg/dL) |
| Rafiullah et al. 2022 [35] | HbA1c, % | Adults with T2DM | KD | 3 months | RD | 6 (n = 388) | MD | -0.61 (-0.82 to -0.40) | < .001 | 44.0 | Moderate | Yes (MCID 0.50%) |
| Castellana et al. 2020 [15] | Body weight, kg | T2DM adults with overweight or obesity | VLCKD | 4–6 weeks | LFD or RD | 2 (n = 142) | MD | -9.33 (-15.45 to -3.22) | < .001 | 0.1 | Moderate | Yes (MCID 4.40 kg) |
| Cao et al. 2021 [39] | Respiratory exchange rate | Athletes | K-LCHF | 4–6 weeks | HCD | 2 (n = 15) | SMD | -2.65 (-3.77 to -1.54) | < .001 | 0.8 | Moderate | N/A |
| Lee et al. 2021 [17] | Total cholesterol, mg/dL | Athletes | K-LCHF | 11–24 weeks | RD | 2 (n = 41) | MD | 1.32 (0.64,1.99) | < .001 | 0 | Moderate | No (10.05 mg/dL) |
Abbreviations: GRADE Grading of Recommendations, Assessment, Development, and Evaluations, HbA1c hemoglobin A1c, HCD high carbohydrate diet, K-LCHF ketogenic low-carbohydrate high-fat diet, KD ketogenic diet, LCD low-calorie diet, LDL-C low-density lipoprotein cholesterol, LFD low-fat diet, MAD modified Atkins diet, MD mean difference, MCID minimally clinically important difference, N/A not applicable, RD regular diet, RR risk ratio, SMD standardized mean difference, T2DM type 2 diabetes mellitus, VLCKD very low-calorie ketogenic diet
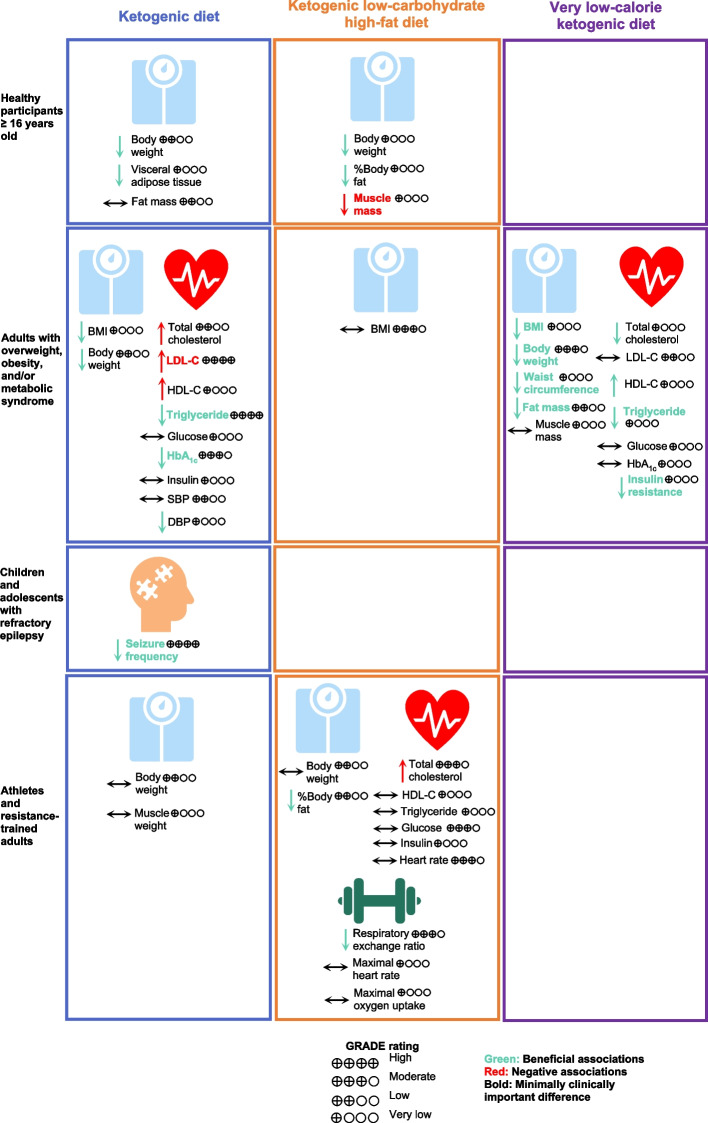
Types of KD showed different effects on health outcomes with changes more than the MCID thresholds in different populations (Fig. 2). KD or MAD for 3–16 months was associated with a 5-times higher proportion of children and adolescents with refractory epilepsy achieving seizure frequency reduction ≥ 50% from baseline compared with a regular diet (RR, 5.11; 95% CI, 3.18 to 8.21) [16]. In healthy participants, K-LCHF for 3–12 weeks could reduce body weight by 3.68 kg (95% CI, -4.45 to -2.90) but also significantly reduced muscle mass by 1.27 kg (95% CI, -1.83 to -0.70, MCID threshold 1.10 kg) [14, 26, 38]. In adults with T2DM, KD for 3–12 months was found to have significant associations with changes more than the MCID thresholds, including reduction of triglyceride and HbA1c; however, KD for 12 months led to a clinically meaningful increase in LDL-C by 6.35 mg/dL (95% CI, 2.02 to 10.69, MCID threshold 3.87 mg/dL) [14, 35]. In adults with overweight or obesity and/or metabolic syndrome, VLCKD for 4–6 weeks demonstrated a clinically meaningful weight loss of 9.33 kg (95% CI, -15.45 to -3.22, MCID threshold 4.40 kg) [14, 15]. VLCKD for 3–96 weeks led to a clinically meaningful improvement in BMI, body weight, waist circumference, triglyceride, fat mass, and insulin resistance, while preserving muscle mass [42].
Fig. 2.
Associations of Types of Ketogenic Diet with Health Outcomes. Abbreviations: BMI, body mass index, DBP, diastolic blood pressure; GRADE, Grading of Recommendations, Assessment, Development, and Evaluations; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model of insulin resistance; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TEI, total energy intake
Sensitivity analyses
Excluding RCTs with small sizes in 7 associations found that the strength of evidence of one association was downgraded to very low quality, i.e., KD for 12 months, and the increase of LDL-C in adults with T2DM compared with a control diet. Another association was downgraded to low quality, i.e., KD for 12 months and the reduction of triglyceride in adults with T2DM compared with the control diet (Additional file 2: Table S6). The remaining associations retained the same rank.
Discussion
This umbrella review was performed to systematically assess the potential associations of KD and health outcomes by summarizing the evidence from meta-analyses of RCTs. Sensitivity analyses were performed to provide additional evidence from high-quality RCTs, which further increased the reliability of results. We identified 115 associations of KD with a wide range of outcomes. Most associations were rated as low and very low evidence according to the GRADE criteria because of serious imprecision and large heterogeneity in findings, and indirectness due to a mix of different interventions and comparators.
Our findings showed that KD or MAD resulted in better seizure control in children and adolescents with medication-refractory epilepsy (approximately a third of cases) for up to 16 months [10, 11, 16]. Anti-epileptic mechanisms of KD remain unknown but are likely multifactorial. Enhanced mitochondrial metabolism and an increase in ketone bodies or reduction in glucose across the blood–brain barrier resulted in synaptic stabilization [48–50]. Other mechanisms include an increase in gamma-aminobutyric acid (GABA) [51], more beneficial gut microbiome [52], less pro-inflammatory markers [53], and epigenetic modifications (e.g. beta-hydroxybutyrate [beta-OHB]) [54].
In adults, KD was associated with improved anthropometric measures, cardiometabolic parameters, and exercise performance. Our findings, however, demonstrated differences in the level of associations with type of KD. On the one hand, VLCKD is very effective in producing weight loss while preserving muscle mass in adults with overweight or obesity, with specific benefits on anthropometric and cardiometabolic parameters [15, 42]. On the other hand, a significant portion of the weight loss seen in K-LCHF was due to muscle mass loss [17, 38]. Overall KD was negatively associated with reduced muscle mass and increased LDL-C and total cholesterol.
Our findings demonstrated that KD could induce a rapid weight loss in the initial phase of 6 months, after which time further weight loss was hardly achieved [35]. Furthermore, weight loss induced by KD is relatively modest and appears comparable to other dietary interventions that are effective for short-term weight loss, e.g., intermittent fastingand Mediterranean diet [55–57].
KD is one of the dietary interventions employed by individuals to achieve rapid weight loss, which usually comes with reduced muscle mass [58]. However, KD has been hypothesized to preserve muscle mass following weight loss based on several mechanisms, including the protective effect of ketones and its precursors on muscle tissue [59–61], and increased growth hormone secretion stimulated by low blood glucose to increase muscle protein synthesis [58, 62, 63].
With regards to KD effects on lipid profiles, our results demonstrate an effective reduction in serum triglyceride levels with 3 months of lowered dietary carbohydrate intake, with even further reduction by month 12 [35]. Triglyceride levels are consistently shown to decrease after KD. Acute ketosis (beta-OHB ≈ 3 mM) due to ketone supplementation also shows decreases in triglycerides, indicating a potential effect of ketones on triglycerides independent of weight loss. One possible mechanism is the decreased very low-density lipoprotein content in the plasma due to low insulin levels. Due to a lack of insulin, lipolysis increases in fat cells [2, 13, 15]. Of note, the converse has also been observed as a phenomenon known as carbohydrate-induced hypertriglyceridemia, whereby higher dietary carbohydrate intake leads to higher serum triglycerides levels, potentially mediated by changes in triglyceride clearance and hepatic de novo lipogenesis rates [64]. Though our aggregate results also confirm an increase in LDL-C and total cholesterol with KD and K-LCHF, respectively, it is important to note that an increase in either of these levels does not necessarily signify a potentially deleterious cardiovascular end-point. This qualification derives from the fact that LDL particles are widely heterogeneous in composition and size, with small dense LDL particles being significantly more atherogenic than larger LDL particles [65]. Our observed aggregate effect of KD on cholesterol levels does not account for the difference in LDL particle size, nor does it distinguish the sources of dietary fat, which can also be a significant effector of LDL particle size distribution and metabolism [66].
Most RCTs of KD were conducted in patients with a limited group of participants, such as those with overweight, obesity, metabolic syndrome, cancer, and refractory epilepsy. In addition, most outcomes measured were limited to only surrogate outcomes. Thus, more clinical trials with a broader scope in populations and outcomes associated with KD would expand the role of KD in a clinical setting. For example, participant selection could be expanded from previous trials to include elderly patients, nonalcoholic fatty live disease (NAFLD) patients, and polycystic ovarian syndrome patients. Outcomes of interest of could be expanded to include (1) clinical outcomes such as cardiovascular events and liver outcomes, (2) short- and long-term safety outcomes such as adverse events (e.g., gastrointestinal, neurological, hepatic, and renal), eating disorder syndrome, sleep parameters, lipid profiles, and thyroid function and (3) other outcomes such as adherence and quality of life. More importantly, long-term studies are needed to investigate the sustainability of the clinical benefits of KD.
Our findings are useful to support the generation of evidence-based recommendations for clinicians contemplating use of KD in their patients, as well as for the general population. We further emphasize the importance of consultation with healthcare professionals before utilizing KD and any other dietary interventions. We demonstrated the benefits of KD on various outcomes in the short term. However, these improvements may prove difficult to sustain in the long term because of challenges in adherence. As for any diet interventions to achieve sustainable weight loss, factors of success include adherence, negative energy balance, and high-quality foods. Thus, communication and education with KD practitioners are important to ensure their adherence to the diet. Some individuals might benefit from switching from KD to other dietary interventions to maintain long-term weight loss.
Limitations
This umbrella review has several limitations. Firstly, we focused on published meta-analyses which confined us from assessing the associations of KD on outcomes and populations that were not included in existing meta-analyses. Secondly, most of the included meta-analyses were rated with AMSTAR-2 as critically low confidence, mainly due to a lack of study exclusion reasons, unexplained study heterogeneity, and unassessed publication bias. However, these domains unlikely affected our findings. Thirdly, we could not perform a dose–response analysis to understand the effects of different levels of carbohydrate intake on health outcomes because of insufficient details of carbohydrate intake reported in the meta-analyses. Fourthly, most RCTs of KD were limited to a relatively small number of participants with a short-term follow-up period, which limited our assessment of sustained beneficial effects after stopping KD. Lastly, due to decreased adherence, carbohydrate intake most likely increased across the course of the trials. For example, subjects in the KD arm of the A TO Z Weight Loss Study [67], started with a carbohydrate intake < 10 g/day but ended at 12 months with a carbohydrate intake accounting for 34% of TEI. In the DIRECT trial, subjects in the KD group started with carbohydrate intake of 20 g/day and ended at 12 months with 40% of TEI from carbohydrate intake [68]. Thus, we cannot be certain how the precise degree of ketosis contributed to the beneficial effects noted.
Conclusions
Beneficial associations of practicing KD were supported by moderate- to high-quality evidence, including weight loss, lower triglyceride levels, decreased HbA1c, RER, and decreased seizure frequency. However, KD was associated with a clinically meaningful increase in LDL-C. Clinical trials with long-term follow-up are warranted to investigate whether these short-term effects of KD will translate to beneficial effects on more long-term clinical outcomes such as cardiovascular events and mortality.
Supplementary Information
Additional file 1. PRISMA 2020 Main Checklist.
Additional file 2: Method S1. Data extraction. Table S1. Difference from original review protocol. Table S2. Search strategy. Table S3. Excluded studies with reasons. Table S4. Quality assessment. Table S5. Summary of associations. Table S6. Sensitivity analyses.
Acknowledgements
The authors would like to acknowledge Thunchanok Ingkaprasert and Wachiravit Youngjanin for their editorial assistance.
Abbreviations
- Beta-OHB
Beta-hydroxybutyrate
- BMI
Body mass index
- DBP
Diastolic blood pressure
- GABA
Gamma-aminobutyric acid
- HDL-C
High-density lipoprotein cholesterol
- HbA1c
Hemoglobin A1c
- HOMA-IR
Homeostatic model assessment of insulin resistance
- K-LCHF
Ketogenic low-carbohydrate high-fat diet
- KD
Ketogenic diets
- LDL-C
Low-density lipoprotein cholesterol
- MAD
Modified Atkins diet
- MCID
Minimally clinically important difference
- NAFLD
Nonalcoholic fatty liver disease
- RCT
Randomized clinical trials
- RER
Respiratory exchange ratio
- SBP
Systolic blood pressure
- T2DM
Type 2 diabetes mellitus
- TEI
Total energy intake
- VLCKD
Very low-calorie ketogenic diet
- VO2 max
Maximal oxygen consumption
Authors’ contributions
CP, PS, SKV, and NC conceived and designed the study protocol. CP, PS, and SKV performed a literature review and data analysis. CP, PS, TP, PP, YYL, KAV, SKV, and NC interpreted the study findings. CP and PS were major contributors to writing the manuscript. All authors read and approved the final manuscript.
Funding
No funding was obtained for the conduct of this study.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chanthawat Patikorn, Email: chanthawat.patikorn@pharm.utah.edu.
Pantakarn Saidoung, Email: pantakarn.saidoung@gmail.com.
Tuan Pham, Email: Tuan.Pham@hsc.utah.edu.
Pochamana Phisalprapa, Email: coco_a105@hotmail.com.
Yeong Yeh Lee, Email: justnleeyy@gmail.com.
Krista A. Varady, Email: varady@uic.edu
Sajesh K. Veettil, Email: Sajesh.Veettil@pharm.utah.edu
Nathorn Chaiyakunapruk, Email: nathorn.chaiyakunapruk@utah.edu.
References
- 1.Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110(7):1178–87. doi: 10.1017/S0007114513000548. [DOI] [PubMed] [Google Scholar]
- 2.Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12(7):2005. doi: 10.3390/nu12072005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkpatrick CF, Bolick JP, Kris-Etherton PM, Sikand G, Aspry KE, Soffer DE, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13(5):689–711.e1. doi: 10.1016/j.jacl.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Dahlin M, Singleton SS, David JA, Basuchoudhary A, Wickström R, Mazumder R, et al. Higher levels of Bifidobacteria and tumor necrosis factor in children with drug-resistant epilepsy are associated with anti-seizure response to the ketogenic diet. eBioMedicine. 2022;80:104061. doi: 10.1016/j.ebiom.2022.104061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crosby L, Davis B, Joshi S, Jardine M, Paul J, Neola M, et al. Ketogenic diets and chronic disease: weighing the benefits against the risks. Front Nutr. 2021;8:702802. doi: 10.3389/fnut.2021.702802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789–796. doi: 10.1038/ejcn.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gershuni VM, Yan SL, Medici V. Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr Nutr Rep. 2018;7(3):97–106. doi: 10.1007/s13668-018-0235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu H, Bi D, Zhang Y, Kong C, Du J, Wu X, et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct Target Ther. 2022;7(1):11. doi: 10.1038/s41392-021-00831-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefevre F, Aronson N. Ketogenic diet for the treatment of refractory epilepsy in children: a systematic review of efficacy. Pediatrics. 2000;105(4):E46. doi: 10.1542/peds.105.4.e46. [DOI] [PubMed] [Google Scholar]
- 10.Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3(2):175–192. doi: 10.1002/epi4.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48(1):43–58. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 12.Yudkoff M, Daikhin Y, Melø TM, Nissim I, Sonnewald U, Nissim I. The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect. Annu Rev Nutr. 2007;27:415–430. doi: 10.1146/annurev.nutr.27.061406.093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10(1):38. doi: 10.1038/s41387-020-00142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldenberg JZ, Day A, Brinkworth GD, Sato J, Yamada S, Jönsson T, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743. doi: 10.1136/bmj.m4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castellana M, Conte E, Cignarelli A, Perrini S, Giustina A, Giovanella L, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21(1):5–16. doi: 10.1007/s11154-019-09514-y. [DOI] [PubMed] [Google Scholar]
- 16.Sourbron J, Klinkenberg S, van Kuijk SMJ, Lagae L, Lambrechts D, Braakman HMH, et al. Ketogenic diet for the treatment of pediatric epilepsy: review and meta-analysis. Childs Nerv Syst. 2020;36(6):1099–1109. doi: 10.1007/s00381-020-04578-7. [DOI] [PubMed] [Google Scholar]
- 17.Lee HS, Lee J. Influences of ketogenic diet on body fat percentage, respiratory exchange rate, and total cholesterol in athletes: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(6):2912. doi: 10.3390/ijerph18062912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dragioti E, Solmi M, Favaro A, Fusar-Poli P, Dazzan P, Thompson T, et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiat. 2019;76(12):1241–1255. doi: 10.1001/jamapsychiatry.2019.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patikorn C, Roubal K, Veettil SK, Chandran V, Pham T, Lee YY, et al. Intermittent Fasting and Obesity-Related Health Outcomes: An Umbrella Review of Meta-analyses of Randomized Clinical Trials. JAMA Netw Open. 2021;4(12):e2139558-e. doi: 10.1001/jamanetworkopen.2021.39558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014;15(4):310–321. doi: 10.1111/obr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulligan AA, Lentjes MA, Luben RN, Wareham NJ, Khaw K-T. Changes in waist circumference and risk of all-cause and CVD mortality: results from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) cohort study. BMC Cardiovasc Disord. 2019;19(1):1–15. doi: 10.1186/s12872-019-1223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayedi A, Khan TA, Aune D, Emadi A, Shab-Bidar S. Body fat and risk of all-cause mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Int J Obes. 2022;46(9):1573–1581. doi: 10.1038/s41366-022-01165-5. [DOI] [PubMed] [Google Scholar]
- 29.Keller HH, Østbye T. Body mass index (BMI), BMI change and mortality in community-dwelling seniors without dementia. J Nutr Health Aging. 2005;9(5):316–320. [PubMed] [Google Scholar]
- 30.Kelley GA, Kelley KS, Stauffer BL. Walking and resting blood pressure: an inter-individual response difference meta-analysis of randomized controlled trials. Sci Prog. 2022;105(2):00368504221101636. doi: 10.1177/00368504221101636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in cochrane reviews. Syst Rev. 2013;2(1):81. doi: 10.1186/2046-4053-2-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA. 2014;312(6):623–630. doi: 10.1001/jama.2014.8166. [DOI] [PubMed] [Google Scholar]
- 33.Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 34.Sainsbury E, Kizirian NV, Partridge SR, Gill T, Colagiuri S, Gibson AA. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;139:239–252. doi: 10.1016/j.diabres.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Rafiullah M, Musambil M, David SK. Effect of a very low-carbohydrate ketogenic diet vs recommended diets in patients with type 2 diabetes: a meta-analysis. Nutr Rev. 2022;80(3):488–502. doi: 10.1093/nutrit/nuab040. [DOI] [PubMed] [Google Scholar]
- 36.Alarim RA, Alasmre FA, Alotaibi HA, Alshehri MA, Hussain SA. Effects of the ketogenic diet on glycemic control in diabetic patients: meta-analysis of clinical trials. Cureus. 2020;12(10):e10796. doi: 10.7759/cureus.10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amini MR, Aminianfar A, Naghshi S, Larijani B, Esmaillzadeh A. The effect of ketogenic diet on body composition and anthropometric measures: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2022;62(13):3644–3657. doi: 10.1080/10408398.2020.1867957. [DOI] [PubMed] [Google Scholar]
- 38.Ashtary-Larky D, Bagheri R, Asbaghi O, Tinsley GM, Kooti W, Abbasnezhad A, et al. Effects of resistance training combined with a ketogenic diet on body composition: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2022;62(21):5717–5732. doi: 10.1080/10408398.2021.1890689. [DOI] [PubMed] [Google Scholar]
- 39.Cao J, Lei S, Wang X, Cheng S. The effect of a ketogenic low-carbohydrate, high-fat diet on aerobic capacity and exercise performance in endurance athletes: a systematic review and meta-analysis. Nutrients. 2021;13(8):2896. doi: 10.3390/nu13082896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HS, Lee J. Effects of combined exercise and low carbohydrate ketogenic diet interventions on waist circumference and triglycerides in overweight and obese individuals: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(2):828. doi: 10.3390/ijerph18020828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.López-Espinoza M, Chacón-Moscoso S, Sanduvete-Chaves S, Ortega-Maureira MJ, Barrientos-Bravo T. Effect of a ketogenic diet on the nutritional parameters of obese patients: a systematic review and meta-analysis. Nutrients. 2021;13(9):2946. doi: 10.3390/nu13092946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muscogiuri G, El Ghoch M, Colao A, Hassapidou M, Yumuk V, Busetto L. European guidelines for obesity management in adults with a very low-calorie ketogenic diet: a systematic review and meta-analysis. Obes Facts. 2021;14(2):222–245. doi: 10.1159/000515381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith ES, Smith HA, Betts JA, Gonzalez JT, Atkinson G. A Systematic review and meta-analysis comparing heterogeneity in body mass responses between low-carbohydrate and low-fat diets. Obesity. 2020;28(10):1833–1842. doi: 10.1002/oby.22968. [DOI] [PubMed] [Google Scholar]
- 44.Yang YF, Mattamel PB, Joseph T, Huang J, Chen Q, Akinwunmi BO, et al. Efficacy of low-carbohydrate ketogenic diet as an adjuvant cancer therapy: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2021;13(5):1388. doi: 10.3390/nu13051388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vargas-Molina S, Gómez-Urquiza JL, García-Romero J, Benítez-Porres J. Effects of the ketogenic diet on muscle hypertrophy in resistance-trained men and women: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(19):12629. doi: 10.3390/ijerph191912629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Rashidy OF, Nassar MF, Abdel-Hamid IA, Shatla RH, Abdel-Hamid MH, Gabr SS, et al. Modified Atkins diet vs classic ketogenic formula in intractable epilepsy. Acta Neurol Scand. 2013;128(6):402–408. doi: 10.1111/ane.12137. [DOI] [PubMed] [Google Scholar]
- 47.Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47(2):421–424. doi: 10.1111/j.1528-1167.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 48.Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60(2):223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 49.Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, et al. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol. 2015;78(1):77–87. doi: 10.1002/ana.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP–dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9(11):1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 51.Wang ZJ, Bergqvist C, Hunter JV, Jin D, Wang DJ, Wehrli S, et al. In vivo measurement of brain metabolites using two-dimensional double-quantum MR spectroscopy—exploration of GABA levels in a ketogenic diet. Magn Reson Med. 2003;49(4):615–619. doi: 10.1002/mrm.10429. [DOI] [PubMed] [Google Scholar]
- 52.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728–41.e13. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dupuis N, Curatolo N, Benoist JF, Auvin S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56(7):e95–e98. doi: 10.1111/epi.13038. [DOI] [PubMed] [Google Scholar]
- 54.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Neill B, Raggi P. The ketogenic diet: Pros and cons. Atherosclerosis. 2020;292:119–126. doi: 10.1016/j.atherosclerosis.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 56.Freire R. Scientific evidence of diets for weight loss: different macronutrient composition, intermittent fasting, and popular diets. Nutrition. 2020;69:110549. doi: 10.1016/j.nut.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Becker A, Gaballa D, Roslin M, Gianos E, Kane J. Novel nutritional and dietary approaches to weight loss for the prevention of cardiovascular disease: ketogenic diet, intermittent fasting, and bariatric surgery. Curr Cardiol Rep. 2021;23(7):1–9. doi: 10.1007/s11886-021-01515-1. [DOI] [PubMed] [Google Scholar]
- 58.Ashtary-Larky D, Bagheri R, Bavi H, Baker JS, Moro T, Mancin L, et al. Ketogenic diets, physical activity and body composition: a review. Br J Nutr. 2022;127(12):1898–1920. doi: 10.1017/S0007114521002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomsen HH, Rittig N, Johannsen M, Møller AB, Jørgensen JO, Jessen N, et al. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: anticatabolic impact of ketone bodies. Am J Clin Nutr. 2018;108(4):857–867. doi: 10.1093/ajcn/nqy170. [DOI] [PubMed] [Google Scholar]
- 60.Koutnik AP, D’Agostino DP, Egan B. Anticatabolic effects of ketone bodies in skeletal muscle. Trends Endocrinol Metab. 2019;30(4):227–229. doi: 10.1016/j.tem.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Parker BA, Walton CM, Carr ST, Andrus JL, Cheung EC, Duplisea MJ, et al. β-hydroxybutyrate elicits favorable mitochondrial changes in skeletal muscle. Int J Mol Sci. 2018;19(8):2247. doi: 10.3390/ijms19082247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Z, Huang L, Waters MJ, Chen C. Insulin and growth hormone balance: implications for obesity. Trends Endocrinol Metab. 2020;31(9):642–654. doi: 10.1016/j.tem.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Møller N, Copeland KC, Nair KS. Growth hormone effects on protein metabolism. Endocrinol Metab Clin North Am. 2007;36(1):89–100. doi: 10.1016/j.ecl.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Parks EJ. Effect of dietary carbohydrate on triglyceride metabolism in humans. J Nutr. 2001;131(10):2772S–S2774. doi: 10.1093/jn/131.10.2772S. [DOI] [PubMed] [Google Scholar]
- 65.Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, et al. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men: prospective results from the Qué bec Cardiovascular Study. Circulation. 1997;95(1):69–75. doi: 10.1161/01.CIR.95.1.69. [DOI] [PubMed] [Google Scholar]
- 66.Dreon DM, Fernstrom HA, Campos H, Blanche P, Williams PT, Krauss RM. Change in dietary saturated fat intake is correlated with change in mass of large low-density-lipoprotein particles in men. Am J Clin Nutr. 1998;67(5):828–836. doi: 10.1093/ajcn/67.5.828. [DOI] [PubMed] [Google Scholar]
- 67.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z weight loss study: a randomized trial. JAMA. 2007;297(9):969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 68.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. PRISMA 2020 Main Checklist.
Additional file 2: Method S1. Data extraction. Table S1. Difference from original review protocol. Table S2. Search strategy. Table S3. Excluded studies with reasons. Table S4. Quality assessment. Table S5. Summary of associations. Table S6. Sensitivity analyses.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.