Abstract
Introduction
Traumatic brain injury is a substantial cause of mortality and morbidity with a higher burden in low and middle-income countries due to healthcare systems that are unable to deliver effectively the acute and long-term care the patients require. Besides its burden, there is little information on traumatic brain injury-related mortality in Ethiopia, especially in the region. Therefore, this study aimed to assess the incidence and predictors of mortality among traumatic brain injury patients admitted to comprehensive specialized hospitals in the Amhara region, northwest Ethiopia, 2022.
Methods
An institution-based retrospective follow-up study was conducted among 544 traumatic brain injury patients admitted from January 1, 2021, to December 31, 2021. A simple random sampling method was used. Data were extracted using a pre-tested and structured data abstraction sheet. Data were entered, coded, and cleaned into EPi-info version 7.2.0.1 software and exported to STATA version 14.1 for analysis. The Weibull regression model was fitted to determine the association between time to death and covariates. Variables with a P-value < 0.05 were declared statistically significant.
Results
The overall incidence of mortality among traumatic brain injury patients was 1.23 per 100 person-day observation [95% (CI: 1.0, 1.5)] with a median survival time of 106 (95% CI: 60, 121) days. Age [AHR: 1.08 (95% CI; 1.06, 1.1)], severe traumatic brain injury [AHR: 10 (95% CI; 3.55, 28.2)], moderate traumatic brain injury [AHR: 9.2 (95% CI 2.97, 29)], hypotension [AHR: 6.9 (95% CI; 2.8, 17.1)], coagulopathy [AHR: 2.55 (95% CI: 1.27, 5.1)], hyperthermia [AHR: 2.79 (95% CI; 1.4, 5.5)], and hyperglycemia [AHR: 2.28 (95% CI; 1.13, 4.6)] were positively associated with mortality while undergoing neurosurgery were negatively associated with mortality [AHR: 0.47 (95% CI; 0.27-0 0.82)].
Conclusion
The overall incidence of mortality was found to be high. Age, severe and moderate traumatic brain injury, hypotension at admission, coagulopathy, presence of associated aspiration pneumonia, undergoing a neurosurgical procedure, episode of hyperthermia, and hyperglycemia during hospitalization were the independent predictors of time to death. Therefore, interventions to reduce mortality should focus on the prevention of primary injury and secondary brain injury.
Keywords: Traumatic brain injury, Incidence, Mortality, Ethiopia
Introduction
Head injury can be defined as a definite history of an upset to the head, a laceration of the scalp, or altered consciousness secondary to physical injury/structural alteration to the skull by any type of external force to the head [1]. Traumatic brain injury (TBI) is a non-congenital injury to the brain from an external mechanical force that leads to permanent or temporary impairment of cognitive, physical, and psychosocial functions [2, 3]. Globally, TBI is a considerable cause of mortality and morbidity across all age groups, with a greater burden in low and middle-income countries (LMICs) due to the high prevalence of risk factors and the inability of health systems to deliver acute and long-term care effectively [4–6]. The incidence of TBI worldwide is rising, mainly owing to injuries associated with the increased use of motor vehicles, particularly in developing countries [7].
The World Health Organization (WHO) global burden of injury estimation ranks injury among the top ten leading causes of death worldwide, of these TBI, is the leading cause of death and disability accounting for about 30% of all injury-related deaths [4, 6, 8, 9]. Current estimates suggest that about 4.48 million people died due to traumas which accounts for 8% of all deaths globally, 38% more than the number of deaths from malaria, tuberculosis, and HIV/AIDS combined [8]. Of these, an estimated 2 million deaths were attributed to the TBI [2, 11], and the burden was concentrated in LMICs due to limited access to advanced life-sustaining measures after trauma [12].
In the United States of America (USA), TBI is the commonest cause of mortality and disabilities [10]. Annually, more than 2.8 million TBI cases were recorded with a 2% of mortality [11, 12]. In Africa, TBI is a hidden epidemic, one-third of all head injury patients suffer from poor outcomes, and those patients with severe head injury have almost twice the risk of dying compared to those in high-income countries [13, 14]. The resource constraints and inconsistencies in clinical personnel training play an important role when considering the treatment of patients with TBI [15]. Given these problems, the probability of dying from TBI is far greater in LMICs, including Ethiopia, with some areas experiencing as high as a 50% mortality rate in moderate-to-severe cases of TBI [19].
Studies suggested that early prevention of TBI is important to save lives, minimize disabilities and reduce healthcare-related costs [16]. Among these prevention of secondary brain injury plays a major role in the reduction of mortality, the common reason behind secondary brain injury is increased intracranial pressure (ICP), if appropriate treatments to maintain ICP level within the normal limit are not initiated timely, brain herniation can occur and lead to death [17].
In Ethiopia, TBI is a significant public health problem with a reported prevalence of 39.7% and it is the leading reason behind mortality and disability [1, 18]. The effects of TBI are not limited to an individual’s health but are also a cause of increased socioeconomic burden to the family as well as to the country in general [19]. The incidence of mortality secondary to TBI is ranging from 2.26 to 2.6 per 100-person day observations in Ethiopia [5] [14]. The federal government of Ethiopia has proclaimed the rules and regulations about prevention strategies, but despite the measures taken so far still mortality and severe disability were significantly associated with TBI [20]. Thus, a thorough understanding of TBI in LMICs including Ethiopia is essential to mitigate TBI-related mortality [15]. Therefore, this study aimed to assess the incidence and predictors of mortality among TBI patients admitted to comprehensive specialized hospitals in the Amhara region.
Methods
Study design and period
A multicenter retrospective follow-up study was employed from Jan.1, 2021, to Dec.31, 2021, and data was extracted from May 15 – June 15/2022.
Study setting
The study was conducted in comprehensive specialized hospitals in the Amhara regional state of Northwest Ethiopia. Amhara region is one of the 12 regional states which is located in the northwestern part of Ethiopia with an estimated area of 159,173.66 square kilometers. The region is administratively organized into 12 zones, three-city administrations, and 183 districts. According to the 2020 Ethiopian fiscal year report. The total population projection of the region is estimated at 22,191,890 (11,317,864 males and 10,874,026 females) and according to Amhara National Regional Health Bureau, based on the Annual Performance Report, the region has 81 Hospitals, 858 Health Centers, and 3560 Health Posts. Among those 81 hospitals in the region, there are 8 comprehensive specialized hospitals. Of these, the University of Gondar, Felege-Hiwot, Tibebe-Ghion, Debre-Markos, and Deberetabor are comprehensive specialized hospitals found in northwest Amhara. Thus, all those five comprehensive specialized hospitals serve the population found in the area [21]. Those five hospitals served approximately 22,127 adult trauma cases a year, out of the total adult trauma cases TBI is estimated to be 5700 / year.
Source populations
All adult patients admitted with traumatic brain injury in Amhara regional state comprehensive specialized hospitals in northwest Ethiopia.
Study populations
Adult TBI patients who were admitted to Amhara regional state comprehensive specialized hospitals from January 1, 2021, to December 31, 2021.
Inclusion and exclusion criteria
All records of adult TBI patients admitted to the comprehensive specialized hospitals of the Amhara region from January 1, 2021, to December 31, 2021, were included in the study. Incomplete charts missed the outcome record, and patients who were transferred in from other institutions were excluded from the study.
Sample size determination and sampling procedure
The sample size was calculated using single population proportion formula by taking the estimated incidence of TBI mortality at 30.4% from a previous study conducted at Felege Hiwot CSH [14]. 95% confidence interval and 4% margin of error. By adding a 10% for possible incomplete charts, the final sample size was 563. The patient chart was selected by simple random sampling, using a computer-generated table of random numbers.
Variables of the study
Incidence of mortality is the dependent variable.
Independent Variables: Socio-demographic factors (age, sex, and residence); injury-related factors (mechanism of injury, type of injury, the occurrence of Co-existing injury); clinical and diagnostic factors (oxygen saturation, pupillary reflex, Admission GCS, Blood pressure, Blood sugar level, CT scan, comorbidity, hemoglobin level, coagulopathy, and temperature); and management-related factors: (Neurosurgery, Mannitol, phenytoin, intubation, and mechanical ventilation)
Operational definitions
Censored
TBI patients survive at the end of the follow-up period, are lost to follow-up, transferred to a different institution, or left against medical advice.
Event
Occurrence of death from the first confirmed diagnosis of traumatic brain injury until the end of the follow-up.
Time to death
Calculated as the number of days between the date of diagnosis of TBI and the date of death.
Mild TBI:- GCS: 13–15; Moderate TBI:-GCS: 9–12; Severe TBI:- GCS: ≤8 [22].
Type of injury
Is coded as penetrating and blunt based on skin integrity [23].
Poly-trauma
Is defined as the trauma of more than two anatomical areas [24].
Hypoglycemia is defined as a random blood glucose concentration level of < 80 mg/dl during hospitalization [25], and hyperglycemia is defined as a random blood sugar level of ≥ 200 mg/dl [26].
Data collection tool and procedure
Data were extracted by using an appropriate data extraction tool by reviewing patient charts. The data extraction sheet was developed in the English language. It contains four socio-demographic-related items, nineteen clinical and radiologic-related items, six injury-related items, and four management-related items with a total of 33 questions.
Data quality control
To ensure the quality of data, the data extraction tool was checked for the existence of variables in the registration format on the patient’s chart via a preliminary chart review of 10 charts at UOGCSH, thus appropriate modifications were made after analyzing the pretest result before the actual data collection. Face validity was done by emergency and critical care experts. On-site Training was given to data collectors and supervisors on data collection tools and procedures for one day for each site. Data collectors were supervised closely by supervisors. The completeness of each abstraction sheet was checked by the principal investigator and supervisors on a daily base.
Data processing and analysis
Data were cleaned, coded, and entered, into EPi-info version 7.2 software and exported to STATA version 14.1 statistical software. Descriptive statistics were expressed by a median with Interquartile Range whereas categorical variables were expressed by the frequency with percentage. The outcome of each participant was dichotomized into censored or event. The Incidence Density Rate of mortality was calculated for the entire follow-up period. Kaplan Meier (KM) survival curve was used to estimate the median survival time and cumulative probability of survival and the KM failure curve together with the log-rank test was fitted to test the presence of difference in the probability of death among the groups. Both bi-variable and multivariable Weibull regression was used to identify predictor variables. Variables having a p-value < 0.25 in the bi-variable analysis were entered into the multivariable analysis and an Adjusted Hazard Ratio (AHR) with 95% Confidence Intervals (CI) was computed to evaluate the strength of association and variables with a p-value less than 0.05 were considered as statistically significant.
Results
Socio-demographic and injury-related factors
A total of 563 records of TBI patients were reviewed. Of these, 544 (96.6%) charts were included in the analysis. The majority of patients were male 466 (85.6%). Three hundred ninety-three (72.24%) of patients were between 18 and 40 years, and 27 (4.96%) patients were above 65 years with a median age of 32 years (IQR: 25–42 years). More than two-thirds of the patients 373(68.57%) were rural residents. Assaults were the leading cause of TBI 327 (60.11%) followed by road traffic incidents 138 (25.37%). One-third of patients, 164 (30.15%) suffered multiple injuries to the chest, face, extremities, abdomen, and pelvis. Of the total patients, 95(17.46%) had associated aspiration pneumonia. The overall median length of stay was 7 days (IQR: 3–14) (Table 1). Clinical and Management-related findings at admission, the median GCS was 13 (IQR, 10–14). Two hundred ninety -five (54.23%), 166 (30.1%), and 83 (15.26%) patients had sustained mild, moderate, and severe TBI respectively. At admission, 21 (3.86%) were hypotensive, 42 (7.72%) were hypoxic, and two-thirds of patients, 352 (64.71%) had a brain CT scan done. Of these, 127 (36.08%) were contusions followed by subdural hematoma 98, (27.84%). Regarding the pupillary reactivity to light, 46 (8.46%) of patients presented with bilateral non-reactive pupils, and 64 (11.76%) had unilateral reactive pupils. Two hundred thirty-five (43.2%), and 441 (81.07%) patients had received mannitol and phenytoin as prophylaxis of increased ICP and anti-seizure, respectively. Among the cohort, 247 (45.4%) of the patients had undergone neurosurgery. One hundred thirty-four (54%) of patients underwent a craniotomy (Table 2).
Table 1.
Socio-demographic and injury-related characteristics of TBI patients admitted in Amhara Region, comprehensive specialized hospitals, 2022 (n = 544)
| Variables | Category | Total (%) | Death (%) | Censored (%) | PDO | Incidence density |
|---|---|---|---|---|---|---|
| Age | 18–40 years | 393(72.2) | 33(8.4) | 360(91.6) | 5103 | 0.0065 |
| 41–64 years | 124(22.8) | 32(25.81) | 92(74.19) | 1283 | 0.025 | |
| >=65 years | 27(4.96) | 16(59.26) | 11(40.74) | 178 | 0.089 | |
| Sex | Male | 466(85.6) | 71(15.24) | 395(84.76) | 5949 | 0.012 |
| Female | 78(14.4) | 10(12.82) | 68(87.18) | 615 | 0.016 | |
| Residence | Urban | 171(34.3) | 20(11.7) | 151(88.3) | 2429 | 0.008 |
| Rural | 373(65.7) | 61(16.31) | 312(83.65) | 4135 | 0.015 | |
| Mechanism of injury | RTA | 138(25.3) | 41(29.71) | 97(70.29) | 1464 | 0.028 |
| Assault | 327(60.1) | 22(6.73) | 305(93.27) | 4060 | 0.005 | |
| Fall | 75(13.8) | 17(22.67) | 58(77.33) | 851 | 0.019 | |
| Other* | 4(0.74) | 1(25) | 3(75) | 189 | 0.005 | |
| Type of head injury | Blunt | 131(24.1) | 18(13.74) | 113(86.26) | 1083 | 0.016 |
| Penetrating | 413(75.9) | 63(15.25) | 350(84.75) | 5481 | 0.011 | |
| Presence of coexisting trauma | Yes | 164(30.2) | 52(31.71) | 112(68.29) | 2122 | 0.025 |
| No | 380(69.8) | 29(7.63) | 351(92.37) | 4442 | 0.006 | |
| Type of coexisting trauma(n = 164) | Maxillofacial | 66(40.2) | 17(25.76) | 49(74.24) | 898 | 0.019 |
| Chest injury | 32(19.5) | 15(46.88) | 17(53.13) | 564 | 0.026 | |
| Abdo. injury | 5(3.1) | 0 | 5(100) | 34 | 0 | |
| Pelvic injury | 10(6.1) | 1(10) | 9(90) | 105 | 0.0095 | |
| Poly-trauma | 51(31.1) | 19(37.25) | 32(62.75) | 521 | 0.036 | |
| Aspiration pneumonia | Yes | 95(17.5) | 53(55.79) | 42(44.21) | 1411 | 0.037 |
| No | 449(82.5) | 28(6. 24) | 421(93.76) | 5153 | 0.005 |
Note: RTA = road traffic accidents; PDO: person, day, observation; *burn, animal bite
Table 2.
Clinical and management-related characteristics of TBI patients admitted in Amhara Region, comprehensive specialized hospitals, 2022
| Variables | Category | Total (%) | Survival status | PDO | Incidence density | |
| Death (%) | Censored (%) | |||||
| Hypotension | Absent | 523(96.1) | 69(13.2) | 454(86.8) | 6362 | 0.011 |
| Present | 21(3.9) | 12(57.14) | 9(42.86) | 202 | 0.059 | |
| Hypoxia | Yes | 42(7.7) | 32(76.19) | 10(23.81) | 574 | 0.055 |
| No | 502(92.2) | 49(9.76) | 453(90.24) | 5990 | 0.008 | |
| Severity of TBI based on admission GCS | Mild TBI | 295(54.2) | 6(2.03) | 289(97.97) | 3159 | 0.002 |
| Moderate TBI | 166(30.5) | 16(9.64) | 150(90.36) | 1796 | 0.009 | |
| Severe TBI | 83(15.3) | 59(71.08) | 24(28.92) | 1609 | 0.036 | |
| Pupillary reactivity to light | Both reactive | 434(79.8) | 23(5.3) | 411(94.7) | 4670 | 0.005 |
| Unilateral reactive | 64(11.7) | 22(34.38) | 42(65.63) | 835 | 0.026 | |
| Both nonreactive | 46(8.5) | 36(78.26) | 10(21.74) | 1059 | 0.034 | |
| Hypoglycemia | Absent | 510(93.7) | 54(10.59) | 456(89.41) | 5893 | 0.009 |
| Present | 34(6.3) | 27(79.41) | 7(20.59) | 671 | 0.04 | |
| Hyperglycemia | Absent | 433(79.6) | 46(10.62) | 387(89.38) | 4860 | 0.009 |
| Present | 111(20.4) | 35(31.53) | 76(68.47) | 1704 | 0.020 | |
| Hypothermia | Absent | 441(81) | 53(12.02) | 388(87.98) | 4607 | 0.012 |
| Present | 103(19) | 28(27.18) | 75(72.82) | 1957 | 0.014 | |
| Fever | Absent | 323(59.4) | 17(5.26) | 306(94.74) | 3882 | 0.004 |
| Present | 221(40.6) | 64(28.26) | 157(71.04) | 2682 | 0.023 | |
| Seizure | Yes | 65(22) | 24(36.92) | 41(63.08) | 721 | 0.033 |
| No | 479(88) | 57(11.9) | 422(88.1) | 5843 | 0.01 | |
| CT scan | Yes | 344(63.2) | 47(13.66) | 297(84.38) | 3892 | 0.012 |
| No | 200(36.8) | 34(17) | 166(83) | 2672 | 0.013 | |
|
Ct finding (N = 344) |
Brain contusion | 125(36.3) | 9(7.2) | 116(92.8) | 1327 | 0.007 |
| Subdural hematoma | 97(28.2) | 16(16.5) | 81(83.5) | 1114 | 0.014 | |
| Epidural hematoma | 62(18) | 5(8) | 57(92) | 693 | 0.007 | |
| DAI | 35(10) | 17(49.6) | 18(51.4) | 483 | 0.035 | |
| Others ** | 25(7.3) | 0 | 25(100) | 275 | 0 | |
| Comorbidity | Yes | 15(2.8) | 6(40) | 9(60) | 146 | 0.041 |
| No | 529(97.2) | 75(14.18) | 454(85.82) | 6418 | 0.012 | |
| Mannitol | Yes | 235(43.2) | 67(28.5) | 168(71.5) | 3519 | 0.019 |
| No | 309(56.8) | 14(4.53) | 295(95.47) | 3045 | 0.005 | |
| Neurosurgery | Yes | 247(45.4) | 24(9.72) | 223(90.28) | 3408 | 0.007 |
| No | 297(54.6) | 57(19.19) | 240(80.81) | 3156 | 0.018 | |
|
Type of neurosurgery (N = 247) |
Craniotomy | 134(54) | 17(12.69) | 117(87.31) | 1680 | 0.01 |
| Burrhole | 42(16.9) | 7(16.67) | 35(83.33) | 718 | 0.01 | |
| Elevation | 65(26.9) | 0 | 65(100) | 938 | 0 | |
| Other* | 7(2.8) | 1(14.29) | 6(85.71) | 77 | 0.013 | |
GCS: Glasgow coma scale,*craniectomy, toilet surgery, debridement, and duraplasty
DAI: diffused axonal injury ** depressed skull fracture, pneumocephalus, subarachnoid hemorrhage
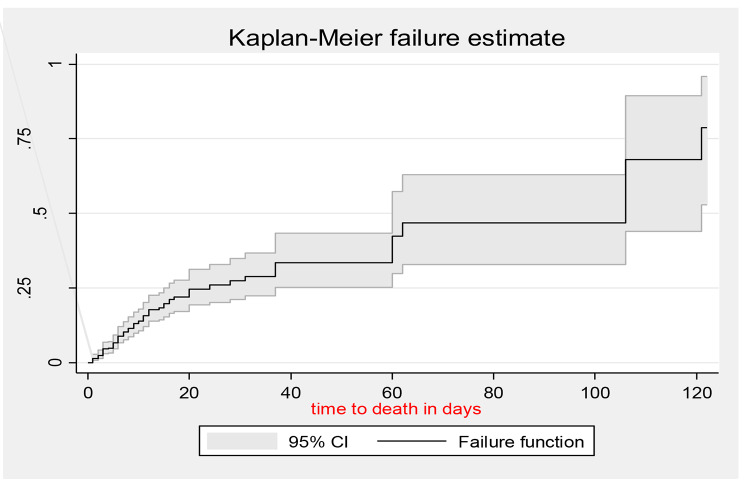
Incidence of mortality
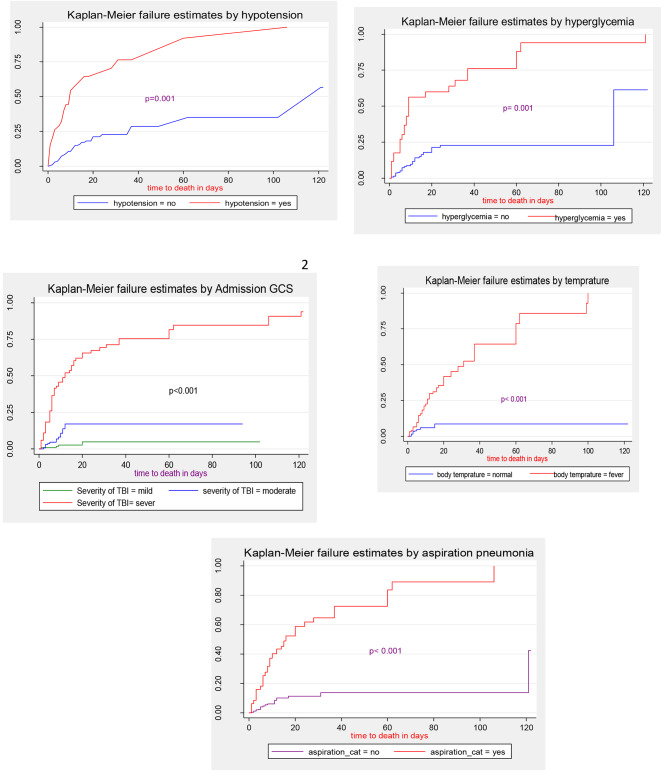
The total time at risk for 544 patients was 6,564 person-days with an incidence rate of 1.23 (95% CI: 1.0–1.5) per 100-person day observation. The maximum number of days of follow-up was 120 days. The overall median survival time was 106 (95% CI: 60–121) days (Fig. 1). Kaplan–Meier failure curve together with the log-rank test was fitted to test for the presence of a difference in the occurrence of death among the categorical variables. The incidence of mortality in patients with severe TBI was 3.66 per 100 person-days it was 0.9 and 0.2 per 100 person-days for moderate and mild TBI, respectively. At seven days of follow-up, the probability of survival for those who sustained mild, moderate, and severe TBI was 96.7%, 82.9%, and 48.1%, respectively. The incidence of mortality was higher among hypotensive patients (Fig. 2).
Fig. 1.
Overall Kaplan-Meier failure estimation of admitted TBI patients in the Amhara region, 2022
Fig. 2.
Kaplan-Meier failure estimate for hypotension, hyperglycemia, Aspiration pneumonia, GCS, and temperature among TBI patients admitted in the Amhara region, 2022
Factors associated with the incidence of mortality
In the bi-variable analysis of baseline clinical and management-related variables (initial GCS, admission systolic BP < 90 mmHg, hypoxia and neurosurgery), age, residence, type of head injury presence of extra-cranial injuries, presence of comorbidity, pupillary reactivity abnormality, episode of hyperglycemia, episode of seizure, presence of aspiration pneumonia, coagulopathy and an episode of hyperthermia, were showed association with time to death at a p-value < 0.25. However, in the final multivariable Weibull regression model; age, initial GCS, admission BP, neurosurgery, episode of hyperglycemia, coagulopathy, presence of associated aspiration pneumonia, type of head injury, and episode hyperthermia were found to be independent predictors of mortality. The hazard of death among patients who underwent neurosurgery was 53% (AHR: 0.47; 95% CI; 0.27-0 0.82) times lower than those who did not undergo neurosurgery. The hazard of death among patients with severe and moderate TBI was 10(AHR: 10; 95% CI; 3.5–28.2) and 9.2 times (AHR: 9.2; [95% CI 2.97-29]) higher than those with mild TBI, respectively. As age increases by one year, the hazard of death among TBI patients increased by 8% (AHR: 1.08; 95% CI; 1.06–1.1). The hazard of death among hypotensive patients was 6.9 (AHR: 6.9 [95% CI; 2.8–17.1]) times higher than those with normotensive. The hazard of death among hyperglycemic patients was 2.28 (AHR: 2.28; [95% CI; 1.13–4.6]) times higher than those with normoglycemic. The hazard of death among hyperthermic patients was 2.79 [AHR: 2.79; [95% CI; 1.4–5.5]) times higher than normothermic patients. The hazard of death among coagulopathic patients was 2.48 (AHR: 2.48; [95%CI 1.19–5.17]) higher than in patients that do not develop coagulopathy (Table 3).
Table 3.
Bi-variable and multivariable Weibull regression analysis for independent predictors of time to death among TBI patients admitted in n Amhara region, 2022
| Variables | Category | Survival status | CHR(95%CI) | AHR(95%CI) | P-value | |
|---|---|---|---|---|---|---|
| Death | Censored | |||||
| Age in years | Cont. | 81(14.8) | 463(85.1) | 1.06(1.04–1.07) | 1.08(1.06–1.1) | < 0.001* |
| Residence | Urban | 20(11.7) | 151(88.3) | 1 | 1 | 1 |
| Rural | 61(16.35) | 312(83.65) | 1.81(1.08-3). | 1.05(0.59–1.88) | 0.847 | |
| Extra-cranial injury | Present | 52(31.71) | 112(68.29) | 3.9(2.5–6.4) | 1(0.58–1.73) | 0.985 |
| Absent | 29(7.63) | 351(92.37) | 1 | 1 | 1 | |
| Type of head injury | Blunt | 18(13.74) | 113(86.26) | 1.4(0.82–2.4) | 3.2(1.5–6.8) | 0.002* |
| Penetrating | 63(15.25) | 350(84.75) | 1 | 1 | 1 | |
| The severity of the head injury | Mild | 6(2.03)) | 289(97.97) | 1 | 1 | 1 |
| Moderate | 16(9.64) | 150(90.36) | 4.5(1.8–11.6) | 9.2(2.97-29) | < 0.001* | |
| Sever | 59(71.08) | 24(28.92) | 23(10–54) | 10(3.6–28.2) | < 0.001* | |
| Pupillary reactivity | Bilateral reactive | 23(5.3%) | 411(94.7%) | 1 | 1 | 1 |
| Unilateral reactive | 22(34.38) | 42(65.63%) | 5.6(3.1–10) | 0.88(0.42–1.85) | 0.749 | |
| Both non-reactive | 36(78.26) | 10(21.74) | 8.9(5.2–15) | 0.74(0.33–1.65) | 0.466 | |
| Seizure | Yes | 24(36.92) | 41(63.08) | 3.9(2.9–6.4) | 1.15(0.51–2.57) | 0.733 |
| No | 57(11.9) | 422(88.1) | 1 | 1 | 1 | |
| Hypoxia | Yes | 32(76.19) | 10(23.81) | 6.6(4.2–10) | 1.46(0.79–2.72) | 0.223 |
| No | 49(9.76) | 453(90.24) | 1 | 1 | 1 | |
| Comorbidity | Yes | 6(40) | 9(60) | 3.4(1.5–7.8) | 0.36(0.11–1.13) | 0.081 |
| No | 75(14.18) | 454(85.82) | 1 | 1 | 1 | |
| Coagulopathy | Yes | 21(52.5) | 19(47.5) | 2.5(1.5–4.15) | 2.48(1.19–5.17) | 0.015 |
| No | 60(11.9) | 444(88.1) | 1 | 1 | 1 | |
| Aspiration pneumonia | Yes | 53(55.79) | 42(44.21) | 7.5(4.7–11.8) | 3.2(1.76–5.9) | < 0.001* |
| No | 28(6.24) | 421(93.76) | 1 | 1 | 1 | |
| Fever | Yes | 64(28.26) | 157(71.04) | 5.7(3.3–9.7) | 2.79(1.4–5.5) | 0.004* |
| No | 17(5.26) | 306(94.74) | 1 | 1 | 1 | |
| Hypotension | Yes | 24(61.5) | 15(38.5) | 5.04(3.1–8.1) | 6.9(2.8–17.1) | < 0.001* |
| No | 57(11.3) | 448(87.7) | 1 | 1 | 1 | |
| Hyperglycemia | No | 46(10.62) | 387(89.38) | 1 | 1 | 1 |
| Yes | 35(31.53) | 76(68.47) | 2.3(1.5–3.7) | 2.28(1.13–4.6) | 0.020* | |
| Neurosurgery | Yes | 24(9.72) | 223(90.28) | 0.4(0.25–0.65) | 0.47(0.27-0 0.82) | 0.008 * |
| No | 57(19.19) | 240(80.81) | 1 | 1 | 1 | |
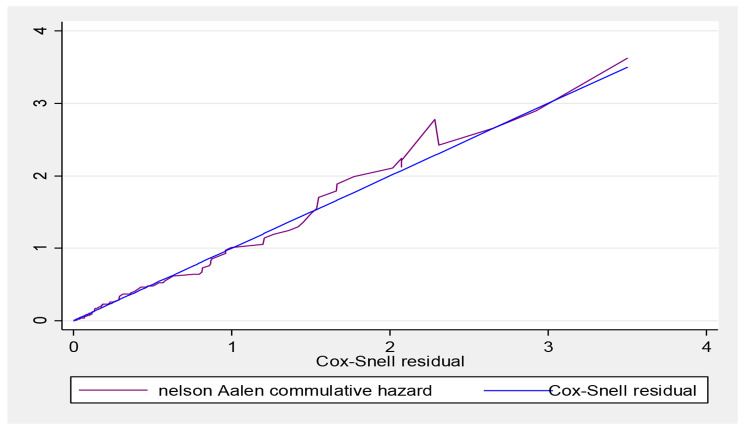
Proportional hazard assumptions were checked both graphically by using a log (-log) plot for each predictor variable and statistically using a Schoenfeld residual test and satisfied at (p-value = 0.9320). Log likely hood and Akaike Information Criteria (AIC) were applied to select the best-fitted model, based on this the Weibull regression was selected (Table 4). The goodness of fit test of the final model was checked by using Cox Snell residuals against the Nelson-Aalen cumulative hazard function. The hazard function follows 45˚ close to the baseline hazard which indicated that the model was well-fitted. For the residual test, it was possible to conclude that the final model fitted well (Fig. 3).
Table 4.
Summary of model comparison based on Akaike information criteria
| Model | Observation | Log likely Hood | DF | AIC | BIC |
|---|---|---|---|---|---|
| Weibull | 544 | -154.72 | 19 | 347.44 | 441.12 |
| Exponential | 544 | -159.94 | 18 | 355.88 | 433.26 |
| Gompertz | 544 | -159.93 | 19 | 357.86 | 439.54 |
| Cox | 544 | -313.13 | 17 | 660.26 | 733.34 |
Fig. 3.
Nelson-Aalen cumulative hazard graph against Cox-Snell residual on TBI patients admitted in Amhara region, 2022
Discussion
TBI is a leading cause of mortality and disability worldwide accounting for about 30% of all injury-related deaths. The overall incidence rate of mortality among TBI patients was found 1.23 (95%CI: 1.0-1.5) per 100 persons, day observation. This finding is lower than studies conducted in Hawasa university hospital’s 2.26 per 100-person day observation [5] and Felegehiwot comprehensive specialized hospital’s 25.53 per 1000-person day observation [14]. This discrepancy could be differences in time and variations in duration of follow-up in this research the total time at risk was 6544 person-days while it was 6,542 person-days and 4032 person-days for Hawasa and Felegehiwot respectively. This study found that 14.89% [95% CI (12.13–18.15%)] of patients died during the follow-up. This finding is in line with a study conducted at Hawasa University hospital12.7% [5]. But this result is lower than studies conducted in Jimma University Specialized Hospital21%[4], India 34.58% [2], Felegehiwot Comprehensive Specialized Hospital30.4%[14], and Qatar27% [27]. This possible discrepancy could be because there is a time difference which will lead to increased access to health institutions, increased awareness about early health-seeking behavior, and advancements in neurosurgical interventions. However, this result is higher than studies conducted in Tikur Anbesa specialized hospital10.3%[3] and China 5%[28]. This possible difference may be poor nursing interventions and a lack of advanced life support after trauma in our setting [29]. Delays in the care of patients with severe head injuries lead to worse outcomes both in terms of survival and functional status. The problem is compounded by a few hospitals providing neurosurgical services in the entire region. This has created even more delays to the optimum care that the patients can get [30]. And also the study period of this study was high in a conflict which may increase trauma admission and may further increase delays to the optimum care that the patients can get. The current finding revealed that age is a predictor of mortality following TBI. This result is supported by studies conducted in Canada [31], Tanzania [32, 33], Rwanda [34], Qatar [27], Hawasa [5], Tikur Anbesa [3], and Felegehiwot comprehensive specialized hospitals [14]. The reason behind this increased mortality could be older age patients have less immunity leading to infection and are also less likely to undergo surgery due to fear of complications related to anesthesia. Also, various cellular and molecular changes occur in the constituents of the brain during the aging process [35]. There is also white matter shrinkage and decreased myelination in the aging brain, which is essential for rapid, integrated neuronal communication [36]. Among injury-related factors, this study found that blunt TBIs have increased the death hazard as compared to penetrating TBIs. This result is in agreement with a study conducted in Uganda [37]. However, this result in on contrary to a study conducted in Texas America [38]. This possible difference could be due to giving less attention to blunt injuries in our setting. Tissue swelling from a traumatic brain injury can increase pressure inside the skull and cause additional damage to the brain leading to ischemia and necrosis [39]. Based on this study, the severity of TBI based on admission GCS was a strong predictor of mortality. This finding is parallel with studies conducted in different countries like Qatar [27], Uganda [37], Tanzania [32], Rwanda [34], Sub-Saharan Africa [15], Hawassa University Hospital [5], TASH [3], and Felegehiwot comprehensive specialized hospital [14]. The possible reason could be those patients with lower GCS scores are unable to protect their airways and they are at risk of aspiration, have poor infection prevention, are not a candidate for neurosurgery, and have less access to intensive care unit settings [40]. This research found that neurosurgical interventions are associated with the greatest decrement in the hazard of death among TBI patients. This finding is supported by different literature conducted in Tanzania [33], Spain [41], Uganda [37], Hawasa [5], and Felegehiwot [14]. This is due to bleeding outside or within the brain can result in a collection of clotted blood (hematoma) that puts pressure on the brain and damages brain tissue, thus surgery relieves pressure inside the skull by draining accumulated blood or creating a window in the skull that provides more room for swollen tissues [19].This study found that Coagulopathy patients have an increased hazard of death. This result is supported by a study conducted in America [38], and China [42]. This is because following trauma there is a massive release of tissue factor, altered protein C homeostasis, micro-particle up-regulation, and platelet hyperactivity which results in dysfunction and severely compromised hemostatic performance leading to death [43]. Hyperglycemia was an independent predictor of mortality following TBI. This finding is supported by a study done in Iran [44] and China [28]. This is true that following damage and stress catechol amines increase glucagon secretion and inhibit insulin secretion this will lead to the development of hyperglycemia which contributes to morbidity and mortality via generating a noxious cellular environment, causing electrolyte irregularities, and depressing immune efficacy [45]. On the other hand, hypotensive patients were at greatest risk of death hazard as compared to normotensive patients. This finding is in line with a study conducted at Hawasa university hospital [5].cerebral perfusion is directly proportional to mean arterial pressure so being hypotensive leads to decreased cerebral perfusion in addition to increased ICP leads to brain hypoxia and necrosis [46]. This study showed that being hyperthermic increases the death hazard as compared to normothermic patients. This result is parallel with a study conducted at Hawasa University hospital [5] and Felegehiwot comprehensive specialized hospital [14]. This is because as the patient becomes febrile metabolism in general increases and minor changes in brain temperature can result in significant changes in neural cell metabolism and therefore in brain function [47]. The presence of aspiration pneumonia was also positively associated with TBI mortality. This finding is supported by a study conducted in Felegehiwot comprehensive specialized hospital [14]. The entry of fluid into the bronchi and alveolar space triggers an anti-inflammatory reaction with the release of pro-inflammatory cytokines, tumor necrosis factor-alpha, and interleukins, which results in the infectious process [48].
Conclusion
The incidence of mortality rate among TBI patients was high. Age, moderate and severe TBI, hypotension at admission, coagulopathy, presence of associated aspiration pneumonia, undergoing the neurosurgical procedure, episode of hyperthermia, and hyperglycemia during hospitalization was the independent predictors of mortality among TBI patients. Therefore, interventions to reduce mortality should focus on the prevention of primary Injury and secondary brain injury by giving special emphasis to the patient with vital sign derangement, and following patients’ vital signs attentively. Better to take care of fluid management and better to give special attention to patient prioritization for neurosurgery.
Acknowledgements
The authors are grateful to the University of Gondar, Felege Hiwot, Tibebe Ghion, Debretabor, and Debre Markos comprehensive specialized hospital directors, data collectors, and study participants.
Abbreviations
- AHR
Adjusted Hazard Ratio
- CHR
Crude Hazard Ratio
- CI
Confidence Interval
- GCS
Glasgow Coma Scale
- LMICs
Low and Middle-Income Countries
- RTA
Road Traffic Accident
- TBI
Traumatic Brain Injury
- UOGCSH
University of Gondar Comprehensive Specialized Hospital
- USA
United States of America
- WHO
World Health Organization
Authors’ contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit it to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
No funding has been received for the conduct of this study and/or the preparation of this manuscript.
Data Availability
All data will be available upon a reasonable request. The reader could contact the corresponding author for the underlying data.
Declarations
Ethical approval and consent to participate
Ethical clearance was obtained from the school of nursing with ref. number S/N/240/2014 on behalf of the University of Gondar, Ethical Review Committee of the College of Medicine and health science. A formal letter of cooperation was written to each hospital. The primary identifiers (name and card number) were not collected. Waiver of informed consent was ascertained from the IRB of the University of Gondar. All information used from the charts is kept confidential in a secret place and on password-protected personal computers and used only for the study purpose. The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tiruye Azene Demlie, Email: tiruyeazene@gmail.com.
Mahlet Temesgen Alemu, Email: 15nemar.tw51@gmail.com.
Mengistu Abebe Messelu, Email: abebemengistu7@gmail.com.
Fasil Wagnew, Email: fasilw.n@gmail.com.
Enyew Getaneh Mekonen, Email: enyewgetaneh12@gmail.com.
References
- 1.Tesfaw A, Eshetu M, Teshome F, Fenta E, Gelaw M, Mihret G, et al. Prevalence of Head Injury among Trauma patients at Debre Tabor Comprehensive Specialized Hospital, North Central Ethiopia. Open Access Surgery. 2021;14:47–54. doi: 10.2147/OAS.S321404. [DOI] [Google Scholar]
- 2.Kamal VK, Agrawal D, Pandey RM. Epidemiology, clinical characteristics and outcomes of traumatic brain injury: evidence from integrated level 1 trauma center in India. J neurosciences rural Pract. 2016;7(04):515–25. doi: 10.4103/0976-3147.188637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landes M, Venugopal R, Berman S, Heffernan S, Maskalyk J, Azazh A. Epidemiology, clinical characteristics and outcomes of head injured patients in an ethiopian emergency center. Afr J Emerg Med. 2017;7(3):130–4. doi: 10.1016/j.afjem.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aenderl I, Gashaw T, Siebeck M, Mutschler W. Head injury–a neglected public health problem: a four-month prospective study at Jimma University Specialized Hospital, Ethiopia. Ethiop J health Sci. 2014;24(1):27–34. doi: 10.4314/ejhs.v24i1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assele DD, Lendado TA, Awato MA, Workie SB, Faltamo WF. Incidence and predictors of mortality among patients with head injury admitted to Hawassa University Comprehensive Specialized Hospital, Southern Ethiopia: a retrospective follow-up study. PLoS ONE. 2021;16(8):e0254245. doi: 10.1371/journal.pone.0254245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 7.Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung Y-C, Punchak M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;130(4):1080–97. doi: 10.3171/2017.10.JNS17352. [DOI] [PubMed] [Google Scholar]
- 8.Haagsma JA, Graetz N, Bolliger I, Naghavi M, Higashi H, Mullany EC, et al. The global burden of injury: incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj Prev. 2016;22(1):3–18. doi: 10.1136/injuryprev-2015-041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Global status report on road safety 2015. World Health Organization; 2015.
- 10.Daugherty J, Waltzman D, Sarmiento K, Xu L. Traumatic brain injury-related deaths by race/ethnicity, sex, intent, and mechanism of injury—United States, 2000–2017. Morb Mortal Wkly Rep. 2019;68(46):1050. doi: 10.15585/mmwr.mm6846a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James SL, Theadom A, Ellenbogen RG, Bannick MS, Montjoy-Venning W, Lucchesi LR, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveillance Summaries. 2017;66(9):1. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emejulu J, Isiguzo C, Agbasoga C, Ogbuagu C. Traumatic brain injury in the accident and emergency department of a tertiary hospital in Nigeria. East and Central African Journal of Surgery. 2010;15(2):28–38. [Google Scholar]
- 14.Amare AT, Tesfaye TD, Ali AS, Woelile TA, Birlie TA, Kebede WM, et al. Survival status and predictors of mortality among traumatic brain injury patients in an ethiopian hospital: a retrospective cohort study. Afr J Emerg Med. 2021;11(4):396–403. doi: 10.1016/j.afjem.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eaton J, Hanif AB, Grudziak J, Charles A, Epidemiology Management, and functional outcomes of traumatic brain Injury in Sub-Saharan Africa. World Neurosurg. 2017;108:650–5. doi: 10.1016/j.wneu.2017.09.084. [DOI] [PubMed] [Google Scholar]
- 16.Asmamaw Y, Yitayal M, Debie A, Handebo S. The costs of traumatic head injury and associated factors at University of Gondar Specialized Referral Hospital, Northwest Ethiopia. BMC Public Health. 2019;19(1):1–7. doi: 10.1186/s12889-019-7800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeNett T, Feltner C. Hypertonic saline versus mannitol for the treatment of increased intracranial pressure in traumatic brain injury. J Am Association Nurse Practitioners. 2021;33(4):283–93. doi: 10.1097/JXX.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 18.Laeke T, Tirsit A, Kassahun A, Sahlu A, Yesehak B, Getahun S, et al. Prospective study of surgery for traumatic brain Injury in Addis Ababa, Ethiopia: Surgical Procedures, Complications, and postoperative outcomes. World Neurosurg. 2021;150:e316–e23. doi: 10.1016/j.wneu.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Iaccarino C, Gerosa A, Viaroli E. Epidemiology of traumatic brain Injury. In: Honeybul S, Kolias AG, editors. Traumatic Brain Injury: Science, Practice, evidence and Ethics. Cham: Springer International Publishing; 2021. pp. 3–11. [Google Scholar]
- 20.Woyessa AH, Heyi WD, Ture NH, Moti BK. Patterns of road traffic accident, nature of related injuries, and post-crash outcome determinants in western Ethiopia - a hospital-based study. Afr J Emerg medicine: Revue africaine de la Med d’urgence. 2021;11(1):123–31. doi: 10.1016/j.afjem.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chanyalew MA, Yitayal M, Atnafu A, Tilahun B. Routine health information system utilization for evidence-based decision making in Amhara national regional state, northwest Ethiopia: a multi-level analysis. BMC Med Inf Decis Mak. 2021;21(1):1–10. doi: 10.1186/s12911-021-01400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savitsky B, Givon A, Rozenfeld M, Radomislensky I, Peleg K. Traumatic brain injury: it is all about definition. Brain Injury.30(10):1194–200. [DOI] [PubMed]
- 23.Surgeons ACo . Advanced Trauma Life Support (ATLS). Introduction. Chicago, United States: American College of Surgeons; 2018. p. xxix. [Google Scholar]
- 24.Duran M, ULUDAG Ö. Mortality analysis of hospitalized trauma patients in the intensive care unit. J Surg Med. 2020;4(11):994–7. doi: 10.28982/josam.780138. [DOI] [Google Scholar]
- 25.Balijepalli C, Druyts E, Siliman G, Joffres M, Thorlund K, Mills EJ. Hypoglycemia: a review of definitions used in clinical trials evaluating antihyperglycemic drugs for diabetes. Clin Epidemiol. 2017;9:291. doi: 10.2147/CLEP.S129268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao C, Zhang D, Sun B, Lan L, Cui W, Xu G, et al. Optimal cut-off points of fasting plasma glucose for two-step strategy in estimating prevalence and screening undiagnosed diabetes and pre-diabetes in Harbin, China. PLoS ONE. 2015;10(3):e0119510. doi: 10.1371/journal.pone.0119510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Menyar A, Consunji R, Abdelrahman H, Latifi R, Wahlen BM, Al-Thani H. Predictors, and Time-Based Hospital Mortality in patients with isolated and Polytrauma Brain Injuries. World J Surg. 2018;42(5):1346–57. doi: 10.1007/s00268-017-4310-2. [DOI] [PubMed] [Google Scholar]
- 28.Gao G, Wu X, Feng J, Hui J, Mao Q, Lecky F, et al. Clinical characteristics and outcomes in patients with traumatic brain injury in China: a prospective, multicentre, longitudinal, observational study. Lancet Neurol. 2020;19(8):670–7. doi: 10.1016/S1474-4422(20)30182-4. [DOI] [PubMed] [Google Scholar]
- 29.De Ramirez SS, Hyder AA, Herbert HK, Stevens K. Unintentional injuries: magnitude, prevention, and control. Annu Rev Public Health. 2012;33:175–91. doi: 10.1146/annurev-publhealth-031811-124558. [DOI] [PubMed] [Google Scholar]
- 30.Laeke T, Tirsit A, Debebe F, Girma B, Gere D, Park KB, et al. Profile of head injuries: prehospital care, diagnosis, and severity in an ethiopian tertiary hospital. World Neurosurg. 2019;127:e186–e92. doi: 10.1016/j.wneu.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 31.Fu WW, Fu TS, Jing R, McFaull SR, Cusimano MD. Predictors of falls and mortality among elderly adults with traumatic brain injury: a nationwide, population-based study. PLoS ONE. 2017;12(4):e0175868. doi: 10.1371/journal.pone.0175868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smart LR, Mangat HS, Issarow B, McClelland P, Mayaya G, Kanumba E, et al. Severe traumatic brain injury at a tertiary referral Center in Tanzania: epidemiology and adherence to Brain Trauma Foundation guidelines. World Neurosurg. 2017;105:238–48. doi: 10.1016/j.wneu.2017.05.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elahi C, Rocha TAH, da Silva NC, Sakita FM, Ndebea AS, Fuller A, et al. An evaluation of outcomes in patients with traumatic brain injury at a referral hospital in Tanzania: evidence from survival analysis. NeuroSurg Focus. 2019;47(5):E6. doi: 10.3171/2019.7.FOCUS19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krebs E, Gerardo CJ, Park LP, Vissoci JRN, Byiringiro JC, Byiringiro F, et al. Mortality-associated characteristics of patients with traumatic brain injury at the University Teaching Hospital of Kigali, Rwanda. World Neurosurg. 2017;102:571–82. doi: 10.1016/j.wneu.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satoh A, Imai S-i. The brain, sirtuins, and ageing. Nat Rev Neurosci. 2017;18(6):362–74. doi: 10.1038/nrn.2017.42. [DOI] [PubMed] [Google Scholar]
- 36.Rivera A, Vanzuli I, Julio Rodríguez Arellano J, Butt A. Decreased regenerative capacity of oligodendrocyte progenitor cells (NG2-glia) in the aging brain: a vicious cycle of synaptic dysfunction, myelin loss, and neuronal disruption? Curr Alzheimer Res. 2016;13(4):413–8. doi: 10.2174/1567205013666151116125518. [DOI] [PubMed] [Google Scholar]
- 37.Abdelgadir J, Smith ER, Punchak M, Vissoci JR, Staton C, Muhindo A, et al. Epidemiology and characteristics of neurosurgical conditions at Mbarara Regional Referral Hospital. World Neurosurg. 2017;102:526–32. doi: 10.1016/j.wneu.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Folkerson LE, Sloan D, Davis E, Kitagawa RS, Cotton BA, Holcomb JB, et al. Coagulopathy as a predictor of mortality after penetrating traumatic brain injury. Am J Emerg Med. 2018;36(1):38–42. doi: 10.1016/j.ajem.2017.06.057. [DOI] [PubMed] [Google Scholar]
- 39.Okidi R, Ogwang DM, Okello TR, Ezati D, Kyegombe W, Nyeko D, et al. Factors affecting mortality after traumatic brain injury in a resource-poor setting. BJS Open. 2019;4(2):320–5. doi: 10.1002/bjs5.50243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agorogianni D, Michalopoulos E, Prantzou A, Liaskou C, Stamou A, Kapadochos T, et al. Clinical indicators as prognostic factors of multi-trauma patients in the Intensive Care Unit. Health & Research Journal. 2021;7(4):206–18. doi: 10.12681/healthresj.28191. [DOI] [Google Scholar]
- 41.Sahuquillo J, Dennis JA. Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst Rev. 2019;12(12):Cd003983. doi: 10.1002/14651858.CD003983.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan Q, Yu J, Wu X, Sun Y-r, Li Z-q, Du Z-y, et al. Prognostic value of coagulation tests for in-hospital mortality in patients with traumatic brain injury. Scand J Trauma Resusc Emerg Med. 2018;26(1):1–9. doi: 10.1186/s13049-017-0471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albert V, Arulselvi S, Agrawal D, Pati HP, Pandey RM. Early posttraumatic changes in coagulation and fibrinolysis systems in isolated severe traumatic brain injury patients and its influence on the immediate outcome. Hematol Oncol Stem Cell Ther. 2019;12(1):32–43. doi: 10.1016/j.hemonc.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Kafaki SB, Alaedini K, Qorbani A, Asadian L, Haddadi K. Hyperglycemia: a predictor of death in severe head injury patients. Clin Med Insights: Endocrinol Diabetes. 2016;9:CMED. doi: 10.4137/CMED.S40330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rau C-S, Wu S-C, Chen Y-C, Chien P-C, Hsieh H-Y, Kuo P-J, et al. Stress-induced hyperglycemia, but not diabetic hyperglycemia, is associated with higher mortality in patients with isolated moderate and severe traumatic brain injury: analysis of a propensity score-matched population. Int J Environ Res Public Health. 2017;14(11):1340. doi: 10.3390/ijerph14111340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinoshita K. Traumatic brain injury: pathophysiology for neurocritical care. J Intensive Care. 2016;4(1):29. doi: 10.1186/s40560-016-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mrozek S, Vardon F, Geeraerts T. Brain temperature: physiology and pathophysiology after brain injury. Anesthesiology research and practice. 2012;2012. [DOI] [PMC free article] [PubMed]
- 48.Sanivarapu RR, Gibson J. Aspiration pneumonia. StatPearls [Internet]: StatPearls Publishing; 2021. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be available upon a reasonable request. The reader could contact the corresponding author for the underlying data.