Abstract
Introduction
Drugs that prevent the onset, slow progression, or improve cognitive and behavioral symptoms of Alzheimer's disease (AD) are needed.
Methods
We searched ClinicalTrials.gov for all current Phase 1, 2 and 3 clinical trials for AD and mild cognitive impairment (MCI) attributed to AD. We created an automated computational database platform to search, archive, organize, and analyze the derived data. The Common Alzheimer's Disease Research Ontology (CADRO) was used to identify treatment targets and drug mechanisms.
Results
On the index date of January 1, 2023, there were 187 trials assessing 141 unique treatments for AD. Phase 3 included 36 agents in 55 trials; 87 agents were in 99 Phase 2 trials; and Phase 1 had 31 agents in 33 trials. Disease‐modifying therapies were the most common drugs comprising 79% of drugs in trials. Twenty‐eight percent of candidate therapies are repurposed agents. Populating all current Phase 1, 2, and 3 trials will require 57,465 participants.
Discussion
The AD drug development pipeline is advancing agents directed at a variety of target processes.
HIGHLIGHTS
There are currently 187 trials assessing 141 drugs for the treatment of Alzheimer's disease (AD).
Drugs in the AD pipeline address a variety of pathological processes.
More than 57,000 participants will be required to populate all currently registered trials.
Keywords: Alzheimer's disease, amyloid, biomarkers, clinical trials, Common Alzheimer's Disease Research Ontology (CADRO), drug development, inflammation, pharmaceutical companies, repurposed drugs, synaptic function, tau
1. INTRODUCTION
Alzheimer's disease (AD) is increasing at an alarming pace as the population of the United States and the world age. There are an estimated 6.2 million individuals with AD dementia in the United States and an estimated 50 million individuals with AD dementia globally. These populations will grow to 12.7 million and 150 million in the United States and globally, respectively, by 2050. 1 , 2 In addition to AD dementia, there are an approximately equal number of individuals with prodromal AD and an even larger number of persons with preclinical AD characterized by normal cognition, biomarkers consistent with AD pathology, and an increased risk for progression to cognitive impairment. 2 , 3 These epidemiologic predictions make it increasingly urgent that new medications to prevent the onset, delay progression, or improve symptoms of AD be found.
The goal of this review is to describe the current AD drug development pipeline; note trends in clinical trial design, clinical outcome measures, and biomarker use in trials; and review which drug mechanisms of action (MoAs) and biological targets are being pursued. Monoclonal antibodies and other biological agents in the current AD pipeline are discussed, small molecules intended to produce disease modification currently in clinical trials are reviewed, and symptomatic agents seeking to produce cognitive enhancement or reduce neuropsychiatric symptoms in AD are reported. This review is based on data derived from the ClinicalTrials.gov registry. The report follows the approach of our previous annual reviews of the AD drug development pipeline. 4 , 5
2. METHODS
The US National Library of Medicine of the National Institutes of Health (NIH) maintains a clinical research registry, ClinicalTrials.gov, which serves as the source of information for this review. The US Food and Drug Administration (FDA) Amendments Act requires that all clinical trials be registered on ClinicalTrials.gov. The “Common Rule” governing ClinicalTrials.gov requires registration for studies that meet the definition of an “applicable clinical trial” (HR3580, 2007). Registration must occur within 21 days of enrolling the first patient in the trial. Studies of compliance with the Common Rule indicate that compliance with the rule is high and most trials are registered appropriately. 6 , 7 The United States has more clinical trials than any other country, ClinicalTrials.gov includes most therapies currently in clinical trials for AD globally, and ClinicalTrials.gov is more comprehensive than any other trial registry. 8 The information in this review can be regarded as comprehensive but not exhaustive.
RESEARCH IN CONTEXT
Systemic Review: Alzheimer's disease (AD) represents a complex disorder for which there are few treatments. Candidate therapies for AD are assessed in clinical trials and are registered on ClinicalTrials.gov. We reviewed clinical trials and the drugs being assessed to understand the flow of drugs from laboratories to the clinic.
Interpretation: There are currently 187 Phase 1, 2, and 3 clinical trials assessing 141 unique drugs. Thirty‐six drugs are being assessed in Phase 3, 87 in Phase 2, and 31 in Phase 1. Transmitter receptors, amyloid, synaptic function, and inflammation are the most common targets of drugs in the pipeline.
Future Directions: Clinical trials represent the only means of generating efficacy and safety data that can lead to drug approval and widespread availability. The AD drug development pipeline includes agents addressing a variety of targets and intended for different phases of AD. Incentives for AD drug development are needed.
The index date for this review is January 1, 2023, and the text and tables apply to the information as registered on ClinicalTrials.gov on this date. We searched all terms related to AD and mild cognitive impairment (MCI) for inclusion in the review. We do not include studies whose participants have dementia of any cause or in which AD is included with other dementias not separated by inclusion and exclusion criteria. We do not include trials in which the MCI is specified to be part of a non‐AD disease such as MCI of Parkinson's disease. We include all trials of agents in Phases 1, 2, and 3. We did not include Phase 4 trials or trials without a phase designation. If a trial is designated as 1/2 or 2/3 we include it with trials of the higher number. We archive information on the trial agent, trial title, trial number assigned on ClinicalTrials.gov, start date, projected primary end date, duration of treatment exposure, number of arms of the study (usually a placebo arm and one or more treatment arms with different doses), whether a biomarker was collected at entry or as an outcome, whether the agent was repurposed, and where the trials were performed. We use the “funder type” trial sponsorship categories specified on ClinicalTrials.gov (the biopharmaceutical industry; public– private partnership; NIH and related including individuals, universities, and organizations; and “other” [non‐NIH] federal entities). We identified “public–private partnerships” as any trial in which a biopharmaceutical company was one of two or more sponsors for the trial. We included trials labeled as recruiting, active but not recruiting (i.e., trials that have completed recruitment and are continuing with the exposure portion of the trial), enrolling by invitation (i.e., open‐label extensions of trials limited to those participating in the double‐blind portion of the trials), and not yet recruiting (i.e., registered on ClinicalTrials.gov but no patients have been enrolled). We note if the trial population comprises participants with preclinical AD (cognitively normal with biomarker evidence of AD or an autosomal dominant AD‐causing mutation participating in AD prevention trials), MCI, AD dementia (mild, moderate, severe), or healthy volunteers. We note the trials listed as completed, terminated, suspended, unknown, or withdrawn since the last index date. The report does not include trials of non‐pharmacologic therapeutic approaches such as exercise trials, cognitive‐behavior therapies, caregiver interventions, supplements, medical foods, or devices. We do not include trials of biomarkers if no intervention is being tested; we note whether biomarkers were collected at trial entry or were included as outcome measures in the intervention trials we report. Cell therapies are included among the interventions described (they are not included in Figure 1).
FIGURE 1.
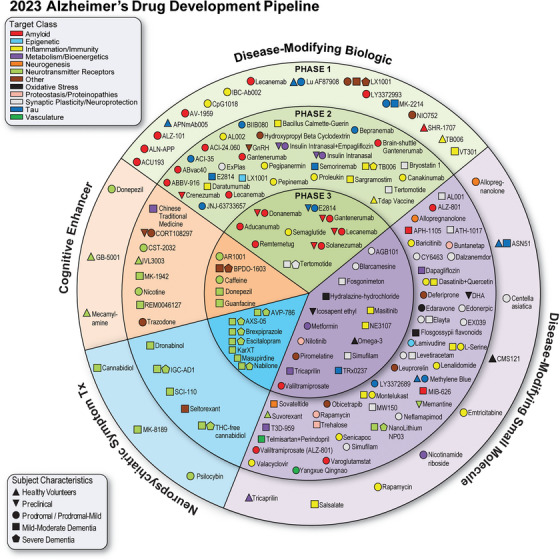
Agents in clinical trials for treatment of Alzheimer's disease in 2023 (from ClinicalTrials.gov as of the index date of January 1, 2023). The inner ring shows Phase 3 agents; the middle ring comprises Phase 2 agents; the outer ring presents Phase 1 therapies; agents in green areas are biologics; agents in purple are disease‐modifying small molecules; agents in orange areas are symptomatic agents addressing cognitive enhancement or behavioral and neuropsychiatric symptoms; the shape of the icon shows the population of the trial; the icon color shows the CADRO‐based class of the agent (“Other” category includes CADRO classes that have three or fewer agents in trials). CADRO, Common Alzheimer's Disease Research Ontology; Tx, treatment. (Figure © J Cummings; M de la Flor, PhD, Illustrator).
We use the Actual Study Start Date as listed on ClinicalTrials.gov for the beginning of the trial and the Estimated Primary Completion Date for the anticipated end of the trial. The total trial duration is the projected period between the actual study start date and the estimated primary completion date. The treatment exposure duration is specified on ClinicalTrials.gov; the recruitment period is calculated as the total trial duration minus the treatment study period.
The Common Alzheimer's Disease Research Ontology (CADRO) of the National Institute on Aging and the Alzheimer's Association; the International Alzheimer's and Related Dementias Research Portfolio (IADRP; iadrp.nia.nih.gov) provides the basis for the description of the biological processes in AD that comprise possible targets for therapeutic intervention. The CADRO Translational Research and Clinical Interventions Category lists potential targets for AD clinical therapies. The targets include amyloid beta; tau; apolipoprotein E (APOE), lipids, and lipoprotein receptors; neurotransmitter receptors; neurogenesis; inflammation; oxidative stress; cell death; proteostasis/proteinopathies; metabolism and bioenergetics; vasculature; growth factors and hormones; synaptic plasticity/neuroprotection; gut–brain axis; circadian rhythm; epigenetic regulators; multi‐target; unknown target; and other. These processes/targets are used to classify the target category of the agents. Some agents may have more than one MoA; for these, we reviewed the literature to identify the putative predominant mechanisms.
Treatments whose purpose is cognitive enhancement or control of neuropsychiatric symptoms without claiming to impact the underlying biological causes of AD are classified as “symptomatic.” Treatments intended to change the biology of AD and slow the course of the disease are listed as “disease modifying.” If the sponsor did not specify the therapeutic purpose, we used the features of the trial (e.g., clinical outcomes, trial duration, use of biomarkers for participant inclusion, use of biomarkers as outcomes, number of participants) to infer if a trial was structured to demonstrate disease modification or symptomatic benefit. We divided disease‐modifying therapies (DMTs) into biologics (e.g., monoclonal antibodies, vaccines, antisense oligonucleotides [ASOs], gene therapy, etc.) and small molecules (e.g., drugs typically taken orally and less than 500 Daltons in molecular weight).
To determine whether an agent is approved for a non‐AD indication and considered a repurposed agent in the pipeline, we used the currently available version of DrugBank (https://go.drugbank.com/).
We downloaded all the original data using the ClinicalTrials.gov application programming interface (API) (https://clinicaltrials.gov/api/gui). We implemented an automated Python script‐based computational database platform to search for the appropriate trials on ClinicalTrials.gov and to interrogate and analyze data from the derived database. As an initial filtering step, we identified all the interventional trials designed with the primary purpose of prevention, treatment, or basic science, and including at least one intervention type including drug, dietary supplement, or biological. We then eliminated all non‐drug trials. Stem cell trials were assembled separately. If questions arose during the analytic process about the nature of the intervention or other trial aspects, they were resolved by expert curation. We generated summary statistics (e.g., mean and count) using the annotated trial data for all analyses.
3. RESULTS
3.1. Overview
There were 187 Phase 1, 2, or 3 clinical trials assessing 141 unique treatments for AD as of the index date of January 1, 2023. There were 36 agents in 55 Phase 3 trials, 87 agents in 99 Phase 2 trials, and 31 agents in 33 Phase 1 trials (some agents are in more than one trial; Figure 1). Among the Phase 1, 2, and 3 trials, the most common agents being studied are DMTs (111 agents; 78% of the total number of drugs in these trials). Symptomatic agents comprise 21% (N = 30) of the pipeline including 15 (11% of all agents in Phase 1, 2, or 3 trials) cognitive enhancers and 15 (11% of all agents in these trials) psychotropic agents. Of the DMTs, there were 49 (44% of DMTs) biologics and 62 (56% of DMTs) small molecules. From the target perspective, 22 (16%) of agents have amyloid, 13 (9%) tau, 24 (17%) inflammation, 18 (13%) synaptic plasticity/neuroprotection, 10 (7%) metabolism and bioenergetics, 7 (5%) oxidative stress, and 4 (3%) proteostasis/proteinopathy as their primary mechanistic targets. Twenty‐eight agents (29%) have neurotransmitters as their biological target; this class includes cognitive‐enhancing agents and drugs being developed to reduce neuropsychiatric symptoms. Sixteen drugs (11%) target processes represented by only one to three agents per CADRO category. Considering DMTs only, 24 (67%) of Phase 3 agents are DMTs; 74 (85%) Phase 2 drugs are DMTs; and 25 (81%) of Phase 1 agents are DMTs. There are 40 repurposed agents in the pipeline comprising 28% of candidate therapies (all phases combined). There are eight ongoing trials involving stem cell therapies. Since January 25, 2022, 31 trials have been completed, 2 were suspended, 15 are of unknown status, and 1 each was terminated or withdrawn. Fifty‐eight new trials (16 in Phase 1, 27 in Phase 2, 15 in Phase 3) have entered the pipeline in the past year (since index date of January 25, 2022).
3.2. Phase 3
Phase 3 has 36 agents in 55 trials (Figure 1, Figure 2, and Table 1). DMTs represent 67% (N = 24) of agents in Phase 3 trials including 9 (25% of the Phase 3 agents) biologics and 15 (42%) small molecules. Five (14% of Phase 3 agents) are putative cognitive‐enhancing agents and seven (19%) drugs target neuropsychiatric symptoms of AD. CADRO mechanisms represented among Phase 3 agents include amyloid (7 agents; 19%), synaptic plasticity/neuroprotection (6; 17%), oxidative stress (3; 8%), metabolism and bioenergetics (3; 8%), tau (2; 6%), inflammation (2; 6%), proteostasis/proteinopathies (1; 3%), and circadian rhythm (1; 3%). Eleven agents in Phase 3 (31%) address transmitter receptor mechanisms. Figures 1 and 2 show the CADRO‐based MOAs of agents in Phase 3. Twelve (33%) of Phase 3 agents are repurposed treatments approved for use in another indication (6 = DMT; 3 = for cognitive enhancement; 3 = for treatment for behavioral symptoms). Six trials that were active in 2022 were completed, one was suspended, and one is of unknown status. Fifteen Phase 3 trials were initiated between January 25, 2022, and January 1, 2023.
FIGURE 2.
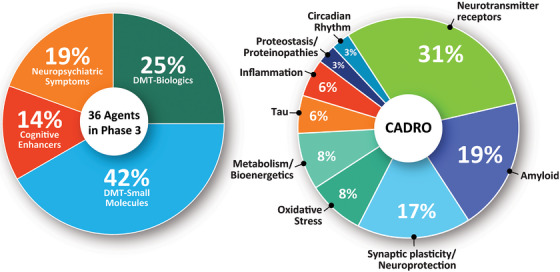
Mechanisms of action of agents in Phase 3 (as classified using the CADRO approach). CADRO, Common Alzheimer's Disease Research Ontology; DMT, disease‐modifying therapy. (Figure © J Cummings; M de la Flor, PhD, Illustrator).
TABLE 1.
Agents in Phase 3 of Alzheimer's disease drug development (ClinicalTrials.gov accessed January 1, 2023).
| Agent | Therapeutic purpose | CADRO target | Mechanism of action | Clinical trial NCT# | Lead sponsor | Start date | Estimated primary completion date |
|---|---|---|---|---|---|---|---|
| Aducanumab | DMT, biologic | Amyloid beta | Anti‐amyloid monoclonal antibody directed at plaques and oligomers | NCT04241068 | Biogen | Mar 2020 | Oct 2023 |
| NCT05310071 | Biogen | Jun 2022 | Dec 2025 | ||||
| AGB101 | DMT, small molecule | Synaptic plasticity/neuroprotection | SV2A modulator; CA3 area downregulation | NCT03486938 | AgeneBio | Jan 2019 | Dec 2022 |
| AR1001 | Sx, cognition | Neurotransmitter receptors | Phosphodiesterase 5 inhibitor increases intracellular cGMP promoting synaptic plasticity | NCT05531526 | AriBio Co., Ltd. | Dec 2022 | Dec 2025 |
| AVP‐786 | Sx, behavior | Neurotransmitter receptors | NMDA receptor antagonist, sigma 1 receptor agonist; serotonin and norepinephrine transporter inhibitor | NCT02446132 | Otsuka Pharmaceutical Development & Commercialization, Inc. | Dec 2015 | Oct 2023 |
| NCT03393520 | Otsuka Pharmaceutical Development & Commercialization, Inc. | Oct 2017 | Jul 2023 | ||||
| NCT04408755 | Otsuka Pharmaceutical Development & Commercialization, Inc. | Jul 2020 | Dec 2024 | ||||
| NCT04464564 | Otsuka Pharmaceutical Development & Commercialization, Inc. | Sep 2020 | Dec 2024 | ||||
| AXS‐05 | Sx, behavior | Neurotransmitter receptors | NMDA receptor antagonist, sigma 1 receptor agonist; serotonin and norepinephrine transporter inhibitor | NCT04947553 | Axsome Therapeutics, Inc. | Jun 2021 | Jun 2023 |
| NCT05557409 | Axsome Therapeutics, Inc. | Sep 2022 | Jun 2025 | ||||
| Blarcamesine (Anavex 2‐73) | DMT, small molecule | Synaptic plasticity/neuroprotection | Sigma‐1 receptor agonist, M2 autoreceptor antagonist | NCT04314934 | Anavex Life Sciences Corp. | Oct 2019 | Jul 2024 |
| BPDO‐1603 | Sx, cognition | Synaptic plasticity/neuroprotection | Undisclosed | NCT04229927 | Hyundai Pharmaceutical Co., LTD. | Feb 2020 | Feb 2022 |
| Brexpiprazole | Sx, behavior | Neurotransmitter receptors | Atypical antipsychotic; D2 receptor partial agonist and serotonin‐dopamine modulator | NCT03620981 | Otsuka Pharmaceutical Co., Ltd. | Aug 2018 | Mar 2023 |
| Caffeine | Sx, cognition | Neurotransmitter receptors | Adenosine antagonist; non‐specific phosphodiesterase inhibitor | NCT04570085 | University Hospital, Lille | Mar 2021 | Nov 2024 |
| Donanemab | DMT, biologic | Aβ | Anti‐amyloid monoclonal antibody specific for pyroglutamate plaque amyloid | NCT04437511 | Eli Lilly and Company | Jun 2020 | Apr 2023 |
| NCT05026866 | Eli Lilly and Company | Aug 2021 | Oct 2027 | ||||
| NCT05108922 | Eli Lilly and Company | Nov 2021 | Sep 2022 | ||||
| NCT05508789 | Eli Lilly and Company | Oct 2022 | Apr 2027 | ||||
| Donepezil | Sx, cognition | Neurotransmitter receptors | Acetylcholinesterase inhibitor; adipokine modulation | NCT04661280 | Assistance Publique—Hôpitaux de Paris | Feb 2022 | Aug 2024 |
| NCT05592678 | The University of Texas Health Science Center at San Antonio | Feb 2023 | Feb 2027 | ||||
| E2814 | DMT, biologic | Tau | Anti‐tau monoclonal antibody | NCT01760005 | Washington University School of Medicine | Dec 2012 | Oct 2027 |
| NCT05269394 | Washington University School of Medicine | Dec 2021 | Jul 2027 | ||||
| Escitalopram | Sx, behavior | Neurotransmitter receptors | Selective serotonin reuptake inhibitor | NCT03108846 | JHSPH Center for Clinical Trials | Jan 2018 | Dec 2021 |
| Fosgonimeton (ATH‐1017) | DMT, small molecule | Synaptic plasticity/neuroprotection | Hepatocyte growth factor (HGF); activates signaling via the HGF/MET receptor system; promotes survival of neurons, enhances hippocampal synaptic plasticity | NCT04488419 | Athira Pharma | Sep 2020 | Sep 2022 |
| Gantenerumab | DMT, biologic | Aβ | Anti‐amyloid monoclonal antibody directed at amyloid oligomers and plaque | NCT01760005 | Washington University School of Medicine | Dec 2012 | Oct 2027 |
| NCT03443973 | Hoffmann‐La Roche | Aug 2018 | Sep 2022 | ||||
| NCT03444870 | Hoffmann‐La Roche | Jun 2018 | Dec 2022 | ||||
| NCT04339413 | Hoffmann‐La Roche | May 2020 | Jan 2023 | ||||
| NCT04374253 | Hoffmann‐La Roche | Feb 2021 | Feb 2023 | ||||
| NCT05256134 | Hoffmann‐La Roche | Apr 2022 | Mar 2023 | ||||
| NCT05552157 | Washington University School of Medicine | Dec 2022 | Nov 2029 | ||||
| Guanfacine | Sx, cognition | Neurotransmitter receptors | Alpha‐2 adrenergic agonist | NCT03116126 | Imperial College London | Jan 2019 | Dec 2022 |
| Hydralazine hydrochloride | DMT, small molecule | Oxidative stress | Free radical scavenger | NCT04842552 | Shahid Sadoughi University of Medical Sciences and Health Services | Aug 2021 | Jun 2023 |
| Icosapent ethyl | DMT, small molecule | Oxidative stress | Purified form of the omega‐3 fatty acid eicosapentaenoic acid (EPA) | NCT02719327 | VA Office of Research and Development | Jun 2017 | Sep 2023 |
| KarXT (Xanomeline + Trospium) | Sx, behavior | Neurotransmitter receptors | Muscarinic cholinergic agonist with peripheral anticholinergic agent | NCT05511363 | Karuna Therapeutics | Aug 2022 | Mar 2025 |
| Lecanemab | DMT, biologic | Aβ | Anti‐amyloid monoclonal antibody directed at amyloid protofibrils and amyloid plaques | NCT01760005 | Washington University School of Medicine | Dec 2012 | Oct 2027 |
| NCT03887455 | Eisai Inc. | Mar 2019 | Sep 2027 | ||||
| NCT04468659 | Eisai Inc. | Jul 2020 | Oct 2027 | ||||
| NCT05269394 | Washington University School of Medicine | Dec 2021 | Jul 2027 | ||||
| Masitinib | DMT, small molecule | Inflammation | Tyrosine kinase inhibitor exhibits neuroprotection via inhibition of mast cell and microglia/macrophage activity | NCT05564169 | AB Science | Nov 2022 | Nov 2025 |
| Masupirdine | Sx, behavior | Neurotransmitter receptors | 5HT6 receptor antagonist | NCT05397639 | Suven Life Sciences Limited | Nov 2022 | Jan 2025 |
| Metformin | DMT, small molecule | Metabolism and bioenergetics | Insulin sensitizer | NCT04098666 | Columbia University | Mar 2021 | Mar 2026 |
| Nabilone | Sx, behavior | Neurotransmitter receptors | Synthetic cannabinoid; cannabinoid (receptor agent); antiemetic | NCT04516057 | Sunnybrook Health Sciences Centre | Feb 2021 | Oct 2025 |
| NE3107 | DMT, small molecule | Inflammation | Beta‐androstenetriol with anti‐inflammatory and insulin signaling effects via ERK 1 and 2 | NCT04669028 | BioVie Inc. | Aug 2021 | Dec 2022 |
| Nilotinib BE | DMT, small molecule | Proteostasis/proteinopathies | Abl tyrosine kinase inhibitor; autophagy enhancer | NCT05143528 | KeifeRx, LLC | Feb 2022 | Dec 2025 |
| Omega‐3 | DMT, small molecule | Oxidative stress | Antioxidant | NCT03691519 | University Hospital, Toulouse | Apr 2018 | Dec 2023 |
| Piromelatine | DMT, small molecule | Circadian rhythm | Melatonin and serotonin receptor agonist | NCT05267535 | Neurim Pharmaceuticals Ltd. | May 2022 | May 2024 |
| Remternetug | DMT, biologic | Aβ | Anti‐amyloid monoclonal antibody targeting pyroglutamate amyloid | NCT05463731 | Eli Lilly and Company | Aug 2022 | Mar 2024 |
| Semaglutide | DMT, biologic | Metabolism and bioenergetics | GLP‐1 agonist; anti‐inflammatory and insulin sensitivity effects | NCT04777396 | Novo Nordisk A/S | May 2021 | Sep 2025 |
| NCT04777409 | Novo Nordisk A/S | May 2021 | Sep 2025 | ||||
| Simufilam (PTI‐125) | DMT, small molecule | Synaptic plasticity/neuroprotection | Filamin A protein inhibitor; stabilizes the interaction of Aβ42 and the α7 nicotinic acetylcholine receptor to decrease tau phosphorylation and improve synaptic function | NCT04994483 | Cassava Sciences, Inc. | Nov 2021 | Oct 2023 |
| NCT05026177 | Cassava Sciences, Inc. | Nov 2021 | Jun 2024 | ||||
| NCT05575076 | Cassava Sciences, Inc. | Nov 2022 | Jul 2026 | ||||
| Solanezumab | DMT, biologic | Aβ | Anti‐amyloid monoclonal antibody directed at amyloid monomers | NCT01760005 | Washington University School of Medicine | Dec 2012 | Oct 2027 |
| NCT02008357 | Eli Lilly and Company | Feb 2014 | Dec 2022 | ||||
| Tertomotide | DMT, biologic | Synaptic plasticity/neuroprotection | Human telomerase reverse transcriptase (hTERT) mimic | NCT05303701 | GemVax & Kael | Jan 2023 | Oct 2025 |
| Tricaprilin | DMT, small molecule | Metabolism and bioenergetics | Caprylic triglyceride; induces ketosis to provide an alternate energy source to glucose and optimize mitochondrial function | NCT04187547 | Cerecin | Jun 2022 | Dec 2023 |
| TRx0237 | DMT, small molecule | Tau | Tau‐aggregation inhibitor | NCT03446001 | TauRx Therapeutics Ltd | Jan 2018 | Mar 2022 |
| Valiltramiprosate (ALZ‐801) | DMT, small molecule | Aβ | Prodrug of tramiprostate | NCT04770220 | Alzheon Inc. | May 2021 | May 2024 |
Abbreviations: Aβ, amyloid beta; CADRO, Common Alzheimer's Disease Research Ontology; cGMP, current good manufacturing practice; DMT, disease‐modifying therapy; GLP‐1, glucagon‐like peptide 1; NCT#, National Clinical Trial number; NMDA, N‐methyl‐D‐aspartic acid; Sx, symptoms.
Five of the trials in Phase 3 are prevention trials enrolling cognitively normal participants, 25 trials enroll early AD defined as MCI/prodromal AD and mild AD dementia (45% of all Phase 3 trials), 11 trials include participants with mild‐to‐moderate AD or moderate AD dementia, 8 enroll moderate‐to‐severe or severe participants, and 6 trials enroll participants with AD dementia of any severity.
Taken together currently active trials in Phase 3 require a total enrollment of 41,864 participants. Prevention trials require 5565 participants with preclinical AD; trials of MCI due to AD or prodromal AD require 2284 participants; trials focusing on early AD (prodromal AD or mild AD dementia) require 20,482 participants; trials for mild‐to‐moderate or moderate AD dementia plan to enroll 6359 participants; and trials of moderate‐to‐severe and severe AD plan enrollment of 4546 participants. Phase 3 DMT trials of biologics require 24,528 participants; DMT small molecule trials will enroll 9450; cognitive enhancer trials plan enrollment of 2360 participants; and trials of drugs being developed for neuropsychiatric syndromes plan enrollment of 5526 participants.
DMT trials assessing biological agents enroll a mean of 1168 participants, DMT trials testing small molecules enroll a mean of 556 individuals, cognitive enhancer trials enroll of a mean of 393 persons per trial, and trials of neuropsychiatric syndrome therapies enroll a mean of 502 participants.
Mean treatment exposure period for prevention trials of DMT biologics was 143 weeks and for DMT small molecules was 78 weeks. DMT trials for symptomatic patients averaged 103 weeks for biologics and 56 weeks for small molecule trials. Cognitive enhancer trials had an average of 25 treatment weeks. Trials for the treatment of neuropsychiatric syndromes had a mean of 19 treatment weeks.
Recruitment is a major challenge for clinical trials. The average recruitment (calculated as the total trial duration minus the treatment period) time for prevention trials of DMT biological agents was 107 and for DMT small molecules was 233 weeks. Non‐prevention DMT trials required 147 weeks for biologics and 99 weeks for small molecules. Cognitive enhancer trials had mean recruitment times of 142 weeks. Recruitment time for trials of treatments for neuropsychiatric syndromes had a mean of 194 weeks.
3.3. Phase 2
Phase 2 has 87 agents in 99 trials (Figure 1, Figure 3, and Table 2). DMTs represent 85% (N = 74) of agents in Phase 2 trials including 31 (36% of the Phase 2 agents) biologics and 43 (49%) small molecules. Eight (9% of Phase 2 agents) are putative cognitive enhancing agents and five (6%) drugs target neuropsychiatric symptoms of AD. CADRO mechanisms represented among Phase 2 treatments include inflammation (17 agents; 20%), synaptic plasticity/neuroprotection (14; 16%), transmitter receptors (12; 14%), amyloid (11 agents; 13%), tau (8; 9%), metabolism and bioenergetics (5; 6%), oxidative stress (3; 3%), proteostasis/proteinopathies (3; 3%), growth factors and hormones (3; 3%), APOE and lipids (3; 3%), vasculature (2; 2%), circadian rhythm (2; 2%), neurogenesis (2; 2%), epigenetic regulators (1; 1%), and cell death (1; 1%). Figures 1 and 3 show the CADRO‐based targets of agents in Phase 2. Twenty‐four (28%) of the Phase 2 agents are repurposed treatments approved for use in another indication (92% = DMT; N = 22), 4% (N = 1) for cognitive enhancement and 4% (N = 1) for treatment for behavioral symptoms. Fourteen trials that were active in 2022 were completed; seven are of unknown status; and one each was suspended, terminated, or withdrawn. Twenty‐seven new Phase 2 trials were initiated between January 25, 2022, and January 1, 2023.
FIGURE 3.
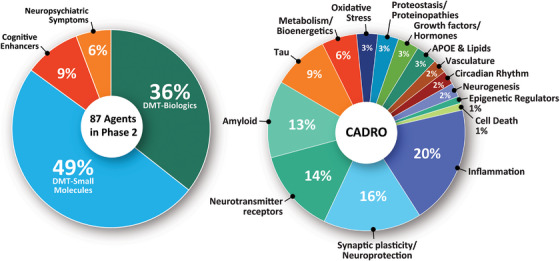
Mechanisms of action of agents in Phase 2. APOE, apolipoprotein E; DMT, disease‐modifying therapy. (Figure © J Cummings; M de la Flor, PhD, Illustrator).
TABLE 2.
Agents in Phase 2 of Alzheimer's disease drug development (ClinicalTrials.gov accessed January 1, 2023).
| Agent | Therapeutic purpose | CADRO target | Mechanism of action | Clinical trial NCT# | Lead sponsor | Start date | Estimated primary completion date |
|---|---|---|---|---|---|---|---|
| ABBV‐916 | DMT, biologic | Aβ | Anti‐amyloid antibody | NCT05291234 | AbbVie | Aug 2022 | Dec 2024 |
| ABvac40 | DMT, biologic | Aβ | Active immunotherapy (SC injection) | NCT03461276 | Araclon Biotech S.L. | Feb 2018 | Dec 2021 |
| ACI‐24.060 | DMT, biologic | Aβ | Vaccine stimulates antibodies against Aβ protein | NCT05462106 | AC Immune SA | Jun 2022 | Jun 2026 |
| ACI‐35 | DMT, biologic | Tau | Active immunotherapy targeting tau (phosphorylated tau) | NCT04445831 | AC Immune SA | Jul 2019 | Oct 2023 |
| AL 001 | DMT, small molecule | Synaptic plasticity/neuroprotection | Lithium inhibits GSL3‐beta activating mTOR to facilitate the Akt signaling pathway | NCT05363293 | Alzamend Neuro, Inc. | May 2022 | Dec 2022 |
| AL002 | DMT, biologic | Inflammation | Monoclonal antibody targeting TREM2 receptors | NCT04592874 | Alector Inc. | Jan 2021 | Dec 2023 |
| Allopregnanolone | DMT, small molecule | Neurogenesis | Allosteric modulator of GABA‐A receptors | NCT04838301 | University of Arizona | Jan 2023 | Jan 2025 |
| APH‐1105 | DMT, small molecule | Aβ | Alpha secretase modulator (amyloid precursor protein secretase modulator) | NCT03806478 | Aphios | Jun 2023 | Sep 2024 |
| Bacillus Calmette‐Guerin | DMT, biologic | Inflammation | Immunomodulation | NCT05004688 | Steven E. Arnold | Mar 2022 | Oct 2023 |
| Baricitinib | DMT, small molecule | Inflammation | Janus kinase (JAK) inhibitor | NCT05189106 | Massachusetts General Hospital | Dec 2022 | Jul 2024 |
| Bepranemab | DMT, biologic | Tau | Anti‐tau monoclonal antibody binding to central region of tau | NCT04867616 | UCB Biopharma SRL | Jun 2021 | Apr 2024 |
| BIIB080 | DMT, biologic | Tau | Antisense oligonucleotide that inhibits translation of tau mRNA into the tau protein | NCT05399888 | Biogen | Aug 2022 | Dec 2026 |
| Brain shuttle gantenerumab | DMT, biologic | Aβ | Monoclonal antibody directed at plaques and oligomers; "brain‐shuttle" gantenerumab | NCT04639050 | Hoffmann‐La Roche | Mar 2021 | Jan 2025 |
| Bryostatin 1 | DMT, biologic | Synaptic plasticity/neuroprotection | Protein kinase C inhibitor | NCT04538066 | Neurotrope Bioscience, Inc. | Aug 2020 | Nov 2022 |
| Buntanetap | DMT, small molecule | Proteostasis/proteinopathies | Reduce amyloid precursor protein (APP) synthesis; selective inhibitor of APP to reduce amyloid; reduces synthesis of tau and alpha‐synuclein proteins | NCT02925650 | Annovis Bio Inc. | Mar 2017 | Dec 2021 |
| Canakinumab | DMT, biologic | Inflammation | Anti‐IL‐1‐beta monoclonal antibody | NCT04795466 | Novartis Pharmaceuticals | Oct 2021 | Feb 2026 |
| Chinese traditional medicine | Sx, cognition | Metabolism and bioenergetics | Three herbs (Rhizoma Acori Tatarinowii, Poria cum Radix Pini, Radix Polygalae) mechanism unknown | NCT05538507 | Peking Union Medical College Hospital | Jun 2022 | Jun 2024 |
| CORT108297 | Sx, cognition | Growth factors and hormones | Selective glucocorticoid receptor antagonist | NCT04601038 | Johns Hopkins University | Jun 2021 | Jun 2023 |
| Crenezumab | DMT, biologic | Aβ | Monoclonal antibody targeting soluble oligomers | NCT01998841 | Genentech, Inc. | Dec 2013 | Mar 2022 |
| CST‐2032 | Sx, cognition | Neurotransmitter receptors | Noradrenergic agonist | NCT05104463 | CuraSen Therapeutics, Inc. | Apr 2022 | Jun 2023 |
| CY6463 | DMT, small molecule | Synaptic plasticity/neuroprotection | Guanylate cyclase positive allosteric modulator | NCT04798989 | Cyclerion Therapeutics | Jun 2021 | Jul 2022 |
| Dalzanemdor | DMT, small molecule | Synaptic plasticity/neuroprotection | Enhances synaptic function through NMDA receptor blockade | NCT05619692 | Sage Therapeutics | Feb 2023 | Dec 2024 |
| Dapagliflozin | DMT, small molecule | Metabolism and bioenergetics | Sodium‐glucose cotransporter 2 (SGLT2) Inhibitor | NCT03801642 | Jeff Burns, MD | Jan 2019 | Oct 2022 |
| Daratumumab | DMT, biologic | Inflammation | Human antibody targeting CD38; immunomodulatory effects | NCT04070378 | Marc L. Gordon, MD | Nov 2019 | Dec 2023 |
| Dasatinib + quercetin | DMT, small molecule | Inflammation | Dasatinib induces apoptosis in senescent cells to allow their removal; quercetin is a flavonoid | NCT04063124 | The University of Texas Health Science Center at San Antonio | Feb 2020 | Dec 2021 |
| NCT04685590 | Wake Forest University Health Sciences | Dec 2021 | Jan 2027 | ||||
| NCT04785300 | James L. Kirkland, MD, PhD | Jul 2022 | Dec 2023 | ||||
| NCT05422885 | Lew Lipsitz | May 2022 | Jun 2023 | ||||
| Deferiprone | DMT, small molecule | Cell death | Iron chelating agent | NCT03234686 | Neuroscience Trials Australia | Jan 2018 | Sep 2022 |
| DHA | DMT, small molecule | Oxidative stress | Omega 3 fatty acid; reduce amyloid production; improve synaptic function; antioxidant | NCT03613844 | University of Southern California | Jul 2018 | May 2024 |
| Dronabinol | Sx, behavior | Neurotransmitter receptors | CB1 and CB2 endocannabinoid receptor partial agonist | NCT02792257 | Johns Hopkins University | Mar 2017 | May 2023 |
| E2814 | DMT, biologic | Tau | Anti‐tau monoclonal antibody | NCT04971733 | Eisai Inc. | Jun 2021 | Sep 2024 |
| Edaravone | DMT, small molecule | Oxidative stress | Pyrazolone free‐radical scavenger | NCT05323812 | Treeway B.V. | Sep 2022 | Jan 2024 |
| Edonerpic | DMT, small molecule | Synaptic plasticity/neuroprotection | Neurotrophic agent; activates sigma‐1 receptor; enhances microglial clearance of Aβ | NCT04191486 | FUJIFILM Toyama Chemical Co., Ltd. | Dec 2019 | Feb 2023 |
| Elayta | DMT, small molecule | Synaptic plasticity/neuroprotection | Sigma 2 receptor antagonist; binds to sigma‐2/PGRMC1 receptor and regulates Aβ oligomer‐mediated synaptic toxicity | NCT03507790 | Cognition Therapeutics | Oct 2018 | Sep 2023 |
| NCT04735536 | Cognition Therapeutics | Aug 2020 | Mar 2023 | ||||
| NCT05531656 | Cognition Therapeutics | Dec 2022 | Aug 2026 | ||||
| EX039 | DMT, small molecule | Synaptic plasticity/neuroprotection | Inhibits D‐amino acids oxidate to increase NMDA receptor activity | NCT05413655 | Excelsior | Aug 2022 | Aug 2024 |
| ExPlas | DMT, biologic | Synaptic plasticity/neuroprotection | Plasma transfusion from exercise‐trained donors | NCT05068830 | Norwegian University of Science and Technology | Sep 2021 | Sep 2024 |
| Flos gossypii flavonoids | DMT, small molecule | Oxidative stress | Anti‐oxidant; anti‐inflammatory | NCT05269173 | Capital Medical University | Oct 2020 | Jun 2024 |
| Fosgonimeton (ATH‐1017) | DMT, small molecule | Synaptic plasticity/neuroprotection | Hepatocyte growth factor (HGF); activates signaling via the HGF/MET receptor system; promotes survival of neurons, enhances hippocampal synaptic plasticity | NCT04886063 | Athira Pharma | Jun 2021 | Apr 2023 |
| Gantenerumab | DMT, biologic | Aβ | Monoclonal antibody directed at plaques and oligomers | NCT04592341 | Hoffmann‐La Roche | Nov 2020 | Dec 2022 |
| GnRH | DMT, biologic | Growth factors and hormones | Antiaging | NCT04390646 | Nelly Pitteloud | Aug 2020 | Dec 2023 |
| Hydroxypropyl Beta Cyclodextrin | DMT, biologic | apoE, lipids, and lipoprotein receptors | Modulates cholesterol transportation with secondary effects on amyloid, tau, and oxidative e stress | NCT05607615 | Cyclo Therapeutics, Inc. | Sep 2022 | Mar 2024 |
| IGC‐AD1 | Sx, behavior | Neurotransmitter receptors | Cannabinoid | NCT05543681 | IGC Pharma LLC | Oct 2022 | Aug 2023 |
| Insulin | DMT, biologic | Metabolism and bioenergetics | Decreases glucose resistance; increase insulin signaling in the brain | NCT05006599 | Wake Forest University Health Sciences | May 2025 | May 2029 |
| Insulin + empagliflozin | DMT, biologic | Metabolism and bioenergetics | SGLT2 inhibitor (empagliflozin) and insulin combination therapy; decrease glucose resistance and increase insulin signaling in the brain | NCT05081219 | Wake Forest University Health Sciences | Oct 2021 | Oct 2026 |
| IVL3003 | Sx, cognition | Neurotransmitter receptors | Cholinesterase inhibitor | NCT05345509 | Inventage Lab., Inc. | Apr 2023 | Mar 2024 |
| JNJ‐63733657 | DMT, biologic | Tau | Monoclonal antibody targeted at soluble tau (mid‐region of tau) | NCT04619420 | Janssen Research & Development, LLC | Jan 2021 | Mar 2025 |
| L‐Serine | DMT, small molecule | Inflammation | Naturally occurring dietary amino acid; inhibits toxic misfolding | NCT03062449 | Aleksandra Stark | Mar 2017 | Dec 2022 |
| Lamivudine | DMT, small molecule | Epigenetic regulators | Human immunodeficiency virus nucleoside analog reverse transcriptase inhibitor | NCT04552795 | Bess Frost, PhD | Feb 2021 | Mar 2023 |
| Lecanemab | DMT, biologic | Aβ | Anti‐amyloid monoclonal antibody directed at amyloid protofibrils and amyloid plaques | NCT01767311 | Eisai Inc. | Dec 2012 | Feb 2025 |
| Lenalidomide | DMT, small molecule | Inflammation | Immunomodulator | NCT04032626 | St. Joseph's Hospital and Medical Center, Phoenix | Jul 2020 | Sep 2023 |
| Leuprorelin | DMT, small molecule | Growth factors and hormones | Gonadotropin releasing hormone (GnRH) receptor agonist | NCT03649724 | Weill Medical College of Cornell University | Nov 2020 | Feb 2025 |
| Levetiracetam | DMT, small molecule | Synaptic plasticity/neuroprotection | SV2A modulator enhancing synaptic plasticity | NCT03489044 | University of Oxford | Oct 2018 | Dec 2022 |
| NCT03875638 | Beth Israel Deaconess Medical Center | Aug 2019 | Aug 2023 | ||||
| NCT04004702 | Walter Reed National Military Medical Center | Jan 2020 | Dec 2024 | ||||
| LX1001 | DMT, biologic | apoE, lipids, and lipoprotein receptors | Adeno‐associated virus (AAV) gene transfer vector expressing the cDNA coding for human APOE ε2 directly to the CNS/CSF of APOE ε4 homozygotes | NCT03634007 | Lexeo Therapeutics | Nov 2019 | Apr 2023 |
| LY3372689 | DMT, small molecule | Tau | O‐GlcNAcase enzyme inhibitor | NCT05063539 | Eli Lilly and Company | Sep 2021 | May 2024 |
| Memantine | DMT, small molecule | Neurotransmitter receptors | NMDA receptor antagonist | NCT05063851 | University of Virginia | Oct 2021 | Sep 2024 |
| Methylene Blue | DMT, small molecule | Tau | Tau protein aggregation inhibitor | NCT02380573 | The University of Texas Health Science Center at San Antonio | Jul 2015 | Apr 2022 |
| MIB‐626 | DMT, small molecule | Aβ | Sirtuin‐nicotinamide adenine dinucleotide stimulator to enhance alpha‐secretase | NCT05040321 | Brigham and Women's Hospital | Dec 2021 | Feb 2024 |
| MK‐1942 | Sx, cognition | Neurotransmitter receptors | Undisclosed | NCT05602727 | Merck Sharp & Dohme LLC | Dec 2022 | May 2026 |
| Montelukast | DMT, small molecule | Inflammation | Leukotriene receptor antagonist (LTRA); anti‐inflammatory effects | NCT03402503 | IntelGenx Corp. | Nov 2018 | Oct 2023 |
| MW150 | DMT, small molecule | Synaptic plasticity/neuroprotection | p38 alpha MAPK kinase inhibitor | NCT05194163 | Neurokine Therapeutics | May 2022 | Aug 2024 |
| NanoLithium NP03 | DMT, small molecule | Neurotransmitter receptors | Ion with effects on amyloid, oxidation, and inflammation | NCT05423522 | Medesis Pharma SA | May 2022 | May 2023 |
| Neflamapimod | DMT, small molecule | Synaptic plasticity/neuroprotection | Selective p38 MAPK alpha inhibitor | NCT03435861 | University Hospital, Toulouse | Oct 2018 | Apr 2021 |
| Nicotine | Sx, cognition | Neurotransmitter receptors | Nicotinic acetylcholine receptor agonist | NCT02720445 | University of Southern California | Jan 2017 | Jul 2023 |
| Obicetrapib | DMT, small molecule | apoE, lipids, and lipoprotein receptors | Cholesteryl ester transfer protein (CETP) inhibitor | NCT05161715 | NewAmsterdam Pharma | Jan 2022 | Jun 2023 |
| Pegipanermin | DMT, biologic | Inflammation | Neutralizes TNF‐alpha | NCT05318976 | Inmune Bio, Inc. | Feb 2022 | Jun 2023 |
| NCT05321498 | Inmune Bio, Inc. | Sep 2022 | Jan 2023 | ||||
| NCT05522387 | Inmune Bio, Inc. | Nov 2022 | Dec 2025 | ||||
| Pepinemab | DMT, biologic | Inflammation | Monoclonal antibody directed at semaphorin 4D; reduces inflammatory cytokine release | NCT04381468 | Vaccinex Inc. | Jul 2021 | Dec 2023 |
| Proleukin | DMT, biologic | Inflammation | IL‐2 immunomodulator | NCT05468073 | Centre Hospitalier St Anne | Oct 2022 | Sep 2025 |
| Rapamycin | DMT, small molecule | Proteostasis/proteinopathies | Autophagy enhancer; mTOR inhibitor; immunomodulator | NCT04629495 | The University of Texas Health Science Center at San Antonio | Aug 2021 | Dec 2023 |
| REM0046127 | Sx, cognition | Neurotransmitter receptors | Modulates Orai calcium (Ca2+) channel activity to normalize neuronal Ca2+ homeostasis | NCT05478031 | reMYND | Jun 2022 | Jun 2023 |
| Sargramostim | DMT, biologic | Inflammation | Hematopoietic growth factor granulocyte macrophage colony stimulating factor; anti‐inflammatory | NCT04902703 | University of Colorado, Denver | Jun 2022 | Jul 2024 |
| SCI‐110 (Dronabinol + PEA) | Sx, behavior | Neurotransmitter receptors | Cannibinoid and palmitoylethanolamide (an endocannabinoid) | NCT05239390 | The Israeli Medical Center for Alzheimer's | Dec 2021 | Jun 2023 |
| Seltorexant | Sx, behavior | Circadian rhythm | Dual orexin receptor antagonist | NCT05307692 | Janssen Research & Development, LLC | May 2022 | Apr 2023 |
| Semorinemab | DMT, biologic | Tau | Anti‐tau monoclonal antibody targeting extracellular tau | NCT03828747 | Genentech, Inc. | Jan 2019 | Jul 2021 |
| Senicapoc | DMT, small molecule | Inflammation | Calcium‐activated potassium channel inhibitor | NCT04804241 | University of California, Davis | Mar 2022 | Dec 2024 |
| Simufilam | DMT, small molecule | Synaptic plasticity/neuroprotection | Filamin A protein inhibitor; stabilizes the interaction of Aβ42 and the α7 nicotinic acetylcholine receptor to decrease tau phosphorylation and improve synaptic function | NCT04388254 | Cassava Sciences, Inc. | Mar 2020 | Jan 2023 |
| NCT05352763 | Cassava Sciences, Inc. | May 2022 | Oct 2025 | ||||
| Sovateltide | DMT, small molecule | Neurogenesis | Endothelin B receptor agonist; augments activity of neuronal progenitor cells, neurovascular repair, and neuroregeneration | NCT04052737 | Pharmazz, Inc. | Mar 2018 | Nov 2022 |
| Suvorexant | DMT, small molecule | Neurotransmitter receptors | Dual orexin receptor antagonist | NCT04629547 | Washington University School of Medicine | May 2022 | May 2026 |
| T3D‐959 | DMT, small molecule | Metabolism and bioenergetics | Dual agonist of peroxisome proliferator activated nuclear receptor delta/gamma (PPARδ/γ); regulates glucose and lipid metabolism | NCT04251182 | T3D Therapeutics, Inc. | Mar 2021 | Apr 2023 |
| TB006 | DMT, biologic | Inflammation | Monoclonal antibody targeting galactose‐specific lectin (galectin) 3, a β‐galactosidase‐binding protein that activates macrophages; anti‐inflammatory | NCT05074498 | TrueBinding, Inc. | Oct 2021 | Oct 2022 |
| NCT05476783 | TrueBinding, Inc. | Sep 2022 | Oct 2024 | ||||
| Tdap | DMT, biologic | Inflammation | Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine to stimulate inflammatory protection | NCT05183516 | Mindful Diagnostics and Therapeutics, LLC | May 2023 | Dec 2023 |
| Telmisartan + perindopril | DMT, small molecule | Vasculature | Angiotensin II receptor blocker, PPAR‐gamma agonist (telmisartan); angiotensin converting enzyme inhibitor (perindopril) | NCT02085265 | Sunnybrook Health Sciences Centre | Mar 2014 | Sep 2023 |
| Tertomotide | DMT, biologic | Synaptic plasticity/neuroprotection | Human telomerase reverse transcriptase (hTERT) mimic | NCT05189210 | GemVax & Kael | Oct 2022 | Jul 2023 |
| THC‐free cannabidiol | Sx, behavior | Neurotransmitter receptors | Cannabinoids | NCT04436081 | Eastern Virginia Medical School | Feb 2021 | Dec 2022 |
| Trazodone | Sx, cognition | Circadian rhythm | Serotonin reuptake inhibitor | NCT05282550 | Johns Hopkins University | Jan 2023 | Mar 2027 |
| Trehalose injection | DMT, small molecule | Proteostasis/proteinopathies | Activates transcription factor EB to increase autophagy | NCT05332678 | Neuroscience Trials Australia | Jan 2023 | Jan 2025 |
| Valacyclovir | DMT, small molecule | Inflammation | Anti‐viral against HSV‐1 and −2; reduces vira‐related “seeding” of amyloid plaque deposition | NCT03282916 | New York State Psychiatric Institute | Feb 2018 | Dec 2023 |
| Valiltramiprosate (ALZ‐801) | DMT, small molecule | Aβ | Aggregation inhibitor | NCT04693520 | Alzheon Inc. | Sep 2020 | Jul 2023 |
| Varoglutamstat | DMT, small molecule | Aβ | Glutaminyl cyclase (QC) enzyme inhibitor to reduce production of pyroglutamate Aβ | NCT03919162 | Vivoryon Therapeutics N.V. | Nov 2021 | May 2023 |
| NCT04498650 | Vivoryon Therapeutics N.V. | Jul 2020 | Jan 2024 | ||||
| Yangxue Qingnao pills | DMT, small molecule | Vasculature | Cerebral blood flow enhancer; traditional Chinese herbal medicine | NCT04780399 | Dongzhimen Hospital, Beijing | Nov 2021 | Mar 2024 |
Abbreviations: Aβ, amyloid beta; Akt, protein kinase B; APOE, apolipoprotein E; CADRO, Common Alzheimer's Disease Research Ontology; CNS, central nervous system; CSF, cerebrospinal fluid; DMT, disease‐modifying therapy; HSV, herpes simplex virus; IL, interleukin; MAPK, mitogen‐activated protein kinase; mTOR, mammalian target or rapamycin; NCT#, National Clinical Trial number; NMDA, N‐methyl‐D‐aspartic acid; PPAR, peroxisome proliferator‐activated receptor; SC, subcutaneous; Sx, symptoms; TNF, tumor necrosis factor.
Four Phase 2 trials enroll preclinical participants, 30 (30% of Phase 2 trials) trials enroll MCI prodromal participants, 29 (29% of Phase 2 trials) trials enroll early AD participants, 27 (27% of Phase 2 trials) trials enroll mild‐to‐moderate or moderate AD dementia, 5 (5%) enroll participants with moderate‐to‐severe and severe AD dementia, and 3 (3%) trials include participants with AD dementia of any severity.
Taken together, currently active trials in Phase 2 trials require a total enrollment of 13,829 participants. DMT trials for biological agents will enroll 5769 participants, trials of small molecule DMTs will enroll 6308 individuals, trials of cognitive enhancing agents will enroll 1282 participants, and trials of drugs being developed for neuropsychiatric syndromes will enroll 470 participants. DMT biological trials have a mean enrollment of 170 participants, DMT small molecule trials have a mean enrollment of 121 participants, trials for cognitive enhancers include a mean of 160 participants, and trials focusing on neuropsychiatric syndromes enroll a mean of 94 participants.
Treatment duration of biologic DMTs in treatment trials for symptomatic AD is 56 weeks and for small molecules in treatments trials is 42 weeks. Treatment exposure in cognitive enhancer trials has a mean of 24 weeks; treatment duration for therapies addressing neuropsychiatric syndromes has a mean exposure period of 8 weeks.
Mean recruitment time for trials of DMT biological agents for symptomatic AD is 104 weeks (on average); recruitment of trials for DMT small molecules took a mean of 125 weeks. Cognitive enhancer trials required a mean recruitment period of 114 weeks. Recruitment for trials of agents addressing behavioral changes in AD had a mean recruitment period of 109 weeks.
3.4. Phase 1
There are 31 agents in 33 Phase 1 trials (Figure 1, Table 3). There are 25 DMTs (81% of Phase 1 agents) in Phase 1 trials including 16 (52% of the Phase 1 agents) biologics and 9 (29%) small molecules. There are three (10% of Phase 1 agents) putative cognitive enhancing agents and three (10%) drugs targeting behavioral symptoms. CADRO mechanisms represented among Phase 1 therapies include amyloid (7 agents; 23%); inflammation (6 agents; 19%); transmitter receptors (6; 19%); tau (4; 13%); metabolism and bioenergetics (2; 6%); and 1 (3%) each for APOE and lipoproteins, neurogenesis, oxidative stress, synaptic plasticity/neuroprotection, and “other.” Seven (23%) of the Phase 1 agents are repurposed treatments approved for use in another indication (four DMTs, two cognitive enhancers, one neuropsychiatric agent). Eleven trials that were active in 2022 were completed and seven are of unknown status. Sixteen new Phase 1 trials have been initiated in the past year.
TABLE 3.
Agents in Phase 1 of Alzheimer's disease drug development (ClinicalTrials.gov accessed January 1, 2023).
| Agent | Therapeutic purpose | CADRO target | Mechanism of action | Clinical trial NCT# | Lead sponsor | Start Date | Estimated Primary Completion Date |
|---|---|---|---|---|---|---|---|
| ACU193 | DMT, biologic | Aβ | Monoclonal antibody targeting soluble AB oligomers | NCT04931459 | Acumen Pharmaceuticals | Jun 2021 | Mar 2023 |
| Allopregnanolone | DMT, small molecule | Neurogenesis | Allosteric modulator of GABA‐A receptors | NCT03748303 | University of Arizona | Oct 2019 | Dec 2022 |
| ALN‐APP | DMT, biologic | Aβ | RNAi to decrease APP and downstream Aβ‐related events | NCT05231785 | Alnylam Pharmaceuticals | Feb 2022 | Jul 2025 |
| ALZ‐101 | DMT, biologic | Aβ | Aβ‐directed vaccine | NCT05328115 | Alzinova AB | Sep 2021 | Jul 2023 |
| APNmAb005 | DMT, biologic | Tau | Anti‐tau antibody | NCT05344989 | APRINOIA Therapeutics, LLC | May 2022 | Jan 2023 |
| ASN51 | DMT, small molecule | Tau | O‐GlcNAcase inhibitor | NCT04759365 | Asceneuron Pty Ltd. | Jun 2021 | Dec 2022 |
| AV‐1959 | DMT, biologic | Aβ | Anti‐amyloid vaccine | NCT05642429 | Institute for Molecular Medicine | Feb 2023 | Feb 2026 |
| Cannabidiol | Sx, behavior | Neurotransmitter receptors | Cannabinoid | NCT04075435 | Mclean Hospital | Jan 2021 | Jan 2023 |
| Centella asiatica | DMT, small molecule | Synaptic plasticity/neuroprotection | Antioxidant and anti‐inflammatory agent with synaptic and neuroprotective effects | NCT05591027 | Oregon Health and Science University | Nov 2022 | Oct 2024 |
| CMS121 | DMT, small molecule | Oxidative stress | Fatty acid synthase inhibitor | NCT05318040 | Virogenics, Inc. | May 2022 | Dec 2022 |
| CpG1018 | DMT, biologic | Inflammation | Toll‐like receptor 9 agonist leading to reduced Aβ plaques and tau pathology | NCT05606341 | NYU Langone Health | Nov 2022 | Nov 2024 |
| Donepezil | Sx, cognition | Neurotransmitter receptors | Cholinesterase inhibitor | NCT04730635 | Merck Sharp & Dohme LLC | Mar 2021 | Jan 2023 |
| Emtricitabine | DMT, small molecule | Inflammation | Nucleoside reverse transcriptase inhibitor (NRTI) | NCT04500847 | Butler Hospital | Dec 2021 | Mar 2023 |
| GB‐5001 | Sx, cognition | Neurotransmitter receptors | Cholinesterase inhibitor | NCT05525780 | G2GBio, Inc. | Aug 2022 | Aug 2023 |
| IBC‐Ab002 | DMT, biologic | Inflammation | Anti‐programmed death‐ligand 1 (PD‐L1) immune checkpoint inhibitor | NCT05551741 | Immunobrain Checkpoint | Oct 2022 | Oct 2024 |
| Lecanemab | DMT, biologic | Aβ | Anti‐amyloid monoclonal antibody | NCT05533801 | Eisai Inc. | Sep 2022 | Feb 2023 |
| Lu AF87908 | DMT, biologic | Tau | Anti‐tau monoclonal antibody | NCT04149860 | H. Lundbeck A/S | Sep 2019 | Jun 2023 |
| LX1001 | DMT, biologic | apoE, lipids, and lipoprotein receptors | Adeno‐associated virus (AAV) gene transfer vector expressing the cDNA coding for human APOE ε2 directly to the CNS/CSF of APOE ε4 homozygotes | NCT05400330 | Lexeo Therapeutics | Nov 2022 | Dec 2027 |
| LY3372993 | DMT, biologic | Aβ | Anti‐amyloid monoclonal antibody | NCT04451408 | Eli Lilly and Company | Jul 2020 | Jan 2024 |
| Mecamylamine | Sx, cognition | Neurotransmitter receptors | Nicotinic antagonist | NCT04129060 | University of Vermont | Mar 2020 | Mar 2024 |
| MK‐2214 | DMT, biologic | Tau | Anti‐tau monoclonal antibody | NCT05466422 | Merck Sharp & Dohme LLC | Sep 2022 | Nov 2024 |
| MK‐8189 | Sx, behavior | Neurotransmitter receptors | PDE10 inhibitor | NCT05227118 | Merck Sharp & Dohme LLC | Jul 2022 | Jan 2023 |
| Nicotinamide riboside | DMT, small molecule | Metabolism and bioenergetics | Mitochondrial function enhancer and antioxidant | NCT04430517 | Mclean Hospital | Mar 2022 | Apr 2025 |
| NIO752 | DMT, biologic | Other | Anti‐tau antisense oligonucleotide | NCT05469360 | Novartis Pharmaceuticals | Sep 2022 | Nov 2023 |
| Psilocybin | Sx, behavior | Neurotransmitter receptors | Psychedelic | NCT04123314 | Johns Hopkins University | Mar 2021 | Dec 2023 |
| Rapamycin | DMT, small molecule | Proteostasis/proteinopathies | Autophagy enhancer; mTOR inhibitor; immunomodulator | NCT04200911 | The University of Texas Health Science Center at San Antonio | Jun 2020 | Jan 2022 |
| Salsalate | DMT, small molecule | Inflammation | Non‐steroidal anti‐inflammatory (NSAID) | NCT03277573 | Adam Boxer | Jul 2017 | Apr 2021 |
| SHR‐1707 | DM, biologic | Aβ | Anti‐amyloid monoclonal antibody | NCT04973189 | Shanghai Hengrui Pharmaceutical Co., Ltd. | May 2021 | Oct 2021 |
| TB006 | DMT, biologic | Inflammation | Monoclonal antibody targeting galactose‐specific lectin (galectin) 3, a β‐galactosidase‐binding protein that activates macrophages; anti‐inflammatory | NCT04920786 | TrueBinding, Inc. | Jun 2021 | Nov 2022 |
| Tricaprilin | DMT, small molecule | Metabolism and bioenergetics | Caprylic triglyceride; induces ketosis to provide an alternate energy source to glucose and optimize mitochondrial function | NCT05028114 | Cerecin | Aug 2021 | Dec 2022 |
| NCT05408780 | Cerecin | Jul 2022 | Oct 2022 | ||||
| NCT05628636 | Cerecin | Nov 2022 | Feb 2023 | ||||
| VT301 | DMT, biologic | Inflammation | Regulatory T cells | NCT05016427 | VTBIO Co. LTD | Nov 2020 | Nov 2021 |
Abbreviations: Aβ, amyloid beta; APOE, apolipoprotein E; CADRO, Common Alzheimer's Disease Research Ontology; DMT, disease‐modifying therapy; mTOR, mammalian target or rapamycin; NCT#, National Clinical Trial number; Sx, symptoms.
Phase 1 trials include both single ascending dose and multiple ascending dose studies and will enroll 1772 participants into currently registered trials. Most Phase 1 participants are healthy volunteers (N = 1165); immunotherapy trials in Phase 1 trial may include participants with AD of a variety of severities. Trials of biological agents in Phase 1 will require 994 participants, trials of small molecule DMTs will enroll 514 participants, trials of cognitive enhancing agents will enroll 204 participants, and trials of drugs addressing neuropsychiatric syndromes plan to enroll 60 participants. The mean number of participants in DMT biological trials is 62, DMT small molecules will enroll a means of 47 participants, trials of cognitive enhancers enroll a mean of 68 participants, and trials of drugs of neuropsychiatric syndromes will enroll a mean of 20 participants.
Treatment exposure for DMT biologics is 45 weeks and for DMT small molecules is 13 weeks. Treatment duration for cognitive enhancers in Phase 1 is typically 6 weeks; the mean duration for neuropsychiatric agents in Phase 1 is 5 weeks.
Recruitment for Phase 1 DMT trials of biologics averages 65 weeks and for DMT small molecules averages 77 weeks. Cognitive enhancer trials require 113 weeks to recruit and trials for drugs addressing neuropsychiatric symptoms have average recruitment periods of 87 weeks.
3.5. Stem cells
There are 8 stem cells trials developing cell therapies for AD (Table 4).
TABLE 4.
Stem cell therapy in clinical trials for Alzheimer's disease (ClinicalTrials.gov accessed January 1, 2023).
| Agent | Phase | Clinical trial NCT# | Sponsor | Start date | Primary completion date |
|---|---|---|---|---|---|
| Allogenic human mesenchymal stem cells | Phase 2 | NCT02833792 | Stemedica Cell Technologies, Inc. | 2016‐06‐01 | 2024‐07‐30 |
| Amniotic and umbilical cord tissue | Phase 1 | NCT03899298 | R3 Stem Cell | 2019‐09‐01 | 2024‐03‐20 |
| Autologous adipose tissue derived mesenchymal stem cells | Phase 2 | NCT04482413 | Nature Cell Co. Ltd. | 2023‐02‐01 | 2024‐05‐30 |
| Human mesenchymal stem cells | Phase 1 | NCT04040348 | Bernard (Barry) Baumel | 2019‐10‐08 | 2023‐05‐01 |
| Human umbilical cord blood derived mesenchymal stem cells | Not applicable | NCT04954534 | Samsung Medical Center | 2021‐07‐12 | 2022‐01‐31 |
| Lomecel‐B (mesenchymal stem cells derived from bone marrow) | Phase 2 | NCT05233774 | Longeveron Inc. | 2021‐12‐28 | 2023‐09‐29 |
| SNK01 (autologous natural killer cells) | Phase 1 | NCT04678453 | NKGen Biotech, Inc. | 2021‐01‐06 | 2022‐12‐01 |
Abbreviation: NCT#, National Clinical Trial number.
3.6. Biomarkers in trials
Biomarkers are commonly collected at study entry and at study termination. For trials with biomarker descriptions on ClinicalTrials.gov, 72 Phase 2 trials and 34 Phase 3 trials require magnetic resonance imaging (MRI) at baseline. Thirty‐one Phase 2 trials and 9 Phase 3 trials collect baseline cerebrospinal fluid (CSF) amyloid; and 29 Phase 2 and 12 Phase 3 collect baseline amyloid positron emission tomography (PET). Plasma amyloid is collected in four Phase 2 and one Phase 3 trial. Tau measures collected at baseline include CSF tau in seven Phase 2 trials and three Phase 3 trials; CSF phospho‐tau (p‐tau) in six Phase 2 trials and two Phase 3 trials; tau PET in six Phase 2 trials and three Phase 3 trials. Twenty Phase 2 trials and 17 Phase 3 trials do not assess biomarkers at baseline.
MRI is the most common biomarker collected as an outcome measure. MRI data are captured as outcome assessments in 27 Phase 2 trials and 10 Phase 3 trials. Amyloid PET and tau PET are commonly collected as outcomes; amyloid PET in seven Phase 2 and thirteen Phase 3 trials and tau PET in six Phase 2 and nine Phase 3 trials. Amyloid and tau measures are collected in CSF and in plasma are collected as outcomes. Thirteen Phase 2 trials and three Phase 3 trials collect CSF amyloid; fifteen Phase 2 trials and six Phase 3 trials collect CSF tau; eleven Phase 2 trials and five Phase 3 trials collect CSF p‐tau. These same three biomarkers are also collected in plasma. Twelve Phase 2 trials and two Phase 3 trials collect plasma amyloid, five Phase 2 trials and one Phase 3 trial collect plasma tau, and eight Phase 2 trials and three Phase 3 trials collect plasma p‐tau. A few trials collect neurofilament light (CSF: six Phase 2 trials, three Phase 3 trials; plasma: four Phase 2 trials, four Phase 3 trials) and glial fibrillary acidic protein (GFAP) in CSF and in plasma (CSF: one Phase 2 trial; plasma: one Phase 3 trial). Electroencephalography (EEG) is collected in seven Phase 2 trials and no Phase 3 trials.
3.7. Trial participants
To fully populate the 187 trials in the pipeline, 57,465 participants are needed. Of these, 41,864 are required for Phase 3 trials; 13,829 for Phase 2 trials; and 1772 for Phase 1 trials. An additional 4632 participants are required for Phase 4 trials or trials with unlabeled phases.
3.8. Trial sponsors
Of the 187 currently active Phase 1, 2, and 3 trials, 108 (58%) are industry sponsored; 17 (9%) are public–private partnerships; 60 (32%) include the NIH, individuals, universities, advocacy groups, and other organizations; and 2 are funded through other US federal agencies. Industry contributes to 67% of all current clinical trials. Thirty‐nine of 55 (71%) Phase 3 trials are industry sponsored, 50 of 99 (51%) Phase 2 trials are sponsored by industry and 19 of 33 (58%) Phase 1 trials are funded through industry. NIH, academic, advocacy, and philanthropic enterprises sponsor 9 of 55 (16%) Phase 3 trials, 41 of 99 (41%) Phase 2 trials, and 10 of 33 (30%) Phase 1 trials.
3.9. Repurposed agents
Repurposed agents represent 28% (56/187) of clinical trials in the current AD pipeline and 28% (40/141) of drugs in the current pipeline. These agents tend to have their greatest role in Phase 2 proof‐of‐concept (POC) trials in which they represent 24 of 99 (24%) drugs. Of all repurposed agents, 60% are in Phase 2 (24/40). There are 12 repurposed agents (33%) in Phase 3 and 7 such agents (23%) in Phase 1.
There are seven (18%) repurposed biological agents addressing disease modification in the pipeline. Of repurposed small molecules, 58% (23/40) target disease modification, 13% (5/40) address neuropsychiatric symptoms, and 13% (5/40) target cognitive enhancement.
Funding of repurposed drugs differs markedly from that of the pipeline generally. Seven of 40 (18%) repurposed drugs in the pipeline are funded by industry; 1 is funded through a public–private partnership; and 32 (80%) are funded through NIH, academic, advocacy, and philanthropic enterprises.
3.10. Global trial distribution
Eighty‐three of 187 (44%) trials are conducted in North America only; 42 of 187 (22%) are conducted only outside of North America; and 46 of 187 (25%) are conducted in both North American and non–North American sites (the data are missing for 16 trials). In Phase 3, 14 of 55 (25%) trials are conducted in North America only, 9 of 55 (16%) are conducted outside of North America only, and 28 of 55 (51%) are conducted in both North American and non–North American sites. Forty‐nine of 99 (49%) Phase 2 trials used North American sites only, 26 of 99 (26%) used only non–North American sites, and 15 of 99 (15%) used both North American and non–North American sites. Phase 1 trials are conducted primarily in North America (20 of 33; 61%), with a substantial number of Phase 1 trials conducted only in non–North American sites (7 of 33; 21%), and few Phase 1 trials involve both North American and non–North American sites (3 of 33; 9%).
4. DISCUSSION
Thirty‐seven new therapies—22 new chemical entities and 15 biologics—were approved by the FDA in 2022 across all therapeutic categories. 9 Five agents are active in the central nervous system (CNS) including one agent for relapsing multiple sclerosis, one treatment for amyotrophic lateral sclerosis, one anticonvulsant, and one therapy for the treatment of insomnia. One biological agent was directed at cerebral adrenoleukodystrophy to slow the progression of neurologic dysfunction. This is the smallest number of new drugs approved since 2016. There were no new treatments approved for AD in 2022. In 2021 and 2023, respectively, aducanumab and lecanemab received accelerated approval for the treatment of AD. Development for AD continues to be challenging with only these two drugs approved since 2003.
Review of the CADRO categories of disease processes that represent drug targets demonstrates that inflammation, amyloid, neurotransmitter receptors, synaptic plasticity, tau biology, oxidation, and proteostasis/proteinopathy represent the major targets for drug development (Figure 4). Targets related to inflammation comprise one of the largest categories in the pipeline, with 16% (N = 25) of agents; in Phase 2, agents addressing inflammatory targets represent 20% of all drugs in development. There has been a consistent number of anti‐inflammatory agents in the pipeline over the past few years. In 2020, there were 20 anti‐inflammatory agents (16.5%); 2021 had 19 agents (15%); and 2022 had 23 agents (16%). Nearly every anti‐inflammatory agent has a different target within the inflammatory cascade (Tables 1, 2, 3). Outcomes of the trials may inform which targets can be modulated with therapeutic benefit and what combinations of targets may be desirable.
FIGURE 4.
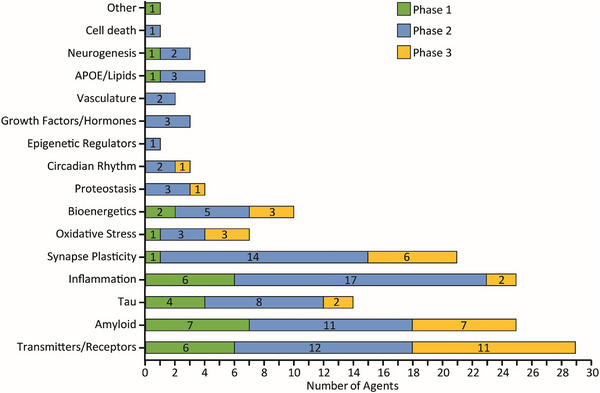
Mechanisms of action of all agents in all phases of clinical trials grouped according to the Common Alzheimer's Disease Research Ontology (CADRO). APOE, apolipoproein E. (Figure © J Cummings; M de la Flor, PhD, Illustrator).
Other common targets of drugs in the pipeline include amyloid 16%, transmitters 20%, synaptic plasticity and circuits 13%, tau targets 9%, and metabolism and bioenergetics drugs 6% (Figures 2, 3, 4). The canonical targets of amyloid and tau comprise 25% of the AD drug development pipeline; the remaining 75% include 14 CADRO categories representing a diverse array of targets among drugs being developed.
Less target diversity is evident in Phase 3 (9 CADRO categories) than Phase 2 (15 categories) reflecting the POC purpose of Phase 2 and the exploration of novel targets at this stage of development. Treatments related to are uncommon in the pipeline despite the influential role played by APOE ε4 in the biology of AD. Cell death as a drug development target is also uncommon, notwithstanding its role in the amyloid, tau, neurodegeneration (AT[N]) approach to AD pathogenesis and biomarkers. Neurotransmitter receptor agents are well represented in the pipeline in part because cognitive enhancing agents and treatments for neuropsychiatric syndromes address transmitter receptor biology. The large number of target processes being addressed by agents in the pipeline will provide important information regarding which processes represent viable targets for AD drug development. Conclusions about the efficacy demonstrated must be considered in the context of the features of the trial, including sample size, target exposure, and population assessed.
Repurposed agents represent 33% of Phase 3 agents, 28% of Phase 2 agents, and 23% of Phase 1 agents. These drugs have been approved for another indication and are being explored for their possible utility in the treatment of AD. Most but not all these drugs are generic. Most repurposed agents are studied in Phase 2 POC trials. In the current pipeline, there are 12 repurposed agents in Phase 3, 24 in Phase 2, and 7 in Phase 1. Development programs for repurposed agents are most likely to be funded by academic (in conjunction with the NIH), advocacy, and philanthropic organizations. Eighty percent of repurposed agents are funded through these mechanisms. The disproportionately smaller role played by the biopharmaceutical industry compared to NIH, academics, advocacy, and philanthropic entities in the development of repurposed agents reflects the difficulty of protecting intellectual property for generic drugs that comprise the foundation for marketability and return on investment. 10 , 11 Deployment and marketing of a successfully developed repurposed compound requires substantial funding. Policy changes, financial incentives, innovation surcharges, and tax advantages will be necessary to attract the funding required to advance repurposed agents to market. 12 , 13 These policy revisions are needed to allow repurposed agents to move beyond their current value in POC trials and become viable therapies for patients.
The globalization of trials is increasingly evident, especially in large late‐stage trials; 51% of Phase 3 trials include both North American and non–North American sites. This reflects the large number of patients and sites required to recruit the large populations required for Phase 3 trials.
Trials increasingly require biological confirmation of the presence of amyloid in participants. Sixty‐four Phase 2 trials and 21 Phase 3 trials require CSF amyloid, CSF amyloid/tau ratios, or amyloid PET at entry. A few trials (four Phase 2; one Phase 3) collect plasma amyloid measures at entry. Tau PET is beginning to have a larger role in clinical trials with six Phase 2 and three Phase 3 trials requiring tau imaging documentation at baseline.
The profile of biomarkers collected as trial outcomes differs from that of biomarkers collected at entry. Outcomes of anti‐amyloid therapies would require amyloid measures as an outcome; non‐amyloid therapeutics might use amyloid for diagnostic confirmation but not as an outcome. There are 20 Phase 2 trials and 16 Phase 3 trials with CSF amyloid or amyloid PET as outcomes. Tau PET is used as an outcome in six Phase 2 trials and nine Phase 3 trials. Biomarkers are playing an increasingly large role in clinical trials in which they may have one or more defined contexts of use including risk determination, diagnosis, monitoring, pharmacodynamic measurement, prognosis determination, response prediction, and safety. 14 , 15 Use of markers in drug development increases the probability of success and is increasingly viewed as a foundational aspect of clinical trials of DMTs. 16
Recruitment is a major challenge for AD clinical trials and a key reason for delay in drug development decisions and advancing new therapies to late‐stage development and possible approval. A Phase 3 biological agent (for symptomatic participants) has a mean treatment duration of 103 weeks and will require, on average, 147 weeks to recruit the participants; a Phase 3 DMT small molecule with a mean treatment period of 56 weeks will require an average of 99 weeks to recruit. The recruitment challenges are more extreme for symptomatic agents. A trial of a cognitive enhancing agent with a mean exposure period of 25 weeks will require an average of 142 weeks for recruitment; trials of treatments for neuropsychiatric symptoms with an average treatment period of 19 weeks require a mean of 194 weeks for recruitment.
The AD drug development pipeline is leading to new therapies. After a 17‐year hiatus in drug approvals, two agents—aducanumab and lecanemab—have entered the market since 2021; brexpiprazole ameliorated agitation in a Phase 3 AD trial; and suvorexant reduced insomnia in AD in a Phase 3 trial. 17 Aducanumab and lecanemab are the first DMTs for AD and among the first DMTs for any neurodegenerative disease. 18 , 19 , 20 Both agents were approved by the FDA using the accelerated pathway based on the reasonable likelihood that amyloid plaque reduction seen on amyloid PET predicts clinical benefit characterized by the slowing of disease progression. 21 A confirmatory trial to demonstrate the clinical efficacy of aducanumab was required by the FDA and a confirmatory trial for lecanemab has been completed. 20 The success of these agents suggests that the understanding of AD‐related biology has progressed sufficiently to allow identification of targets whose modulation ameliorates clinical decline. In addition to improvements in the definition of targets, the availability of more informative biomarkers, identification of candidate drugs with more promising pharmacokinetic and pharmacodynamic characteristics, better definition of appropriate trial populations, and improved trial conduct contributed to the recent successes and provide the foundation for additional productive drug development programs. 22 The recently approved therapies provide treatment for a relatively limited segment (e.g., early AD) of the large and growing AD population. They represent initial steps in the march toward more comprehensive treatments with multiple therapeutic options for those with or at risk for AD.
CONFLICT OF INTEREST STATEMENT
J.C. has provided consultation to Acadia, Alkahest, AlphaCognition, AriBio, Avanir, Axsome, Behren Therapeutics, Biogen, Biohaven, Cassava, Cerecin, Cortexyme, Diadem, EIP Pharma, Eisai, GemVax, Genentech, Green Valley, Grifols, Janssen, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Ono, Otsuka, PRODEO, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, United Neuroscience, and Unlearn AI pharmaceutical, assessment, and investment companies. J.C. is supported by NIGMS grant P20GM109025. NINDS grant U01NS093334. NIA grant R01AG053798. NIA grant P20AG068053. NIA grant R35AG71476. and the Alzheimer's Disease Drug Discovery Foundation (ADDF). J.C. owns the copyright of the Neuropsychiatric Inventory. G.L. is a full‐time employee of Biogen. K.Z. is the CEO of CNS Innovations. Y.Z. and J.F. declare no competing interests. F.C. is supported by the NIA under Award Numbers U01AG073323, R01AG076448, R01AG082211, R01AG066707, 3R01AG066707‐01S1, 3R01AG066707‐02S1, R56AG074001, and R35AG71476. F.C. declares no other competing interests. Author disclosures are available in thesupporting information.
CONSENT STATEMENT
Not applicable. All data are from an anonymized publicly available clinical trial registry (ClinicalTrials.gov). No individual patient‐level data are available on the registry.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
J.C. is supported by NIGMS grant P20GM109025, NINDS grant U01NS093334, NIA grant R01AG053798, NIA grant P20AG068053, NIA grant R35AG71476, and the Alzheimer's Disease Drug Discovery Foundation (ADDF). F.C. is supported by the NIA under Award Numbers U01AG073323, R01AG076448, R01AG082211, R01AG066707, 3R01AG066707‐01S1, 3R01AG066707‐02S1, R56AG074001, and R35AG71476.
Cummings J, Zhou Y, Lee G, Zhong K, Fonseca J, Cheng F. Alzheimer's disease drug development pipeline: 2023. Alzheimer's Dement. 2023;9:e12385. 10.1002/trc2.12385
REFERENCES
- 1. Alzheimer's Association . 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327‐406. [DOI] [PubMed] [Google Scholar]
- 2. Gustavsson A, Norton N, Fast T, et al. Global estimates on the number of persons across the Alzheimer's disease continuum. Alzheimers Dement. 2023; 19(2):658‐670. [DOI] [PubMed] [Google Scholar]
- 3. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer's disease. Lancet. 2021;S0140‐6736(20):32205‐32204. [Google Scholar]
- 4. Cummings J, Lee G, Ritter A, et al. Alzheimer's disease drug development pipeline: 2020. Alzheimers Dement. 2020;6:e12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cummings J, Lee G, Zhong K, et al. Alzheimer's disease drug development pipeline: 2021. Alzheimers Dement. 2021;7:e12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lassman SM, Shopshear OM, Jazic I, et al. Clinical trial transparency: a reassessment of industry compliance with clinical trial registration and reporting requirements in the United States. BMJ Open. 2017;7:e015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phillips AT, Desai NR, Krumholz HM, et al. Association of the FDA Amendment Act with trial registration, publication, and outcome reporting. Trials. 2017;18:333‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Venugopal N, Saberwal G. A comparative analysis of important public clinical trial registries, and a proposal for an interim ideal one. PLoS One. 2021;16:e0251191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mullard A. 2022 FDA approvals. Nat Rev Drug Discov. 2023;22:83‐88. [DOI] [PubMed] [Google Scholar]
- 10. Krishnamurthy N, Grimshaw AA, Axson SA, et al. Drug repurposing: a systematic review on root causes, barriers and facilitators. BMC Health Serv Res. 2022;22:970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41‐58. [DOI] [PubMed] [Google Scholar]
- 12. Robinson JC. An innovation surcharge to fund the repurposing of generic drugs. JAMA. 2022. Online ahead of print. doi: 10.1001/jama.2022.21250 [DOI] [PubMed] [Google Scholar]
- 13. Verbaanderd C, Rooman I, Huys I. Exploring new uses for existing drugs: innovative mechanisms to fund independent clinical research. Trials. 2021;22:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cummings J, Kinney J. Biomarkers for Alzheimer's disease: context of use, qualification, and roadmap for clinical implementation. Medicina. 2022; 58(7):952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Califf RM. Biomarker definitions and their applications. Exp Biol Med. 2018;243:213‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morgan P, Brown DG, Lennard S, et al. Impact of a five‐dimensional framework on R&D productivity at AstraZeneca. Nat Rev Drug Discov. 2018;17:167‐181. [DOI] [PubMed] [Google Scholar]
- 17. Herring WJ, Ceesay P, Snyder E, et al. Polysomnographic assessment of suvorexant in patients with probable Alzheimer's disease dementia and insomnia: a randomized trial. Alzheimers Dement. 2020;16:541‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9:197‐210. [DOI] [PubMed] [Google Scholar]
- 19. Swanson CJ, Zhang Y, Dhadda S, et al. A randomized, double‐blind, phase 2b proof‐of‐concept clinical trial in early Alzheimer's disease with lecanemab, an anti‐Abeta protofibril antibody. Alzheimers Res Ther. 2021;13:80‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9‐21. [DOI] [PubMed] [Google Scholar]
- 21. Dunn B, Stein P, Cavazzoni P. Approval of aducanumab for Alzheimer disease‐the FDA's perspective. JAMA Intern Med. 2021;181:1276‐1278. [DOI] [PubMed] [Google Scholar]
- 22. Cummings J, Feldman HH, Scheltens P. The “rights” of precision drug development for Alzheimer's disease. Alzheimers Res Ther. 2019;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


