Abstract
Gynecomastia is the benign enlargement of breast’s the glandular tissue in male population. Gynecomastia can involve fatty and/or glandular tissue. At the basis of pediatric gynecomastia there is a multifactorial imbalance in the ratio of estrogen to androgens tissue levels. In more than 95% of the cases gynecomastia development is idiopathic. Secondary causes of gynecomastia in adolescents are relatively rare (less than 5%) and may arise from uncommon pathological conditions. Gynecomastia is self-limited and regresses in 1-3 years in 84%, 47% and 20% of adolescents with mild, moderate and severe gynecomastia. The correct first line of therapy is observation and reassurance in the treatment of mild cases. In order to manage adolescent gynecomastia is advised to adopt a tailored therapy. Despite gynecomastia is a common condition only few adolescents need cosmetic or antalgic treatment. Medical therapy should be considered in patient with emotional distress or psychological limitation on normal activities. Finally, if gynecomastia does not go in remission after two years surgical procedures should be performed. The aim of this article is to be an updated discussion of pubertal gynecomastia, in particular the surgical aspect, and report our surgical experience with a retrospective study. In conclusion surgical treatment of this condition is a quiet rare procedure but, in according to global literature we demonstrated that it is a safe surgery with low rate of complications. (www.actabiomedica.it)
Keywords: Gynecomastia, Primary Gynecomastia
Introduction
Gynecomastia is the benign enlargement of breast’s the glandular tissue in male population. (1) Gynecomastia can involve fatty and/or glandular tissue. Gynecomastia patients can occur in atypical ductal hyperplasia (ADH) but this condition does not pose the same risk as ADH in women. (2) Some authors have drawn distinction between true gynecomastia and pseudogynecomastia. (3) In fact, pseudogynecomastia is related with only fatty hypertrophy and exhibits a pattern of increasing superimposable to the obesity rate. (4) True gynecomastia has a trimodal age distribution (neonatal, pubertal and elderly). (5) Among them, pubertal gynecomastia has been reported to have an incidence rate up to 65% in adolescent male. (6) Gynecomastia etiology is multifactorial, and the most frequent cause of this pathology is idiopathic. (3) Primary pubertal regresses spontaneously in about 90% of the patients in 6 months to 3 years. (1) This means that in case of primary pubertal gynecomastia the correct conduct is observation and reassurance without specific treatment. (4) However, it is important to remember that various pathologies can lead to the development of gynecomastia by disrupting the delicate hormone balance with a androgen deficiency or an estrogen excess (7) (Table 1).
Table 1.
Pathologic causes of gynecomastia (8).
|
Endocrine Testicular
|
|
Systemic Chronic liver disease Chronic kidney disease Refeeding syndrome Malnutrition |
|
Neoplasms Breast carcinoma Testicular tumors
Lung carcinoma Liver carcinoma hCG-secreting tumors |
|
Infections HIV |
|
Other Obesity |
It is important for the patient to undergo to a primary care physician visit and in selected cases to an endocrinologist evaluation before the plastic surgeon workup. Surgical approach has to be considered if gynecomastia last for 1 year at least. Surgical treatments for gynecomastia involve four general approaches. 1 excision of the breast tissue, 2 suction-assisted lipectomy, 3 skin resection, 4 any combination thereof. (4)
The objective of this study is to report the characteristics of adolescent gynecomastia patients who received surgical treatments at our hospital and discuss the long-term surgical outcomes of these patients.
Etiologies and pathogenesis
At the basis of pediatric gynecomastia there is a multifactorial imbalance in the ratio of estrogen to androgens tissue levels. In fact, estrogens stimulate the proliferation of the breast mass, while androgens inhibit it; normal male breast tissue has receptor for both, like female tissue. (9)
It appears to be a local imbalance between estrogen stimulation and the inhibitory action of androgens on breast tissue proliferation, although the majority of adolescents with gynecomastia have normal estrogen levels. (10)
In more than 95% of the cases gynecomastia development is idiopathic. Secondary causes of gynecomastia in adolescents are relatively rare (less than 5%) and may arise from uncommon pathological conditions. (11) Although these conditions are less common that idiopathic gynecomastia it is important to differentiate them with appropriates clinical assessment and diagnostic procedures.
Classification of gynecomastia
Actually, there is not a univocal classification system of classification of gynecomastia. The most common classification is Simon’s one.
Simon et al. identified four grades of gynecomastia:
Grade I: small enlargement without skin excess
Grade IIa: moderate enlargement without skin excess
Grade IIb: moderate enlargement with minor skin excess
Grade III: Marked enlargement with excess skin.
This classification despite analyzes patients only by two features (breast mass and skin excess) can make an effective surgical algorithm tata avoids the misclassification of the type of gynecomastia and guides to the most appropriate surgical management.
Progression of disease and diagnostic path
The first step on diagnostic algorithm is to discriminate gynecomastia from pseudogynecomastia by radiological imaging. Ultra-sound examination is the primary imaging tool for diagnosis and classification of gynecomastia. Mammography is universal reserved only in the case of suspected sonographic malignant finds. (12) Once it is described the presence of true gynecomastia serum liver function has to be investigated. (13) If liver function is normal or red flags, like pain, are present more serum valuation has to be performed: blood levels of testosterone, LH, FSH, 17β -estradiol, βhCG and thyroid function. (14) Also karyotype has to be investigate in order to exclude genetic chromosomal syndromes. (15) Low levels of testosterone and elevated E2/testosterone ratio suggest androgenic deficit syndrome. (16) Elevated levels of βhCG prompt the hypothesis that gynecomastia is a symptom of paraneoplastic syndrome of cancer secrete βhCG. (17) A testicular sonographic examination has to be performed in order to rule out the presence of gonadal tumor. This typology of tumors is not limited to gonadal tissue but it is possible to find its primary origin extra-gonadal. (18) In the majority of patient blood exams reveal high levels of E2, normal levels of testosterone and resulting E2/testosterone ratio imbalance. These results are typical of an increase in aromatization process. This upsurge is determined by a wide range of conditions: obesity, Sertoli cells tumor, adolescent idiopathic gynecomastia, hereditary conditions. Red flags for endocrinopathy include abnormal testicular examination, galactorrhea, features of Klinefelter syndrome (small testicles, eunuchoism, and behavioral problems for examples), rapid gynecomastia progression and abnormal funduscopic testing. (19) De Sanctis et al. revealed that adolescent idiopathic gynecomastia is self-limited and regresses in 1-3 years in 84%, 47% and 20% of adolescents with mild, moderate and severe gynecomastia. (10) The correct first line of therapy is observation and reassurance in the treatment of mild cases. (4) In order to manage adolescent gynecomastia is advise to adopt a tailored therapy. Despite gynecomastia is a common condition only few adolescents need cosmetic or antalgic treatment. Medical therapy should be considered in patient with emotional distress or psychological limitation on normal activities. Finally, if gynecomastia does not go in remission after two years surgical procedures should be performed. Timing for perform surgery procedure is important, in fact premature surgical intervention is related to breast regrown. (1)
Medical treatment
Pharmacological treatment aims to correct hormonal imbalance of gynecomastia by three possible paths: 1 blocking the effects of estrogens on the breast tissue (examples: tamoxifen, raloxifene, clomiphene), 2 managing androgens (example: danazol) and 3 inhibiting estrogen production (examples: anastrozole, testolactone). (20) Medicinal therapy is most effective if used in recent gynecomastia (up to 2 years). After two years the stroma become more fibrotic and conservative therapy in ineffective. (9) Due to its spontaneous regression data on efficacy of pharmacological therapy of adolescent gynecomastia is mostly limited to case reports and smalls case series without control group, which give a less level of significance. (21) In conclusion pharmacological treatment of pubertal gynecomastia is more effective if given in early stage of the condition and it is more successful in moderate enlargement and it should be started immediately in case of painful gynecomastia.
Surgical treatment
Propose surgical therapy of pubertal gynecomastia should be considered in non-obese male adolescent after a 12-months history of condition, breast pain, breast tenderness or significant psychosocial distress with social life limitation. However, obesity is not an absolute contraindication to surgical approach. (11) Kasielska-Trojan et al. demonstrated that patients with gynecomastia had a life quality improved significantly after surgery procedures. (22) It is important to exclude adolescent breast cancer before performing the surgical treatment although it is a rare condition. (7, 23) In fact Lu et al. encountered one case of undiagnosed performing breast liposuction. (24) A wide range of plastic surgery techniques have been studied in order to treat gynecomastia. Plastic surgeon should offer a tailored surgery to individual patients, according to physical aspects. The most used technique is liposuction that minimizes the scarring and reduces days in hospital. (25) This technique is effective in mild and localized gynecomastia, with 92% satisfied results. (26) In patients with an high component of hypertrophic breast tissue (grade IIa and IIb of Simon’s classification) it is mandatory to perform a reduction of both skin and breast tissue using surgical excision. In women with hypertrophy of breast the most common techniques are the inverted T incision and vertical incision techniques, but they are inadequate in used in masculinization of thorax. (27) In pediatric gynecomastia mastectomy is performed commonly with peri-areolar incision. This technique left only minor scars and the remnant skin flap become less visible with the healing. (28) Combined technique (liposuction + mastectomy) is more effective than singles procedures. (29) In order to reduce complication some authors suggest that vacuum-assisted minimally invasive surgery should be performed, but there is not still evidences in pediatric use. (30) Tarallo et al. reported a new technique for correction of gynecomastia called “the round-the-clock” that leads only a 3 mm scar each side. (31) Further studies have to pe performed in order to demonstrate the efficacy in adolescent gynecomastia. Endoscope-assisted subcutaneous mastectomy offers a smaller incision than peri-areolar incision, but this technique did not totally eliminate a potential complication of the scar on a visible part of the chest. (32) Some surgeons (33) affirm that it is possible to perform a more aesthetic body contouring leaving inside the patient and shaping part of mammary gland by “superior dynamic flap method”. This technique can be customized in order to correct asymmetry of the chest. This technique has not been studied yet in paediatric population and our opinion is that it is better to perform total asportation of gland in adolescents in order to avoid recurrences. Gynecomastia surgery is a relatively low-risk procedure associated with minimal morbidity. Only 1.5% of patients is associated with postoperative superficial surgical site complications. Hematoma is the most frequent early complication. (34) Some authors affirm that it is possible to reduce the frequency of hematoma using the external quilting suture technique, (35) but there is no evidence in effectiveness in pediatric population. Some authors (36, 37) affirm that drains are not useful in the prevention of hematoma and other complications after mastectomy procedure. This theory is not universally accepted and our equips support a safer approach positioning drains after the asportation of mammary glands. (38) Others possible complication, less common, are seroma, infections, under-resection, unaesthetic scarring, and over-resection with saucer-type deformity. It is important to inform pediatric patients and their parents about these complications, albeit rare. In the post-operatory, like in women mastectomy procedures, patients should wear a compression garment at all times until post-operative visit. (39) After the first week post operatory wearing the garments could be reduced to 18 hours/day. Garments apply pressure to reduce swelling as well as contour the surgical site. Post-Operative garments are usually worn between 6-8 weeks depending on healing. Rest is the most important post operatory advice. Patients should not perform strenuous activity (anything that increases your blood pressure) for the first 2 weeks. Exercise can be practiced after 2 weeks, if there are not involved muscles of chest. Pectoralis’ and upper limbs’ exercises can be started at least 8 weeks after surgery.
Liposuction
Patient is settled in supine position and after surgical field preparation the mammary region is infiltrated with local anesthesia in tumescent solution of 10 cc of 2% Mepicain withandrenaline in 2000 cc of saline solution. Via a stab incision at 8 o’ clock in the anterior axillary fold, liposuction of the breast is performed drawing fatty tissue by a 3 mm or 4 mm cannula.
Resection of fibro-stromal and glandular component
The subcutaneous mastectomy procedures were performed by using Webster method with peri-areolar incision. We did not find skin redundancy. We performed Davidson concentric circles technique if skin reduction was present. Each mammary tissue was examined to achieve histopathological diagnosis. Follow-through alternate liposuction and resections are repeated as necessary to achieve complete asportation of subcoutaneous tissue. In order to prevent hematoma a suction drain was positioned in each mastectomy.
Our experience
Between September 2003 and December 2019 we treated 18 adolescents (average age 15 years and 4 months, 14-18 years). 13 of our patients were treated bilaterally and 5 were treated monolaterally with a total of 31 breasts. We included in the study patient with more than 12 months gynecomastia history and no satisfactory response to medical treatment. Patients had grade 2 gynecomastia (Simon classification). We obtained written informed consent from all patients. Every surgical procedure was performed under general anesthesia, and antibiotic prophylaxis was routinely administered at induction. All surgeries were performed by the same operator. The surgical technique involves two steps: liposuction of fatty tissue and resection of fibro-stromal and glandular component with round-block masropexy (Figs. 1-6).
Figure 1.
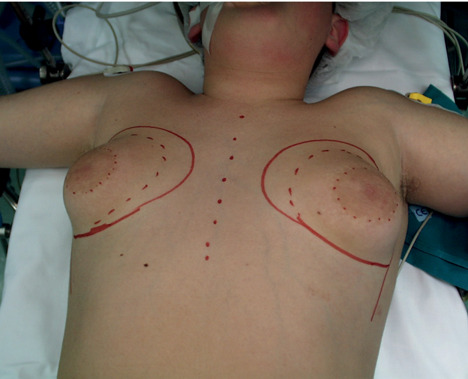
Preoperative drawing of gynecomastia.
Figure 6.
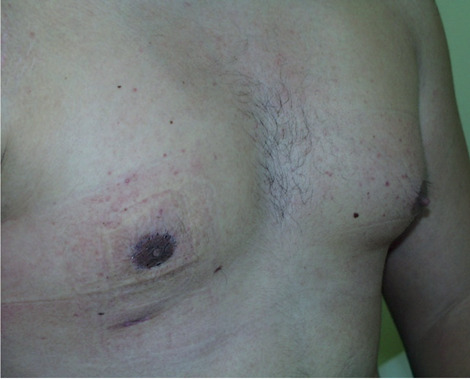
Post operative result.
Figure 2.
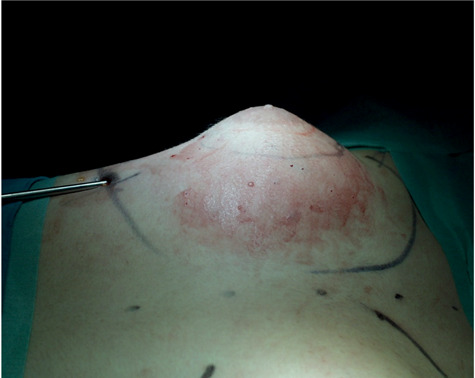
Lipoaspiration.
Figure 3.
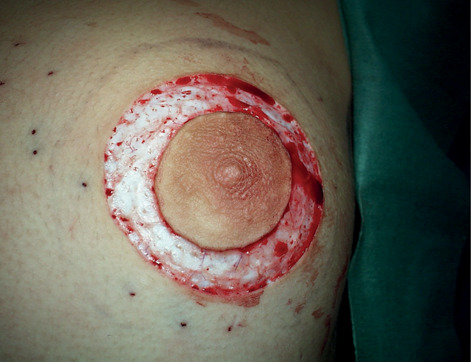
Round block disepitelisation.
Figure 4.
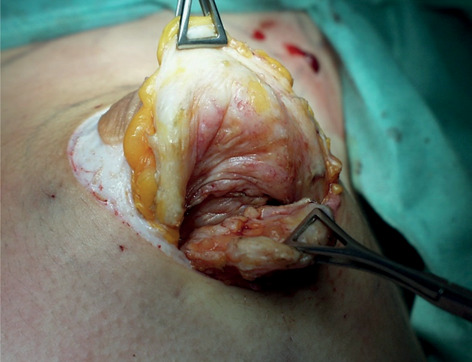
Gland asportation.
Figure 5.
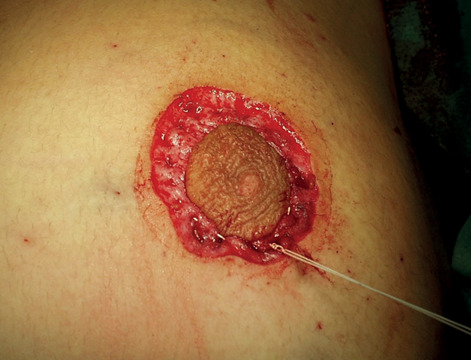
Round block suture.
Results
Of 14 patient who underwent the procedure, the average age at surgery was 15 years and 4 months with a follow-up period ranging 12 to 18 months. Gynecomastia was mono-lateral in 5 patients and bilateral in 13 patients. Family history of gynecomastia was not exhibited by any patients. No patients presented risk factors for gynecomastia as drug abuse (cannabis, cocaine), antiepileptic drugs, anabolic steroids, anti-androgens drugs, history of prolactinoma or hypogonadotrophic hypogonadism; so we classified all cases of gynecomastia as idiopathic. all patients underwent surgical operation under general anesthesia. Mean surgical time was 73 ± 20 minutes and postoperative stay was 2 ± 1 days. In every case histopathological examination excluded diagnosis of malignancy. We did not report any intraoperative or early post-operative complications. Our equips contacted by phone patients in order to discover their level of aesthetic satisfaction: all patients were reachable; 17 patients reached on the phone expressed satisfaction with final aesthetic result (94,44%), 1 was unsatisfied. The cause of his disappointment was due an enlargement of the scar. No more late complications were registered.
Discussion
Gynecomastia is a benign clinical finding characterized by enlargement of the male breast, due to proliferation of glandular tissue.1 This condition differs from pseudogynecomastia (fatty breasts) due to increased local fat deposition without glandular enlargement. (3) Gynecomastia can be seen as part of the normal physiological development in the new- born and adolescent boys. In paediatric population more the 95% of cases of gynecomastia are idiopathic, unlike adult population in which secondary gynecomastia is more frequent. (40) In our experience all patients were affected by idiopathic gynecomastia. Despite secondary causes of gynecomastia in adolescents are relatively rare, it may arise from uncommon pathological conditions. Hence, it is fundamental to follow a diagnostic path involving blood hormonal test (LH, FSH, estradiol, testosterone, prolactin, dehydroepiandrosterone and human chorionic gonadotropin) and endocrinologist valuation. (41) The majority of boys affected by gynecomastia are asymptomatic, while those referred to the specialist present persistently tenderness of the breasts, palpable lump or unsatisfactory body image with important psychological repercussions. (42) The timing of the onset of gynecomastia is very important: the greatest psychological impact occurs with onset in adolescence as compared with young childhood. (43) Sonography is widely used in all breast’s diseases. Typical findings in case of gynecomastia include hypoechoic retroareolar masses (nodular, poorly defined or flame- shaped), with increased anteroposterior depth at the nipple. (44) This evaluation is able to settle the composition of the breast (fat tissue and glandular tissue). Mammography is used in case of suspicion of cancer, with sensitivity of 90% and specificity of 92%. (45) In our experience all patient underwent sonography, and none underwent mammography due to the absence of malignant features. Our accurate preoperative diagnostic path (medical history, physical examination, ultrasound and hormonal profile) allowed us to exclude from surgery patients affected by secondary gynecomastia. Pubertal gynecomastia is self-limited and regresses in 1-3 years in 84%, 47% and 20% of adolescents with mild, moderate and severe gynecomastia. (10) Asymptomatic adolescent with less than 2 years disease’s history do not require treatment if diagnostic path does not reveal any underlying disease: reassurance and periodic follow-up visits are recommended (every 3-6 months). (46) If patient’s condition of breast is caused by an underlying hormonal disorder, its treatment is generally sufficient to cause regression of gynecomastia. (47) If gynecomastia either persists or becomes more severe and symptomatic with reduced quality of life (pain, unsatisfactory body image with psychological distress), pharmacological and surgical treatment should be considered, especially if the patient is compliant. (48) The aim of medical treatment of gynecomastia is the correction of estrogen-androgen imbalance by different pathways and can be useful if performed during the early proliferative phase without stromal hyalinization and fibrosis. Medical treatment is actually controversial: there is no consensus regarding the drug of choice and the optimal duration of treatment. Surgery is the resolutive treatment for gynecomastia. Different surgical techniques share the same goals of restoring a pleasant chest shape with limited scar extension and the choice of surgeon depends on the severity of breast enlargement, the presence of skin excess and surgeon and patient preference. (49) All patients we treated presented Simon’s grade II B gynecomastia (moderate breast enlargement with minor skin redundancy). Hence, we performed Davidson technique to remove excess skin. In this technique drains were always used in order to minimize hematoma’s risk. Our surgical technique has enabled us to remove excess of parenchymal tissue with limited injury of mammary region and low incidence of local complications. We had only one minor complication: we observed a diastase scar in 1 breast (3,2%). This situation was probably caused by a too early resumption of sport activities (20 days). No hematoma was registered. We did not observe complications as wound infection, necrosis of nipple-areolar complex or wound dehiscence. Postoperative course of our patients was regular with rapid discharge and median postoperative stay was 2 ± 1 days. Gynecomastia does not improve patients’ risk for breast cancer. (50) Instead, patients affected by Klinefelter syndrome have high risk of cancer, with a 50-fold higher risk that among men in the general population. (48) Despite in pediatric population cancer risk is low we performed routine histopathological analysis of all mammary specimens in order to exclude malignancy. Given that premise, the biggest problem of patients affected by gynecomastia is unsatisfactory body image that leads to a self-limitation of daily activities. The assessment of patient satisfaction with final aesthetic result is fundamental: 94,4% of our patients expressed full satisfaction with final results. The main causes of patient’s dissatisfaction are breast asymmetry and hypertrophic scars. (48)
Conclusion
Pubertal gynecomastia is a common condition in young children and adolescents and this condition can be self-limiting. In case of persistence and correlated symptomatology responsible for reduced quality of life treatment should be performed. Surgical correction is more effective than medical therapy and in some cases, it is the only therapeutic option. Preoperative assessment (medical history, physical examination, ultrasound, and hormonal profile and endocrinological valuation) is mandatory in order to discover secondary causes of gynecomastia and set up specific treatment. Several surgical techniques are described for correcting gynecomastia. If performed by experienced plastic and pediatric surgeon surgical treatment of gynecomastia is safe and permits to reach satisfactory aesthetic results with minimal complications.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Author Contributions: Conceptualization:
Massimo Pinelli, Pierluca Ceccarelli; Writing original draft preparation: Massimo Pinelli, Federico De Maria, Giorgio De Santis; Data analysis Barbara Pedrieri, Maria Anastasia Bianchini, Lorenzo Iughetti.
References
- Braunstein GD. Gynecomastia. N Engl J Med. 2007;357(12):1229–1237. doi: 10.1056/NEJMcp070677. doi:10.1056/NEJMcp070677. [DOI] [PubMed] [Google Scholar]
- Coopey SB, Kartal K, Li C, et al. Atypical ductal hyperplasia in men with gynecomastia: what is their breast cancer risk? Breast Cancer Res Treat. 2019;175(1):1–4. doi: 10.1007/s10549-018-05117-4. doi:10.1007/s10549-018-05117-4. [DOI] [PubMed] [Google Scholar]
- Rahmani S, Turton P, Shaaban A, Dall B. Overview of Gynecomastia in the Modern Era and the Leeds Gynaecomastia Investigation Algorithm. Breast J. 2011;17(3):246–255. doi: 10.1111/j.1524-4741.2011.01080.x. doi:10.1111/j.1524-4741.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- Waltho D, Hatchell A, Thoma A. Gynecomastia Classification for Surgical Management. Plast Reconstr Surg. 2017;139(3):638e–648e. doi: 10.1097/PRS.0000000000003059. doi:10.1097/PRS.0000000000003059. [DOI] [PubMed] [Google Scholar]
- Choi BS, Lee SR, Byun GY, Hwang SB, Koo BH. The Characteristics and Short-Term Surgical Outcomes of Adolescent Gynecomastia. Aesthetic Plast Surg. 2017;41(5):1011–1021. doi: 10.1007/s00266-017-0886-z. doi:10.1007/s00266-017-0886-z. [DOI] [PubMed] [Google Scholar]
- Kumanov P, Deepinder F, Robeva R, Tomova A, Li J, Agarwal A. Relationship of Adolescent Gynecomastia with Varicocele and Somatometric Parameters: A Cross-Sectional Study in 6200 Healthy Boys. J Adolesc Heal. 2007;41(2):126–131. doi: 10.1016/j.jadohealth.2007.03.010. doi:10.1016/j.jadohealth.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Chang YW, Oh JH, Hwang J, Hong SS, Kim HJ. Breast lesions in children and adolescents: Diagnosis and management. Korean J Radiol. 2018;19(5):978–991. doi: 10.3348/kjr.2018.19.5.978. doi:10.3348/kjr.2018.19.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalfi A, Sommer N. Gynecomastia. In: Chung KC, editor. Grabb and Smith’s Plastic Surgery. Eight edit. Wolters Kluwer; 2020. pp. 575–581. [Google Scholar]
- Narula HS, Carlson HE. Gynaecomastia—pathophysiology, diagnosis and treatment. Nat Rev Endocrinol. 2014;10(11):684–698. doi: 10.1038/nrendo.2014.139. doi:10.1038/nrendo.2014.139. [DOI] [PubMed] [Google Scholar]
- Bidlingmaier F, Knorr D. Plasma testosterone and estrogens in pubertal gynecomastia. Zeitschrift für Kinderheilkd. 1973;115(1):89–94. doi: 10.1007/BF00438995. doi:10.1007/BF00438995. [DOI] [PubMed] [Google Scholar]
- Rosen H, Webb ML, DiVasta AD, et al. Adolescent Gynecomastia: not only an obesity issue. Ann Plast Surg. April 2010:1. doi: 10.1097/SAP.0b013e3181dba827. doi:10.1097/SAP.0b013e3181dba827. [DOI] [PubMed] [Google Scholar]
- Telegrafo M, Introna T, Coi L, et al. Breast US as primary imaging modality for diagnosing gynecomastia. G di Chir. 2016;37(3):118–122. doi: 10.11138/gchir/2016.37.3.118. doi:10.11138/gchir/2016.37.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JW, Toll GD, Russfield AB, Reiss AM, Quinby WC, Baker WH. Sexual precocity attributable to ectopic gonadotropin secretion by hepatoblastoma. Am J Med. 1973;54(3):390–403. doi: 10.1016/0002-9343(73)90034-x. doi:10.1016/0002-9343(73)90034-X. [DOI] [PubMed] [Google Scholar]
- Fauci Anthony, Kasper Dennis, Hauser Stephen, Longo Dan, Jameson Larry, Loscalzo J. Harrison-Manuale Di Medicina. XIX 2017. (Casa Editrice Ambrosiana, ed) 2017 [Google Scholar]
- Kanakis GA, Nordkap L, Bang AK, et al. EAA clinical practice guidelines—gynecomastia evaluation and management. Andrology. 2019;7(6):778–793. doi: 10.1111/andr.12636. doi:10.1111/andr.12636. [DOI] [PubMed] [Google Scholar]
- Castro-Magana M, Angulo M, Uy J. Male Hypogonadism with Gynecomastia Caused by Late-Onset Deficiency of Testicular 17-Ketosteroid Reductase. N Engl J Med. 1993;328(18):1297–1301. doi: 10.1056/NEJM199305063281802. doi:10.1056/NEJM199305063281802. [DOI] [PubMed] [Google Scholar]
- Pusl T, Stoemmer P. Gynecomastia: Look Beyond the Obvious. Am J Med. 2017;130(10):e439–e440. doi: 10.1016/j.amjmed.2017.04.021. doi:10.1016/j.amjmed.2017.04.021. [DOI] [PubMed] [Google Scholar]
- Rehman T, Hameed A, Beharry N, Parcq J, Du Bano G. An unusual cause of gynaecomastia in a male. Endocrinol Diabetes Metab Case Reports. 2019;2019 doi: 10.1530/EDM-19-0060. doi:10.1530/EDM-19-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantopoulos S, Bao Y. Gynecomastia and Premature Thelarche: A Guide for Practitioners. Pediatr Rev. 2007;28(9):e57–e68. doi: 10.1542/pir.28-9-e57. doi:10.1542/pir.28-9-e57. [DOI] [PubMed] [Google Scholar]
- Nordt CA, DiVasta AD. Gynecomastia in adolescents. Curr Opin Pediatr. 2008;20(4):375–382. doi: 10.1097/MOP.0b013e328306a07c. doi:10.1097/MOP.0b013e328306a07c. [DOI] [PubMed] [Google Scholar]
- Kanakis GA, Nordkap L, Bang AK, et al. EAA clinical practice guidelines—gynecomastia evaluation and management. Andrology. 2019;7(6):778–793. doi: 10.1111/andr.12636. doi:10.1111/andr.12636. [DOI] [PubMed] [Google Scholar]
- Kasielska-Trojan A, Antoszewski B. Gynecomastia Surgery-Impact on Life Quality: A Prospective Case-Control Study. Ann Plast Surg. 2018;80(2):194. doi: 10.1097/SAP.0000000000001173. doi:10.1097/SAP.0000000000001173. [DOI] [PubMed] [Google Scholar]
- Fagerlund A, Lewin R, Rufolo G, Elander A, Santanelli di Pompeo F, Selvaggi G. Gynecomastia: A systematic review. J Plast Surg Hand Surg. 2015;49(6):311–318. doi: 10.3109/2000656X.2015.1053398. doi:10.3109/2000656X.2015.1053398. [DOI] [PubMed] [Google Scholar]
- Tu L-C, Tung K-Y, Chen H-C, Huang W-C, Hsiao H-T. Eccentric Mastectomy and Zigzag Periareolar Incision for Gynecomastia. Aesthetic Plast Surg. 2009;33(4):549–554. doi: 10.1007/s00266-008-9285-9. doi:10.1007/s00266-008-9285-9. [DOI] [PubMed] [Google Scholar]
- Rohrich RJ, Ha RY, Kenkel JM, Adams WP. Classification and Management of Gynecomastia: Defining the Role of Ultrasound-Assisted Liposuction. Plast Reconstr Surg. 2003;111(2):909–923. doi: 10.1097/01.PRS.0000042146.40379.25. doi:10.1097/01.PRS.0000042146.40379.25. [DOI] [PubMed] [Google Scholar]
- Abdelrahman I, Steinvall I, Mossaad B, Sjoberg F, Elmasry M. Evaluation of Glandular Liposculpture as a Single Treatment for Grades I and II Gynaecomastia. Aesthetic Plast Surg. 2018;42(5):1222–1230. doi: 10.1007/s00266-018-1118-x. doi:10.1007/s00266-018-1118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cely AMG, Triana CE, Triana LM. Thorax masculinization in a transsexual patient: Inferior pedicle mastectomy without an inverted T scar. Arch Plast Surg. 2019;46(3):262–266. doi: 10.5999/aps.2018.00108. doi:10.5999/aps.2018.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J-E, Ko CW, Yang JD, Lee JS. Combined surgical and medical treatment in an adolescent with severe gynecomastia due to excessive estradiol secretion: a case report. BMC Pediatr. 2019;19(1):515. doi: 10.1186/s12887-019-1887-7. doi:10.1186/s12887-019-1887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Byun IH, Lee WJ, Rah DK, Kim JY, Lee DW. Surgical Management of Gynecomastia: Subcutaneous Mastectomy and Liposuction. Aesthetic Plast Surg. 2016;40(6):877–884. doi: 10.1007/s00266-016-0705-y. doi:10.1007/s00266-016-0705-y. [DOI] [PubMed] [Google Scholar]
- Yao Y, Yang Y, Liu J, Wang Y, Zhao Y. Vacuum-assisted minimally invasive surgery—An innovative method for the operative treatment of gynecomastia. Surgery. 2019;166(5):934–939. doi: 10.1016/j.surg.2019.04.032. doi:10.1016/j.surg.2019.04.032. [DOI] [PubMed] [Google Scholar]
- Tarallo M, Di Taranto G, Fallico N, Ribuffo D. The round-the-clock technique for correction of gynecomastia. Arch Plast Surg. 2019;46(3):221–227. doi: 10.5999/aps.2018.00472. doi:10.5999/aps.2018.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qutob O, Elahi B, Garimella V, Ihsan N, Drew P. Minimally invasive excision of gynaecomastia – a novel and effective surgical technique. Ann R Coll Surg Engl. 2010;92(3):198–200. doi: 10.1308/003588410X12628812458815. doi:10.1308/003588410X12628812458815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harinatha S. Male Gynecomastia Correction by Superior Dynamic Flap Method: A Consistent and Versatile Technique. World J Plast Surg. 2020;9(1):33–38. doi: 10.29252/wjps.9.1.33. doi:10.29252/wjps.9.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora Y, Mittal RR, Williams EA, Thaller SR. Barriers to the Effective Management of Gynecomastia in Adolescents. J Craniofac Surg. 2019;30(8):2381–2384. doi: 10.1097/SCS.0000000000005999. doi:10.1097/SCS.0000000000005999. [DOI] [PubMed] [Google Scholar]
- Murugesan L, Karidis A. External Quilting: New Technique to Avoid Haematoma in Gynaecomastia Surgery. Aesthetic Plast Surg. 2020;44(1):45–51. doi: 10.1007/s00266-019-01537-9. doi:10.1007/s00266-019-01537-9. [DOI] [PubMed] [Google Scholar]
- Jackson PC, MacInnes EG, Nicholson JK, Brayshaw I, Relton S, Achuthan R. Mastectomy Without Drains Reduces Cost with No Detriment to Patient Outcome. Cureus. 2019;11(7):e5160. doi: 10.7759/cureus.5160. doi:10.7759/cureus.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin M, Sutcu M, Cigsar B, Karacaoglan N. Necessity of Suction Drains in Gynecomastia Surgery. Aesthetic Surg J. 2014;34(4):538–544. doi: 10.1177/1090820X14526598. doi:10.1177/1090820X14526598. [DOI] [PubMed] [Google Scholar]
- Chao JW, Raveendran JA, Maly C, Rogers G, Boyajian M, Oh AK. Closed-Suction Drains After Subcutaneous Mastectomy for Gynecomastia: Do They Reduce Complications? Aesthetic Plast Surg. 2017;41(6):1291–1294. doi: 10.1007/s00266-017-0959-z. doi:10.1007/s00266-017-0959-z. [DOI] [PubMed] [Google Scholar]
- Hansdorfer-Korzon R, Teodorczyk J, Gruszecka A, Lass P. Are compression corsets beneficial for the treatment of breast cancer-related lymphedema? New opportunities in physiotherapy treatment – a preliminary report. Onco Targets Ther. April 2016;2089 doi: 10.2147/OTT.S100120. doi:10.2147/OTT.S100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Webb ML, DiVasta AD, et al. Adolescent Gynecomastia. Ann Plast Surg. April 2010:1. doi: 10.1097/SAP.0b013e3181dba827. doi:10.1097/SAP.0b013e3181dba827. [DOI] [PubMed] [Google Scholar]
- Bembo SA, Carlson HE. Gynecomastia: its features, and when and how to treat it. Cleve Clin J Med. 2004;71(6):511–517. doi: 10.3949/ccjm.71.6.511. doi:10.3949/ccjm.71.6.511. [DOI] [PubMed] [Google Scholar]
- Al-Allak A, Govindarajulu S, Shere M, Ibrahim N, Sahu AK, Cawthorn SJ. Gynaecomastia: A decade of experience. Surg. 2011;9(5):255–258. doi: 10.1016/j.surge.2010.10.004. doi:10.1016/j.surge.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Schonfeld WA. Gynecomastia in Adolescence: Effect on Body Image and Personality Adaptation. Psychosom Med. 1962;24(4):379–389. doi: 10.1097/00006842-196200700-00008. doi:10.1097/00006842-196200700-00008. [DOI] [PubMed] [Google Scholar]
- Dialani V, Baum J, Mehta TS. Sonographic Features of Gynecomastia. J Ultrasound Med. 2010;29(4):539–547. doi: 10.7863/jum.2010.29.4.539. doi:10.7863/jum.2010.29.4.539. [DOI] [PubMed] [Google Scholar]
- Hines SL, Yasrebi M, Tan WW, Perez EA, DePeri ER. The Role of Mammography in Male Patients With Breast Symptoms. Mayo Clin Proc. 2007;82(3):297–300. doi: 10.4065/82.3.297. doi:10.4065/82.3.297. [DOI] [PubMed] [Google Scholar]
- Jones DJ, Holt SD, Surtees P, Davison DJ, Coptcoat MJ. A comparison of danazol and placebo in the treatment of adult idiopathic gynaecomastia: results of a prospective study in 55 patients. Ann R Coll Surg Engl. 1990;72(5):296–298. http://www.ncbi.nlm.nih.gov/pubmed/2221763. [PMC free article] [PubMed] [Google Scholar]
- Lemaine V, Cayci C, Simmons P, Petty P. Gynecomastia in Adolescent Males. Semin Plast Surg. 2013;27(01):056–061. doi: 10.1055/s-0033-1347166. doi:10.1055/s-0033-1347166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longheu A, Medas F, Corrias F, et al. Surgical management of gynecomastia: Experience of a general surgery center. G di Chir. 2016;37(4):150–154. doi: 10.11138/gchir/2016.37.4.150. doi:10.11138/gchir/2016.37.4.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova A, Moschella F. Algorithm for clinical evaluation and surgical treatment of gynaecomastia. J Plast Reconstr Aesthetic Surg. 2008;61(1):41–49. doi: 10.1016/j.bjps.2007.09.033. doi:10.1016/j.bjps.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Olsson H, Bladstrom A, Alm P. Male gynecomastia and risk for malignant tumours – a cohort study. BMC Cancer. 2002;2(1):26. doi: 10.1186/1471-2407-2-26. doi:10.1186/1471-2407-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]


