Abstract
Non-gay identifying men who have sex with men and women (MSMW) are an important subgroup of men who have sex with men (MSM) and have been underrepresented in studies of MSM that only use gay venues to draw their samples. We assessed heterosexual and drug use risks of MSMW who use drugs in a sample of male entrants to the Mount Sinai Beth Israel drug treatment programs from 2005 to 2018. Blood samples were collected and tested for HIV and HSV-2 infections. Among HIV seronegative participants, MSMW had significantly greater odds of sharing used needles with others, and reporting unprotected sex with female casual partners and female commercial sex partners, compared to their counterparts who reported sex with women exclusively (MSWE). Although not recruited from gay venues, MSMW had a significantly higher HIV prevalence than MSWE (23% vs. 10%, p < 0.001). Interventions that are specifically tailored to HIV prevention among MSMW are needed to ameliorate the prevalence of HIV risks and infection.
Keywords: Non-gay identifying MSMW, HIV risk behaviors, Injection drug use
Resumen
Los hombres que no se que identifican como gay y que tienen sexo con hombres y mujeres (HSHM) son una importante subgrupo de hombres que tienen sexo con hombres (HSH) y han sido subrepresentados en estudios de HSH que solo usan lugares gay para extraer sus muestras. Evaluamos los riesgos heterosexuales y de uso de drogas de los HSHM que usan drogas en una muestra de hombres participantes en los programas de tratamiento de drogas en Mount Sinai Beth Israel desde 2005 hasta 2018. Se recogieron muestras de sangre y se analizaron infecciones por VIH y VHS-2. Entre el VIH participantes seronegativos, HSHM tuvo significativamente mayores probabilidades de compartir utilizados agujas con otros, y reportar relaciones sexuales sin protección con parejas ocasionales y parejas sexuales comerciales femeninas, en comparación con sus contrapartes que informaron haber tenido relaciones sexuales exclusivamente con mujeres (HSEM). Aunque no fue reclutado de lugares gay, HSHM tuvo un prevalencia del VIH significativamente mayor que la HSEM (23% frente a 10%, p <0,001). Intervenciones que se adaptan específicamente a la prevención del VIH entre los HSHM son necesarios para mejorar la prevalencia de los riesgos y la infección de VIH.
Introduction
Improved targeting of HIV prevention efforts and HIV testing are crucial to ending HIV epidemics, especially among populations with disparately high HIV prevalence and incidence rates [1–4]. Men who have sex with men (MSM) have had a disproportionate burden of HIV infection since the epidemic began in the early 1980s [5–7]. Despite the recent worldwide successes in HIV prevention, the rates of HIV infection in the MSM population remain high and new and re-emerging HIV epidemics have been observed in several countries [8, 9]. In New York City, although the overall HIV incidence among MSM has been decreasing, new HIV cases among MSM constitute 71% of new diagnoses among males [10].
To surveil HIV epidemics in hidden populations, sampling techniques that can effectively reach subgroups of at-risk populations are needed [11]. Certain characteristics of some sampling techniques can introduce bias in samples. For example, recruiting from venues that are not frequented by all subgroup members of the population may result in under representing those subgroups [11]. With respect to studies of HIV risk behaviors among MSM, sampling from gay-venues may have resulted in overlooking non-gay identifying men who have sex with both men and women (MSMW) [12–14]. HIV risk behaviors vary across subgroups of MSM populations. Differences exist in both sexual identity (e.g., gay vs. bisexual) and sexual behavior (e.g., sexual intercourse with men exclusively vs. sexual intercourse with men and women). For example, men who have sex with men exclusively have different risks than MSMW [15]. MSMW may be less likely to engage in condomless anal receptive sex compared to men who have sex with men exclusively [15, 16], but more likely to engage in condomless anal insertive sex with casual partners [17]. MSMW are an important and sizeable subgroup of MSM who may be more difficult to reach, less likely to disclose their sexual behavior [18, 19], and less likely to have been tested for HIV [20]. Among HIV seropositive people who inject drugs, MSMW have been more likely to engage in condomless sex with women compared to men who have sex with women exclusively (MSWE) [16]. These characteristics may put MSMW at a greater risk of HIV acquisition and transmission than men who have sex with women exclusively (MSWE).
Synergistic epidemics—syndemics—such as alcohol and substance use among MSMW, compound the challenges of the HIV epidemic [6]. Among people who use drugs, MSMW, compared to MSM who only have sex with men, have been reported to be more likely to engage in transactional/commercial sex and to use drugs and alcohol when engaging in sex [21]. These differences suggest HIV prevention programs that are directed towards MSMW should be specifically tailored to their needs in order to improve effectiveness [22]. However, none of the 41 evidence-based HIV interventions listed by Centers for Disease Control and Prevention (CDC) were specifically tailored to MSMW [23].
The aim of this study is to assess HIV injection and heterosexual risk behaviors of non-gay identifying MSMW, in comparison with MSWE, among persons who use drugs. Men who have sex with men exclusively could not be included in the analyses, because we did not collect heterosexual risk behaviors data with respect to same-sex partners during the main period of the study. We hypothesized that HIV seronegative MSMW have higher rates of injection risk behaviors and heterosexual risk behaviors compared to their MSWE counterparts.
Methods
This study, including the procedure for obtaining informed consent, was approved by the Mount Sinai Beth Israel Institutional Review Board.
Participants
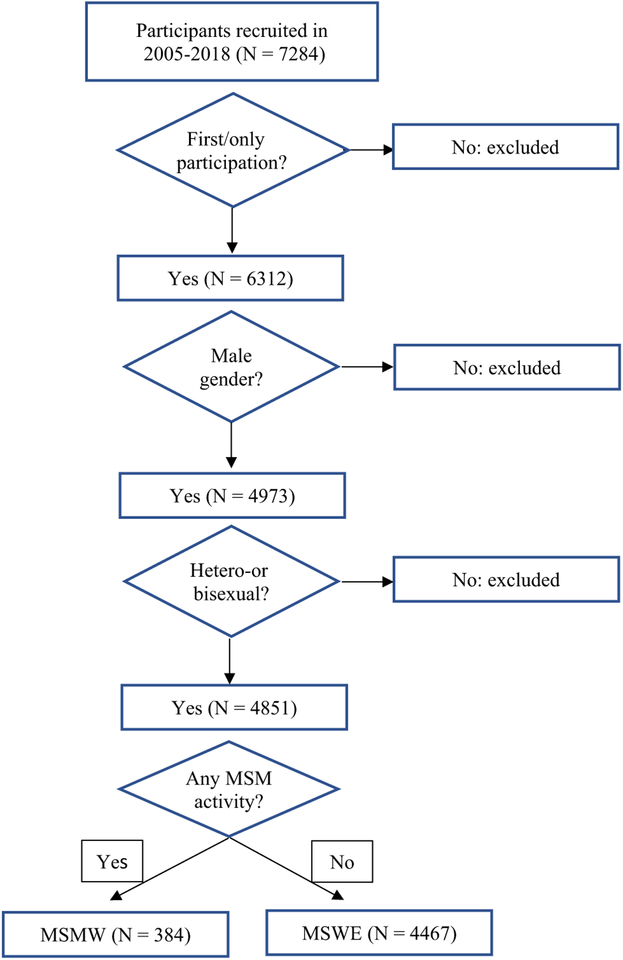
Data were collected as part of a serial cross-sectional study of risk factors for HIV among entrants to the Mount Sinai Beth Israel detoxification and methadone maintenance treatment programs in New York City. Trained interviewers visited the admissions wards of the programs and examined the intake records to identify patients admitted in the previous 3 days. Patients who were admitted for drug treatment other than alcohol and marijuana were approached for participation in the study. The study was fully described to each potential participant and a signed informed consent was obtained. Eligibility criteria included a minimum of 18 years of age, using illicit drugs (other than alcohol and marijuana) in the previous 6 months, and no participation in the study in the previous year. All eligible patients were approached, 95% of whom agreed to participate in the study. Participants were compensated for their time with two $10 MetroCards. For the current study, only male entrants to the drug treatment programs who did not identify as gay and entered the programs between January 2005 and February 2018 were included. For persons who had participated in the study in multiple years, we only included the record from their first participation. Figure 1 presents the flowchart of how the analytic sample was obtained.
Fig. 1.
Flowchart of the analytic sample of participants entering Mount Sinai Beth Israel drug-treatment program in NYC (2005–2018)
Measures
A questionnaire was administered, in a private interview room, by trained interviewers to collect demographic data as well as information regarding drug use, sexual identity, sexual behaviors, history of HIV-testing and utilizing HIV prevention and treatment services, general health status, and history of antiretroviral use. We used lifetime report of sexual activity with men and women to classify participants as MSMW or MSWE. We asked “How many men have you ever had any kind of sex with?” We also asked “How many women have you ever had any kind of sex with?” All male participants who reported sex with men and women and did not identify as gay (i.e., selected “heterosexual”, “bisexual”, or “other”, and did not select “gay”, or “transgender”, in response to sexual identity/orientation item) were classified as MSMW. None of the male participants who reported sex with both men and women identified as gay. Males who reported sex exclusively with women and did not identify as gay were classified as MSWE. Frequencies of alcohol and drug use, as well as frequencies of anal and vaginal sex with specific types (i.e., primary, casual, and commercial) of opposite sex partners and condom use with each type of opposite sex partner were assessed for the 6-month period prior to the interview. Primary partner was defined as “someone who is a regular, steady sex partner.” Casual partner was defined as someone “you had sex with other than any primary partners,” and not including “partners who gave you, or got from you, drugs, money, or other goods for sex.” Commercial sex partner was defined as a sex partner “who gave you drugs, money, or other goods for sex.” Frequencies of sex with different types of same-sex partners and condom use with those partners during the 3 months prior to the interview were only assessed in some years of the full study.
After the questionnaire was completed, pretest HIV counseling was conducted and blood samples were collected. HIV testing was conducted at the New York City Department of Health laboratory using a commercial, enzyme-linked, immunosorbent assays (EIA) with Western blot confirmation (BioRad Genetic Systems HIV-1–2+0 EIA and HIV-1 Western Blot, BioRad Laboratories, Hercules, CA). HSV-2 testing was performed by BioReference Laboratories using the Focus HerpeSelect 1 and 2 ELISA (enzyme-linked immunosorbent assay). The laboratory used an index value of 1.1 or greater for classifying a subject as HSV-2 seropositive and reported results as positive/negative for all assays. The HSV-2 reports also included index values for assays after 2006. Because there is debate about the appropriate index value for seropositivity for the Focus, we also examined the HSV-2 results using an index value of 3.0 for HSV-2 assays after 2006.
Statistical Analyses
All analyses were conducted using Stata 15 [24]. For testing differences in bivariate proportions and distribution of categorical variables we used the Chi squared test. Potential differences in continuous variables were tested by t test (when comparing two groups), or by analysis of variance (when comparing multiple groups). In the latter case, when a significant difference was observed, we used the Bonferroni test for multiple post hoc comparisons.
We used logistic regression analysis to test the hypothesis that HIV seronegative MSMW, in comparison to MSWE group, have higher rates of sexual and injection risk behaviors for HIV infection. Previous findings indicate that HIV positive individuals and those who believe they are likely to be HIV positive reduce their transmission risk behaviors [25]. Therefore, we stratified the logistic models of HIV risk behaviors by HIV serostatus. Injection risk outcome variables included injecting with a needle someone else had used, lending a needle that was already used to other injectors, and sharing needles with others who were not in the participant’s close network of friends, relatives, or sex partners—thereby increasing the potential exposure risk. Heterosexual risk variables included reporting multiple sex partners. Other sexual risk outcome variables were analyzed by each partner type (i.e., primary, casual, and commercial sexual partner). To assess whether any condomless sex had occurred, we included in the denominator those who had abstained from sexual intercourse, as well as those who had engaged in sex with or without using condoms. We refer to this variable as “condomless sex.” To assess whether condoms were consistently used during sexual intercourse, we included in the denominator only those who had reported sexual activity. We refer to this variable as “consistent condom use.”
Because certain drugs and alcohol are associated with sexual risk behaviors, alcohol and drug use variables (including the interaction of each of them with MSM status) were included as covariates in the models. Specific drugs used in the previous 6 months were included as dichotomous variables. Alcohol use was measured as “at-risk drinking” and defined as 14 or more drinks per week [26]. The association of each potential predictor or important factor with the outcome variable was first tested in univariate logistic models. All potential variables were then entered into the model and tested using backward elimination, following Agresti’s approach [27] until we arrived at the final model.
Results
The sample consisted of 4851 participants. Table 1 presents demographic, drug use characteristics, heterosexual risk behaviors, prior year HIV testing, HSV-2 and HIV infection among the MSMW and MSWE groups. There was no difference between MSMW and MSWE with respect to average age (43, SD = 9 vs. 44, SD = 10, respectively; t = 0.57, p = 0.57) or proportion of participants who injected drugs (45%). Nor was there a significant difference in race/ethnicity or the specific drugs injected. There were, however, significant differences in non-injecting drug and alcohol use.
Table 1.
Demographic, drug use characteristics, and sexual risk behaviors of non-gay identifying MSMW and MSWE entering Mount Sinai Beth Israel drug-treatment program in NYC (2005–2018)
| MSWE N (%) |
MSMW N (%) |
Chi square (df); p-value | |
|---|---|---|---|
| Total (N = 4851) | 4467 (100) | 384 (100) | |
| Race/ethnicity | 4.0 (3); 0.26 | ||
| White | 698 (16) | 73 (19) | |
| Black | 2006 (45) | 171 (45) | |
| Latino | 1655 (37) | 129 (34) | |
| Other | 108 (2) | 11 (3) | |
| Homelessness | 2139 (48) | 203 (53) | 3.5 (1); 0.06 |
| Injection status | 0.01 (2); 0.94 | ||
| Injected drugs | 2021 (45) | 173 (45) | |
| Did not inject drugs | 2446 (55) | 211 (55) | |
| Non-injection drug use | |||
| Intranasal speedball | 494 (11) | 54 (15) | 3.8 (1); 0.05 |
| Intranasal heroin | 2270 (53) | 155 (43) | 13.2 (1); < 0.001 |
| Intranasal cocaine | 1543 (36) | 144 (40) | 2.2 (1); 0.14 |
| Crack cocaine | 2172 (50) | 244 (67) | 38.3 (1); < 0.001 |
| At-risk drinking | 2011 (45) | 197 (51) | 5.6 (1); < 0.05 |
| Multiple sex partners | 1348 (30) | 128 (33) | 1.7 (1); 0.2 |
| Condomless sex w/ | |||
| Primary female partner | 1853/4450 (42) | 107/383 (28) | 27.5 (1); < 0.001 |
| Casual female partner | 754/4821 (17) | 84/383 (22) | 6.0 (1); < 0.05 |
| Commercial female partner | 36/4817 (1) | 16/383 (4) | 37.4 (1); < 0.001 |
| Consistent condom use w/ | |||
| Primary female partner | 528/2383 (22) | 28/135 (21) | 0.2 (1); 0.7 |
| Casual female partner | 534/1288 (41) | 44/129 (34) | 2.6 (1); 0.11 |
| Commercial female partner | 22/64 (34) | 2/19 (11) | 4.1 (1); < 0.05 |
| HIV testing last year | 3319/4191 (79) | 254/320 (79) | 0.01 (1); 0.94 |
| Serology | |||
| HSV-2+ | 1735/3968 (44) | 183/348 (53) | 10.2 (1); 0.001 |
| HIV+ | 425/4135 (10) | 81/357 (23) | 50.6 (1); < 0.001 |
Bivariate Comparisons of Injection Risk Behaviors Among HIV Negatives
Twenty-four percent of MSMW who injected drugs did so with a used needle, compared to 16% of MSWE (Chi square = 6.8 (1), p = 0.009). Nineteen percent of MSMW, compared to 13% of MSWE (Chi square = 4.4 (1), p = 0.04), lent needles they had already used to others.
Bivariate Comparisons of Heterosexual Risk Behaviors Among HIV Negatives
Substantial majorities (59% and 72%) of the MSMW and MSWE participants reported sexual intercourse with female partners in the 6 months prior to the interview. MSMW were significantly more likely to report condomless sex with female casual partners and commercial partners.
HIV and HSV-2 Infections
Both HSV-2 and HIV were more prevalent among MSMW (53% and 23%, respectively), compared to MSWE (44% and 10%, respectively). The prevalence of HSV-2/HIV co-infection was also greater among MSMW than among MSWE (17% vs. 7%, Chi square = 53.5 (1), p < 0.001).
Logistic Models of Injection Risk Behaviors
All logistic models were stratified by HIV serostatus, as described in the Methods section. Table 2 presents the results of multivariable logistic models of injection risk behaviors among HIV seronegative participants. In univariate analyses, among HIV seronegative participants who injected drugs, MSMW had greater odds of injecting with needles that were already used by other injectors (OR 1.79, 95% CI 1.16–2.78, p < 0.01) and greater odds of lending their already used needles to others (OR 1.85, 95% CI 1.17–2.94, p < 0.01). After controlling for the effect of age, race/ethnicity, daily injection, and injecting cocaine—all of which were significantly associated with the outcome variable—HIV seronegative MSMW had almost twice the odds of the MSWE to inject with a used needle (AOR 1.93, 95% CI 1.21–3.07, p < 0.01). MSMW also had greater odds of lending needles they had already used to other injectors (AOR 2.05, 95% CI 1.26–3.35, p < 0.01), compared to MSWE after controlling for the effect of other covariates, including age, race/ethnicity, daily injection, injecting heroin, and injecting cocaine. Among HIV seropositives, there was no significant difference between MSMW and MSWE in any of the injection risk behaviors (AOR 2.4, 95% CI 0.78–7.43, p = 0.13, for receptive sharing; and AOR 1.7, 95% CI 0.35–8.20, p = 0.52, for distributive sharing).
Table 2.
Odds ratios for injection risk behaviors among HIV seronegative, non-gay identifying MSMW and MSWE entering Mount Sinai Beth Israel drug-treatment program in NYC (2005–2018)
| Injecting with used needles/syringes | Lending used needles/syringes | |||||
|---|---|---|---|---|---|---|
| OR (95% CI) |
AOR (95% CI) |
p | OR (95% CI) |
AOR (95% CI) |
p | |
| Non-gay MSMW | 1.79 (1.16–2.78) | 1.93 (1.21–3.07) | < 0.01 | 1.85 (1.17–2.94) | 2.05 (1.26–3.35) | < 0.01 |
| MSWE (ref) | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Age | 0.96 (0.95–0.97) | 0.98 (0.96–0.99) | < 0.01 | 0.96 (0.94–0.97) | 0.97 (0.96–0.99) | < 0.01 |
| Race/ethnicity | ||||||
| Black | 0.45 (0.30–0.69) | 0.84 (0.50–1.38) | 0.48 | 0.64 (0.42–0.96) | 1.21 (0.73–2.03) | 0.46 |
| Latino | 1.14 (0.88–1.47) | 0.96 (0.71–1.29) | 0.76 | 0.99 (0.75–1.31) | 0.88 (0.64–1.21) | 0.42 |
| Whites (ref) | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Injecting heroin | 3.83 (2.30–6.37) | 0.83 (0.47–1.47) | 0.53 | 2.50 (1.55–4.02) | 0.51 (0.29–0.90) | < 0.05 |
| Injecting cocaine | 2.19 (1.69–2.84) | 1.65 (1.26–2.15) | < 0.01 | 2.34 (1.77–3.10) | 1.73 (1.30–2.33) | < 0.01 |
| Daily injection | 2.56 (1.77–3.68) | 2.50 (1.72–3.63) | < 0.01 | 3.18 (2.08–4.87) | 3.53 (2.25–5.52) | < 0.01 |
Logistic Models of Heterosexual Risk Behaviors
Odds ratios (OR) from univariate logistic analyses and adjusted odds ratios (AOR) from multivariable models of condomless sex, stratified by HIV serostatus, are presented in Tables 3 and 4 (AOR cells, in the tables, without values for corresponding variables indicate that univariate associations were not statistically significant and were not included in the multivariable models). After adjusting for the effect of age, race/ethnicity, methadone, and intranasal heroin, MSMW had significantly lower odds of reporting any condomless sex with a female primary partner (AOR 0.59, 95% CI 0.45–0.77, p < 0.01), compared to MSWE. Consistent condom use among those reporting sex with a female primary partner did not significantly vary by MSMW status (OR 0.71, CI 0.40–1.27; p = 0.25).
Table 3.
Odds ratios for condomless sex with female primary, casual, and commercial partners of HIV seronegative, non-gay identifying MSMW and MSWE entering Mount Sinai Beth Israel drug-treatment program in NYC (2005–2018)
| Condomless sex primary female partner | Condomless sex casual female partner | Condomless sex commercial female partner | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
AOR (95% CI) |
p | OR (95% CI) |
AOR (95% CI) |
p | OR (95% CI) |
AOR (95% CI) |
p | ||
| Non-gay MSMW | 0.60 (0.46–0.78) | 0.59 (0.45–0.77) | < 0.01 | 1.62 (1.22–2.14) | 1.48 (1.10–1.99) | < 0.01 | 6.38 (3.42–11.89) | 5.97 (3.17–11.24) | < 0.01 | |
| MSWE (ref) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Age | 0.98 (0.97–0.98) | 0.97 (0.96–0.98) | < 0.01 | 0.98 (0.97–0.98) | 0.97 (0.96–0.98) | < 0.01 | 1.00 (0.97–1.03) | – | 0.91 | |
| Race/ethnicity | ||||||||||
| Black | 0.97 (0.85–1.10) | 1.66 (1.35–2.03) | < 0.01 | 1.29 (1.10–1.51) | 1.11 (0.86–1.42) | 0.44 | 1.41 (0.80–2.50) | – | 0.24 | |
| Latino | 1.24 (1.09–1.42) | 1.59 (1.31–1.93) | < 0.01 | 0.64 (0.53–0.76) | 0.73 (0.57–0.93) | < 0.01 | 0.93 (0.51–1.68) | – | 0.81 | |
| Whites (ref) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Injection drug use | 0.96 (0.85–1.09) | – | 0.56 | 0.87 (0.74–1.03) | – | 0.10 | 0.47 (0.25–0.89) | 0.53 (0.27–1.01) | 0.06 | |
| No injection drug use (ref) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Intranasal heroin | 1.31 (1.15–1.49) | 1.29 (1.13–1.47) | < 0.01 | 0.76 (0.65–0.90) | 0.81 (0.67–0.97) | < 0.05 | 0.88 (0.50–1.56) | – | 0.66 | |
| Street methadone | 1.45 (1.24–1.70) | 1.39 (1.18–1.64) | < 0.01 | 1.03 (0.85–1.26) | – | 0.76 | 1.12 (0.57–2.21) | – | 0.74 | |
| Intranasal speedball | 1.24 (1.02–1.51) | 1.04 (0.82–1.31) | 0.76 | 1.50 (1.19–1.88) | 1.57 (1.22–2.03) | < 0.01 | 2.82 (1.48–5.38) | 2.72 (1.41–5.26) | < 0.01 | |
| Crack cocaine | 0.92 (0.81–1.05) | – | 0.22 | 1.80 (1.52–2.12) | 1.51 (1.25–1.81) | < 0.01 | 2.30 (1.23–4.30) | 1.29 (0.66–2.51) | 0.46 | |
| At-risk drinking | 0.85 (0.75–0.96) | 0.90 (0.78–1.02) | 0.10 | 1.49 (1.27–1.75) | 1.34 (1.13–1.60) | < 0.01 | 3.56 (1.85–6.87) | 2.82 (1.45–5.49) | < 0.01 | |
AOR cells without values for corresponding variables indicate that univariate associations were not statistically significant
Table 4.
Odds ratios for condomless sex with female primary, casual, and commercial partners of HIV seropositive, non-gay identifying MSMW and MSWE entering Mount Sinai Beth Israel drug-treatment program in NYC (2005–2018)
| Condomless sex primary female partner | Condomless sex casual female partner | Condomless sex commercial female partner | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
AOR (95% CI) |
p | OR (95% CI) |
AOR (95% CI) |
p | OR (95% CI) |
AOR (95% CI) |
p | |
| Non-gay MSMW | 0.63 (0.31–1.27) | – | 0.19 | 1.14 (0.51–2.56) | – | 0.74 | 15.82 (0.64–391.81) | – | 0.92 |
| MSWE (ref) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||
| Age | 0.96 (0.93–0.99) | 0.95 (0.93–0.98) | < 0.01 | 0.94 (0.91–0.98) | 0.92 (0.89–0.96) | < 0.01 | 0.91 (0.74–1.12) | – | 0.37 |
| Race/ethnicity | |||||||||
| Black | 1.67 (1.03–2.73) | 1.63 (0.61–4.42) | 0.34 | 1.97 (1.01—3.85) | 1.41 (0.40–4.90) | 0.57 | 2.27 (0.09–55.98) | – | 0.62 |
| Latino | 0.66 (0.40–1.10) | 0.91 (0.32–2.59) | 0.86 | 0.51 (0.25–1.06) | 0.59 (0.15–2.30) | 0.78 | 0.63 (0.03–15.44) | – | 0.77 |
| Whites (ref) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||
| Injection drug use | 0.70 (0.44–1.13) | 1.02 (0.60–1.73) | 0.95 | 0.63 (0.33–1.20) | – | 0.16 | 0.39 (0.02–9.71) | – | 0.57 |
| No injection drug use (ref) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||
| Intranasal heroin | 0.94 (0.58–1.52) | – | 0.78 | 0.72 (0.37–1.42) | – | 0.35 | 0.48 (0.02–11.74) | – | 0.65 |
| Street methadone | 1.26 (0.66–2.39) | – | 0.49 | 0.62 (0.21–1.79) | – | 0.38 | 1.96 (0.08–48.57) | – | 0.68 |
| Intranasal speedball | 0.63 (0.26–1.53) | – | 0.31 | 0.66 (0.20–2.23) | – | 0.51 | 2.85 (0.12–70.96) | – | 0.52 |
| Crack cocaine | 0.87 (0.54–1.41) | – | 0.58 | 2.03 (0.97–4.25) | 1.46 (0.65–3.27) | 0.34 | 1.87 (0.08–46.10) | – | 0.70 |
| At-risk drinking | 1.00 (0.64–1.60) | – | 0.97 | 1.29 (0.70–2.38) | – | 0.42 | 3.33 (0.14–82.03) | – | 0.46 |
AOR cells without values for corresponding variables indicate that univariate associations were not statistically significant
Among HIV seronegatives, MSMW had significantly greater odds of reporting condomless sex with female casual sex partners (AOR 1.48, 95% CI 1.10–1.99, p < 0.01), compared to MSWE. Similarly, HIV seronegative MSMW reporting sex with a causal sex partner had lower odds of consistently using condoms during sexual intercourse (AOR 0.61, CI 0.39–0.95; p = 0.03). MSMW had more than 6 times the odds of MSWE to report any condomless sex with a female commercial sex partner (AOR 5.97, 95% CI 3.17–11.24, p < 0.01). None of the MSMW, compared to 34% of MSWE, who reported sex with a female commercial sex partner reported consistently using condoms with their female commercial sex partner. Among HIV seropositives, there were no significant differences between MSMW and MSWE in any of the heterosexual risk behaviors.
Discussion
The vast majority of studies that assess HIV risk among MSM do not distinguish between gay and non-gay identifying populations which may have different prevalence of HIV risk behaviors and sexually transmitted infections [15]. To improve prevention efforts among MSM, it is important to address the specific patterns of HIV risk in different MSM groups. Non-gay identifying MSMW are an important and sizeable proportion of MSM with higher rates of HIV and risk behaviors associated with transmission of HIV compared to MSWE.
This study found that even among people who use drugs and enter a drug treatment program in New York City, a population with an already increased level of HIV risk behaviors and infection, MSMW have a greater prevalence of risk factors for HIV, additional risk of HIV seropositivity, and twice the proportion of HIV positives as MSWE. MSMW who injected drugs had almost twice the odds of MSWE injectors to inject with used needles and to lend their already used needles to others.
A significantly greater proportion of HV seronegative MSMW engaged in unprotected sex with female casual and commercial sex partners and had a greater prevalence of HSV-2—a risk factor for HIV transmission [28–30]. All of these factors suggest that female partners of MSMW are at an increased risk of HIV infection, because HIV seronegative MSMW, compared to MSME, are at a greater risk of HIV infection and, therefore, of transmitting recent HIV infections to sexual partners.
Differences in HIV risk behaviors among different subgroups of drug users entering treatment programs in New York City persist in spite of equal or greater use of many HIV prevention and intervention programs intended to reduce HIV risk behaviors. These observations point to the need for interventions that are more precisely tailored to the needs of non-gay identifying MSMW who use drugs. Improved interventions that better target drug and alcohol use among MSMW would be expected to result in decreased substance use. Such interventions will need to facilitate HIV-testing among MSMW and address the concurrent drug and alcohol use with sexual activity, both of which may be closely related to sexual identity and disclosure [31, 32].
Limitations
The study reported here has several limitations. The participants of this study represented a non-random sample of MSMW and MSWE who use drugs and enter drug treatment. As a result, the findings of this study are not meant to be generalized to the MSMW population as whole. Also, we used lifetime report of sexual activity with men and women to classify our participants as MSMW or MSWE. Others have reported that longer recall windows are associated with a higher proportion of MSMW [15]. Moreover, we measured drug and alcohol use and heterosexual risk behaviors, but we do not have any data regarding the concurrent use of drugs and alcohol with heterosexual risk behaviors for the period of study reported here. Additionally, because the original risk factors study was designed to assess risk behaviors primarily of people who use drugs, we do not have more precise data regarding sexual risk behaviors with male partners, including condom use with same-sex partners. Data regarding condom use with same-sex partners were not collected in the main study. Another limitation of the study is that we did not measure the impact of Pre-Exposure Prophylaxis (PrEP) and Treatment as Prevention (TasP) on the risk behaviors of our sample. Furthermore, although the measures in our questionnaire had construct validity we did not use standardized scales with known reliability and validity. Finally, we did not collect data regarding socioeconomic status and did not adjust the associations for its potential effect. However, the available data, reflecting multiple injection and heterosexual risk behaviors, as well as the consumption of alcohol and various drugs, show a consistent pattern of differences between MSMW and MSWE that are associated with the acquisition and transmission of HIV.
Conclusions
A greater proportion of MSMW are infected with HIV and HSV-2 compared to MSWE who enter drug treatment programs in New York City. To sustain the trajectory of ending HIV epidemics, local, state, and national strategies need to incorporate interventions that specifically target these key populations with persistently higher infection rates. Drug treatment programs are the most likely opportunity for linking this population (i.e., MSMW who seek drug treatment) with HIV treatment and care, and for providing interventions that can reduce at-risk drinking, cocaine and crack use, and injection and sexual risk behaviors.
Acknowledgments
This research was supported by R01DA003574, and R01DA0357407 from the US National Institute on Drug Abuse. The funding agency had no role in the design, conduct, data analysis or report preparation for the study.
Footnotes
Conflict of interest All Authors declare that they have no conflict of interest.
Informed Consent This study, including the procedure for obtaining informed consent, was approved by the Mount Sinai Beth Israel Institutional Review Board.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. [Google Scholar]
- 2.Valdiserri RO, Holtgrave DR. Ending America’s HIV epidemic: why the national HIV/AIDS strategy still matters. AIDS Behav. 2018;22(7):2033–41. [DOI] [PubMed] [Google Scholar]
- 3.New York City Department of Health and Mental Hygiene. Ending the epidemic: overview, New York. 2017. https://www1.nyc.gov/assets/doh/downloads/pdf/ah/ete-strategy.pdf. Accessed 13 Nov 2018.
- 4.New York State Department of Health Aids Institute. Ending the AIDS epidemic in New York State. 2018. https://www.health.ny.gov/diseases/aids/ending_the_epidemic/. Accessed 13 Nov 2018.
- 5.World Health Organization. Prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender people: Recommendations for a public health approach. Prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender people: recommendations for a public health approach. Genva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 6.Sullivan P, Hamouda O, Delpech V, Geduld J, Prejean J, Semaille C, et al. Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996–2005. Ann Epidemiol. 2009;19:423–31. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control Prevention. HIV/AIDS surveillance report, 2006. Vol. 18. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2007. [Google Scholar]
- 8.Jaffe HW, Valdiserri RO, De Cock KM. The reemerging HIV/AIDS epidemic in men who have sex with men. JAMA. 2007;298(20):2412–4. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Gu X, Tao X, Qian Y, Babu GR, Wang G, et al. Prevalence and trends of HIV, syphilis, and HCV in migrant and resident men who have sex with men in Shandong, China: results from a serial cross-sectional study. PloS ONE. 2017;12(1):e0170443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.New York City Department of Health and Mental Hygiene. New York City HIV/AIDS surveillance slide sets New York. 2017. https://www1.nyc.gov/assets/doh/downloads/pdf/dires/hiv-aids-in-msm.pdf. Accessed 13 Nov 2018.
- 11.Magnani R, Sabin K, Saidel T, Heckathorn D. Review of sampling hard-to-reach and hidden populations for HIV surveillance. AIDS. 2005;19:S67–72. [DOI] [PubMed] [Google Scholar]
- 12.Mills T, Paul J, Stall R, Pollack L, Paul J, Binson D, et al. Health-related characteristics of men who have sex with men: a comparison of those living in “gay ghettos” with those living elsewhere. Am J Public Health. 2001;91:980–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Cai R, Chen L, Cai W, Yang Z, Richardus JH, et al. A comparison between respondent-driven sampling and time-location sampling among men who have sex with men in Shenzhen, China. Arch Sex Behav. 2015;44(7):2055–65. [DOI] [PubMed] [Google Scholar]
- 14.Paz-Bailey G, Miller W, Shiraishi RW, Jacobson JO, Abimbola TO, Chen SY. Reaching men who have sex with men: a comparison of respondent-driven sampling and time-location sampling in Guatemala City. AIDS Behav. 2013;17(9):3081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman MR, Wei C, Klem ML, Silvestre AJ, Markovic N, Stall R. HIV infection and sexual risk among men who have sex with men and women (MSMW): a systematic review and meta-analysis. PloS ONE. 2014. 10.1371/journal.pone.0087139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight KR, Shade SB, Purcell DW, Rose CD, Metsch LR, Latka MH, et al. Sexual transmission risk behavior reported among behaviorally bisexual HIV-positive injection drug-using men. JAIDS J Acquir Immune Defic Syndr. 2007;46:S80–7. [DOI] [PubMed] [Google Scholar]
- 17.Feinstein BA, Moran KO, Newcomb ME, Mustanski B. Differences in HIV risk behaviors between self-identified gay and bisexual young men who are HIV-negative. Arch Sexual Behav. 2018. 10.1007/s10508-018-1148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benoit E, Pass M, Randolph D, Murray D, Downing MJ Jr. Reaching and engaging non-gay identified, non-disclosing Black men who have sex with both men and women. Cult Health Sex. 2012;14(9):975–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel K, Schrimshaw EW, Lekas H-M, Parsons JT. Sexual behaviors of non-gay identified non-disclosing men who have sex with men and women. Arch Sex Behav. 2008;37(5):720–35. [DOI] [PubMed] [Google Scholar]
- 20.Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med. 2006;145(6):416–25. [DOI] [PubMed] [Google Scholar]
- 21.Friedman MR, Kurtz SP, Buttram ME, Wei C, Silvestre AJ, Stall R. HIV risk among substance-using men who have sex with men and women (MSMW): findings from South Florida. AIDS Behav. 2014;18(1):111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beyrer C, Baral SD, Van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman MR, Dodge BM. The role of syndemic in explaining health disparities among bisexual men: a blueprint for a theoretically informed perspective. In: Wright ER, Carnes N, editors. Understanding the HIV/AIDS epidemic in the United States: the role of syndemics in the production of health disparities. Cham: Springer International Publishing; 2016. p. 71–98. [Google Scholar]
- 24.StataCorp. Stata statistical software: release 15. Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 25.Des Jarlais DC, Perlis T, Arasteh K, Hagan H, Milliken J, Braine N, et al. “Informed altruism” and “partner restriction” in the reduction of HIV infection in injecting drug users entering detoxification treatment in New York City, 1990–2001. J Acquir Immune Defic Syndr. 2004;35(2):158–66. [DOI] [PubMed] [Google Scholar]
- 26.Dawson DA, Grant BF, Li TK. Quantifying the risks associated with exceeding recommended drinking limits. Alcohol Clin Exp Res. 2005;29(5):902–8. [DOI] [PubMed] [Google Scholar]
- 27.Agresti A Categorical data analysis. New York: Wiley; 2003. [Google Scholar]
- 28.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2—seropositive persons: a meta-analysis. J Infect Dis. 2002;185(1):45–52. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, et al. Persistence of HIV-1 receptor–positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15(8):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Des Jarlais DC, Arasteh K, McKnight C, Perlman DC, Feelemyer J, Hagan H, et al. HSV-2 Co-infection as a driver of HIV transmission among heterosexual non-injecting drug users in New York City. PloS ONE. 2014. 10.1371/journal.pone.0087993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harawa NT, Williams JK, Ramamurthi HC, Manago C, Avina S, Jones M. Sexual behavior, sexual identity, and substance abuse among low-income bisexual and non-gay-identifying African American men who have sex with men. Arch Sex Behav. 2008;37(5):748–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernstein KT, Liu K-L, Begier EM, Koblin B, Karpati A, Murrill C. Same-sex attraction disclosure to health care providers among New York City men who have sex with men: implications for HIV testing approaches. Arch Intern Med. 2008;168(13):1458–64. [DOI] [PubMed] [Google Scholar]