Abstract
Background
Many patients with Crohn’s disease (CD) require fecal diversion. To understand the long-term outcomes, we performed a multicenter review of the experience with retained excluded rectums.
Methods
We reviewed the medical records of all CD patients between 1990 and 2014 who had undergone diversionary surgery with retention of the excluded rectum for at least 6 months and who had at least 2 years of postoperative follow-up.
Results
From all the CD patients in the institutions’ databases, there were 197 who met all our inclusion criteria. A total of 92 (46.7%) of 197 patients ultimately underwent subsequent proctectomy, while 105 (53.3%) still had retained rectums at time of last follow-up. Among these 105 patients with retained rectums, 50 (47.6%) underwent reanastomosis, while the other 55 (52.4%) retained excluded rectums. Of these 55 patients whose rectums remained excluded, 20 (36.4%) were symptom-free, but the other 35 (63.6%) were symptomatic. Among the 50 patients who had been reconnected, 28 (56%) were symptom-free, while 22(44%) were symptomatic. From our entire cohort of 197 cases, 149 (75.6%) either ultimately lost their rectums or remained symptomatic with retained rectums, while only 28 (14.2%) of 197, and only 4 (5.9%) of 66 with initial perianal disease, were able to achieve reanastomosis without further problems. Four patients developed anorectal dysplasia or cancer.
Conclusions
In this multicenter cohort of patients with CD who had fecal diversion, fewer than 15%, and only 6% with perianal disease, achieved reanastomosis without experiencing disease persistence.
Keywords: Crohn’s Disease, Rectum, Surgery, Perianal disease
Introduction
Crohn’s disease (CD) is a chronic progressing and debilitating disease.1 Despite significant progress made in the treatment of CD, surgery remains an integral part of the overall treatment plan in patients with aggressive CD.2 In some patients, exclusion of the rectum along with fecal diversion is needed as a temporary or permanent measure to control aggressive disease, including perianal complications.3,4
CD patients who undergo a diverting ileostomy or colostomy often choose to keep this anatomy or may experience other delays in reconstructive operations, and hence may spend considerable periods of time with their rectums excluded from the intestinal stream.5 The fate of the excluded rectum is a particularly important issue because it determines the chance of a successful reconnection. Furthermore, carcinoma and other complications in the excluded rectal stump are persistent risks.6,7
Previous studies of the fate of the excluded rectum in patients with CD have reported adverse outcomes including persistent disease activity, diversion proctitis, and dysplasia or cancer.8–12 However, a number of these reports have experienced selection and recall bias, short-term follow-up, or exclusive focus on cancer. Furthermore, even those few studies that have avoided these pitfalls date mostly from the prebiologic era.
We therefore assembled a multicenter international cohort (Consortium to Assess the Prognosis of the Excluded Rectum) recording a full range of outcomes of excluded rectums in CD from the biologic era. Specifically, we have tabulated not only instances of cancer, but also other potential adverse results like bleeding, strictures, fistulae, abscesses, drainage, or other problems requiring local or systemic therapy, corrective operations, or even subsequent proctectomy.
Methods
This was a multicenter retrospective international cohort study that was approved by the Institutional Review Board at each respective institution. The cohorts were composed of CD patients followed at the respective institutions between 1990 and 2014, who had undergone diversionary surgery and who had retained excluded rectums for at least 6 months postoperatively. This latter stipulation was intended to eliminate most of those cases in whom a second-stage proctectomy or reanastomosis had been preplanned at the time of initial operation. Patients were identified using the International Classification of Diseases–Ninth Revision, International Classification of Diseases–10th Revision, clinical modification codes, and procedural codes. Patients who did not fulfill the inclusion criteria, who had ulcerative colitis or indeterminate colitis, or who did not have at least 2 years of postoperative follow-up were excluded. All but 2 of the patients had undergone colectomy at the time of the initial diversion.
The individual cohorts and numbers of cases from each center are outlined in Table 1. As a group, these 8 centers constituted a Consortium to Assess Prognosis of the Excluded Rectum. Electronic medical records for all eligible patients were retrieved and reviewed by the study investigators to confirm eligibility of patients and to ascertain detailed medical and surgical data for each patient.
Table 1.
Baseline characteristics of all patients that met inclusion criteria
| General Characteristics | Overall Cohort (N = 197) |
|---|---|
| Sex | |
| Male | 86 (44) |
| Female | 111 (56) |
| Smoking status | |
| Current | 21 (11) |
| Former | 42 (21) |
| Never | 115 (58) |
| Unknown | 19 (10) |
| Localization of CD before surgery | |
| L1 | 11 (6) |
| L2 | 48 (24) |
| L3 | 129 (66) |
| Unknown | 9 (5) |
| Disease behavior before surgery | |
| B1 | 28 (14) |
| B2 | 50 (25) |
| B3 | 101 (51) |
| Unknown | 18 (9) |
| Perineal disease | 125 (64) |
| History of bowel surgery | 89 (45) |
| Extraintestinal manifestation | 33 (17) |
| SPA | 20 |
| Erythema nodosum | 4 |
| Pyoderma gangrenosum | 3 |
| PSC | 4 |
| Uveitis | 1 |
| Episcleritis | 1 |
| CD duration before surgery, y | 11.54 (0-31) |
| Previous exposure to tumor necrosis factor inhibitors | 137 (70) |
| Previous exposure to azathioprine/6-mercaptopurine | 111 (56) |
| Previous exposure to vedolizumab | 14 (7) |
| Age at surgery, y | 35.8 (13-80) |
| Follow-up period, y | 9.2 (2-42) |
Values are n (%), n, or median (interquartile range).
Abbreviations: CD, Crohn’s disease; PSC, primary sclerosing cholangitis; SPA, spondyloarthritis.
Variables
Baseline information was obtained from the medical charts including sex, smoking status, disease duration prior to diverting surgery, and duration of clinical follow-up. Disease characteristics prior to diverting surgery were collected including presence of perianal disease, presence of extraintestinal manifestation, prior exposure to azathioprine or 6-mercaptopurine, prior exposure to tumor necrosis factor inhibitors, prior exposure to vedolizumab, and prior history of bowel surgery. Disease localization and disease behavior prior to surgery were characterized based on the Montreal classification for CD.13 Age at time of diverting surgery was also recorded, and indications for diversion and rectal exclusion were assessed. Primary outcomes were determined at times of last follow-up, at least 2 years postdiversion. Rate of subsequent proctectomy, rate of rectal retention, outcomes of retained rectums whether excluded or reconnected, and symptoms reported in each group were reviewed and tabulated.
Statistical Analysis
We used categorical data in this study. All categorical variables were described in the form of proportions. Continuous variables were used for descriptive purposes and were reported as mean or median. As needed for continuous variables, comparisons were done using 2-sample t tests.
Results
A total of 197 patients meeting the inclusion criteria were identified across all academic institutions participating in this study. At Mount Sinai Hospital, 91 patients were found to meet inclusion criteria; 10 patients did not have sufficient follow-up data and were excluded, leaving a cohort of 81 patients. From the University of Chicago, 50 CD patients met the inclusion criteria. From the Humanitas University Rozzano Milano, 22 CD patients met the inclusion criteria. From the rest of the Mount Sinai Health System, excluding the main hospital, 69 CD patients were identified, of whom 21 met inclusion criteria. From Utrecht University, 6 CD patients met the inclusion criteria. From Weill Cornell Medical Center, 6 CD patients met the inclusion criteria. At the Northwell Health System, 9 CD patients were identified, of whom 6 met all inclusion criteria. From the NYU Health system, 5 CD patients met the inclusion criteria. Thus, overall, we identified 197 CD patients who had undergone fecal diversion, had retained an excluded rectum for at least 6 months, and had postoperative follow-ups of at least 2 years.
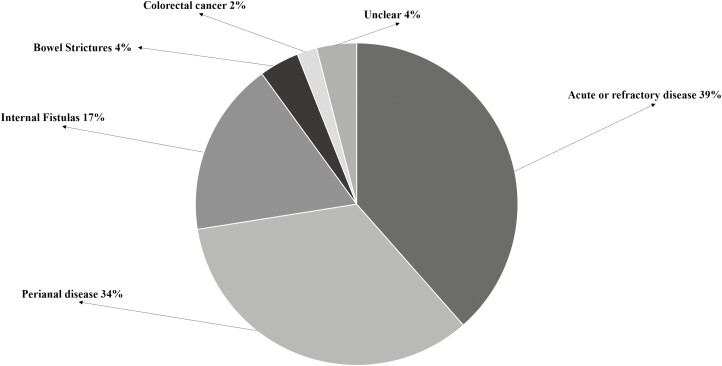
Baseline characteristics are listed in Table 1. Baseline characteristics were very similar across all centers and are reported in detail as a Supplementary Table. Primary indications for diversionary surgery with rectal exclusion are shown in Figure 1. About a third of the patients had undergone surgery for severe perianal disease 67 (34%), with another third for acute refractory bowel disease (n = 75, 38.5%); 35 (17.5%) for internal fistulas; 8 (4%) for bowel strictures; and 4 (2%) for known colon cancer.
Figure 1.
Primary indications for rectal exclusion.
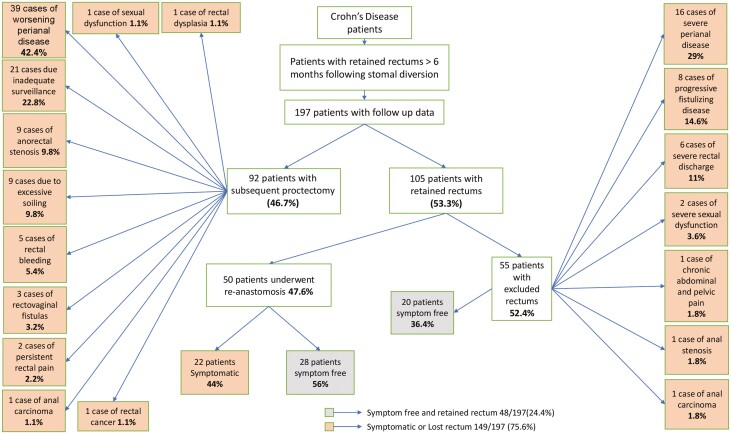
Primary outcomes are outlined in Figure 2. Of the 197 patients, 92 (46.7%) underwent subsequent proctectomy and the other 105 (53.3%) retained their rectums. With respect to the indication for subsequent proctectomy among the 92 patients who ultimately required this procedure, 39 (42.4%) patients needed subsequent proctectomy for worsening perianal disease, 21 (22.8%) for inadequate cancer surveillance in the rectal stump, 9 (9.8%) for severe anorectal stenosis, 9 (9.8%) on account of excessive soiling, 5 (5.4%) because of rectal bleeding, 3 (3.2%) owing to rectovaginal fistulas, 2 (2.2%) for persistent rectal pain, and 1 (1.1%) each for sexual dysfunction, rectal dysplasia, rectal cancer, and anal carcinoma.
Figure 2.
Primary outcomes for all patients who underwent diversionary surgery and who had retained excluded rectums for at least 6 months postoperatively.
With regard to the indication for initial diversion, patients in whom the indication for initial diversion was perianal disease, their likelihood of requiring subsequent proctectomy was 44 (66%) of 67 vs 48 (37%) of 130 among those who had undergone subsequent proctectomy for other indications (P = .0001). The details are outlined in Table 2.
Table 2.
Outcomes of patients who underwent diversion surgery for perianal disease vs those who underwent diversion surgery for all other indications
| Indication for Diversion Surgery | Number of Patients | Subsequent Proctectomy Outcome | Retained Excluded Rectum Outcome | Reconnected Rectum Outcome | Percentage of Patients Who Underwent Subsequent Proctectomy |
|---|---|---|---|---|---|
| Mount Sinai Hospital | |||||
| Perianal disease | 26 | 14 | 7 | 5 | 54% |
| All other indications | 55 | 23 | 18 | 14 | 42% |
| University of Chicago | |||||
| Perianal disease | 21 | 14 | 3 | 4 | 67% |
| All other indications | 29 | 10 | 5 | 14 | 34% |
| Humanitas University Rozzano Milano | |||||
| Perianal disease | 6 | 4 | 1 | 1 | 67% |
| All other indications | 16 | 6 | 4 | 6 | 37% |
| Mount Sinai Health System | |||||
| Perianal disease | 9 | 9 | 0 | 0 | 100% |
| All other indications | 12 | 2 | 6 | 4 | 17% |
| Utrecht University | |||||
| Perianal disease | 2 | 2 | 0 | 0 | 100% |
| All other indications | 4 | 4 | 0 | 0 | 100% |
| Weill Cornell Medical Center | |||||
| Perianal disease | 1 | 0 | 1 | 0 | 0% |
| All other indications | 5 | 3 | 2 | 0 | 60% |
| Northwell Health system | |||||
| Perianal disease | 2 | 1 | 0 | 1 | 50% |
| All other indications | 4 | 0 | 3 | 1 | 0% |
| NYU Health system | |||||
| Perianal disease | 0 | 0 | 0 | 0 | 0% |
| All other indications | 5 | 0 | 5 | 0 | 0% |
| Total | |||||
| Perianal disease | 67 | 44 | 12 | 11 | 66% |
| All other indications | 130 | 48 | 43 | 39 | 37% |
Among the 44 patients who had undergone initial rectal exclusion for perianal disease and who had gone on to subsequent proctectomy, 30 (68%) required rectal excision for ongoing perianal disease vs the other 14 (32%) whose subsequent proctectomy was performed for different reasons other than ongoing perianal disease (P = .0008).
Focusing on the 105 patients who retained their rectums, 50 (47.6%) had undergone reanastomosis and 55 (52.4%) continued to have their rectums excluded. Of those 50 patients who underwent reanastomosis, 22 (44%) were still symptomatic, while 28 (56%) were symptom-free at the time of last follow-up. Among the 55 patients who continued to have an excluded rectum, 20 (36.4%) were symptom-free, but the other 35 (63.6%) continued to experience problems: 16 (29%) had severe perianal disease, 8 (14.6%) had progressive fistulizing disease, 6 (11%) reported severe rectal discharge, 2 (3.6%) experienced severe sexual dysfunction, and 1 (1.8%) each had anal stenosis, chronic abdominal and pelvic pain, and anal carcinoma.
In summary, the goal of the original diversionary surgery to retain the rectum and be symptom-free was achieved in 48 (24.4%) of 197 patients, while 149 (75.6%) either ultimately lost their rectums or remained symptomatic with retained rectums, either reconnected or excluded.
Moreover, when considering the “ideal outcome” that patients and clinicians might wish for, to be symptom-free with a reconnected rectum, only 28 (14.2%) of 197 of patients ultimately achieved that outcome. On further analysis, we found that only 4 (5.9%) of 67 patients whose initial surgical indication had been perianal disease attained symptom-free reanastomosis, vs 24 of 130 (18.4%) whose initial diverting operation had been for other reasons attained symptom-free reanastomosis (P = .017).
Finally, it is noteworthy that 4 (2%) patients in our cohort developed neoplasia: 1 case of rectal dysplasia, 1 case of rectal carcinoma, and 2 cases of anal carcinomas. The patient who developed rectal dysplasia had undergone initial diversion for rectal cancer; the other 3 had no prior neoplasia in their backgrounds.
Discussion
In this international multicenter cohort of patients with CD and fecal diversion, almost half of those who carried a retained rectum for over 6 months ultimately required subsequent proctectomy, and fewer than 15% ever reached an outcome of reanastomosis without ongoing problems. All but 2 of the patients had undergone colectomy at the time of the initial diversion. Diversions without colectomy were performed only as temporizing measures and resulted in reanastomosis within 6 months in all but 2 patients. Hence, all but 2 of our 197 cases had subtotal colectomies at the time of diversion because simple diversion alone was not expected to definitively resolve their distal colonic disease or perianal lesions.
These data are not only generally consistent among our several different cohorts, but the proctectomy figures are also in substantial agreement with prior reports. For example, one such study followed 69 patients with CD who underwent diversionary surgery between 1962 and 1997 and found that 37 (54%) patients required subsequent proctectomy within 2 years.14 Five other studies of such cases have also reported subsequent proctectomy rates of 30% to 68%.3,15–18
Most recently, our colleagues in Utrecht published a landmark study of 167 patients with CD (none included in the present study), in whom a rectal stump had been left in situ for more than 12 months. Among the 105 patients in their cohort who responded to a questionnaire, 44 (42%) had undergone subsequent proctectomy.19 Our findings are also in line with those reported in a more recent study.20
In addition to tabulating the ultimate rate of subsequent proctectomy in our series, we have also focused on the symptomatic outcomes of the individual patients. While about half of our total cohort were able to retain their rectums over the long term, only about half of those rectums remained symptom-free, including those reanastomosed and those permanently excluded. Of course, as the Utrecht group has pointed out,19 many symptoms may simply reflect diversion proctitis and need not necessarily be disabling, but in this present series virtually all still required some ongoing treatment.
Equally noteworthy, it would appear from our data that the long-term fate of the excluded rectum depends to a significant extent on the original indication for the exclusion. The likelihood of requiring subsequent proctectomy was nearly twice as great when the initial reason for rectal diversion was perianal disease, rather than for other indications (P = .0001). Moreover, if the indication for diversion had been perianal disease, the need for proctectomy was also dictated by perianal disease more than by other complications (P = .0008). With regard to the ideal outcome of ultimate restoration of bowel continuity without ongoing symptoms, the likelihood of such an outcome was lowest (5.9%) among patients who had had perianal disease as their initial surgical indications as compared with others (18.4%) (P = .017).
The strengths of this study are its multicenter design that included 8 major academic centers from the United States and Europe; the size of our overall cohort; the comprehensively inclusive and unselected composition of the databases, the uniformity of the selection criteria from the databases, the consistency of findings across the different cohorts, the inclusion of cases from the biologic era only, and the mean 10-year duration of the patient follow-up. This study, however, also entails substantial limitations. Prominent among them is the failure to include follow-up data on those patients who had undergone either early or delayed completion proctectomy, rendering it impossible to compare outcomes in patients with retained vs resected rectums. Also obvious is the inability to evaluate any of the medical therapies given to these patients, which might well have influenced their long-term outcomes. Moreover, other limitations of our study include its retrospective design, the small numbers in some of the subsets of the cohort, and the fact that most of the participating sites were tertiary referral centers, which might introduce selection bias. Nonetheless, our study provides real-life data for both patients and clinicians regarding the outcomes they can realistically anticipate from retention of excluded rectums for more than 6 months following initial diversionary surgery. It also calls attention to the presence of perianal disease as a relatively poor prognostic factor for the most desirable outcomes of rectal exclusion. Indeed, prior studies have likewise observed the low rate of successful restoration of bowel continuity following diversion for perianal CD.21
The neoplasia outcomes in 4 of these patients are important and are a reminder to clinicians that the retained but excluded rectum requires attention and monitoring. Future efforts will be needed to define how such surveillance would best be performed; in the meantime, a careful examination and individualized approach to follow-up seems prudent.
Conclusions
In our retrospective multicenter cohort study of CD patients with fecal diversion for over 6 months with an excluded rectum, fewer than 15%, and only 6% with perianal disease, achieved reanastomosis without experiencing disease persistence.
Supplementary Material
Acknowledgments
We are grateful to the Information Technology Data Warehouse team at the Icahn School of Medicine at Mount Sinai for invaluable assistance in data retrieval. Special thanks also to Inga Peter of the Department of Genetics and Genomic Sciences and the Icahn Institute for Data Science and Genomic Technology for making available personnel and Institutional Review Board support from her laboratory. These data were presented in part at the following conferences: ECCO, Copenhagen, Denmark, 2019; DDW, San Diego, CA, USA, 2019; the 6th Annual IBD Conference at Icahn School of Medicine at Mount Sinai New York, USA. 2019; ACG, Virtual 2020.
Contributor Information
Gassan Kassim, Division of Gastroenterology and Hepatology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Clara Yzet, Division of Gastroenterology and Hepatology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Nilendra Nair, Division of Gastroenterology and Hepatology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Anketse Debebe, Division of Gastroenterology and Hepatology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Alexa Rendon, Division of Gastroenterology and Hepatology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Jean-Frédéric Colombel, Division of Gastroenterology and Hepatology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Cindy Traboulsi, University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA.
David T Rubin, University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA.
Annalisa Maroli, Department of Biomedical Sciences, IRCCS Humanitas Research Hospital, Humanitas University, Milan, Italy.
Elisabetta Coppola, Department of Biomedical Sciences, IRCCS Humanitas Research Hospital, Humanitas University, Milan, Italy.
Michele M Carvello, Department of Biomedical Sciences, IRCCS Humanitas Research Hospital, Humanitas University, Milan, Italy.
Nadat Ben David, Department of Biomedical Sciences, IRCCS Humanitas Research Hospital, Humanitas University, Milan, Italy.
Francesca De Lucia, Department of Biomedical Sciences, IRCCS Humanitas Research Hospital, Humanitas University, Milan, Italy.
Matteo Sacchi, Department of Biomedical Sciences, IRCCS Humanitas Research Hospital, Humanitas University, Milan, Italy.
Silvio Danese, Department of Biomedical Sciences, IRCCS Humanitas Research Hospital, Humanitas University, Milan, Italy.
Antonino Spinelli, Department of Biomedical Sciences, IRCCS Humanitas Research Hospital, Humanitas University, Milan, Italy.
Meike M C Hirdes, Division of Gastroenterology and Hepatology, University Medical Center Utrecht, Utrecht, the Netherlands.
Joren ten Hove, Division of Gastroenterology and Hepatology, University Medical Center Utrecht, Utrecht, the Netherlands.
Bas Oldenburg, Division of Gastroenterology and Hepatology, University Medical Center Utrecht, Utrecht, the Netherlands.
Aurada Cholapranee, Division of Gastroenterology and Hepatology, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA.
Maxine Riter, Division of Gastroenterology and Hepatology, Weill Cornell Medical College, NY, USA.
Dana Lukin, Jill Roberts Center for IBD, Weill Cornell Medicine, NY, USA.
Ellen Scherl, Jill Roberts Center for IBD, Weill Cornell Medicine, NY, USA.
Esen Eren, Inflammatory Bowel Disease Center at NYU Langone Health, NYU Grossman School of Medicine, New York, NY, USA.
Keith S Sultan, Division of Gastroenterology and Hepatology, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA.
Jordan Axelrad, Inflammatory Bowel Disease Center at NYU Langone Health, NYU Grossman School of Medicine, New York, NY, USA.
David B Sachar, Division of Gastroenterology and Hepatology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Author Contribution
Research coordination and data extraction and consolidation at individual centers (A.D., A.R., N.N., C.T., A.C., A.M., E.C., M.M.C., M.S., F.D.L., N.B.D., J.t.H., M.R., E.E.). Advanced data analysis at individual centers (C.Y., M.M.C.H., D.L.). Supervising principal investigators at individual centers (D.T.R., A.S., S.D., B.O., K.S., J.A., E.S.). Oversight and consultation (J.-F.C.). Critical Review and editing of final manuscript (J.-F.C., D.T.R., K.S.S.). Principal investigator, overall data consolidation and analysis, manuscript author (G.K.). Research question, conception, design, analysis, and overall study supervision (D.B.S.).
Funding
This work was supported by the Oppenheimer-Ginzburg Research Fund of the Icahn School of Medicine at Mount Sinai, granted by the Shendell Foundation.
Conflicts of Interest
The authors have no conflicts of interest to disclose. The current manuscript, including related data and figures, has not been previously published and is not under consideration elsewhere.
References
- 1. Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn’s disease. Relationship between the clinical pattern and prognosis. Gastroenterology. 1985;88:1818–1825. [DOI] [PubMed] [Google Scholar]
- 2. Bernstein CN, Loftus EV Jr, Ng SC, et al.; Epidemiology and Natural History Task Force of the International Organization for the Study of Inflammatory Bowel Disease (IOIBD). Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61:622–629. [DOI] [PubMed] [Google Scholar]
- 3. Gu J, Valente MA, Remzi FH, Stocchi L. Factors affecting the fate of faecal diversion in patients with perianal Crohn’s disease. Colorectal Dis. 2015;17:66–72. [DOI] [PubMed] [Google Scholar]
- 4. Geoghegan JG, Carton E, O’Shea AM, et al. Crohn’s colitis: the fate of the rectum. Int J Colorectal Dis. 1998;13:256–259. [DOI] [PubMed] [Google Scholar]
- 5. Nordenvall C, Myrelid P, Ekbom A, et al. Probability, rate and timing of reconstructive surgery following colectomy for inflammatory bowel disease in Sweden: a population-based cohort study. Colorectal Dis. 2015;17:882–890. [DOI] [PubMed] [Google Scholar]
- 6. Bafford AC, Latushko A, Hansraj N, et al. The use of temporary fecal diversion in colonic and perianal crohn’s disease does not improve outcomes. Dig Dis Sci. 2017;62:2079–2086. [DOI] [PubMed] [Google Scholar]
- 7. Dharmaraj R, Nugent M, Simpson P, et al. Outcomes after fecal diversion for colonic and perianal Crohn disease in children. J Pediatr Surg. 2018;53:472–476. [DOI] [PubMed] [Google Scholar]
- 8. Chaikhouni A, Regueyra FI, Stevens JR. Adenocarcinoma in perineal fistulas of Crohn’s disease. Dis Colon Rectum. 1981;24:639–643. [DOI] [PubMed] [Google Scholar]
- 9. Guillem JG, Roberts PL, Murray JJ, et al. Factors predictive of persistent or recurrent Crohn’s disease in excluded rectal segments. Dis Colon Rectum. 1992;35:768–772. [DOI] [PubMed] [Google Scholar]
- 10. Korelitz BI, Cheskin LJ, Sohn N, Sommers SC. The fate of the rectal segment after diversion of the fecal stream in Crohn’s disease: its implications for surgical management. J Clin Gastroenterol. 1985;7:37–43. [DOI] [PubMed] [Google Scholar]
- 11. Sjödahl RI, Myrelid P, Söderholm JD. Anal and rectal cancer in Crohn’s disease. Colorectal Dis. 2003;5:490–495. [DOI] [PubMed] [Google Scholar]
- 12. Winther KV, Bruun E, Federspiel B, et al. Screening for dysplasia and TP53 mutations in closed rectal stumps of patients with ulcerative colitis or Crohn disease. Scand J Gastroenterol. 2004;39:232–237. [DOI] [PubMed] [Google Scholar]
- 13. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamamoto T, Keighley MR. Long-term outcome of total colectomy and ileostomy for Crohn disease. Scand J Gastroenterol. 1999;34:280–286. [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto T, Allan RN, Keighley MR. Effect of fecal diversion alone on perianal Crohn’s disease. World J Surg. 2000;24:1258–1262; discussion 1262. [DOI] [PubMed] [Google Scholar]
- 16. Edwards CM, George BD, Jewell DP, et al. Role of a defunctioning stoma in the management of large bowel Crohn’s disease. Br J Surg. 2000;87:1063–1066. [DOI] [PubMed] [Google Scholar]
- 17. Régimbeau JM, Panis Y, Cazaban L, et al. Long-term results of faecal diversion for refractory perianal Crohn’s disease. Colorectal Dis. 2001;3:232–237. [DOI] [PubMed] [Google Scholar]
- 18. Hong MK, Craig Lynch A, Bell S, et al. Faecal diversion in the management of perianal Crohn’s disease. Colorectal Dis. 2011;13:171–176. [DOI] [PubMed] [Google Scholar]
- 19. Ten Hove JR, Bogaerts JMK, Bak MTJ, et al. Malignant and nonmalignant complications of the rectal stump in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:377–384. [DOI] [PubMed] [Google Scholar]
- 20. Lightner AL, Buhulaigah H, Zaghiyan K, et al. Is intestinal diversion an effective treatment for distal crohn’s disease? Inflamm Bowel Dis. 2022;28:547–552. [DOI] [PubMed] [Google Scholar]
- 21. Burke JP. Role of fecal diversion in complex Crohn’s disease. Clin Colon Rectal Surg. 2019;32:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.