Abstract
Objective
To determine whether growth measures at birth differ between offspring of pregnant women with epilepsy and healthy pregnant women.
Study design
The Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study is a National Institutes of Health–funded, prospective, observational, multicenter investigation of pregnancy outcomes for mothers and their infants. Between 2012 and 2016, pregnant women with epilepsy and healthy pregnant women were enrolled at 20 US epilepsy centers. Pregnant women with epilepsy were exposed to various antiepileptic drugs. The main outcome measure was small for gestational age at birth. Principal univariate and multivariate analyses compared outcomes between pregnant women with epilepsy and healthy pregnant women. Secondary analyses focused on outcomes among mothers receiving different antiepileptic drug therapies.
Results
In total, 345 infants were born to 331 pregnant women with epilepsy and 106 infants were born to 102 healthy pregnant women. No differences were seen between infants born to pregnant women with epilepsy vs healthy pregnant women in preterm births, major congenital malformations, 5-minute Apgar <6, special care nursery or neonatal intensive care unit admission, gestational age, or any growth measure. There was no difference in the rates of small for gestational age status among infants born to pregnant women with epilepsy vs healthy pregnant women; however, infants born to mothers receiving topiramate had lower birth weight z scores and lamotrigine higher birth weight z scores compared with other monotherapies. The greatest rate of special care nursery or neonatal intensive care unit admission was observed among those on oxcarbazepine monotherapy.
Conclusions
Maternal treatment with antiepileptic drugs, overall, appears unassociated with adverse early neonatal outcomes. However, specific monotherapies appear to affect fetal growth with, on average, the greatest reduction in birth weight z score observed among infants born to pregnant women with epilepsy exposed to topiramate monotherapy.
Trial registration
Keywords: epilepsy, pregnancy, anticonvulsants, newborn, neonate, outcomes
Improvements in obstetric care have led to successful childbearing among a greater number of women who have chronic illnesses, including epilepsy. The effects of antiepileptic drugs on the fetus and newborn are important in determining short- and long-term health outcomes. The majority of studies to date have focused on associations of selected antiepileptic drug monotherapies or antiepileptic drug polytherapy on the risk of major congenital anomalies or neurodevelopmental outcomes. The multicenter observational study, Neurodevelopmental Effects of Antiepileptic Drugs (NEAD), conducted in the US and United Kingdom between 1999 and 2004, found transiently reduced Apgar scores among infants born to mothers treated with valproate or phenytoin and elevated risk of being born small for gestational age (SGA) among those born to mothers receiving valproate or carbamazepine.1
The Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study is a continuation of the NEAD study, enrolling a new cohort. The primary goals of the MONEAD study are to delineate multiple maternal and child outcomes in pregnant women with epilepsy (eg, seizure frequencies, obstetrical complications, neonatal complications, and neurodevelopmental outcomes in the children) compared with control groups of nonpregnant women with epilepsy and healthy pregnant women. With respect to neonatal outcomes, our primary hypothesis was that children of pregnant women with epilepsy would have greater rates of SGA related to antiepileptic drug type. In this article, we present our findings on neonatal outcomes, including birth weight, head circumference, Apgar scores, preterm birth, and likelihood of receiving newborn intensive care.
Methods
The MONEAD study is a prospective, observational, multicenter investigation of pregnancy outcomes for both pregnant women with epilepsy and their children. The study's primary outcomes are maternal seizures, obstetric complications, maternal depression, neonatal outcomes, and neurodevelopmental outcomes. The sample sizes were powered to assess these primary outcomes. The current analyses focus on neonatal outcomes among infants born to pregnant women with epilepsy vs healthy pregnant women in the MONEAD study. Mothers were enrolled in the study between December 2012 and January 2016. The 20 clinical sites at US epilepsy centers were selected because they specialize in management of women with epilepsy during childbearing years. These included Augusta University, Columbia University, Emory University, Geisinger Clinic, Brigham and Women's Hospital of Harvard University, Henry Ford Health System, Johns Hopkins University, Minnesota Epilepsy Group, New York University, Northwell Health, Northwestern University, Stanford University, University of Alabama, University of Arizona, University of Cincinnati, University of Miami, University of Pittsburgh, University of Southern California, University of Washington, and Wake Forest University.
Inclusion criteria for pregnant women with epilepsy were ages 14-45 years and ≤20 weeks of gestational age. Exclusion criteria included history of psychogenic nonepileptic spells, expected IQ < 70 (confirmed with the Peabody Picture Vocabulary Test-IV as an IQ estimate on enrollment and later measured more specifically by the Wechsler Adult Intelligence Scale, Third Edition), other major medical illness, progressive cerebral disease, and change in antiepileptic drug treatment(s) in pregnancy before enrollment. In addition to pregnant women with epilepsy, the MONEAD Study also enrolled 2 control groups: healthy pregnant women and nonpregnant women with epilepsy; enrollment criteria of these control groups were adjusted during the study to maintain relative similarity to the pregnant women with epilepsy. In addition, the children born to the pregnant women with epilepsy and healthy pregnant women, their fathers, and maternal relatives were enrolled. Data were collected from subjects and their medical records.
This study is registered on clinicaltrials.gov as NCT01730170. It was approved by the institutional review boards of individual sites. Signed informed consent was obtained in advance of study procedures from all mothers who agreed to participate.
For the neonatal report, the primary outcome was the occurrence of SGA newborns born to pregnant women with epilepsy vs healthy pregnant women. Secondary outcomes for study newborns were birth weight z scores, birth head circumference z scores, preterm birth, low Apgar score at 5 minutes, and need for and duration of special care nursery or neonatal intensive care unit (NICU) admission.
Statistical Analyses
Birth weight and head circumference z scores were derived using growth curves from Olsen et al.2 Using the growth curve percentiles from Olsen et al, children with a birth weight lower than the 10th percentile were classified as SGA and children with a birth weight higher than the 90th percentile were classified as large for gestational age.
Pregnant women with epilepsy and healthy pregnant women who gave birth while enrolled in the study and their children who were enrolled at birth were included in the analysis population. Demographic and outcome variables for children and mothers were summarized with counts and percentages for categorical variables and mean, SD, median, minimum, and maximum for continuous variables. For categorical variables, differences between study groups (pregnant women with epilepsy and healthy pregnant women) were assessed with the use of Pearson χ2 tests or Fisher exact tests for variables with low counts. Differences for continuous variables were assessed with the use of Student t tests for normally distributed variables or Wilcoxon rank-sum tests for non-normally distributed variables. Normality was assessed visually with the use of QQ-plots.
For children of pregnant women with epilepsy, the antiepileptic drug group was categorized based on the mother's prescribed antiepileptic drug at the time of enrollment into the following 5 groups: lamotrigine monotherapy, levetiracetam monotherapy, all other monotherapy, polytherapy, and no antiepileptic drug. The Fisher exact test was used to assess the association between antiepileptic drug group and categorical outcome variables. The association between antiepileptic drug group and both birth weight z score and head circumference z score was assessed with the use of ANOVA models. The Tukey honest significant difference adjustment was used to correct for multiple pair-wise comparisons between antiepileptic drug groups. A follow-up analysis was performed in children of women on monotherapy with the use of an ANOVA model to assess the association between the specific antiepileptic drug at enrollment and birth weight z score. Birth weight z score grouped by antiepileptic drug for monotherapy or antiepileptic drug group for polytherapy and no antiepileptic drug was visualized using box plots.
All outcome analyses were also run adjusting for folate use, alcohol use during pregnancy, and child's race. The analyses of head circumference were also adjusted for mother's and father's head circumference separately and together in the same model.
Results
Demographics for the MONEAD study pregnant women with epilepsy and healthy pregnant women are shown in Table I. The 331 pregnant women with epilepsy included in this analysis delivered 345 infants (7 pairs of twins). The 102 healthy pregnant women enrolled had 106 infants (2 pairs of twins). The group of pregnant women with epilepsy differed from healthy pregnant women in race (pregnant women with epilepsy: 85% White, 7% African American; healthy pregnant women: 71% White, 17% African American; P = .002), periconceptional folate use (pregnant women with epilepsy 85%; healthy pregnant women 67%; P < .001), and self-reported alcohol intake during pregnancy (pregnant women with epilepsy 24%; healthy pregnant women 34%; P = .031).
Table I.
Demographics for pregnant women with epilepsy and healthy pregnant women∗
| Demographics | Pregnant women with epilepsy | Healthy pregnant women | P value† |
|---|---|---|---|
| Total enrolled | 331 | 102 | |
| Mean age, y (SD) | 31 (5) | 30 (5) | .23 |
| Mean head circumference, cm (SD) | 55.8 (2.2) | 55.6 (2.3) | .54 |
| Education, No. (%) | .19 | ||
| No college degree | 99 (30) | 31 (30) | |
| College degree | 149 (45) | 37 (36) | |
| Advanced degree | 83 (25) | 34 (33) | |
| Race, No. (%) | .002 | ||
| White | 281 (85) | 72 (71) | |
| Black or African American | 22 (7) | 17 (17) | |
| Other/unknown | 28 (8) | 13 (13) | |
| Ethnicity, No. (%) | .48 | ||
| Hispanic or Latino | 64 (19) | 23 (23) | |
| Non-Hispanic or Non-Latino | 267 (81) | 79 (77) | |
| Antiepileptic drug category at enrollment, No. (%) | – | ||
| Antiepileptic drug monotherapy | 250 (76) | – | |
| Antiepileptic drug polytherapy | 66 (20) | – | |
| Without antiepileptic drug | 15 (5) | – | |
| Seizure types‡ | – | ||
| Generalized | 106 (32) | – | |
| Focal§ | 202 (61) | – | |
| Unclassified | 26 (8) | – | |
| Births | 1.00 | ||
| Singletons | 317 | 98 | |
| Twins | 28 (14 pairs) | 8 (4 pairs) | |
| Total births | 345 | 106 | |
| Periconceptional folate use, No. (%) | <.001 | ||
| Yes | 283 (85) | 68 (67) | |
| No | 48 (15) | 34 (33) | |
| Smoking,¶ No. (%) | .59 | ||
| Yes | 21 (6) | 5 (5) | |
| No | 310 (94) | 97 (95) | |
| Alcohol use,¶ No. (%) | .03 | ||
| Yes | 78 (24) | 35 (34) | |
| No | 253 (76) | 67 (66) | |
| Illicit drug¶∗∗ use, No. (%) | .75 | ||
| Yes | 10 (3) | 4 (4) | |
| No | 319 (97) | 97 (96) | |
| Missing | 2 | 1 |
Only women who gave birth during the study were included.
P values from t test (age, head circumference), Pearson χ2 (education, race, ethnicity, folate use, gestational hypertension, preeclampsia, smoking, alcohol use), and the Fisher exact test (births, illicit drug use).
Three subjects reported multiple seizure types, 2 reported generalized and focal seizures, and 1 reported generalized and unclassified seizures. Percentages represent the proportion of subjects who have that seizure type and total may sum to more than 100%.
Incudes focal to bilateral tonic clonic seizure.
Self-reported use of smoking, alcohol, and illicit drug use at any time during pregnancy.
Illicit drug use includes use of marijuana at any time during pregnancy.
Categorical neonatal variables are reported in Table II, and, similar to maternal demographics, showed that infants born to pregnant women with epilepsy were more likely than healthy pregnant women to be White (80.6% vs 67.0%) and less likely to be African American (5.2% vs 14.2%; P = .003). No differences were observed between infants of pregnant women with epilepsy vs healthy pregnant women on other demographics or outcomes, including ethnicity, 5-minute Apgar score <6, preterm birth, major malformations, NICU admission, and duration of NICU admission for those requiring a NICU stay. There was no difference in the rates of SGA status among infants born to pregnant women with epilepsy vs healthy pregnant women (5.5% vs 8.8%; P = .24 [raw]; P = .16 [adjusted]). Further, we found no significant association between seizures during pregnancy and SGA (data not shown). Rates of breastfeeding are reported elsewhere.3
Table II.
Categorical variable summary, children of pregnant women with epilepsy and healthy pregnant women
| Demographics | Children of pregnant women with epilepsy N = 345 |
Children of healthy pregnant women N = 106 |
P value |
|
|---|---|---|---|---|
| No. (%) | No. (%) | Raw∗ | Adjusted† | |
| Infant sex | .37 | – | ||
| Male | 164 (47.5) | 56 (52.8) | ||
| Female | 181 (52.5) | 50 (47.2) | ||
| Infant race | .003 | – | ||
| White | 278 (80.6) | 71 (67.0) | ||
| Black or African American | 18 (5.2) | 15 (14.2) | ||
| Other/unknown | 49 (14.2) | 20 (18.9) | ||
| Infant ethnicity | .79 | – | ||
| Non-Hispanic or non-Latino | 274 (79.4) | 83 (78.3) | ||
| Hispanic or Latino | 71 (20.6) | 23 (21.7) | ||
| Outcome variables | ||||
| SGA‡ | .24 | .16 | ||
| Yes | 18 (5.5) | 9 (8.8) | ||
| No | 310 (94.5) | 93 (91.2) | ||
| Missing | 17 | 4 | ||
| 5-min Apgar < 6 | 1.00 | – | ||
| Yes | 2 (0.6) | 0 (0.0) | ||
| No | 316 (99.4) | 97 (100.0) | ||
| Missing | 27 | 9 | ||
| Premature | .86 | .70 | ||
| Not premature (>36 wk) | 306 (88.7) | 95 (89.6) | ||
| Premature (≤36 wk) | 39 (11.3) | 11 (10.4) | ||
| Prematurity | .26 | – | ||
| Late (34-36 wk gestational age) | 27 (69.2) | 9 (81.8) | ||
| Moderate (28-33 wk gestational age) | 11 (28.2) | 1 (9.1) | ||
| Extreme (<28 wk gestational age) | 1 (2.6) | 1 (9.1) | ||
| Major malformations | .18 | .12 | ||
| Yes | 18 (5.2) | 2 (1.9) | ||
| No | 327 (94.8) | 104 (98.1) | ||
| NICU admission | .53 | .40 | ||
| Yes | 53 (16.4) | 13 (13.0) | ||
| No | 271 (83.6) | 87 (87.0) | ||
| Missing | 21 | 6 | ||
P values from the Fisher exact test.
Adjusted P values from logistic regression adjusted for periconceptual folate use, alcohol use during pregnancy, and child's race. Major malformations not adjusted for folate use, because all malformations were born to mothers who indicated folate use. Adjusted models not run for demographic variables or outcome variables with low cell counts.
Based on growth curves from Olsen et al. SGA defined as weight for gestational age <10th percentile.
Table III summarizes continuous outcome variables for infants born to pregnant women with epilepsy and healthy pregnant women, including gestational age, 5-minute Apgar scores, and birth weight and head circumference and their respective z scores. No significant differences were observed between groups in any of these measures. Weight and head circumference z-score analyses also showed no differences in unadjusted analyses or when adjusted for periconceptual folate use, alcohol use during pregnancy, and the child's race.
Table III.
Continuous variable summary, children of pregnant women with epilepsy and healthy pregnant women
| Descriptive variables | Children of pregnant women with epilepsy N = 345 |
Children of healthy pregnant women N = 106 |
P value |
|||||
|---|---|---|---|---|---|---|---|---|
| N | Mean ± SD | Median [min, max] | N | Mean ± SD | Median [min, max] | Raw∗ | Adjusted† | |
| Week of gestation | 345 | 38.39 ± 2.08 | 39 [24, 42] | 106 | 38.58 ± 1.98 | 39 [26, 43] | .61 | – |
| Apgar score, 5 min | 318 | 8.78 ± 0.68 | 9 [2, 10] | 97 | 8.82 ± 0.54 | 9 [6, 9] | .47 | – |
| Birth weight, g | 329 | 3207 ± 600 | 3240 [620, 4895] | 104 | 3253 ± 623 | 3272 [1075, 5025] | .81 | – |
| Birth weight, z score‡ | 328 | −0.06 ± 0.81 | −0.09 [–2.25, 2.71] | 102 | −0.09 ± 0.95 | −0.12 [–2.64, 3.60] | .80 | .72 |
| Head circumference, cm | 283 | 33.76 ± 1.91 | 34 [23, 39] | 90 | 33.98 ± 1.74 | 34 [26, 37] | .23 | – |
| Head circumference, z score‡ | 282 | −0.07 ± 0.93 | −0.18 [–3.40, 2.62] | 88 | –-0.04 ± 0.83 | 0.03 [–2.28, 1.52] | .72 | .70 |
max, maximum; min, minimum.
P values from t test (birth weight z score, head circumference z score) or Wilcoxon rank-sum test (week of gestation, birth weight, head circumference, Apgar score).
Adjusted P values from ANCOVA model adjusted for periconceptual folate use, alcohol use during pregnancy, and child's race.
z scores based on growth curves from Olsen et al.
The types of maternal epilepsy and the distributions of specific antiepileptic drugs used in pregnant women with epilepsy have been described previously.4,5 In previous analyses of the MONEAD population, Meador et al found the majority (74%) of pregnant women with epilepsy were receiving monotherapy.4 The most common antiepileptic drug monotherapy treatments were lamotrigine and levetiracetam (42.1% and 37.5% of monotherapies, respectively). The most common antiepileptic drug polytherapy was the combination of lamotrigine and levetiracetam (42.9% of polytherapies).
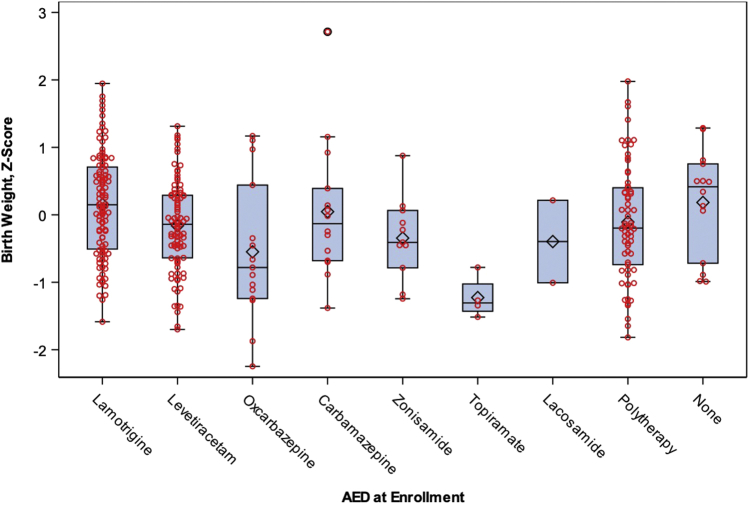
When we evaluated neonatal outcomes by antiepileptic drug group at maternal enrollment (Table IV), variability in SGA was observed (P = .04) with a greater rate of SGA among the other monotherapy group. There were no statistically significant differences among antiepileptic drug groups for rates of prematurity or NICU admission. However, it was observed that for infants born to mothers in the other monotherapy group (excluding lamotrigine and levetiracetam), 23.1% required special care nursery or NICU admission compared with 15.7% and 11.5% for infants born to mothers on lamotrigine and levetiracetam, respectively. This difference seemed largely due to the high rate of NICU admission observed among a modest number of infants whose mothers were treated with oxcarbazepine (7 of 15 infants; 46.7%). Among monotherapy antiepileptic drug regimens, topiramate was associated with lower birth weight z scores (mean ± SD: −1.23 ± 0.32, n = 4) and lamotrigine (mean ± SD: 0.15 ± 0.77, n = 106) was linked with greater z scores compared with other antiepileptic drugs (Figure).
Table IV.
Outcome variable summary by antiepileptic drug group at enrollment, children of pregnant women with epilepsy
| Outcome variables | Antiepileptic drug group |
P value |
|||||
|---|---|---|---|---|---|---|---|
| LTG monotherapy (N = 110) | LEV monotherapy (N = 97) | Other monotherapy (N = 54) | Polytherapy (N = 69) | No antiepileptic drug (N = 15) | Raw∗ | Adjusted† | |
| SGA,‡ No. (%) | .04 | – | |||||
| Yes | 1 (0.9) | 6 (6.7) | 5 (10.0) | 6 (8.7) | 0 (0.0) | ||
| No | 105 (99.1) | 83 (93.3) | 45 (90.0) | 63 (91.3) | 14 (100.0) | ||
| Missing | 4 | 8 | 4 | 0 | 1 | ||
| Premature, No. (%) | .57 | .34 | |||||
| Premature (≤36 wk) | 9 (8.2) | 11 (11.3) | 7 (13.0) | 11 (15.9) | 1 (6.7) | ||
| Not premature (>36 wk) | 101 (91.8) | 86 (88.7) | 47 (87.0) | 58 (84.1) | 14 (93.3) | ||
| Prematurity, No. (%)§ | .18 | – | |||||
| Late (34-36 wk gestational age) | 8 (88.9) | 8 (72.7) | 3 (42.9) | 8 (72.7) | 0 (0.0) | ||
| Moderate (28-33 wk gestational age) | 1 (11.1) | 2 (18.2) | 4 (57.1) | 3 (27.3) | 1 (100.0) | ||
| Extreme (<28 wk gestational age) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| NICU admission, No. (%) | .46 | .43 | |||||
| Yes | 16 (15.7) | 10 (11.5) | 12 (23.1) | 13 (18.8) | 2 (14.3) | ||
| No | 86 (84.3) | 77 (88.5) | 40 (76.9) | 56 (81.2) | 12 (85.7) | ||
| Missing | 8 | 10 | 2 | 0 | 1 | ||
| Birth weight, z score¶ | |||||||
| No. | 106 | 89 | 50 | 69 | 14 | ||
| Mean ± SD | 0.15 ± 0.77 | −0.16 ± 0.67 | −0.34 ± 0.93 | −0.12 ± 0.87 | 0.18 ± 0.79 | .002∗∗ | .002∗∗ |
| Head circumference, z score¶ | |||||||
| No. | 89 | 73 | 47 | 61 | 12 | ||
| Mean ± SD | 0.04 ± 0.91 | −0.08 ± 0.89 | −0.37 ± 1.09 | −0.07 ± 0.88 | 0.19 ± 0.84 | .13 | .09 |
LEV, levetiracetam; LTG, lamotrigine; other monotherapy, all other monotherapy antiepileptic drugs.
P values from the Fisher exact test for categorical variables and ANOVA model for continuous variables.
Adjusted for periconceptual folate use, alcohol use during pregnancy, and child's race using logistic regression (premature and NICU admission) or ANCOVA models (birth weight, z score and head circumference, z score). Adjusted models not run for outcomes with low cells counts.
Based on growth curves from Olsen et al. SGA defined as weight for gestational age <10th percentile.
Number of infants born premature (≤36 weeks) used as denominator for calculating percentages.
z scores based on growth curves from Olsen et al.
After we adjusted for multiple pairwise comparisons using Tukey honestly significant difference, LTG monotherapy was significantly higher than LEV monotherapy and other monotherapy in both the ANOVA model and adjusted ANCOVA model.
Figure.
Box plot for birth weight z score by antiepileptic drug. Based on growth curves from Olsen et al2; antiepileptic drug at enrollment, pregnant women with epilepsy on monotherapy only.
Discussion
MONEAD, a multicenter observational study, compares pregnancies of pregnant women with epilepsy and healthy pregnant women, focusing on maternal, fetal, and neonatal outcomes. With respect to neonatal outcomes, the MONEAD primary hypothesis was that children of pregnant women with epilepsy would have greater rates of SGA, related to antiepileptic drug type and exposure in a concentration dependent manner. Previous analyses of fetal outcomes of the NEAD6 and MONEAD populations5 revealed greater risk of serious adverse fetal outcomes (ie, fetal losses and major congenital malformations combined) among pregnant women with epilepsy compared with healthy pregnant women and these varied across antiepileptic drugs. In contrast, our current analyses of neonatal outcomes showed generally favorable outcomes with exceptions for 2 specific monotherapies: topiramate was associated with substantially lower birth weight z score than other monotherapies or polytherapy, and oxcarbazepine was linked with a greater likelihood of NICU or special care nursery admission than other monotherapies. These findings need to be interpreted with caution, given the small sample sizes for topiramate and oxcarbazepine monotherapy. Other specific adverse neonatal outcomes were not different across groups.
Strengths of the current study include the large overall study population across a wide geographic region and the detailed collection of relevant clinical data, including information on all known confounding factors for primary and most secondary analyses. The main limitations are that this is an observational study, rather than a randomized clinical trial, although a randomized clinical trial in the treatment of women with epilepsy during pregnancy would be unethical. In addition, there are small sample sizes in some subgroups analyzed in secondary outcomes, particularly those relating to monotherapy subgroups. One pregnant women with epilepsy taking topiramate at the time of enrollment was no longer taking the medication at the time of delivery.
Differential effects of antiepileptic drug treatment in pregnancy have been reported previously. In a recent population-based cohort study of more than 1.4 million births in Sweden,7 infants born to pregnant women with epilepsy were found to be at greater risk than those born to healthy pregnant women for congenital malformation, preterm birth, being SGA at birth, asphyxia-related complications, lower Apgar scores 4-6 at 5 minutes, neonatal hypoglycemia, and respiratory distress syndrome. A number of studies have noted the most prominent increase in major congenital malformations to be among infants born to mothers receiving valproic acid treatment. 8, 9, 10, 11, 12 Additional antiepileptic drugs, such as phenobarbital, phenytoin, and some others, and greater dosing levels of selected antiepileptic drugs also appear to contribute to increased risk of major malformations.13, 14, 15
A relatively common finding has been the association of antenatal antiepileptic drug exposure with increased risks of impaired fetal growth,16 lower birth weight for gestational age,17 and smaller head circumference at birth,18 especially with polytherapy.19 In NEAD, a 25-center study in the US and United Kingdom of 329 pregnant women with epilepsy,1 valproate and carbamazepine were found to be associated with SGA status at birth, and infants born to mothers receiving valproate and phenytoin groups exhibited transiently reduced Apgar scores. Among babies born to mothers enrolled in the North American Antiepileptic Drug Registry between 1997 and 2016, topiramate and zonisamide were noted to be associated with increased likelihood of lower mean birth weight and lesser mean length.20,21 A large recent study of a national Norwegian cohort (1999-2011; n = 771 412) also found associations with antenatal topiramate exposure and risk of microcephaly and SGA birth weight.22 We also found an association with topiramate use and a lower weight for gestation age z score; however, we did not observe the association with zonisamide reported by this study or by Norwegian investigators who conducted a single-county study between 1989 and 2012.23
There are several potential explanations for our failure to find previously reported associations between infants born to pregnant women with epilepsy in rates of SGA to maternal zonisamide treatment or microcephaly to topiramate exposure. The first concerns potential effects of trends in antiepileptic drug use over time in polytherapy vs monotherapy, doses of antiepileptic drugs, as well as in potential modifying factors in their relationships to birth weight, such as smoking. The discrepancies also might relate to the study population: previous studies were largely conducted using data from large national registries whereas our study population consisted of volunteers to participate in research who were receiving regular obstetrical care at academic medical centers. These populations likely differed in demographics and health vulnerabilities. In addition, some adverse outcomes in our study population might have been averted due to the consistency and quality of obstetrical care. In addition, we chose to use as a basis of weight for gestational age, growth curves for a contemporary US neonatal population and to analyze using both z scores as well as proportions in each treatment group designated “SGA.” Finally, small sample sizes in some of our treatment groups might have led to an inability to detect some effects, such as differences in head circumferences.
Fetal growth and SGA status at birth can be influenced by more than 40 factors,24 including fetal syndromes or malformations, parental anthropometrics,25 maternal weight gain during pregnancy,26 environmental exposures,27 and maternal health factors, including smoking, presence or absence of diabetes mellitus, chronic hypertension, or pregnancy-induced hypertension. Among pregnant women with epilepsy, seizures during pregnancy also have been found to be associated with SGA status at birth28; however, this was not found in analyses of the MONEAD study population. Further information regarding seizures during pregnancy and other obstetrical factors will be reported in a separate publication. For the purposes of our evaluation, we assessed other maternal health factors and found no difference between pregnant women with epilepsy and healthy pregnant women in pregnancy smoking, weight gain, diabetes, or hypertension. Combined fetal losses and major congenital malformations occurred more often in pregnant women with epilepsy; the occurrence of major congenital malformations alone did not differ, although the sample sizes were small.5 Mechanisms by which antiepileptic drugs might influence fetal growth include predisposition to hypertension in pregnancy, detected in a recent population-based study conducted in Norway,29 and adverse placental effects, shown recently in experimental studies of valproic acid exposure.30 The way in which topiramate contributes to diminished fetal growth is unknown. Weight loss is a known side effect of topiramate treatment in adults, although the precise mechanism of action for this effect has not been described. Fetal growth restriction most often is the result of specific maternal health conditions, placental malfunction, or fetal genetic abnormalities. A direct effect of growth-inhibiting exposures on the fetus is possible, however, is not commonly recognized. There are no published reports of placental abnormalities associated with topiramate treatment, nor are their reports of increase in bleeding complications of mother or newborn.31 We likewise found no adverse effect of the medication on maternal health. Laboratory data suggest that topiramate interferes with lipolysis in a specific type of adipocyte.32 Investigation in a rodent model showed topiramate associated impairment of skeletal ossification33; therefore, it is possible topiramate has a direct effect on fetal growth through these or other as yet-unidentified mechanisms.
Substantially impaired fetal growth may have significant health and/or developmental implications. SGA status is a major risk factor for adverse neonatal and long-term health outcomes. Fetal growth restriction frequently prompts preterm delivery because it often is a marker of fetal compromise. Even if not born preterm in the initial neonatal period, the SGA infant is at risk of hypoglycemia, feeding intolerance, poor growth, polycythemia, leukopenia, and thrombocytopenia. The infant born preterm and SGA also is at increased risk of serious gastrointestinal complications and of developing chronic lung disease. Even with improved postnatal growth, the infant born SGA remains at increased risk of impaired childhood growth, hypertension, and neurodevelopmental disability, compared with appropriate size for gestational age newborns.
In conclusion, our findings add to the increasing information on neonatal outcomes among infants born to pregnant women with epilepsy. Overall, the antiepileptic drugs most often used among subjects in this study appear to be associated with few adverse neonatal outcomes, with the exception of a possible increased risk of low birth weight for gestational age among infants born to mothers on topiramate monotherapy and suggested elevated risk of newborn intensive or intermediate care admission among infants whose mothers were being treated with oxcarbazepine monotherapy at enrollment. Further investigation of these findings is warranted given the relatively small sample sizes in which they were observed. Future analyses will evaluate the relationship of neonatal outcomes and the amount of antiepileptic drug exposure in a concentration-dependent manner.
Data Statement
Data sharing statement available at www.jpeds.com.
Acknowledgments
We thank the children and families who have given their time to participate in the MONEAD Study. We thank all the members of the MONEAD Study Group (Appendix) for their contributions through participation in these research endeavors. We also thank Bridget Thompson, Annie Ulchak Ritchings, and Irene Miller for clerical support.
Footnotes
Funding and disclosures information is available at www.jpeds.com
Contributor Information
Linda J. Van Marter, Email: lvanmarter@bwh.harvard.edu.
MONEAD Investigator Group:
Anto Bagic, Gregory Barkley, Jennifer Cavitt, Jennifer DeWolfe, Jacqueline French, Evan Gedzelman, Elizabeth Gerard, Sean Hwang, Laura Kalayjian, Gregory Krauss, David Labiner, Paul McCabe, John Miller, Alison Pack, Patricia Penovich, Maria Sam, Enrique Serrano, and Suzanne Strickland
Funding and Conflicts of Interest Disclosures
Supported by National Institutes of Health (NIH) National Institute of Neurological Disorders & Stroke (NINDS), Eunice Kennedy Shriver National Institute of Child Health and Human Development (#U01-NS038455 [to K.M. and P.P.], U01-NS050659 [to R.M.], and 2U01-NS038455 [to K.M., P.P., and R.M]). The NIH had a role in the analyses and writing of the manuscript but had no role in the design, conduct of the study, or decision to submit for publication. K.M. has received research support from the NIH and Sunovion Pharmaceuticals; has received travel support from UCB Pharma; and the Epilepsy Study Consortium pays Stanford University for his research consultant time related to Eisai, GW Pharmaceuticals, NeuroPace, Novartis, Supernus, Upsher-Smith Laboratories, UCB Pharma, and Vivus Pharmaceuticals. P.P. has received research support from the NIH and the Epilepsy Foundation; and has received honoraria and travel support from American Epilepsy Society, American Academy of Neurology, Epilepsy Foundation, NIH, and academic institutions for CME lectures. L.V.M. has received support from Shire Pharmaceuticals (now Takeda) for travel to several investigator meetings. T.M. has received research support from the NIH and NxPrenatal; and serves on advisory boards for Hoffman La Roche and Mirvie. A.H. is employed by the NINDS. R.M. declares no conflicts of interest.
Supplementary Data
Appendix
Additional MONEAD Study Site Investigators:
Anto Bagic, MD (University of Pittsburgh), Gregory Barkley, MD (Henry Ford Hospital), Jennifer Cavitt, MD (University of Cincinnati), Jennifer DeWolfe, MD (University of Alabama), Jacqueline French, MD (New York University), Evan Gedzelman, MD (Emory University), Elizabeth Gerard, MD (Northwestern University), Sean Hwang, MD (North Shore Long Island Jewish Health System), Laura Kalayjian, MD (University of Southern California), Gregory Krauss, MD (Johns Hopkins Medical Center), David Labiner, MD (University of Arizona Health Sciences), Paul McCabe, MD (Geisinger Medical Center), John Miller, MD (University of Washington), Alison Pack, MD (Columbia University), Patricia Penovich, MD (Minnesota Epilepsy Group), Maria Sam, MD (Wake Forest University), Enrique Serrano, MD (University of Miami), Suzanne Strickland, MD (Georgia Regents University).
References
- 1.Pennell P.B., Klein A.M., Browning N., Baker G.A., Clayton-Smith J., Kalayjian L.A., et al. Differential effects of antiepileptic drugs on neonatal outcomes. Epilepsy Behav. 2012;24:449–456. doi: 10.1016/j.yebeh.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen I.E., Groveman S.A., Lawson M.L., Clark R.H., Zemel B.S. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaum A.K., Meador K.J., Karanam A., Brown C., May R.C., Gerard E.E., et al. Antiepileptic drug exposure in infants of breastfeeding mothers with epilepsy. JAMA Neurol. 2020;77:441–450. doi: 10.1001/jamaneurol.2019.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meador K.J., Pennell P.B., May R.C., Gerard E., Kalayjian L., Velez-Ruiz N., et al. Changes in antiepileptic drug-prescribing patterns in pregnant women with epilepsy. Epilepsy Behav. 2018;84:10–14. doi: 10.1016/j.yebeh.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meador K.J., Pennell P.B., May R.C., Van Marter L., McElrath T.F., Brown C., et al. Fetal loss and malformations in the MONEAD study of pregnant women with epilepsy. Neurology. 2020;94:e1502–e1511. doi: 10.1212/WNL.0000000000008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meador K.J., Baker G.A., Finnell R.H., Kalayjian L.A., Liporace J.D., Loring D.W., et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407–412. doi: 10.1212/01.wnl.0000227919.81208.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Razaz N., Tomson T., Wikstrom A.K., Cnattingius S. Association between pregnancy and perinatal outcomes among women with epilepsy. JAMA Neurol. 2017;74:983–991. doi: 10.1001/jamaneurol.2017.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harden C.L., Meador K.J., Pennell P.B., Hauser W.A., Gronseth G.S., French J.A., et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:133–141. doi: 10.1212/WNL.0b013e3181a6b312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mawer G., Briggs M., Baker G.A., Bromley R., Coyle H., Eatock J., et al. Pregnancy with epilepsy: obstetric and neonatal outcome of a controlled study. Seizure. 2010;19:112–119. doi: 10.1016/j.seizure.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez-Diaz S., Smith C.R., Shen A., Mittendorf R., Hauser W.A., Yerby M., et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78:1692–1699. doi: 10.1212/WNL.0b013e3182574f39. [DOI] [PubMed] [Google Scholar]
- 11.Bromley R.L., Weston J., Marson A.G. Maternal use of antiepileptic agents during pregnancy and major congenital malformations in children. JAMA. 2017;318:1700–1701. doi: 10.1001/jama.2017.14485. [DOI] [PubMed] [Google Scholar]
- 12.Tomson T., Battino D., Bonizzoni E., Craig J., Lindhout D., Perucca E., et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530–538. doi: 10.1016/S1474-4422(18)30107-8. [DOI] [PubMed] [Google Scholar]
- 13.Tomson T., Battino D., Bonizzoni E., Craig J., Lindhout D., Sabers A., et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10:609–617. doi: 10.1016/S1474-4422(11)70107-7. [DOI] [PubMed] [Google Scholar]
- 14.Tomson T., Battino D., Bonizzoni E., Craig J., Lindhout D., Perucca E., et al. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866–872. doi: 10.1212/WNL.0000000000001772. [DOI] [PubMed] [Google Scholar]
- 15.Veroniki A.A., Cogo E., Rios P., Straus S.E., Finkelstein Y., Kealey R., et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med. 2017;15:95. doi: 10.1186/s12916-017-0845-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viale L., Allotey J., Cheong-See F., Arroyo-Manzano D., McCorry D., Bagary M., et al. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015;386:1845–1852. doi: 10.1016/S0140-6736(15)00045-8. [DOI] [PubMed] [Google Scholar]
- 17.Hvas C.L., Henriksen T.B., Ostergaard J.R., Dam M. Epilepsy and pregnancy: effect of antiepileptic drugs and lifestyle on birthweight. BJOG. 2000;107:896–902. doi: 10.1111/j.1471-0528.2000.tb11089.x. [DOI] [PubMed] [Google Scholar]
- 18.Hiilesmaa V.K., Teramo K., Granstrom M.L., Bardy A.H. Fetal head growth retardation associated with maternal antiepileptic drugs. Lancet. 1981;2:165–167. doi: 10.1016/s0140-6736(81)90354-8. [DOI] [PubMed] [Google Scholar]
- 19.Almgren M., Kallen B., Lavebratt C. Population-based study of antiepileptic drug exposure in utero--influence on head circumference in newborns. Seizure. 2009;18:672–675. doi: 10.1016/j.seizure.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Diaz S., Mittendorf R., Smith C.R., Hauser W.A., Yerby M., Holmes L.B., et al. Association between topiramate and zonisamide use during pregnancy and low birth weight. Obstet Gynecol. 2014;123:21–28. doi: 10.1097/AOG.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Diaz S., McElrath T.F., Pennell P.B., Hauser W.A., Yerby M., Holmes L.B., et al. Fetal growth and premature delivery in pregnant women on antiepileptic drugs. Ann Neurol. 2017;82:457–465. doi: 10.1002/ana.25031. [DOI] [PubMed] [Google Scholar]
- 22.Veiby G., Daltveit A.K., Engelsen B.A., Gilhus N.E. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol. 2014;261:579–588. doi: 10.1007/s00415-013-7239-x. [DOI] [PubMed] [Google Scholar]
- 23.Farmen A.H., Grundt J., Tomson T., Nakken K.O., Nakling J., Mowinchel P., et al. Intrauterine growth retardation in foetuses of women with epilepsy. Seizure. 2015;28:76–80. doi: 10.1016/j.seizure.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Kramer M.S. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 25.Albouy-Llaty M., Thiebaugeorges O., Goua V., Magnin G., Schweitzer M., Forhan A., et al. Influence of fetal and parental factors on intrauterine growth measurements: results of the EDEN mother-child cohort. Ultrasound Obstet Gynecol. 2011;38:673–680. doi: 10.1002/uog.9006. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein R.F., Abell S.K., Ranasinha S., Misso M., Boyle J.A., Black M.H., et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317:2207–2225. doi: 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snijder C.A., Roeleveld N., Te Velde E., Steegers E.A., Raat H., Hofman A., et al. Occupational exposure to chemicals and fetal growth: the Generation R Study. Hum Reprod. 2012;27:910–920. doi: 10.1093/humrep/der437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y.H., Chiou H.Y., Lin H.C., Lin H.L. Affect of seizures during gestation on pregnancy outcomes in women with epilepsy. Arch Neurol. 2009;66:979–984. doi: 10.1001/archneurol.2009.142. [DOI] [PubMed] [Google Scholar]
- 29.Danielsson K.C., Borthen I., Morken N.H., Gilhus N.E. Hypertensive pregnancy complications in women with epilepsy and antiepileptic drugs: a population-based cohort study of first pregnancies in Norway. BMJ Open. 2018;8:e020998. doi: 10.1136/bmjopen-2017-020998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubinchik-Stern M., Shmuel M., Bar J., Kovo M., Eyal S. Adverse placental effects of valproic acid: Studies in perfused human placentas. Epilepsia. 2018;59:993–1003. doi: 10.1111/epi.14078. [DOI] [PubMed] [Google Scholar]
- 31.Panchaud A., Cohen J.M., Patorno E., Huybrechts K.F., Desai R.J., Gray K.J., et al. Anticonvulsants and the risk of perinatal bleeding complications: a pregnancy cohort study. Neurology. 2018;91:e533–e542. doi: 10.1212/WNL.0000000000005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins G.P., Souza C.O., Marques S., Luciano T.F., DASP B.L., Rosa J.C., et al. Topiramate effects lipolysis in 3T3-L1 adipocytes. Biomed Rep. 2015;3:827–830. doi: 10.3892/br.2015.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fadel R.A., Sequeira R.P., Abu-Hijleh M.F., Obeidat M., Salem A.H. Effect of prenatal administration of therapeutic doses of topiramate on ossification of ribs and vertebrae in rat fetuses. Rom J Morphol Embryol. 2012;53:321–327. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.