Abstract
Infection by human adeno-associated virus type 2 (AAV2) is a possible protective factor in the development of cervical carcinomas associated with human papillomaviruses (HPV). The replicative proteins of AAV2 (Rep) have been implicated in the inhibition of papillomavirus replication and transforming activities, although the molecular events underlying these effects are poorly understood. We observed that each of the four forms of AAV2 Rep inhibited the E1- and E2-driven replication of oncogenic HPV type 16 (HPV16). Rep40, corresponding to the C-terminal domain of all Rep proteins, inhibited both HPV DNA replication and HPV16 E2-mediated transactivation. Rep40 specifically bound the N-terminal transactivation domain of HPV16 E2 both in vitro and in vivo. This interaction was found to specifically disrupt the binding of E2 to the cellular transcriptional coactivator p300. Accordingly, the inhibitory effect of Rep on HPV16 E2 transactivation was rescued by the overexpression of p300. These data indicate a novel role of Rep in the down-regulation of papillomaviruses through inhibition of complex formation between the HPV16 E2 transcriptional activator and its cellular coactivator, p300.
Adeno-associated virus (AAV) type 2 (AAV2) is a helper-dependent human parvovirus with a single-stranded DNA genome coding for two genes, rep and cap. Four overlapping nonstructural proteins, Rep78, Rep68, Rep52, and Rep40, are the products of the rep gene (49). The two major forms of Rep (Rep78 and Rep68) bind to specific sites within the inverted terminal repeats (48, 61, 73), have helicase and endonuclease activities (28, 74), and are needed for the initial steps of DNA replication (23, 70). The two major forms of Rep are also required for site-specific integration of the viral genome into human chromosome 19 (30, 38). The two minor forms of Rep (Rep52 and Rep40) do not bind the inverted terminal repeats and are dispensable for viral DNA replication and site-specific integration (29, 52).
Rep proteins are involved in the regulation of gene expression from homologous AAV2 promoters (34). These promoters are up-regulated by Rep in the presence of adenovirus infection (46, 47), while in the absence of helper virus, the effect of Rep is inhibitory (4, 32, 71). Several heterologous promoters, including viral and proto-oncogene promoters, have also been shown to be down-regulated by Rep, suggesting a Rep-induced pleiotropic effect on gene expression (21, 26, 33, 58). In addition, Rep proteins have been shown to inhibit the replication of a number of DNA viruses, including adenoviruses, herpesviruses, and papillomaviruses (11, 19, 20). While this inhibitory effect can be partially ascribed to the above-mentioned down-modulation of transcription by Rep, a more general effect on DNA replication may also be involved. Accordingly, it has been demonstrated that Rep inhibits cellular DNA replication, herpesvirus-induced amplification of chromosomally integrated simian virus 40 DNA (3), and bovine papillomavirus (BPV) DNA amplification (22). Taken together, these activities have led to the notion that AAV2 possesses broad oncosuppressive and antiproliferative functions.
The interaction of AAV2 and human papillomaviruses (HPV) appears to have special significance, given the large amount of both clinical and molecular data that indicate that AAV2 is an inhibitor of HPV replication and HPV-induced cellular transformation both in vivo and in vitro. In vitro, AAV2 infection inhibits BPV and HPV type 16 (HPV16) cellular transformation as well as BPV DNA replication through the activity of Rep (15, 20, 22). In vivo, an inverted statistical correlation was observed between the occurrence of cervical cancer and the levels of anti-AAV antibodies in serum (45). Finally, it was reported that AAV2 particles could be detected in cervical biopsies, demonstrating the possible colocalization of both AAV2 and HPV in the same tissues in vivo (14, 69, 75, 76). Despite this large body of evidence, few insights are available to date about the molecular mechanisms by which AAV2 inhibits HPV replication and gene expression. Recent data indicate that Rep78 may directly bind the papillomavirus DNA upstream regulatory region (URR), exerting its inhibitory activities by preventing the accessibility of the URR sequence to other cognate factors (80). Furthermore, Rep78 has also been shown to disrupt the binding of the TATA box-binding protein (TBP) to the TATA box of the p97 promoter of HPV16 (65).
Papillomavirus DNA replication has an absolute requirement for two virus-encoded proteins, E1 and E2 (12, 72, 79). E1 is a phosphoprotein that has ATPase and helicase activities (8, 27, 60) and that binds the origin of replication (ori) within the viral URR with a low affinity (5, 66, 77). E2 binds E1 and the origin of replication at specific sites and strengthens the E1-ori interaction by forming an E1-E2-ori ternary complex (50, 59). E2 is also a transcription factor involved in the modulation of viral promoter activity (6, 55). The protein can be divided into two functional domains separated by a hinge motif (17). The N-terminal transactivation domain is believed to recruit transcription factors to ori and to the promoter, while the C-terminal domain binds the responsive elements present in the URR and is required for dimerization. The transcription and replication activities of E2 are mediated by its interactions with several cellular proteins. These proteins include cellular transcription factor Sp1 (37), TBP (63), and the recently described transcriptional regulator AMF-1 (9). Of particular interest is the recent observation that the N terminus of E2 also interacts with the CREB-binding protein (CBP) (35) which, together with its closely related homologue p300, is a multifunctional transcriptional coactivator involved in the regulation of several cellular and viral transcription factors.
Here we show that both HPV16 DNA replication and HPV16 E2 (16E2)-driven transcriptional activation are inhibited by all four forms of AAV2 Rep. The inhibition of E2-dependent transcriptional activation involves the binding of Rep to the N-terminal activation domain of 16E2, resulting in the specific displacement of p300 from this region.
MATERIALS AND METHODS
Plasmids.
Plasmid phisRep68, a derivative of pET-16b (Novagen, Milwaukee, Wis.) used for the expression of N-terminally His-tagged Rep68, was obtained from M. Linden (Mt. Sinai School of Medicine, New York, N.Y.). phis-Rep78 was derived from phis-Rep68 by remodeling of the 3′ end of the gene. pGEX-R68N expressing glutathione S-transferase (GST) fused to the N termini of Rep78 and Rep68 (amino acids [aa] 1 to 224) was obtained by PCR. pGEX-Rep52 and pGEX-Rep40 were also obtained by PCR and express GST fused to Rep52 and Rep40, respectively. pcDNA3-Rep78 and pcDNA3-Rep68 were obtained by subcloning of Rep78 and Rep68 from phis-Rep78 and phis-Rep68, respectively, into pcDNA3.1 (Invitrogen, Carlsbad, Calif.). pcDNA3-Rep52 and pcDNA3-Rep40 were obtained by subcloning of the 3′ ends of the genes from the unique SacI sites present within Rep78 and Rep68, respectively, into pcDNA3.1. Plasmids pSP6-16E2, pSP6-16E2Δ156–159, and pSP6-16E1, used for in vitro transcription and translation of 16E2, 16E2Δ156–159 (a mutant 16E2 protein lacking aa 156 to 159 within the activation domain of 16E2), and 16E1, respectively, as well as pGEX2T-16E2, pGEX2T-16E2N, and pGEX2T-16E2C, used for expression and purification of GST-based fusion proteins, were described previously (64). pTKM.32 was obtained from F. Thierry (68). p16URR:TK:CAT, pJ4Ω.16E2, and pCGE1BΔE5 were described previously (6, 57). pCMVβp300 and pcDNA3-AMF-1 were kind gifts from D. M. Livingston (Dana Farber Cancer Institute, Boston, Mass.) and E. J. Androphy (Tufts University School of Medicine), respectively. pcDNA3-p300 was described previously (43). Plasmid pEGFP-N1, containing the enhanced green fluorescent protein (EGFP) under the control of the cytomegalovirus (CMV) promoter, was purchased from Clontech (Palo Alto, Calif.).
HPV16 replication assays.
Transient replication assays were performed with 293 cells transfected by the calcium phosphate method with 1 μg of the pCGE1BΔE5 vector, expressing 16E1 and 16E2, together with 3 μg of the replicon p16URR:TK:CAT and 3 μg of the various forms of Rep. Three days posttransfection, low-molecular-mass DNA was isolated by the Hirt extraction procedure. Samples were digested overnight with DpnI to remove the unreplicated input methylated DNA. Total digestion products were separated on an 0.8% agarose gel, blotted on Hybond-N+ (Amersham International plc, Little Chalfont, United Kingdom), and subsequently hybridized to a 32P-labeled replicon probe generated by random priming as previously described (57).
E2-dependent transcription assay.
U2OS cells were transfected by the calcium phosphate method with reporter plasmid p16URR:TK:CAT or pTKM.32 together with the 16E2-expressing construct pJ4Ω.16E2, the various forms of Rep, and p300 (pCMVβp300). Chloramphenicol acetyltransferase (CAT) assays were routinely performed with 1 to 5 μg of total protein extract, estimated by the Bio-Rad (Richmond, Calif.) protein assay as previously described (6). Following extraction with ethyl acetate, samples were analyzed by thin-layer chromatography and quantified with an Instant Imager (Packard, Meriden, Conn.). Transfection efficiencies were monitored by transfecting a LacZ expression plasmid on parallel plates.
Expression and purification of His and GST fusion proteins.
Exponentially growing cultures of Escherichia coli BL21(DE3)pLysS* (Promega, Madison, Wis.), harboring phis-Rep68, were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h at 30°C. Bacterial pellets were resuspended in lysis buffer (10 mM phosphate buffer [pH 8], 500 mM NaCl, 1% Tween 20, 5% glycerol, 10 mM β-mercaptoethanol), frozen-thawed three times, and sonicated. Cleared lysates were loaded on Ni-nitrilotriacetic acid (NTA) beads (Qiagen GmbH, Hilden, Germany) and thoroughly washed with lysis buffer at pH 6. His-Rep68 was eluted with the same buffer containing 600 mM imidazole, extensively dialyzed in 300 mM NaCl–10 mM Tris-HCl (pH 8), and kept frozen until use.
All other fusion proteins were immobilized on beads and kept frozen in lysis buffer until use. Briefly, bacterial cultures were induced as described for His-Rep68, and pellets were lysed in 20 mM Tris-HCl (pH 7.6)–500 mM NaCl–10% glycerol–0.02% Triton X-100, washed extensively, and resuspended at 50% (vol/vol) in the same buffer.
Coimmunoprecipitation.
Expression plasmids pJ4Ω.16E2 and pcDNA3-Rep68 were cotransfected in 293 cells by the calcium phosphate method. Cells were lysed in radioimmunoprecipitation assay buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS] phosphate-buffered saline plus protease inhibitors) on ice for 5 min and disrupted by repeated aspiration through a 21-gauge needle. One milliliter of whole-cell lysate was precleared with 5 μl of serum from a control rabbit together with 50 μl of a 50% slurry of protein A beads (Amersham). E2 protein was immunoprecipiated with 5 μl of a rabbit antiserum raised against GST-E2. Beads were washed three times with radioimmunoprecipitation assay buffer and heated in sample buffer before being loaded on an SDS–8% polyacrylamide gel. Proteins were blotted on Hybond-C (Amersham) and immunoblotted with a Rep-specific rabbit serum generously provided by J. Kleinschmidt (DFKZ, Heidelberg, Germany) by use of an ECL kit from Amersham.
Transcription-translation of proteins and in vitro binding assay.
35S-labeled proteins were produced in vitro by using a coupled transcription-translation system (Promega TNT) according to the manufacturer's instructions. Recombinant proteins immobilized on beads were pretreated with 0.25 U of DNase I per μl and 0.2 μg of RNase I per μl for 1 h at 25°C in 50 mM Tris-HCl (pH 8)–5 mM MgCl2–2.5 mM CaCl2–100 mM NaCl–5% glycerol–1 mM dithiothreitol to remove bacterial nucleic acids. The proteins were subsequently washed twice with 1 M NaCl, equilibrated with NETN buffer (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) supplemented with 0.2 mg of ethidium bromide per ml to reduce further nonspecific interactions between proteins and residual bacterial DNA, and resuspended in the same buffer. Labeled in vitro-translated (IVT) proteins (200 to 500 cpm) were added to 1 to 5 μg of proteins immobilized on beads in a final volume of 50 μl. The reaction mixture was incubated for 1 h at 4°C on a rotating wheel, and the beads were subsequently washed three times with NETN buffer supplemented with ethidium bromide, three times with NETN buffer, and once with 150 mM NaCl–10 mM Tris-HCl (pH 8). The beads were then heated in Laemmli buffer and separated by SDS-polyacrylamide gel electrophoresis. Dried gels were quantified with Instant Imager.
RESULTS
HPV16 replication is inhibited by the C terminus of AAV2 Rep proteins.
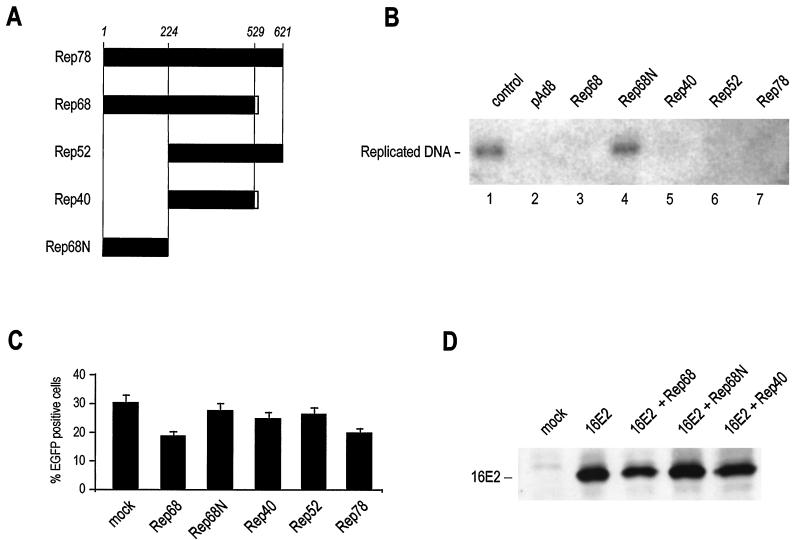
In order to assess whether the replication of oncogenic HPV16 DNA is inhibited by the Rep proteins of AAV, as observed for BPV (22), we analyzed the extent of replication of an HPV16 ori-containing plasmid in the presence of different Rep variants. Transfection of human 293 cells with the viral URR together with a plasmid encoding 16E1 and 16E2 resulted in the formation of newly replicated viral DNA which could be distinguished from the input plasmid by DpnI digestion followed by Southern blotting (Fig. 1B, lane 1). Cotransfection of pAd-8, which contains the entire AAV-2 Rep and Cap open reading frames, resulted in the complete inhibition of viral replication (Fig. 1B, lane 2). The AAV-2 Rep open reading frame gives rise to four overlapping proteins, as shown in Fig. 1A. Cotransfection of each of these variants also resulted in the complete inhibition of HPV DNA replication (Fig. 1B), indicating that the inhibitory effect resided in a C-terminal region common to all four Rep proteins. Accordingly, the first N-terminal 224 amino acids of Rep (Rep68N), which are shared only by Rep78 and Rep68, did not inhibit HPV DNA replication (Fig. 1B, lane 4).
FIG. 1.
Rep proteins inhibit HPV16 replication. (A) Schematic representation of Rep proteins from AAV2. AAV2 has four overlapping Rep proteins: the two major products Rep78 and Rep68 and the two minor variants Rep52 and Rep40, which represent the C-terminal portions of Rep78 and Rep68, respectively. In this work, the N terminus of Rep78 or Rep68 (amino acids 1 to 224) was also expressed independently and termed Rep68N. All constructs were either used as GST fusion proteins in the in vitro binding studies or transiently expressed from a CMV promoter in cells. (B) Rep proteins inhibit HPV16 replication. 293 cells were transfected with an HPV16 ori-containing plasmid together with 16E1, 16E2, and the indicated Rep variants. Low-molecular-weight DNA was digested with DpnI, and replicated DNA was detected by Southern blot hybridization. (C) Short-term Rep expression does not induce nonspecific cellular toxicity. To verify that the observed inhibition of HPV16 replication by the Rep forms shown in panel B was not related to nonspecific cytotoxic effects, the indicated Rep plasmids were transfected with 1 μg of plasmid pEGFP-N1, expressing EGFP under the control of the CMV promoter. The number of fluorescent cells was measured after 48 h. Experiments were performed in triplicate; the mean and standard deviation values are shown for each point. (D) Changes in 16E2 expression do not account for Rep-induced inhibition of HPV16 replication. Western blotting experiments with anti-16E2 antibodies were performed 48 h after transfection of the indicated Rep forms.
Two control experiments were performed to assess the potential interference of the Rep proteins, particularly of the larger variants Rep78 and Rep68, with the HPV DNA replication assay. In the first experiment, we transfected the same amounts of Rep variants with 1 μg of a plasmid expressing EGFP under the control of the CMV promoter and measured the number of fluorescent cells by flow cytometry at 48 h after transfection. As shown in Fig. 1C, EGFP expression was readily detected in all the experimental samples and was only modestly decreased (less than 30%) in cultures transfected with Rep78 and Rep68 but not in those expressing Rep40, in which HPV DNA replication was completely inhibited. In the second control experiment, we assessed by immunoblotting the expression of 16E2 coexpressed with Rep68, Rep68N, and Rep40 (Fig. 1D). Again, only Rep68 slightly reduced the expression of 16E2.
These results indicate that an activity exerted by the C terminus of Rep and common to all four variants negatively interferes with HPV 16 DNA replication and that this inhibition does not result from a nonspecific effect of Rep on cell metabolism or expression of the transfected genes.
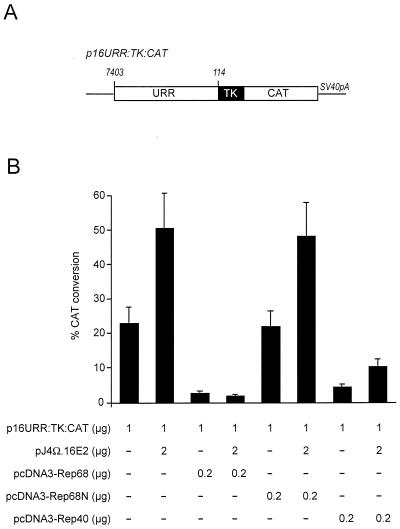
Rep68 and Rep40 inhibit 16E2-mediated transcription from the HPV16 URR.
Since viral DNA replication is dependent upon both the viral E1 and the viral E2 proteins, we next investigated the effects of Rep upon E2 transcriptional activity. Cells were cotransfected with a 16E2 expression plasmid together with the different Rep expression plasmids in the presence of an E2-responsive promoter comprising the HPV16 enhancer linked to the herpes simplex virus type 1 thymidine kinase promoter (Fig. 2A). Expression of both Rep68 and Rep40 inhibited 16E2-mediated transactivation of this promoter (Fig. 2B). Both Rep proteins also inhibited basal expression of this promoter, suggesting that they interfere with a pathway of transcriptional activation which is common to both the basal and the 16E2-induced promoters. In keeping with the DNA replication data, the N-terminal portion of Rep78 or Rep68 inhibited neither 16E2-mediated transactivation nor basal promoter activity.
FIG. 2.
Inhibition of 16E2 transcriptional activation function by Rep68 and Rep40. (A) Schematic representation of the reporter construct for CAT assays. The CAT reporter gene is under the control of the thymidine kinase (TK) promoter and is followed by the simian virus 40 polyadenylation site (SV40pA). The E2-responsive enhancer consists of the HPV16 URR from nucleotides 7403 to 114 (6). (B) CAT assay. The E2-dependent CAT reporter was cotransfected in U2OS cells with or without a 16E2-expressing vector, as indicated. Full-length Rep68 as well as Rep40 inhibited basal and E2-mediated stimulation of CAT activity. Expression of the N terminus of Rep68 or Rep78 did not show any inhibitory activity. The data represent the average of three independent experiments and are reported as means and standard deviations.
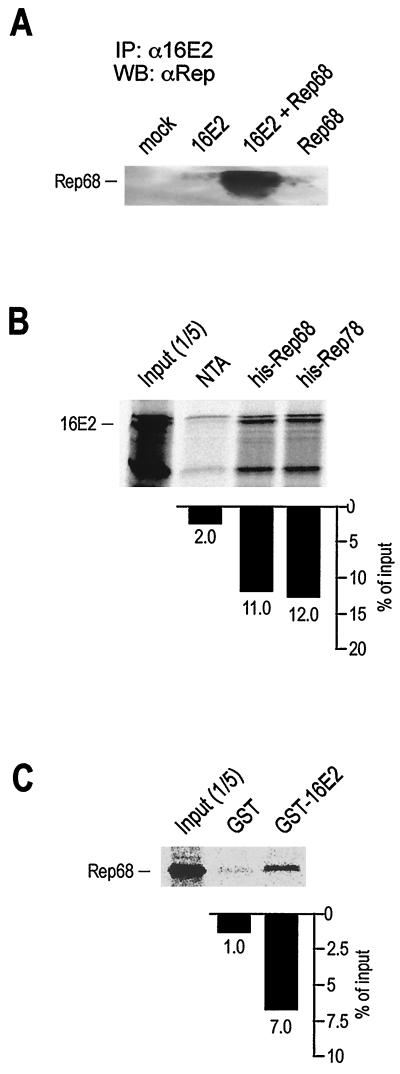
Rep proteins associate with 16E2.
The marked inhibition of 16E2-dependent replication and transcription activities shown in Fig. 1 and 2 indicates that a specific effect on E2 activity is restricted to the C terminus of Rep. To assess the possibility that Rep could physically associate with E2, a coimmunoprecipitation study was carried out (Fig. 3A). A plasmid expressing 16E2 was transiently cotransfected with a Rep68 expression plasmid in 293 cells. Negative controls included cells either mock transfected or transfected with each single construct. Selective immunoprecipitation of E2 from the cell lysate resulted in the coprecipitation of Rep68, thus indicating that 16E2 and Rep68 associate inside these cells. To further explore the interaction between 16E2 and Rep, the association of 35S-labeled IVT 16E2 with recombinant His-Rep68 immobilized on Ni-NTA beads was measured in pulldown assays. In each of these experiments, we used a fixed amount of IVT protein and a matched amount of each fusion protein. IVT 16E2 strongly bound to His-Rep78 and His-Rep68 (Fig. 3B), compared to Ni-NTA alone. To avoid nonspecific binding, the beads were treated with nucleases and washed at a high salt concentration prior to the binding assays (see Materials and Methods). In addition, the intercalating agent ethidium bromide was also used during the incubation step to disrupt any residual nonspecific protein-nucleic acid interaction. To further confirm the specificity of the interaction between 16E2 and Rep68, a GST-16E2 fusion protein was immobilized on glutathione beads and used to pull down IVT Rep68. Consistent with the results of Fig. 3B, IVT Rep68 strongly associated with GST-16E2 (Fig. 3C).
FIG. 3.
16E2 binds to Rep. (A) 16E2 and Rep68 coimmunoprecipitate in vivo. 293 cells were transfected with expression constructs for 16E2 and Rep68 alone or in combination, as indicated. At 48 h after transfection, cleared lysates were immunoprecipitated with an anti-16E2 antiserum as described in Materials and Methods. Bound proteins were washed and loaded onto an SDS–10% polyacrylamide gel. The gel was blotted on Hybond-C membranes and probed with an anti-Rep antiserum. IP, immunoprecipitation; WB, Western blotting; α, antiserum. (B) Rep68 and Rep78 expressed as His-tagged fusion proteins bind IVT 16E2. Each binding reaction mixture contained 2 to 5 μg of His fusion protein immobilized on Ni-NTA beads and 35S-labeled IVT 16E2 in NETN buffer. The percentage of bound 16E2 is indicated. (C) 16E2 expressed as a GST fusion protein binds IVT Rep68. Each binding reaction mixture contained 2 to 5 μg of GST-16E2 immobilized on gluthatione-CL4 beads and 35S-labeled IVT Rep68 in NETN buffer. After binding at 4°C, beads were extensively washed prior to being loaded onto an SDS–10% polyacrylamide gel.
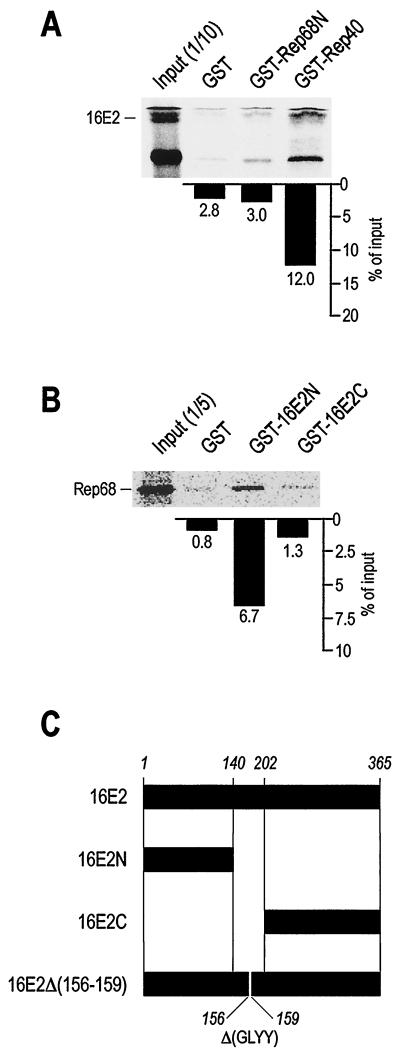
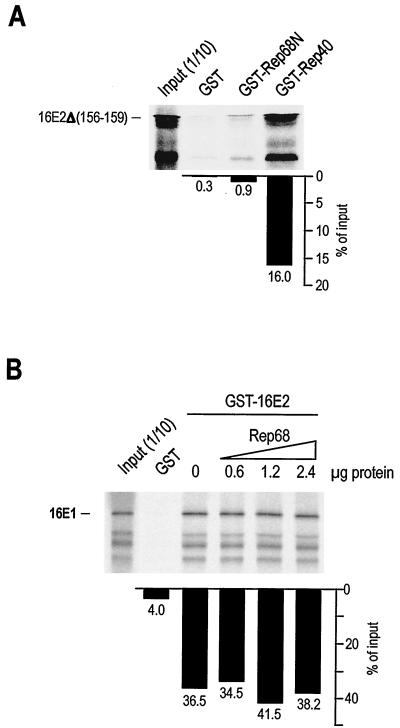
The C terminus of Rep68 (Rep40) binds to the N terminus of 16E2.
To characterize in more detail the interaction of Rep68 with 16E2, we separately cloned and expressed the N-terminal portion of the Rep protein (aa 1 to 224), common to the two major proteins Rep78 and Rep68, and the C-terminal portion, corresponding to Rep40 (aa 225 to 536 of Rep68) (Fig. 1A). 16E2 was also split into two portions, an N-terminal region (aa 1 to 140), corresponding to the transactivation domain, and a C-terminal region (aa 202 to 365), corresponding to the ori-binding and dimerization domains (Fig. 4C). As shown in Fig. 4A and B, the C terminus of Rep68 (Rep40) strongly associates with the N terminus of 16E2 in GST pulldown assays. Taken together, these results indicate that the N-terminal transactivation domain of 16E2 associates with a C-terminal region of Rep which is common to all four Rep proteins.
FIG. 4.
Interaction between 16E2 and Rep involves the N terminus of 16E2 and the C terminus of Rep. (A) IVT 16E2 associates with GST-Rep40 but not with GST-Rep68N or GST. (B) IVT Rep68 associates with GST-16E2N but not with 16E2C or GST. Binding reactions in panels A and B were carried out as described in the legend to Fig. 3. (C) Schematic representation of 16E2 and the constructs used in this study. The N-terminal activation domain (amino acids 1 to 140) and the C-terminal DNA binding and dimerization domain (amino acids 202 to 365) were used as GST fusion proteins and as IVT products. A 16E2 deletion mutant (amino acids 156 to 159) which has lost the ability to bind 16E1 is also indicated [16E2Δ(156–159)].
Binding of Rep to 16E2 does not disrupt the interaction of 16E1 with 16E2.
The possibility existed that the interaction of Rep with the transactivation domain of 16E2 disrupted 16E1 binding, thus affecting efficient replication of viral DNA. To address this possibility, we investigated whether a mutant 16E2 protein lacking a region within the activation domain of 16E2 (aa 156 to 159) which is required for efficient binding to 16E1 (16E2Δ156–159) (64) was capable of associating with Rep68. As shown in Fig. 5A, 16E2Δ156–159 was capable of binding GST-Rep40 as efficiently as the wild-type protein (compare Fig. 5A with Fig. 4A). Furthermore, since other domains within the N terminus of 16E2 have been described to be involved in the interaction with 16E1 (25, 56), we conducted an experiment where the interaction of IVT 16E1 with GST-16E2 was challenged with increasing amounts of recombinant His-Rep68. As shown in Fig. 5B, increasing concentrations of Rep68 did not disrupt the interaction of 16E2 with 16E1. These data appear to rule out the possible involvement of 16E1 in the mechanism of Rep inhibition of HPV DNA replication and favor the hypothesis that Rep may inhibit the interaction of the activation domain of 16E2 with other cellular factors that are required for efficient replication of HPV16 DNA and for E2-mediated transcriptional activation.
FIG. 5.
Binding of Rep to 16E2 does not interfere with the E2-E1 interaction. (A) IVT 16E2Δ156–159, a mutant 16E2 form with a four-amino-acid deletion which impairs its capacity to bind 16E1, associates in vitro with GST-Rep40 but not with GST-Rep68N or GST. Binding reactions were carried out as described in the legend to Fig. 3. (B) The association of GST-16E2 with IVT 16E1 is not affected by increasing concentrations of recombinant Rep68. The binding reaction was carried out as described in the legend to Fig. 3, except for the addition of increasing concentrations of affinity-purified His-Rep68 protein.
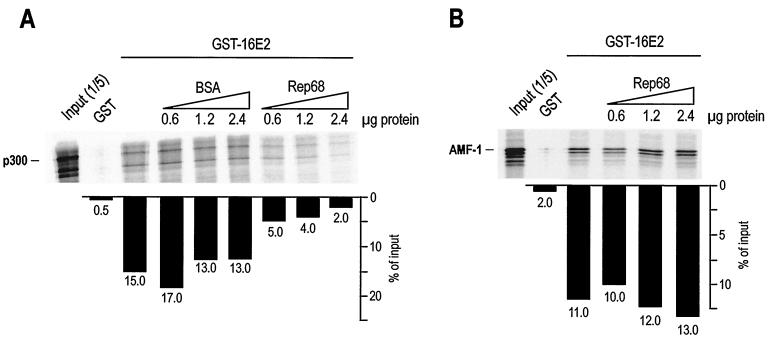
AAV Rep68 disrupts the binding of 16E2 to p300.
The N-terminal activation domain of 16E2 is a region highly conserved among the 86 known papillomavirus types. Recently, it has been reported that this region interacts with the transcriptional coactivator CBP. This nuclear protein and its closely related homologue p300 act at several cellular and viral promoters by coactivating transcription and promoting chromatin remodeling through intrinsic histone acetyltransferase activity (2, 13, 39, 53). Recruitment of CBP also has been shown recently to mediate transcriptional activation by 16E2 (35). Given these considerations, we explored the possibility that Rep could functionally and physically interfere with the interaction between 16E2 and p300. As shown in Fig. 6A, IVT full-length p300 specifically associated with GST-16E2 on beads. Increasing concentrations of free recombinant His-Rep68 as opposed to bovine serum albumin control could disrupt this interaction. Inhibition of the 16E2-p300 interaction by Rep68 was specific, since another protein which interacts with the same domain of 16E2, the cellular AMF-1 factor (9), could not be displaced from 16E2 on beads by the same concentrations of Rep. In the same set of experiments, we were not able to detect a direct association of Rep68 with p300 (data not shown). Although we cannot formally exclude the possibility that other components in the IVT mixture mediated binding, the above-reported data suggest a model by which the association of 16E2 with p300 is specifically disrupted by AAV Rep68 binding to the N terminus of 16E2.
FIG. 6.
Rep disrupts the binding of 16E2 to p300. (A) Increasing concentrations of Rep68 disrupt the association of GST-16E2 with IVT p300. The binding reaction was carried out as described in the legend to Fig. 5B. The same concentrations of bovine serum albumin (BSA) were used as a control. (B) Increasing concentrations of Rep68 do not disrupt the association of GST-16E2 with IVT AMF-1, a cellular factor known to associate with the N terminus of E2. The binding reaction was carried out as described in the legend to Fig. 5B.
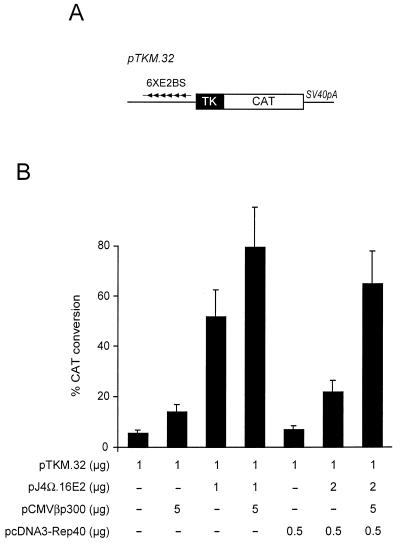
p300 rescues 16E2 transcriptional activity from Rep40 inhibition.
Since p300 plays a role in E2 transcriptional activation and having shown that Rep can inhibit the association between E2 and p300, we proceeded to investigate whether ectopic expression of p300 could overcome the inhibitory effects of Rep. Cells were transfected with a minimal promoter containing six E2-binding sites (Fig. 7A) together with expression vectors for p300 and/or Rep40. In agreement with previous observations, coexpression of p300 with 16E2 markedly increased 16E2 transcriptional activity. This effect was E2 dependent, since p300 alone had a minimal effect on the basal activity of the promoter. In addition, Rep40 dramatically inhibited E2 transcriptional activity, in agreement with the results shown in Fig. 2. Coexpression of p300 with Rep40 overcame Rep40 inhibition of E2 transcriptional activity. These results demonstrate that the most likely mechanism by which Rep inhibits E2 transcriptional activity is through inhibition of E2-p300 complex formation, which in turn can be overcome by ectopic expression of p300.
FIG. 7.
Rep inhibits p300-mediated stimulation of E2 transactivation. (A) Schematic representation of the reporter construct. The pTKM.32 construct contains only six E2-binding sites (6XE2BS) and a thymidine kinase (TK) promoter upstream of the CAT reporter gene followed by the simian virus 40 polyadenylation site (SV40pA) (68). (B) CAT assay. Cells transfected with the minimal E2-responsive promoter shown in panel A were cotransfected with plasmids expressing E2, Rep40, and p300, as indicated. Rep40 inhibited E2 activity, an effect that could be rescued by cotransfecting p300. Data are reported as means and standard deviations.
DISCUSSION
Several lines of evidence indicate the existence of a bidirectional interplay between the oncogenic papillomavirues and AAV. While papillomaviruses might serve as helpers for AAV replication (54, 75), AAV inhibits BPV replication and HPV16 and HPV18 oncogenic transformation (20, 24). The interaction between these two types of viruses probably has an important consequence at the clinical level. Both papillomaviruses and AAV infect cells in the anogenital tract (14, 40, 69, 76); however, while the former are positively associated with the development of cervical cancer, the latter appears to be a protective factor for this disease (15, 45, 62). In addition to these clinical considerations, the study of the molecular basis of the interaction between these two types of viruses can aid in an understanding of the respective molecular mechanisms of DNA replication and gene expression.
In AAV, the major players in the down-regulation of papillomavirus replication and transformation are the nonstructural Rep proteins (20, 22). The initial aim of this study was to gain insight into the mechanisms of Rep-mediated inhibition of human oncogenic HPV16 replication. We initially found that all four forms of Rep were capable of inhibiting HPV16 DNA replication, thus restricting this activity within the Rep40 product. As shown in Fig. 1C and D, the expression of both 16E1 and 16E2 in these experiments was driven by the CMV immediate-early promoter, which is only marginally affected by Rep (19, 58, 78). It may therefore be concluded that the effects of Rep were actually on DNA replication itself and not on the expression of the two HPV replicative proteins. Recent data suggest that Rep78 is able to physically interact with the HPV16 URR by binding a 44-bp region that includes functional Sp1- and E2-binding motifs, as well as part of the origin of replication (80). This interaction was reported to be dependent on the integrity of the N-terminal region of Rep. Similarly, Rep68 and Rep78 and, to a lesser extent, Rep52 were also reported to inhibit the basal transcriptional activity of the HPV18 URR promoter (26). The mechanism of this inhibition was reported to involve the direct binding of Rep to the promoter DNA sequence or competition of Rep78 with the binding of TBP to the TATA box (65, 80).
These effects are clearly different from those observed here. In our conditions, inhibition of HPV DNA replication was also obtained by using Rep proteins truncated in the N-terminal domain and was fully reproducible with Rep40 and Rep52, corresponding to the C-terminal portions of Rep68 and Rep78, respectively. Accordingly, inhibition of URR transcription was also obtained with both Rep68 and Rep40, again indicating that this is a function of the C-terminal portion of Rep. In addition, these proteins inhibited not only the basal activity of the URR promoter but also specifically transactivation by the 16E2 protein. In contrast, the N terminus of Rep was inactive in both the DNA replication and the E2 transactivation assays. Furthermore, none of the Rep variants showed increased cytotoxic or cytostatic effects in a cell survival and colony formation assay with both 293 and U2OS cells (data not shown).
The inhibition of E2 transactivation by Rep40 suggested a physical interaction between the two proteins. Rep and E2 are indeed capable of interacting in vivo and in vitro, as shown by coimmunoprecipitation and pulldown experiments. In keeping with the functional results, when we mapped the domains of the two proteins involved in this interaction, we found that the C-terminal domain of Rep, namely, Rep40, was the minimal domain capable of associating with the N-terminal activation domain of 16E2. At least two experimental results indicated that the interaction between Rep and E2 does not interfere with the binding of E2 to E1. First, a mutant 16E2 protein (16E2Δ156–159) that was unable to associate with E1 in vitro was still fully competent for binding Rep. Second, the in vitro interaction between GST-16E2 and IVT E1 could not be disrupted by increasing amounts of recombinant Rep68. These data indicate that Rep-mediated inhibition of E2 activity involves the interaction of other factors with the activation domain of E2.
One of the factors recently identified as binding to the N-terminal domain of E2 is the human transcriptional coactivator p300 homologue CBP (35), a protein with histone acetyltransferase (2, 53) and factor acetyltransfrase (7, 18, 41, 44, 51) activities. p300 and its closely related homologue CBP are two evolutionary conserved proteins acting as molecular bridges between transcription factors and components of the basal transcriptional machinery (13, 39). In the last few years, a growing number of cellular transcription factors have been identified for their capacity to interact with these two proteins (for a recent review, see reference 10). Given the pivotal role of p300 or CBP in the control of gene expression, it is not surprising that several viruses encode proteins targeting the two factors. Besides HPV E2, among these viral products are adenovirus E1A (13), human T-cell leukemia virus type 1 Tax (16), human immunodeficiency virus type 1 Tat (43), and simian virus 40 large T antigen (1).
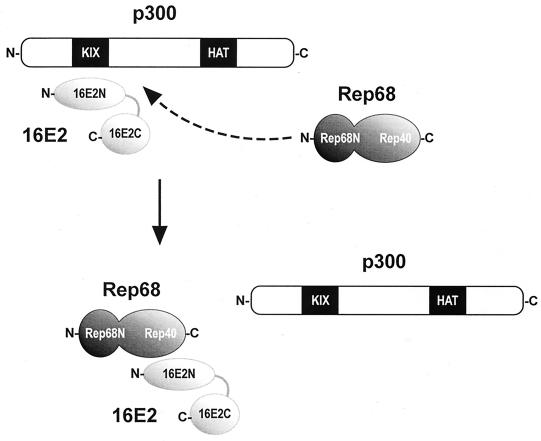
AAV Rep does not directly associate with p300 (data not shown) but clearly inhibits E2 functions by interfering with the recruitment of p300 by E2. In vitro, the association of 16E2 with p300 was specifically disrupted by purified Rep68; and in vivo, the inhibition of 16E2 transactivation by Rep68 and Rep40 could be rescued by overexpression of p300. A model can be envisaged where E2, along with E1, modulates chromatin structure at the URR of the viral genome by recruiting cellular macromolecular complexes that integrate transcription and replication activities. These complexes are likely to contain basal transcription and replication factors, chromatin remodeling factors, and transcriptional coactivators (31, 42, 67). Among those, E1 has already been shown to associate with the ini1-hSNF5 component of the ATP-dependent SWI-SNF chromatin remodeling complex (36). In this context, the role of AAV2 Rep appears to be that of a molecular dissector capable of affecting both the replication and the transcription activities of E2 by disrupting its interaction with p300 (Fig. 8).
FIG. 8.
A model for Rep-mediated inhibition of E2 activity. The N-terminal activation domain of 16E2 recruits the transcriptional coactivator p300 through its KIX domain (35). AAV2 Rep proteins that bind 16E2 specifically disrupt this interaction. Rep-mediated squelching of the 16E2-p300 interaction accounts for the inhibition of E2-mediated transactivation and HPV16 replication.
How do the findings described here integrate in a more extended model that could explain the general down-modulating activities of Rep for transcription from several cellular promoters as well as for cellular DNA replication? The involvement of Rep in a pathway involving p300 or CBP recruitment is intriguing in this respect, since many of the described down-modulating activities of Rep for general transcription and replication could be explained by interference with this pathway. Whether Rep, besides HPV 16E2, interacts with nuclear proteins whose activities are common to the transcription of cellular genes and chromosomal DNA replication remains a topic for future investigation.
ACKNOWLEDGMENTS
This work was supported by grants from Telethon Italy to M.G. (A.104), from Associazione Italiana per la Ricerca sul Cancro to L.B., and from the National Research Programme on AIDS of the Istituto Superiore di Sanità to A.M.
We thank E. J. Androphy for pcDNA3-AMF-1, M. Linden for phis-Rep68, F. Thierry for pTKM.32, D. M. Livingston for pCMVβp300, and J. Kleinschmidt for the antiserum against Rep. We are grateful to B. Boziglav and M. E. Lopez for excellent technical assistance.
REFERENCES
- 1.Avantaggiati M L, Carbone M, Graessmann A, Nakatani Y, Howard B, Levine A S. The SV40 large T antigen and adenovirus E1a oncoproteins interact with distinct isoforms of the transcriptional co-activator, p300. EMBO J. 1996;15:2236–2248. [PMC free article] [PubMed] [Google Scholar]
- 2.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 3.Bantel-Schaal U, zur Hausen H. Adeno-associated viruses inhibit SV40 DNA amplification and replication of herpes simplex virus in SV40-transformed hamster cells. Virology. 1988;164:64–74. doi: 10.1016/0042-6822(88)90620-4. [DOI] [PubMed] [Google Scholar]
- 4.Beaton A, Palumbo P, Berns K I. Expression from the adeno-associated virus p5 and p19 promoters is negatively regulated in trans by the rep protein. J Virol. 1989;63:4450–4454. doi: 10.1128/jvi.63.10.4450-4454.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blitz I L, Laimins L A. The 68-kilodalton E1 protein of bovine papillomavirus is a DNA binding phosphoprotein which associates with the E2 transcriptional activator in vitro. J Virol. 1991;65:649–656. doi: 10.1128/jvi.65.2.649-656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouvard V, Storey A, Pim D, Banks L. Characterization of the human papillomavirus E2 protein: evidence of trans-activation and trans-repression in cervical keratinocytes. EMBO J. 1994;13:5451–5459. doi: 10.1002/j.1460-2075.1994.tb06880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 8.Bream G L, Ohmstede C A, Phelps W C. Characterization of human papillomavirus type 11 E1 and E2 proteins expressed in insect cells. J Virol. 1993;67:2655–2663. doi: 10.1128/jvi.67.5.2655-2663.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breiding D E, Sverdrup F, Grossel M J, Moscufo N, Boonchai W, Androphy E J. Functional interaction of a novel cellular protein with the papillomavirus E2 transactivation domain. Mol Cell Biol. 1997;17:7208–7219. doi: 10.1128/mcb.17.12.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown C E, Lechner T, Howe L, Workman J L. The many HATs of transcription coactivators. Trends Biochem Sci. 2000;25:15–19. doi: 10.1016/s0968-0004(99)01516-9. [DOI] [PubMed] [Google Scholar]
- 11.Casto B C, Armstrong J A, Atchison R W, Hammon W M. Studies on the relationship between adeno-associated virus type 1 (AAV-1) and adenoviruses. II. Inhibition of adenovirus plaques by AAV; its nature and specificity. Virology. 1967;33:452–458. doi: 10.1016/0042-6822(67)90120-1. [DOI] [PubMed] [Google Scholar]
- 12.Chiang C M, Ustav M, Stenlund A, Ho T F, Broker T R, Chow L T. Viral E1 and E2 proteins support replication of homologous and heterologous papillomaviral origins. Proc Natl Acad Sci USA. 1992;89:5799–5803. doi: 10.1073/pnas.89.13.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 14.Friedman-Einat M, Grossman Z, Mileguir F, Smetana Z, Ashkenazi M, Barkai G, Varsano N, Glick E, Mendelson E. Detection of adeno-associated virus type 2 sequences in the human genital tract. J Clin Microbiol. 1997;35:71–78. doi: 10.1128/jcm.35.1.71-78.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georg-Fries B, Biederlack S, Wolf J, zur Hausen H. Analysis of proteins, helper dependence, and seroepidemiology of a new human parvovirus. Virology. 1984;134:64–71. doi: 10.1016/0042-6822(84)90272-1. [DOI] [PubMed] [Google Scholar]
- 16.Giebler H A, Loring J E, van Orden K, Colgin M A, Garrus J E, Escudero K W, Brauweiler A, Nyborg J K. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giri I, Yaniv M. Structural and mutational analysis of E2 trans-activating proteins of papillomaviruses reveals three distinct functional domains. EMBO J. 1988;7:2823–2829. doi: 10.1002/j.1460-2075.1988.tb03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 19.Heilbronn R, Burkle A, Stephan S, zur Hausen H. The adeno-associated virus rep gene suppresses herpes simplex virus-induced DNA amplification. J Virol. 1990;64:3012–3018. doi: 10.1128/jvi.64.6.3012-3018.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermonat P L. Adeno-associated virus inhibits human papillomavirus type 16: a viral interaction implicated in cervical cancer. Cancer Res. 1994;54:2278–2281. [PubMed] [Google Scholar]
- 21.Hermonat P L. Down-regulation of the human c-fos and c-myc proto-oncogene promoters by adeno-associated virus Rep78. Cancer Lett. 1994;81:129–136. doi: 10.1016/0304-3835(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 22.Hermonat P L. Inhibition of bovine papillomavirus plasmid DNA replication by adeno-associated virus. Virology. 1992;189:329–333. doi: 10.1016/0042-6822(92)90710-7. [DOI] [PubMed] [Google Scholar]
- 23.Hermonat P L, Labow M A, Wright R, Berns K I, Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984;51:329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermonat P L, Meyers C, Parham G P, Santin A D. Inhibition/stimulation of bovine papillomavirus by adeno-associated virus is time as well as multiplicity dependent. Virology. 1998;247:240–250. doi: 10.1006/viro.1998.9256. [DOI] [PubMed] [Google Scholar]
- 25.Hibma M H, Raj K, Ely S J, Stanley M, Crawford L. The interaction between human papillomavirus type 16 E1 and E2 proteins is blocked by an antibody to the N-terminal region of E2. Eur J Biochem. 1995;229:517–525. doi: 10.1111/j.1432-1033.1995.0517k.x. [DOI] [PubMed] [Google Scholar]
- 26.Horer M, Weger S, Butz K, Hoppe-Seyler F, Geisen C, Kleinschmidt J A. Mutational analysis of adeno-associated virus Rep protein-mediated inhibition of heterologous and homologous promoters. J Virol. 1995;69:5485–5496. doi: 10.1128/jvi.69.9.5485-5496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes F J, Romanos M A. E1 protein of human papillomavirus is a DNA helicase/ATPase. Nucleic Acids Res. 1993;21:5817–5823. doi: 10.1093/nar/21.25.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Im D S, Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell. 1990;61:447–457. doi: 10.1016/0092-8674(90)90526-k. [DOI] [PubMed] [Google Scholar]
- 29.Im D S, Muzyczka N. Partial purification of adeno-associated virus Rep78, Rep52, and Rep40 and their biochemical characterization. J Virol. 1992;66:1119–1128. doi: 10.1128/jvi.66.2.1119-1128.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotin R M, Siniscalco M, Samulski R J, Zhu X D, Hunter L, Laughlin C A, McLaughlin S, Muzyczka N, Rocchi M, Berns K I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon H, Imbalzano A N, Khavari P A, Kingston R E, Green M R. Nucleosome disruption and enhancement of activator binding by a human SW1/SNF complex. Nature. 1994;370:477–481. doi: 10.1038/370477a0. [DOI] [PubMed] [Google Scholar]
- 32.Kyostio S R, Owens R A, Weitzman M D, Antoni B A, Chejanovsky N, Carter B J. Analysis of adeno-associated virus (AAV) wild-type and mutant Rep proteins for their abilities to negatively regulate AAV p5 and p19 mRNA levels. J Virol. 1994;68:2947–2957. doi: 10.1128/jvi.68.5.2947-2957.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labow M A, Graf L H, Jr, Berns K I. Adeno-associated virus gene expression inhibits cellular transformation by heterologous genes. Mol Cell Biol. 1987;7:1320–1325. doi: 10.1128/mcb.7.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labow M A, Hermonat P L, Berns K I. Positive and negative autoregulation of the adeno-associated virus type 2 genome. J Virol. 1986;60:251–258. doi: 10.1128/jvi.60.1.251-258.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee D, Lee B, Kim J, Kim D W, Choe J. cAMP response element-binding protein-binding Protein binds to human papillomavirus E2 protein and activates E2-dependent transcription. J Biol Chem. 2000;275:7045–7051. doi: 10.1074/jbc.275.10.7045. [DOI] [PubMed] [Google Scholar]
- 36.Lee D, Sohn H, Kalpana G V, Choe J. Interaction of E1 and hSNF5 proteins stimulates replication of human papillomavirus DNA. Nature. 1999;399:487–491. doi: 10.1038/20966. [DOI] [PubMed] [Google Scholar]
- 37.Li R, Knight J D, Jackson S P, Tjian R, Botchan M R. Direct interaction between Sp1 and the BPV enhancer E2 protein mediates synergistic activation of transcription. Cell. 1991;65:493–505. doi: 10.1016/0092-8674(91)90467-d. [DOI] [PubMed] [Google Scholar]
- 38.Linden R M, Ward P, Giraud C, Winocour E, Berns K I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1996;93:11288–11294. doi: 10.1073/pnas.93.21.11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundblad J R, Kwok R P, Laurance M E, Harter M L, Goodman R H. Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature. 1995;374:85–88. doi: 10.1038/374085a0. [DOI] [PubMed] [Google Scholar]
- 40.Malhomme O, Dutheil N, Rabreau M, Armbruster-Moraes E, Schlehofer J R, Dupressoir T. Human genital tissues containing DNA of adeno-associated virus lack DNA sequences of the helper viruses adenovirus, herpes simplex virus or cytomegalovirus but frequently contain human papillomavirus DNA. J Gen Virol. 1997;78:1957–1962. doi: 10.1099/0022-1317-78-8-1957. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Balbas M A, Bauer U M, Nielsen S J, Brehm A, Kouzarides T. Regulation of E2F1 activity by acetylation. EMBO J. 2000;19:662–671. doi: 10.1093/emboj/19.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marzio G, Giacca M. Chromatin control of HIV-1 gene expression. Genetica. 1999;106:125–130. doi: 10.1023/a:1003797332379. [DOI] [PubMed] [Google Scholar]
- 43.Marzio G, Tyagi M, Gutierrez M I, Giacca M. HIV-1 tat transactivator recruits p300 and CREB-binding protein histone acetyltransferases to the viral promoter. Proc Natl Acad Sci USA. 1998;95:13519–13524. doi: 10.1073/pnas.95.23.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marzio G, Wagener C, Gutierrez M I, Cartwright P, Helin K, Giacca M. E2F family members are differentially regulated by reversible acetylation. J Biol Chem. 2000;275:10887–10892. doi: 10.1074/jbc.275.15.10887. [DOI] [PubMed] [Google Scholar]
- 45.Mayor H D, Drake S, Stahmann J, Mumford D M. Antibodies to adeno-associated satellite virus and herpes simplex in sera from cancer patients and normal adults. Am J Obstet Gynecol. 1976;126:100–104. doi: 10.1016/0002-9378(76)90472-5. [DOI] [PubMed] [Google Scholar]
- 46.McCarty D M, Christensen M, Muzyczka N. Sequences required for coordinate induction of adeno-associated virus p19 and p40 promoters by Rep protein. J Virol. 1991;65:2936–2945. doi: 10.1128/jvi.65.6.2936-2945.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarty D M, Ni T H, Muzyczka N. Analysis of mutations in adeno-associated virus Rep protein in vivo and in vitro. J Virol. 1992;66:4050–4057. doi: 10.1128/jvi.66.7.4050-4057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarty D M, Ryan J H, Zolotukhin S, Zhou X, Muzyczka N. Interaction of the adeno-associated virus Rep protein with a sequence within the A palindrome of the viral terminal repeat. J Virol. 1994;68:4998–5006. doi: 10.1128/jvi.68.8.4998-5006.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mendelson E, Trempe J P, Carter B J. Identification of the trans-acting Rep proteins of adeno-associated virus by antibodies to a synthetic oligopeptide. J Virol. 1986;60:823–832. doi: 10.1128/jvi.60.3.823-832.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohr I J, Clark R, Sun S, Androphy E J, MacPherson P, Botchan M R. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science. 1990;250:1694–1699. doi: 10.1126/science.2176744. [DOI] [PubMed] [Google Scholar]
- 51.Munshi N, Merika M, Yie J, Senger K, Chen G, Thanos D. Acetylation of HMG I(Y) by CBP turns off IFN beta expression by disrupting the enhanceosome. Mol Cell. 1998;2:457–467. doi: 10.1016/s1097-2765(00)80145-8. [DOI] [PubMed] [Google Scholar]
- 52.Ni T H, Zhou X, McCarty D M, Zolotukhin I, Muzyczka N. In vitro replication of adeno-associated virus DNA. J Virol. 1994;68:1128–1138. doi: 10.1128/jvi.68.2.1128-1138.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 54.Ogston P, Raj K, Beard P. Productive replication of adeno-associated virus can occur in human papillomavirus type 16 (HPV-16) episome-containing keratinocytes and is augmented by the HPV-16 E2 protein. J Virol. 2000;74:3494–3504. doi: 10.1128/jvi.74.8.3494-3504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phelps W C, Howley P M. Transcriptional trans-activation by the human papillomavirus type 16 E2 gene product. J Virol. 1987;61:1630–1638. doi: 10.1128/jvi.61.5.1630-1638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piccini A, Storey A, Massimi P, Banks L. Mutations in the human papillomavirus type 16 E2 protein identify multiple regions of the protein involved in binding to E1. J Gen Virol. 1995;76:2909–2913. doi: 10.1099/0022-1317-76-11-2909. [DOI] [PubMed] [Google Scholar]
- 57.Piccini A, Storey A, Romanos M, Banks L. Regulation of human papillomavirus type 16 DNA replication by E2, glucocorticoid hormone and epidermal growth factor. J Gen Virol. 1997;78:1963–1970. doi: 10.1099/0022-1317-78-8-1963. [DOI] [PubMed] [Google Scholar]
- 58.Rittner K, Heilbronn R, Kleinschmidt J A, Sczakiel G. Adeno-associated virus type 2-mediated inhibition of human immunodeficiency virus type 1 (HIV-1) replication: involvement of p78rep/p68rep and the HIV-1 long terminal repeat. J Gen Virol. 1992;73:2977–2981. doi: 10.1099/0022-1317-73-11-2977. [DOI] [PubMed] [Google Scholar]
- 59.Seo Y S, Muller F, Lusky M, Gibbs E, Kim H Y, Phillips B, Hurwitz J. Bovine papilloma virus (BPV)-encoded E2 protein enhances binding of E1 protein to the BPV replication origin. Proc Natl Acad Sci USA. 1993;90:2865–2869. doi: 10.1073/pnas.90.7.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seo Y S, Muller F, Lusky M, Hurwitz J. Bovine papilloma virus (BPV)-encoded E1 protein contains multiple activities required for BPV DNA replication. Proc Natl Acad Sci USA. 1993;90:702–706. doi: 10.1073/pnas.90.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Snyder R O, Im D S, Muzyczka N. Evidence for covalent attachment of the adeno-associated virus (AAV) rep protein to the ends of the AAV genome. J Virol. 1990;64:6204–6213. doi: 10.1128/jvi.64.12.6204-6213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sprecher-Goldberger S, Thiry L, Lefebvre N, Dekegel D, Halleux F D. Complement-fixation antibodies to adenovirus-associated viruses, cytomegaloviruses and herpes simplex viruses in patients with tumors and in control individuals. Am J Epidemiol. 1971;94:351–358. doi: 10.1093/oxfordjournals.aje.a121330. [DOI] [PubMed] [Google Scholar]
- 63.Steger G, Ham J, Lefebvre O, Yaniv M. The bovine papillomavirus 1 E2 protein contains two activation domains: one that interacts with TBP and another that functions after TBP binding. EMBO J. 1995;14:329–340. doi: 10.1002/j.1460-2075.1995.tb07007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Storey A, Piccini A, Massimi P, Bouvard V, Banks L. Mutations in the human papillomavirus type 16 E2 protein identify a region of the protein involved in binding to E1 protein. J Gen Virol. 1995;76:819–826. doi: 10.1099/0022-1317-76-4-819. [DOI] [PubMed] [Google Scholar]
- 65.Su P F, Chiang S Y, Wu C W, Wu F Y. Adeno-associated virus major Rep78 protein disrupts binding of TATA-binding protein to the p97 promoter of human papillomavirus type 16. J Virol. 2000;74:2459–2465. doi: 10.1128/jvi.74.5.2459-2465.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun S, Thorner L, Lentz M, MacPherson P, Botchan M. Identification of a 68-kilodalton nuclear ATP-binding phosphoprotein encoded by bovine papillomavirus type 1. J Virol. 1990;64:5093–5105. doi: 10.1128/jvi.64.10.5093-5105.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Syntichaki P, Topalidou I, Thireos G. The Gcn5 bromodomain coordinates nucleosome remodelling. Nature. 2000;404:414–417. doi: 10.1038/35006136. [DOI] [PubMed] [Google Scholar]
- 68.Thierry F, Dostatni N, Arnos F, Yaniv M. Cooperative activation of transcription by bovine papillomavirus type 1 E2 can occur over a large distance. Mol Cell Biol. 1990;10:4431–4437. doi: 10.1128/mcb.10.8.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tobiasch E, Rabreau M, Geletneky K, Larue-Charlus S, Severin F, Becker N, Schlehofer J R. Detection of adeno-associated virus DNA in human genital tissue and in material from spontaneous abortion. J Med Virol. 1994;44:215–222. doi: 10.1002/jmv.1890440218. [DOI] [PubMed] [Google Scholar]
- 70.Tratschin J D, Miller I L, Carter B J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984;51:611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tratschin J D, Tal J, Carter B J. Negative and positive regulation in trans of gene expression from adeno-associated virus vectors in mammalian cells by a viral rep gene product. Mol Cell Biol. 1986;6:2884–2894. doi: 10.1128/mcb.6.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ustav M, Stenlund A. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 1991;10:449–457. doi: 10.1002/j.1460-2075.1991.tb07967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker S L, Wonderling R S, Owens R A. Mutational analysis of the adeno-associated virus Rep68 protein: identification of critical residues necessary for site-specific endonuclease activity. J Virol. 1997;71:2722–2730. doi: 10.1128/jvi.71.4.2722-2730.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker S L, Wonderling R S, Owens R A. Mutational analysis of the adeno-associated virus type 2 Rep68 protein helicase motifs. J Virol. 1997;71:6996–7004. doi: 10.1128/jvi.71.9.6996-7004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walz C, Deprez A, Dupressoir T, Durst M, Rabreau M, Schlehofer J R. Interaction of human papillomavirus type 16 and adeno-associated virus type 2 co-infecting human cervical epithelium. J Gen Virol. 1997;78:1441–1452. doi: 10.1099/0022-1317-78-6-1441. [DOI] [PubMed] [Google Scholar]
- 76.Walz C M, Anisi T R, Schlehofer J R, Gissmann L, Schneider A, Muller M. Detection of infectious adeno-associated virus particles in human cervical biopsies. Virology. 1998;247:97–105. doi: 10.1006/viro.1998.9226. [DOI] [PubMed] [Google Scholar]
- 77.Wilson V G, Ludes-Meyers J. A bovine papillomavirus E1-related protein binds specifically to bovine papillomavirus DNA. J Virol. 1991;65:5314–5322. doi: 10.1128/jvi.65.10.5314-5322.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wonderling R S, Kyostio S R, Walker S L, Owens R A. The Rep68 protein of adeno-associated virus type 2 increases RNA levels from the human cytomegalovirus major immediate early promoter. Virology. 1997;236:167–176. doi: 10.1006/viro.1997.8724. [DOI] [PubMed] [Google Scholar]
- 79.Yang L, Li R, Mohr I J, Clark R, Botchan M R. Activation of BPV-1 replication in vitro by the transcription factor E2. Nature. 1991;353:628–632. doi: 10.1038/353628a0. [DOI] [PubMed] [Google Scholar]
- 80.Zhan D, Santin A D, Liu Y, Parham G P, Li C, Meyers C, Hermonat P L. Binding of the human papillomavirus type 16 p97 promoter by the adeno-associated virus Rep78 major regulatory protein correlates with inhibition. J Biol Chem. 1999;274:31619–31624. doi: 10.1074/jbc.274.44.31619. [DOI] [PubMed] [Google Scholar]