Abstract
Background
To date, it is not fully understood to what extent COVID-19 has burdened society in Japan. This study aimed to estimate the total disease burden due to COVID-19 in Japan during 2020–2021.
Methods
We stratify disease burden estimates by age group and present it as absolute Quality Adjusted Life Years (QALYs) lost and QALYs lost per 100,000 persons. The total estimated value of QALYs lost consists of (1) QALYs lost brought by deaths due to COVID-19, (2) QALYs lost brought by inpatient cases, (3) QALYs lost brought by outpatient cases, and (4) QALYs lost brought by long-COVID.
Results
The total QALYs lost due to COVID-19 was estimated as 286,782 for two years, 114.0 QALYs per 100,000 population per year. 71.3% of them were explained by the burden derived from deaths. Probabilistic sensitivity analysis showed that the burden of outpatient cases was the most sensitive factor.
Conclusions
The large part of disease burden due to COVID-19 in Japan from the beginning of 2020 to the end of 2021 was derived from Wave 3, 4, and 5 and the proportion of QALYs lost due to morbidity in the total burden increased gradually. The estimated disease burden was smaller than that in other high-income countries. It will be our future challenge to take other indirect factors into consideration.
Keywords: COVID-19, Disease burden, Japan
Introduction
Coronavirus disease 2019 (COVID-19) caused by the SARS-CoV-2 virus, has become a global health threat since the beginning of 2020 [1], [2], [3]. In Japan, it was first detected in early 2020 [4].
This emerging infectious disease became one of the most pressing concerns for the Japanese general population and the Ministry of Health, Labour and Welfare (MHLW) Japan in early 2020 and the Prime Minister of Japan declared the state of emergency on 7th April 2020 for seven prefectures including the Tokyo metropolitan area [5], [6], [7]. MHLW recommended to avoid Three Cs (Closed spaces, Crowded places, and Close-contact settings) to prevent COVID-19 transmission [8] and behaviour of the general population drastically changed. The number of healthcare facility visits and the consumption of antimicrobials decreased substantially after the emergence of COVID-19 in Japan. Previous studies reported that the number of outpatient visits decreased by 22% and antimicrobial use decreased by 21% in 2020 compared with 2019 [9], [10]. In short, the COVID-19 pandemic changed the Japanese.
way of life substantially.
To date, it is not fully understood to what extent this novel emerging disease has burdened society. A quantification of the observed burden, despite the great efforts that were made to minimise it, is a first step towards understanding how pandemic management can be improved.
Compared with other high-income countries (HICs), the cumulative incidence of COVID-19 cases, hospitalisations and deaths has been comparatively small in Japan, at least until the end of the year 2021[11]. For instance, the United Kingdom reported 952.6 cumulative hospitalizations per 100,000 population [12] and the United States reported 11,700.6 cumulative hospitalizations per 100,000 population at the end of 2021[13], while Japan reported 1,706.1 cumulative hospitalizations per 100,000 population in the same period. As for deaths, Japan reported lower rates (14.6 deaths per 100,000 population) compared with the UK and the US (218.1 and 247.8 deaths per 100,000 population, respectively) and the average of the world (69.1 deaths per 100,000 population) [14].
In order to learn from the crisis and be better prepared for future pandemics, we aim to assess the burden caused by COVID-19 in more detail. We can classify the disease burden directly caused by COVID-19 into four categories;.
-
(i)
Quality Adjusted Life Year (QALY) losses caused by fatal cases
-
(ii)
QALY losses caused by outpatient cases
-
(iii)
QALY losses caused by mild to severe inpatient cases
-
(iv)
QALY losses caused by long-COVID [15]
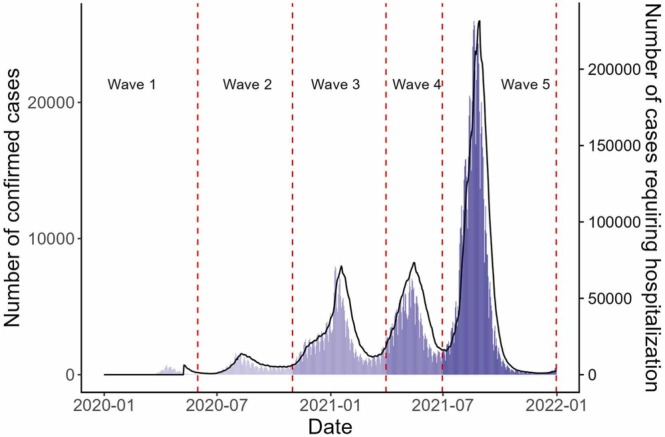
Since they can be both interpreted as indicators of pandemic management, here we aim to document both the cumulative and the chronological, per-wave, disease burden caused by COVID-19. The COVID-19 epidemic in Japan was characterised by five waves in 2020 and 2021. We adopted a previously proposed classification of waves observed in Japan [16], as follows (see also Fig. 2):
Fig. 2.
Daily number of confirmed COVID-19 cases and hospitalisations. Wave 1; 01/01/2020–05/31/2020, Wave 2; 06/01/2020–10/31/2020, Wave 3; 11/01/2020–03/31/2021, Wave 4; 4/1/2021–6/30/2021, Wave 5; 7/1/2021–12/31/2021. Bars represent the daily number of confirmed cases. The black line represents the number of cases requiring hospitalization. Vertical lines represent the delimitation of five epidemic waves.
(i) First wave (Wave 1), 01/01/2020–05/31/2020;.
(ii) Second wave (Wave 2), 06/01/2020–10/31/2020;.
(iii) Third wave (Wave 3), 11/01/2020–03/31/2021;.
(iv) Fourth wave (Wave 4), 4/1/2021–6/30/2021;.
(v) Fifth wave (Wave 5), 7/1/2021–12/31/2021.
The Japanese government had implemented different non-pharmaceutical interventions (NPIs) in each period and the guidelines for clinical management of COVID-19 cases grew gradually, implying the characteristics of the burden in each wave are expected to be different.
The main objective of this study is to assess the disease burden caused by COVID-19 in Japan between the beginning of 2020 and the end of 2021 in order to enable comparisons over time, with other diseases and with other countries.
Methods
Settings
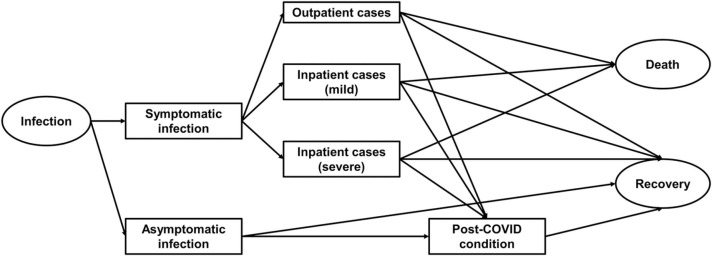
We constructed a progression pathway model of COVID-19 infection ( Fig. 1), in which two types of infection; symptomatic and asymptomatic, and three degrees of severity were defined; outpatient cases, inpatient cases (mild), and inpatient cases (severe). The definition of “severe” inpatient cases varied by prefecture because each prefecture defined the severity of inpatient COVID-19 cases by its own criteria. A large part of prefectures defined “severe” cases as patients admitted to an intensive care unit (ICU) or patients requiring mechanical ventilation. Additionally, we assumed that any type of symptomatic infection could lead to long-COVID [17], [18], [19]. In line with previous studies [17], [20], we defined long-COVID patients as presenting with COVID-19 symptoms for longer than four weeks from symptom onset. Outpatient cases were defined based on the cases reported to MHLW. Physicians and hospitals were required to report any case diagnosed as COVID-19 to local authorities and local authorities had to report the number of cases to MHLW. The outpatient cases we obtained from MHLW were basically patients with a definite diagnosis of COVID-19 that were not and did not need to be admitted to any healthcare facility. The numbers of both mild and severe inpatient cases were also extracted from MHLW reports. Inpatient cases include both hospitalised patients and patients who should have been, but could not be admitted, due to healthcare capacity and other reasons. These patients have convalesced in isolation, most often receiving on-site treatment in their own home. The final stage of each infected case was “Death” or “Recovery”. We assumed that all symptomatic infections (both outpatient and inpatient cases), including long-COVID episodes, and deaths contributed to the disease burden, whereas asymptomatic infections did not. The latter assumption is justified based on the fact that our study estimates disease burden observed over the past two years, does not apply a model to make forward projections, and estimates long-COVID cases by applying earlier derived proportions, which are defined relative to symptomatic cases.
Fig. 1.
Progression pathway diagram of COVID-19 infection.
We estimated the disease burden due to COVID-19 in Japan for the period from the beginning of 2020 to the end of 2021 because the first case of COVID-19 in Japan was detected on 15th January 2020 [4], and by the end of 2021 80.4% of the population had received their primary course of COVID-19 vaccination. Additionally, the less severe Omicron variant of concern (VOC), became dominant early in 2022, and its clinical, detection and epidemiological characteristics were quite different from other strains.
Data sources
We used open data sources for the daily number of confirmed cases and deaths[21]. Demographic data were sourced from official statistics [22]. Disutility of each health status was presented as QALYs lost per episode and defined according to values from the literature [20], [23], [24]. For instance, if a person got COVID-19 but was not admitted to any hospital and did not present long-COVID symptoms (i.e., just an outpatient case), his or her QALY loss was assumed to have a distribution with median 0.033 (see Table 1). We calculated the proportion of acute symptomatic COVID-19 cases that gives rise to long-COVID using the pooled data derived from meta-analyses [25], [26]. As a result, we estimated it 16.6% in adults and 3.9% in children. An overview of these parameters is shown in Table 1.
Table 1.
Details of parameters included in the model.
| Parameters (sample size) | Value | Distribution | Reference |
|---|---|---|---|
| Disutility (QALYs lost) per person | Median [IQR] | ||
| Outpatient case (n = 138) | 0.033 [0.017–0.059] | Lognormal (mean = −5.187, SD = 0.034) |
[23] |
| Inpatient case (n = 405) | 0.439 [0.420–0.457] | Normal (mean = 0.439, SD = 0.027) |
[24] |
| Long-COVID | 0.013 [0.007–0.029] | Lognormal (mean = −4.209, SD = 2.597) |
[20] |
| Proportion with long-COVID | |||
| In adults (n = 3,288) | 0.166 | Binomial | [25] |
|
In children (under 20, n = 580,467) |
0.039 | Binomial | [26] |
IQR: interquartile range
Estimation of disease burden
We stratify the disease burden estimates by age group and present it as absolute QALYs lost [27], [28] and QALYs lost per 100,000 persons. QALYs lost due to premature mortality were calculated as the remaining life expectancy at the time of death per fatal case, i.e., the number of life years lost (LYL). We used nine age groups, in accordance with available COVID-19 mortality statistics: < 10 years, 10–19 years, 20–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years, 80–89 years, and ≥ 90 years, and assumed deaths reported within a given age group occurred at the mid-point of the age interval, in line with previous studies [29], [30]. Since there is no information about the age of each fatal case and only the number of deaths in each age group was available, we used the mid-point of the age interval as the mean age of fatal cases in each age group to estimate the disease burden at population level. For instance, we assume that the mean age of fatal cases in 60–69 years was 64.5 years.
QALYs lost due to inpatient cases were calculated by multiplying the total number of mild/severe inpatient cases with the disutility per COVID-19 inpatient case. Similarly, QALYs lost due to outpatient and long-COVID cases were calculated by multiplying the total number of outpatient and long-COVID cases with the respective disutilities per case (see Table 1).
The total disease burden of COVID-19 was expressed as the sum of the above, i.e., the QALYs lost due to disease in inpatient cases, outpatient cases and due to premature deaths.
Two-sided p values of < 0.05 were considered to show statistical significance. All analyses were conducted by R, version 4.1.3 [31].
Sensitivity analysis
We conducted probabilistic sensitivity analysis to acknowledge uncertainty and examine the robustness of our results. We ran 1,000 simulations of the disease burden estimation with different sets of parameter values derived from the defined ranges and distributions. The range of each parameter, distribution, and the references we used to determine it are available in Table 1. We considered one year of life lost as one quality-unadjusted LYL in the main analysis. In sensitivity analysis, we estimated the quality-adjusted LYL according to the method described by Briggs et al. [32], using the population norm for health-related quality of life of Japan, as reported by Shiroiwa et al. [33].
Assessment of the influence of variables
To assess which variable in the model has larger impact in our model, the influence of each parameter on the total disease burden was evaluated by a linear regression analysis, with 1000 simulation results as an independent variable and 1000 parameter sets (i.e., “disutility of outpatient case”, “disutility of long COVID”, “disutility of inpatient case”, “proportion of Long COVID in adults”, and “proportion of Long COVID in children”) as dependent variables. Multicollinearity of variables was evaluated by variance inflation factor (VIF) and VIF > 3 was regarded as significant multicollinearity.
Ethics approval
All data used in this study are publicly available. As such, the datasets used in our study were de-identified and fully anonymized. Therefore, this study did not require specific ethical approval.
Results
In total, 1,728,228 COVID-19 cases were confirmed in Japan from the beginning of 2020 to the end of 2021. Among them, 232,495 cases were observed in 2020 and 1,495,733 cases in 2021. A relatively small number of cases was observed in Waves 1 and 2 (99,959 cases and 1,765 deaths) in Japan, and more than half of the total cases between 2020 and 2021 were observed in Wave 5 (931,393 cases), although the number of deaths in Wave 5 was comparatively small (3,762 deaths). Similarly, the maximum number of cases requiring hospitalization or any other medical care at their home or any accommodations in Wave 1 was 6,250, while in Wave 5 it was 231,596. Fig. 2 shows the epidemic curve of confirmed cases and number of cases requiring hospitalization or any other medical care at their home or any accommodations from 2020 to 2021. When the life years lost were adjusted by the age-specific population norm for health-related quality of life (as shown in the Supplementary file), the interpretation of these results did not change.
More than a half of total cases were observed in Wave 5 (931,393 cases). The Delta variant of concern (VOC) was dominant in this period[34], [35], as it outcompeted the less transmissible Alpha VOC in July 2021.
The number of fatal cases was 3,095 in 2020 and 14,520 in 2021. The case- fatality ratio (CFR) was 0.013 in 2020 and 0.0097 in 2021, respectively. The estimated average age of fatal cases was 80.2 (Wave 1 and 2), 82.3 (Wave 3), 81.5 (Wave 4), and 75.8 (Wave 5) years.
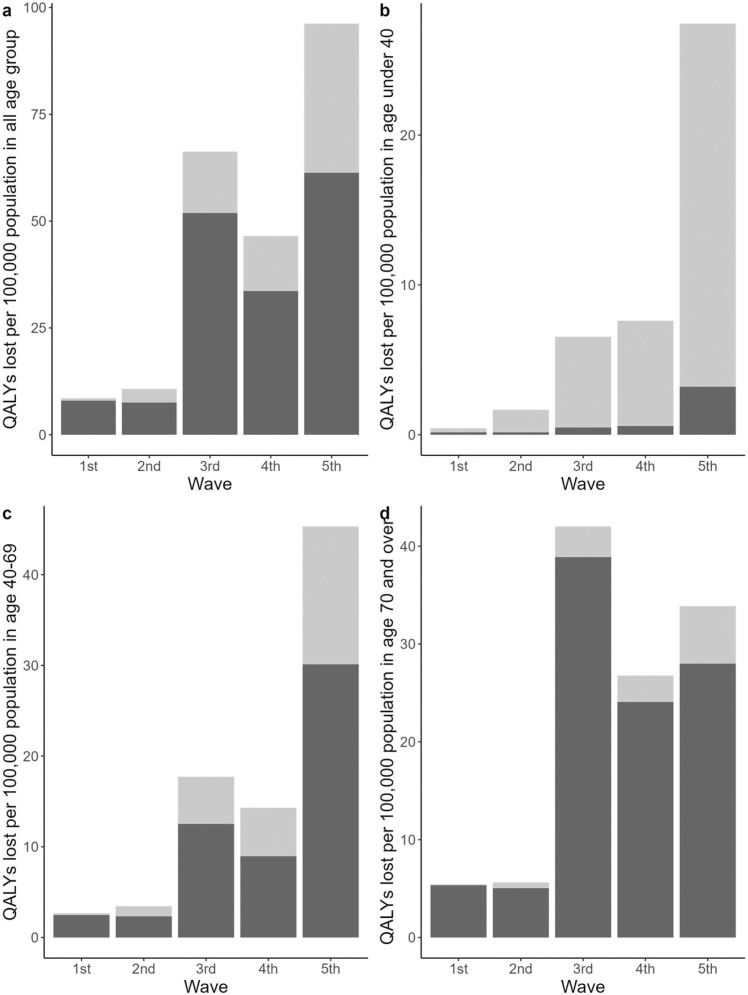
Total QALYs lost due to COVID-19 were estimated as 286,781 over two years, or an average of 114.0 QALYs per 100,000 population per year. The observed disease burden differed substantially between waves: from 8.5 QALYs per 100,000 population in Wave 1, up to 96.2 QALYs per 100,000 population in Wave 5. Table 2 shows the disease burden of each epidemic wave. Fig. 3 shows the evolution of QALYs lost per 100,000 population in the total population and by broad age group (under 40, 40–69, and over 69) over the waves. The disease burden in younger age groups gradually increased during the study period with 36.4%, 47.6%, 36.6%, 45.0% and 68.2% of the QALYs lost occurring in the age groups younger than 70 in Waves 1 through 5, respectively. When life years lost are adjusted for quality these percentages become38.7%, 50.7%, 39.6%, 48.3%, and 70.7%, respectively.
Table 2.
Breakdown of QALYs lost population in each epidemic wave.
| Outpatient | Inpatient | Severe | Death | Long-COVID | Total | |
|---|---|---|---|---|---|---|
| Wave 1 | 550 (0.4) |
88.4 (0.07) |
5.8 (0.005) |
10,054 (8.0) |
30.6 (0.02) |
10,729 (8.5) |
| Wave 2 | 2,748 (2.2) |
1,047 (0.8) |
26.6 (0.02) |
9,494 (7.5) |
173 (0.1) |
13,489 (10.7) |
| Wave 3 | 12,289 (9.8) |
4,899 (3.9) |
111 (0.1) |
65,302 (51.9) |
741 (0.6) |
83,342 (66.2) |
| Wave 4 | 10,707 (8.5) |
4,737 (3.8) |
111 (0.1) |
42,310 (33.6) |
619 (0.5) |
58,484 (46.5) |
| Wave 5 | 30,736 (24.4) |
11,289 (9.0) |
168 (0.1) |
77,145 (61.3) |
1,691 (1.3) |
121,030 (96.2) |
Unit: QALYs lost. Numbers represent the total QALYs lost and those in brackets represent QALYs lost per 100,000.
Fig. 3.
Comparison of QALYs lost per 100,000 population in each epidemic wave. Wave 1; 01/01/2020–05/31/2020, Wave 2; 06/01/2020–10/31/2020, Wave 3; 11/01/2020–03/31/2021, Wave 4; 4/1/2021–6/30/2021, Wave 5; 7/1/2021–12/31/2021. Light grey bars represent QALYs lost due to morbidity and dark grey bars represent QALYs lost due to mortality. QALYs; Quality-adjusted life years. Panel a: Disease burden in total population. Panel b: Disease burden in population under 40. Panel c: Disease burden in population between age 40 and 69. Panel d: Disease burden in population 70 and over.
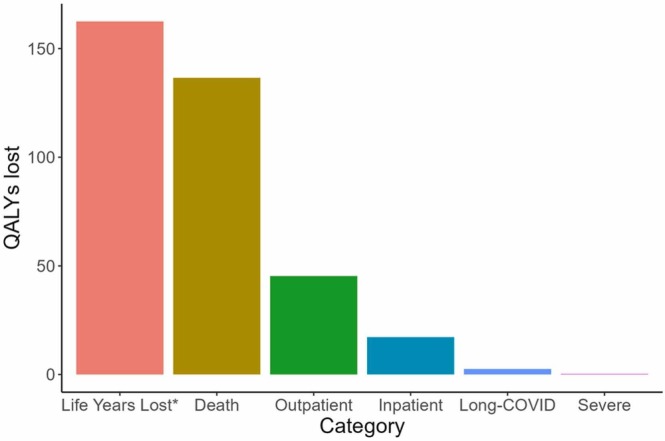
More than 70% of QALYs were lost due to premature mortality (204,437.2 out of 286,781.7, 71.3%), while nearly 20% were lost due to morbidity in outpatient cases (57,031.5 QALYs lost, 19.9%), and only a small part of the burden came from morbidity in severe cases (422.5 QALYs lost, 0.1%). Long-COVID accounts for 3.4% of the total disease burden (9,791.7 QALYs lost). Fig. 4 shows the breakdown of disease burden attributed to each clinical status.
Fig. 4.
Breakdown of disease burden per 100,000 population by clinical manifestation of COVID-19. QALYs; Quality-adjusted life years. *Count without quality adjustment (i.e., assuming life years lost due to premature mortality would have been lived in perfect health).
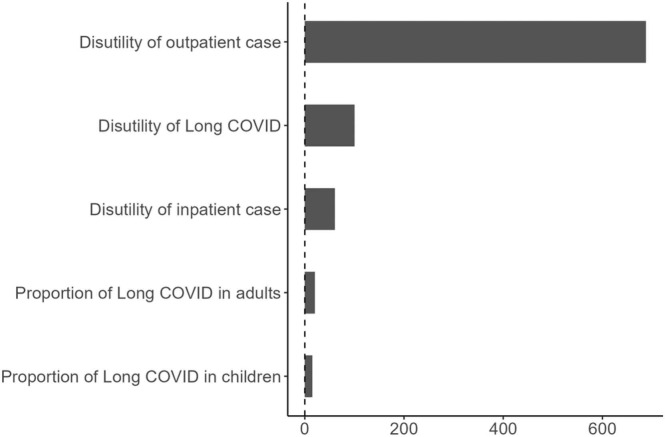
Probabilistic sensitivity analysis showed the disease burden directly due to COVID-19 ranged between 226,883 and 512,236 (median 242,834, IQR 236,108 to 254,488) QALYs, or between 90.1 and 203.6 (median 96.5, IQR 93.8–101.1) QALYs per 100,000 population. The disutility per outpatient case was the most influential parameter for the estimated QALY losses due to morbidity, whereas the disutility per long-COVID patient was the second most influential. Fig. 5 shows the influence of these and the other input parameters by their coefficient in a linear regression analysis using 1,000 input parameter sets and 1,000 associated QALY estimates. Clearly, accurate estimates for the disutilities per outpatient and per long-COVID patient are important to estimate the burden of disease from COVID-19.
Fig. 5.
Linear regression coefficient of each parameter included in the probabilistic sensitivity analysis. X axis represents coefficient value of each parameter.
Discussion
To the best of our knowledge, this is the first study which estimated the disease burden directly due to COVID-19 in Japan in the first two years of the pandemic. The disease burden brought by this emerging infectious disease during the first two years was smaller than in most other high-income countries (HICs). For instance, McDonald et al. reported that the total disease burden per-capita in the Netherlands in 2020 due to COVID-19 was 1,640 Disability-adjusted life-years (DALYs) per 100,000 population [36]. This number is more than ten times higher than that of our study. Other countries, e.g., Scotland and Malta, also reported similar size of burden in 2020 [37], [38]. Germany and Denmark reported smaller burden, however, the results were still much larger than ours (368 and 520 DALYs/100,000, respectively) [39], [40].
An obvious difference between previous studies and ours is that we used QALYs instead of DALYs to express burden of disease. Many guidelines advocate the use of a combined measure of morbidity and mortality as preferred outcome in economic evaluation, and the majority of country-specific guidelines, including those for Japan, prescribe the use of QALYs [41], although some influential generic guidelines such as the The International Decision Support Initiative (iDSI) [42] and the 2019 WHO guide for economic evaluation of vaccinations [43] indicate that the choice between these outcome measures may depend on the analyst’s preference and of the specific intervention under study. QALYs were used in our study to include the disease burden attributable to long-COVID based on a previous observational Japanese study using the EQ-5D-3 L questionnaire [20]. Although this choice may limit the comparability with studies using DALYs as an outcome, it allows us to attain our primary objective, i.e., assessing the disease burden by clinical manifestation, wave and age group, and have a basis to start from to assess the QALY impact of interventions (as one would for economic evaluation). Furthermore, DALYs were conceived in a very similar manner as QALYs, and applied studies using both measures have reported relatively small differences [27].
As for the total disease burden, this could be simply attributed to the relatively small number of confirmed cases and deaths per population in Japan [11]. Mortality of COVID-19 cases was smaller in Japan than in most other HICs during the study period [21], [44], [45], [46]. With regard to the number of deaths, the total number of all-cause excess deaths from the beginning of 2020 to the end of 2021 was estimated at between 11,014 and 58,905, while all-cause exiguous deaths in the same period was between 9,069 and 45,185 [47]. Considering these numbers included the influence of diseases other than COVID-19, the number of indirect deaths due to COVID-19 might not change the total burden estimates substantially. One can speculate about the reasons why the COVID-19 case and death toll tended to be lower in Japan than in many other HICs. Basically, people who presented fever or any symptoms suspicious of COVID-19 were required to visit one of the designated medical facilities and physicians who diagnosed COVID-19 had to report all COVID-19 cases they diagnosed, implying the potential risk of underestimating the number of cases was low. The cumulative number of COVID-19 cases in Japan increased most steeply after the emergence of the Omicron VOC, and the proportion of the Japanese population with non-vaccine induced immunity against COVID-19 remained low, even in 2021 [48]. Urashima et al. investigated the reason of low excess mortality due to COVID-19 in Japan, and reported that three factors were strongly associated with low excess mortality [49]. The percentage of fully vaccinated people, gross domestic product (GDP) per capita, and life expectancy at age 60 years. Japan is one of few countries which showed high vaccine coverage, high GDP, and long life-expectancy at age 60 years. It is sure that Japan has experienced an extremely aging society, however at the same time, it means that elder people in Japan live healthier life than those in other countries. It is noteworthy that Urashima et al. could not control for social contact pattern differences and differences in voluntary behavioural change and adherence to non-pharmaceutical interventions, which may all have age-specific influences on the exposure of the elderly part of the population to viral pathogens. Nevertheless, these factors pointed out by them might contribute to mitigate the disease burden due to COVID-19.
Considering the breakdown of QALYs lost, the part of the burden attributable to fatal cases was smaller than that in previous studies. Although only 71.3% of QALYs lost was attributed to fatal cases in our study, more than 90% of the total burden was attributed to LYL in previous studies in other countries [36], [37], [38], [39], [40]. It may be partly explained by some studies not including the burden of long-COVID. Furthermore, we have used a simplified approach by counting each lost life year as one, implying that each of these life years was assumed to be lived in perfect health. We also made estimates accounting for a quality adjustment in the life years gained, and as expected, this leads to lower estimates of the QALYs lost due to premature mortality.
The probabilistic sensitivity analysis acknowledges parametric uncertainty and indicates a relatively wide range for the total disease burden. This might be attributed to uncertainty in the estimation of the burden of outpatient cases and long-COVID because we defined the range of disease burden caused by these two statuses according to empirical data in our previous studies [20], [23], then small sample sizes affected their uncertainty. Additionally, it would also be due to the fact that there is no single established definition of long-COVID. Tsuzuki et al. defined long-COVID as four weeks or longer duration of symptoms after diagnosis of COVID-19 [20], however, WHO defines post COVID-19 condition as the status “usually 3 months from the onset of COVID-19 with symptoms and that last for at least 2 months and cannot be explained by an alternative diagnosis” [50]. With changing definitions, the proportion of symptomatic cases incurring long-COVID varies significantly. In addition, disutility of each long-COVID case varies substantially by study [51], [52], [53] which will bring further uncertainty.
There are several limitations in this study, similar to the previous studies. First, our results did not consider how many cases were unreported. As described above, all diagnosed COVID-19 cases including asymptomatic ones had to be reported in Japan, nevertheless some level of underreporting seems inevitable. For instance, McKenzie et al. insisted that there might be 1.77 times higher number of cases than reported during the same period (from the beginning of 2020 to the end of 2021) [54]. It is difficult for us to estimate the precise cumulative incidence of COVID-19 because we have very little evidence on the seroprevalence of SARS-CoV-2 in Japan [48], [54]. However, most of such unreported cases were expected to be “asymptomatic” because they had to be reported if they presented any suspicious symptoms. Since our objective is to assess the disease burden directly attributable due to COVID-19, we can ignore such cases, although they may have given rise to anxiety, especially in 2020, and have an impact on mental wellbeing in both the infected person and their direct contacts in and outside the household.
Second, we did not take the burden to the healthcare systems into consideration. Japanese government decided to admit all the patients diagnosed with COVID-19 regardless of its severity in the early phase of the pandemic [5]. As a result, a large number of tertiary hospitals could not offer normal healthcare due to high burden of the management of COVID-19 cases. This would have brought additional burden to society as previous studies reported [55], [56]. Nevertheless, we believe that this additional burden to the healthcare system did not contribute to increase the total disease burden, because excess mortality attributable to the causes other than COVID-19 was smaller than before during the study period [47], and no large catch-up effect due to postponed care has been detected in mortality statistics to date. In addition, there was only a very small number of cases in Japan in the early phase of COVID-19 pandemic. At this stage, there was a possible overestimation of the disease burden because some mild cases were admitted to tertiary hospitals without individual clinical reason. However, its impact should be very small because the number of cases were also very small compared with later periods. Furthermore, even if health disutility caused by such admissions were small, productivity losses were present as well as potential mental distress because the patients had to stay at healthcare facilities and they could not live their usual life. In addition, as in any country, there might exist regional differences in the accessibility to healthcare resources in Japan, which could affect the reported number of inpatient cases and consequently the estimated burden associated with inpatient cases. Considering these factors, biases caused by the healthcare policy in the early phase of the pandemic is likely to have had only a small impact on the main results.
Third, we did not evaluate counter-factual scenarios, and therefore do not attempt to estimate the impact of pharmaceutical and non-pharmaceutical interventions (PIs and NPIs). As for PIs, we did not take the benefit of vaccination into consideration, although mRNA vaccines obviously reduced the observed burden of COVID-19 in 2021 [57], [58], [59]. The data presented here could be used as a baseline to study counterfactual scenarios in future studies. The Japanese government had implemented a “state of emergency” declaration as a kind of NPI, recommending to avoid social contacts, with the aim to avoid lockdown, like in other countries [60]. Such NPIs might contribute to decrease the number of COVID-19 cases, but may cause mental ill-health due to reduced social contacts as well as restrict economic activities (e.g., dine outside, go travel, and so forth). In other words, NPIs can mitigate the disease burden in exchange for economic and potentially also higher mental health burden. We have scarce evidence about both the economic and mental health aspects of these non-pharmaceutical countermeasures so far and a counterfactual scenario about the burden of disease and these economic and mental health impacts in case we had not implemented such NPIs. Although it seems clear that the total disease burden due to COVID-19 in Japan was relatively smaller than in most other HICs, the impact of these aspects of NPIs remains a topic on research, as it is in many other countries.
Fourth, we did not distinguish between primary and secondary infections in unvaccinated persons and breakthrough infections in vaccinated persons. The cases reported in 2021, included breakthrough infections, but we lacked the information to specify these cases. Al-Aly et al. showed that the risk of death and post-acute sequelae were higher in unvaccinated cases than breakthrough infections [61], and the total burden might become slightly smaller if we consider the impact of breakthrough infection. Nevertheless, the number of breakthrough infection can be considered smaller than the normal ones, then its impact also should be a small one.
Fifth, in line with all other studies known to us, we exclude other indirect aspects of the disease burden such as that caused by isolation, imposed on patients’ family members, and so forth, because the number of isolated persons and the number of patients’ household members are not well documented. Although our previous studies had estimated the per-patient impact of these aspects [23], [62], future work will attempt to combine these results with the estimated number of isolated people and household members of infected cases as part of a more general assessment of the indirect burden.
Last, we did not include the year 2022 in our analysis. Due to the rapid spread of the Omicron VOC, the number of COVID-19 patients in Japan in 2022 was far higher than in the previous two years. Its population-level disease burden is therefore also likely to have increased in 2022. Additional analyses using also 2022 data are essential to undertake in the near future.
Conclusions
This study aimed to estimate the disease burden due to COVID-19 in Japan from the beginning of 2020 to the end of 2021, by using QALYs lost as outcome measure. To our knowledge, the present study is the first report from Japan which tried to estimate the disease burden caused by this emerging infectious disease in a quantitative manner.
Most of the disease burden in Japan during the study period was incurred in 2021 during waves 3, 4, and 5. The proportion of the total burden due to non-fatal disease increased gradually and this was probably due to the lower mortality by COVID-19 in the latter half of the study period, especially in the elderly. It also shows that younger people contributed more to the disease burden as SARS-CoV-2 circulated more in the general population from 2021 onwards. The estimated disease burden in Japan was smaller than in most other HICs and this can be attributed to the small number of confirmed cases in Japan.
Our results may contribute to make more precise estimation of the disease burden due to COVID-19 and this would be useful when considering the trade-offs between specific economic and mental health burden impacts of countermeasures against COVID-19 and their benefit for overall population health and overall economic activity. Future research may further explore the underlying reasons for the lower cumulative incidence of COVID-19 cases in Japan, while taking contextual factors into consideration, thus contributing to more optimally designed countermeasures against future pandemics.
Ethics approval
This study analysed data which were publicly available. As such, the datasets used in our study were de-identified and fully anonymized in advance, then therefore this study did not require ethical approval.
Funding
This research was funded by JSPS KAKENHI [Grant number 20K10546 and 23H03175]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CRediT authorship contribution statement
Shinya Tsuzuki: Conceptualization, Funding acquisition, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft preparation. Philippe Beutels: Supervision, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ST reports payment for supervising medical articles outside this study from Gilead Sciences, Inc. PB reports grants from Pfizer, GlaxoSmithKline, and the European Commission IMI, unrelated to this work.
Acknowledgment
PB acknowledges support from the Epipose project from the European Union’s SC1-PHE-CORONAVIRUS-2020 programme, project number 101003688, during the conduct of the study.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jiph.2023.05.025.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministry of Health, Labour and Welfare. Current status of the novel coronavirus infection and the response of the MHLW. n.d. https://www.mhlw.go.jp/stf/newpage_09290.html (accessed June 1, 2022).
- 5.Ministry of Health, Labour and Welfare. Information list for local authorities and healthcare facilities: COVID-19 2020. https://www.mhlw.go.jp/stf/seisakunitsuite/newpage_00024.html (accessed August 17, 2021).
- 6.Matsunaga N., Hayakawa K., Terada M., Ohtsu H., Asai Y., Tsuzuki S., et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: Report of the COVID-19 REGISTRY JAPAN. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue H. Japanese strategy to COVID-19: How does it work. Glob Health Med. 2020;2:131–132. doi: 10.35772/ghm.2020.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Health, Labour and Welfare. Information on health and medical consultation n.d. https://www.mhlw.go.jp/stf/covid-19/kenkou-iryousoudan_00006.html (accessed February 23, 2022).
- 9.Yamaguchi S., Okada A., Sunaga S., Kurakawa K.I., Yamauchi T., Nangaku M., et al. Impact of COVID-19 pandemic on healthcare service use for non-COVID-19 patients in Japan: retrospective cohort study. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-060390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ono A., Koizumi R., Tsuzuki S., Asai Y., Ishikane M., Kusama Y., et al. Antimicrobial use fell substantially in Japan in 2020—The COVID-19 pandemic may have played a role. Int J Infect Dis. 2022 doi: 10.1016/j.ijid.2022.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coronavirus (COVID-19) Cases - Our World in Data n.d. https://ourworldindata.org/covid-cases (accessed February 24, 2022).
- 12.Healthcare in the UK | Coronavirus in the UK n.d. https://coronavirus.data.gov.uk/details/healthcare (accessed December 3, 2022).
- 13.United States - COVID-19 Overview - Johns Hopkins. Johns Hopkins Coronavirus Resource Center n.d. https://coronavirus.jhu.edu/region/united-states (accessed December 3, 2022).
- 14.Idogawa M., Tange S., Nakase H., Tokino T. Interactive web-based graphs of coronavirus disease 2019 cases and deaths per population by country. Clin Infect Dis. 2020;71:902–903. doi: 10.1093/cid/ciaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayakawa K., Asai Y., Matsunaga N., Tsuzuki S., Terada M., Suzuki S., et al. Evaluation of the representativeness of data in the COVID-19 Registry Japan during the first six waves of the epidemic. Glob Health Med. 2022 doi: 10.35772/ghm.2022.01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyazato Y., Morioka S., Tsuzuki S., Akashi M., Osanai Y., Tanaka K., et al. Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Kessel S.A.M., Olde Hartman T.C., Lucassen P.L.B.J., van Jaarsveld C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: a systematic review. Fam Pract. 2022;39:159–167. doi: 10.1093/fampra/cmab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng N., Zhao Y.-M., Yan W., Li C., Lu Q.-D., Liu L., et al. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: call for research priority and action. Mol Psychiatry. 2023;28:423–433. doi: 10.1038/s41380-022-01614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuzuki S., Miyazato Y., Terada M., Morioka S., Ohmagari N., Beutels P. Impact of long-COVID on health-related quality of life in Japanese COVID-19 patients. Health Qual Life Outcomes. 2022;20:125. doi: 10.1186/s12955-022-02033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ministry of Health, Labour and Welfare. Visualizing the data: information on COVID-19 infections n.d. https://covid19.mhlw.go.jp/extensions/public/en/index.html (accessed February 24, 2022).
- 22.National Institute of Population and Social Security Research. Population Statistics 2020. http://www.ipss.go.jp/syoushika/tohkei/Popular/Popular2014.asp?chap=5&title1=%87X%81D%8E%80%96S%81E%8E%F5%96%BD (accessed December 20, 2020).
- 23.Tsuzuki S., Ohmagari N., Beutels P. The burden of isolation to the individual: a comparison between isolation for COVID-19 and for other influenza-like illnesses in Japan. Epidemiol Infect. 2022:150. doi: 10.1017/S0950268821002569. e5-undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glick H.A., Miyazaki T., Hirano K., Gonzalez E., Jodar L., Gessner B.D., et al. One-year quality of life post–pneumonia diagnosis in Japanese adults. Clin Infect Dis. 2021;73:283–290. doi: 10.1093/cid/ciaa595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson E.J., Williams D.M., Walker A.J., Mitchell R.E., Niedzwiedz C.L., Yang T.C., et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. 2022:13. doi: 10.1038/s41467-022-30836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wulf Hanson S., Abbafati C., Aerts J.G., Al-Aly Z., Ashbaugh C., Ballouz T., et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022 doi: 10.1001/jama.2022.18931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Augustovski F., Colantonio L.D., Galante J., Bardach A., Caporale J.E., Zarate V., et al. Measuring the benefits of healthcare: DALYs and QALYs - does the choice of measure matter? A case study of two preventive interventions. Int J Health Policy Manag. 2017;7:120–136. doi: 10.15171/ijhpm.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drummond M.F., Sculpher M.J., Claxton K., Stoddart G.L., Torrance G.W. Methods for the Economic Evaluation of Health Care Programmes. fourth ed. Oxford University Press; Oxford: 2015. [Google Scholar]
- 29.Tsuzuki S., Baguelin M., Pebody R., van Leeuwen E. Modelling the optimal target age group for seasonal influenza vaccination in Japan. Vaccine. 2020;38:752–762. doi: 10.1016/j.vaccine.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Tsuzuki S., Schwehm M., Eichner M. Simulation studies to assess the long-term effects of Japan’s change from trivalent to quadrivalent influenza vaccination. Vaccine. 2018;36:624–630. doi: 10.1016/j.vaccine.2017.12.058. [DOI] [PubMed] [Google Scholar]
- 31.R Core Team. R: A Language and Environment for Statistical Computing 2018.
- 32.Briggs A.H., Goldstein D.A., Kirwin E., Meacock R., Pandya A., Vanness D.J., et al. Estimating (quality‐adjusted) life‐year losses associated with deaths: with application to COVID‐19. Health Econ. 2021;30:699–707. doi: 10.1002/hec.4208. [DOI] [PubMed] [Google Scholar]
- 33.Shiroiwa T., Noto S., Fukuda T. Japanese population norms of EQ-5D–5L and health utilities index mark 3: disutility catalog by disease and symptom in community settings. Value Health. 2021;24:1193–1202. doi: 10.1016/j.jval.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Shoji K., Akiyama T., Tsuzuki S., Matsunaga N., Asai Y., Suzuki S., et al. Clinical characteristics of COVID-19 in hospitalized children during the Omicron variant predominant period. J Infect Chemother. 2022;28:1531–1535. doi: 10.1016/j.jiac.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoji K., Akiyama T., Tsuzuki S., Matsunaga N., Asai Y., Suzuki S., et al. Comparison of the clinical characteristics and outcomes of COVID-19 in children before and after the emergence of Delta variant of concern in Japan. J Infect Chemother. 2022;28:591–594. doi: 10.1016/j.jiac.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mcdonald S.A., Lagerweij G.R., De Boer P., De Melker H.E., Pijnacker R., Mughini Gras L., et al. The estimated disease burden of acute COVID-19 in the Netherlands in 2020, in disability-adjusted life-years. Eur J Epidemiol. 2022 doi: 10.1007/s10654-022-00895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wyper G.M.A., Fletcher E., Grant I., Mccartney G., Fischbacher C., Harding O., et al. Measuring disability-adjusted life years (DALYs) due to COVID-19 in Scotland, 2020. Archives of Public Health 2022;80. https://doi.org/10.1186/s13690–022-00862-x. [DOI] [PMC free article] [PubMed]
- 38.Cuschieri S., Calleja N., Devleesschauwer B., Wyper G.M.A. Estimating the direct Covid-19 disability-adjusted life years impact on the Malta population for the first full year. BMC Public Health. 2021:21. doi: 10.1186/s12889-021-11893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rommel A., von der Lippe E., Plass D., Ziese T., Diercke M., an der Heiden M., et al. The COVID-19 Disease Burden in Germany in 2020. Dtsch Arztebl International 2021;118:145–51. [DOI] [PMC free article] [PubMed]
- 40.Pires S.M., Redondo H.G., Espenhain L., Jakobsen L.S., Legarth R., Meaidi M., et al. Disability adjusted life years associated with COVID-19 in Denmark in the first year of the pandemic. BMC Public Health. 2022:22. doi: 10.1186/s12889-022-13694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y., Feng H., Qu J., Luo X., Ma W., Tian J. A systematic review of pharmacoeconomic guidelines. J Med Econ. 2018;21:85–96. doi: 10.1080/13696998.2017.1387118. [DOI] [PubMed] [Google Scholar]
- 42.Health Technology Assessment Toolkit. IDSI 2017. https://www.idsihealth.org/htatoolkit/ (accessed December 8, 2022).
- 43.World Health Organization. WHO guide for standardization of economic evaluations of immunization programmes, 2nd ed n.d. https://www.who.int/publications-detail-redirect/who-guide-for-standardization-of-economic-evaluations-of-immunization-programmes-2nd-ed (accessed December 8, 2022).
- 44.Eurosurveillance | Estimates of mortality attributable to COVID-19: a statistical model for monitoring COVID-19 and seasonal influenza, Denmark, spring 2020 n.d. https://www.eurosurveillance.org/content/10.2807/1560–7917. ES.2021.26.8.2001646 (accessed February 26, 2021). [DOI] [PMC free article] [PubMed]
- 45.Lipsitch M. Estimating case fatality rates of COVID-19. Lancet Infect Dis. 2020;20:775. doi: 10.1016/S1473-3099(20)30245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pastor-Barriuso R., Pérez-Gómez B., Hernán M.A., Pérez-Olmeda M., Yotti R., Oteo-Iglesias J., et al. Infection fatality risk for SARS-CoV-2 in community dwelling population of Spain: nationwide seroepidemiological study. BMJ. 2020;371 doi: 10.1136/bmj.m4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawashima T., Nomura S., Tanoue Y., Yoneoka D., Eguchi A., Ng C.F.S., et al. Excess All-Cause Deaths during Coronavirus Disease Pandemic, Japan, January–May 2020 - Volume 27, Number 3—March 2021 - Emerging Infectious Diseases journal - CDC n.d. https://doi.org/10.3201/eid2703.203925. [DOI] [PMC free article] [PubMed]
- 48.Sugiyama A., Okada F., Abe K., Imada H., Ouoba S., E.B., et al. A longitudinal study of anti-SARS-CoV-2 antibody seroprevalence in a random sample of the general population in Hiroshima in 2020. Environ Health Prev Med. 2022;27 doi: 10.1265/ehpm.22-00016. 30–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urashima M., Tanaka E., Ishihara H., Akutsu T. Association between life expectancy at age 60 years before the COVID-19 pandemic and excess mortality during the pandemic in aging countries. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.37528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021 2021. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed September 19, 2022).
- 51.Sandmann F.G., Tessier E., Lacy J., Kall M., Van Leeuwen E., Charlett A., et al. Long-Term Health-Related Quality of Life in Non-Hospitalized Coronavirus Disease 2019 (COVID-19) Cases With Confirmed Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in England: Longitudinal Analysis and Cross-Sectional Comparison W. Clinical Infectious Diseases 2022. https://doi.org/10.1093/cid/ciac151. [DOI] [PMC free article] [PubMed]
- 52.Malik P., Patel K., Pinto C., Jaiswal R., Tirupathi R., Pillai S., et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J Med Virol. 2022;94:253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malinowska A., Muchlado M., Ślizień Z., Biedunkiewicz B., Heleniak Z., Dębska-Ślizień A., et al. Post-COVID-19 sydrome and decrease in health-related quality of life in kidney transplant recipients after SARS-COV-2 infection-A cohort longitudinal study from the North of Poland. J Clin Med. 2021;10:5205. doi: 10.3390/jcm10215205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mckenzie L., Shoukat A., Wong K.O., Itahashi K., Yasuda E., Demarsh A., et al. Inferring the true number of SARS-CoV-2 infections in Japan. J Infect Chemother. 2022 doi: 10.1016/j.jiac.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verelst F., Kuylen E., Beutels P. Indications for healthcare surge capacity in European countries facing an exponential increase in coronavirus disease (COVID-19) cases, March 2020. Eurosurveillance. 2020;25:2000323. doi: 10.2807/1560-7917.ES.2020.25.13.2000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCabe R., Kont M.D., Schmit N., Whittaker C., Løchen A., Baguelin M., et al. Modelling intensive care unit capacity under different epidemiological scenarios of the COVID-19 pandemic in three Western European countries. Int J Epidemiol. 2021 doi: 10.1093/ije/dyab034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. New Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chemaitelly H., Almukdad S., Ayoub H.H., Altarawneh H.N., Coyle P., Tang P., et al. Covid-19 vaccine protection among children and adolescents in Qatar. New Engl J Med. 2022;387:1865–1876. doi: 10.1056/nejmoa2210058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lauring A.S., Tenforde M.W., Chappell J.D., Gaglani M., Ginde A.A., Mcneal T., et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022 doi: 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Machida M., Wada K. Public health responses to COVID-19 in Japan. Glob Health Med. 2022;4:78–82. doi: 10.35772/ghm.2022.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Aly Z., Bowe B., Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuzuki S., Yoshihara K. The characteristics of influenza-like illness management in Japan. BMC Public Health. 2020;20:568. doi: 10.1186/s12889-020-08603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material