Abstract
Cell surface heparan sulfate (HS) serves as an initial receptor for many different viruses, including herpes simplex virus types 1 and 2 (HSV-1 and 2, respectively). Glycoproteins C and B (gC and gB) are the major components of the viral envelope that mediate binding to HS. In this study, purified gB and gC homologous proteins as well as purified HSV-1 and HSV-2 virions were compared for the ability to bind isolated HS receptor molecules. HSV-1 gC and HSV-2 gC bound comparable amounts of HS. Similarly, HSV-1 gB and its HSV-2 counterpart showed no difference in the HS-binding capabilities. Despite the similar HS-binding potentials of gB and gC homologs, HSV-1 virions bound more HS than HSV-2 particles. Purified gC and gB proteins differed with respect to sensitivity of their interaction with HS to increased concentrations of sodium chloride in the order gB-2 > gB-1 > gC-1 > gC-2. The corresponding pattern for binding of whole HSV virions to cells in the presence of increased ionic strength of the medium was HSV-2 gC-neg1 > HSV-1 gC−39 > HSV-1 KOS 321 > HSV-2 333. These results relate the HS-binding activities of individual glycoproteins with the cell-binding abilities of whole virus particles. In addition, these data suggest a greater contribution of electrostatic forces for binding of gB proteins and gC-negative mutants compared with binding of gC homologs and wild-type HSV strains. Binding of wild-type HSV-2 virions was the least sensitive to increased ionic strength of the medium, suggesting that the less extensive binding of HS molecules by HSV-2 than by HSV-1 can be compensated for by a relatively weak contribution of electrostatic forces to the binding. Furthermore, gB and gC homologs exhibited different patterns of sensitivity of binding to cells to inhibition with selectively N-, 2-O-, and 6-O-desulfated heparin compounds. The O-sulfate groups of heparin were found to be more important for interaction with gB-1 than gB-2. These results indicate that HSV-1 and HSV-2 differ in their interaction with HS.
Cell surface carbohydrate moieties frequently serve as initial receptors for viruses. Their location at the cell periphery and their abundant expression, ubiquity, and frequency of negative charge load make them well suited for electrostatic attraction of viruses to the cell membrane. This event is followed by an initial weak virus-cell contact that concludes in multiple interactions between numerous copies of the viral attachment proteins and receptor molecules. Such a cascade of events is of great importance in the stable cell adherence of complex pathogens such as enveloped viruses. One example of a viral receptor of carbohydrate nature is heparan sulfate (HS) proteoglycan, the molecule first identified to serve as initial receptor for herpes simplex virus types 1 and 2 (HSV-1 and -2) by WuDunn and Spear (52). At least two proteins of both HSV-1 and HSV-2 envelope, glycoprotein C (designated gC-1 for HSV-1 and gC-2 for HSV-2) and glycoprotein B (gB-1 and gB-2, respectively), were demonstrated to be able to bind heparin (10, 14, 49), a molecule related to HS. It has been reported that in HSV-1, gC-1 played a key role in the adsorption of wild-type HSV-1 to cells (14), whereas gB-1 mediated binding of gC-null mutants (15). In contrast, gC-2 was not found to be a key attachment protein of HSV-2, and consequently a greater contribution of gB for HSV-2 than HSV-1 binding has been suggested (10). In addition to providing sites for initial virus binding, HS was reported to promote HSV-1-induced cell-to-cell fusion (42). Furthermore, HS modified by 3-O-sulfotransferase isomer 3 was recently reported to be able to interact with HSV-1 gD, an event that triggered the virus entry into cell (43).
Although both HSV-1 and HSV-2 target HS as a receptor molecule, these viruses have been reported to exhibit a number of type-specific differences in their interactions with host cells. In particular, HSV-2 infection of cells was more efficiently inhibited than that of HSV-1 by polyanionic substances such as heparin, dextran sulfate, agar inhibitors, and chondroitin sulfate B (19). In contrast, polycationic substances such as neomycin and poly-l-lysine more efficiently inhibited infection by HSV-1 than by HSV-2 strains (24, 25), and this phenotype was mapped to the N-terminal region of gC-2 (2, 26, 37). Moreover, HSV-2 exhibited greater sensitivity than HSV-1 as regards inhibition of viral binding by O-desulfated heparins, a type-specific phenotype that was attributed to gC-2 (13). Furthermore, HSV-1 but not HSV-2 infection of mutant HS- and chondroitin sulfate-deficient cells was stimulated by dextran sulfate, and it was demonstrated that gB-1 mediated this ability whereas the presence of gC-2 exerted an inhibitory effect (5). Finally, HSV-1-infected but not HSV-2-infected cells bound the C3b component of complement, and gC-1 was identified to act as a receptor for C3b (8). Interestingly, whereas HSV-2-infected cells bound no C3b, purified gC-2 exhibited such activity (6, 34), indicating that differences in the presentation and/or quantity of gC-1 and gC-2 on virus-infected cells or on the virion surface may account for type-specific phenotypes with respect to C3b binding and sensitivity to polyanionic and polycationic substances.
The carbohydrate moieties of HS proteoglycans, i.e., HS chains, provide receptor sites for HSV, and a single chain may contain up to several hundred saccharides. For example, HS chains that occur in green monkey kidney (GMK AH1) and human epidermoid carcinoma (HEp-2) cells averaged 30 kDa (7) and 105 kDa (33) in apparent molecular mass, which correspond to approximately 55 nm (120 sugar residues per chain) and 190 nm (420 sugar residues), respectively, in chain length. Simple calculation indicates that HEp-2 cell-specific HS could theoretically adhere to almost half of the virion circumference. HS chains are composed of alternating glucosamine and uronic acid residues. These sugar residues can be modified by N-, 6-O-, and 3-O-sulfation (glucosamine) and by 2-O-sulfation (uronic acid). In contrast to heparin, which could be described as a continuous block of extensively sulfated saccharides, there is a nonuniform distribution of sulfate groups in HS, with an overall tendency to form clusters. Consequently, HS chains are thought to possess a domain-like structure where blocks of weakly sulfated saccharides alternate with stretches of moderately to highly sulfated sugar residues (for a review, see reference 40). According to the protein-polyelectrolyte interaction theory (32), the binding of protein to HS relies on the release of cations, most notably sodium ions, from polyanionic HS chains by positively charged components of the protein. The protein-HS complexes can dissociate at specific ionic strength of the medium, and the HS-binding proteins significantly differ with regard to this parameter. An overall tendency is that the higher the concentration of sodium ions necessary to break the protein-HS complex, the lower the contribution of electrostatic forces for the binding. For example, for the interaction of fibroblast growth factor with heparin that dissociates in the presence of more than 1 M NaCl, it was shown that only approximately 30% of the binding energy resulted from pure electrostatic forces (50). However, most of the proteins elute from heparin/HS chains at relatively low ionic strength of the medium, and the contribution of electrostatic forces to their binding is significant.
In this investigation, we compared purified gB and gC homologs as well as whole HSV virions for the ability to bind isolated HS receptor molecules. Our results revealed that HSV-2 virions bound less HS than did HSV-1 particles, but the contribution of relatively nonspecific electrostatic forces was greater for HSV-1 than for HSV-2 binding to cells.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney (GMK AH1) cells were cultivated in Eagle's minimum essential medium (EMEM) supplemented with 2% calf serum, 0.05% Primaton RT substance, and antibiotics. Baby hamster kidney (BHK) cells were propagated in Glasgow minimum essential medium supplemented with 8% calf serum, 8% tryptose phosphate broth, 1 mM l-glutamine, and antibiotics. Human epidermoid carcinoma (HEp-2) cells were grown in EMEM supplemented with 8% fetal bovine serum. The HSV strains used were HSV-1 KOS 321, a plaque-purified isolate of wild-type strain KOS (17), an HSV-1 KOS gC-null mutant designated gC−39 (16), HSV-2 strain 333 (4), and a local clinical isolate of HSV-2 designated B4327UR (22). The former three strains were kindly provided by J. Glorioso (University of Pittsburgh School of Medicine, Pittsburgh, Pa.). The gB and gC genes of strains KOS 321 and 333 have been sequenced (18, 38, 46, 48). For the B4327UR isolate, the coding sequence for gC-2 is available (EMBL database, accession number AJ297389). For preparation of the HSV-2 gC-negative mutant, plaques produced by HSV-2 333 were immunostained by using rabbit polyclonal anti-gC sera R65 and R118 (a generous gift from G. Cohen and R. Eisenberg, University of Pennsylvania, Philadelphia), and then several unstained plaques were selected for purification to the point of homogeneity. One clone, designated HSV-2 gC-neg1, was sequenced by PCR and appeared to carry a frameshift mutation, due to an insertion of cytosine within a run of five cytosines that precedes codon 166. This alteration gave rise to a stop codon at triplet 252 resulting in a premature truncation of gC-2.
Virus purification.
The virus was purified from infectious culture media of GMK AH1 cells. For preparation of radiolabeled virus, the cells were infected at an multiplicity of infection of 3 PFU per cell; after an adsorption period of 2 h at 37°C, the cells were washed and then incubated for 48 h at 37°C in EMEM supplemented with [methyl-3H]thymidine (25 μCi/ml). The media were clarified by centrifugation at 1,000 × g for 25 min and then by centrifugation at 5,000 × g for 10 min. Extracellular virus was pelleted from the resulting supernatant by centrifugation at 22,000 × g for 2 h. The pellet was covered with phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4) and left overnight at 4°C. The virus was purified from the pelleted material by centrifugation through a three-step discontinuous sucrose gradient (23). The relative number of virions in purified HSV-1 and HSV-2 preparations was determined by quantitating amounts of the major viral capsid protein (VP5) as described by WuDunn and Spear (52). Briefly, serial twofold dilutions of purified virions were electrophoresed under reducing conditions on a 4 to 12% NuPAGE Bis-Tris precast gel and stained with colloidal blue kit as instructed by the manufacturer (Novex, San Diego, Calif.). Relative amounts of VP5 were determined by comparing the densities of the stained proteins.
Purification of viral glycoproteins.
Confluent monolayers of GMK AH1 cells (for gC-1, gB-1, and gB-2 purification) or BHK and HEp-2 cells (for gC-2 purification) in roller bottles were infected with strain KOS 321 or B4327UR; when the cytopathic effect was pronounced, the cells and media were harvested. The virus was spun down from the media by centrifugation at 130,000 × g for 1 h, and both the virus pellet and infected cells were stored at −70°C. The infected cells and/or viral pellet were lysed with cold 0.02 M Tris-HCl buffer (pH 7.5) containing 1% sodium deoxycholate, 1% Nonidet P-40, 2 mM EDTA, 2 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, and 2 mM Nα-p-tosyl-l-lysine chloromethyl ketone. The mixture was dispersed vigorously with a pipette and left on ice for 1 h. Unsolubilized material was pelleted by centrifugation at 130,000 × g for 1 h; the supernatant was preadsorbed on an immunosorbent column containing anti-gE monoclonal antibody B1E6 (to prevent possible contamination of gC preparations with gE) and then passed through columns containing monoclonal antibody C4H11 (anti-gC-1) or B11D8 (anti-gB) (1). For purification of gC-2, rabbit anti-HSV gC polyclonal serum K65 was used to prepare an immunosorbent column (6). Subsequently the columns were washed with 0.02 M Tris-HCl (pH 7.5) containing 0.1% Nonidet P-40, 0.5 M NaCl, and 2 mM EDTA. To minimize the amount of detergent in purified viral proteins, the columns were washed with detergent-free washing buffer just before elution with 0.1 M glycine-HCl (pH 2.4). Following neutralization of the eluent with 1 M Tris-HCl (pH 8.0), the material was centrifuged to near dryness over a microcentrifugal concentrator with a 30-kDa cutoff (PallGelman Sciences, Lund, Sweden), then resuspended in PBS, and centrifuged again. The final product was suspended in a small volume of PBS and stored at −70°C. Protein concentration was determined according to standard Lowry method (DC protein assay kit; Bio-Rad).
Isolation of HS chains.
Nearly confluent monolayers of HEp-2 or GMK AH1 cells were labeled for 48 h with N235SO4 (50 μCi/ml; specific activity, 1,325 Ci/mmol; NEN Life Science Products, Boston, Mass.) in low-sulfate EMEM (10% original sulfate concentration) supplemented with 10% fetal calf serum and antibiotics. Cell-associated HS chains were purified by the method of Lyon et al. (30).
Modified heparin compounds.
Modifications were performed on bovine lung heparin which had been isolated and purified as previously described (27). Selective 2-O-desulfation was performed by lyophilization at pH 12.5 as previously described (21). N-desulfation was performed on the pyridiminium salt of heparin in dimethyl sulfoxide-H2O (19:1) at 50°C for 90 min (20). Preferential 6-O-desulfation was performed in dimethyl sulfoxide-methanol (9:1) at 93°C for 2 h (35). O-desulfated heparin chains were N-resulfated (28), whereas N-desulfated heparin was N-acetylated (3).
Neuraminidase and glycosidase treatment.
Five-microgram aliquots of purified gC-1 and gC-2 in 0.1 M sodium phosphate buffer (pH 7.3) were treated for 2 h at 36°C with 5 mU of neuraminidase, 5 mU of both neuraminidase and O-glycosidase, and 250 mU of endoglycosidase F/N-glycosidase F (all enzymes from Boehringer Mannheim Scandinavia AB, Bromma, Sweden). Products of mock and enzymatic digestions were subjected to electrophoresis under reducing conditions on a 10% acrylamide tris-glycine precast gel and then stained with a colloidal blue kit (Novex). Purified HSV-1 and HSV-2 in 0.2 ml of PBS were incubated with 20 mU of neuraminidase for 1 h at 36°C.
Binding of glycosaminoglycans to HSV proteins and HSV virions.
Equal quantities of purified gB and gC proteins (adjusted according to protein contents) or the same relative number of HSV-1 and HSV-2 particles (quantification based on relative amounts of the VP5 protein) were diluted in 0.2 ml of PBS supplemented with 0.05% bovine serum albumin (PBS-BSA) and then mixed with ca. 5,000 cpm of GMK AH1 or HEp-2 cell-specific 35S-HS. Following incubation for 2 h at room temperature, the amounts of bound glycosaminoglycan were determined by the nitrocellulose membrane filtration method (31). The binding of viral proteins to HS in the presence of increased concentrations of sodium chloride was assayed in a similar manner, except that the protein-HS mixtures were incubated in phosphate buffer in which the concentration of sodium chloride was adjusted to required values with a 2 M solution of sodium chloride in phosphate buffer.
Viral plaque assay in the presence of modified heparin compounds.
Serial fivefold dilutions of a specific compound in 2 ml of cold Hanks balanced salt solution (without phenol red and glucose) was mixed with 200 μl of the same medium containing ca. 200 PFU of either HSV-1 or HSV-2 and incubated for 10 min at 4°C (cold room). Confluent, dense monolayers (3 days old) of GMK AH1 cells in six-well plates, precooled for 20 min at room temperature and for 20 min at 4°C, were washed with 2 ml of cold Hanks medium; then 1-ml portions of the virus-heparin mixture were added. Following adsorption for 1 h at 4°C, the cells were washed twice with 2 ml of cold Hanks medium and overlaid with 4 ml of EMEM containing 1% methylcellulose, 2% fetal calf serum, and antibiotics. The plaques were stained with crystal violet solution after 3 days of incubation at 37°C. In a set of similar experiments, incubation of the virus-heparin mixture in EMEM and subsequent virus adsorption to cells were carried out at 37°C. The concentrations of heparin preparations that inhibited the number of viral plaques by 50% (IC50s) were interpolated from the dose-response curves.
Binding of purified viral proteins to cells in the presence of modified heparin compounds.
The effect of selectively or preferentially N-, 2-O-, or 6-O-desulfated heparin preparations on binding of purified gC-1, gC-2, gB-1, and gB-2 to GMK AH1 cells was tested by an enzyme-linked immunosorbent assay (ELISA)-attachment method as described previously (29, 47). Both preincubation of glycoproteins with heparin compounds and their attachment to GMK AH1 cells were carried out at 4°C.
Enzymatic digestion of cells.
Confluent monolayers of 3-day-old GMK AH1 cells in six-well plates were washed twice with 2 ml of Hanks medium, and 1-ml portions of the same medium containing various numbers of heparinase units were added. Following incubation at 37°C for 1 h and at 4°C for 20 min, the cells were washed twice with 2 ml of cold Hanks medium, and ca. 200 PFU of the virus in 1 ml of Hanks medium was added. Following virus adsorption for 1 h at 4°C, the cells were washed twice with 2 ml of Hanks medium and overlaid with 3 ml of 1% methylcellulose solution. The plaques were stained with crystal violet solution after 3 days of incubation at 37°C.
Binding of virus to cells at increased ionic strength of the medium.
Confluent, dense monolayers of 3-day-old GMK AH1 cells in 24-well plates were precooled for 30 min at room temperature and for 30 min at 4°C. The cells were washed twice with 0.5 ml of cold Hanks medium supplemented with various concentrations of sodium chloride and 1% BSA (H-NaCl-BSA). Subsequently 0.2 ml of H-NaCl-BSA containing the same number of relative VP5 units of [3H]thymidine-labeled KOS 321, 333, gC−39, or gC-neg1 was added. Following adsorption for 20 min at 4°C, the cells were washed once with 0.5 ml of H-NaCl-BSA and twice with 0.5 ml of normal Hanks solution. The cells were then lysed with a 5% solution of sodium dodecyl sulfate and transferred to scintillation vials for quantification of radioactivity. The binding of nonlabeled virus to cells was assayed in a similar manner. Briefly, confluent, dense monolayers of 3-day-old GMK AH1 cells in six-well plates were precooled for 30 min at room temperature and for 30 min at 4°C. The cells were washed twice with 2 ml of cold Hanks medium supplemented with various concentrations of sodium chloride (H-NaCl). Subsequently 1 ml of H-NaCl containing approximately 200 PFU of an HSV strain was added and left for adsorption for 20 min at 4°C. The cells were washed once with 2 ml of H-NaCl and twice with 2 ml of normal Hanks solution. The cells were then overlaid with 1% methylcellulose solution and incubated for 2 (HSV-2 strains) or 3 (HSV-1 strains) days at 37°C for the development of viral plaques.
RESULTS
HS-binding capabilities of purified gC and gB homologs of HSV and purified HSV virions.
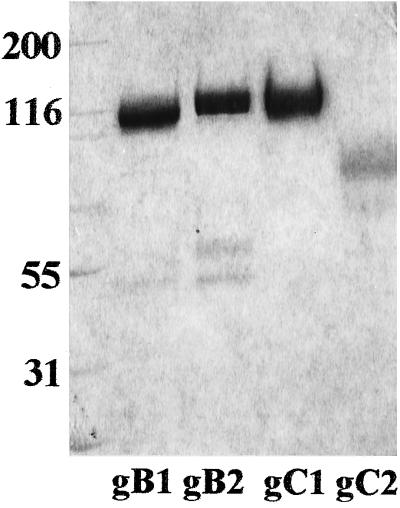
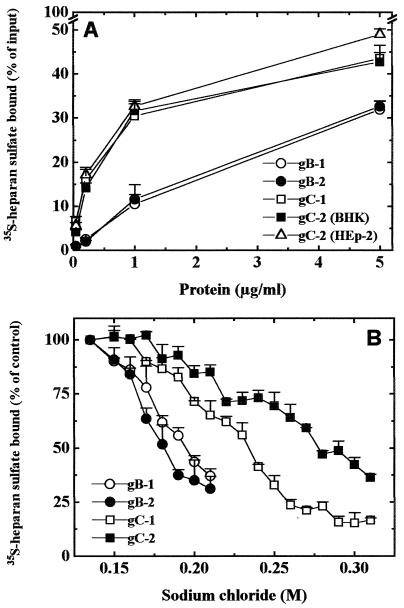
To study the relative contribution of gC and gB homologs for HSV-1 and HSV-2 attachment to cells, the physical abilities of gC and gB proteins to bind isolated HS receptor molecules were first assayed. Electrophoretic profiles of the viral proteins used are shown in Fig. 1. The presence of two extra bands (ca. 45 and 55 kDa) in the preparation of gB-2 might be explained by the fact that up to 30% of mature gB-2 (110 kDa) could be cleaved during in vitro processing. However, due to the presence of disulfide bonds between Cys1-Cys8 and Cys2-Cys7, these two parts were purified together by immunoaffinity chromatography but separated when electrophoresed under reducing conditions (36). To compare the HS-binding capabilities of gB-1, gB-2, gC-1, and gC-2 (purified from infected BHK cells), stocks of purified proteins were diluted to the same protein contents and then mixed with GMK AH1 cell-specific 35S-labeled HS (Fig. 2A). Purified gB-1 and gB-2 bound similar amounts of HS. The same was observed for gC-1 and gC-2. Based on these results, the HS-binding capacity of gB and gC homologs, i.e., the quantity of 35S-HS per molecule of the protein, was calculated. At low protein concentration, i.e., 0.2 μg, gB-1 bound 1.26 × 10−10, gB-2 bound 1.00 × 10−10, gC-1 bound 4.28 × 10−10, and gC-2 bound 3.54 × 10−10 cpm of HS per protein molecule. The corresponding values calculated for a high protein concentration, i.e., 5 μg, were as follows: 6.19 × 10−11 cpm of HS per gB-1 molecule, 6.51 × 10−11 per gB-2 molecule, 4.6 × 10−11 per gC-1 molecule, and 4.27 × 10−11 per gC-2 molecule. These results indicated that at a relatively low protein concentration, gB homologs bound three to four times less HS per molecule than gC proteins, whereas at a high protein concentration, gB-1 and gB-2 bound more HS per molecule than gC-1 and gC-2. To examine whether biological functions of viral glycoproteins could be affected by the cell substratum used for virus multiplication, one of the glycoproteins used, gC-2, was also extracted from HEp-2 and GMK AH1 cells. The electrophoretic mobility of gC-2 extracted from HEp-2 cells was comparable to that shown in Fig. 1 for BHK cell-derived gC-2, whereas the apparent molecular mass of gC-2 purified from GMK AH1 cells was ca. 2 to 3 kDa less than those of two other preparations of gC-2 (data not shown). gC-2 derived from HEp-2 cells bound slightly more HS than the corresponding protein extracted from BHK cells (Fig. 2A). In addition, at a concentration of 5 μg, gC-2 purified from GMK AH1 and HEp-2 cells bound comparable amounts of HS.
FIG. 1.
Electrophoretic analysis of affinity-purified gB-1, gB-2, gC-1, and gC-2. Glycoproteins were subjected to electrophoresis under reducing conditions on a 10% acrylamide tris-glycine precast gel and stained with a colloidal Coomassie blue kit.
FIG. 2.
(A) HS-binding capabilities of purified gB-1, gB-2, gC-1, and gC-2. 35S-labeled HS from GMK AH1 cells was incubated for 2 h at room temperature with purified viral proteins. Bound HS was trapped on nitrocellulose filters. Values shown are averages of four individual determinations from two separate experiments. Data for gC-2 (HEp-2) and the rest of the proteins were obtained in experiments done at different times. (B) Binding of purified gB-1, gB-2, gC-1, and gC-2 to HS in the presence of increasing concentrations of sodium chloride. Purified proteins were incubated with 35S-labeled HS from GMK AH1 in phosphate buffer supplemented with specific concentrations of sodium chloride. The rest of the procedure was as described for panel A. Values shown are averages of two individual determinations from two separate experiments.
Differences in the HS-binding capacities of gB and gC molecules observed at various protein concentrations inspired us to investigate the effect of increased sodium chloride concentration on binding of purified proteins to HS (Fig. 2B). This assay is a measure of specificity and reflects the relative contribution of electrostatic forces to the formation of protein-HS complexes (see the introduction). The concentrations of sodium chloride that reduced the binding of viral proteins to HS by 50% were 0.18 M for gB-2, 0.19 M for gB-1, 0.23 M for gC-1, and 0.27 M for gC-2. These data indicated that there was a greater contribution of relatively nonspecific electrostatic forces for binding of gB homologs than gC molecules. This could, at least in part, explain the differences in the HS-binding capacity of gB molecules when tested at relatively low and high concentrations of protein.
In addition to examining HS-binding abilities of gB and gC homologs, we compared purified, whole HSV-1 and HSV-2 virions for this ability. Based on the VP5 contents in different HSV-1 and HSV-2 preparations, stocks of purified virus were adjusted to contain the same relative number of virus particles and then tested for the ability to bind GMK AH1 cell-specific and HEp-2 cell-specific 35S-labeled HS (Table 1). HSV-1 virions bound more GMK AH1 cell-specific HS than HSV-2 particles. The same was observed when HEp-2 cell-specific HS was used. In addition, we were interested in whether differential binding of HS by HSV-1 and HSV-2 virions could be related to HSV type-specific differences that affect their sensitivity to polyanions. It was reported (19) that many different polyanionic substances such as heparin, dextran sulfate, agar inhibitors, or dermatan sulfate inhibited HSV-2 infection of cells more efficiently than HSV-1 infection of cells. We sought to examine whether this observation could be extended to a biologically important polyanionic ligand for HSV, HS. To this end, two different preparations of HS, from human aorta (a gift from W. Murphy, Melbourne, Australia) and from bovine kidney (Seikagaku, Tokyo, Japan), were tested for the ability to inhibit HSV-1 and HSV-2 infection of GMK AH1 cells. Virus adsorption to cells in the presence of exogenously added HS was carried out at 4°C. IC50s of human aorta HS were 20 and 3 μg/ml for HSV-1 and HSV-2, respectively. The corresponding values for bovine kidney HS were >500 and 70 μg/ml for HSV-1 and -2, respectively. Thus, as was observed for other polyanions (19), less HS was necessary to inhibit HSV-2 than HSV-1 infection of cells. This difference could, at least in part, be attributed to our observation that HSV-2 virions bound less HS than HSV-1 particles (Table 1).
TABLE 1.
HS-binding abilities of purified HSV-1 and HSV-2 virions
| Source of HSa | Virus | Mean bound HS (% of input 35S-labeled material) ± SD
|
||
|---|---|---|---|---|
| 8b | 1.6b | 0.32b | ||
| GMK cells | HSV-1 | 19.3 ± 1.4 | 13.3 ± 0.6 | 6.4 ± 0.7 |
| HSV-2 | 7.3 ± 0.6 | 4.9 ± 1.0 | 3.0 ± 0.2 | |
| HEp-2 cells | HSV-1 | 24.8 ± 1.7 | 13.3 ± 0.9 | 5.4 ± 0.4 |
| HSV-2 | 10.7 ± 0.6 | 4.3 ± 0.2 | 1.9 ± 0.2 | |
Two separate experiments were carried out in duplicate for each HS preparation.
Relative number of viral VP5 units.
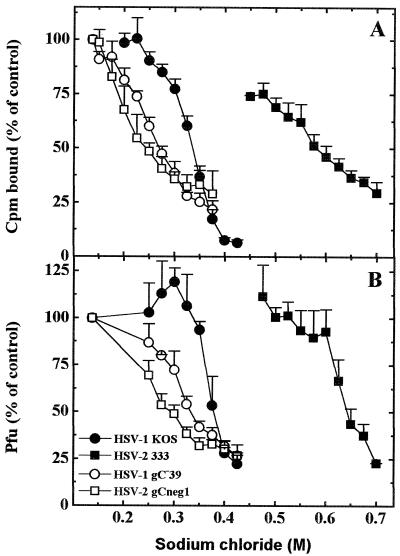
Knowing that the binding of viral gB-1, gB-2, gC-1, and gC-2 to HS molecules was prevented by different concentrations of sodium chloride, we sought to determine whether the binding of whole virus particles to cells would be affected similarly by the increased ionic strength of the medium. To this end, purified radiolabeled virions of HSV-1 KOS 321, HSV-1 gC−39, HSV-2 333, and HSV-2 gC-neg1 were compared for the ability to attach to cells in the presence of increasing concentrations of sodium chloride. It should be noted that this experiment required exposure of cells to hypertonic concentrations of sodium chloride. To protect the cells, all experiments were conducted at 4°C, and the total exposure of cells to increased concentrations of sodium chloride did not exceed 40 min. Further growth of GMK AH1 cells was not affected by this treatment. The concentration of sodium chloride necessary to prevent the binding of HSV-2 gC-neg1 by 50% was 0.24 M (Fig. 3A). The corresponding values were 0.27 M for HSV-1 gC−39, 0.34 M for HSV-1 KOS 321, and 0.58 M for HSV-2 333. Similar tendencies were observed with nonlabeled virus preparations (Fig. 3B). In this experiment, following virus adsorption to cells at 4°C in the presence of sodium chloride, the cells were washed and incubated at 37°C for viral plaques to develop. Differences shown in Fig. 3 cannot be attributed to a direct virus inactivation by hypertonic solutions of sodium chloride because incubation of HSV-1 KOS 321 for 15 min at 4°C in 0.9 M sodium chloride and subsequent restoration of isotonicity did not reduce viral infectivity. These results indicated that a hierarchy of sensitivity of virus binding to increased ionic strength of the medium, i.e., HSV-2 gC-neg1 > HSV-1 gC−39 > HSV-1 KOS 321 > HSV-2 333, correlated with corresponding sensitivities of purified viral proteins, i.e., gB-2 > gB-1 > gC-1 > gC-2 (compare Fig. 2B with Fig. 3).
FIG. 3.
HSV binding to cells in the presence of increasing concentrations of sodium chloride. Purified radiolabeled (A) or nonlabeled (B) HSV-1 KOS 321, HSV-1 gC−39, HSV-2 333, and HSV-2 gC-neg1 were incubated with GMK AH1 cells for 20 min at 4°C in the presence specific concentrations of sodium chloride. Experiments were carried out at 4°C (cold room). Sodium chloride solutions were left on cells for a maximum of 40 min. For further details, see Materials and Methods. Values shown are averages of four individual determinations from two separate experiments.
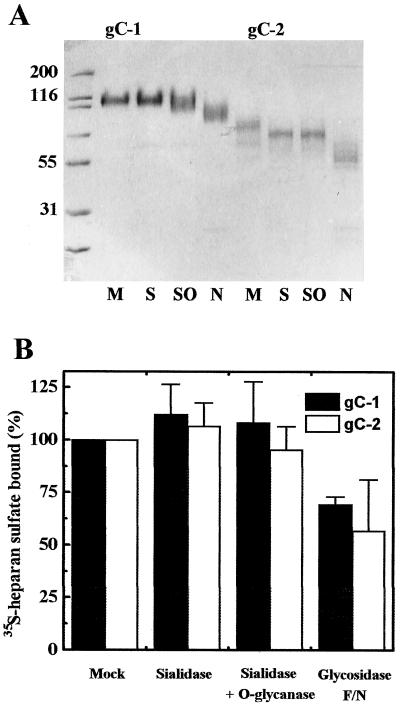
In an attempt to explain the less efficient binding of HS by HSV-2 than HSV-1 virions, we considered the possibility that due to differences in presentation of protein in its purified versus virion-associated form, some glycans may differently modulate the binding of HS in these two systems. To this end, purified gC proteins were treated with neuraminidase, neuraminidase and O-glycanase, and endoglycosidase F/N and then tested for binding of HS. Electrophoretic mobility and HS-binding capabilities of enzyme-treated proteins are shown in Fig. 4. Although we are aware that some sugar residues might not be eliminated by these treatments, removal of terminal sialic acid residues slightly increased HS-binding capabilities of gC-1 and gC-2, whereas further removal of a population of O-glycans specifically cleaved by O-glycanase had little or no effect. In contrast, endoglycosidase F/N-treated gC-1 and gC-2 bound ca. 40 to 50% less HS than untreated controls. Because neuraminidase-treated proteins exhibited slightly enhanced HS-binding capabilities, we examined the possibility that sialic acid may shield the binding of HS by HSV-2 virions. Neuraminidase-treated and mock-treated HSV-1 virions bound 24.1% ± 1.9% and 20.9% ± 1.7% of applied HS, respectively; the corresponding values for HSV-2 virions were 7.7% ± 0.7% and 5.6% ± 0.6%. Thus, as was found with purified proteins (Fig. 4B), HS-binding capabilities of HSV-1 and HSV-2 virions were only slightly augmented by neuraminidase treatment.
FIG. 4.
Carbohydrate modification and HS binding by gC-1 and gC-2. (A) Electrophoretic analysis of mock (M)-, sialidase (S)-, sialidase and O-glycanase (SO)-, and endoglycosidase F/N (N)-treated gC-1 and gC-2. Sizes are indicated in kilodaltons. (B) Binding of HS by sialidase and/or glycosidase-treated gC-1 and gC-2. Results are expressed as percent bound HS relative to mock-treated controls. For further details, see the legend to Fig. 2A.
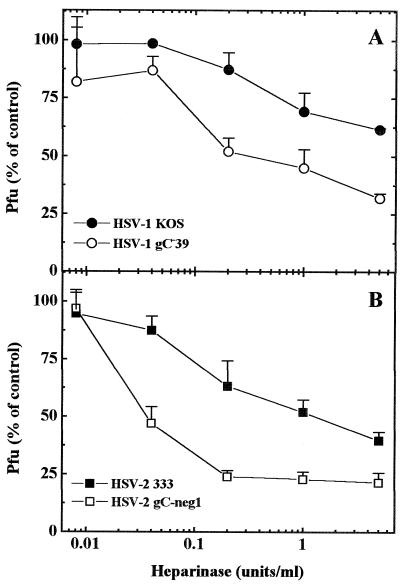
It was reported (10) that wild-type HSV-2 and a gC-null mutant did not differ in their binding to cells, and consequently gB-2 was suggested as a dominant HSV-2 attachment protein. Our data suggest that gC-2 mediated binding of HSV-2 virions that, at least to some extent, appeared to be resistant to increased ionic strength. To further explore the extent of manifestation of gC-2 functions in the HSV-2 background, HSV-2 wild-type and gC-neg1 mutant strains were compared for the ability to form plaques on heparinase-treated GMK AH1 cells. With HSV-1, we invariably observed that when assayed on cells that had been pretreated with relatively low doses of heparinase, the gC-null mutant produced fewer plaques than the wild-type strain (Fig. 5A). This suggested that gC-1 can facilitate virus binding when the density of the HS receptor is reduced. As seen in Fig. 5B, the same was observed for the HSV-2 gC-negative mutant and its wild-type parent. This difference suggests that at least under these specific conditions, the presence of gC-2 is beneficial for HSV-2 virions.
FIG. 5.
Infection of heparinase-treated cells by HSV wild-type and gC-negative mutant strains. GMK AH1 cells in six-well plates cells were pretreated with the indicated number of heparinase Sigma units (1 IU corresponds to approximately 600 Sigma units) and then approximately 200 PFU of KOS 321, gC−39, or 333 or gC-neg1 was added. Values shown for HSV-2 are means of six replicates from three separate experiments. For HSV-1, the results represent means of two replicates from a single experiment.
Interaction of gB and gC homologs with cell surface.
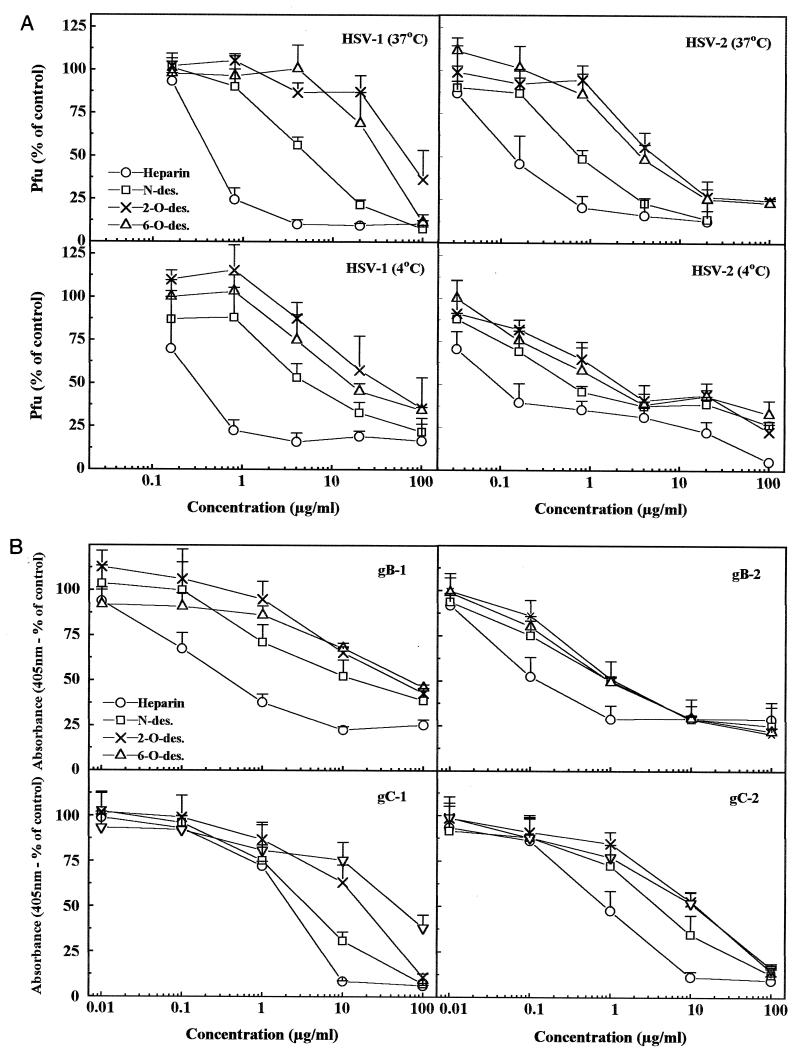
In addition to examining a direct binding of purified proteins to isolated HS chains, we attempted to search for individual specificities in interactions of these proteins with the cell surface. To this end, we tested the effects of selectively or preferentially N-, 2-O-, and 6-O-desulfated preparations of heparin on binding of purified gB-1, gB-2, gC-1, and gC-2 to GMK AH1 cells (Fig. 6B); similar experiments with whole HSV-1 and HSV-2 were also carried out (Fig. 6A). Compositional analysis of the products of N- and 2-O-desulfation of heparin revealed selective removal of N- and 2-O-sulfate groups, respectively (7). In contrast, 6-O-desulfation is a preferential process since in addition to 6-O-sulfate groups, approximately 20 to 30% of 2-O-sulfates are lost. We reasoned that if the sulfate group of a particular type is nonessential for interaction, then its selective removal would impair little or not at all the ability of such modified heparin to compete with cell surface HS for binding to the isolated viral proteins or whole virions. In line with this interpretation, ratios of IC50 of modified heparin to native heparin were calculated for each sample (Table 2). These ratios were higher for gB-1 than gB-2, suggesting that N-, 2-O-, and 6-O-sulfate groups of heparin could be more important for interaction with gB-1 than gB-2, or that the latter could show preference for any two types of sulfates. Little differences were observed between gC-1 and gC-2 (Fig. 6; Table 2). However, an overall pattern of sensitivity to modified heparins of gC homologs was different from that of gB molecules. In particular, less heparin (determined as IC50) was needed to inhibit gB than gC binding; however, a significant proportion of binding of gB to cells was insensitive to heparin. In addition, we found some similarities between gB-2 and whole HSV-2 (with binding step at 4°C) patterns of sensitivity to modified heparins (similar IC50s and incomplete inhibition of binding). Thus, our results suggest that all three kinds of sulfate groups played a role in interaction with both types of HSV in the order of significance 2-O-sulfates = 6-O-sulfates > N-sulfates. Because IC50 ratios of modified heparins to native heparin were higher for HSV-1 than HSV-2, it is likely that 6-O-, 2-O-, and to some extent N-sulfate groups could be more important for interaction with HSV-1 than HSV-2 (Table 2). A similar observation regarding these two viruses was made earlier by others (13).
FIG. 6.
Sensitivities of HSV-1, HSV-2, and purified gC and gB homologs to selectively desulfated heparin compounds. (A) HSV-1 and HSV-2 were preincubated for 10 min at either 4 or 37°C in the presence of fivefold-increasing concentrations of N-, 2-O-, or 6-O-desulfated samples of heparin. Mixtures were transferred to GMK AH1 cells and held for 1 h at either 4 or 37°C. The number of PFU is expressed as a percentage of the average number of plaques formed in the absence of competitor. Two to three separate experiments were carried out in duplicate for each sample. (B) Purified gB-1, gB-2, gC-1, and gC-2 were preincubated for 10 min at 4°C in the presence of 10-fold-increasing concentrations of modified heparin samples. The mixtures were transferred to GMK AH1 cells in 96-well plates and left for attachment for 1 h at 4°C. Bound glycoproteins were detected by an ELISA-based procedure. Results are means of six replicates from two separate experiments.
TABLE 2.
Sensitivities of HSV-1, HSV-2, and purified viral proteins to modified heparin compounds
| Prepn | IC50 (μg/ml)
|
|||
|---|---|---|---|---|
| Heparin | N-desulfated heparin | 2-O-desulfated heparin | 6-O-desulfated heparin | |
| HSV-1, 37°C | 0.4 | 5 (13a) | 60 (150) | 30 (75) |
| HSV-2, 37°C | 0.1 | 0.8 (8) | 5 (50) | 3 (30) |
| HSV-1, 4°C | 0.3 | 5 (17) | 30 (100) | 10 (33) |
| HSV-2, 4°C | 0.09 | 0.6 (7) | 2 (22) | 1 (11) |
| gB-1 | 0.4 | 10 (25) | 50 (125) | 60 (150) |
| gB-2 | 0.1 | 0.9 (9) | 0.9 (9) | 1 (10) |
| gC-1 | 2 | 3 (2) | 10 (5) | 40 (20) |
| gC-2 | 0.8 | 3 (4) | 10 (13) | 10 (13) |
Ratio of modified heparin to native heparin.
DISCUSSION
A number of type-specific differences have been reported for the early interaction of HSV-1 and HSV-2 with cells. In addition to having distinct preferential tropisms, these two viruses differ in their sensitivity to polyanions (19) and polycations (24, 25), preference for specific structural features of HS/heparin chains (13), binding to the C3b component of complement (8), and stimulation of viral infection in glycosaminoglycan-deficient cells by dextran sulfate (5). In the present investigation, we observed another type-specific difference: purified HSV-2 virions bound fewer isolated HS molecules than HSV-1 virions. Because the structures of HS chains produced in particular cells are thought to be similar (for a review, see reference 40), it is likely that physical abilities to bind HS are greater for HSV-1 than HSV-2. However, the possibility that decreased binding of HS by HSV-2 could be attributed to the affinity of the virus for some rare structural features of HS chains cannot be excluded. In addition, the binding experiments in the presence of increased ionic strength revealed that the binding of HSV-1 to cells is more dependent on relatively nonspecific electrostatic forces than the attachment of HSV-2 virions. gC-2 appeared to mediate this characteristic binding of HSV-2 to cells. The binding of HSV gC-negative mutants as well as that of their putative attachment proteins, (gB homologs) was found to rely on a substantial contribution of electrostatic forces. Such the binding is known to be critically dependent on the ligand concentration, a feature that may explain decreased infectivity of the gC-negative mutant on heparinase-treated cells or efficient binding of HS by gB homologs that occurred only at a high concentration of protein.
In addition, our data could provide an explanation of other HSV type-specific phenotypes, especially those associated with greater sensitivity to polyanions of HSV-2 than of HSV-1. Because a likely mechanism of virus-HS interaction is binding of negatively charged sulfate/carboxylate groups of HS chains to positively charged HS-binding domains in the viral envelope glycoprotein gC and/or gB, our results suggest some basic differences in an overall positive charge or a distribution of charges on the surfaces of HSV-1 and HSV-2. In a viral plaque assay, we observed that HSV-2 was more sensitive than HSV-1 to different HS preparations. Hutton et al. (19) found that HSV-2 strains were more sensitive than HSV-1 strains to several polyanionic substances of carbohydrate nature such as heparin, dextran sulfate, and dermatan sulfate. This type-specific difference could, at least in part, be related to our observation that HSV-2 virions bound less HS than did HSV-1 virions. Thus, irrespective of the competition capacity of a particular compound, less HS or other polyanions might be necessary to saturate or block the HS-binding sites on the HSV-2 than on the HSV-1 virion surface.
Both gB and gC molecules of the virus envelope could mediate binding to HS (10, 14, 15). To understand the relative contribution of gB and gC for HSV attachment to cells, we tested the binding of purified gB-1, gB-2, gC-1, and gC-2 to isolated HS receptor molecules. gB-1 and gB-2 did not differ with respect to the amount of HS bound. Similarly, gC-1 and gC-2 bound comparable amounts of HS (the HEp-2 cell-extracted gC-2 bound slightly more HS than gC-1); however, these proteins exhibited greater HS-binding capacities than gB homologs when tested at a relatively low concentration of protein. Interestingly, even though the potentials to bind to HS were similar for gB homologs and for the gC homolog, purified HSV-1 virions, as noted before, bound more HS than HSV-2 virions. Similar observations on the binding of the C3b component of complement by purified viral components and by HSV-infected cells were made earlier by others (6, 8, 34). While purified gC-1 and gC-2 bound C3b efficiently (6), HSV-1-infected but not HSV-2-infected cells exhibited this activity (8). These data together with the observation that the gC-2-negative HSV-2 mutant bound to cells as efficiently as the wild-type gC-2-positive strain (10) suggest that HS- and C3b-binding potentials of gC-2 are not fully exhibited when this protein is present in the HSV-2 background. In contrast to HSV-2-infected cells, transfected cells expressing gC-2 on their surfaces bound C3b efficiently, suggesting that an HSV-2-specific protein(s) may shield and/or interact with gC-2, thus reducing its functions, although induction of C3b receptor as a result of relative overexpression of gC-2 on transfected cells compared to virus-infected cells cannot be excluded (41).
In our attempt to explain the reduced binding of HS by HSV-2 virions compared to HSV-1 particles, we focused on the structural differences between gC-1 and gC-2. In gC-1, a major part of the HS-binding domain was localized around the base of a small amino-terminal Cys127-Cys144 loop (39) and was found to comprise several positively charged amino acids scattered between residues 129 through 160 (51; K. Mårdberg, E. Trybala, J. C. Glorioso, and T. Bergström, submitted for publication). Positively charged amino acids in the gC-1 segment delimited by Lys114 and Asp128 can also contribute to the virus binding to cells (49; Mårdberg et al., submitted). An entire gC-1 segment that is located N terminally to the HS-binding domain (amino acids 26 through 113) represents in fact a mucin-like region with 22 predicted O-glycosylation (12) and 5 N-glycosylation (9) sites. Of these, no more than 13 O-glycosylation sites and at least 4 N-glycosylation sites are occupied in baculovirus-expressed gC-1 (39). The HS-binding domain of gC-2 has not been localized. However, by analogy to gC-1, positively charged amino acids scattered around the base of Cys96-Cys113 loop, and a cluster of basic residues between Arg66 and Lys72 are likely candidates. In contrast to gC-1, the N-terminal part of gC-2 may not possess the mucin-like structure (39), as in this region there are only four predicted O-glycosylation sites (12), and none of them were detected to be occupied in baculovirus-expressed gC-2 (39). Instead, positively charged amino acids are scattered throughout the N-terminal part of gC-2 (48). With these differences in mind, we tested how different glycans would modify HS-binding abilities of gC-1 and gC-2. Although electrophoretic analysis suggested that gC-2 might contain more sialic acid residues than gC-1, removal of this sugar had only a slight enhancing effect on the binding of HS by both gC-1 and gC-2. Similarly, neuraminidase-treated HSV-2 virions bound only slightly more HS molecules than the sham-treated virions, suggesting that sialic acid was not the factor that markedly masked this activity in HSV-2 virions. It was reported that neuraminidase treatment of either HSV-1-infected cells (44) or purified gC-1 and gC-2 (6) substantially enhanced binding of complement component C3b. This treatment, however, did not unmask C3b receptor on HSV-2-infected cells (44). Further, we found that O-glycanase treatment had little or no effect on HS binding by gC-1 and gC-2, whereas removal of N-glycans decreased binding activity by 40 to 50%. Similar results were reported for studies on the effect of removal of these glycans on binding of gC-1 to cells (49). As no enhancing effects were observed, it is unlikely that either O- or N-linked glycans of gC-2 could down-modulate the binding of HS by HSV-2 virions. However, apart from modulation of biological activities, the mucin-like domains may influence the structure of a protein. Thus, one can speculate that due to absence of a mucin-like region in gC-2, this protein may not adopt a long and slender morphology as was reported for gC-1 (45), a structural feature that could affect its attachment functions. In addition, one cannot exclude that the N-terminal part of gC-2 could be a target for nonspecific interactions due to the presence of multiple basic amino acids (calculated isoelectric points for gC-2 and gC-1 are 10.34 and 7.48, respectively). In this regard, either expression of a limited number of gC-2 copies or occurrence of gC-2 in complexes with other HSV-2 proteins could be of benefit. Thus, differences in presentation and/or quantity of gC copies in the virus envelope may account for the reduced binding of HS by HSV-2 virions compared to HSV-1 particles.
In this study, we observed that HSV-1 and HSV-2 virions differed with respect to the quantity of HS chains bound. Herold et al. (13) observed some qualitative differences between susceptibility of HSV-1 and HSV-2 to modified heparin compounds. HSV-1 appeared to be less sensitive than HSV-2 to O-desulfated heparin preparations, suggesting that 2-O- and 6-O-sulfate groups of heparin or HS are important determinants for interaction with HSV-1 (13). Here we confirmed this observation and extended this approach to compare the abilities of selectively N-, 2- O-, and 6-O-desulfated heparin preparations to inhibit binding to cells of purified gB-1, gB-2, gC-1, and gC-2. As noted before, O-sulfate groups of heparin appeared to be more important for interaction with gB-1 than gB-2. In addition the patterns of sensitivity to modified heparins of gB homologs were distinct from those of gC proteins. This finding, together with the observation that gB homologs bound fewer HS than gC molecules, suggests that these proteins may contribute different functions in early virus-cell interaction. Presumably these functions are balanced differently in the two types of HSV. Given the presence in the HSV-1 envelope of approximately 3,200 and 4,900 copies of gB-1 and gC-1, respectively (11), and the length of HS chains exceeding 40 nm, multiple gC-1 and gB-1 molecules are likely to interact with single HS chain. It is noteworthy that gB, gC, and another HSV-1 protein gD were reported to function as oligomeric complexes in the virus envelope (11). It has recently been reported that in addition to gB and gC, gD-1 bound HS chains that had been modified by 3-O-sulfotransferase isoform 3 and that this event triggered HSV-1 entry into CHO cells (43). Prior gC- and/or gB-mediated immobilization of HS chains on the virus surface may help gD recognize the specific binding site on the chain.
ACKNOWLEDGMENTS
We thank Dorothe Spillmann (Uppsala University, Uppsala, Sweden) for modified heparin compounds and Gary Cohen and Roselyn Eisenberg (University of Pennsylvania) for polyclonal rabbit sera.
This work was supported by grants (all to T.B.) from the Stiftelsen för Strategisk Forskning “Glycoconjugates in Biological Systems,” Swedish Medical Research Council (no. 11225), Sahlgren's University Hospital Läkarutbildningsavtal, and Scandinavian Society for Antimicrobial Chemotherapy.
REFERENCES
- 1.Bergström T, Sjögren-Janson E, Jeansson S, Lycke E. Mapping neuroinvasiveness of the herpes simplex virus type 1 encephalitis-inducing strain 2762 by the use of monoclonal antibodies. Mol Cell Probes. 1992;6:41–49. doi: 10.1016/0890-8508(92)90070-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camdadelli-Fiume G, Stripe D, Boscaro A, Avitable E, Foa-Tomasi L, Barker D, Roizman B. Glycoprotein C-dependent attachment of herpes simplex virus to susceptible cells leading to productive infection. Virology. 1990;178:213–222. doi: 10.1016/0042-6822(90)90396-9. [DOI] [PubMed] [Google Scholar]
- 3.Danishefsky I, Steiner H. Investigations on the chemistry of heparin. V. Disaccharides obtained after partial hydrolysis. Biochim Biophys Acta. 1965;101:37–45. doi: 10.1016/0926-6534(65)90028-x. [DOI] [PubMed] [Google Scholar]
- 4.Duff R, Rapp F. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol. 1971;233:48–50. doi: 10.1038/newbio233048a0. [DOI] [PubMed] [Google Scholar]
- 5.Dyer A P, Banfield B W, Martindale D, Spannier D-M, Tufaro F. Dextran sulfate can act as an artificial receptor to mediate a type-specific herpes simplex virus infection via glycoprotein B. J Virol. 1997;71:191–198. doi: 10.1128/jvi.71.1.191-198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenberg R J, Ponce de Leon M, Friedman H M, Fries L, Frank M M, Hastings J, Cohen G H. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus type 1 and 2. Microb Pathog. 1987;3:423–425. doi: 10.1016/0882-4010(87)90012-x. [DOI] [PubMed] [Google Scholar]
- 7.Feyzi E, Trybala E, Bergström T, Lindahl U, Spillmann D. Structural requirement of heparan sulfate for interaction with herpes simplex type 1 virions and isolated glycoprotein C. J Biol Chem. 1997;272:24850–24857. doi: 10.1074/jbc.272.40.24850. [DOI] [PubMed] [Google Scholar]
- 8.Friedman H M, Cohen G H, Eisenberg R J, Seidel C, Cines D B. Glycoprotein C of herpes simplex virus type 1 acts as a receptor for C3b component of complement on infected cells. Nature. 1984;309:633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- 9.Frink R J, Eisenberg R J, Cohen G H, Wagner E K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983;45:634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber S I, Belval B J, Herold B C. Differences in the role of glycoprotein C of HSV-1 and HSV-2 in viral binding may contribute to serotype differences in cell tropism. Virology. 1995;214:29–39. doi: 10.1006/viro.1995.9957. [DOI] [PubMed] [Google Scholar]
- 11.Handler C G, Eisenberg R J, Cohen G H. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J Virol. 1996;70:6067–6075. doi: 10.1128/jvi.70.9.6067-6070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen J E, Lund O, Engelbrecht J, Bohr H, Nielsen J O, Hansen J E. Prediction of O-glycosylation in mammalian proteins: specificity patterns of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase. Biochem J. 1995;308:801–813. doi: 10.1042/bj3080801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herold B C, Gerber S I, Belval B J, Siston A M, Shulman N. Differences in the susceptibility of herpes simplex virus types 1 and 2 to modified heparin compounds suggest serotype differences in viral entry. J Virol. 1996;70:3461–3469. doi: 10.1128/jvi.70.6.3461-3469.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herold B C, WuDunn D, Visalli R J, Sumarski N, Brandt C, Spear P G. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulfate and glycoprotein B. J Gen Virol. 1994;75:1211–1222. doi: 10.1099/0022-1317-75-6-1211. [DOI] [PubMed] [Google Scholar]
- 16.Holland T C, Homma F L, Marlin S D, Levine M, Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984;52:566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland T C, Marlin S D, Levine M, Glorioso J. Antigenic variants of herpes simplex virus selected with glycoprotein-specific monoclonal antibodies. J Virol. 1983;45:672–682. doi: 10.1128/jvi.45.2.672-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homma F L, Purifoy D J M, Glorioso J C, Levine M. Molecular basis of glycoprotein C-negative phenotypes of herpes simplex virus type 1 mutants selected with a virus-neutralizing monoclonal antibody. J Virol. 1986;58:281–289. doi: 10.1128/jvi.58.2.281-289.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutton R D, Ewert D L, French G R. Differentiation of types 1 and 2 of herpes simplex virus by plaque inhibition with sulfated polyanions. Proc Soc Exp Biol Med. 1973;142:27–29. doi: 10.3181/00379727-142-36950. [DOI] [PubMed] [Google Scholar]
- 20.Inoue Y, Nagasawa K. Selective N-desulfation of heparin with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1976;46:87–95. doi: 10.1016/s0008-6215(00)83533-8. [DOI] [PubMed] [Google Scholar]
- 21.Jaseja M, Rej R N, Sauriol F, Perlin A. Novel regio- and stereoselective modifications of heparin in alkaline solution. Nuclear magnetic resonance spectroscopic evidence. Can J Chem. 1989;67:1449–1456. [Google Scholar]
- 22.Jeansson S, Molin L. On the occurrence of genital herpes simplex virus infection. Clinical and virological findings and relation to gonorrhoea. Acta Dermatol Venereol. 1974;54:479–485. [PubMed] [Google Scholar]
- 23.Karger A, Mettenleiter T C. Glycoproteins gIII and gp50 play a dominant roles in the biphasic attachment of pseudorabies virus. Virology. 1993;194:654–664. doi: 10.1006/viro.1993.1305. [DOI] [PubMed] [Google Scholar]
- 24.Langeland N, Moore L J, Holmsen H, Haarr L. Interaction of polylysine with the cellular receptor for herpes simplex virus type 1. J Gen Virol. 1988;69:1137–1145. doi: 10.1099/0022-1317-69-6-1137. [DOI] [PubMed] [Google Scholar]
- 25.Langeland N, Moore A L J, Lillehaug J R, Haarr L. Evidence that neomycin inhibits binding of herpes simplex virus type 1 to cellular receptor. J Virol. 1987;61:3388–3393. doi: 10.1128/jvi.61.11.3388-3393.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langeland N, Oyan A M, Marsden H S, Cross A, Glorioso J C, Moore L, Haarr L. Localization on the herpes simplex virus type 1 genome of a region encoding proteins involved in adsorption to cellular receptor. J Virol. 1990;64:1271–1277. doi: 10.1128/jvi.64.3.1271-1277.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindahl U, Cifonelli J A, Lindahl B, Rodén L. The role of serine in the linkage of heparin to protein. J Biol Chem. 1965;240:2817–2820. [PubMed] [Google Scholar]
- 28.Lloyd A G, Embery G, Fowler L J. Studies on heparin degradation. I. Preparation of [35S]sulphamate derivatives for studies on heparin degrading enzymes of mammalian origin. Biochem Pharmacol. 1971;20:637–648. doi: 10.1016/0006-2952(71)90150-x. [DOI] [PubMed] [Google Scholar]
- 29.Lycke E, Johansson M, Svennerholm B, Lindahl U. Binding of herpes simplex virus to cellular heparan sulphate, an initial step in the adsorption process. J Gen Virol. 1991;72:1131–1137. doi: 10.1099/0022-1317-72-5-1131. [DOI] [PubMed] [Google Scholar]
- 30.Lyon M, Deakin J A, Mizuno K, Nakamura T, Gallagher J T. Interaction of hepatocyte growth factor with heparan sulfate. Elucidation of the major heparan sulfate structural determinants. J Biol Chem. 1994;269:11216–11223. [PubMed] [Google Scholar]
- 31.Maccarana M, Lindahl U. Mode of interaction between platelet factor 4 and heparin. Glycobiology. 1993;3:271–277. doi: 10.1093/glycob/3.3.271. [DOI] [PubMed] [Google Scholar]
- 32.Manning G S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978;11:179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- 33.McClain D S, Fuller A O. Cell-specific kinetics and efficiency of herpes simplex virus type 1 entry are determined by two distinct phases of attachment. Virology. 1994;198:690–702. doi: 10.1006/viro.1994.1081. [DOI] [PubMed] [Google Scholar]
- 34.McNearney T A, Odell C, Holers V M, Spear P G, Atkinson J P. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J Exp Med. 1987;166:1525–1535. doi: 10.1084/jem.166.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagasawa K, Inoue Y, Kamata T. Solvolytic desulfation of glycosaminoglycuronan sulfates with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1977;58:47–55. doi: 10.1016/s0008-6215(00)83402-3. [DOI] [PubMed] [Google Scholar]
- 36.Norais N, Tang D, Kaur S, Chamberlain S H, Masiarz F R, Burke R L, Marcus F. Disulfide bonds of herpes simplex virus type 2 glycoprotein gB. J Virol. 1996;70:7379–7387. doi: 10.1128/jvi.70.11.7379-7387.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oyan A M, Dolter K E, Langeland N, Goins W F, Glorioso J C, Haarr L, Crumpacker C S. Resistance of herpes simplex virus type 2 to neomycin maps to N-terminal portion of glycoprotein C. J Virol. 1993;67:2434–2441. doi: 10.1128/jvi.67.5.2434-2441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins A K, Dorney D J, Wathen M W, Whealy M E, Gold C, Watson R J, Holland L E, Weed S E, Levine M, Glorioso J C, Enquist L W. The pseudorabies virus gII gene is closely related to the gB glycoprotein gene of herpes simplex virus. J Virol. 1987;61:2691–2701. doi: 10.1128/jvi.61.9.2691-2701.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rux A H, Moore W T, Lambris J D, Abrams W R, Peng C, Friedman H M, Cohen G H, Eisenberg R J. Disulfide bond structure determination and biochemical analysis of glycoprotein C from herpes simplex virus. J Virol. 1996;70:5455–5465. doi: 10.1128/jvi.70.8.5455-5465.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salmivirta M, Lidholt K, Lindahl U. Heparan sulfate: a piece of information. FASEB J. 1996;10:1270–1279. doi: 10.1096/fasebj.10.11.8836040. [DOI] [PubMed] [Google Scholar]
- 41.Seidel-Dugan C, Ponce de Leon M, Friedman H M, Fries L F, Frank M M, Cohen G H, Eisenberg R J. C3b receptor activity on transfected cells expressing glycoprotein C of herpes simplex virus types 1 and 2. J Virol. 1988;62:4027–4036. doi: 10.1128/jvi.62.11.4027-4036.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shieh M T, Spear P G. Herpesvirus-induced cell fusion that is dependent on cell surface heparan sulfate or soluble heparin. J Virol. 1994;68:1224–1228. doi: 10.1128/jvi.68.2.1224-1228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shukla D, Liu J, Blaiklock P, Shworak N W, Bai X, Esko J D, Cohen G H, Eisenberg R J, Rosenberg R D, Spear P G. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 44.Smiley M L, Friedman H M. Binding of complement component C3b to glycoprotein C is modulated by sialic acid on herpes simplex virus type 1-infected cells. J Virol. 1985;55:857–861. doi: 10.1128/jvi.55.3.857-861.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stannard L M, Fuller A O, Spear P G. Herpes simplex virus glycoproteins associated with different morphological entities projecting from the virus envelope. J Gen Virol. 1987;68:715–725. doi: 10.1099/0022-1317-68-3-715. [DOI] [PubMed] [Google Scholar]
- 46.Stuve L L, Brown-Shimer S, Pachl C, Najarian R, Dina D, Burke R L. Structure and expression of the herpes simplex virus type 2 glycoprotein gB gene. J Virol. 1987;61:326–335. doi: 10.1128/jvi.61.2.326-335.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svennerholm B, Jeansson S, Vahlne A, Lycke E. Involvement of glycoprotein C (gC) in adsorption of herpes simplex virus type 1 (HSV-1) to cells. Arch Virol. 1991;120:273–279. doi: 10.1007/BF01310482. [DOI] [PubMed] [Google Scholar]
- 48.Swain M A, Peet R W, Galloway D A. Characterization of the gene encoding herpes simplex virus type 2 glycoprotein C and comparison with the type 1 counterpart. J Virol. 1985;53:561–569. doi: 10.1128/jvi.53.2.561-569.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tal-Singer R, Peng C, Ponce de Leon M, Abrams W R, Banfield B W, Tufaro F, Cohen G H, Eisenberg R J. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol. 1995;69:4471–4483. doi: 10.1128/jvi.69.7.4471-4483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson L D, Pantoliano M W, Springer B A. Energetic characterization of the basic fibroblast growth factor-heparin interaction: identification of the heparin binding domain. Biochemistry. 1994;33:3831–3840. doi: 10.1021/bi00179a006. [DOI] [PubMed] [Google Scholar]
- 51.Trybala E, Bergström T, Svennerholm B, Jeansson S, Glorioso J C, Olofsson S. Localization of a functional site of herpes simplex virus type 1 glycoprotein C involved in binding to cell surface heparan sulfate. J Gen Virol. 1994;75:743–752. doi: 10.1099/0022-1317-75-4-743. [DOI] [PubMed] [Google Scholar]
- 52.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]