Abstract
Streptococcus pyogenes genotype emm1 is a successful, globally distributed epidemic clone that is regarded as inherently virulent. An emm1 sublineage, M1UK, that produces increased levels of SpeA toxin was associated with increased scarlet fever and invasive infections in England in 2015/2016. Defined by 27 SNPs in the core genome, M1UK is now dominant in England. To more fully characterize M1UK, we undertook comparative transcriptomic and proteomic analyses of M1UK and contemporary non-M1UK emm1 strains (M1global). Just seven genes were differentially expressed by M1UK compared with contemporary M1global strains. In addition to speA, five genes in the operon that includes glycerol dehydrogenase were upregulated in M1UK (gldA, mipB/talC, pflD, and phosphotransferase system IIC and IIB components), while aquaporin (glpF2) was downregulated. M1UK strains have a stop codon in gldA. Deletion of gldA in M1global abrogated glycerol dehydrogenase activity, and recapitulated upregulation of gene expression within the operon that includes gldA, consistent with a feedback effect. Phylogenetic analysis identified two intermediate emm1 sublineages in England comprising 13/27 (M113SNPs) and 23/27 SNPs (M123SNPs), respectively, that had failed to expand in the population. Proteomic analysis of invasive strains from the four phylogenetic emm1 groups highlighted sublineage-specific changes in carbohydrate metabolism, protein synthesis and protein processing; upregulation of SpeA was not observed in chemically defined medium. In rich broth, however, expression of SpeA was upregulated ~10-fold in both M123SNPs and M1UK sublineages, compared with M113SNPs and M1global. We conclude that stepwise accumulation of SNPs led to the emergence of M1UK. While increased expression of SpeA is a key indicator of M1UK and undoubtedly important, M1UK strains have outcompeted M123SNPs and other emm types that produce similar or more superantigen toxin. We speculate that an accumulation of adaptive SNPs has contributed to a wider fitness advantage in M1UK on an inherently successful emm1 streptococcal background.
Keywords: Streptococcus pyogenes, superantigen, scarlet fever, genomics, proteome
Data Summary
RNA-seq: all new RNA-seq data are uploaded to the European Nucleotide Archive under project reference PRJEB58303. Genomic data: all genomes listed are available on the European Nucleotide Archive using accession numbers as listed in the appendix. Proteomic data are available as a Microbiology Society FigShare item [1]: https://doi.org/10.6084/m9.figshare.22138172.v1
Impact Statement.
Although the major Streptococcus pyogenes reservoir is in children with pharyngitis and skin infections, S. pyogenes can lead to rarer, invasive infections that are rapidly progressive and associated with high mortality and morbidity. Emm1 S. pyogenes strains are the single most frequent genotype causing invasive infections in high-income countries and are established worldwide as an epidemic clone. The M1UK S. pyogenes emm1 sublineage, which is defined by 27 new SNPs in the core genome, and characterized by increased scarlet fever toxin SpeA production, emerged and rose to dominance over a period of 5–6 years since initial recognition, outcompeting other emm1 strains in England. Increased dominance of emm1 among invasive infections in the winter of 2022/23, on a background of already-increased numbers of S. pyogenes infections, points to a key shift in host–pathogen interaction. We hypothesize that a combination of pathogen fitness, virulence and host susceptibility have coalesced to account for the excess of circulating S. pyogenes and emm1 invasive infections. In this paper we undertake a systems-based evaluation of M1UK in comparison to older non-M1UK emm1 strains, and identify a number of pathways that are altered in addition to the previously reported increased SpeA expression. The emergence of a new sublineage within an already virulent clone requires ongoing surveillance, and more detailed investigation of the likely mechanisms leading to increased fitness. The capacity of S. pyogenes to cause outbreaks at a national scale highlights a potential need to consider strain-specific public health guidance, underlining the inherent virulence of this exclusively human pathogen.
Introduction
The modern-day Streptococcus pyogenes emm1 genotype emerged in the 1980s and spread globally to become the leading cause of invasive S. pyogenes infection throughout the developed world [2, 3]. The lineage expanded following a recombination event that conferred increased expression of the NADase/streptolysin O (nga/slo) toxin gene locus, and was associated with specific prophage content, including a prophage encoding the superantigen, streptococcal pyrogenic exotoxin A (SpeA) [2]. More recently, during a period of increased scarlet fever activity in England, a new sublineage of emm1 S. pyogenes (M1UK) was detected and found to have expanded [4]. These M1UK strains were strongly associated with not only sore throats and scarlet fever, but also increases in cases of invasive infection [4]. The earliest M1UK strain detected to date was in a collection of non-invasive infection isolates from London in 2010, while the first invasive strains were detected in England in 2012. By 2016, the M1UK sublineage represented around 80 % of all invasive emm1 isolates in England [4]; this rose to 91 % by the end of 2020 [5]. Despite differing from older emm1 strains by just 27 core genome SNPs, the new sublineage was characterized by a ten-fold increase in transcription and 9.5-fold increase in median production of the superantigen SpeA. Since 2019, the M1UK lineage has been identified elsewhere in Europe and North America [6–8].
Emm1 strains are the single most dominant cause of invasive S. pyogenes infection in developed countries. In this work, we set out to characterize the wider phenotype of the new sublineage M1UK, and to compare M1UK strains with minor sublineages that appeared briefly as intermediates, although did not expand to the extent of M1UK. We also examined natural mutants of M1UK and the minor sublineages that provide insight into the cost–benefit balance of the changes in this new highly successful group of S. pyogenes M1T1 strains.
Methods
Bacterial strains
S. pyogenes strains used are outlined in Tables S1 and S2, available in the online version of this article; strains stored in 20 % glycerol were streaked onto Columbia blood agar (CBA) prior to broth culture. S. pyogenes were cultured in Todd Hewitt Broth (THB; Oxoid) or chemically defined medium (CDM) comprising iron, phosphate, magnesium, manganese, sodium acetate, calcium, sodium bicarbonate, l-cysteine, bases, vitamins and amino acids, with different carbon sources (Table S3) at 37 °C in 5 % CO2.
RNA sequencing
RNA was extracted from four different S. pyogenes strains from each lineage (Table S1), cultured in THB for 6 h corresponding to late-log growth phase using methods as previously described [4]. RNA sequencing (RNA-seq) of M1global and M1UK RNA was undertaken by Novogene and by the MRC London Institute of Medical Sciences (LMS). Data (deposited in project PRJEB58303) were analysed according to published guidelines [9]. Briefly, read quality was accessed using FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/), filtered and trimmed using trimmomatic [10], and mapped against the MGAS5005 (CP000017) reference genome using bowtie2 [11] with the highest sensitivity options. The resulting alignments were converted to sorted BAM files using vcftools [12]. Initial visualizations of the sequencing mapping were performed using the Integrative Genomics Viewer (IGV) [13] including confirmation of gldA disruption. The mapped RNA-seq reads were then transformed into a fragment count per gene per sample using the HT-seq [14] package. Exploratory data analysis (principal component analysis and heatmap of sample-to-sample distances) of the RNA-seq data was implemented and plotted using the DESeq2 package [15]. Differential expression analysis in each dataset was performed using three different R packages (DESeq2 [15], EdgeR [16] and limma (https://bioconductor.riken.jp/packages/3.0/bioc/html/limma.html)) with a log2fold change of 0.5 and P-adj <0.05 for M1global vs. M1UK, and a log2fold change of 1 and P-adj <0.05 for M1H1488∆gldA vs. M1H1488. Only genes differentially expressed (DE) in at least two of the three softwares used were considered as DE genes and used in analysis. Prophage regions were predicted using phaster [17], and curated by visual assessment and blast alignment.
Gene transcription studies
Specific transcript abundance was evaluated by real time quantitative reverse transcription PCR (qRT-PCR) using a plasmid standard for each gene and compared with the housekeeping gene proS. For the gldA operon plasmid standard, single amplicons were amplified to create a single linear insert (proS-gldA-mipB-pflD-PTS subunit IIC) that was TA-cloned into plasmid pCR2.1. For glpF2 and speA, the plasmid standard comprised just glpF2 and proS, or speA and proS respectively. cDNA synthesis from S. pyogenes RNA was undertaken as previously reported prior to RT-PCR [4]; primers are listed in Table S4. Comparisons were subject to analysis in GraphPad Prism v9. Non-parametric (Mann Whitney U) or t-tests were used; P<0.05 was considered significant.
Genetic manipulation
The gene encoding gldA was mutated by allelic replacement using the suicide vector pUCMUT. A 541 bp fragment upstream of the gldA gene was amplified (forward primer: 5′-AGCGAATTCTCGCCCAAGATTACGAAGG-3′, reverse primer: 5′-GGGGTACCCGTTGAACTCCTTTATCTGTGATT-3′) incorporating 5′ EcoRI and 3′ KpnI restriction sites (shown in italics), and cloned into the suicide vector pUCMUT to produce vector pUCMUTgldAUP. A 532 bp fragment downstream of the gldA gene was amplified (forward primer: 5′-AACTGCAGCTATTGCAGAGCTGGTGCT-3′, reverse primer: 5′ -ACGCGTCGACCGAGTCGATAGGCTAACC-3′) incorporating 5′ PstI and 3′ SalI restriction sites (shown in italics) and cloned into PstI/SalI digested pUCMUTgldAUP to create pUCMUTgldAKO. The construct was introduced into S. pyogenes M1global strains H1488 (M1H1488) and BHS162 (M1BHS162) by electroporation and crossed into the chromosome by homologous recombination. Transformants were selected using kanamycin (400 µg ml−1). Successful disruption of the gldA gene and insertion of the kanamycin resistance cassette was confirmed by PCR, DNA sequencing and whole genome sequencing of mutated strains M1H1488ΔgldA and M1BHS162ΔgldA (isolate identifiers H1589 and H2151 respectively).
GldA activity assay
Cell-free extracts were prepared from bacteria cultured overnight in chemically defined medium containing either 0.5 % glucose or 0.5 % glycerol to an A 600 of 0.6–0.7 (or as close to this as feasible). Bacteria were washed, centrifuged and kept on ice for 1 h within an anaerobic jar, then suspended in 10 mM Tris buffer, pH 9. Cells were disrupted by agitation in three 60 s bursts with 0.1 mm glass beads. Beads were allowed to settle, and the supernatant fluid was centrifuged for 30 s at 14 000 g . GldA results in conversion of glycerol+NAD to dihydroxyacetone+NADH+H+. GldA activity was derived from the increase in absorbance at 340 nm resulting from the reduction of NAD; one unit reduces one micromole of NAD per minute at 25 °C and pH 10.0 under the specific conditions used [18].
Phylogenetic analysis
Emm1 genomes used in phylogenetic analysis were from the UK and are listed in the Supplementary Information. These comprise sequenced non-invasive emm1 isolates (n=139) [4]; sequenced invasive emm1 isolates (n=40) from two studies [4, 19]; 64 invasive emm1 isolates from the British Society for Antimicrobial Chemotherapy (BSAC) collection [20]; and 23 emm1 isolates from a hospital outbreak study [21]. Two new emm1 genomes were sequenced from an additional outbreak and are available from the European nucleotide archive (Project PRJEB36425: ERS4267588 and ERS4267589). Raw reads were trimmed using trimmomatic version 0.36 [10] with default parameters. SNP calling was performed by mapping trimmed reads to the complete emm1.0 MGAS5005 (CP000017) reference genome using Snippy v4.6.0 (https://github.com/tseemann/snippy), with a minimum coverage of 10, minimum fraction of 0.9 and minimum vcf variant call quality of 100. Gubbins v2.4.1 [22] was used to identify and remove recombinant regions from the resulting full genome alignment file. A maximum likelihood phylogeny was created from core SNPs using the general time-reversible (GTR) model of nucleotide substitution with the gamma distributed rate heterogeneity implemented in FastTree v2.1.10–4 [23] Phylogenetic trees were visualized using FigTree v1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/) and Microreact (https://microreact.org/showcase) and edited using INKSCAPE (https://inkscape.org/pt/).
SpeA production
In preliminary work SpeA production by 135 non-invasive strains previously evaluated by transcription [4] was evaluated. SpeA production by 40 invasive S. pyogenes isolates was undertaken using cell-free culture supernatants from isolates cultured in THB for 6 h (overnight when testing n=135 non-invasive isolates), concentrated 5× using Amicon filters, western blotting using a rabbit polyclonal antibody to rSpeA and comparison with standard concentrations of rSpeA expressed from Escherichia coli as previously reported [4]. Supernatants with undetectable levels of SpeA were assigned an arbitrary value of half that of the lowest concentration detectable (usually 12.5 ng ml−1).
Proteomics
In pilot studies (Experiment 1), five strains were randomly selected from each of M1UK or M1global, cultured in 50 ml CDM to an A 600 of 1.2–1.4 for 6 h at 37 °C with 5 % CO2, and then cytosolic, cell wall and supernatant fractions were prepared for proteomic analysis. For proteomic analysis of sublineages (Experiment 2, cytosolic fractions only), strains were randomly selected from five phylogenetic branches within each lineage (five strains per sublineage, four sublineages in total). The supernatant fraction was removed, syringe filtered (Minisart 0.2 µm filter; Sartorius) and proteins precipitated overnight at 4 °C using 10 % trichloroacetic acid precipitation. Cell-wall proteins were extracted from the bacterial pellet using 1 ml of 30 % raffinose, centrifugation at 10 000 r.p.m. for 5 min, followed by resuspending the pellet in 1 ml of cell wall extraction buffer (960 µl 30 % raffinose, 10 µl of 1 M Tris-HCl pH 8, 10 µl of 10 kU ml–1 mutanolysin, 10 µl of 100 mg ml−1 lysozyme and 10 µl protease inhibitor cocktail III; Avantor VWR), followed by incubation at 37 °C for 3 h with occasional turning, and then aspiration of cell wall extract supernatant after centrifugation at 13 000 r.p.m. for 10 min. The residual cytosolic fraction was further mechanically lysed via bead beating for three cycles for 45 s (Lysing Matrix B from MP Bio). The samples of each cellular fraction then underwent centrifugal concentration using 3 kDa filters and buffer was exchanged (Amicon Ultra-15; Millipore) with 50 mM Tris buffer at pH 8. The samples were then submitted to the Proteomics Facility of the National Phenome Centre (London, UK) for LC mass spectrometry (MS). Precipitated samples were dissolved in 8 M urea and 100 mM ammonium bicarbonate (AmBic) by sonicating for 10 min in a water bath. Total protein was determined in all samples by a protein assay (Protein Assay II; BioRad) according to the manufacturer’s instructions. In total, 20 µg of protein was digested by the addition of 40 mM chloracetamide, 10 mM TCEP (Bondbreaker; ThermoScientific) and 0.2 µg trypsin in 100 mM AmBic. Proteins in 8 M urea were diluted to 1 M urea prior to the addition of trypsin and all samples left overnight at 37 °C. Desalting was performed by acidifying samples to 0.5 % trifluroacetic acid (TFA) and adding them to a pre-equilibrated uElution HLB desalting plate (Waters), washing (3×100 µl) with 0.5 % TFA and eluting with 80 % acetonitrile (3×50 µl). All washes were drawn through the plate under vacuum. Desalted peptides were dried completely at 45 °C in a vacuum-centrifuge.
For MS analysis, proteins were dissolved in 0.1 % formic acid by sonicating in a water bath for 10 min. In total, 0.5 µg of peptides was separated over 90 min over a gradient of 10–40 % acetonitrile at 0.5 µl min−1 (0.1 % formic acid) (M-Class UHPLC; Waters) by trapping (nanoE MZ Sym 5 µm 20 mm; Waters) and eluting across a 20 cm C18 column (nanoE MZ HSS T3 1.8 µm 75 µm×200 mm; Waters) and analysed by high definition mass spectrometry (HDMSe) (Synapt G2S; Waters). Data were acquired over 50–2000mz for both low and high energy (switching every 1 s) in positive resolution mode. Lock mass (Leu-enkephalin) was acquired every 60 s. For fragmentation, a ramped collision energy was used between 19 and 45 eV. Data were searched and processed using Progenesis QI for Proteomics (FigShare) [24].
DE proteins with a fold change threshold of 1.5 (P value threshold 0.05) were visualized on volcano plots. Data comparisons are provided in the accompanying supporting Excel file [24]. Enrichment analysis and protein-protein interactions were performed using STRING (https://string-db.org/), a database able to predict direct (physical) and indirect (functional) associations based on collected data across a range of experimental and in silico protein interactions. Proteins with a percentage identity >90 % and a ‘combined interaction score’ >0.7 were used to create a protein network in which the interaction between two proteins was inferred based on the information available in the STRING database and colour coded accordingly.
Results
Transcriptome of M1UK S. pyogenes
When comparing four TH broth-cultured M1UK and four M1global S. pyogenes , significant differential expression of just seven genes was observed (Table 1). As expected, transcription of speA was upregulated ~5-fold in M1UK strains compared with other M1global strains; ~10-fold increased speA transcription by M1UK has previously been confirmed by qRT-PCR of RNA from 135 emm1 isolates [4]. Unexpectedly, transcription of glpF2, a putative aquaporin (identified as Spy1573 in emm1 reference strain MGAS5005), was 5-fold downregulated in M1UK strains. Bioinformatic analysis of the S. pyogenes aquaporin gene glpF2 demonstrated similarity to the glpF3 family of Lactobacillus plantarum reported to be associated with both glycerol and water, but also dihydroxyacetone (DHA) transport [25].
Table 1.
Differentially expressed genes comparing four M1UK and four M1global strains
|
Gene ID |
Gene name |
Description |
Average log2fold change |
Average P adj |
Strand |
|---|---|---|---|---|---|
|
M5005_Spy0996 |
speA2 |
Enterotoxin |
2.361 |
3.731E-09 |
+ |
|
M5005_Spy1573 |
glpF2 |
Glycerol uptake facilitator protein |
−2.423 |
5.143E-09 |
− |
|
M5005_Spy1741 |
gldA |
Glycerol dehydrogenase |
1.024 |
0.0006 |
− |
|
M5005_Spy1742 |
mipB |
Transaldolase |
1.043 |
0.0002 |
− |
|
M5005_Spy1743 |
pflD |
Formate acetyltransferase |
0.963 |
0.0007 |
− |
|
M5005_Spy1744 |
na |
PTS system, cellobiose-specific IIC component |
0.653 |
0.0061 |
− |
|
M5005_Spy1745 |
na |
PTS system, cellobiose-specific IIB component |
0.749 |
0.0204 |
− |
The remaining five DE transcripts that were upregulated in M1UK represented consecutive ORFs in an apparent operon that includes glycerol dehydrogenase (gldA), pyruvate formate lyase (pflD) and a transaldolase-like protein (talC or mipB) as well as phosphotransferase (PTS) system IIC and IIB component genes, annotated as cellobiose-specific.
GldA operon
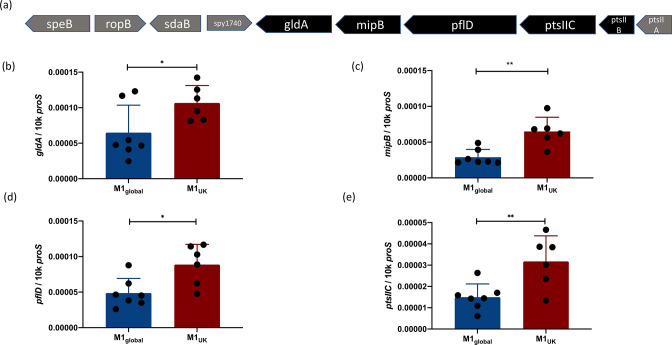
A single SNP in the glycerol dehydrogenase gene gldA among all M1UK strains is known to introduce a premature stop codon at position 175 of the 362 residue enzyme and is predicted to result in a truncated protein with abrogated enzyme activity [4]. The gene gldA is the final ORF in the sequence of genes that was found to be differentially expressed (Fig. 1a). Differential expression of genes comprising the apparent operon was confirmed using qRT-PCR (Fig. 1b–e). Transcription of the aquaporin gene was evaluated in three strains from each lineage, and although non-significant, there was a 2-fold reduction in transcription in M1UK (Fig. S1).
Fig. 1.
The genes within the pflD-mipB-gldA operon are upregulated in M1UK. Five adjacent genes were found to be upregulated in M1UK compared to M1global (a). Genes upregulated in RNA-seq are shown in black and include two components of a PTS annotated as PTS system (cellobiose) subunits IIC and IIB. qRT-PCR using RNA from M1global (n=7) and M1UK (n=6) strains indicating transcription of gldA (b); mipB, also known as talC (c); pflD (d); and PTS subunit IIC (e). Data points (black dots) represent different strains that were each tested in technical triplicates and expressed as copies per 10000 copies of proS. Error bars show sd of the mean.**P<0.01 using an unpaired t-test; *P<0.05.
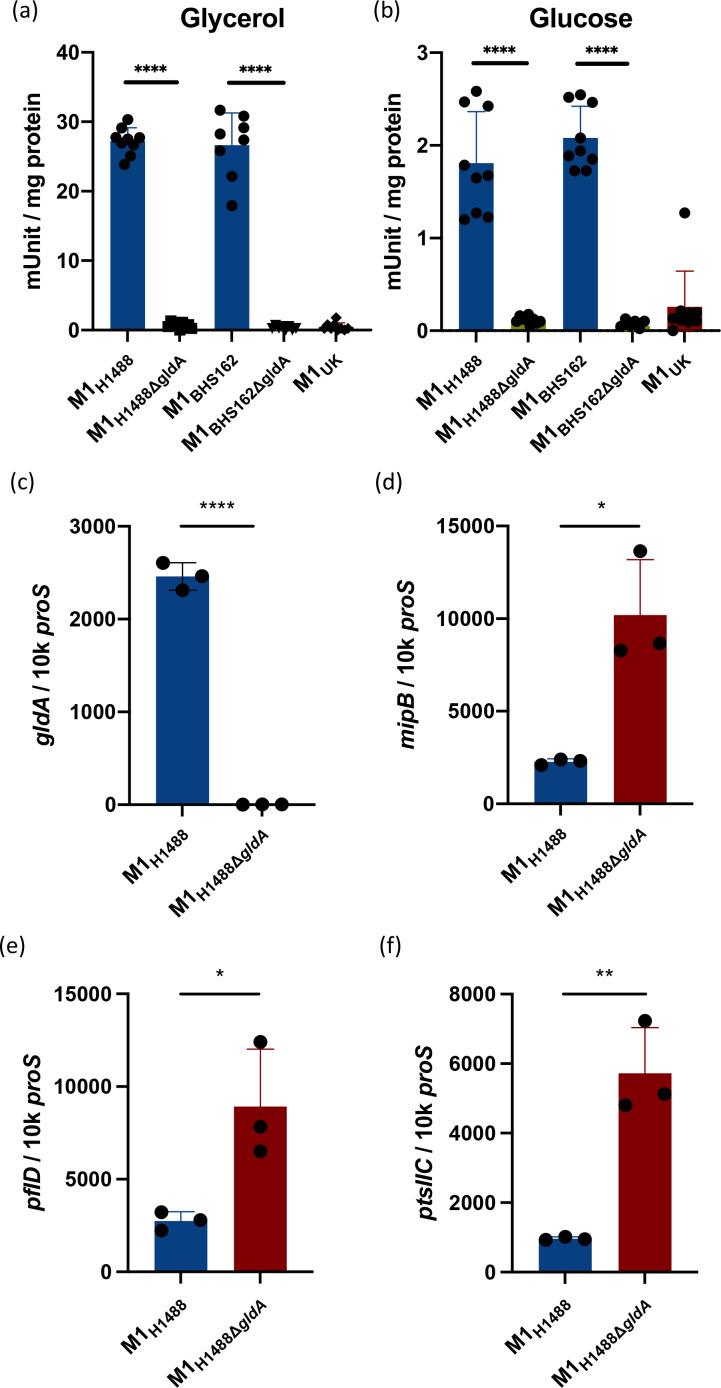
We hypothesized that the loss of GldA enzyme activity may in some way feedback on transcription of the adjacent PTS subunit EII genes, as well as mipB and pflD. To determine the impact of isolated loss of GldA function in S. pyogenes , gldA was disrupted through allelic replacement in M1global strain M1H1488 to create M1H1488ΔgldA. A GldA enzyme activity assay was undertaken, in the presence of glycerol and glucose, to confirm that enzyme function was present in the parent strain, but abrogated in the mutant (Fig. 2a, b); this was replicated using a second pair of isogenic M1global strains (M1BHS162 and M1BHS162ΔgldA). By comparison, M1UK strain BHS581 demonstrated barely detectable GldA activity, similar to the knockouts. RNA from M1H1488 and the isogenic M1H1488ΔgldA was subject to RNA-seq to compare the wider transcriptome of S. pyogenes in the absence of a functional gldA gene. There were almost no changes in the transcriptome except in the genes of the putative pflD–mipB–gldA operon; deletion of gldA abrogated transcription of gldA as expected, but was associated with a clear increase in transcription of pflD, mipB and the adjacent PTS system cellobiose-specific IIC genes. (Table 2). Upregulation of two genes adjacent to one another, Spy0123 and Spy0124 (including sloR), was also observed.
Fig. 2.
Loss of GldA function is associated with upregulated expression of adjacent genes. Glycerol dehydrogenase activity in S. pyogenes M1global is abrogated following inactivation of the gldA gene, to the level observed in M1UK. Activity in the parent strain was greatest when cultured in the presence of glycerol (a) and less in glucose (b). Data show eight or nine individual reactions for each strain. Deletion of the gldA gene resulted in markedly reduced transcription of gldA (c), and upregulated transcription of mipB (d), pflD (e) and PTS component IIC (f). Data show three biological replicates per strain. Error bars show sd of the mean. *P<0.05; **P<0.01; ****P<0.0001 using a Mann–Whitney U test (a, b) and unpaired t-test (c–f).
Table 2.
RNA-seq comparison of gldA-mutant S. pyogenes and parent strain
|
Gene ID |
Gene name |
Description |
Average log2 fold change* |
Average P adj |
Strand |
|---|---|---|---|---|---|
|
M5005_Spy0008 |
divIC |
Cell division protein |
−1.003 |
0.032 |
+ |
|
M5005_Spy0123 |
na |
Translation initiation inhibitor |
1.152 |
0.016 |
+ |
|
M5005_Spy0124 |
sloR |
Transcriptional regulator |
1.250 |
0.0009 |
|
|
M5005_Spy1166 |
na |
Hypothetical protein |
−1.094 |
0.0001 |
− |
|
M5005_Spy1258 |
na |
Putative cytosolic protein |
−1.361 |
0.019 |
− |
|
M5005_Spy1541 |
na |
Hypothetical protein |
−1.030 |
0.018 |
− |
|
M5005_Spy1741 |
gldA |
Glycerol dehydrogenase |
−9.212 |
1.111E-05 |
− |
|
M5005_Spy1742 |
mipB |
Transaldolase |
1.367 |
1.91E-03 |
|
|
M5005_Spy1743 |
pflD |
Formate acetyltransferase |
1.394 |
0.0019 |
|
|
M5005_Spy1744 |
na |
PTS system cellobiose-specific IIC |
1.036 |
0.0036 |
− |
*Comparison is made between M1H1488ΔgldA and parent strain M1H1488; only genes differentially expressed by at least log2 fold value of 1.0 are shown, P<0.05. Genes from the same predicted operon are shaded in grey.
Significant downregulation of gldA transcription, and upregulation of the adjacent genes was confirmed by qRT-PCR (Fig. 2c–f). Taken together, the data suggested that loss of GldA activity led to upregulation of the entire operon that is concerned with metabolism of DHA, fructose 1,6 phosphate and pyruvate. S. pyogenes has been reported to use a number of carbon sources; however, under conditions where emm1 S. pyogenes grew well in CDM supplemented with glucose, we were unable to demonstrate any growth in CDM supplemented with glycerol alone, consistent with other reports [26] (data not shown). Informatic analysis of publicly available genomes from a range of bacterial species demonstrated remarkable conservation of the genes and organization of this region in all members of the Streptococcaciae compared with other species (Fig. S2).
Intermediate sublineages of emm1 S. pyogenes
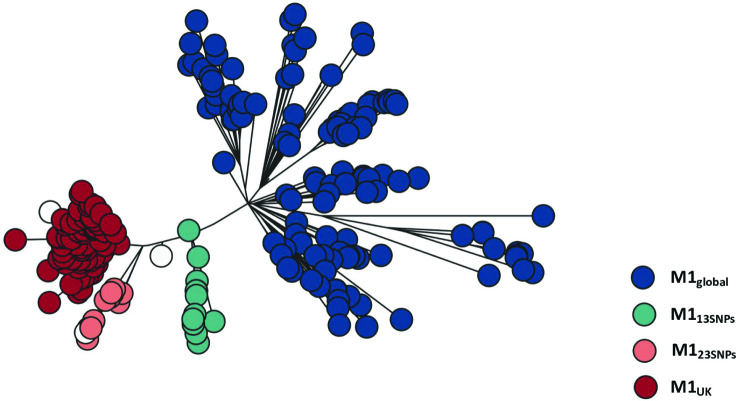
M1UK strains are distinguished from older emm1 strains by the presence of 27 SNPs [4] (Table 3). Although a number of additional indels are common in M1UK, only the 27 SNPs define the new lineage. When analysing genomes from S. pyogenes strains isolated in the UK, we identified small numbers of strains with either 13 or 23 of the 27 SNPs [4]. All emm1 sublineages except M1global possessed three SNPs in the transcriptional regulator RofA, but the gldA stop codon is present only in strains with 23 or 27 SNPs. (Table 3). We analysed our original non-invasive S. pyogenes whole genome sequences alongside other sequenced UK emm1 strains (Table S2) and enriched for sublineages by including 10 invasive isolates from each of the following groups: M1global, M113SNPs, M123SNPs and M1UK (Fig. 3). As reported before, the earliest M1UK strain identified was 2010, but the earliest M113SNPs strain was 2005, from the BSAC collection [20].
Table 3.
SNPs in sublineages
|
Position in MGAS5005 |
Gene locus |
Gene |
Product |
S/NS |
Ref |
SNP |
M113 SNPs |
M119SNPs∗ |
M123SNPs |
M127 SNPs† |
|---|---|---|---|---|---|---|---|---|---|---|
|
115 646 |
M5005_Spy0106 |
rofA |
Transcriptional regulator |
ns |
C |
T |
T |
T |
T |
T |
|
116 162 |
M5005_Spy0106 |
rofA |
Transcriptional regulator |
ns |
A |
C |
C |
C |
C |
C |
|
116 163 |
M5005_Spy0106 |
rofA |
Transcriptional regulator |
ns |
C |
A |
A |
A |
A |
A |
|
250 832 |
M5005_Spy0243 |
ABC transporter-associated protein |
S |
T |
C |
T |
T |
C |
C |
|
|
513 254 |
M5005_Spy0525 |
Galactose-6-phosphate isomerase LacB |
ns |
G |
T |
T |
T |
T |
T |
|
|
528 360 |
Intergenic |
– |
– |
A |
T |
A |
T |
T |
T |
|
|
563 631 |
M5005_Spy0566 |
sagE |
Streptolysin S putative self-immunity protein |
ns |
G |
A |
G |
G |
A |
A |
|
613 633 |
M5005_Spy0609 |
Phosphoglycerol transferase |
ns |
T |
C |
C |
C |
C |
C |
|
|
626 494 |
M5005_Spy0623 |
Methyltransferase |
S |
G |
A |
A |
A |
A |
A |
|
|
661 707 |
M5005_Spy0656 |
trmD |
tRNA (guanine-N (1)-)-methyltransferase |
ns |
G |
A |
G |
A |
A |
A |
|
730 823 |
M5005_Spy0727 |
recJ |
ssDNA-specific exonuclease |
ns |
C |
T |
T |
T |
T |
T |
|
784 467 |
M5005_Spy0779 |
Putative membrane spanning protein |
S |
T |
C |
T |
T |
C |
C |
|
|
819 098 |
M5005_Spy0825 |
murB |
UDP-N-acetylenolpyruvoylglucosamine reductase |
ns |
G |
A |
G |
A |
A |
A |
|
923 079 |
M5005_Spy0933 |
Putative NADH-dependent flavin oxidoreductase |
ns |
G |
A |
G |
A |
A |
A |
|
|
942 633 |
M5005_Spy0951 |
pstB |
Phosphate transport ATP-binding protein |
ns |
G |
T |
G |
G |
G |
T |
|
983 438 |
Intergenic/within ssrA |
– |
– |
G |
C |
G |
G |
C |
C |
|
|
1 082 253 |
M5005_Spy1108 |
metK2 |
S-Adenosylmethionine synthetase |
ns |
C |
T |
T |
T |
T |
T |
|
1 238 124 |
M5005_Spy1282 |
msrA |
Peptide methionine sulphoxide reductase |
ns |
G |
A |
A |
A |
A |
A |
|
1 238 673 |
M5005_Spy1283 |
tlpA |
Thiol:disulphide interchange protein |
ns |
G |
A |
A |
A |
A |
A |
|
1 251 193 |
M5005_Spy1293 |
Hypothetical protein |
ns |
G |
A |
G |
G |
G |
A |
|
|
1 373 176 |
M5005_Spy1400 |
PTS system, galactose-specific IIB component |
ns |
C |
A |
C |
C |
C |
A |
|
|
1 407 497 |
M5005_Spy1439 |
Portal protein |
ns |
C |
T |
T |
T |
T |
T |
|
|
1 446 116 |
M5005_Spy1490 |
3-Oxoacyl-[acyl-carrier protein] reductase |
S |
C |
T |
T |
T |
T |
T |
|
|
1 535 209 |
Intergenic |
– |
– |
A |
G |
A |
A |
A |
G |
|
|
1 702 540 |
M5005_Spy1714 |
gldA |
Glycerol dehydrogenase |
STOP |
C |
T |
C |
T |
T |
T |
|
1 734 749 |
M5005_Spy1772 |
Glutamate formimidoyltransferase |
ns |
G |
A |
A |
A |
A |
A |
|
|
1 828 734 |
M5005_Spy1860 |
Putative membrane spanning protein |
ns |
G |
A |
G |
A |
A |
A |
*Single strain with 19 of the 27 SNPs that characterize M1UK (not a sublineage).
†Lineage with 27 SNPs is equivalent to M1UK.
Fig. 3.
Phylogeny of M1UK, M1global and two intermediate sublineages. Maximum likelihood phylogenetic tree reconstructed from core SNPs (without recombination regions) of 269 invasive and non-invasive emm1 S. pyogenes isolate genomes representative of four main groups (M1global, M113SNPs, M123SNPs, M1UK). The phylogenetic tree is coloured as described in the key. White bubbles represent isogenic strains from two distinct outbreaks with 26 and 22 SNPs, respectively, and one invasive isolate with 19 SNPs. Isolate whole genome sequences used in the phylogenetic tree are listed in Table S2.
SpeA expression by sublineages
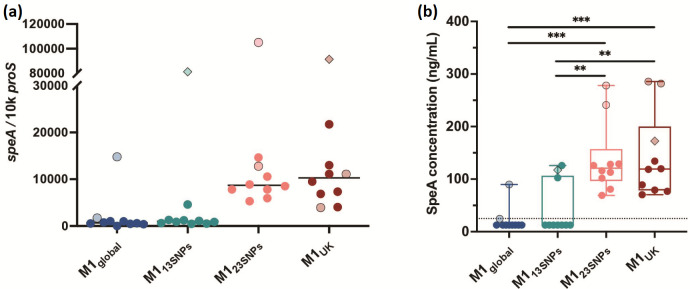
Previous comparison had demonstrated ~10-fold greater speA gene transcription by non-invasive M1UK isolates compared to non-invasive M1global strains [4]; we first established that SpeA protein expression was similarly elevated in the same large panel of non-invasive isolates (Fig. S3). There was an indication that SpeA production was not increased in a small number of strains from intermediate lineages. To better understand the impact of the step-wise changes in SNP content, we examined speA gene transcription and protein production in a new set of strains. To include sufficient numbers of intermediate sublineage isolates, we used 40 strains from a larger national collection of invasive emm1 S. pyogenes that had been submitted to the reference laboratory and were previously sequenced [4, 19].
Transcription of speA was low in all M1global and M113SNPs strains, except for the occasional strain with a mutation in covRS, a two-component system regulator known to suppress virulence factors, but which can undergo mutation to confer a more invasive phenotype in emm1 and other S. pyogenes strains. In contrast, transcription of speA was high in all invasive strains with 23 or 27 SNPs (Fig. 4a). Likewise, SpeA protein production differed markedly between the sublineages; again SpeA production was greatest in all invasive isolates with 23 or 27 SNPs and was hard to detect in all M1global and M113SNPs (Fig. 4b). Indeed, the amount of SpeA produced routinely by M1UK strains was similar to that produced by M1global strains with mutations in covRS, a regulator that is known to repress speA in emm1 [27]. We did not detect a difference in production of other virulence factors such as SpyCEP, SpeB or M protein, in broth culture (data not shown). We concluded that the genetic changes required for basal increased speA expression in M1UK resided in M123SNPs but not M113SNPs.
Fig. 4.
SpeA expression is increased in M123SNPs and M1UK sublineages. (a) Transcription of speA using 10 isolates from each sublineage is shown (total n=40). Each dot represents a single isolate (measured in triplicate) with light shading/fine black outline indicating the presence of a covRS mutation. Two isolates possessed both covRS and rgg4 mutations (diamond shapes). Solid line represents the median. There was no statistically significant difference between the sublineages in speA transcription, largely related to the outlying covRS mutants in each sublineage. Excluding isolates with covRS mutations a difference was observed between M1global and either M123SNPs or M1UK (P<0.0001), and a difference between between M113SNPs and either M123SNPs or M1UK (P=0.0002). Median fold difference M1UK versus M1global was 15.2 including all strains tested. (b) SpeA protein production by 40 invasive isolates (10 in each sublineage) cultured for 6 h in THB. Limit of detection was 25 ng ml−1. Isolates with undetectable SpeA were assigned a value of 12.5 ng ml−1. Median fold difference M1UK versus M1global was 9.5 including all strains tested. Box plot shows median and range. A multiple comparisons test was made using one-way ANOVA (Tukey’s): **P<0.01; ***P<0.001.
Isogenic isolates that differed by just single SNPs were available from two outbreak settings. Interestingly, in both settings, a single isolate was identified wherein a single SNP from the 27 SNPs that define M1UK reverted to the wild-type. In one daycare centre outbreak, a non-invasive isolate exhibited only 26 of the 27 SNPs but was otherwise identical to an invasive isolate from the same cluster; in this case, the SNP in trmD, a tRNA (guanine-N (1)-)-methyltransferase, had reverted to the wild-type. This isolate made as much SpeA as the isolate with 27 SNPs.
In a separate hospital outbreak associated with a fatal case of invasive infection caused by the M123SNPs sublineage [21], one isolate from a healthcare worker was identical to five other isolates in the cluster, apart from one single SNP. This single SNP represented one of the 23 SNPs but is present in both M113SNPs and M123SNPs, a phage portal protein (Spy1439). This isolate also produced the same amount of SpeA as the parent M123SNPs strain, demonstrating the SNPs that were dispensible for increased SpeA expression.
A review of published UK emm1 genome sequences [20] identified a single strain with 19 of the 27 SNPs among emm1 bloodstream isolates. Unlike the sublineage that possessed 23 SNPs, this M119SNPs strain did not produce detectable quantities of SpeA, pointing to an influential role for the four SNPs that differentiate M119SNPs and the M123SNPs sublineage in SpeA expression. Of these four SNPs, two were synonymous SNPs and unlikely to affect phenotype; one was a non-synonymous SNP in sagE; while the final change was an SNP that appeared to be intergenic in annotated emm1 S. pyogenes genomes but lies within the start of the tmRNA ssrA [28] upstream of the phage insertion and start site of speA (Spy0996 in MGAS5005). RNA-seq read abundance in this region did not show a difference between M1global and M1UK strains, with the exception of the gene encoding SpeA. Abundance of reads in the ‘paratox’ (Spy0995) gene, which is transcribed on the opposite strand to speA, was increased in two of four M1UK strains, but this finding was not consistent.
Proteomic analysis of S. pyogenes emm1 sublineages
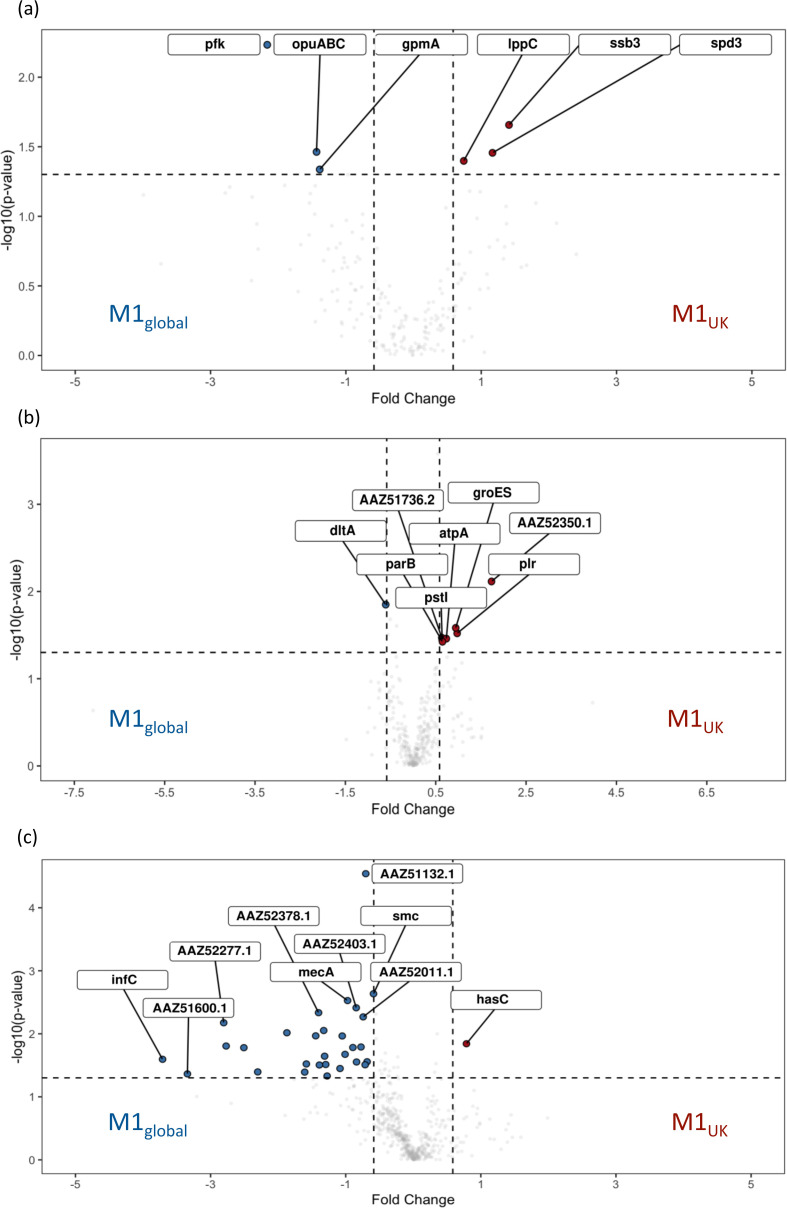
To screen for lineage-specific differences in proteomes, cell wall, cytosolic and supernatant fractions of five randomly selected M1UK isolates were compared with five M1global following culture in CDM. Though SpeA was detected, a significant difference between M1UK and M1global supernatants was not observed when strains were cultured in CDM, in contrast to results (reported above) in THB, pointing to a role for specific culture conditions in induction of SpeA. CDM supernatant from M1UK strains demonstrated increased phage-encoded DNase (Spd3), acid phosphatase (LppC) and a DNA binding protein compared with M1global. However, CDM supernatant from M1global demonstrated increased phosphoglycerate mutase and phosphofructokinase, both of which are linked to carbohydrate utilization pathways in S. pyogenes (Figs 5a and S4A) [29]. Cell wall fractions demonstrated a small number of proteins that were differentially expressed in M1UK strains. These included a more than 3-fold increase in PrsA2 (Spy1732, AAZ52350.1), which controls protein folding and may operate at the ExPortal [30], and almost 2-fold increases in GAPDH and the 10 kDa chaperonin GroES compared with M1global (Figs 5b and S4B).
Fig. 5.
Volcano plots comparing proteins differentially produced by M1UK vs. M1global cultured in CDM. Log2 fold change is shown on the x-axis. Specific fractions examined were supernatant (a), cell wall (b) and cytosol (c). Proteins increased in M1UK are shown on the right in red. Those increased in M1global are shown on the left in blue. Comparisons are listed in the supporting Excel file (Figshare) [24].
In M1global strains, several cytosolic proteins were increased compared to M1UK, including those encoded by adjacent genes Spy0438 (rnc, ribonuclease III) and Spy0439 (smc) as well as mecA, coding for an adapter protein and negative regulator of competence; the greatest fold changes were, however, seen in InfC, an Initiation Factor 3, SatD, and a number of proteins linked to protein secretion (SecA), maintenance of ribosomal function and RNA. String analysis highlighted links between phosphoentomutase (DeoB), protein synthesis pathways (GidA) and acid tolerance (SatD) (Figs 5c and S4C).
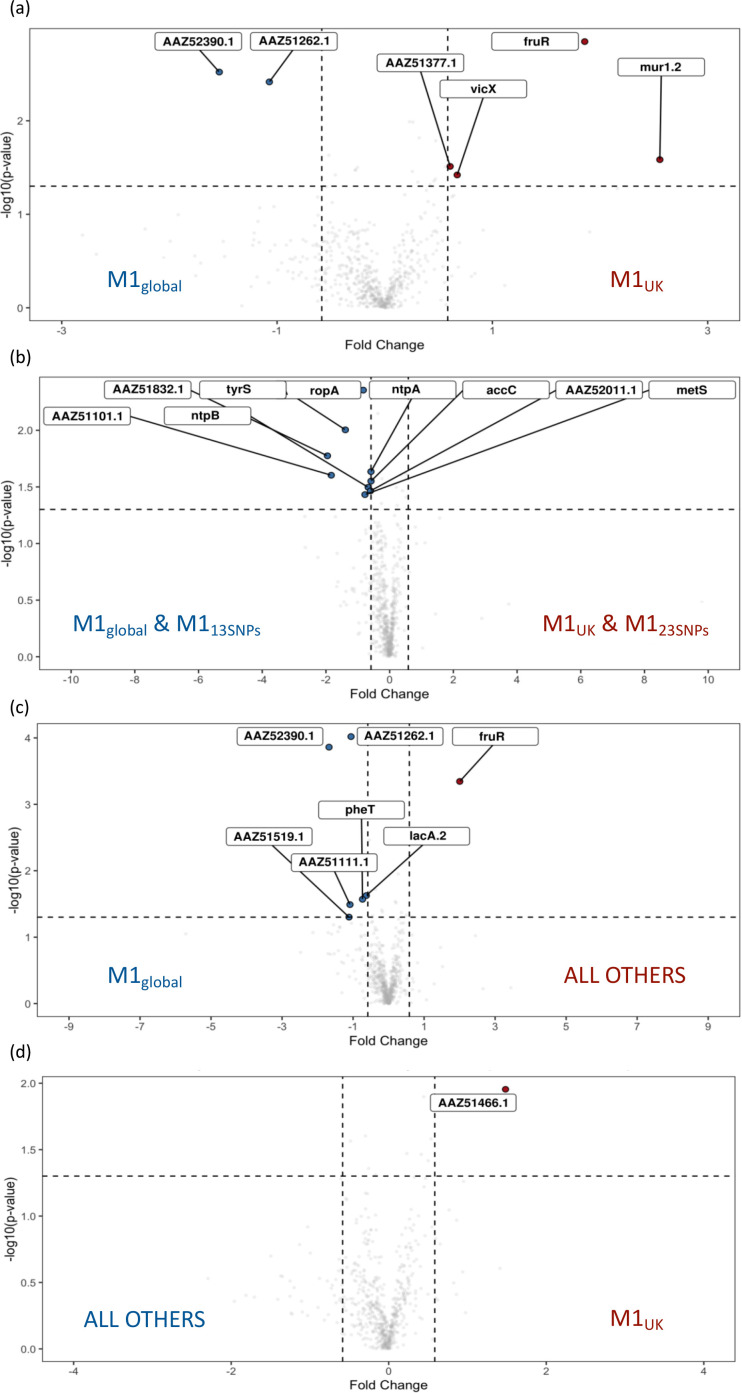
To screen for differences between all four sublineages (two intermediate and two major sublineages), five isolates from each intermediate sublineage (M113SNPs and M123SNPs) were randomly selected from appropriate phylogenetic branches as well as five new isolates from each of M1UK and M1global. Fresh cytosolic fractions of the four phylogenetic groups (20 strains) were prepared and subject to new proteomic analysis. The data were then analysed by comparing groups in different combinations. When cytosolic preparations from all four sublineages were compared with one another, FruR production was increased in M123SNPs in comparison to other lineages, and lowest in M1global, while a network of ribosomal proteins was increased in M113SNPs (Fig. S5A). Comparison of cytosolic preparations from new M1UK and M1global strains did not identify the same DE features seen previously; however, a negative regulator of competence, MecA, again was increased in M1global strains although only by 1.3-fold (Fig. S5B). The biggest fold change was a 3.6-fold increase in FruR and 5.87-fold increase in Mur1.2, a potential autolysin (adjacent to a PTS fructose-specific IIABC system and fruR), in M1UK isolates (Fig. 6a). As M1UK and M123SNPs strains had demonstrated comparable SpeA production, we proceeded to determine if there was commonality between these two sublineages by comparing cytosolic proteomes of [M1UK and M123SNPs] with [M1global and M113SNPs]. NtpA and B, a V type ATPase, was increased in [M1global and M113SNPs]. Proteins linked to ligase activity were found to be enriched in string analysis and highest in [M1global and M113SNPs] (Figs 6b and S5C). When considering M1global compared with all other three ‘new’ lineages, carbohydrate metabolism genes were further highlighted, specifically PTS system and disaccharide metabolic processes (Fig. S5D). As observed, FruR was four-fold increased in non-M1global strains, with increased FruA in M1global strains; a similar pattern was seen for LacR and LacA1/LacA2 (Figs 6c and S5D). A glutamate formiminotransferase (MGAS5005_Spy1772) was also increased in M1global strains compared with non-M1global. Finally, comparing cytosolic proteins in M1UK with all other lineages, just one protein was clearly relatively increased in M1UK, and this was Spy0848 (PpnK), an ATP-NAD kinase (Figs 6d and S5E).
Fig. 6.
Volcano plots comparing proteins differentially produced when considering different pairings of M1global, M113SNPs, M123SNPs and M1UK cultured in CDM. Log2 fold change is shown on the x-axis. Cytosolic fractions only were compared as follows: (a) M1UK vs. M1global, (b) [M1UK + M123SNPs] vs. [M1global + M113SNPs], (c) all other sublineages vs. M1global, and (d) M1UK vs. all other sublineages. Comparisons are listed in the supporting Excel file (Figshare) [24].
Discussion
M1UK is now the dominant S. pyogenes emm1 lineage in the UK, having expanded during an earlier upsurge in scarlet fever in 2014–2016 [4, 5]. Importantly, emm1 strains are inherently virulent and represent the single most frequent emm type causing invasive infections in the UK [31, 32]. As such, any change in the emm1 lineage that results in increased fitness is of relevance to public health. In this first systematic study to characterize the changes in M1UK and its associated lineages, we have confirmed the speA over-expression phenotype, and demonstrated that increased SpeA production is restricted to M1UK and an increasingly rare sublineage M123SNPs. The phenotype is manifest in broth culture but not CDM. M1UK is defined by just 27 SNPs in the core genome, including three SNPs in a stand-alone regulator rofA and a stop codon in glycerol dehydrogenase, gldA. RNA-seq demonstrated a difference in expression of the operon that includes gldA and a PTS system EIIC and B which represents a combined phosphate and sugar transporter, pointing to a potential shift in metabolism in the new lineage. This was accompanied by a sharp reduction in transcripts for the aquaporin gene glpF2. Preliminary proteomic analysis of strains by sublineage identified altered carbohydrate pathways related to fructose that may well be important.
Alterations in expression of gldA, mipB, pflD, and the adjacently encoded PTS system impact on the glycolytic Embden–Meyerhof–Parnas pathway [29] which, in S. pyogenes, relies on the phospho-enolpyruvate PTS system for acquisition of sugars other than glucose, and for transfer of phosphate ions required for carbon catabolite repression and gene regulation [33].
The results indicate that both the premature stop codon in gldA present in M1UK and allelic replacement of gldA impact glycerol dehydrogenase activity and result in upregulation of the other genes in the operon, mipB and pflD. These are involved in the glycolytic pathway required for generation and metabolism of pyruvate from glucose; the changes in carbohydrate metabolism are supported by proteomic findings that indicate alterations in fructose pathways. Interestingly, increased transcription in this operon was accompanied by increased transcription of adjacent PTS components IIC and IIB when comparing M1UK with M1global, and following experimental deletion of gldA. This PTS is annotated as being a cellobiose transporter; systematic experimental disruption of PTS EII systems in S. pyogenes has not shown an essential role for these genes, but the precise sugar transported is not known [33].
The role of GldA in S. pyogenes has not been experimentally examined previously; GldA is reported to catalyse the conversion of glycerol to DHA under microaerophilic or anaerobic conditions, but it is clear that GldA may also undertake a reverse role, which is to catalyse DHA to glycerol. This may be of importance since an absence of gldA activity may lead to a build-up of DHA, which when converted to methylglyoxal can be toxic [34]. Upregulation of the PTS system is of interest since these are recognized to be key players in a phosphorelay process that maintains central carbon catabolite repression of many virulence systems in S. pyogenes [33, 35].
The marked ~5-fold downregulation of aquaporin glpF2 (Spy1573) transcription in M1UK compared to M1global was unexpected but may represent an adaptation to the metabolic changes that have arisen in M1UK. There are few reports, if any, relating to glpF2 in S. pyogenes but there is evidence of functional links to the pflD-containing operon in enterococci [36]. Notably one of the intergenic SNPs that defines M1UK is 39 bp from the start codon of the Spy1573 gene, though the significance of this is not yet known. Aquaporins are membrane proteins that function as channels for water and other uncharged solutes in all forms of life. While mostly considered as channels for water or glycerol in bacteria, potentially important to osmoregulation, aquaporins also can function as a channel for DHA. Indeed, there are similarities between glpF2 of streptococci and glpF3 of Lactobacillus plantarum that points to a possibility for action as a channel for DHA or similar molecules [25]. Research undertaken in the related Lactococcus lactis has also identified marked downregulation of glpF2 following osmotic stress [37]. Taken together it would seem that the downregulation of glpF2 may be a necessary adaptation for M1UK S. pyogenes , although it may also confer an as-yet unknown advantage.
The upregulation of SpeA expression by M1UK is clearly of importance to virulence, particularly in interaction with the human host, and those who have not yet mounted an immune response to the secreted toxins of this species. There is good evidence that superantigens such as SpeA undermine development of the adaptive host immune response to S. pyogenes through promotion of a dysregulated T cell response associated with B cell death [38, 39]. SpeA has also been shown to promote carriage of S. pyogenes in the nasopharynx of transgenic mice [40]. To date, the expression of SpeA has only been measured in broth culture and we do not know if the upregulation in M1UK might differ in vivo. Recent epidemiological studies found a high (44%) secondary infection rate in schoolchildren and household contacts of a case of scarlet fever caused by M1UK, pointing to a potential transmission advantage compared with other S. pyogenes lineages [41]. We identified sublineage-specific altered expression of SpeA, allowing us to highlight the genetic changes likely to account for this. Importantly, the three SNPs identified in the major regulator rofA do not alone account for the SpeA phenotype since these SNPs are present in M113SNPs, although we cannot discount a role for these in the wider success of this lineage. While the genetic changes required for increased SpeA expression do not reside in M113SNPs, they do reside in M123SNPs, and strains with reversion of single SNPs pointed to a potential key role for the ssrA SNP in SpeA upregulation. The amount of SpeA made by M1UK and M123SNPs was augmented to the level of M1global covRS mutants yet presumably without the fitness burden of covRS mutation that might impair pharyngeal carriage [42].
There are a number of limitations to our study. First, investigation of the gldA operon is in its early stages; it is possible that the premature stop codon mutation in gldA confers an additional phenotype that is not recapitulated by gldA gene deletion, while the metabolic pathways that include GldA, MipB and PflD are not fully understood. The roles of GlpF2 and the PTS EII system that is upregulated are also not understood; any role in transfer of DHA, for example, has not been experimentally addressed. The proteomic studies are preliminary and require both validation and repetition using richer media, but have provided a rationale for further study of the role of sugar metabolism in emm1 S. pyogenes . Finally, the role of specific SNPs would necessarily require experimental proof.
Several European countries are, at the time of writing, affected by epidemic waves of invasive S. pyogenes disease, notably in England, where the leading cause of invasive infection is emm1, underlining the importance of understanding pathogenicity and transmission [32, 43]. Importantly, however, despite the enhanced production of SpeA by M123SNPs, this intermediate sublineage did not expand in the manner seen for M1UK in England, and was not detected at all in a 2020 systematic evaluation of >300 invasive emm1 isolates from England [5]. This suggests that the fitness of M1UK has required the additional acquisition of four further SNPs. These include three non-synonymous SNPs in phosphate transport ATP binding protein, pstB; a PTS galactose-specific IIB component gene; a hypothetical protein; as well as the intergenic SNP upstream to glpF2. The amount of SpeA produced by M1UK strains remains an order of magnitude lower than the amount produced by the historic emm1 strain NCTC8198 that was used for erythrogenic toxin production, and can produce 2000 ng ml−1 [44]. Despite this, the new M1UK lineage has outcompeted M123SNPs and has replaced older strains, suggesting that the added fitness of M1UK may lie beyond the ability to make SpeA.
Supplementary Data
Funding information
This work was supported by the UK Medical Research Council (grant number MR/P022669/1) and the NIHR Imperial Biomedical Research Centre. H.K.L. is a UKRI-MRC CMBI Clinical Research Training Fellow, C.E.T. is a Royal Society and Wellcome Trust Sir Henry Dale Research Fellow (208765/Z/17/Z), and E.J. is a Rosetrees/Stoneygate Imperial College Research Fellow (M683).
Acknowledgements
The authors acknowledge support from the National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Healthcare Associated Infections and Antimicrobial Resistance and the NIHR Imperial Biomedical Research Centre. The authors gratefully acknowledge the support of colleagues in the UKRI-MRC LMS and National Phenome Centre in facilitating sequencing and proteomics, and colleagues in the UKHSA Reference laboratory.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: CBA, Columbia blood agar; CDM, chemically defined medium; DE, differentially expressed; DHA, dihydroxyacetone; HDMSe, High definition mass spectrometry; PTS, phosphotransferase system; qRT-PCR, quantitative reverse transcription PCR; RNA-seq, RNA sequencing; TFA, trifluroacetic acid; THB, Todd–Hewitt broth.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Five supplementary figures and four supplementary tables are available with the online version of this article.
References
- 1.Li HK, Zhi X, Vieira A, Whitwell HJ, Schricker A, et al. 2023. Characterisation of emergent toxigenic M1UK Streptococcus pyogenes and associated sublineages. Microbiology Society. Figure. [DOI] [PMC free article] [PubMed]
- 2.Nasser W, Beres SB, Olsen RJ, Dean MA, Rice KA, et al. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci U S A. 2014;111:E1768–76. doi: 10.1073/pnas.1403138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, et al. Evolutionary origin and emergence of A highly successful clone of serotype M1 group A Streptococcus involved multiple horizontal gene transfer events. J Infect Dis. 2005;192:771–782. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- 4.Lynskey NN, Jauneikaite E, Li HK, Zhi X, Turner CE, et al. Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: a population-based molecular epidemiological study. Lancet Infect Dis. 2019;19:1209–1218. doi: 10.1016/S1473-3099(19)30446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhi X, Li HK, Li H, Loboda Z, Charles S. Emerging Invasive Group A Streptococcus M1UK Lineage Detected by Allele-Specific PCR, 2020. Emerg Infect Dis. 2023 doi: 10.3201/eid2905.221887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rümke LW, de Gier B, Vestjens SMT, van der Ende A, van Sorge NM, et al. Dominance of M1UK clade among Dutch M1 Streptococcus pyogenes . Lancet Infect Dis. 2020;20:539–540. doi: 10.1016/S1473-3099(20)30278-4. [DOI] [PubMed] [Google Scholar]
- 7.Demczuk W, Martin I, Domingo FR, MacDonald D, Mulvey MR. Identification of Streptococcus pyogenes M1UK clone in Canada. Lancet Infect Dis. 2019;19:1284–1285. doi: 10.1016/S1473-3099(19)30622-X. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Nanduri SA, Van Beneden CA, Beall BW. M1UK lineage in invasive group A Streptococcus isolates from the USA. Lancet Infect Dis. 2020;20:538–539. doi: 10.1016/S1473-3099(20)30279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conesa A, Madrigal P, Tarazona S, Gomez-Cabrero D, Cervera A, et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016;17:13. doi: 10.1186/s13059-016-0881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clausen PTLC, Aarestrup FM, Lund O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinformatics. 2018;19:1–8. doi: 10.1186/s12859-018-2336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danecek P, Auton A, Abecasis G, Albers CA, Banks E, et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Love M, Anders S, Huber W. Differential analysis of count data–the deseq2 package. Genome Biol. 2014;15:10–1186. doi: 10.1186/s13059-014-0550-8. [DOI] [Google Scholar]
- 16.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arndt D, Grant JR, Marcu A, Sajed T, Pon A, et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin EC, Magasanik B. The activation of glycerol dehydrogenase from Aerobacter aerogenes by monovalent cations. J Biol Chem. 1960;235:1820–1823. [PubMed] [Google Scholar]
- 19.Kapatai G, Coelho J, Platt S, Chalker VJ. Whole genome sequencing of group A Streptococcus: development and evaluation of an automated pipeline for emm gene typing. PeerJ. 2017;5:e3226. doi: 10.7717/peerj.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner CE, Holden MTG, Blane B, Horner C, Peacock SJ, et al. The emergence of successful Streptococcus pyogenes lineages through convergent pathways of capsule loss and recombination directing high toxin expression. mBio. 2019;10:e02521-19. doi: 10.1128/mBio.02521-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma H, Ong MR, Ready D, Coelho J, Groves N, et al. Real-time whole genome sequencing to control a Streptococcus pyogenes outbreak at a national orthopaedic hospital. J Hosp Infect. 2019;103:21–26. doi: 10.1016/j.jhin.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Croucher NJ, Page AJ, Delaney AJ, Keane JA, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015;43:e15. doi: 10.1093/nar/gku1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figshare Item comprising supplementary data items. n.d. [DOI]
- 25.Bienert GP, Desguin B, Chaumont F, Hols P. Channel-mediated lactic acid transport: a novel function for aquaglyceroporins in bacteria. Biochem J. 2013;454:559–570. doi: 10.1042/BJ20130388. [DOI] [PubMed] [Google Scholar]
- 26.Sundar GS, Islam E, Braza RD, Silver AB, Le Breton Y, et al. Route of glucose uptake in the group A Streptococcus impacts SLS-mediated hemolysis and survival in human blood. Front Cell Infect Microbiol. 2018;8:71. doi: 10.3389/fcimb.2018.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006;2:e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosinski-Chupin I, Sauvage E, Fouet A, Poyart C, Glaser P. Conserved and specific features of Streptococcus pyogenes and Streptococcus agalactiae transcriptional landscapes. BMC Genomics. 2019;20:236. doi: 10.1186/s12864-019-5613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pancholi V, Caparon M. M. In: Streptococcus Pyogenes: Basic Biology to Clinical Manifestations [Internet] Ferretti JJ, Stevens DL, Fischetti VA, editors. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. Streptococcus pyogenes metabolism.https://www.ncbi.nlm.nih.gov/books/NBK333417/ [PubMed] [Google Scholar]
- 30.Wu ZY, Campeau A, Liu CH, Gonzalez DJ, Yamaguchi M, et al. Unique virulence role of post-translocational chaperone PrsA in shaping Streptococcus pyogenes secretome. Virulence. 2021;12:2633–2647. doi: 10.1080/21505594.2021.1982501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson GE, Pondo T, Toews KA, Farley MM, Lindegren ML, et al. Epidemiology of invasive group A Streptococcal infections in the United States, 2005-2012. Clin Infect Dis. 2016;63:478–486. doi: 10.1093/cid/ciw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.UK Health Security Agency Group A Streptococcal infections: second update on seasonal activity in England 2022. [ December 16; 2022 ]. www.gov.uk/government/publications/group-a-streptococcal-infections-activity-during-the-2022-to-2023-season/group-a-streptococcal-infections-second-update-on-seasonal-activity-in-england-2022-to-2023 n.d. accessed.
- 33.Sundar GS, Islam E, Gera K, Le Breton Y, McIver KS. A PTS EII mutant library in group A Streptococcus identifies A promiscuous man-family PTS transporter influencing SLS-mediated hemolysis. Mol Microbiol. 2017;103:518–533. doi: 10.1111/mmi.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subedi KP, Kim I, Kim J, Min B, Park C. Role of GldA in dihydroxyacetone and methylglyoxal metabolism of Escherichia coli K12. FEMS Microbiol Lett. 2008;279:180–187. doi: 10.1111/j.1574-6968.2007.01032.x. [DOI] [PubMed] [Google Scholar]
- 35.Rom JS, Hart MT, McIver KS. PRD-Containing Virulence Regulators (PCVRs) in pathogenic bacteria. Front Cell Infect Microbiol. 2021;11:772874. doi: 10.3389/fcimb.2021.772874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doi Y, Ikegami Y. Pyruvate formate-lyase is essential for fumarate-independent anaerobic glycerol utilization in the Enterococcus faecalis strain W11. J Bacteriol. 2014;196:2472–2480. doi: 10.1128/JB.01512-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Meulen SB, de Jong A, Kok J. Early transcriptome response of Lactococcus lactis to environmental stresses reveals differentially expressed small regulatory RNAs and tRNAs. Front Microbiol. 2017;8:1704. doi: 10.3389/fmicb.2017.01704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies FJ, Olme C, Lynskey NN, Turner CE, Sriskandan S. Streptococcal superantigen-induced expansion of human tonsil T cells leads to altered T follicular helper cell phenotype, B cell death and reduced immunoglobulin release. Clin Exp Immunol. 2019;197:83–94. doi: 10.1111/cei.13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dan JM, Havenar-Daughton C, Kendric K, Al-Kolla R, Kaushik K, et al. Recurrent group A Streptococcus tonsillitis is an immunosusceptibility disease involving antibody deficiency and aberrant TFH cells. Sci Transl Med. 2019;11:eaau3776. doi: 10.1126/scitranslmed.aau3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasper KJ, Zeppa JJ, Wakabayashi AT, Xu SX, Mazzuca DM, et al. Bacterial superantigens promote acute nasopharyngeal infection by Streptococcus pyogenes in a human MHC Class II-dependent manner. PLoS Pathog. 2014;10:e1004155. doi: 10.1371/journal.ppat.1004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordery R, Purba AK, Begum L, Mills E, Mosavie M, et al. Frequency of transmission, asymptomatic shedding, and airborne spread of Streptococcus pyogenes in schoolchildren exposed to scarlet fever: a prospective, longitudinal, multicohort, molecular epidemiological, contact-tracing study in England, UK. Lancet Microbe. 2022;3:e366–e375. doi: 10.1016/S2666-5247(21)00332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alam FM, Turner CE, Smith K, Wiles S, Sriskandan S. Inactivation of the CovR/S virulence regulator impairs infection in an improved murine model of Streptococcus pyogenes naso-pharyngeal infection. PLoS One. 2013;8:e61655. doi: 10.1371/journal.pone.0061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization Increase in invasive Group A streptococcal infections among children in Europe, including fatalities. [ December 14; 2022 ]. www.who.int/europe/news/item/12-12-2022-increase-in-invasive-group-a-streptococcal-infections-among-children-in-europe--including-fatalities#:~:text=The%20observed%20increases%20reported%20to,during%20the%20COVID%2D19%20pandemic n.d. accessed.
- 44.Sriskandan S, Moyes D, Buttery LK, Krausz T, Evans TJ, et al. Streptococcal pyrogenic exotoxin A release, distribution, and role in a murine model of fasciitis and multiorgan failure due to Streptococcus pyogenes . J Infect Dis. 1996;173:1399–1407. doi: 10.1093/infdis/173.6.1399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Li HK, Zhi X, Vieira A, Whitwell HJ, Schricker A, et al. 2023. Characterisation of emergent toxigenic M1UK Streptococcus pyogenes and associated sublineages. Microbiology Society. Figure. [DOI] [PMC free article] [PubMed]