Abstract
Background
Mannose-binding lectin (MBL) is a member of innate immunity and acts with MASP (MBL-associated serine protease) to activate the lectin pathway of the complement system. MBL gene polymorphisms are associated with susceptibility to infectious diseases. This study investigated whether MBL2 genotype, serum MBL levels, and serum MASP-2 levels affect the course of SARS-CoV-2 infection.
Methods and results
Pediatric patients diagnosed with COVID-19 by positive real-time polymerase chain reaction (PCR) were included in the study. Single nucleotide polymorphisms in the promoter and exon 1 in the MBL2 gene (rs11003125, rs7096206, rs1800450, rs1800451, rs5030737) were identified by a PCR and restriction fragment length polymorphisms analysis. Serum MBL and MASP-2 levels were measured by ELISA. COVID-19 patients were divided into asymptomatic and symptomatic. Variables were compared between these two groups. A total of 100 children were included in the study. The mean age of the patients was 130 ± 67.2 months. Of the patients, 68 (68%) were symptomatic, and 32 (32%) were asymptomatic. The polymorphisms in the − 221nt and − 550nt promoter regions did not differ between groups (p > 0.05). All codon 52 and codon 57 genotypes were determined as wild-type AA. AB genotypes were found 45.6% in symptomatic patients while 23.5% in asymptomatics. Moreover, BB genotype was detected 9.4% in symptomatic and 6.3% in asymptomatic patients (p < 0.001). B allele was more frequent in symptomatic patients (46.3%) compared to asymptomatic patients (10.9%). (p < 0.001). Serum MBL and MASP-2 levels did not differ statistically between the groups (p = 0.295, p = 0.073).
Conclusion
These findings suggest that codon 54 polymorphism in the MBL2 gene exon-1 region can be associated with the symptomatic course of COVID-19.
Keywords: SARS-CoV-2, Mannose-binding lectin, Gen, Polymorphisms, Pediatrics
Introduction
Coronavirus disease (COVID-19) presents different clinical findings, including acute upper respiratory tract infection, pneumonia, and gastrointestinal or nervous system symptoms. According to the clinical signs, COVID-19 can be asymptomatic or symptomatic, with fever, cough, sore throat, headache, muscular pain, and diarrhea [1]. Although it is known that the clinical course of COVID-19 is milder in children than in adults, some patients need intensive care due to respiratory failure, while others may not have any symptoms [2]. Therefore, many studies focused on understanding the clinical, genetic, epigenetic, and demographic factors that can affect the course of the disease [3, 4]. It has been determined that severe COVID-19 is high in children with comorbidities such as obesity, diabetes, heart disease, and chronic lung diseases [5]. However, these factors alone do not predict the risk of severe COVID-19 in children.
On the other hand, the role of genetic factors in susceptibility to COVID-19 infection is still unclear. Many polymorphism studies have been conducted on the genetic mechanisms of critical illness in COVID-19 [6]. Human angiotensin-converting enzyme 2 (ACE2) is one of the most studied genes that play a role in binding Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) spike protein. It was reported that the polymorphisms of the ACE2 gene could help better understand the pathophysiology and clinical outcomes of the patients [7]. Moreover, angiotensin system polymorphisms (ACE1, ACE2, AGT, AGTR1) were studied and evaluated to show that these polymorphisms can be a predictive point for the SARS-CoV-2 infection [8]. Another group worked on the association between human leukocyte antigen polymorphisms and COVID-19 [9].
Mannose-binding lectin (MBL) is a critical molecule function in innate immunity and plays an essential role in the primary part of the host defense [10]. MBL binds to microbial surfaces and activates the complement system via MBL-associated serine protease (MASP)-1 and MASP-2. MBL protein is encoded by the MBL2 (OMIM: 154,545) gene, located at chromosome 10 [11, 12]. Polymorphism in the promoter and coding regions of the MBL gene affects the susceptibility of patients to infectious diseases. The association between MBL2 gene polymorphisms and Hepatitis B, Hepatitis C, human immunodeficiency virus, and SARS-CoV has been reported [13–16]. It is known that the expression of MBL is more important in children than in adults. Low serum levels of MBL and low expressed MBL2 gene has caused increased infectious diseases [17].
This study aimed to determine the association between MBL promoter and codon 52, 54, 57 polymorphisms, serum MBL levels, and serum MASP-2 levels with the clinical course of the SARS-CoV-2 infection in pediatric patients.
Materials and methods
Study design and study population
This is a cross-sectional study involving patients who applied to the pediatric clinic of Izmir Health Sciences University Tepecik Training and Research Hospital suspected of COVID-19.
One hundred pediatric patients aged one month to 18 years diagnosed with COVID-19 in the first wave of the pandemic with positive real-time polymerase chain reaction (Bio-Speedy SARS CoV-2 double Gene RT-qPCR Kit) and agreed to participate in the study were included. The clinical data of the patients, age, gender, and comorbidity were obtained from the medical records. Patients were grouped into two main groups, symptomatic and asymptomatic, and all results were compared between these two groups. An informed consent form was obtained from the parents/guardians of the children before initiating the study.
Genotyping of MBL gene
Genomic DNA extraction
Genomic DNA extraction was performed by Exgene Clinic SV mini kit (GeneAll, Korea) from whole blood according to the manufacturer’s instructions.
PCR-RFLP analysis
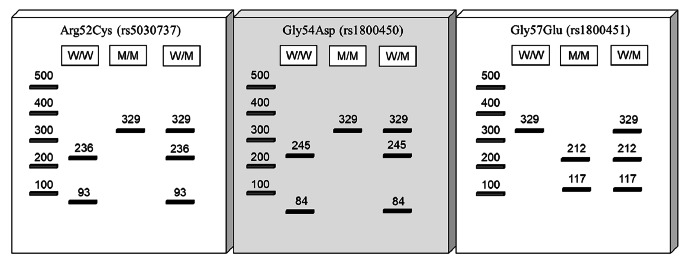
Genotyping of MBL promoter 550 (H/L) and 221 (X/Y) variants (rs11003125 and rs7096206) was performed by allele-specific PCR. LY, LX, and HY promoter regions were amplified in PCR reactions ending with allele-specific base primers. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis was used for genotyping codon 54 (Gly54Asp-rs1800450), codon 57 (Gly57Glu-rs1800451), and codon 52 (Arg52Cys-rs5030737) single nucleotide polymorphisms (SNPs) in MBL2 gene. Primer pairs used for genotyping were selected according to Shawky et al. [18] to amplify template DNA (120ng), 2.5 µl buffer, 2 µl MgCl2, 1 µlM of each primer, 0,5 µl dNTP, 0,25 µl Taq polymerase was added to the reaction mixture with a total volume of 25 µl. PCR conditions were as follows: initial denaturation at 94 0 C for 5 min, 30 cycles of denaturation at 94 0 C for 30 s, annealing at 61 0 C for 30 s, extension at 72 0 C for 30 s, followed by a final extension at 72 0 C for 5 min. Analysis of the PCR products was determined by electrophoresis on 1, 5% agarose gels stained with ethidium bromide. The genetic polymorphisms in codons 52, 54, and 57 were performed by digestion of the PCR products by BanI, MwoI, and MboII, respectively. RFLP results were evaluated according to the fragments shown in Table 1.
Table 1.
Enzymes and fragments of RFLP genotyping
| Enzyme | Polymorphism | RFLP condition | Wild-type homozygous W/W (bp) | Mutant homozygous M/M (bp) | Wild mutant heterozygous W/M (bp) |
|---|---|---|---|---|---|
| BanI | Gly54Asp (rs1800450) | 4 h at 37 °C | 245 + 84 | 329 | 329 + 245 + 84 |
| MboII | Gly57Glu (rs1800451) | 4 h at 37 °C | 329 | 212 + 117 | 329 + 212 + 117 |
| MwoI | Arg52Cys (rs5030737) | overnight at 37 °C | 236 + 93 | 329 | 329 + 236 + 93 |
Measurement of MBL and MASP levels
Peripheral blood was taken from each patient in tubes containing a gel separator and coagulation activator, centrifuged at 4500 rpm for 5 min, and separated serums. The human MBL and MASP-2 ELISA kits measured serum MBL and MASP-2 levels in samples from patients (BT LAB, China). The procedures were carried out as directed by the manufacturer, and serum MBL and MASP-2 levels were compared between groups.
Statistical analysis
We performed the statistical analysis with the statistical software SPSS version 24.0 (IBM Corporation, Armonk, NY, USA). Demographic and clinical data were analyzed descriptively and reported as proportions of total patients. The mean ± standard deviation or median and range (minimum value-maximum value) were used depending on whether the data were parametric. The categorical data used numbers (n) and percentages (%). Numerical data with normal distribution were compared with the independent groups’ t-test (independent samples t-test). Data not showing normal distribution were compared with the Mann-Whitney U test. Categorical variables were compared using the chi-square test or Fisher’s exact test. Binary logistic regression analyses were performed to examine the effects of combined genotypes and expression features on the clinical severity of COVID-19 and serum MBL levels. The statistical significance level was accepted as p < 0.05.
Results
One hundred pediatric patients, 50 (50%) girls and 50 (50%) boys were included in the study. The mean age of the patients was 130 ± 67.2 months. Sixty-eight (68%) patients were symptomatic, and 32 (32%) were asymptomatic. Of the symptomatic patients, 45 had mild, 22 had moderate, and one had severe COVID-19. There were not critically ill COVID-19 patients in the study. Most of the patients (88%) had no comorbidities. The most common comorbidities were asthma (4%), while the others were epilepsy (3%), endocrine disorders (3%), hypogammaglobinemia (1%), and celiac disease (1%). While there was one patient (8.3%) with comorbidity in the asymptomatic patient group, 11 (91.6%) patients were in the symptomatic group. Of the patients included in the study, 16% had COVID-19 pneumonia, while 3% required oxygen therapy.
This study analyzed two MBL promoters and three exons 1 polymorphisms. At promoter regions, the allele frequencies of -550 (H/L) and − 221 (X/Y) were evaluated according to the symptomatic and asymptomatic patients and shown in Table 2. Table 3 demonstrated the frequency of promoter haplotypes between symptomatic and asymptomatic patients. There was no significant difference between these two groups (All p > 0.05). One rare haplotype HX was not considered.
Table 2.
Frequency of MBL gene promoter polymorphisms
| Position | Variant allele | Asymptomatic n = 64 (%) |
Symptomatic n = 136 (%) |
p |
|---|---|---|---|---|
| -550 | H | 23 (35.9) | 62 (45.6) | 0.198 |
| L | 41(64.1) | 74 (54.4) | ||
| -221 | X | 18 (28.1) | 35 (25.7) | 0.721 |
| Y | 46 (71.9) | 101 (74.3) |
Table 3.
Promoter haplotype frequency among symptomatic and asymptomatic patients
| HY n (%) |
LY n (%) |
LX n (%) |
|
|---|---|---|---|
| Asymptomatic | 19 (24.8) | 17 (34.7) | 13 (30.2) |
| Symptomatic | 48 (71.6) | 32 (65.3) | 30 (69.8) |
| p | 0.266 | 0.571 | 0.742 |
| Total | 67 | 49 | 43 |
The alleles of the codons 52, 54, and 57 were determined by the RFLP method using the restriction enzymes MwoI, BanI, and MboII, respectively. The results were evaluated according to Fig. 1. All codon 52 and codon 57 genotypes were determined as wild-type homozygote AA. At the same time, both wild (A) and mutant alleles (B) were evaluated for codon 54 according to the RFLP results and shown in Table 4.
Fig. 1.
Assessment of the RFLP results in agarose gel electrophoresis
Table 4.
Genotypes of codon 54
| Genotypes | Asymptomatic n = 32 (%) |
Symptomatic n = 68 (%) |
p |
|---|---|---|---|
| AA | 27 (84.4) | 21 (30.9) | < 0.001 |
| AB | 3 (9.4) | 31 (45.6) | |
| BB | 2 (6.3) | 16 (23.5) | |
| Alleles | n = 64 (%) | n = 136 (%) | < 0.001 |
| A | 57 (89.1) | 73 (53.7) | |
| B | 7 (10.9) | 63 (46.3) |
It was evaluated that AB and BB genotypes were detected more in symptomatic patients (45.6% and 23.5%) than in asymptomatic (9.4% and 6.3%) (p < 0.001). According to allele frequencies, the B allele was found to be more frequent in symptomatic patients (46.3%) than in asymptomatic patients (10.9%) (p < 0,001).
Serum MBL and MASP levels were determined by ELISA, and the results were evaluated according to symptomatic and asymptomatic patients, as shown in Table 5. There was no statistically significant difference between groups (p = 0,295; p = 0,073). The association between codon 54 polymorphism and serum MBL and MASP-2 levels were shown in Table 6. However, no statistically significant correlation was detected (p = 0,575; p = 0,904).
Table 5.
Serum MBL and MASP-2 levels according to the clinical features of the patients
| Clinical features of the patients | |||
|---|---|---|---|
| Asymptomatic | Symptomatic | p | |
| MBL [mean (SD), ng/ml] | 3.53 ± 1.52 | 3.40 ± 1.11 | 0.295 |
| MASP-2 [mean (SD), ng/ml] | 96.41 ± 96.68 | 88.95 ± 59.73 | 0.073 |
Table 6.
Serum MBL and MASP-2 levels, according to the Codon 54 polymorphisms
| Codon 54 Genotypes | ||||
|---|---|---|---|---|
| AA | AB | BB | p | |
| MBL [mean (SD), ng/ml] | 3.46 ± 1.35 | 3.54 ± 1.34 | 3.22 ± 0.76 | 0.575 |
| MASP-2 [mean (SD), ng/ml] | 93.45 ± 85.95 | 72.00 ± 12.72 | 77.14 ± 36.93 | 0.904 |
Both codon and promoter polymorphisms and combined genotypes can affect serum MBL levels. We divided the haplotypes into A/A, A/B, and B/B groups in which wild-type homozygous and wild/mutant heterozygous or mutant/mutant homozygotic alleles were included, respectively. When we combined the HY, LY, and LX promoter haplotypes with codon 54 polymorphisms as A (wild type) and B (mutant type), we obtained six genotypes shown in Table 7. In a preliminary study, these alleles were associated with promoter expression levels as high, medium, and low [18].
Table 7.
Combined genotypes, expression features and clinical status
| Genotypes | Features | Asymp. n = 32 (%) |
Symp. n = 68 (%) |
p | Mean serum MBL levels (ng/ml) |
|---|---|---|---|---|---|
|
A/A group Two wild alleles | |||||
| YA/YA | High-expression promoter | 18 (56.3) | 10 (14.7) | 0.028 | 3.43 |
| YA/XA |
High-expression promoter Low-expression promoter |
6 (18.8) | 7 (10.3) | 0.069 | 3.48 |
| XA/XA | Low expression promoter | 3 (9.4) | 4 (5.9) | 0.215 | 3.39 |
|
A/B group One wild, one mutant allele | |||||
| YA/B | High-expression promoter | 2 (6.2) | 30 (44.1) | 0.01 | 3.44 |
| XA/B | Low-expression promoter | 1 (3.1) | 1 (1.5) | 0.15 | 3.19 |
| B/B | Two defective alleles | 2 (6.2) | 16 (23.5) | 0.007 | 3.47 |
Asymp: asymptomatic, Symp: symptomatic
According to the multivariate analysis, YA/YA, YA/B, and B/B genotypes were found significantly different between asymptomatic and symptomatic patients (p < 0.05), while YA/XA, XA/XA, and XA/B genotypes were not (p > 0.05). But there was no association between combined genotypes and serum MBL levels.
Discussion
MBL is a serum protein that mainly has a role in the innate immune response by binding the surface of the microorganisms and activating the complement system [10]. While MBL distinguishes the ligands of the microorganisms, MASP-2 can be activated and starts an antibody-dependent pathway of the complement system [11]. A recent study reported that MBL recognized the SARS-CoV-2 spike protein and showed antiviral activity against the virus in vitro [19].
The MBL2 gene is located on chromosome 10, and serum MBL concentrations mainly depend on the two promoter polymorphisms at -550 (H/L) and − 221 (X/Y) regions [20]. According to these polymorphisms, the haplotype variants are maintained as HY, LY, and LX, associated with the high, medium, and low expression profiles of the MBL gene, respectively [20]. Moreover, three polymorphisms on codons 52, 54, and 57 were determined, while the wild-type allele, referred to as A, D, B, and C alleles, were used to show 52-Arg/Cys, 54-Gly/Asp, 57-Gly/Glu polymorphisms [20, 21]. It was reported that these variants could vary due to different populations, and the allele frequencies could be different [22]. Glesse et al. observed that HY haplotypes were detected more frequently than other haplotypes in the African population [23]. Therefore, the most restricted part of the polymorphism studies is the ethnic origins of the population groups.
It was shown that MBL gene mutations were frequently detected in pediatric patients with respiratory system infections [24, 25]. Although several studies demonstrated an association between MBL polymorphism, serum concentration, and viral infections, no published data includes SARS-CoV-2-infected children. Therefore, this is the first study to determine the association between MBL gene polymorphisms and the clinical severity of the SARS-CoV-2 infection in the Turkish pediatric population.
Our results showed no statistically significant difference in the frequency of H, L, X, and Y variant alleles and haplotypes of the promoter region between the asymptomatic and symptomatic groups. Likewise, it has been established that there was no association between MBL2 promoter polymorphisms and both clinical severity and susceptibility to COVID-19 [19, 26].
In this study we demonstrated that the codon 54 genotypes AB and BB were more frequent in symptomatic patients than in asymptomatic patients. B allele was more common in symptomatic patients. Previous studies showed that the B allele was significantly associated with susceptibility to SARS-CoV infection [16, 27, 28]. However, Yuan et al. showed no correlation between SARS infection and B alleles [29]. Considering SARS-CoV-2, Medetalibeyoglu et al. reported that the BB genotype in COVID-19 cases was detected more than in controls [30]. Moreover, they said that BB or AB genotypes were higher risk factors for patients that need intensive care [30]. Similarly, Speletas et al. found that patients carrying the B allele required more frequent hospitalization due to pneumonia requiring supplemental oxygen administration [26]. A recent review reported that B allele frequencies were associated with the number of COVID-19 cases and deaths per country [31].
Contrary to these studies, in one study, the incidence of AB genotype and B allele was higher in healthy individuals and patients with SARS-CoV-2 PCR positive but without lung involvement [32]. In another study, no difference was found in codon 54 polymorphism in individuals who were healthy and infected with SARS-CoV-2 [19].
Both promoter and codon polymorphisms can control serum MBL levels [21]. In our study, combinations of genotypes were divided into groups responsible for the high, medium, and low serum MBL levels. According to these results, YA/YA, YA/B, and B/B genotypes were detected significantly more in symptomatic patients than in asymptomatic patients. This can be due to the codon 54 B allele that symptomatic patients mostly had. However, the genotypes did not affect mean serum MBL levels, and there was no difference between the symptomatic and asymptomatic patients. According to our results, mean serum MBL levels were not affected by the haplotypes, and a preliminary study confirmed this data [33]. Many studies have also underlined that serum MBL levels are affected by factors such as age, oxidative stress, vitamin D levels, and diet [34, 35].
MASP-2 is associated with the serum levels of the MBL, and lectin complements the MBL-MASP complex have a role together [11]. This is a protease enzyme encoded by the MASP-2 gene in viral infections like hepatitis C and increasing levels of MASP-2 were reported [36]. However, Wang et al. found no correlation between polymorphisms of MASP-2 and coronavirus infection [37]. Our study detected no association between serum MASP-2 levels and symptomatic and asymptomatic patients. Moreover, no significant relation was found between codon 54 genotypes and serum MASP-2 levels. It is known that several clinical factors can affect serum protein levels. Therefore, further studies are needed with a huge number of patients.
Our results showed that MBL2 polymorphisms on exon 1 or promoter region could not explain serum MBL, and MASP-2 levels of SARS-CoV-2 infected children. Allele frequencies and combined haplotypes should be analyzed to determine the clinical severity of COVID-19-infected pediatric patients.
Our study had some limitations. The essential limit was the size of the subject. Therefore, we could not evaluate the patients according to the severity of the symptoms.
Conclusion
MBL gene codon 54 B allele variant was detected more in symptomatic pediatric patients when compared with asymptomatic pediatric patients. There can be a possible interaction with MBL genotypes containing the B allele, indicating that it could be used as a molecular biomarker in pediatric COVID-19 patients.
Acknowledgements
None.
Authorship Contributions
DY: Design, Supervision, Fundings, Critical Review. MS: Supervision, Materials, Data Collection, Analysis. AS: Data Collection, Literature Review, Writing. BCA: Analysis, Literature Review, Writing. HIKC: Data Collection, Analysis, Literature Review. YEK: Data Collection, Literature Review. GU: Data Collection, Literature Review. AKA: Supervision, Critical Review. NY: Materials, Data Collection, Critical Review. IP: Supervision, Critical Review.
Funding
The Growing Child Association (Turkey) supported the study.
Data sharing statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Declarations
Ethics Committee approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Izmir Health Sciences University Tepecik Training and Research Hospital (2020/7–17).
Consent to participate
Informed consent was obtained from the parents/legally authorized representatives of all participants included in the study.
Competing interests
Dilek Yilmaz is a Growing Child Association board member. For the remaining authors, none were declared.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 2.Du W, Yu J, Wang H, et al. Clinical characteristics of COVID-19 in children compared with adults in Shandong Province, China. Infection. 2020;48(3):445–452. doi: 10.1007/s15010-020-01427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Öztürk R, Taşova Y, Ayaz A. COVID-19: pathogenesis, genetic polymorphism, clinical features and laboratory findings. Turk J Med Sci. 2020;50(SI–1):638–657. doi: 10.3906/sag-2005-287. [DOI] [PubMed] [Google Scholar]
- 4.Severe Covid-19 GWAS Group. Ellinghaus D, Degenhardt F, et al. Genomewide Association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020;383(16):1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JH, Choi SH, Yun KW. Risk factors for severe COVID-19 in children: a systematic review and Meta-analysis. J Korean Med Sci. 2020;37(5):e35. doi: 10.3346/jkms.2022.37.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaltoum ABO. Mutations and polymorphisms in genes involved in the infections by COVID-19: a review. Gene Rep. 2021;23:101062. doi: 10.1016/j.genrep.2021.101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devaux CA, Rolain JM, Raoult D. ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect. 2020;53(3):425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cafiero C, Rosapepe F, Palmirotta R, et al. Angiotensin system polymorphisms in SARS-CoV-2 positive patients: Assessment between symptomatic and asymptomatic patients: a pilot study. Pharmgenomics Pers Med. 2021;14:621–629. doi: 10.2147/PGPM.S303666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorente L, Martín MM, Franco A, et al. HLA genetic polymorphisms and prognosis of patients with COVID-19. Polimorfismos genéticos de los HLA y pronóstico de pacientes con COVID-19. Med Intensiva (Engl Ed) 2021;45(2):96–103. doi: 10.1016/j.medin.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiel S, Holmskov U, Hviid L et al (1992) The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol 1992;90(1):31–35. 10.1111/j.1365-2249.1992.tb05827.x [DOI] [PMC free article] [PubMed]
- 11.Thiel S, Vorup-Jensen T, Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 12.Kuhlman M, Joiner K, Ezekowitz RA. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733–1745. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koutsounaki E, Goulielmos GN, Koulentaki M, et al. Mannose-binding lectin MBL2 gene polymorphisms and outcome of hepatitis C virus-infected patients. J Clin Immunol. 2008;28(5):495–500. doi: 10.1007/s10875-008-9201-8. [DOI] [PubMed] [Google Scholar]
- 14.Erdemir G, Ozkan TB, Ozgur T, et al. Mannose-binding lectin gene polymorphism and chronic hepatitis B infection in children. J Gastroenterol. 2015;21(2):84–89. doi: 10.4103/1319-3767.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amoroso A, Berrino M, Boniotto M, et al. Polymorphism at codon 54 of mannose-binding protein gene influences AIDS progression but not HIV infection in exposed children. AIDS. 1991;13:863–864. doi: 10.1097/00002030-199905070-00019. [DOI] [PubMed] [Google Scholar]
- 16.Ip WK, Chan KH, Law HK, et al. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005;191(10):1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carmolli M, Duggal P, Haque R, et al. Deficient serum mannose-binding lectin levels and MBL2 polymorphisms increase the risk of single and recurrent Cryptosporidium infections in young children. J Infect Dis. 2009;200(10):1540–1547. doi: 10.1086/606013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shawky RM, El-Fattah SM, Kamal TM, et al. Genotyping of mannose-binding lectin (MBL2) codon 54 and promoter alleles in egyptian infants with acute respiratory tract infections. Egyp J Med Hum Gen. 2014;15(1):31–38. doi: 10.1016/j.ejmhg.2013.10.002. [DOI] [Google Scholar]
- 19.Stravalaci M, Pagani I, Paraboschi EM, et al. Recognition and inhibition of SARS-CoV-2 by humoral innate immunity pattern recognition molecules. Nat Immunol. 2022;23(2):275–286. doi: 10.1038/s41590-021-01114-w. [DOI] [PubMed] [Google Scholar]
- 20.Garred P, Larsen F, Seyfarth J, et al. Mannose-binding lectin and its genetic variants. Genes Immun. 2006;7(2):85–94. doi: 10.1038/sj.gene.6364283. [DOI] [PubMed] [Google Scholar]
- 21.Madsen HO, Garred P, Thiel S, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155(6):3013–3020. doi: 10.4049/jimmunol.155.6.3013. [DOI] [PubMed] [Google Scholar]
- 22.Klaassen K, Stankovic B, Zukic B, Kotur N, Gasic V, Pavlovic S, Stojiljkovic M (2020 Oct) Functional prediction and comparative population analysis of variants in genes for proteases and innate immunity related to SARS-CoV-2 infection. Infect Genet Evol 84:104498. 10.1016/j.meegid.2020.104498Epub 2020 Aug 7. PMID: 32771700; PMCID: PMC7410821 [DOI] [PMC free article] [PubMed]
- 23.Glesse N, Monticielo OA, Mattevi VS, et al. Association of mannose-binding lectin 2 gene polymorphic variants with susceptibility and clinical progression in systemic lupus erythematosus. Clin Exp Rheumatol. 2011;29(6):983–990. [PubMed] [Google Scholar]
- 24.Cedzynski M, Szemraj J, Swierzko AS, et al. Mannan-binding lectin insufficiency in children with recurrent infections of the respiratory system. Clin Exp Immunol. 2004;136:304–311. doi: 10.1111/j.1365-2249.2004.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuolivirta K, He Q, Gröndahl-Yli-Hannuksela K, et al. Mannose-binding lectin gene polymorphisms in infants with bronchiolitis and post-bronchiolitis wheezing. Allergol Int. 2012;61(2):305–309. doi: 10.2332/allergolint.11-OA-0385. [DOI] [PubMed] [Google Scholar]
- 26.Speletas M, Dadouli K, Syrakouli A, et al. MBL deficiency-causing B allele (rs1800450) as a risk factor for severe COVID-19. Immunol. 2021;226(6):152136. doi: 10.1016/j.imbio.2021.152136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Zhou G, Zhi L, et al. Association between mannose-binding lectin gene polymorphisms and susceptibility to severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005;192(8):1355–1361. doi: 10.1086/491479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu X, Chong WP, Zhai Y, et al. Functional polymorphisms of the CCL2 and MBL genes cumulatively increase susceptibility to severe acute respiratory syndrome coronavirus infection. J Infect. 2015;71(1):101–109. doi: 10.1016/j.jinf.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan FF, Tanner J, Chan PK, et al. Influence of Fcγ RIIA and MBL polymorphisms on severe acute respiratory syndrome. Tissue Antigens. 2005;66:291–296. doi: 10.1111/j.1399-0039.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medetalibeyoglu A, Bahat G, Senkal N, et al. Mannose binding lectin gene 2 (rs1800450) missense variant may contribute to development and severity of COVID-19 infection. Infect Genet Evol. 2021;89:104717. doi: 10.1016/j.meegid.2021.104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monticelli M, Mele BH, Andreotti G, et al. Why does SARS-CoV-2 hit in different ways? Host genetic factors can influence the acquisition or the course of COVID-19. Eur J Med Genet. 2021;64(6):104227. doi: 10.1016/j.ejmg.2021.104227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pehlivan S, Köse M, Mese S, et al. Investigation of MBL2 and NOS3 functional gene variants in suspected COVID-19 PCR (-) patients. Pathog Glob Health. 2022;116(3):178–184. doi: 10.1080/20477724.2021.1984726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das BK, Panda AK. MBL-2 polymorphisms (codon 54 and Y-221X) and low MBL levels are associated with susceptibility to multi-organ dysfunction in P. falciparum malaria in Odisha, India. Front Microbiol. 2015;6:778. doi: 10.3389/fmicb.2015.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallenbach S, Thiel S, Aebi C, et al. Serum concentrations of lectin-pathway components in healthy neonates, children and adults: mannan-binding lectin (MBL), M-, L-, and H-ficolin, and MBL-associated serine protease-2 (MASP-2) Pediatr Allergy Immunol. 2011;22(4):424–430. doi: 10.1111/j.1399-3038.2010.01104.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen M, Deng J, Su C, et al. Impact of passive smoking, cooking with solid fuel exposure, and MBL/MASP-2 gene polymorphism upon susceptibility to tuberculosis. Int J Infect Dis. 2014;29:1–6. doi: 10.1016/j.ijid.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Tulio S, Faucz FR, Werneck RI, et al. MASP2 gene polymorphism is associated with susceptibility to hepatitis C virus infection. Hum Immunol. 2011;72(10):912–915. doi: 10.1016/j.humimm.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Yan J, Shi Y, et al. Lack of association between polymorphisms of MASP2 and susceptibility to SARS coronavirus infection. BMC Infect Dis. 2009;9:51. doi: 10.1186/1471-2334-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.