Abstract
Despite the importance of chemotherapy-associated adverse events in oncology practice and the broad range of interventions available to mitigate them, limited systematic efforts have been made to identify, critically appraise and summarize the totality of evidence on the effectiveness of these interventions. Herein, we review the most common long-term (continued beyond treatment) and late or delayed (following treatment) adverse events associated with chemotherapy and other anticancer treatments that pose major threats in terms of survival, quality of life and continuation of optimal therapy. These adverse effects often emerge during and continue beyond the course of therapy or arise among survivors in the months and years following treatment. For each of these adverse effects, we discuss and critically evaluate their underlying biological mechanisms, the most commonly used pharmacological and non-pharmacological treatment strategies, and evidence-based clinical practice guidelines for their appropriate management. Furthermore, we discuss risk factors and validated risk-assessment tools for identifying patients most likely to be harmed by chemotherapy and potentially benefit from effective interventions. Finally, we highlight promising emerging supportive-care opportunities for the ever-increasing number of cancer survivors at continuing risk of adverse treatment effects.
Subject terms: Cancer, Cancer therapy, Outcomes research
The effective management of treatment-related events remains an unmet need in oncology. The authors of this Review discuss the underlying biological mechanisms, risk factors, most commonly used pharmacological and non-pharmacological management strategies, and clinical practice guidelines for the most common long-term (continuing beyond treatment) and late or delayed (following treatment) adverse events associated with chemotherapy and other anticancer treatments.
Key points
Chemotherapy-associated adverse events (CAAEs) and those associated with other anticancer treatments place a high burden on patients, with a direct effect on symptoms, health-related quality of life and function, but also compromising treatment intensity and continuation, potentially increasing the risk of cancer recurrence.
Similar to acute CAAEs, long-term and late CAAEs are frequently multifactorial in nature, and often adversely affect multiple dimensions, including physical, emotional and social functioning domains.
Innovative symptom monitoring, patient communication, and educational tools and supportive-care interventions have been developed to reduce the risk of complications from CAAEs and to improve health-related quality of life among cancer survivors.
Further research addressing the underlying pathophysiology, emerging biomarkers, supportive-care therapies and patient risk stratification for CAAEs is needed to further enhance personalized care for patients receiving chemotherapy.
The necessary coordination of multidisciplinary care and routine engagement of patient advocates to empower patients are essential for promoting good-health practices to reduce present and future risks of disease recurrence and additional malignancies and to minimize complications associated with comorbidities.
Introduction
Systemic therapy with cytotoxic agents (referred to as chemotherapy in this Review) continues to be a mainstay of cancer treatment alone or in combination with systemic targeted, immunotherapeutic agents and/or radiotherapy for most human malignancies. Patients receiving chemotherapy are at risk for a range of adverse events that are associated with considerable morbidity, mortality and treatment-related costs. Treatment-associated adverse events have a direct effect on patient symptoms and health-related quality of life (HR-QOL) but can also compromise the administration of further treatment to prevent disease recurrence. In 2022, we reviewed the most common acute chemotherapy-associated adverse events (CAAEs) with major effects on the aforementioned outcomes and recommended mitigation strategies1. Herein, we discuss additional adverse events that can present during treatment and persist as long-term sequelae in many cancer survivors such as cancer-related fatigue (CRF), chemotherapy-induced peripheral neuropathy (CIPN), chemotherapy-related cognitive impairment (CRCI), ovarian failure or infertility. We also discuss late or delayed adverse events, which first appear months or years following treatment, such as cardiotoxicity and secondary malignancies. Additional long-term effects of chemotherapy and other cancer therapies include a range of emotional effects such as anxiety, depression and fear of cancer recurrence (FCR). The prevalence of these adverse effects varies across toxicities and depends on both patient-related and treatment-related factors. We have selected these toxicities based on the available literature — including the published results from the 2021–2022 patient survey from the National Coalition for Cancer Survivorship2 — detailing these as the most prevalent toxicities reported by cancer survivors. Furthermore, although often not discussed, the increasing financial burden (or ‘financial toxicity’) associated with anticancer treatment remains an important concern. We discuss risk factors for each of these adverse events along with recommendations drawn from evidence-based guidelines for their mitigation. Although the long-term and late adverse effects discussed herein are often associated with chemotherapy, they can also occur with other forms of anticancer treatment, including endocrine therapy, targeted agents and immunotherapies. Finally, we highlight the fact that optimal implementation of the recommendations aimed at improving HR-QOL in survivors requires consistent care coordination and overall general health promotion among all health-care stakeholders.
Cancer-related fatigue
Background
CRF is one the most burdensome and long-lasting complications of cancer and cancer treatment3. CRF is commonly experienced by patients as a persistent sense of physical, emotional and/or cognitive exhaustion. The reported prevalence of fatigue during cancer treatment depends upon patient characteristics and type of treatment received as well as on the assessment tool used, and can range from 25% to nearly 100%, with 30–60% of patients reporting moderate-to-severe fatigue during treatment4. CRF commonly first occurs during treatment with increased cumulative intensity after repeat exposure to anticancer therapies. These symptoms often improve within months of ending therapy. In some patients, however, symptoms can persist well beyond the completion of treatment5 for years and have been reported in a variety of malignancies, with a prevalence of 23–49% in cancer survivors6–9. CRF is rarely improved by rest or sleep and is one of the most common distressing symptoms experienced by patients with cancer receiving chemotherapy, affecting HR-QOL and, when severe, treatment administration10–15.
The precise aetiology of CRF remains unclear. Possible mechanisms include dysregulation of various pathways, including 5-HT neurotransmitters, vagal signalling, muscle and ATP metabolism, the hypothalamic–pituitary–adrenal axis, circadian rhythms, and activation of pro-inflammatory cytokine cascades16–18. Strong associations between sleep alterations and affective symptoms in patients with cancer have been reported. Impaired sleep, mood and lower physical activity at the last treatment are predictive of persistent CRF 1 year after completion of treatment in cancer survivors19,20.
Cancer survivors with CRF have significantly higher serum levels of pro-inflammatory cytokine markers than survivors without fatigue or healthy individuals21. Cytokines such as C-reactive protein (CRP), IL-1 receptor antagonist protein, IL-1β, IL-6, interferons, soluble TNF receptor 2 (sTNFR2) and TNF can induce neuroinflammatory changes that promote CRF, although the exact mechanisms remain to be fully elucidated4,21,22. Increased cytokine activity might be the result of tissue damage after chemotherapy, and whether this cytokine activity induces or just exacerbates CRF is not fully understood. Serum levels of these cytokines (particularly sTNFR2), can remain persistently elevated in patients who have completed primary therapy and continue to experience CRF12,23.
Risk factors
CRF has multiple and complex causes, including demographic, clinical, biological, behavioural and socioeconomic factors4,24 (Fig. 1a). Emerging evidence suggests an underlying inflammatory aetiology for CRF, with elevated serum levels of inflammatory mediators in patients prior to, during and following treatment. Disruptions in the central and/or peripheral nervous systems could also induce CRF via ATP dysregulation or build-up of metabolic by-products in the neuromuscular junction or muscle21. Cancer stage, advancing age, cumulative dose of cytotoxic chemotherapy, use of multi-agent regimens and chronic comorbidities are common risk factors for CRF10,13. In a study, women with breast cancer who had received chemotherapy reported more severe fatigue and worse HR-QOL than those with no history of cancer15. Moreover, women with worse sleep quality, menopausal symptoms and/or a psychiatric disorder were more likely to have severe CRF15. In another study, individuals reporting higher levels of life-stress exposure during childhood and/or adulthood had increased levels of CRF25. Across studies, however, the most consistent predictor of CRF following treatment is the presence of fatigue prior to therapy. In addition, those reporting greater intensity of fatigue before chemotherapy experience greater CRF4. Other predictors of persistent severe fatigue include variables such as age, obesity, tobacco use, receipt of endocrine therapy and presence of other symptoms (pain, insomnia and depression)26,27. A predictive model of severe CRF validated in women with breast cancer was described in 2022 (ref. 28); independent risk factors for severe CRF were severe pretreatment fatigue, younger age, higher body mass index, current smoking, worse anxiety, insomnia and pain at diagnosis. Identifying other contributors to CRF, such as anaemia, sleep issues, depression, malnutrition, involuntary weight loss or gain, thyroid imbalances, and other comorbidities, is always important to enable additional interventions that might ameliorate CRF. CRF can arise together with other CAAEs such as sleep disturbances, depression and anxiety29–31.
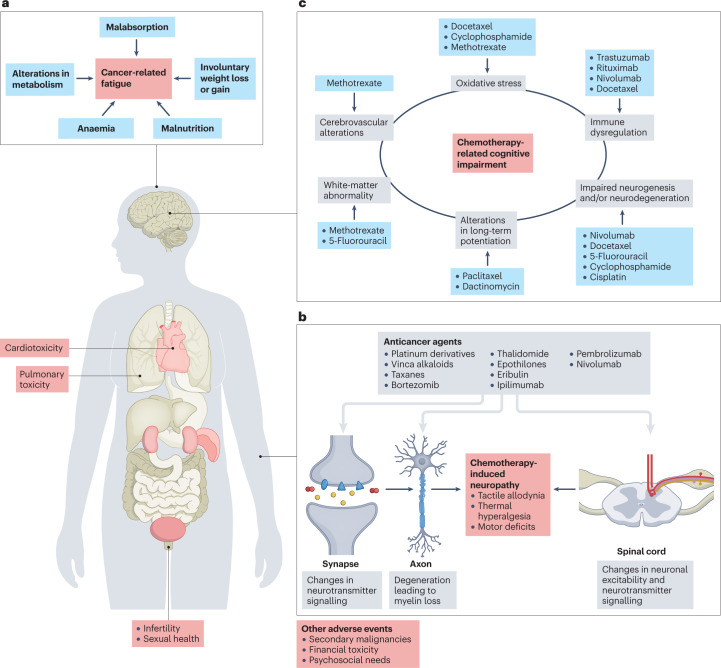
Fig. 1. Long-term treatment-related adverse events in patients with cancer.
a, Fatigue is the most common symptom reported by patients with cancer receiving chemotherapy and it has multiple risk factors24. b, Cancer-induced peripheral neuropathy most commonly manifests as either positive or negative sensory symptoms and pain, although autonomic and motor symptoms can also appear in patients with severe cancer-induced peripheral neuropathy52,53. c, Chemotherapy-related cognitive impairment is caused by various pathophysiological mechanisms activated by certain therapeutic agents. Importantly, fatigue, depression and sleep disturbances can contribute to chemotherapy-related cognitive impairment85. These and other adverse events can compromise patient quality of life and their willingness to continue effective therapy. Part a is adapted with permission from ref. 24, Taylor & Francis. Part b is adapted from ref. 53, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Part c is adapted with permission from ref. 85, Elsevier.
Management and guideline recommendations
Clinical practice guidelines for the management of CRF are available from the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO), all of which recommend prompt assessment as part of routine cancer care and potential underlying medical conditions10–13. All patients with cancer should be serially screened for the presence and severity of CRF during and after completion of treatment. Screening should be conducted using brief and fully validated tools. More than 26 different scales for assessing fatigue have been developed32. The Numerical Rating Scale13 and the Brief Fatigue Inventory, which integrates the assessment of CRF severity and its effect on essential functional domains33, are among the most commonly used owing to their simplicity of use. Other scales validated for this purpose include the Functional Assessment of Cancer Therapy – Fatigue and the Multidimensional Fatigue Symptom Inventory34–36.
Once CRF is identified and other contributing factors are ruled out, appropriate supportive efforts (including referral to oncological rehabilitation) can be helpful for better managing this toxicity and maintaining HR-QOL. Psychostimulants (including methylphenidate, dexmethylphenidate, modafinil, armodafinil and dexamphetamine) have been evaluated in multiple randomized controlled trials (RCTs). Although no consistent evidence supports the superiority of these agents over placebo, some studies of methylphenidate and modafinil reported positive effects on symptoms of CRF37,38. In addition, no benefit was shown from the use of the acetylcholinesterase inhibitor donepezil39. Given the high cost and toxicities associated with these pharmacological agents (including exacerbation of sleep alterations), their routine use is not the current standard of care for the management of CRF10,12,13.
In two RCTs involving patients with advanced-stage cancer, short-term use of corticosteroids was associated with some improvement in symptoms, although adverse events from these agents (such as anxiety, insomnia, immunosuppression and potential diabetes) tend to be a concern and need to be individually considered for each patient40–42. In another RCT, exercise plus dexamethasone had beneficial effects in patients with advanced-stage cancer, but further studies are needed43.
Finally, although the results of a meta-analysis support the safety and effectiveness of ginseng supplements, the fact that these studies involved both patients with advanced-stage cancer and survivors not on therapy, along with the limited number of high-quality studies available, means that insufficient evidence is available for the routine use of these supplements to manage CRF44. In summary, given the limitations of the existing data, no pharmacological agent or supplement is recommended for the routine management of CRF.
Emerging strategies
Given the limited efficacy of currently available pharmacological interventions, other management strategies are needed for the often-debilitating effects of CRF. A meta-analysis of 113 studies concluded that physical activity and/or psychological interventions were the approaches that most improve CRF symptoms45. The analysis included 51 studies in which patients were receiving primary treatment (surgery, chemotherapy and/or radiotherapy) during the study intervention and 45 studies in which patients had already completed primary treatment. Exercise seems to be the most effective intervention for patients with acute or delayed CRF receiving primary treatment, whereas exercise plus psychological interventions would be most beneficial after treatment completion. Accumulating evidence supports the use of multidisciplinary exercise-based programmes to improve patient outcomes, although their routine implementation remains challenging46. Additional research is needed to generate evidence on the potential role of complementary and integrative therapies (such as acupuncture, stress reduction and touch therapies), in the management of CRF47. CRF can follow distinct trajectories depending on the degree of fatigue at different times after cancer diagnosis. The growing recognition of these trajectories will probably lead to innovative interventions adapted to personalized fatigue trajectories to address this common problem3. Furthermore, evidence supports a role for cognitive–behavioural therapy and mindfulness‐based therapies in the mitigation of CRF12,48,49. Notably, these interventions can be delivered in person or through online platforms such as eHealth and mHealth45,50,51.
Chemotherapy-induced peripheral neuropathy
Background
CIPN is a common CAAE that generally presents with symptoms, including but not limited to pain, tingling, numbness and increased sensitivity to temperature (Fig. 1b), present in as many as 68% of patients at the end of the first month of chemotherapy and in 30% at 6 months52–54. CIPN can negatively affect cancer outcomes by leading to reduced dosing or even premature cessation of chemotherapy in patients with a high symptom burden. In at least one-third of patients with acute CIPN, permanent functional impairments can occur that, in some patients, seriously affect HR-QOL and functional well-being54. In addition, ototoxicity is yet another complication of neurotoxic drugs (particularly platinum-based agents)55,56. Neurotoxic injury associated with CIPN is mediated by signalling pathways involved in inflammation, apoptosis and neurodegeneration57,58; however, despite the identification of these biological targets, their translation into mechanistic-based clinical interventions remains challenging59–61.
Risk factors
Risk assessment for CIPN includes the consideration of other comorbidities such as pre-existing neuropathy, diabetes or family history of neuropathy58. For older patients (≥65 years of age), a comprehensive geriatric assessment can help to estimate the probability of CAAEs, including CIPN. CIPN is frequently associated with treatment with specific chemotherapeutic agents such as vinca alkaloids (for example, vincristine), taxanes (paclitaxel and docetaxel) and platinum-based agents (cisplatin, carboplatin and oxaliplatin)53 (Fig. 1b). In patients at high risk of treatment-related complications, the substitution of these agents for less neurotoxic drugs should be considered as part of a shared decision-making process between patients and clinicians62. Ongoing research on CIPN risk factors includes identification of genetic predictors and blood-based biomarkers to improve risk calculation in a useful way for both clinicians and patients58.
Management and guideline recommendations
One of the main challenges in the management of patients with CIPN is the lack of validated standardized measures for routine evaluation of neuropathy in clinical practice. Notable differences exist between patient self-reporting and clinician reporting of CIPN symptoms, with the latter often leading to under-representation of symptom severity63–65.
Both the ASCO and ESMO guidelines from 2020 have confirmed the lack of efficacious evidenced-based interventions for the prevention of CIPN60,61. Indeed, the RCTs performed thus far have failed to definitively demonstrate that any strategy can prevent CIPN. For example, in two placebo-controlled trials testing whether the antioxidant calmangafodipir prevents CIPN, patients receiving this agent were more likely to develop serious hypersensitivity reactions and CIPN; therefore, the trials were consequently discontinued66. Hence, early recognition and a high index of suspicion remain crucial for limiting the burden of CIPN, although these strategies remain hindered by the underestimation of symptoms by clinicians and limited routine implementation of regular standardized assessment tools.
For patients with CIPN symptoms, clinicians should discuss dose delays and/or reductions, or a change of treatment regimen as part of a shared decision-making strategy during treatment to help to reduce the chance of chronic persistent CIPN once treatment ends, which would lead to more-permanent chronic injury. Decision-making should weigh the potential benefits of cancer therapy against the risk of permanent injury and functional deficits60,62. Duloxetine is the only drug established in an RCT to provide benefit in patients with established painful CIPN. Patients receiving duloxetine as part of their initial 5-week CIPN management plan reported a greater decrease in average pain compared with those who received placebo (1.06 versus 0.34; P = 0.003) and were more likely to report decreased pain (59% versus 38%)67. The effect size attributable to duloxetine was 0.513, which means that this drug is only modestly effective for the management of painful CIPN. Moreover, this drug is also associated with high costs as well as other toxicities (such as nausea, constipation and xerostomia) in some patients67. The data currently available on supplements (including vitamins B6 and B12, glutamine, and thiamine, among others) is insufficient to recommend them for the treatment of CIPN60,61. Although multivitamin use has been associated with improved CIPN outcomes in retrospective studies, their use might be a surrogate for other related behaviours that are the actual drivers of CIPN reduction68. Supplements (such as multivitamins) can also adversely affect both CIPN and cancer outcomes and increase the financial burden on patients69,70.
Emerging strategies
Early phase studies of non-pharmacological strategies, including acupuncture, physical exercise, cryotherapy and/or compression, and Scrambler therapy (an approach that involves electrical stimulation), for the prevention or treatment of CIPN have shown promising results in terms of tolerability. Large-scale RCTs testing these strategies are ongoing. In one small sham-controlled trial, weekly electro-acupuncture was not efficacious for the prevention of CIPN71. In another RCT, an 8-week course of acupuncture intervention for the treatment of CIPN resulted in clinically meaningful and statistically significant improvements in several neuropathy-related sensory symptoms compared to usual care (all P ≤ 0.03)72. Acupuncture has a favourable toxicity profile but also major limitations, including often high costs, limited access to services and time devoted to repeated treatments. Several clinical trials testing compression therapy using surgical gloves for the prevention of CIPN have had potentially promising results in some patients73,74; however, not all studies have shown consistent results75. Larger, well-powered studies are needed60,75. Potential adverse effects include the risk of frostbite from cooling, patient discomfort and vasospasm, which is of particular concern in patients who have received oxaliplatin. The results of other studies suggest that physical exercise might mitigate CIPN through attenuation of pro-inflammatory signalling pathways, although further evidence supporting this approach is needed76–79. Finally, considering the tremendous effect of CIPN on HR-QOL, the collection of patient-reported outcomes is imperative and treatment decisions regarding toxicity must be shared within a multidisciplinary team.
Chemotherapy-related cognitive impairment
Background
CRCI is experienced by ~35% of survivors of all cancer types and stages80,81, and can negatively affect the functional ability and HR-QOL of patients and their families82,83. This CAAE, commonly referred to as ‘chemobrain’, involves loss of memory and other cognitive changes associated with the previous administration of chemotherapy. The mechanisms of CRCI are complex and affect multiple neural pathways. Notably, the most frequent impairments are processing speed, learning and memory, and executive function84. CRCI has been reported in patients with haematological or solid tumour malignancies receiving a variety of anticancer agents, including taxanes, anthracyclines and certain antimetabolites (such as methotrexate or 5-fluorouracil)85–89 (Fig. 1c).
The effect of CRCI is complicated by the disease status in a patient and concurrent or subsequent cancer therapies90. CRCI affects patient well-being, HR-QOL, and human relationships and can have substantial financial sequelae for individuals unable to return to their pre-cancer work situation91,92. The recognition of CRCI as a true CAAE is limited, and this situation is further compounded by evidence showing that many patient-reported symptoms are not fully captured by most neuropsychological tests84,90.
Risk factors
CRCI is a multifactorial disorder with risk factors that include the type of cancer, anticancer therapies received and comorbidities53. Indeed, non-cancer-related comorbidities (such as those associated with ageing) can further affect cognitive function and result in decreased recovery of cognitive function after therapy88,93. Evidence indicates that micronutrients and malnutrition have a role in cognitive performance94. Moreover, anaemia is associated with CRCI in observational clinical studies, although the use of erythropoietin as a treatment for this CAAE is not currently recommended95.
In a cohort of long-term survivors of breast cancer who had received chemotherapy, those harbouring the ε4 allele of APOE (involved in lipid metabolism and neuronal function) had worse cognitive decline in several domains than those with other alleles96,97. In another study involving patients with breast cancer receiving chemotherapy, patients with the single-nucleotide polymorphism rs165599 in COMT (involved in dopamine degradation) had an increased prevalence of CRCI98. Pro-inflammatory cytokines (such as IL-1β, IL-6, TNF, IL-10 and BDNF99–101) have been related to CRCI symptoms in observational studies, although the exact mechanisms of neurotoxicity remain under investigation. Larger prospective studies are needed to validate these associations before such biomarkers can be routinely used to risk-stratify patients.
Management and guideline recommendations
The management of CRCI should include routine screening for cognitive symptoms as part of ongoing cancer care. Such screening procedures can include common probing questions listed in the NCCN Guidelines on Survivorship102. More comprehensive assessment of cognitive symptoms using the EORTC QLQ-C30 (ref. 103) and FACT-COG104 scales should become a routine approach for the optimal management of CRCI. Other symptoms that contribute to CRCI (such as fatigue, mood disturbances and sleep alterations) can confound the diagnosis and management of this common CAAE. Once CRCI is diagnosed, current recommended management strategies include patient education, cognitive training, rehabilitation105,106, exercise107,108, mind–body interventions (such as yoga, qigong and tai chi) and pharmacological therapies, personalized to the unique symptom burden of each patient and their other comorbidities80. The available evidence supports clinical implementation of cognitive rehabilitation to improve cognitive symptoms as well as the potential benefits of mind–body interventions on this CAAE; however, additional validation studies are needed to confirm the effectiveness of both strategies for the treatment of CRCI105,106,108. The utility of pharmacological agents in this setting has not been consistently demonstrated; further research is needed before agents, such as modafinil, donepezil and memantine, can be routinely used39,109,110.
Emerging strategies
Given the increasing use of eHealth, mHealth and other similar tools to improve symptom management, preliminary studies have suggested the benefit of incorporating a computer-based cognitive rehabilitation programme in the management of CRCI, with improvement in patient self-reported cognitive symptoms111,112. Mechanistic understanding of CRCI obtained through the use of novel imaging techniques might lead to improved management strategies in the future. Given the complexity of CRCI, intervention studies focused on the management of this CAAE would be most effective if they combined pharmacological and non-pharmacological treatment strategies, including exercise and cognitive-based interventions. Further studies also need to involve patients with metastatic disease and address the potential role of validated biomarkers in improving the risk stratification of patients for CRCI.
Cardiotoxicity
Background
The cardiotoxicity of several anticancer therapies in combination with multiple risk factors common to heart disease and cancer, particularly in an ageing population, has led to a dramatic rise in cardiac complications in cancer survivors102. These complications include acute life-threatening toxicities, such as coronary vasospasms, arrythmias113 and myocarditis, and long-term cardiovascular complications, including cardiomyopathies, and thus an increased risk of cardiovascular events in cancer survivors113. Consequently, cardio-oncology has emerged as a specialty that precisely addresses this increased risk of cardiovascular complications and the associated need for multidisciplinary management114.
Risk factors
The mechanisms of chemotherapy-related cardiac damage are diverse and depend on the drug, cumulative level of exposure, and host factors and susceptibilities115. Patients at high risk for cardiovascular complications include those receiving high-dose anthracyclines (for example, doxorubicin ≥250 mg/m2 or epirubicin ≥600 mg/m2), high-dose mediastinal radiation therapy encompassing the heart in the treatment field116, and other predisposing drugs (including but not limited to 5-fluorouracil, doxorubicin, trastuzumab, and VEGF inhibitors and other anti-angiogenic agents). Additional risk factors for cardiovascular toxicity include age >60 years, smoking, hypertension, diabetes, dyslipidaemia, chronic renal failure, obesity and previous cardiovascular disease114. The latest guidelines from the Task Force on Cardio-Oncology of the European Society of Cardiology propose strategies for triaging patients on the basis of risk stratification117.
Management and guideline recommendations
Cardiovascular monitoring, including screening with strain imaging echocardiography in patients with cancer receiving treatment and who are at high risk of cardiac complications, is a key focus area of the European Society of Cardiology, ESMO and ASCO guidelines117–119. Priority areas include the management of complications related to high-risk anticancer agents such as anthracyclines, HER2-targeted agents, VEGF inhibitors, BCR–ABL1 tyrosine kinase inhibitors, BTK inhibitors and immune-checkpoint inhibitors (ICIs). Various cardiovascular risks and risk factors should be actively managed before, during and after anticancer therapy and discussed with patients to enable shared decision-making. Furthermore, health-promotion strategies, such as a healthy diet and exercise, need to be addressed120,121. Patients reporting toxicities should promptly receive care from cardiologists, and multidisciplinary management with oncology teams is encouraged. The specific role and timing for blood-based cardiac monitoring, including natriuretic peptide B and high-sensitivity troponin testing, in asymptomatic patients undergoing cardiotoxic treatments are yet to be determined114, although guidelines such as those from ESMO118 and ASCO119 discuss the promising emerging data for their use. Changes in the levels of high-sensitivity troponin might be predictive of future cardiotoxicity in patients treated with anthracyclines, although further validation is needed122,123. Along with cardiac imaging, these biomarkers should be promptly drawn in patients who are symptomatic while undergoing cardiotoxic therapy114. The ASCO guidelines recommend using the cardioprotectant dexrazoxane and treatment with a continuous infusion or liposomal formulation of doxorubicin to prevent cardiotoxicity in patients allocated to receive high-dose anthracyclines119.
Emerging strategies
Several studies have evaluated risk-reduction or cardioprotective strategies prior to the onset of cardiotoxicity in patients receiving anthracycline-based and HER2-targeted agents. Despite modest efficacy, the routine use of cardioprotective medications (such as β-blockers124, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers125, aldosterone antagonists126 and statins127) are not yet routinely prescribed and require further validation, although intervention studies have shown promise124,125,128,129. Better risk stratification to identify those patients most in need of cardioprotective strategies, with more sophisticated cardiac imaging and the use of validated cardiac toxicity risk tools and biomarkers, should enable pragmatic and targeted personalized prevention and therapeutic interventions124,125,128,129.
Pulmonary toxicity
Background
Chemotherapy-induced pulmonary toxicity (CIPT) can result from direct effects or from secondary infectious complications in the lung owing to therapy-related immunosuppression. In this Review, we focus on the direct pulmonary toxicity from chemotherapy that can manifest as both long-term and delayed lung disease in cancer survivors130–132.
The risk of pulmonary toxicity in patients receiving anticancer therapy is variable and depends on the specific agents used, their dosages and the presence of pre-existing lung disease; in general, the incidence is estimated to be 6–7%133. The most common forms of CIPT include interstitial pneumonitis (caused by anthracyclines, oxaliplatin, gemcitabine, ifosfamide, fludarabine or irinotecan), diffuse alveolar damage (from gemcitabine, etoposide or oxaliplatin) and non-cardiogenic pulmonary oedema (from gemcitabine, cytarabine or vinblastine). The pathogenesis of CIPT is poorly understood and involves direct injury to pneumocytes, endothelial dysfunction, oxidative stress-induced injury and dysregulation of the immune system (characterized by cytokine release and recruitment of inflammatory cells)134.
Risk factors
While some lung injury reactions in patients receiving chemotherapy are related to the total dose (such as those from bleomycin), others are mostly idiosyncratic. Although information on specific risk factors for CIPT is limited, assessment of a previous or current history of smoking and lung conditions existing prior to chemotherapy is recommended. Baseline pulmonary function tests might enable identification of patients at high risk of such toxicity, especially in those allocated to receive known causative agents such as bleomycin, gemcitabine, busulfan, methotrexate, all-trans retinoic acid and etoposide135. CIPT is a major limitation, in particular in patients receiving bleomycin, which has been used to treat Hodgkin lymphoma and germ-cell tumours136. Bleomycin-induced lung injury has four different subtypes: acute chest pain syndrome during infusion, hypersensitivity pneumonitis, cryptogenic organizing pneumonia and chronic progressive pulmonary fibrosis. Risk factors for bleomycin-induced lung toxicity include advancing age, renal insufficiency, total dose >400 units (although lower doses might also result in CIPT), and concurrent administration of radiation or other chemotherapeutic agents137–140.
Management and guideline recommendations
The clinical manifestations of CIPT are generally non-specific, including cough with or without sputum production, dyspnoea and hypoxaemia. The time of onset of this toxicity is variable, but it generally occurs within weeks to a few months from the start of initial therapy — except for the delayed fibrosis seen with bleomycin and nitrosoureas141. Acute chest pain syndrome (which occurs during bleomycin infusion), hypersensitivity pneumonitis and diffuse alveolar damage can present with more rapidly progressing symptoms142. Meanwhile, indolent dyspnoea on exertion several months after treatment can be a symptom of chronic progressive pulmonary fibrosis and chronic usual interstitial pneumonia143. Pretreatment pulmonary function tests are performed to assess baseline lung function and guide decisions on whether to administer agents known to cause CIPT. However, routine screening for lung toxicity, especially in patients receiving non-bleomycin agents, is not common practice144. The FDA label for bleomycin recommends monitoring of lung diffusing capacity for carbon monoxide at baseline and monthly thereafter to detect toxicities, and treatment discontinuation when the lung diffusing capacity for carbon monoxide falls below 30–35% of the pretreatment value. Frequent chest radiography every 1–2 weeks is also recommended by the FDA but these short intervals are seldom followed in routine practice. Diagnosis is usually made through imaging, such as CT scans and increasingly by high-resolution CT scans, to identify various abnormalities that can range between ground-glass opacities, consolidation, interlobular septal thickening and centrilobular nodules depending on the clinical syndrome134. Invasive diagnostic modalities (including bronchoscopy, cytological analysis of bronchoalveolar lavage and lung biopsy) might be required to rule out other confounding aetiologies.
Pulmonary symptom assessment and monitoring during anticancer treatment and having a high index of suspicion of pulmonary toxicities, especially in patients at high risk of such complications, are important components of management to identify serious toxicities in a timely fashion. Drug discontinuation, glucocorticoid therapy and supportive care are key elements in the management of CIPT145. In general, suspicion of substantial lung toxicity requires the discontinuation of the triggering chemotherapeutic agent. For most antineoplastic agents, no specific treatment has proven effective besides timely drug discontinuation. Although data on the use of steroids is based on experience146, severe respiratory compromise owing to CTCAE grade 3–4 CIPT is often treated with prednisone 1 mg/kg daily. Meanwhile, intravenous glucocorticoids (such as methylprednisolone 1 g daily for 3 days) followed by a long taper can be used in patients who have impending respiratory failure or require mechanical ventilation. Respiratory support with supplemental oxygen, inhaled bronchodilators and mechanical ventilation (if clinically indicated) are other important aspects of management for serious lung complications. The American Thoracic Society and several other professional organizations updated their guidelines for the management of progressive pulmonary fibrosis in patients with interstitial lung disease in 2022 (refs. 117,147). CIPT has gained increased attention with the advent of lung toxicities from other anticancer agents such as ICIs, which can cause immune-related pneumonitis, and antibody–drug conjugates (ADCs), which can cause interstitial lung disease that can be life-threatening148–150.
Emerging strategies
With the incorporation of ICIs and ADCs into the treatment of many cancer types and the continued use of chemotherapeutic agents, new strategies for preventing and treating CIPT are needed. Novel agents currently approved for the treatment of idiopathic pulmonary fibrosis, such as the multi-tyrosine kinase inhibitor nintedanib as well as the antifibrotic agent pirfenidone, have shown benefit in animal models and promising preliminary patient case reports, for example, in patients with lymphomas and bleomycin-induced pneumonitis with substantial fibrosis burden. These agents can also prevent poor outcomes from CIPT and, therefore, may have a role in the routine management of CIPT in the future151,152.
Sexual health
Background
Sexual function can be affected by cancer, cancer therapies and ageing, and thus related concerns are common among cancer survivors153. Survivors across multiple cancer types and disease stages commonly report sexual dysfunction, which often persists during and after cancer treatment, affecting patient HR-QOL154. Despite multiple studies highlighting the wide-ranging effects of different anticancer treatment modalities on sexual dysfunction, interventions to address these issues are not frequently used in routine practice. Common sexual concerns reported by female cancer survivors include low desire, arousal issues, reduced lubrication, difficulty achieving orgasm and pelvic pain155. Male cancer survivors (in particular those receiving androgen-deprivation therapy) most commonly report loss of libido, erectile dysfunction and incontinence. Additionally, chemotherapy (particularly multi-agent regimens) is associated with a higher prevalence of sexual dysfunction in male survivors of testicular cancer156–158. Male cancer survivors often report the desire for assistance in addressing issues regarding desire, erection and ejaculation159. Despite the importance of sexual health, this area of cancer care is often not part of the patient–clinician dialogue and is frequently unaddressed. While 87% of patients report that their cancer treatment has affected their sex life, only 27.9% report being asked about it. Women are asked less frequently than men (22% versus 53%)160. Post-treatment sexual health issues have been reported that can be long-lasting and seem to have a multidimensional nature.
Additionally, the sexual health-care needs of persons from sexual and gender minorities (LGBTQ+) are often not addressed in routine oncology or survivorship care. Anatomical examination involving organs that no longer align with the identity of transgender individuals is a particularly difficult aspect that can be fraught with emotional challenges160.
Risk factors
In premenopausal women, chemotherapy and gonadotropin-releasing hormone (GnRH) agonists can induce a sudden decline in hormone levels, referred to as treatment-induced menopause, and can lead to many sexual concerns reported by survivors. Endocrine therapies, such as aromatase inhibitors and tamoxifen, along with ovarian suppression from GnRH agonists can also lead to treatment-induced menopause161,162. Persisting sexual dysfunction can be present in some long-term survivors well beyond discontinuation of drug therapy163. In men, androgen-deprivation therapy, multi-agent chemotherapy and medical comorbidities often exacerbate sexual dysfunction. Data from a meta-analysis of 43 studies addressing sexual function in male cancer survivors showed a high prevalence of erectile dysfunction (40%)164.
Management and guideline recommendations
Addressing sexual concerns in cancer survivors requires a multidisciplinary approach addressing both physical and psychosocial aspects. In female survivors, physical concerns include dyspareunia owing to genitourinary syndrome of menopause, for which interventions include long-acting vaginal moisturizers containing polycarbophil or hyaluronic acid and, in those with refractory symptoms, local vaginal oestrogen, the oestrogen precursor dehydroepiandrosterone or its analogous prasterone, or the selective oestrogen receptor modulator ospemifene165. Despite concerns about the potential for systemic absorption of topical hormonal therapies, the evidence available indicates that a small amount of absorption occurs and, thus, women receiving aromatase inhibitors might be particularly vulnerable to this effect166. Although studies testing these topical hormonal therapies are not powered to link their use with an increased risk of cancer recurrence, the effect of untreated genitourinary syndrome of menopause on patient HR-QOL must be considered167. Pelvic floor physical therapy is recommended for women with persistent dyspareunia not responding to pharmacological therapies. For male survivors with erectile dysfunction, early penile rehabilitation, penile prosthesis, treatment with phosphodiesterase type 5 inhibitors and testosterone repletion can be considered168,169. For both men and women, psychosocial support is paramount to addressing unmet needs and aiding in recovery.
Emerging strategies
In addition to novel therapies, the management of cancer-related sexual problems requires the integration of multidisciplinary teams, including clinicians with expertise in sexual health, pelvic floor rehabilitation, penile rehabilitation and psychosocial oncology165,169. Physician awareness also needs to be improved through educational efforts to ensure that sexual health issues are discussed during routine oncology visits.
Infertility
Background
The effect of chemotherapy on fertility varies depending on the cancer drug, dose and pretreatment fertility of the patient170–173. In male individuals, chemotherapy can reduce sperm count and motility, and DNA integrity. In female individuals, it can reduce the number of primordial follicles and induce hormonal changes that result in premature ovarian failure174.
Risk factors
Multiple risk factors have been identified as risk factors for fertility loss, including age175, genetic predisposition176–178 and cumulative dose of chemotherapy173,175. The type of chemotherapy regimen also affects fertility loss, and the highest risk is conferred by alkylating agents (such as cisplatin, cyclophosphamide and ifosfamide) and stem cell transplantation179,180. The ovarian reserve (that is, the remaining reproductive potential) of a female survivor depends on age, genetic predisposition, prior cancer history and anticancer treatments175,179,181. Serological antibody testing for anti-Müllerian hormone (AMH) can help to evaluate ovarian reserve in cancer survivors. Although AMH is not a precise predictor of future pregnancy nor the health of remaining follicles182, it is increasingly being used as a complementary tool in decisions about the management and treatment of female infertility183,184.
Management and guideline recommendations
ASCO guidelines recommend discussing the risk of infertility and fertility preservation options with both male and female patients of childbearing age prior to the start of chemotherapy as an essential component of cancer survivorship care delivery185. Similar recommendations are included in the latest guidelines from ESMO and the European Society of Human Reproduction and Embryology173,186. Sperm, oocyte and embryo cryopreservation and, most recently, ovarian tissue cryopreservation are all now considered standard practice187. Developments in fertility preservation from the past few years include random start ovarian stimulation for oocyte cryopreservation, which can be performed at any time during the ovarian cycle and thus provides added flexibility and reduces delays in the start of chemotherapy188. Increasing evidence suggests that administering GnRH agonists concurrently with chemotherapy could improve regular menses189. Nevertheless, protection of ovaries with GnRH should not be used as a replacement for oocyte harvesting and in vitro fertilization for those cancer survivors who would like to preserve fertility given the limited availability of efficacy data172,173.
Emerging strategies
Testicular tissue cryopreservation should only be offered as part of clinical trial protocols. Ongoing research is leading to advances in the construction and cryopreservation of artificial ovaries, in a process that entails grafting isolated follicles embedded in a biological scaffold, as an additional option for fertility restoration190. Although ovarian stimulation has been shown to be safe in patients with cancer in terms of disease recurrence191, an emerging technology, involving immature oocyte retrieval from ovaries without ovarian stimulation followed by in vitro maturation and vitrification, presents a promising new option that could further reduce delays in the start of anticancer therapy192. Financial counselling and improvements in coverage of out-of-pocket costs are key next steps for ensuring that all patients with cancer who are of reproductive age have equitable access to these much-needed services.
Secondary malignancies
Background
The risk of secondary malignancy after achieving initial cancer control remains one of the most serious and yet infrequently discussed late effects of anticancer treatment, which often manifests years later. In fact, secondary malignant neoplasms might account for 10–20% of all cancers reported to the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program data base193. Secondary malignancies can be especially life-threatening as they commonly have high-risk features compared with those in patients with the corresponding de novo acute myeloid leukaemia, myelodysplastic syndrome (MDS), or myeloproliferative neoplasms and are associated with worse outcomes. Advances in oncology have resulted in an increased number of long-term survivors and, consequently, the incidence of secondary malignancies is also rising. Therefore, appropriate management of secondary malignancies is becoming increasingly important193,194.
Risk factors
Treatment-related secondary malignancies might be associated with the same aetiological factors that led to the primary cancer such as smoking, diet, alcohol or hereditary syndromes195. In a large retrospective study, survivors of cancers associated with smoking, obesity, infection or alcohol consumption had a higher risk of secondary malignancy with the same aetiology. Moreover, secondary malignancies associated with the aforementioned risk factors were among those with the highest mortality rates196,197. The mutagenic and immunosuppressive effects of chemotherapy and radiotherapy also increase the risk of secondary malignancies193,198. The chemotherapeutic agents most commonly associated with secondary malignancies include alkylating agents, platinum-based agents and topoisomerase II inhibitors. The link between chemotherapy exposure and secondary malignancies was first investigated in patients with haematological malignancies developing acute leukaemias, MDS, or myeloproliferative neoplasms199,200 and has been demonstrated in numerous subsequent studies201–207 with a fourfold to fivefold increased risk of leukaemia compared with the general population208. Alkylating agents have been associated with an increased risk of various secondary solid tumour types, including those in the gastrointestinal and genitourinary tracts, lung cancer, and sarcomas193,209,210. Although not the focus of this Review, a substantially increased risk of secondary malignancies following exposure to ionizing radiation has also been reported; in this regard, cytotoxic agents, radiation, and various environmental and lifestyle exposures have important additive or even synergistic interactions195,211,212.
Several observational studies have suggested that patients with cancer receiving chemotherapy and supportive care with GM-CSF or G-CSF have a greater incidence of acute myeloid leukaemia or MDSs than those not receiving such growth factors213,214. However, the results of subsequent systematic reviews and meta-analyses of RCTs of chemotherapy with and without GM-CSF or G-CSF suggest that growth factor support enables the administration of a greater relative dose intensity of chemotherapeutic agents known to be leukaemogenic, possibly explaining the small increase in the risk of secondary malignancies, along with improvements in overall survival, reduced cancer recurrence and reduced occurrence of fatal infections194,215. Therefore, the independent contribution of these growth factors to a greater risk of leukaemia and MDS remains unclear194,215.
Overall, the risk of secondary malignancies (including leukaemias) following cytotoxic chemotherapy must be considered in perspective with the urgency of treating the initial malignancy. The cumulative risk of secondary malignancies following current standard-of-care chemotherapy seems to develop over a period of 2–10 years and rarely exceeds 1% across various studies194,215. While in patients with non-haematological malignancies, the risk of chemotherapy-related secondary acute myeloid leukaemia disappears beyond 10 years, among patients with lymphomas and, possibly, those with myeloma, a threefold to sixfold risk remains, and 5-year survival appears to be <10% among patients with cytogenetically confirmed secondary malignancies216. By contrast, the risk of death from an untreated primary malignancy during this time period would be considerably greater in most settings.
Management
The close relationship between cumulative chemotherapy dose intensity and treatment efficacy, while increasing the risk of secondary malignancies, limits the use of dose de-escalation interventions, which might increase the risk of recurrence of the primary malignancy. To the greatest possible extent, clinicians should avoid therapeutic approaches involving concurrent chemotherapy and radiotherapy, which considerably increase the risk of secondary malignancies217. Certain standard-of-care treatment regimens might be using drug doses or durations beyond those required for effective cancer control; comparative studies of lower doses, different schedules or a reduced number of chemotherapeutic agents have been and are being conducted.
The aforementioned retrospective study of secondary malignancies highlights the importance of surveillance and prevention efforts197. The results of this study also reinforce the importance of encouraging a healthy lifestyle among cancer survivors as well as individuals at high risk of cancer but not yet diagnosed with the disease. Initiatives to reduce tobacco and alcohol use, promotion of healthy diets and regular exercise, and the development of more effective treatments for obesity are recommended because they might reduce the risk of secondary malignancies197,218.
Emerging strategies
Thus far, perhaps the most promising strategy to prevent chemotherapy-related secondary malignancies is the identification of non-cytotoxic anticancer agents such as novel targeted agents or immunotherapies associated with a lower or no known risk of secondary malignancies but capable of providing similarly effective cancer treatment. The development of such therapies is being urgently pursued to provide alternatives to many currently used cytotoxic agents without forgetting the continued investigation of de-escalation approaches that maintain the therapeutic safety and efficacy of these current regimens. ADCs are a potentially less leukaemogenic approach to deliver a cytotoxic payload, often with a more favourable adverse-effect profile219. Perhaps most importantly, patients should be counselled on lifestyle factors and, thus, avoid tobacco and alcohol, which can enhance the carcinogenic effects of cancer chemotherapy. Patients should also be encouraged to have a healthy diet and exercise to the greatest possible extent.
Anxiety, distress, depression and fear of recurrence
Background
Emotional distress is frequently reported by patients with cancer across the disease continuum, including those undergoing chemotherapy; anxiety and depression are the most frequent psychiatric diagnoses220. Notably, 33–45% of patients with cancer report a substantial level of distress221. Emotional symptoms might be prompted or exacerbated during anticancer treatment and can result in HR-QOL impairment and decreased adherence to treatment. In recognition of the high prevalence and the pervasive nature of distress, anxiety and depression, routine screening of patients for these emotional symptoms is recommended throughout the cancer trajectory, particularly if disease status changes or they transition to palliative or end-of-life care. To raise awareness of the importance of identifying the emotional needs of patients, screening guidelines were developed and implemented by several organizations, including the Institute of Medicine222, the NCCN223, the Commission on Cancer of the American College of Surgeons and ASCO224, for the screening, assessment and care of emotional distress. Quality indicators have subsequently been validated and implemented; for example, the Quality Oncology Practice Initiative from ASCO suggests assessing the emotional well-being of patients within 1 month of the first oncology office visit225. FCR, which an expert panel defined as the “fear, worry, or concern relating to the possibility that cancer will come back or progress”226, is one of the most common unmet needs reported by patients but is often misdiagnosed as depression or anxiety and can subsequently be mistreated. No formal guidelines for FCR screening exist at present.
Risk factors
Various risk factors for distress have been identified, including a history of psychiatric disorder or substance use, a history of depression and/or suicide attempt, a history of trauma and/or abuse (physical, sexual, emotional and/or verbal), cognitive impairment, severe comorbid illness, social issues (including, among others, family conflicts, inadequate social support, social isolation, financial problems, immigration status and current substance use), younger age (<65 years), and uncontrolled symptoms223. Studies have also included non-married status and socioeconomic disadvantage as possible risk factors.
In addition, certain cancer types can be associated with higher rates of distress, including pancreatic, head and neck cancers, and testicular cancer; the latter is associated with the highest rates of psychiatric disorders in the literature227,228. In a meta-analysis of 28 studies, patients with cancer types generally associated with poor prognoses (such as pancreatic, liver and biliary tract, and lung cancers) and advanced-disease stage were those with the highest risk of suicide mortality229. Furthermore, evidence suggests that patients who have received a combination of chemotherapy, radiotherapy and surgery are at the highest risk of developing a psychiatric disorder, whereas those who have received radiotherapy alone tend to have the lowest risk227,228.
Management and guideline recommendations
Existing guidelines recommend the use of validated screening tools to identify common unmet needs, determine the severity of the emotional symptoms and help guide treatment planning; several measures have been validated in this setting. Whereas brief tools are the most acceptable for health-care staff and patients alike, they should assess, at a minimum, emotional, physical and practical domains; examples include the Distress Thermometer or the Edmonton Symptom Assessment Scale223–225 (Fig. 2).
Fig. 2. Addressing psychosocial needs in cancer survivors.
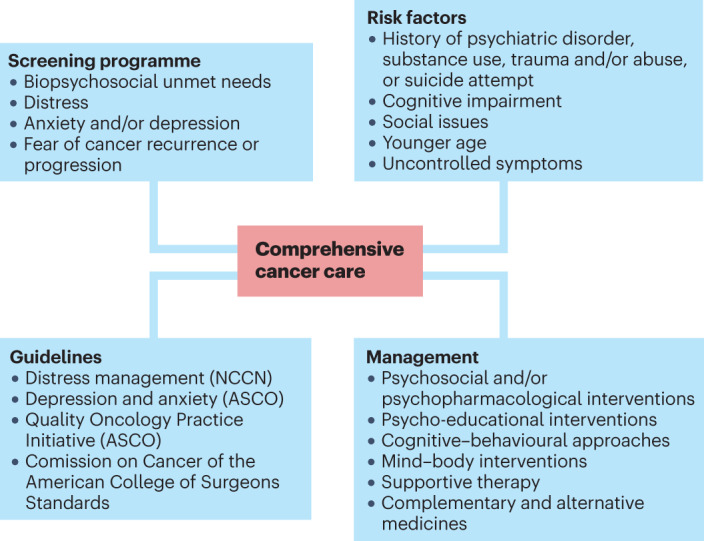
Guidelines recommend the use of validated screening tools to identify common unmet psychosocial oncology needs, determine the severity of the associated symptoms and guide treatment planning. ASCO, American Society of Clinical Oncology; NCCN, National Comprehensive Cancer Network.
Identifying psychosocial needs can help clinical teams to determine the characteristics of distress (psychiatric disorder versus mild symptoms), the most effective treatment and appropriate referrals225. The literature supports the use of psychosocial and psychopharmacological treatments for the management of cancer-related distress. Several targeted psychosocial interventions have demonstrated efficacy in treating emotional symptoms, including psychoeducation, cognitive–behavioural approaches (such as cognitive–behavioural therapy, cognitive–existential therapy, problem-solving therapy and systematic desensitization), mind–body modalities (such as mindfulness-based therapy, relaxation with guided imagery, physical exercise and hypnosis), supportive therapy (for example, supportive-expressive therapy and supportive counselling), and complementary and alternative medicine (for example, acupuncture)230–232.
Emerging strategies
The advent of electronic symptom reporting tools, such as eHealth and mHealth, has expanded access to supportive care services. Multiple interventions based on these tools are now available that have demonstrated efficacy in the management of emotional and physical symptoms. These programmes can also be used to favour effective communication between patients and their medical care team, promote health literacy, provide psychosocial information and education, support patients and caregiver groups, and monitor patient-reported outcomes as well as help patients to self-manage their emotional and physical symptoms30,121,233–258.
Financial burden and toxicity
Background
Health-care costs related to hospitalization, diagnosis and administration of pharmacological agents have rapidly increased over the past few decades in the USA and worldwide. No setting has witnessed the skyrocketing increases in health-care costs more clearly than oncology treatment with chemotherapy as well as novel targeted agents and immunotherapies259,260. The USA is the only high-income country (HIC) in which life expectancy has actually decreased over the past few years despite dramatically increasing health-care costs that far outpace those in other HICs261. While the USA is perhaps the most egregious example of the adverse impact of increasing health-care costs, this represents a major global problem that is beyond the scope of this Review262,263.
Risk factors
In addition to the failure of policymakers in the USA to tackle the overall health economics crisis and rapidly escalating costs, health insurance companies are increasingly exacerbating costs for patients through the introduction of marginal coverage policies with large deductibles, frequent prior approval requirements, upfront coverage denials and low co-pay caps, all of which fail to address the high costs associated with cancer care. In a study at a large health-care centre in the USA, 26% of patients with cancer reported what they described as financial toxicity264. The National Cancer Institute defines financial toxicity as “[the] problems a patient has related to the cost of medical care”265, noting the fact that patients with cancer are more likely to experience financial toxicity than the general population. Financial toxicity can have a deep and lasting effect on patient willingness to continue expensive treatments that could result in greater patient debt or even bankruptcy263. In addition, financial toxicity might be associated with poor physical and emotional health, reduced survival and HR-QOL (the latter, in part, owing to poor social engagement), anxiety and depression, as well as suicidal ideation266.
Patients with cancers at more advanced stages, major medical conditions, from minority groups, with lower educational levels and/or who are survivors of childhood cancer tend to be at the greatest risk of financial toxicity266,267. Moreover, among 9 million individuals aged ≥50 years and newly diagnosed with cancer followed in the Health Retirement Study, over 42% had depleted their life savings by the second year after diagnosis; older women, patients on Medicaid or uninsured, those with major comorbidities, and those with progressive cancer were the most affected268.
Management
Strategies to mitigate the increasing costs of therapeutic agents and their effect on patients include the wide-scale use of generic medications and the introduction of biosimilars in the past decade in an effort to compete with highly expensive novel therapies269–271, although their mitigating effect on patient financial burden has been limited thus far269,270. Subsequent efforts have focused on greater price transparency for drugs, tests and hospitalization as well as novel payment and reimbursement models, including various proposals for more value-based pricing. ASCO and ESMO have developed scales to define the value of oncology treatments272–274, which are yet to demonstrate an effect on pricing and clinical practice272,275. Patient and health-care provider initiatives have included petitions calling for lower prices for cancer drugs and for the government of the USA to negotiate drug pricing similarly to other HICs276. Finally, the importance of the financial burden of cancer care on patients and their families has prompted the common use of the term financial toxicity, likening it to other adverse events associated with cancer treatment and thus with an increasing call to discuss its effect on patients early in their management187,277,278.
To better measure and follow the factors associated with financial toxicity, several instruments have been developed and validated, including but not limited to the Comprehensive Score for Financial Toxicity, the Breast Cancer Finances Survey inventory and the Socioeconomic Wellbeing Scale279. The large variation in insurance coverage associated with different health-care plans has proved challenging to both patients and providers attempting to advise their patients about the financial effect of various therapeutic options; this situation has resulted in increased reliance on trained financial counsellors, further expanding the staff needed for appropriate cancer care.
Emerging strategies
Ultimately, health care must be recognized as a human right280. As we undertake a concerted effort to align health-care delivery with our moral, ethical and social understanding of fairness, compassion and justice, the medical community has an urgent obligation to eliminate financial toxicity, which inflicts incalculable harm on the most vulnerable members of our society263.
Conclusions
The trajectory of various toxicities associated with oncology treatments is often unique to each cancer type and, most importantly, to each patient with cancer or cancer survivor. In 2022, we reviewed the most common major acute CAAEs1; however, the separation of acute toxicities from late effects is often not entirely clear, and knowledge about the long-term effects of certain early adverse events remains limited. For example, CRF, febrile neutropenia (often with serious hospitalizations owing to infection) and CIPN most commonly manifest early during treatment and, yet, can have persistent long-term effects for some survivors years after the initial cancer treatment or can be ongoing for those cancer survivors who remain on treatment. Sexual and reproductive effects can also often manifest early and continue to affect HR-QOL and intimate relationships decades later. Alternatively, several toxicities mostly manifest as late effects (for example, bleomycin-induced lung toxicity or cardiovascular disease) and reduce survival; yet, some of these toxicities remain poorly studied.
Given the complexity of toxicity management in cancer survivors, additional education and awareness of all cancer-related long-term toxicities and how they can present and affect survivorship is a key component of education for all specialties of medicine, including primary care providers, to ensure the best patient outcomes. Care coordination is a key component of survivorship care; in this regard, primary care providers are key stakeholders in ensuring that cancer survivors are receiving optimal care234. Care coordination is of utmost importance, particularly in survivors with multiple medical comorbidities resulting from chronic health conditions, cancer treatment complications and ageing235.
Risk-stratified personalized care is the future of effective survivorship care delivery245. Decisions regarding the model of health-care delivery need to be tailored to the type of cancer, treatment exposure, comorbidities and other patient risk factors242. The increased use of telehealth services, catalysed by the COVID-19 pandemic, has improved access to survivorship care in certain populations, although disparities remain246,247.
Central to the appropriate coordination of survivorship care among disciplines is overall health promotion focusing on lifestyle behaviours that can improve physical and emotional well-being and overall HR-QOL as well as mitigate the risk of cancer recurrence248–250. Many cancer survivors continue to have lifestyle habits that have negative effects on overall health and increase the risk or severity of CAAEs, including tobacco use, excess alcohol consumption, sedentary lifestyle, obesity and unhealthy diets251. Lifestyle changes (such as smoking cessation, dietary management and exercise) are needed to improve health outcomes and are addressed by multiple implementation science initiatives121,250. Regular exercise can improve outcomes associated with a cancer diagnosis and treatment, including CRF, HR-QOL, physical function and depressive symptoms252–257. An overall framework of health promotion is needed from diagnosis through acute treatment and on to survivorship and end-of-life care. Importantly, the outcomes of patients with cancer are influenced by their socioeconomic environment (or social determinants of health). Across different communities, health outcomes can be disproportionately affected by social determinants of health, including economic security, education, health-care access and/or quality, and community context30,281.
The successful execution of health-promotion initiatives requires investigations of social determinants of health, health-care delivery and coordination challenges, and must integrate patient preferences within established frameworks for implementation and dissemination science258,282,283. Finally, more research is needed to design better interventions to mitigate CAAEs as the number of cancer survivors continues to grow globally. Additionally, the implementation of existing validated interventions will continue to serve this growing population while we all strive to improve care models and management strategies.
Author contributions
All the authors contributed to all aspects of the preparation of the article.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks M. Lambertini and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
M.B.L. is on the physician advisory board for Infinite Strength, Project Life and The Right Dose; has been a consultant for AstraZeneca, Lilly Novartis, Pfizer and Sanofi; and reports institutional research funding from Pfizer. N.M.K. reports being a consultant for BeyondSpring, BMS, G1 Therapeutics, Invitae, Janssen, Pfizer, Sandoz, Seattle Genetics, Spectrum and Total Health, all outside the submitted work. G.H.L. reports institutional research funding from Amgen; honoraria for lectures from ER Squibb, Frensenius Kabi, Kallyope, Merck, Partners Healthcare, Sandoz, Samsung and Seattle Genetics; and honoraria for consulting from BeyondSpring, G1 Therapeutics and Jazz Pharm. A.D. and C.B. declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nicole M. Kuderer, Email: nkuderer@advancecancerresearch.org
Gary H. Lyman, Email: glyman@fredhutch.org
References
- 1.Kuderer NM, Desai A, Lustberg MB, Lyman GH. Mitigating acute chemotherapy-associated adverse events in patients with cancer. Nat. Rev. Clin. Oncol. 2022;19:681–697. doi: 10.1038/s41571-022-00685-3. [DOI] [PubMed] [Google Scholar]
- 2.National Coalition for Cancer Survivorship. State of Survivorship Survey: 2022https://canceradvocacy.org/2022-state-of-cancer-survivorship-survey/ (2022).
- 3.Vaz-Luis I, et al. Long-term longitudinal patterns of patient-reported fatigue after breast cancer: a group-based trajectory analysis. J. Clin. Oncol. 2022;40:2148–2162. doi: 10.1200/JCO.21.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bower JE. Cancer-related fatigue — mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thong MSY, et al. Cancer-related fatigue: causes and current treatment options. Curr. Treat. Options Oncol. 2020;21:17. doi: 10.1007/s11864-020-0707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smeland KB, et al. Chronic fatigue is highly prevalent in survivors of autologous stem cell transplantation and associated with IL-6, neuroticism, cardiorespiratory fitness, and obesity. Bone Marrow Transpl. 2019;54:607–610. doi: 10.1038/s41409-018-0342-y. [DOI] [PubMed] [Google Scholar]
- 7.Reinertsen KV, et al. Predictors and course of chronic fatigue in long-term breast cancer survivors. J. Cancer Surviv. 2010;4:405–414. doi: 10.1007/s11764-010-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husson O, et al. Variation in fatigue among 6011 (long-term) cancer survivors and a normative population: a study from the population-based PROFILES registry. Support. Care Cancer. 2015;23:2165–2174. doi: 10.1007/s00520-014-2577-5. [DOI] [PubMed] [Google Scholar]
- 9.Steen R, et al. A study of chronic fatigue in Norwegian cervical cancer survivors. Gynecol. Oncol. 2017;146:630–635. doi: 10.1016/j.ygyno.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Berger AM, et al. Cancer-related fatigue, version 2.2015. J. Natl Compr. Canc. Netw. 2015;13:1012–1039. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger AM, Mooney K. Dissemination and implementation of guidelines for cancer-related fatigue. J. Natl Compr. Canc. Netw. 2016;14:1336–1338. doi: 10.6004/jnccn.2016.0144. [DOI] [PubMed] [Google Scholar]
- 12.Bower JE, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology clinical practice guideline adaptation. J. Clin. Oncol. 2014;32:1840–1850. doi: 10.1200/JCO.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabi A, et al. Cancer-related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann. Oncol. 2020;31:713–723. doi: 10.1016/j.annonc.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen PB, et al. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J. Pain Symptom Manag. 1999;18:233–242. doi: 10.1016/S0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 15.Broeckel JA, et al. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J. Clin. Oncol. 1998;16:1689–1696. doi: 10.1200/JCO.1998.16.5.1689. [DOI] [PubMed] [Google Scholar]
- 16.Ryan JL, et al. Mechanisms of cancer-related fatigue. Oncologist. 2007;12:22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 17.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav. Immun. 2013;30:S48–S57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, et al. Prediction of breast cancer treatment-induced fatigue by machine learning using genome-wide association data. JNCI Cancer Spectr. 2020;4:pkaa039. doi: 10.1093/jncics/pkaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charalambous A, et al. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support. Care Cancer. 2019;27:2747–2753. doi: 10.1007/s00520-019-04746-9. [DOI] [PubMed] [Google Scholar]
- 20.Goedendorp MM, et al. Development of fatigue in cancer survivors: a prospective follow-up study from diagnosis into the year after treatment. J. Pain Symptom Manag. 2013;45:213–222. doi: 10.1016/j.jpainsymman.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 21.O’Higgins CM, et al. The pathophysiology of cancer-related fatigue: current controversies. Support. Care Cancer. 2018;26:3353–3364. doi: 10.1007/s00520-018-4318-7. [DOI] [PubMed] [Google Scholar]
- 22.Eyob T, et al. Impact of chemotherapy on cancer-related fatigue and cytokines in 1312 patients: a systematic review of quantitative studies. Curr. Opin. Support. Palliat. Care. 2016;10:165–179. doi: 10.1097/SPC.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 23.Dantzer R, et al. The neuroimmune basis of fatigue. Trends Neurosci. 2014;37:39–46. doi: 10.1016/j.tins.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inglis JE, et al. Nutritional interventions for treating cancer-related fatigue: a qualitative review. Nutr. Cancer. 2019;71:21–40. doi: 10.1080/01635581.2018.1513046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bower JE, Crosswell AD, Slavich GM. Childhood adversity and cumulative life stress: risk factors for cancer-related fatigue. Clin. Psychol. Sci. 2014;2:108–115. doi: 10.1177/2167702613496243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bower JE, et al. Do all patients with cancer experience fatigue? A longitudinal study of fatigue trajectories in women with breast cancer. Cancer. 2021;127:1334–1344. doi: 10.1002/cncr.33327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bower JE, et al. Fatigue after breast cancer treatment: biobehavioral predictors of fatigue trajectories. Health Psychol. 2018;37:1025–1034. doi: 10.1037/hea0000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Meglio A, et al. Development and validation of a predictive model of severe fatigue after breast cancer diagnosis: toward a personalized framework in survivorship care. J. Clin. Oncol. 2022;40:1111–1123. doi: 10.1200/JCO.21.01252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammermuller C, et al. Depression, anxiety, fatigue, and quality of life in a large sample of patients suffering from head and neck cancer in comparison with the general population. BMC Cancer. 2021;21:94. doi: 10.1186/s12885-020-07773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown LF, Kroenke K. Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics. 2009;50:440–447. doi: 10.1016/S0033-3182(09)70835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong TS, et al. Association of genetic variants with fatigue in patients with malignant glioma. Neurooncol. Pract. 2018;5:122–128. doi: 10.1093/nop/npx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jean-Pierre P, et al. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist. 2007;12:11–21. doi: 10.1634/theoncologist.12-S1-11. [DOI] [PubMed] [Google Scholar]
- 33.Mendoza TR, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(SICI)1097-0142(19990301)85:5<1186::AID-CNCR24>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.Yellen SB, et al. Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. J. Pain Symptom Manag. 1997;13:63–74. doi: 10.1016/S0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 35.Stein KD, et al. Further validation of the multidimensional fatigue symptom inventory-short form. J. Pain Symptom Manag. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hann DM, et al. Measurement of fatigue in cancer patients: development and validation of the fatigue symptom inventory. Qual. Life Res. 1998;7:301–310. doi: 10.1023/A:1008842517972. [DOI] [PubMed] [Google Scholar]
- 37.Chow R, et al. Cancer-related fatigue-pharmacological interventions: systematic review and network meta-analysis. BMJ Support. Palliat. Care. 2021 doi: 10.1136/bmjspcare-2021-003244. [DOI] [PubMed] [Google Scholar]
- 38.Popescu RA, et al. Supportive care: low cost, high value. Am. Soc. Clin. Oncol. Educ. Book. 2021;41:1–11. doi: 10.1200/EDBK_320041. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence JA, et al. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J. Cancer Surviv. 2016;10:176–184. doi: 10.1007/s11764-015-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruera E, et al. Donepezil for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J. Clin. Oncol. 2007;25:3475–3481. doi: 10.1200/JCO.2007.10.9231. [DOI] [PubMed] [Google Scholar]
- 41.Paulsen O, et al. Efficacy of methylprednisolone on pain, fatigue, and appetite loss in patients with advanced cancer using opioids: a randomized, placebo-controlled, double-blind trial. J. Clin. Oncol. 2014;32:3221–3228. doi: 10.1200/JCO.2013.54.3926. [DOI] [PubMed] [Google Scholar]
- 42.Yennurajalingam S, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J. Clin. Oncol. 2013;31:3076–3082. doi: 10.1200/JCO.2012.44.4661. [DOI] [PubMed] [Google Scholar]
- 43.Yennu SVV, et al. High/low dose dexamethasone with physical activity for cancer-related fatigue in patients with advanced cancer: a phase II randomized double blind study. J. Clin. Oncol. 2019;37(Suppl. 31):110. doi: 10.1200/JCO.2019.37.31_suppl.110. [DOI] [Google Scholar]
- 44.Sadeghian M, et al. Ginseng and cancer-related fatigue: a systematic review of clinical trials. Nutr. Cancer. 2021;73:1270–1281. doi: 10.1080/01635581.2020.1795691. [DOI] [PubMed] [Google Scholar]
- 45.Mustian KM, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961–968. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dennett AM, et al. Multidisciplinary, exercise-based oncology rehabilitation programs improve patient outcomes but their effects on healthcare service-level outcomes remain uncertain: a systematic review. J. Physiother. 2021;67:12–26. doi: 10.1016/j.jphys.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 47.David A, Hausner D, Frenkel M. Cancer-related fatigue-is there a role for complementary and integrative medicine? Curr. Oncol. Rep. 2021;23:145. doi: 10.1007/s11912-021-01135-6. [DOI] [PubMed] [Google Scholar]
- 48.Gielissen MF, et al. Effects of cognitive behavior therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavior therapy: a randomized controlled trial. J. Clin. Oncol. 2006;24:4882–4887. doi: 10.1200/JCO.2006.06.8270. [DOI] [PubMed] [Google Scholar]
- 49.Poort H, et al. Cognitive behavioral therapy or graded exercise therapy compared with usual care for severe fatigue in patients with advanced cancer during treatment: a randomized controlled trial. Ann. Oncol. 2020;31:115–122. doi: 10.1016/j.annonc.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Seiler A, et al. eHealth and mHealth interventions in the treatment of fatigued cancer survivors: a systematic review and meta-analysis. Psychooncology. 2017;26:1239–1253. doi: 10.1002/pon.4489. [DOI] [PubMed] [Google Scholar]
- 51.Foster C, et al. A web-based intervention (RESTORE) to support self-management of cancer-related fatigue following primary cancer treatment: a multi-centre proof of concept randomised controlled trial. Support. Care Cancer. 2016;24:2445–2453. doi: 10.1007/s00520-015-3044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maihofner C, et al. Chemotherapy-induced peripheral neuropathy (CIPN): current therapies and topical treatment option with high-concentration capsaicin. Support. Care Cancer. 2021;29:4223–4238. doi: 10.1007/s00520-021-06042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salat K. Chemotherapy-induced peripheral neuropathy: part 1-current state of knowledge and perspectives for pharmacotherapy. Pharmacol. Rep. 2020;72:486–507. doi: 10.1007/s43440-020-00109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seretny M, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 55.Coradini PP, et al. Ototoxicity from cisplatin therapy in childhood cancer. J. Pediatr. Hematol. Oncol. 2007;29:355–360. doi: 10.1097/MPH.0b013e318059c220. [DOI] [PubMed] [Google Scholar]
- 56.Landier W. Ototoxicity and cancer therapy. Cancer. 2016;122:1647–1658. doi: 10.1002/cncr.29779. [DOI] [PubMed] [Google Scholar]
- 57.Hu S, et al. Recent developments of novel pharmacologic therapeutics for prevention of chemotherapy-induced peripheral neuropathy. Clin. Cancer Res. 2019;25:6295–6301. doi: 10.1158/1078-0432.CCR-18-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan A, et al. Biological predictors of chemotherapy-induced peripheral neuropathy (CIPN): MASCC neurological complications working group overview. Support. Care Cancer. 2019;27:3729–3737. doi: 10.1007/s00520-019-04987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.St Germain DC, et al. Chemotherapy-induced peripheral neuropathy: identifying the research gaps and associated changes to clinical trial design. Cancer. 2020;126:4602–4613. doi: 10.1002/cncr.33108. [DOI] [PubMed] [Google Scholar]
- 60.Loprinzi CL, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J. Clin. Oncol. 2020;38:3325–3348. doi: 10.1200/JCO.20.01399. [DOI] [PubMed] [Google Scholar]
- 61.Jordan B, et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO clinical practice guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020;31:1306–1319. doi: 10.1016/j.annonc.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Hertz DL, et al. Patient-centric decision framework for treatment alterations in patients with chemotherapy-induced Peripheral Neuropathy (CIPN) Cancer Treat. Rev. 2021;99:102241. doi: 10.1016/j.ctrv.2021.102241. [DOI] [PubMed] [Google Scholar]
- 63.Mizrahi D, et al. Development and consensus process for a clinical pathway for the assessment and management of chemotherapy-induced peripheral neuropathy. Support. Care Cancer. 2022;30:5965–5974. doi: 10.1007/s00520-022-07024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan AC, et al. Chemotherapy-induced peripheral neuropathy-patient-reported outcomes compared with NCI-CTCAE grade. Support. Care Cancer. 2019;27:4771–4777. doi: 10.1007/s00520-019-04781-6. [DOI] [PubMed] [Google Scholar]
- 65.McCrary JM, et al. Optimal clinical assessment strategies for chemotherapy-induced peripheral neuropathy (CIPN): a systematic review and Delphi survey. Support. Care Cancer. 2017;25:3485–3493. doi: 10.1007/s00520-017-3772-y. [DOI] [PubMed] [Google Scholar]
- 66.Pfeiffer P, et al. Calmangafodipir for prevention of oxaliplatin-induced peripheral neuropathy: two placebo-controlled, randomized phase 3 studies (POLAR-A/POLAR-M) JNCI Cancer Spectr. 2022;6:pkac075. doi: 10.1093/jncics/pkac075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith EM, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309:1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zirpoli GR, et al. Supplement use and chemotherapy-induced peripheral neuropathy in a cooperative group trial (S0221): the DELCaP study. J. Natl Cancer Inst. 2017;109:djx098. doi: 10.1093/jnci/djx098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hershman DL, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J. Clin. Oncol. 2013;31:2627–2633. doi: 10.1200/JCO.2012.44.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ambrosone CB, et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221) J. Clin. Oncol. 2020;38:804–814. doi: 10.1200/JCO.19.01203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenlee H, et al. Randomized sham-controlled pilot trial of weekly electro-acupuncture for the prevention of taxane-induced peripheral neuropathy in women with early stage breast cancer. Breast Cancer Res. Treat. 2016;156:453–464. doi: 10.1007/s10549-016-3759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu W, et al. Acupuncture for chemotherapy-induced peripheral neuropathy in breast cancer survivors: a randomized controlled pilot trial. Oncologist. 2020;25:310–318. doi: 10.1634/theoncologist.2019-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsuyuki S, et al. Evaluation of the effect of compression therapy using surgical gloves on nanoparticle albumin-bound paclitaxel-induced peripheral neuropathy: a phase II multicenter study by the Kamigata Breast Cancer Study Group. Breast Cancer Res. Treat. 2016;160:61–67. doi: 10.1007/s10549-016-3977-7. [DOI] [PubMed] [Google Scholar]
- 74.Tsuyuki S, et al. Effectiveness and safety of surgical glove compression therapy as a prophylactic method against nanoparticle albumin-bound-paclitaxel-induced peripheral neuropathy. Breast. 2019;47:22–27. doi: 10.1016/j.breast.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 75.Kanbayashi Y, et al. Comparison of the efficacy of cryotherapy and compression therapy for preventing nanoparticle albumin-bound paclitaxel-induced peripheral neuropathy: a prospective self-controlled trial. Breast. 2020;49:219–224. doi: 10.1016/j.breast.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanzawa-Lee GA, et al. Exercise effects on chemotherapy-induced peripheral neuropathy: a comprehensive integrative review. Cancer Nurs. 2020;43:E172–E185. doi: 10.1097/NCC.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 77.Kleckner IR, et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support. Care Cancer. 2018;26:1019–1028. doi: 10.1007/s00520-017-4013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Streckmann F, et al. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann. Oncol. 2014;25:493–499. doi: 10.1093/annonc/mdt568. [DOI] [PubMed] [Google Scholar]
- 79.Streckmann F, et al. Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med. 2014;44:1289–1304. doi: 10.1007/s40279-014-0207-5. [DOI] [PubMed] [Google Scholar]
- 80.Mayo SJ, et al. Cancer-related cognitive impairment in patients with non-central nervous system malignancies: an overview for oncology providers from the MASCC Neurological Complications Study Group. Support. Care Cancer. 2021;29:2821–2840. doi: 10.1007/s00520-020-05860-9. [DOI] [PubMed] [Google Scholar]
- 81.Janelsins MC, et al. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry. 2014;26:102–113. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams AM, Zent CS, Janelsins MC. What is known and unknown about chemotherapy-related cognitive impairment in patients with haematological malignancies and areas of needed research. Br. J. Haematol. 2016;174:835–846. doi: 10.1111/bjh.14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lange M, et al. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019;30:1925–1940. doi: 10.1093/annonc/mdz410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wefel JS, et al. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 85.Mounier NM, et al. Chemotherapy-induced cognitive impairment (CICI): an overview of etiology and pathogenesis. Life Sci. 2020;258:118071. doi: 10.1016/j.lfs.2020.118071. [DOI] [PubMed] [Google Scholar]
- 86.Janelsins MC, et al. Longitudinal changes in cognitive function in a nationwide cohort study of patients with lymphoma treated with chemotherapy. J. Natl Cancer Inst. 2021;114:47–59. doi: 10.1093/jnci/djab133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Magnuson A, et al. Cognitive function in older adults with cancer: assessment, management, and research opportunities. J. Clin. Oncol. 2021;39:2138–2149. doi: 10.1200/JCO.21.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mayo SJ, et al. Predictors of the trajectory of cognitive functioning in the first 6 months after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 2020;55:918–928. doi: 10.1038/s41409-019-0746-3. [DOI] [PubMed] [Google Scholar]
- 89.Mayo SJ, et al. Late cognitive outcomes among allogeneic stem cell transplant survivors: follow-up data from a 6-year longitudinal study. Support. Care Cancer. 2021;29:2621–2630. doi: 10.1007/s00520-020-05761-x. [DOI] [PubMed] [Google Scholar]
- 90.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J. Clin. Oncol. 2012;30:3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Von Ah D, et al. Cancer, cognitive impairment, and work-related outcomes: an integrative review. Oncol. Nurs. Forum. 2016;43:602–616. doi: 10.1188/16.ONF.602-616. [DOI] [PubMed] [Google Scholar]
- 92.Duijts SF, et al. Cancer-related cognitive impairment and patients’ ability to work: a current perspective. Curr. Opin. Support. Palliat. Care. 2017;11:19–23. doi: 10.1097/SPC.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 93.Mandelblatt JS, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J. Clin. Oncol. 2014;32:1909–1918. doi: 10.1200/JCO.2013.54.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huskisson E, Maggini S, Ruf M. The influence of micronutrients on cognitive function and performance. J. Int. Med. Res. 2007;35:1–19. doi: 10.1177/147323000703500101. [DOI] [PubMed] [Google Scholar]
- 95.Mancuso A, et al. Correlation between anemia and functional/cognitive capacity in elderly lung cancer patients treated with chemotherapy. Ann. Oncol. 2006;17:146–150. doi: 10.1093/annonc/mdj038. [DOI] [PubMed] [Google Scholar]
- 96.Mandelblatt JS, et al. Cancer-related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study. J. Clin. Oncol. 2018;36:JCO1800140. doi: 10.1200/JCO.18.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahles TA, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 98.Cheng H, et al. The COMT (rs165599) gene polymorphism contributes to chemotherapy-induced cognitive impairment in breast cancer patients. Am. J. Transl. Res. 2016;8:5087–5097. [PMC free article] [PubMed] [Google Scholar]
- 99.Lyon DE, et al. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J. Neuroimmunol. 2016;301:74–82. doi: 10.1016/j.jneuroim.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pomykala KL, et al. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013;7:511–523. doi: 10.1007/s11682-013-9243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yap NY, et al. Associations of plasma brain-derived neurotrophic factor (BDNF) and Val66Met polymorphism (rs6265) with long-term cancer-related cognitive impairment in survivors of breast cancer. Breast Cancer Res. Treat. 2020;183:683–696. doi: 10.1007/s10549-020-05807-y. [DOI] [PubMed] [Google Scholar]
- 102.Tevaarwerk A, et al. Survivorship, version 1.2021. J. Natl Compr. Canc Netw. 2021;19:676–685. doi: 10.6004/jnccn.2021.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giesinger JM, et al. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J. Clin. Epidemiol. 2016;69:79–88. doi: 10.1016/j.jclinepi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 104.Henneghan AM, et al. Measuring self-reported cancer-related cognitive impairment: recommendations from the Cancer Neuroscience Initiative Working Group. J. Natl Cancer Inst. 2021;113:1625–1633. doi: 10.1093/jnci/djab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fernandes HA, Richard NM, Edelstein K. Cognitive rehabilitation for cancer-related cognitive dysfunction: a systematic review. Support. Care Cancer. 2019;27:3253–3279. doi: 10.1007/s00520-019-04866-2. [DOI] [PubMed] [Google Scholar]
- 106.Vardy JL, et al. A randomised controlled trial evaluating two cognitive rehabilitation approaches for cancer survivors with perceived cognitive impairment. J. Cancer Surviv. 2022 doi: 10.1007/s11764-022-01261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Campbell KL, et al. The effect of exercise on cancer-related cognitive impairment and applications for physical therapy: systematic review of randomized controlled trials. Phys. Ther. 2020;100:523–542. doi: 10.1093/ptj/pzz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Farahani MA, et al. The effect of mind-body exercise on cognitive function in cancer survivors: a systematic review. Can. Oncol. Nurs. J. 2022;32:38–48. doi: 10.5737/236880763213848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kohli S, et al. The effect of modafinil on cognitive function in breast cancer survivors. Cancer. 2009;115:2605–2616. doi: 10.1002/cncr.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown PD, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro-oncology. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dos Santos M, et al. Cognitive rehabilitation program to improve cognition of cancer patients treated with chemotherapy: a 3-arm randomized trial. Cancer. 2020;126:5328–5336. doi: 10.1002/cncr.33186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bray VJ, et al. Evaluation of a web-based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J. Clin. Oncol. 2017;35:217–225. doi: 10.1200/JCO.2016.67.8201. [DOI] [PubMed] [Google Scholar]
- 113.Desai A, et al. Takotsubo cardiomyopathy in cancer patients. Cardiooncology. 2019;5:7. doi: 10.1186/s40959-019-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alexandre J, et al. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European Cardio-Oncology Guidelines. J. Am. Heart Assoc. 2020;9:e018403. doi: 10.1161/JAHA.120.018403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020;17:474–502. doi: 10.1038/s41569-020-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Belzile-Dugas E, Eisenberg MJ. Radiation-induced cardiovascular disease: review of an underrecognized pathology. J. Am. Heart Assoc. 2021;10:e021686. doi: 10.1161/JAHA.121.021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marinescu DC, et al. Integration and application of Clinical Practice Guidelines for the diagnosis of idiopathic pulmonary fibrosis and fibrotic hypersensitivity pneumonitis. Chest. 2022;162:614–629. doi: 10.1016/j.chest.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 118.Curigliano G, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Armenian SH, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 120.Ligibel JA, Bohlke K, Alfano CM. Exercise, diet, and weight management during cancer treatment: ASCO Guideline Summary and Q&A. JCO Oncol. Pract. 2022;18:695–697. doi: 10.1200/OP.22.00277. [DOI] [PubMed] [Google Scholar]
- 121.Campbell KL, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zardavas D, et al. Role of troponins I and T and N-terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early-stage human epidermal growth factor receptor 2-positive breast cancer receiving trastuzumab: a herceptin adjuvant study cardiac marker substudy. J. Clin. Oncol. 2017;35:878–884. doi: 10.1200/JCO.2015.65.7916. [DOI] [PubMed] [Google Scholar]
- 123.Lenihan DJ, et al. The utility of point-of-care biomarkers to detect cardiotoxicity during anthracycline chemotherapy: a feasibility study. J. Card. Fail. 2016;22:433–438. doi: 10.1016/j.cardfail.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 124.Kalay N, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 2006;48:2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 125.Heck SL, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): extended follow-up of a 2x2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Circulation. 2021;143:2431–2440. doi: 10.1161/CIRCULATIONAHA.121.054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Akpek M, et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur. J. Heart Fail. 2015;17:81–89. doi: 10.1002/ejhf.196. [DOI] [PubMed] [Google Scholar]
- 127.Shahid I, et al. Meta-analysis evaluating the use of statins to attenuate cardiotoxicity in cancer patients receiving anthracyclines and trastuzumab-based chemotherapy. Am. J. Cardiol. 2021;156:142–145. doi: 10.1016/j.amjcard.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 128.Cardinale D, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 129.Livi L, et al. Cardioprotective strategy for patients with nonmetastatic breast cancer who are receiving an anthracycline-based chemotherapy: a randomized clinical trial. JAMA Oncol. 2021;7:1544–1549. doi: 10.1001/jamaoncol.2021.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dhamija E, et al. Chemotherapy-induced pulmonary complications in cancer: significance of clinicoradiological correlation. Indian J. Radiol. Imaging. 2020;30:20–26. doi: 10.4103/ijri.IJRI_178_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Libura J, et al. Risk of chemotherapy-induced pulmonary fibrosis is associated with polymorphic tumour necrosis factor-a2 gene. Eur. Respir. J. 2002;19:912–918. doi: 10.1183/09031936.02.00238102. [DOI] [PubMed] [Google Scholar]
- 132.Maldonado, F., Limper, A. H. & Cass, A. S. Pulmonary Toxicity Associated with Systemic Antineoplastic Therapy: Clinical Presentation, Diagnosis, and Treatmenthttps://www.uptodate.com/contents/pulmonary-toxicity-associated-with-systemic-antineoplastic-therapy-clinical-presentation-diagnosis-and-treatment (2021).
- 133.Fujimoto D, et al. Characteristics and prognostic impact of pneumonitis during systemic anti-cancer therapy in patients with advanced non-small-cell lung cancer. PLoS One. 2016;11:e0168465. doi: 10.1371/journal.pone.0168465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vahid B, Marik PE. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest. 2008;133:528–538. doi: 10.1378/chest.07-0851. [DOI] [PubMed] [Google Scholar]
- 135.Limper AH. Chemotherapy-induced lung disease. Clin. Chest Med. 2004;25:53–64. doi: 10.1016/S0272-5231(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 136.Jules-Elysee K, White DA. Bleomycin-induced pulmonary toxicity. Clin. Chest Med. 1990;11:1–20. doi: 10.1016/S0272-5231(21)00668-7. [DOI] [PubMed] [Google Scholar]
- 137.Blum RH, Carter SK, Agre K. A clinical review of bleomycin — a new antineoplastic agent. Cancer. 1973;31:903–914. doi: 10.1002/1097-0142(197304)31:4<903::AID-CNCR2820310422>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 138.Kawai K, et al. Serum creatinine level during chemotherapy for testicular cancer as a possible predictor of bleomycin-induced pulmonary toxicity. Jpn. J. Clin. Oncol. 1998;28:546–550. doi: 10.1093/jjco/28.9.546. [DOI] [PubMed] [Google Scholar]
- 139.O’Sullivan JM, et al. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann. Oncol. 2003;14:91–96. doi: 10.1093/annonc/mdg020. [DOI] [PubMed] [Google Scholar]
- 140.Haugnes HS, et al. Pulmonary function in long-term survivors of testicular cancer. J. Clin. Oncol. 2009;27:2779–2786. doi: 10.1200/JCO.2008.18.5181. [DOI] [PubMed] [Google Scholar]
- 141.O’Driscoll BR, et al. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N. Engl. J. Med. 1990;323:378–382. doi: 10.1056/NEJM199008093230604. [DOI] [PubMed] [Google Scholar]
- 142.White DA, et al. Acute chest pain syndrome during bleomycin infusions. Cancer. 1987;59:1582–1585. doi: 10.1002/1097-0142(19870501)59:9<1582::AID-CNCR2820590909>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 143.Uzel I, et al. Delayed onset bleomycin-induced pneumonitis. Urology. 2005;66:195. doi: 10.1016/j.urology.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 144.FDA. Bleomycin Sulfate for Injection, USP Labelhttps://www.accessdata.fda.gov/drugsatfda_docs/label/2010/050443s036lbl.pdf (2010).
- 145.Freites-Martinez A, et al. Using the common terminology criteria for adverse events (CTCAE – Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr. 2021;112:90–92. doi: 10.1016/j.ad.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 146.Camus P, et al. Drug-induced and iatrogenic infiltrative lung disease. Clin. Chest Med. 2004;25:479–519. doi: 10.1016/j.ccm.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 147.Raghu G, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022;205:e18–e47. doi: 10.1164/rccm.202202-0399ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Desai A, et al. Antibody-drug conjugates: a promising novel therapeutic approach in lung cancer. Lung Cancer. 2022;163:96–106. doi: 10.1016/j.lungcan.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 149.Thompson JA, et al. Management of immunotherapy-related toxicities, version 1.2019. J. Natl Compr. Canc Netw. 2019;17:255–289. doi: 10.6004/jnccn.2019.0013. [DOI] [PubMed] [Google Scholar]
- 150.Tarantino P, et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: a review. JAMA Oncol. 2021;7:1873–1881. doi: 10.1001/jamaoncol.2021.3595. [DOI] [PubMed] [Google Scholar]
- 151.Flaherty KR, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 152.King TE, Jr., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 153.Bober SL, Varela VS. Sexuality in adult cancer survivors: challenges and intervention. J. Clin. Oncol. 2012;30:3712–3719. doi: 10.1200/JCO.2012.41.7915. [DOI] [PubMed] [Google Scholar]
- 154.Melisko ME, Narus JB. Sexual function in cancer survivors: updates to the NCCN Guidelines for Survivorship. J. Natl Compr. Canc Netw. 2016;14:685–689. doi: 10.6004/jnccn.2016.0193. [DOI] [PubMed] [Google Scholar]
- 155.Smith T, Kingsberg SA, Faubion S. Sexual dysfunction in female cancer survivors: addressing the problems and the remedies. Maturitas. 2022;165:52–57. doi: 10.1016/j.maturitas.2022.07.010. [DOI] [PubMed] [Google Scholar]
- 156.van Basten JP, et al. Sexual dysfunction in nonseminoma testicular cancer patients is related to chemotherapy-induced angiopathy. J. Clin. Oncol. 1997;15:2442–2448. doi: 10.1200/JCO.1997.15.6.2442. [DOI] [PubMed] [Google Scholar]
- 157.Pallotti F, et al. Long-term follow up of the erectile function of testicular cancer survivors. Front. Endocrinol. 2019;10:196. doi: 10.3389/fendo.2019.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jonker-Pool G, et al. Sexual functioning after treatment for testicular cancer: comparison of treatment modalities. Cancer. 1997;80:454–464. doi: 10.1002/(SICI)1097-0142(19970801)80:3<454::AID-CNCR13>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 159.Barkatz J, et al. Sexual dysfunctions of patients treated with orchidectmoy, chemotherapy and retroperitoneal lymphadenectomy, need for systematic andrological care? [French] Bull. Cancer. 2019;106:915–922. doi: 10.1016/j.bulcan.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 160.Katz A, Agrawal LS, Sirohi B. Sexuality after cancer as an unmet need: addressing disparities, achieving equality. Am. Soc. Clin. Oncol. Educ. Book. 2022;42:1–7. doi: 10.1200/EDBK_100032. [DOI] [PubMed] [Google Scholar]
- 161.Robinson PJ, et al. Aromatase inhibitors are associated with low sexual desire causing distress and fecal incontinence in women: an observational study. J. Sex. Med. 2017;14:1566–1574. doi: 10.1016/j.jsxm.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 162.Baumgart J, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162–168. doi: 10.1097/GME.0b013e31826560da. [DOI] [PubMed] [Google Scholar]
- 163.Smedsland SK, et al. Sexual activity and functioning in long-term breast cancer survivors; exploring associated factors in a nationwide survey. Breast Cancer Res. Treat. 2022;193:139–149. doi: 10.1007/s10549-022-06544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pizzol D, et al. Prevalence of erectile dysfunction in male survivors of cancer: a systematic review and meta-analysis of cross-sectional studies. Br. J. Gen. Pract. 2021;71:e372–e380. doi: 10.3399/bjgp20X714197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carter J, et al. Interventions to address sexual problems in people with cancer: American Society of Clinical Oncology Clinical Practice Guideline Adaptation of Cancer Care Ontario Guideline. J. Clin. Oncol. 2018;36:492–511. doi: 10.1200/JCO.2017.75.8995. [DOI] [PubMed] [Google Scholar]
- 166.Cold S, et al. Systemic or vaginal hormone therapy after early breast cancer: a Danish observational cohort study. J. Natl Cancer Inst. 2022;114:1347–1354. doi: 10.1093/jnci/djac112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.ACOG Committee Opinion No. 659. The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet. Gynecol. 2016;127:e93–e96. doi: 10.1097/AOG.0000000000001351. [DOI] [PubMed] [Google Scholar]
- 168.Chung E, Brock G. Sexual rehabilitation and cancer survivorship: a state of art review of current literature and management strategies in male sexual dysfunction among prostate cancer survivors. J. Sex. Med. 2013;10:102–111. doi: 10.1111/j.1743-6109.2012.03005.x. [DOI] [PubMed] [Google Scholar]
- 169.Wittmann D, et al. Guidelines for sexual health care for prostate cancer patients: recommendations of an International Panel. J. Sex. Med. 2022;19:1655–1669. doi: 10.1016/j.jsxm.2022.08.197. [DOI] [PubMed] [Google Scholar]
- 170.Lee SJ, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 171.Paoli D, et al. Spermatogenesis in Hodgkin’s lymphoma patients: a retrospective study of semen quality before and after different chemotherapy regimens. Hum. Reprod. 2016;31:263–272. doi: 10.1093/humrep/dev310. [DOI] [PubMed] [Google Scholar]
- 172.Oktay K, Harvey BE, Loren AW. Fertility preservation in patients with cancer: ASCO Clinical Practice Guideline Update Summary. J. Oncol. Pract. 2018;14:381–385. doi: 10.1200/JOP.18.00160. [DOI] [PubMed] [Google Scholar]
- 173.Lambertini M, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020;31:1664–1678. doi: 10.1016/j.annonc.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 174.Spears N, et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update. 2019;25:673–693. doi: 10.1093/humupd/dmz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Poorvu PD, et al. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectr. 2019;3:pkz008. doi: 10.1093/jncics/pkz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.van Dorp W, et al. Genetic variation may modify ovarian reserve in female childhood cancer survivors. Hum. Reprod. 2013;28:1069–1076. doi: 10.1093/humrep/des472. [DOI] [PubMed] [Google Scholar]
- 177.Su HI, et al. Association of cyclophosphamide drug-metabolizing enzyme polymorphisms and chemotherapy-related ovarian failure in breast cancer survivors. Fertil. Steril. 2010;94:645–654. doi: 10.1016/j.fertnstert.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reimer T, et al. SLCO1B1*5 polymorphism (rs4149056) is associated with chemotherapy-induced amenorrhea in premenopausal women with breast cancer: a prospective cohort study. BMC Cancer. 2016;16:337. doi: 10.1186/s12885-016-2373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gracia CR, et al. Impact of cancer therapies on ovarian reserve. Fertil. Steril. 2012;97:134–40.e1. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Thomas-Teinturier C, et al. Ovarian reserve after treatment with alkylating agents during childhood. Hum. Reprod. 2015;30:1437–1446. doi: 10.1093/humrep/dev060. [DOI] [PubMed] [Google Scholar]
- 181.Partridge AH, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil. Steril. 2010;94:638–644. doi: 10.1016/j.fertnstert.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 182.Pilsgaard F, et al. The use of anti-Müllerian hormone for controlled ovarian stimulation in assisted reproductive technology, fertility assessment and -counseling. Acta Obstet. Gynecol. Scand. 2018;97:1105–1113. doi: 10.1111/aogs.13334. [DOI] [PubMed] [Google Scholar]
- 183.Dewailly D, Laven J. AMH as the primary marker for fertility. Eur. J. Endocrinol. 2019;181:D45–D51. doi: 10.1530/EJE-19-0373. [DOI] [PubMed] [Google Scholar]
- 184.Moolhuijsen LME, Visser JA. Anti-Mullerian hormone and ovarian reserve: update on assessing ovarian function. J. Clin. Endocrinol. Metab. 2020;105:3361–3373. doi: 10.1210/clinem/dgaa513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oktay K, et al. Fertility preservation in patients with cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018;36:1994–2001. doi: 10.1200/JCO.2018.78.1914. [DOI] [PubMed] [Google Scholar]
- 186.ESHRE Guideline Group on Female Fertility Preservation et al. ESHRE guideline: female fertility preservation. Hum. Reprod. Open. 2020;2020:hoaa052. doi: 10.1093/hropen/hoaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J. Clin. 2018;68:153–165. doi: 10.3322/caac.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.von Wolff M, et al. Timing of ovarian stimulation in patients prior to gonadotoxic therapy: an analysis of 684 stimulations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;199:146–149. doi: 10.1016/j.ejogrb.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 189.Lambertini M, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. J. Clin. Oncol. 2018;36:1981–1990. doi: 10.1200/JCO.2018.78.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen J, et al. Construction and cryopreservation of an artificial ovary in cancer patients as an element of cancer therapy and a promising approach to fertility restoration. Hum. Fertil. 2022;25:651–661. doi: 10.1080/14647273.2021.1885756. [DOI] [PubMed] [Google Scholar]
- 191.Arecco L, et al. Safety of fertility preservation techniques before and after anticancer treatments in young women with breast cancer: a systematic review and meta-analysis. Hum. Reprod. 2022;37:954–968. doi: 10.1093/humrep/deac035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chian RC, Uzelac PS, Nargund G. In vitro maturation of human immature oocytes for fertility preservation. Fertil. Steril. 2013;99:1173–1181. doi: 10.1016/j.fertnstert.2013.01.141. [DOI] [PubMed] [Google Scholar]
- 193.Wood ME, et al. Second malignant neoplasms: assessment and strategies for risk reduction. J. Clin. Oncol. 2012;30:3734–3745. doi: 10.1200/JCO.2012.41.8681. [DOI] [PubMed] [Google Scholar]
- 194.Lyman GH, et al. Overall survival and risk of second malignancies with cancer chemotherapy and G-CSF support. Ann. Oncol. 2018;29:1903–1910. doi: 10.1093/annonc/mdy311. [DOI] [PubMed] [Google Scholar]
- 195.Travis LB. Therapy-associated solid tumors. Acta Oncol. 2002;41:323–333. doi: 10.1080/028418602760169361. [DOI] [PubMed] [Google Scholar]
- 196.Ganz PA, Casillas JN. Incorporating the risk for subsequent primary cancers into the care of adult cancer survivors: moving beyond 5-year survival. J. Am. Med. Assoc. 2020;324:2493–2495. doi: 10.1001/jama.2020.23410. [DOI] [PubMed] [Google Scholar]
- 197.Sung H, et al. Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. J. Am. Med. Assoc. 2020;324:2521–2535. doi: 10.1001/jama.2020.23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Areethamsirikul N, Reece DE. The risk of secondary primary malignancies after therapy for multiple myeloma. Leuk. Lymphoma. 2015;56:3012–3021. doi: 10.3109/10428194.2014.974043. [DOI] [PubMed] [Google Scholar]
- 199.Castro GA, Church A, Pechet L, Snyder LM. Leukemia after chemotherapy of Hodgkin’s disease. N. Engl. J. Med. 1973;289:103–104. doi: 10.1056/NEJM197307122890215. [DOI] [PubMed] [Google Scholar]
- 200.Preisler HD, Lyman GH. Acute myelogenous leukemia subsequent to therapy for a different neoplasm: clinical features and response to therapy. Am. J. Hematol. 1977;3:209–218. doi: 10.1002/ajh.2830030301. [DOI] [PubMed] [Google Scholar]
- 201.Crump M, et al. Risk of acute leukemia following epirubicin-based adjuvant chemotherapy: a report from the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2003;21:3066–3071. doi: 10.1200/JCO.2003.08.137. [DOI] [PubMed] [Google Scholar]
- 202.Smith RE, et al. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J. Clin. Oncol. 2003;21:1195–1204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 203.Praga C, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J. Clin. Oncol. 2005;23:4179–4191. doi: 10.1200/JCO.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 204.Leone G, et al. Therapy-related leukemia and myelodysplasia: susceptibility and incidence. Haematologica. 2007;92:1389–1398. doi: 10.3324/haematol.11034. [DOI] [PubMed] [Google Scholar]
- 205.Le Deley MC, et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J. Clin. Oncol. 2007;25:292–300. doi: 10.1200/JCO.2006.05.9048. [DOI] [PubMed] [Google Scholar]
- 206.Patt DA, et al. Acute myeloid leukemia after adjuvant breast cancer therapy in older women: understanding risk. J. Clin. Oncol. 2007;25:3871–3876. doi: 10.1200/JCO.2007.12.0832. [DOI] [PubMed] [Google Scholar]
- 207.Josting A, et al. Secondary myeloid leukemia and myelodysplastic syndromes in patients treated for Hodgkin’s disease: a report from the German Hodgkin’s Lymphoma Study Group. J. Clin. Oncol. 2003;21:3440–3446. doi: 10.1200/JCO.2003.07.160. [DOI] [PubMed] [Google Scholar]
- 208.Morton LM, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975–2008. Blood. 2013;121:2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Travis LB, et al. Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin’s lymphoma. J. Natl Cancer Inst. 1995;87:524–530. doi: 10.1093/jnci/87.7.524. [DOI] [PubMed] [Google Scholar]
- 210.Mudie NY, et al. Risk of second malignancy after non-Hodgkin’s lymphoma: a British Cohort Study. J. Clin. Oncol. 2006;24:1568–1574. doi: 10.1200/JCO.2005.04.2200. [DOI] [PubMed] [Google Scholar]
- 211.Travis LB, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J. Natl Cancer Inst. 2002;94:182–192. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 212.Travis LB, et al. Cancer survivorship–genetic susceptibility and second primary cancers: research strategies and recommendations. J. Natl Cancer Inst. 2006;98:15–25. doi: 10.1093/jnci/djj001. [DOI] [PubMed] [Google Scholar]
- 213.Relling MV, et al. Granulocyte colony-stimulating factor and the risk of secondary myeloid malignancy after etoposide treatment. Blood. 2003;101:3862–3867. doi: 10.1182/blood-2002-08-2405. [DOI] [PubMed] [Google Scholar]
- 214.Hershman D, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J. Natl Cancer Inst. 2007;99:196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 215.Lyman GH, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: a systematic review. J. Clin. Oncol. 2010;28:2914–2924. doi: 10.1200/JCO.2009.25.8723. [DOI] [PubMed] [Google Scholar]
- 216.Smith SM, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102:43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 217.Schaapveld M, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N. Engl. J. Med. 2015;373:2499–2511. doi: 10.1056/NEJMoa1505949. [DOI] [PubMed] [Google Scholar]
- 218.Perez DG, Loprinzi C, Ruddy KJ. Lifestyle factors can lead to multiple cancers over a lifetime-here we go again. JAMA Oncol. 2021;7:505–506. doi: 10.1001/jamaoncol.2020.7360. [DOI] [PubMed] [Google Scholar]
- 219.Verma S, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bergerot CD, Pal SK. Shining a light on the psychological burden of cancer. Nat. Med. 2022;28:637–638. doi: 10.1038/s41591-022-01763-w. [DOI] [PubMed] [Google Scholar]
- 221.Zabora J, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::AID-PON501>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 222.Institute of Medicine (US) Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs (National Academies Press, 2008). [PubMed]
- 223.Riba MB, et al. Distress management, version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc Netw. 2019;17:1229–1249. doi: 10.6004/jnccn.2019.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Andersen BL, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J. Clin. Oncol. 2014;32:1605–1619. doi: 10.1200/JCO.2013.52.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jacobsen PB, Wagner LI, New A. Quality standard: the integration of psychosocial care into routine cancer care. J. Clin. Oncol. 2012;30:1154–1159. doi: 10.1200/JCO.2011.39.5046. [DOI] [PubMed] [Google Scholar]
- 226.Lebel S, et al. From normal response to clinical problem: definition and clinical features of fear of cancer recurrence. Support. Care Cancer. 2016;24:3265–3268. doi: 10.1007/s00520-016-3272-5. [DOI] [PubMed] [Google Scholar]
- 227.Bergerot CD, et al. Fear of cancer recurrence or progression: what is it and what can we do about it? Am. Soc. Clin. Oncol. Educ. Book. 2022;42:1–10. doi: 10.1200/EDBK_100031. [DOI] [PubMed] [Google Scholar]
- 228.Chang WH, Lai AG. Cumulative burden of psychiatric disorders and self-harm across 26 adult cancers. Nat. Med. 2022;28:860–870. doi: 10.1038/s41591-022-01740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Heinrich M, et al. Suicide risk and mortality among patients with cancer. Nat. Med. 2022;28:852. doi: 10.1038/s41591-022-01745-y. [DOI] [PubMed] [Google Scholar]
- 230.Li M, Fitzgerald P, Rodin G. Evidence-based treatment of depression in patients with cancer. J. Clin. Oncol. 2012;30:1187–1196. doi: 10.1200/JCO.2011.39.7372. [DOI] [PubMed] [Google Scholar]
- 231.Traeger L, et al. Evidence-based treatment of anxiety in patients with cancer. J. Clin. Oncol. 2012;30:1197–1205. doi: 10.1200/JCO.2011.39.5632. [DOI] [PubMed] [Google Scholar]
- 232.Bower JE, et al. Targeting depressive symptoms in younger breast cancer survivors: the pathways to wellness randomized controlled trial of mindfulness meditation and survivorship education. J. Clin. Oncol. 2021;39:3473–3484. doi: 10.1200/JCO.21.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.McAlpine H, et al. A systematic review of types and efficacy of online interventions for cancer patients. Patient Educ. Couns. 2015;98:283–295. doi: 10.1016/j.pec.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 234.Dossett LA, et al. The primary care provider (PCP)-cancer specialist relationship: a systematic review and mixed-methods meta-synthesis. CA Cancer J. Clin. 2017;67:156–169. doi: 10.3322/caac.21385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Snyder CF, et al. Comorbid condition care quality in cancer survivors: role of primary care and specialty providers and care coordination. J. Cancer Surviv. 2015;9:641–649. doi: 10.1007/s11764-015-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Barone BB, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Griffiths RI, Keating NL, Bankhead CR. Quality of diabetes care in cancer: a systematic review. Int. J. Qual. Health Care. 2019;31:75–88. doi: 10.1093/intqhc/mzy124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Halpern MT, et al. Models of cancer survivorship care: overview and summary of current evidence. J. Oncol. Pract. 2015;11:e19–e27. doi: 10.1200/JOP.2014.001403. [DOI] [PubMed] [Google Scholar]
- 239.Chan RJ, et al. Outcomes of cancer survivorship education and training for primary care providers: a systematic review. J. Cancer Surviv. 2022;16:279–302. doi: 10.1007/s11764-021-01018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Earle CC, et al. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J. Clin. Oncol. 2003;21:1447–1451. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 241.Khan NF, Carpenter L, Watson E, Rose PW. Cancer screening and preventative care among long-term cancer survivors in the United Kingdom. Br. J. Cancer. 2010;102:1085–1090. doi: 10.1038/sj.bjc.6605609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Nekhlyudov L, O’Malley DM, Hudson SV. Integrating primary care providers in the care of cancer survivors: gaps in evidence and future opportunities. Lancet Oncol. 2017;18:e30–e38. doi: 10.1016/S1470-2045(16)30570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bober SL, et al. Caring for cancer survivors: a survey of primary care physicians. Cancer. 2009;115:4409–4418. doi: 10.1002/cncr.24590. [DOI] [PubMed] [Google Scholar]
- 244.Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J. Clin. Oncol. 2009;27:2489–2495. doi: 10.1200/JCO.2008.20.3232. [DOI] [PubMed] [Google Scholar]
- 245.Alfano CM, et al. Building personalized cancer follow-up care pathways in the United States: lessons learned from implementation in England, Northern Ireland, and Australia. Am. Soc. Clin. Oncol. Educ. Book. 2019;39:625–639. doi: 10.1200/EDBK_238267. [DOI] [PubMed] [Google Scholar]
- 246.Jones JM, et al. Readdressing the needs of cancer survivors during COVID-19: a path forward. J. Natl Cancer Inst. 2021;113:955–961. doi: 10.1093/jnci/djaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Qian AS, et al. Disparities in telemedicine during COVID-19. Cancer Med. 2022;11:1192–1201. doi: 10.1002/cam4.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Nekhlyudov L, et al. Developing a quality of cancer survivorship care framework: implications for clinical care, research, and policy. J. Natl Cancer Inst. 2019;111:1120–1130. doi: 10.1093/jnci/djz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chan RJ, et al. Effectiveness and implementation of models of cancer survivorship care: an overview of systematic reviews. J. Cancer Surviv. 2021;17:197–221. doi: 10.1007/s11764-021-01128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ. Res. 2003;18:156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- 251.Demark-Wahnefried W, et al. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J. Clin. Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Brown JC, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol. Biomark. Prev. 2011;20:123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 253.Sweegers MG, et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2018;52:505–513. doi: 10.1136/bjsports-2017-097891. [DOI] [PubMed] [Google Scholar]
- 254.Buffart LM, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2017;52:91–104. doi: 10.1016/j.ctrv.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 255.Brown JC, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta-analysis. PLoS One. 2012;7:e30955. doi: 10.1371/journal.pone.0030955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Dalla Via J, Daly RM, Fraser SF. The effect of exercise on bone mineral density in adult cancer survivors: a systematic review and meta-analysis. Osteoporos. Int. 2018;29:287–303. doi: 10.1007/s00198-017-4237-3. [DOI] [PubMed] [Google Scholar]
- 257.Mercier J, Savard J, Bernard P. Exercise interventions to improve sleep in cancer patients: a systematic review and meta-analysis. Sleep Med. Rev. 2017;36:43–56. doi: 10.1016/j.smrv.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 258.Mitchell SA, Chambers DA. Leveraging implementation science to improve cancer care delivery and patient outcomes. J. Oncol. Pract. 2017;13:523–529. doi: 10.1200/JOP.2017.024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ramsey SD, Lyman GH, Bangs R. Addressing skyrocketing cancer drug prices comes with tradeoffs: pick your poison. JAMA Oncol. 2016;2:425–426. doi: 10.1001/jamaoncol.2015.5813. [DOI] [PubMed] [Google Scholar]
- 260.Desai AP, et al. Economic cost and sustainability of oral therapies in precision oncology. JCO Oncol. Pract. 2022;18:e1247–e1254. doi: 10.1200/OP.21.00847. [DOI] [PubMed] [Google Scholar]
- 261.Time. How Healthcare Costs Hurt American Workers and Benefit the Wealthyhttps://time.com/5785945/health-care-problems-america/ (20 February 2020).
- 262.Lyman GH. Counting the costs of cancer care. Lancet Oncol. 2013;14:1142–1143. doi: 10.1016/S1470-2045(13)70480-7. [DOI] [PubMed] [Google Scholar]
- 263.Lyman GH, Kuderer N. Financial toxicity, financial abuse, or financial torture: let’s call it what it is! Cancer Invest. 2020;38:139–142. doi: 10.1080/07357907.2020.1735084. [DOI] [PubMed] [Google Scholar]
- 264.Knight TG, et al. Financial toxicity in adults with cancer: adverse outcomes and noncompliance. J. Oncol. Pract. 2018 doi: 10.1200/JOP.18.00120. [DOI] [PubMed] [Google Scholar]
- 265.National Cancer Institute. Financial Toxicity. NCI Dictionary of Cancer Terms.https://www.cancer.gov/publications/dictionaries/cancer-terms/def/financial-toxicity (2023).
- 266.Huang IC, et al. Determinants and consequences of financial hardship among adult survivors of childhood cancer: a report from the St. Jude lifetime cohort study. J. Natl Cancer Inst. 2019;111:189–200. doi: 10.1093/jnci/djy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.de Souza JA, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: the validation of the COmprehensive Score for financial Toxicity (COST) Cancer. 2017;123:476–484. doi: 10.1002/cncr.30369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Gilligan AM, et al. Death or Debt? National estimates of financial toxicity in persons with newly-diagnosed cancer. Am. J. Med. 2018;131:1187–1199.e5. doi: 10.1016/j.amjmed.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 269.Lyman GH, Zon R, Harvey RD. Rationale, opportunities, and reality of biosimilar medications. N. Engl. J. Med. 2018;379:694–695. doi: 10.1056/NEJMc1808348. [DOI] [PubMed] [Google Scholar]
- 270.Lyman GH, Henk HJ. Association of generic imatinib availability and pricing with trends in tyrosine kinase inhibitor use in patients with chronic myelogenous leukemia. JAMA Oncol. 2020;6:1969–1971. doi: 10.1001/jamaoncol.2020.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rodriguez G, et al. ASCO policy statement on biosimilar and interchangeable products in oncology. JCO Oncol. Pract. 2023 doi: 10.1200/OP.22.00783. [DOI] [PubMed] [Google Scholar]
- 272.Schnipper LE, et al. Updating the American Society of Clinical Oncology value framework: revisions and reflections in response to comments received. J. Clin. Oncol. 2016;34:2925–2934. doi: 10.1200/JCO.2016.68.2518. [DOI] [PubMed] [Google Scholar]
- 273.Cherny NI, et al. ESMO-magnitude of clinical benefit scale version 1.1. Ann. Oncol. 2017;28:2340–2366. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 274.Cherny NI, et al. Comparative assessment of clinical benefit using the ESMO-magnitude of clinical benefit scale version 1.1 and the ASCO value framework net health benefit score. J. Clin. Oncol. 2019;37:336–349. doi: 10.1200/JCO.18.00729. [DOI] [PubMed] [Google Scholar]
- 275.Schnipper LE, et al. American society of clinical oncology statement: a conceptual framework to assess the value of cancer treatment options. J. Clin. Oncol. 2015;33:2563–2577. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tefferi A, et al. In support of a patient-driven initiative and petition to lower the high price of cancer drugs. Mayo Clin. Proc. 2015;90:996–1000. doi: 10.1016/j.mayocp.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Altice CK, et al. Financial hardships experienced by cancer survivors: a systematic review. J. Natl Cancer Inst. 2017;109:djw205. doi: 10.1093/jnci/djw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Desai A, Gyawali B. Financial toxicity of cancer treatment: moving the discussion from acknowledgement of the problem to identifying solutions. EClinicalMedicine. 2020;20:100269. doi: 10.1016/j.eclinm.2020.100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Witte J, et al. Methods for measuring financial toxicity after cancer diagnosis and treatment: a systematic review and its implications. Ann. Oncol. 2019;30:1061–1070. doi: 10.1093/annonc/mdz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.United Nations. Universal Declaration of Human Rightshttps://www.un.org/en/about-us/universal-declaration-of-human-rights (1948).
- 281.National Academies of Sciences, Engineering, and Medicine. Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health (National Academies Press, 2019). [PubMed]
- 282.Moullin JC, et al. A systematic review of implementation frameworks of innovations in healthcare and resulting generic implementation framework. Health Res. Policy Syst. 2015;13:16. doi: 10.1186/s12961-015-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Colquhoun H, et al. Towards a common terminology: a simplified framework of interventions to promote and integrate evidence into health practices, systems, and policies. Implement. Sci. 2014;9:51. doi: 10.1186/1748-5908-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]