Abstract
BACKGROUND
Patients diagnosed as having multiple sclerosis (MS) experience a wide range of symptoms requiring pharmacologic management, and many do not achieve adequate symptom control. The purpose of this study was to evaluate the role of medical cannabis (MC) as part of a comprehensive treatment plan for patients with MS.
METHODS
A retrospective medical record review of 141 patients with MS receiving MC for symptom management was conducted. Data were collected for up to 4 follow-up appointments after initiation of MC. Outcomes included changes in MS symptoms, medication changes, adverse events, and changes in cognition and mobility.
RESULTS
Patients experienced extensive MS symptom improvement after initiation of MC, with alleviation of pain (72% of patients) and spasticity (48% of patients) and improvement in sleep (40% of patients) the most common. There was a significant reduction in concomitant opioid use after initiating MC as evidenced by a significant decrease in daily morphine milligram equivalents among patients prescribed opioid analgesics (P = .01). Decreases in muscle relaxant use and benzodiazepine use did not reach significance (P > .05). The most common adverse reaction to MC was fatigue (11% of patients).
CONCLUSIONS
In many patients with MS, MC was well tolerated, eased pain and spasticity, improved sleep and other symptoms, and reduced use of concomitant opioid analgesics. Prospective studies are needed to further investigate the role of MC in the treatment of patients with MS.
Keywords: medical marijuana, multiple sclerosis, cannabis, spasticity
Multiple sclerosis (MS), a chronic demyelinating autoimmune disease of the central nervous system (CNS), is a leading cause of nontraumatic neurologic disability in young adults.1,2 More than 700,000 people in the United States live with MS, and the prevalence is increasing across North America.1,3 Progress has been made in the development of disease-modifying treatments that slow disease progression and preserve nerve function, but treatment of MS symptoms remains complex. Common MS symptoms include muscle spasms, impaired gait, pain, tremor, depression, fatigue, and cognitive dysfunction.4 A comprehensive treatment plan for many patients with MS includes disease-modifying treatments and multiple pharmacologic agents to manage symptoms; as a result, polypharmacy plagues this patient population.5–8 Despite the high frequency of opioid use to alleviate pain and other medications to manage MS symptoms, many patients continue to suffer.9,10 New therapies to manage MS symptoms, especially those that address more than 1 symptom, are urgently needed.
Medical cannabis (MC) has recently generated interest as a therapy for neurologic disorders, including MS. Medical cannabis varies widely in composition and includes pharmacologically active cannabinoids.11,12 Δ9-Tetrahydrocannabinol (THC) is the primary cannabinoid found in MC, and it exerts antinociceptive and psychotropic effects by binding 2 types of G-protein–coupled receptors: cannabinoid types 1 (CB1) and 2 (CB2).13 The CB1 receptors are abundant in the CNS and are also found in the peripheral nervous system, and the CB2 receptors are predominantly located in peripheral inflammatory and immune pathways.14 Cannabidiol (CBD) is another prevalent cannabinoid in MC with different pharmacodynamic effects, many of which remain unclear. It exhibits effects on G-protein–coupled receptors (including opioid receptors CB1 and CB2) and serotonergic receptors and is known to enhance endogenous cannabinoid actions and interact with cytochrome P450 enzymes. Cannabidiol has been shown to provide pain relief, reduce inflammation, and act as a potent antioxidant.15,16 There is evidence that CBD may potentiate the beneficial effects of THC, resulting in synergistic effects when the 2 are administered in combination. In part, this synergy may be due to counteracting effects of THC and CBD on CB1 receptors in the CNS, resulting in enhanced tolerability to THC’s psychotropic effects.16,17
Recent systematic reviews concluded that MC may be effective at relieving spasticity and pain in patients with MS, including a systematic review by the American Academy of Neurology.18,19 A double-blind, placebo-controlled study of oral cannabis extract containing THC and CBD with 279 patients with MS found that those in the cannabis group experienced muscle stiffness relief at almost twice the rate as the placebo group (29.4% and 15.7%, respectively). This study also found a higher rate of pain relief in the cannabis group. Adverse events (AEs) that occurred in more than 10% of patients and more frequently in the cannabis group included dizziness, dry mouth, urinary tract infection, weakness, fatigue, and headache; however, these effects were mild to moderate in 95% of patients.20 Similarly, 630 patients with MS participated in a study that examined the impact of oral cannabis extract containing THC and CBD in a 2:1 ratio, THC alone, and placebo on spasticity and pain. There was no evidence of a treatment effect on change in overall spasticity scores using the Ashworth scale; however, there was an observed effect on patient-reported symptoms, including spasticity, pain, tremor, and bladder symptoms. Common AEs in the cannabis extract and THC groups included gastrointestinal issues, vision issues, dizziness, dry mouth, and sleepiness, all of which were minor.21
Despite evidence that supports the use of MC for MS, issues of legality limit access to MC in the United States. Although none are approved by the Food and Drug Administration (FDA) to treat MS symptoms, there are 3 cannabinoid products that are federally legal to prescribe in the United States: dronabinol (Marinol [AbbVie Inc] and Syndros [Benuvia Therapeutics, Inc]), nabilone (Cesamet [Valeant Pharmaceuticals International]; discontinued in the United States), and CBD (Epidiolex [Jazz Pharmaceuticals, Inc]).22–24 Approved in several countries, including Canada and much of Europe, Sativex [GW Pharma Ltd] is a combination of THC and CBD labeled specifically to treat spasticity induced by MS; however, it is not approved in the United States.25 The status of MC as a federal schedule I controlled substance that is not approved by the FDA for MS treatment limits the use of MC in patients with MS and restricts opportunities for clinical research.
As evidence supporting MC grows, legislation has been passed to support its use in the United States. The Agriculture Improvement Act of 2018 removed low-THC derivatives of cannabis from the definition of marijuana in the Controlled Substances Act.26 In addition, individual states have passed laws that permit the use of cannabis products for legitimate medical indications. New York State (NYS) passed the Compassionate Care Act in July 2014 that legalized MC for specific medical conditions, including MS. Recently, NYS expanded these regulations; as of January 2022, providers can certify patients for MC for any condition at their clinical discretion.27 A total of 38 states in the United States have passed legislation permitting use of MC for patients with MS.28
As MC use increases with state access programs, clinical research describing optimal MC doses, THC to CBD ratios, and administration routes to relieve symptoms in patients with MS is lacking. In addition, patterns of concomitant medication use by patients with MS initiating MC for symptom relief require investigation. The present study expands on current literature concerning MC use by patients with MS to provide symptom relief. Unlike previous research, this study describes concomitant medication changes after MC initiation and reviews patterns of THC to CBD ratio and formulation changes to increase our understanding of MC use in patients with MS.
METHODS
A retrospective medical record review was conducted at a large outpatient neurologic practice in NYS. The primary objective of this study was to evaluate patients diagnosed as having MS who received MC for symptom management. Outcomes included changes in concomitant medications, AEs, patient-reported symptomatic improvements, MC THC to CBD ratio and formulation changes, and mobility and cognition changes. This study was approved by WCG Institutional Review Board. Research was conducted according to the principles of the Declaration of Helsinki and parts 50 and 56 of the US 21 Code of Federal Regulations.
Patients were included if they were at least 18 years old, were diagnosed as having MS, were certified to receive MC, and used MC consistently from initiation until at least 1 follow-up appointment. Baseline and follow-up visits occurred between March 21, 2016, and October 31, 2018. Patients started MC between March 21, 2016, and January 23, 2018.
Data were collected from each patient’s electronic health record at certification and follow-up. Baseline data were collected from each patient’s most recent appointment within 6 months preceding certification. Follow-up data were collected for up to 4 appointments immediately after initiation of MC. Data collection stopped after discontinuation of therapy, after the first 4 follow-up visits, or at the end of the data collection period (October 31, 2018), whichever came first.
Data collected at each visit included changes in MC formulation (eg, tincture, vapor) and MC THC to CBD ratio (eg, 20:1, 1:1); vital signs; changes in mobility and cognition as measured by the Timed 25-Foot Walk test (T25FW), the Montreal Cognitive Assessment (MoCA), and the Mini-Mental State Examination (MMSE); symptomatic changes perceived by the patient and/or clinician; and AEs from MC use. The T25FW is a test of maximum walking speed for a short distance; results of this test are associated with clinical outcomes in patients with MS.29 The MoCA and MMSE are scales to evaluate cognitive function and are commonly used in patients with MS.30 All AEs were evaluated using the Naranjo scale to determine relatedness to MC.31,32 Any AEs scored as at least “possibly” related to MC (total Naranjo score ≥ 1) were included. Severity of AEs were rated as follows: mild if no intervention was required, moderate if an intervention was made due to the AE or the AE had a meaningful effect on daily life, or severe if the AE caused an emergency department visit, hospitalization, or permanent disability.
Medication changes were recorded for MC and other medications associated with MS symptom treatment: muscle relaxants, opioids, and benzodiazepines. Opioid use was converted to daily morphine milligram equivalents (MMEs) and recorded at each visit.33 Daily opioid use was based on maximum prescribed daily dose or patient-reported use. Benzodiazepine changes were recorded at each visit as lorazepam milligram equivalents (LMEs).34
Results of the Zung Self-Rating Anxiety Scale (ZSRAS) and the Beck Depression Inventory (BDI) were reviewed in a subset of patients after initiating MC to characterize the patient population.35,36 These rating scales were administered in spring 2018 in the normal course of clinical practice, regardless of the patient’s number of follow-up visits at this time. Furthermore, a post hoc analysis was conducted to examine the relationship between baseline MC THC to CBD ratio/formulation and risk of discontinuing MC treatment before 4 follow-up visits.
Statistical Analysis
A Wilcoxon signed rank test was used to compare the T25FW and MoCA/MMSE scores at baseline with the most recent available measurement using the last-observation-carried-forward method. The MMSE scores were converted to equivalent MoCA scores for analysis.37 The McNemar test was used to compare the prevalence of opioid, benzodiazepine, and muscle relaxant use at initiation with the prevalence at the last available time point. A paired-samples t test using the last-observation-carried-forward method was used to assess changes in body mass index (BMI)/weight and to describe changes in MMEs and LMEs in patients treated with opioids and benzodiazepines, respectively. Baseline characteristics, symptom outcomes, AEs, ZSRAS/BDI results, and other outcomes were reported using descriptive statistics. A logistic regression analysis was performed post hoc to examine the relationship between baseline MC THC to CBD ratio/formulation at initial certification and discontinuing treatment before 4 follow-up visits. Analyses were performed using Microsoft Excel (Microsoft Corp), GraphPad (GraphPad Software, Inc), and R version 4.1.0.
RESULTS
An electronic health record query and subsequent screening resulted in 141 patients eligible for study inclusion (FIGURE S1, available online at IJMSC.org). Participants were primarily female (n = 98; 70%), with a mean ± SD age of 51 ± 12 years. Approximately one-third of participants had a history of recreational cannabis use (n = 44; 31%), and 40% (n = 57) were receiving disability benefits. Indications for MC use included chronic pain (n = 113; 80%) and/or spasticity (n = 54; 38%).
The mean ± SD length of time between each follow-up visit was 113 ± 61 days, and the mean ± SD length of time from MC initiation (or certification if initiation date was unavailable) to last follow-up visit was 340 ± 153 days. FIGURE S2 displays the rate of dropout from treatment across the first 4 follow-up visits. Patients returned for a mean ± SD of 3 ± 1 follow-up visits.
TABLE 1 includes AEs and reasons for discontinuing MC therapy. No severe AEs were reported due to MC use. At the end of the study (October 31, 2018), 11 patients were in treatment but had not yet completed 4 follow-up visits; after removing these patients from analysis, 48% of patients (n = 62) discontinued MC treatment before the fourth follow-up visit.
TABLE 1.
Adverse Drug Events and Reasons for MC Discontinuation
| Participants, No. (%) | |||
|---|---|---|---|
| Mild severity | Moderate severity | Total | |
| Adverse drug events | |||
| Fatigue | 14 (10) | 1 (1) | 15 (11) |
| Dizziness | 2 (1) | 0 | 2 (1) |
| Euphoria or cognitive impairment | 5 (4) | 1 (1) | 6 (4) |
| Unpleasant taste | 3 (2) | 0 | 3 (2) |
| Increased appetite | 2 (1) | 0 | 2 (1) |
| Stomach upset | 2 (1) | 0 | 2 (1) |
| Throat discomfort | 1 (1) | 1 (1) | 2 (1) |
| Cough | 1 (1) | 0 | 1 (1) |
| Muscle spasms | 1 (1) | 0 | 1 (1) |
| Anxiety | 0 | 1 (1) | 1 (1) |
| “Burning” sensation on tongue/lips | 1 (1) | 0 | 1 (1) |
| Dry mouth | 1 (1) | 0 | 1 (1) |
| Experienced any adverse drug event | 31 (22) | 4 (3) | 34 (24) |
| Reason for MC discontinuation (n = 130)a | |||
| Lost to follow-up | 35 (27) | ||
| Cost | 14 (11) | ||
| Lack of efficacy | 8 (6) | ||
| Adverse events | 4 (3) | ||
| Unknown | 1 (1) | ||
| Subtotal | 62 (48) | ||
MC, medical cannabis.
Patients (n = 11) who were in treatment as of the end of the study period (October 31, 2018) but had not yet completed 4 follow-up visits were removed from this analysis.
Results for the THC to CBD ratio and formulation analysis are shown in FIGURE 1. The most frequently certified primary MC ratio at baseline was 1:1 (65% of patients), followed by 20:1 (34% of patients); however, by the fourth follow-up visit, patients were more frequently certified for 20:1 (56% of patients) than for 1:1 (32% of patients). Formulations for primary MC remained relatively consistent throughout the baseline visit and the first 4 follow-up visits, with patients most frequently certified to receive a tincture. Many patients added additional MC to be used as needed for breakthrough symptoms (notated as “PRN” therapy); the most common MC THC to CBD ratio/formulation PRN was 20:1 vapor.
FIGURE 1.
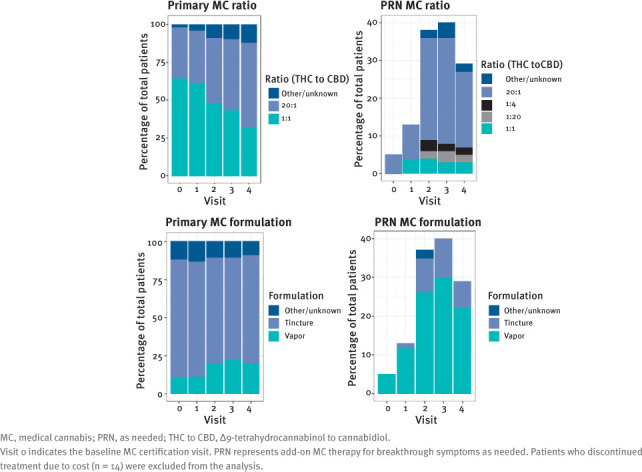
MC THC to CBD Ratio and Formulation Changes During Treatment
Details regarding changes in weight, BMI, T25FW scores, and MoCA/MMSE scores are shown in TABLE 2. There was no significant change in weight (P = .8), BMI (P = .6), T25FW scores (P = .2), or MoCA/MMSE scores (P = .4) from baseline to the last follow-up visit. The T25FW results were not recorded for 104 participants at baseline, and an additional 9 participants did not complete the test in any follow-up visits; therefore, 28 participants were included in the T25FW analysis. Similarly, MoCA or MMSE scores were available at baseline and at least 1 follow-up visit for only 13 patients.
TABLE 2.
Changes After Taking MC for Study Participants
| Baseline | Last follow-up | P value | |
|---|---|---|---|
| Outcome, mean ± SD | |||
| Weight, lba | 182 ± 50 | 182 ± 50 | .8b |
| BMIa | 28 ± 8 | 29 ± 7 | .6b |
| T25FW score (n = 28)c | 9.3 ± 5.1 | 8.8 ± 6.7 | .2b |
| MoCA score (n = 13)d | 26 ± 3 | 25 ± 4 | .4b |
| Medication changes | |||
| Opioid users, No. (%) | 50 (35) | 43 (30) | .1e |
| Decreased | NA | 16 | NA |
| Increased | NA | 7 | NA |
| Discontinued | NA | 11 | NA |
| Initiated | NA | 4 | NA |
| Opioid MMEs, mean ± SD, mg (n = 53)f | 51 ± 68 | 40 ± 67 | .01b |
| Benzodiazepine users, No. (%) | 61 (43) | 55 (39) | .2e |
| Decreased | NA | 11 | NA |
| Increased | NA | 8 | NA |
| Discontinued | NA | 10 | NA |
| Initiated | NA | 4 | NA |
| Daily LMEs, mean ± SD, mg (n = 64)g | 3.5 ± 3.5 | 3.1 ± 3.5 | .07b |
| Muscle relaxant users, No. (%) | 66 (47) | 61 (43) | .4e |
| Decreased | NA | 13 | NA |
| Increased | NA | 4 | NA |
| Discontinued | NA | 12 | NA |
| Initiated | NA | 7 | NA |
| Patient-reported improvement after MC, No. (%) | |||
| Pain relief | 101 (72) | ||
| Decreased muscle spasticity | 68 (48) | ||
| Improved sleep | 57 (40) | ||
| Improved gait | 15 (11) | ||
| Anxiety relief | 15 (11) | ||
| Decreased headache | 13 (9) | ||
| Improved mood | 12 (9) | ||
| Increased quality of life | 10 (7) | ||
| Otherh | 20 (14) |
BMI, body mass index; LME, lorazepam milligram equivalent; MC, medical cannabis; MME, morphine milligram equivalent; MoCA, Montreal Cognitive Assessment; NA, not applicable; T25FW, Timed 25-Foot Walk test.
Weight and BMI were unknown for 3 patients at baseline; these patients were excluded from the analysis.
Last-observation-carried-forward analysis was performed.
The T25FW results were not recorded for 104 patients at baseline, and an additional 9 patients did not complete the test in any follow-up visits; therefore, 28 patients were included in the analysis.
The Mini-Mental State Examination scores were converted to equivalent MoCA scores and included in this analysis. The MoCA or Mini-Mental State Examination scores were recorded at baseline and at 1 or more follow-up visits for 13 patients.
The McNemar test was performed.
Excludes buprenorphine due to lack of a validated conversion factor per current Centers for Disease Control and Prevention guidelines.49 A patient was excluded from MME analysis due to unknown opioid medication dose.
Lorazepam equivalents were unknown for 1 benzodiazepine user; this patient was excluded from the analysis.
Other patient-reported symptom improvement included improved balance (n = 3), focus (n = 3), numbness (n = 3), tremor (n = 3), nausea (n = 3), restless legs (n = 2), energy (n = 1), memory (n = 1), and seizures (n = 1).
Table 2 displays changes in concomitant medications during MC treatment. There was a significant reduction in daily opioid MMEs after starting MC (P = .01). Concomitant benzodiazepine use decreased overall, with mean daily LMEs reduced from 3.5 mg to 3.1 mg after starting MC, but this did not reach significance (P = .07). The number of patients who used muscle relaxants reduced from 66 to 61 after starting MC, but this decrease was also not significant (P = .4).
In addition, Table 2 details subjective symptomatic improvement reported by patients after initiating MC. Notably, 72% of patients reported pain relief and 48% reported decreased muscle spasticity. Furthermore, patients reported improved sleep (40%), gait (11%), anxiety (11%), and quality of life (7%).
Detailed results of the BDI and ZSRAS are presented in TABLE S1. While taking MC, 48 patients completed the BDI with a mean ± SD score of 14.5 ± 11, indicating mild mood disturbance.35 The mean ± SD ZSRAS score from 46 patients after starting MC was 39.5 ± 7. Scoring of this scale designates the average score of the population to be normal.36
Post hoc analysis was performed to evaluate the relationship between baseline MC THC to CBD ratio/formulation and discontinuing MC treatment. Patients who discontinued MC treatment due to cost were excluded (n = 14). There were no specific baseline ratios or formulations associated with discontinuing treatment before 4 follow-up visits (P > .05).
DISCUSSION
The results of this study indicate that use of MC to alleviate symptoms of MS is largely efficacious, with improvement in pain (72% of patients), muscle spasticity (48% of patients), and sleep disturbance (40% of patients) frequently reported. These findings are consistent with previously described studies that found that MC significantly improved pain in patients with MS and consistent with a meta-analysis that found that a 1:1 THC to CBD preparation effectively reduced muscle spasticity in patients with MS.20,21,38 Although outside the scope of the present study, it would be beneficial for future studies to capture the specific type of pain being treated by MC (eg, neuropathic pain, musculoskeletal pain) because this information may be helpful to clinicians. It is unclear how much the improvements in pain and spasticity mediated the reported improvements in sleep disturbance, as pain has been associated with decreased sleep quality in patients with MS.39 Resolution of sleep disturbances has been a common finding in studies of MC in patients with MS, even when changes in sleep were not the primary end point.20,21,40
More than half of opioid users at baseline were able to discontinue (n = 11; 22%) or decrease (n = 16; 32%) opioid use after starting MC. The mean daily MME was significantly reduced from the initial visit (51 mg) to the last follow-up visit (40 mg). This is consistent with previous literature showing that MC legalization is associated with decreased opioid use and that MC use is associated with decreased opioid use in patients with chronic pain.41–43 These findings indicate that MC may represent an alternative analgesic to opioids for some patients. It is possible that the opioid-sparing effect of MC is due to synergistic analgesic effects of MC and opioids, a phenomenon that has been previously described.44,45
Patients with MS with higher levels of spasticity are more likely to experience depression, anxiety, fatigue, and pain.46 There was a trend toward decreased concomitant use of muscle relaxants after starting MC, but this was not significant (P = .4). Unfortunately, there is no clear method to convert muscle relaxant use to dose equivalents across different medications. It is possible that analysis of dose amount of muscle relaxants may yield additional insights. There was also a trend toward lower prevalence of concomitant benzodiazepine use after starting MC, but this was not significant (P = .2). When the mean daily LMEs were examined, the change from baseline approached significance (P = .07). A larger sample size of patients with MS using MC in addition to muscle relaxants and/or benzodiazepines could better describe these relationships.
The present study examined the relationship between MC use and T25FW and MoCA/MMSE scores. There was a trend toward decreased T25FW and MoCA/MMSE scores after starting MC, but these relationships were not statistically significant. However, only a small portion of the patient population had scores for these measures recorded in their medical records; only 28 patients could be included in the T25FW analysis and 13 in the MoCA/MMSE analysis due to data availability. Therefore, it is not clear whether these trends are representative of the MS population studied. Furthermore, there are several confounding factors that may have impacted these results; analysis taking these confounders into consideration was outside the scope of this study.
A significant gap in current MC research pertains to optimal dosing regimens and routes of administration. This study provides a description of common MC THC to CBD ratios and formulations used by patients with MS. Findings indicate the need for therapy individualization, as approximately 71% of patients had an MC ratio change, formulation change, and/or addition of MC ratio/formulation PRN. Patients should be monitored closely by their clinical care team when initiating MC therapy to optimize efficacy and minimize AEs.
Fatigue was the most common AE observed in the present study (11% of patients). This finding is similar to a previous study of MC use in patients with MS that found that 14% of patients who received cannabis extracts experienced fatigue.20 Furthermore, there were no severe AEs experienced by patients, and only 3% of patients experienced a moderate AE, indicating that MC was well tolerated. It is important to note that the design of the present study was limited to the information present in each patient’s medical record. Mild AEs may not have been significant enough for the patient and/or provider to record, potentially resulting in underreporting of AEs.
Excluding loss to follow-up, cessation of MC treatment before 4 follow-up visits occurred principally due to cost, followed by lack of efficacy and AEs. Commercial insurance companies do not pay for MC in NYS; therefore, the out-of-pocket cost incurred is prohibitive to many patients. As previously stated, there are no established dosing guidelines for MC in patients with MS, and it is unclear whether patients who did not experience improvement or who experienced AEs may have benefitted from different dosing strategies. A low rate of MC discontinuation due to AEs occurred; of patients who discontinued MC, only 3% did so due to AEs.
A cross-sectional sample of a small subset of patients who completed the ZSRAS and the BDI while treated with MC illustrates that most fell into the normal range on the ZSRAS. No patients experienced marked to severe or extreme anxiety. The mean BDI score of 14.5 falls within the range of “mild mood disturbance.”35 It is not possible to assess causality owing to the absence of pre-MC measures. Nonetheless, it helps to characterize the mental health of patients with MS while using MC. A 2017 study investigated MC and opioid use as it related to anxiety and depression and concluded that patients using MC had lower rates of both mood disorders compared with patients using opioids.47 Depression is a prevalent comorbidity in those with MS, affecting up to 50% of patients.48 Additional controlled, prospective research is needed to elucidate any potential impact of MC on mood and anxiety in patients with MS.
The present findings support the current literature findings that cannabis products have a positive impact in the treatment of several MS-related symptoms.20,21,40 A strength of this study is that it describes the impact of MC on patients with MS in a real-world clinical setting. This study also adds to the current literature by describing different formulations and THC to CBD ratios used by patients with MS. However, this study’s retrospective design is limiting; opportunities for prospective research evaluating MC are restricted by US federal regulations. Another study limitation is that follow-up was restricted to the first 4 clinic visits after MC initiation; further long-term follow-up may yield additional insights. A high rate of loss to follow-up was observed that must be taken into consideration when interpreting the reasons for MC treatment discontinuation and reporting of AEs. Prospective, placebo-controlled studies are needed to explore the efficacy and safety of MC as a treatment for MS symptoms. Structured assessments at predefined intervals would better assess the effect of MC on patients with MS.
This study shows that MC may help decrease chronic pain, muscle spasticity, and sleep disturbances in patients with MS, and patients may also be able to reduce opioid use during MC treatment. Users of MC may require individualized therapy to balance efficacy and AEs. Prospective studies of the effects of MC on MS symptoms and opioid use would be beneficial, and, ultimately, could influence future legislation as it pertains to the legalization of MC in the United States.
PRACTICE POINTS
Medical cannabis may be an effective and well-tolerated adjunctive therapy for patients with multiple sclerosis with chronic pain and muscle spasticity.
Medical cannabis treatment may allow for dose reduction or discontinuation of opioid analgesics in patients with multiple sclerosis.
Cost is a barrier to medical cannabis treatment for many patients.
Supplementary Material
ACKNOWLEDGMENTS:
The authors thank Christopher C. Ralyea Jr, MBA, for reviewing the manuscript; Erica S. Westphal, BA, BS, for assistance in protocol development; and Se Ra Kim, PharmD; Kaitlyn A. Reinhardt, PharmD; Allyson M. Bonnoni, and Rufina Tsur-Tsar, BS, MS, for assistance with data collection.
Funding Statement
FUNDING/SUPPORT: This project was made possible by support from the Harry Dent Family Foundation, Inc.
Footnotes
FINANCIAL DISCLOSURES: Ms McCormack is a speaker for EMD Serono, Biogen, and Genentech. Dr Mechtler has received personal compensation for consulting, serving on a scientific advisory board, speaking, research affiliation, or other activities from Alder Pharmaceuticals, Allergan, Amgen, Avanir, Biohaven, Boston Biomedical, Inc, CellDex, DelMar Pharmaceuticals, electroCore, Jushi, Novartis, Orbis Pharmaceuticals, Promius, and Teva Pharmaceuticals. Dr Mechtler also has a financial interest in Jushi. No Jushi products were used by patients in this study. The other authors declare no conflicts of interest.
PRIOR PRESENTATION: This work was previously presented at the Consortium of Multiple Sclerosis Centers Annual Conference; May 2018; Nashville, Tennessee; at the American Academy of Neurology Annual Meeting; May 2019; Philadelphia, Pennsylvania; and at the College of Psychiatric and Neurologic Pharmacists Annual Meeting; April 2022; San Antonio, Texas.
REFERENCES
- 1.Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol . 2010;9(5):520–532. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 2.Browne P, Chandraratna D, Angood C et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology . 2014;83(11):1022–1024. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallin MT, Culpepper WJ, Campbell JD et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology . 2019;92(10):e1029–e1040. doi: 10.1212/WNL.0000000000007035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med . 2020;133(12):1380–1390.e2. doi: 10.1016/j.amjmed.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olek MJ, Narayan RN, Frohman EM, Frohman TC. Symptom management of multiple sclerosis in adults. UpToDate. Accessed July 17, 2021. https://www.uptodate.com/contents/symptom-management-of-multiple-sclerosis-in-adults. [Google Scholar]
- 6.Rae-Grant A, Day GS, Marrie RA et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology . 2018;90(17):777–788. doi: 10.1212/WNL.0000000000006722. [DOI] [PubMed] [Google Scholar]
- 7.Olek MJ. Multiple sclerosis. Ann Intern Med . 2021;174(6):ITC81–ITC96. doi: 10.7326/AITC202106150. [DOI] [PubMed] [Google Scholar]
- 8.Thelen J, Zvonarev V, Lam S, Burkhardt C, Lynch S, Bruce J. Polypharmacy in multiple sclerosis: current knowledge and future directions. Mo Med . 2021;118(3):239–245. [PMC free article] [PubMed] [Google Scholar]
- 9.Hugos CL, Joos S, Sajeev N, Norton J, Samiee S, Cameron M. One in five (20%) people with multiple sclerosis use prescription opioids. Neurology . 2021;96(15 Supplement):4926. [Google Scholar]
- 10.Hadjimichael O, Kerns RD, Rizzo MA, Cutter G, Vollmer T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain . 2007;127(1–2):35–41. doi: 10.1016/j.pain.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Lewis MM, Yang Y, Wasilewski E, Clarke HA, Kotra LP. Chemical profiling of medical cannabis extracts. ACS Omega . 2017;2(9):6091–6103. doi: 10.1021/acsomega.7b00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberbarnscheidt T, Miller N. Pharmacology of marijuana. J Addict Res Ther . 2017;S11:012. doi: 10.4172/2155-6105.1000S11-012. [DOI] [Google Scholar]
- 13.Costa B. On the pharmacological properties of Δ9-tetrahydrocannabinol (THC) Chem Biodivers . 2007;4(8):1664–1677. doi: 10.1002/cbdv.200790146. [DOI] [PubMed] [Google Scholar]
- 14.Brown AJ. Novel cannabinoid receptors. Br J Pharmacol . 2007;152(5):567–575. doi: 10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mlost J, Bryk M, Starowicz K. Cannabidiol for pain treatment: focus on pharmacology and mechanism of action. Int J Mol Sci . 2020;21(22):8870. doi: 10.3390/ijms21228870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devinsky O, Cilio MR, Cross H et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia . 2014;55(6):791–802. doi: 10.1111/epi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage . 2010;39(2):167–179. doi: 10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen S, Germanos R, Weier M et al. The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: a systematic review of reviews. Curr Neurol Neurosci Rep . 2018;18(2):8. doi: 10.1007/s11910-018-0814-x. [DOI] [PubMed] [Google Scholar]
- 19.Koppel BS, Brust JC, Fife T et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology . 2014;82(17):1556–1563. doi: 10.1212/WNL.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG;, MUSEC Research Group Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry . 2012;83(11):1125–1132. doi: 10.1136/jnnp-2012-302468. [DOI] [PubMed] [Google Scholar]
- 21.Zajicek J, Fox P, Sanders H et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet . 2003;362(9395):1517–1526. doi: 10.1016/S0140-6736(03)14738-1. [DOI] [PubMed] [Google Scholar]
- 22.AbbVie Inc; 2017. Marinol. Prescribing information. Accessed December 2, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf. [Google Scholar]
- 23.Valeant Pharmaceuticals International; 2006. Cesamet. Prescribing information. Accessed December 2, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2006/018677s011lbl.pdf. [Google Scholar]
- 24.Jazz Pharmaceuticals, Inc; 2022. Epidiolex. Prescribing information. Accessed December 2, 2022. https://pp.jazzpharma.com/pi/epidiolex.en.USPI.pdf. [Google Scholar]
- 25.GW Pharma Ltd; 2019. Sativex. Product monograph. Accessed December 2, 2022. https://www.bayer.com/sites/default/files/2020-11/sativex-pm-en.pdf. [Google Scholar]
- 26.Abernethy A. Hemp production and the 2018 Farm Bill. Updated July 25, 2019. Accessed January 12, 2022. https://www.fda.gov/news-events/congressional-testimony/hemp-production-and-2018-farm-bill-07252019.
- 27.Summary of Medical Cannabis Data Management System (MCDMS) enhancements. New York State Office of Cannabis Management. January 25, 2022. Accessed January 12, 2022. https://cannabis.ny.gov/system/files/documents/2022/01/Summary_MCDMS_Enhancements.pdf.
- 28. Legal recreational marijuana states and DC. ProCon/Encyclopaedia Britannica Inc. Updated June 22, 2021. Accessed July 20, 2021. https://www.procon.org/legal-recreational-marijuana-states-and-dc.
- 29.Bethoux FA, Palfy DM, Plow MA. Correlates of the Timed 25 Foot Walk in a multiple sclerosis outpatient rehabilitation clinic. Int J Rehabil Res . 2016;39(2):134–139. doi: 10.1097/MRR.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosca EC, Simu M. Montreal cognitive assessment for evaluating cognitive impairment in multiple sclerosis: a systematic review. Acta Neurol Belg . 2020;120(6):1307–1321. doi: 10.1007/s13760-020-01509-w. [DOI] [PubMed] [Google Scholar]
- 31.Naranjo CA, Busto U, Sellers EM et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther . 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 32. LiverTox Clinical and Research Information on DrugInduced Liver Injury . National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Adverse Drug Reaction Probability Scale (Naranjo) in drug induced liver injury. [PubMed] [Google Scholar]
- 33. Morphine milligram equivalents (MME) calculator. MDCalc. Accessed September 13, 2021. https://www.mdcalc.com/morphine-milligram-equivalents-mme-calculator.
- 34. Benzodiazepine conversion calculator. MDCalc. Accessed September 13, 2021. https://www.mdcalc.com/benzodiazepine-conversion-calculator.
- 35.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry . 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 36.Zung WW. From art to science: the diagnosis and treatment of depression. Arch Gen Psychiatry . 1973;29(3):328–337. doi: 10.1001/archpsyc.1973.04200030026004. [DOI] [PubMed] [Google Scholar]
- 37.van Steenoven I, Aarsland D, Hurtig H et al. Conversion between Mini-Mental State Examination, Montreal Cognitive Assessment, and Dementia Rating Scale-2 scores in Parkinson’s disease. Mov Disord . 2014;29(14):1809–1815. doi: 10.1002/mds.26062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wade DT, Collin C, Stott C, Duncombe P. Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler . 2010;16(6):707–714. doi: 10.1177/1352458510367462. [DOI] [PubMed] [Google Scholar]
- 39.Bøe Lunde HM, Aae TF, Indrevåg W et al. Poor sleep in patients with multiple sclerosis. PLoS One . 2012;7(11):e49996. doi: 10.1371/journal.pone.0049996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinkle L, Domen CH, Shelton I, Sillau S, Nair K, Alvarez E. Exploring cannabis use by patients with multiple sclerosis in a state where cannabis is legal. Mult Scler Relat Disord . 2019;27:383–390. doi: 10.1016/j.msard.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Boehnke KF, Litinas E, Clauw DJ. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain . 2016;17(6):739–744. doi: 10.1016/j.jpain.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Shah A, Hayes CJ, Lakkad M, Martin BC. Impact of medical marijuana legalization on opioid use, chronic opioid use, and high-risk opioid use. J Gen Intern Med . 2019;34(8):1419–1426. doi: 10.1007/s11606-018-4782-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lake S, Walsh Z, Kerr T et al. Frequency of cannabis and illicit opioid use among people who use drugs and report chronic pain: a longitudinal analysis. PLoS Med . 2019;16(11):e1002967. doi: 10.1371/journal.pmed.1002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Cowan A, Inan S et al. Opioid-sparing effects of cannabinoids on morphine analgesia: participation of CB1 and CB2 receptors. Br J Pharmacol . 2019;176(17):3378–3389. doi: 10.1111/bph.14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiese B, Wilson-Poe AR. Emerging evidence for cannabis’ role in opioid use disorder. Cannabis Cannabinoid Res . 2018;3(1):179–189. doi: 10.1089/can.2018.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milinis K, Tennant A, Young C, Group TS. Spasticity in multiple sclerosis: associations with impairments and overall quality of life. Mult Scler Relat Disord . 2016;5:34–39. doi: 10.1016/j.msard.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Feingold D, Brill S, Goor-Aryeh I, Delayahu Y, Lev-Ran S. Depression and anxiety among chronic pain patients receiving prescription opioids and medical marijuana. J Affect Disord . 2017;218:1–7. doi: 10.1016/j.jad.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 48.Feinstein A. Multiple sclerosis and depression. Mult Scler . 2011;17(11):1276–1281. doi: 10.1177/1352458511417835. [DOI] [PubMed] [Google Scholar]
- 49. CDC clarifies opioid guideline dosage thresholds. American Academy of Family Physicians. January 12, 2018. Accessed December 15, 2021. https://www.aafp.org/news/health-of-the-public/20180112cdcopioidclarify.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


