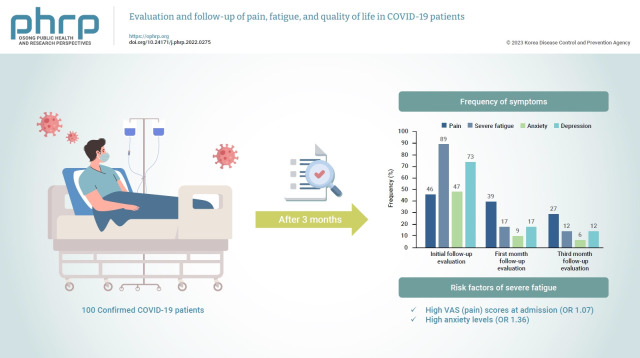
Abstract
Objectives
We evaluated pain, fatigue, anxiety, depression, and quality of life in patients hospitalized for coronavirus disease 2019 (COVID-19) and observed them over a period of 3 months. We also investigated the relationship of these symptoms to age, sex, disease severity, and levels of anxiety and depression.
Methods
The study included 100 confirmed COVID-19 patients (i.e., positive on a polymerase chain reaction test) between the ages of 18 and 75 years. Pain (visual analog scale [VAS]), fatigue (fatigue severity scale), anxiety, and depression (hospital anxiety and depression scales) were evaluated on the first day of hospitalization and at 1-month and 3-month follow-ups. The short form-12 questionnaire was used to measure quality of life at the 1-month and 3-month follow-ups.
Results
No differences were found in pain, fatigue, anxiety levels, depression levels, and quality of life according to disease severity. High VAS scores at hospital admission were related to continued pain at the 3-month follow-up (odds ratio [OR], 1.067; p<0.001). High VAS (OR, 1.072; p=0.003) and anxiety levels (OR, 1.360; p=0.007) were related to severe fatigue at the 3-month evaluation.
Conclusion
Pain, fatigue, anxiety, and depression appear to be long-term sequelae of COVID-19 and can affect quality of life. High VAS and anxiety levels were found to be associated with long-term fatigue.
Keywords: COVID-19, Fatigue, Pain
Graphical abstract
Introduction
On February 11, 2020, the World Health Organization (WHO) named a new disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), coronavirus disease 2019 (COVID-19), and declared the COVID-19 pandemic on March 11, 2020 [1]. Since then, the virus has spread rapidly around the world. As of June 2022, more than 535 million people had been infected with COVID-19, and 6 million had died [2].
COVID-19 can affect all age groups, from infants to the elderly, and manifests as a viral disease with a wide range of clinical symptoms, of which fatigue, fever, and cough are the most common [3]. The most frequent musculoskeletal symptoms are myalgia, arthralgia, back pain, and fatigue, which can be seen from the onset of symptoms through the most severe stages of the disease [4‒6]. Symptoms such as fatigue and dyspnea have been shown to persist beyond the acute stage to long after discharge from the hospital, emphasizing the importance of long-term follow-up and the rehabilitation therapy needs of COVID-19 patients [7]. References to long-term COVID or post-COVID syndrome have come to the fore. The National Institute for Health and Care Excellence defines post-COVID syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19 that last more than 12 weeks and cannot be explained by another diagnosis” [8].
The current literature supports the premise that pain in COVID-19 patients is due, at least in part, to the neurotropism of SARS-CoV-2. Pain can be caused by the interaction of the virus with the angiotensin-converting enzyme isoform 2 receptors in spinal neurons and microglia, by immune system-mediated inflammation, or by viral damage [9].
Pain and fatigue dramatically affect quality of life. In one study, the quality of life was found to be worse in COVID-19 patients with persistent pain [10], but the factors associated with these persistent symptoms were unclear. In this study, we evaluated musculoskeletal symptoms such as pain and fatigue, levels of anxiety and depression, and the quality of life in patients hospitalized for COVID-19 and followed them for 3 months. We also investigated how their symptoms were linked to age, sex, disease severity, and levels of anxiety and depression.
Materıals and Methods
Participants
This was a single-center cohort study that included confirmed COVID-19 (i.e., polymerase chain reaction positive) patients between ages 18 and 75 years who were hospitalized at the Afyonkarahisar Health Sciences University Hospital, Pandemic Service between February15, 2021 and July 15, 2021 and voluntarily agreed to participate.
We excluded patients who were unwilling, illiterate, or pregnant and patients with a history of chronic pain or fatigue during the past 6 months (before COVID-19). We also excluded critically ill patients with respiratory failure requiring mechanical ventilation, shock, or organ failure requiring admission to the intensive care unit (ICU). Patients who were later taken to the ICU or who could not be reached during the follow-up period were excluded.
A total of 242 patients were hospitalized with a diagnosis of COVID-19 at the Afyonkarahisar Health Sciences University Hospital between February 1, 2021 and July 1, 2021. Forty-two patients were >75 years old, 19 did not want to participate, 26 had chronic fatigue and pain symptoms, 8 were illiterate, and 6 were pregnant. Thus, 141 patients were assessed for eligibility. Of the 141 patients, 21 could not be reached for follow-up by telephone, 5 had communication problems due to language differences, and 15 were transferred to the ICU. Therefore, the remaining 100 patients were contacted and completed the questionnaire (Figure 1).
Figure 1.
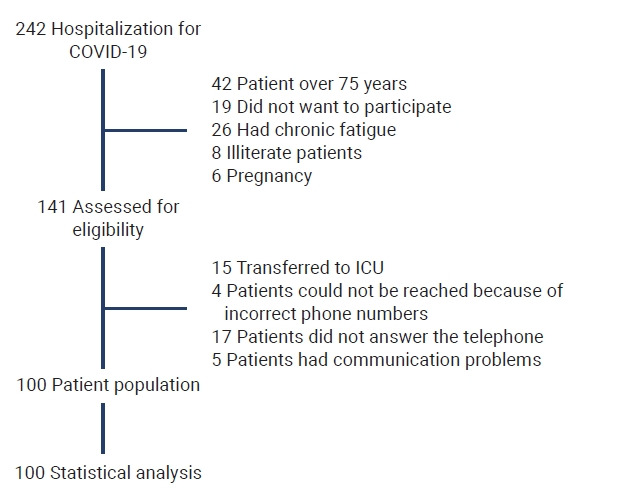
Diagram of patient sample selection and exclusions (boxes on the right).
COVID-19, coronavirus disease 2019; ICU, intensive care unit.
Assessments
Pain
For the pain assessment, we used the visual analog scale (VAS), which is easy to understand, apply, and interpret. It provides valid and reliable data in a short time. Using a visual linear scale of 100 mm, the patient was told that point 0 represented no pain and point 100 represented the most severe pain they had ever felt in their life. The patient was asked to put a mark on the point corresponding to his/her pain. Patients who marked a VAS score of more than 4 mm were considered to have pain [11]. In addition, we determined the location of their pain (neck, back, low back, upper extremity, lower extremity).
Fatigue
Fatigue was evaluated using the Turkish version of the fatigue severity scale (FSS). The FSS consists of 9 items, and each item is scored on a 7-point scale from 1 to 7 (1, strongly disagree; 7, strongly agree). The total score is calculated by deriving the arithmetic mean. In our study, patients were considered to have severe fatigue if the total FSS score was ≥4 [12].
Anxiety and depression
We used the hospital anxiety and depression scale (HADS) to determine the risk of anxiety and depression in patients and to determine its level. The HADS consists of 2 subscales to evaluate anxiety and depression, and the cutoff scores in the Turkish version are 10 for the anxiety subscale and 7 for the depression subscale [13].
Quality of life
The short form-12 (SF-12) was used to evaluate patients’ quality of life. The SF-12, a shortened and simplified version of the SF-36, is an easy-to-administer questionnaire. It assesses the 2 main components of general health, physical and mental health, and consists of 12 questions. High scores indicate good health.
Patients’ demographic information was obtained including comorbidities, initial COVID-19 symptoms, vaccination status, and drugs used by the patient. Pain, fatigue, anxiety, and depression were evaluated on the first day of hospitalization (initial) and at the 1-month and 3-month follow-ups. The 1-month and 3-month patient evaluations were conducted over the telephone. In these phone interviews, answers to the SF-12 questionnaire were used to measure the patients’ quality of life, pain, fatigue, anxiety, and depression.
A patient’s COVID-19 illness was classified as mild, moderate, or severe according to the severity of their symptoms. Levels of pain, fatigue, anxiety, depression, and quality of life were compared according to disease severity. The disease severity classification was determined based on the clinical management guidelines for COVID-19 published by the WHO. Patients without evidence of pneumonia or hypoxia were classified as mild cases. Patients with clinical signs and symptoms of pneumonia but no signs of severe pneumonia and no need for oxygen were classified as moderate cases. Patients with signs and symptoms of pneumonia, a respiratory rate of more than 30 breaths per minute, severe respiratory distress, or a saturated oxygen level in room air of <90% were considered severe cases [14].
The correlations between symptoms and quality of life were investigated. In addition, the factors that could predict pain and fatigue at the 3-month follow-up were assessed.
Statistical Analysis
The statistical analysis was performed using IBM SPSS ver. 20.0 (IBM Corp.). The distribution of continuous variables was evaluated using the Shapiro-Wilk test. For descriptive statistics, the number of units, percent, and median (range) values were given. For comparisons of 2 independent groups using nonparametric data, the Mann-Whitney U-test was applied. For a comparison of 3 independent groups using nonparametric data, the Kruskal-Wallis test was used. A Spearman correlation analysis was used to evaluate the associations between quantitative variables. Prior to logistic regression analysis, we used the chi-square test, Student t-test, and analysis of variance to identify factors related to long-term pain and fatigue with p<0.1. These factors were then analyzed using logistic regression analysis. A p-value less than 0.05 was considered to indicate statistical significance.
Ethical Approval and Ministry Permission
We received approval for this study from the Afyonkarahisar Health Sciences University, Clinical Research Ethics Committee (January 8, 2021; Protocol 2021/57), as well as permission from the Ministry of Health of the Republic of Turkey. The ClinicalTrials.gov identifier number for the study is NCT04454333. The study was conducted following the principles of the Declaration of Helsinki. Written informed consent for the study was obtained.
Results
The patients’ demographic data, comorbidities, initial symptoms, pain sites, drugs used, and vaccination status are presented in Table 1. The frequencies of pain, severe fatigue, anxiety, and depression are shown in Figure 2.
Table 1.
Demographic information, comorbidities, initial symptoms, pain sites, medications, and vaccination status of patients hospitalized for COVID-19
| Variable | Value (n=100) |
|---|---|
| Age (y), mean±SD (range) | 53.45±12.76 (20‒75) |
| Sex (male:female) | 49:51 |
| Education | |
| No formal education | 3 |
| Primary school | 39 |
| High school | 33 |
| University | 25 |
| Comorbidities | |
| None | 31 |
| Hypertension | 5 |
| Diabetes mellitus | 10 |
| Coronary artery disease | 1 |
| Chronic respiratory disease | 1 |
| Other | 24 |
| Multiple | 28 |
| Initial symptoms | |
| Fever | 34 |
| Cough | 65 |
| Sore throat | 21 |
| Headache | 26 |
| Musculoskeletal pain | 46 |
| Fatigue | 63 |
| Loss of taste and smell | 8 |
| Pain sites | |
| Cervical | 66 |
| Back | 77 |
| Low back | 55 |
| Upper extremity | 64 |
| Lower extremity | 68 |
| Analgesic | |
| NSAID | 43 |
| Paracetamol | 35 |
| Opioids | 3 |
| None | 14 |
| Drug | |
| Favipiravir | 93 |
| None | 7 |
| Corticosteroids | 69 |
| Vaccination status | |
| None | 75 |
| 1 Dose Sinovaca) | 4 |
| 2 Doses Sinovac | 15 |
| 1 Dose BioNTechb) | 1 |
| 2 Doses BioNTech | 1 |
| 2 Doses Sinovac, 1 dose BioNTech | 3 |
| 2 Doses Sinovac, 2 doses BioNTech | 1 |
COVID-19, coronavirus disease 2019; n, number of patients; SD, standard deviation; NSAID, nonsteroidal anti-inflammatory drug.
CoronaVac,
mRNA BNT162b2.
Figure 2.
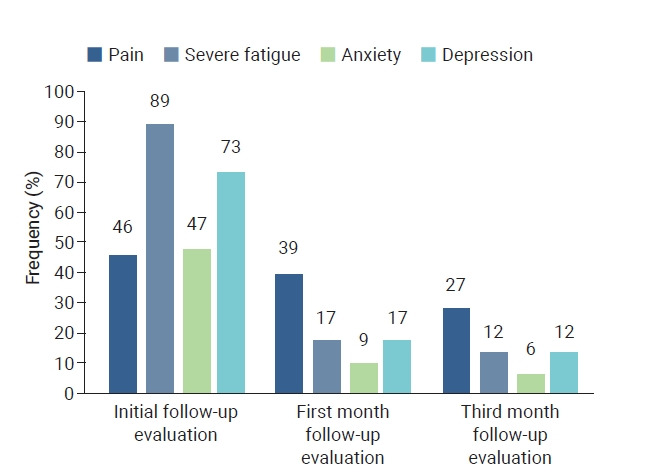
Frequency of symptoms at initial, 1-month, and 3-month follow-up evaluations.
The mean VAS, FSS, HADS-anxiety, HADS-depression, and SF-12 scores for female and male patients at their initial, 1-month, and 3-month follow-up evaluations are given in Table 2. The VAS score was higher in females than in males in both the 1-month and 3-month follow-ups (p=0.040, p=0.007, respectively). The mean VAS, FSS, HADS-anxiety, HADS-depression, and SF-12 scores showed statistically significant changes at 1 month and 3 months, respectively (Table 2).
Table 2.
Mean VAS, FSS, HADS-anxiety, HADS-depression, and SF-12 scores at initial, 1-month, and 3-month follow-up evaluations in female and male COVID-19 patients
| Evaluation time | Male (n=49) | Female (n=51) | p |
|---|---|---|---|
| Age (y) | 54.49±13.27 | 52.45±12.31 | 0.42 |
| VAS | |||
| Initial | 24.48±32.58a) | 37.64±37.04a) | 0.071 |
| 1 Month | 11.22±18.1 | 20.39±23.36b) | 0.040 |
| 3 Months | 2.75±8.35b) | 8.33±12.35c) | 0.007 |
| p* | <0.001 | <0.001 | |
| FSS | |||
| Initial | 4.99±1.88a) | 5.11±1.55a) | 0.825 |
| 1 Month | 3.32±1.83b) | 3.54±1.77b) | 0.508 |
| 3 Months | 2.38±1.85c) | 2.71±1.63c) | 0.229 |
| p* | <0.001 | <0.001 | |
| HADS-anxiety | |||
| Initial | 9.93±5.19a) | 9.92±5.29a) | 0.975 |
| 1 Month | 4.18±.08b) | 4.76±4.28b) | 0.390 |
| 3 Months | 2.42±3.98c) | 2.76±3.78c) | 0.409 |
| p* | <0.001 | <0.001 | |
| HADS-depression | |||
| Initial | 10.28±5.88a) | 10.27±5.83a) | 0.981 |
| 1 Month | 3.95±4.43b) | 4.29±4.15b) | 0.413 |
| 3 Months | 2.87±5.16c) | 2.49±3.67c) | 0.243 |
| p* | <0.001 | <0.001 | |
| SF-12 | |||
| 1 Month | 74.57±15.71 | 75.31±15.03 | 0.970 |
| 3 Months | 86.83±16.36 | 87.68±15.58 | 0.869 |
| p * | <0.001 | <0.001 |
Data are presented as mean±standard deviation.
VAS, visual analog scale (for measuring pain); FSS, fatigue severity scale; HADS, hospital anxiety depression scale; SF-12, short form-12 (quality of life questionnaire); COVID-19, coronavirus disease 2019.
Different letters in the same column indicate within-group differences.
Significance level of intragroup data; p-values <0.05 indicate statistical significance.
There were no differences in pain severity, fatigue, anxiety, or depression according to disease severity (p>0.05). When males and females were examined separately, the 3-month VAS value in the moderate disease category was significantly higher in females than in males (p=0.009). In patients with severe disease, anxiety and depression scores were significantly higher in females than in males at the 3-month follow-up (p=0.038, p=0.010, respectively) (Table 3).
Table 3.
Comparison of fatigue, anxiety, depression, and quality of life according to disease severity and sex
| Variable | Evaluation time | Mild disease (n=43) |
Moderate disease (n=47) |
Severe disease (n=10) |
p | |||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |||
| Age (y) | Initial | 49.8±13.3 | 46.8±12.0 | 56.4±12.2 | 55.4±10.7 | 63.8±11.9 | 65.0±9.8 | 0.001 |
| p a) | 0.458 | 0.864 | 0.914 | 0.041b) | ||||
| 0.003c) | ||||||||
| 0.188d) | ||||||||
| VAS | Initial | 17.1±28.9 | 26.6±33.5 | 28.6±33.7 | 44.2±38.2 | 35.0±40.7 | 57.5±39.5 | 0.056 |
| p a) | 0.324 | 0.134 | 0.476 | |||||
| 1 Month | 8.8±16.9 | 11.6±18.2 | 12.7±20.1 | 25.4±23.8 | 14.2±16.3 | 37.5±33.0 | 0.074 | |
| p a) | 0.591 | 0.067 | 0.257 | |||||
| 3 Months | 2.9±7.8 | 3.9±10.2 | 3.4±9.9 | 11.0±11.9 | 0.0±0.0 | 16.3±19.7 | 0.115 | |
| p a) | 0.968 | 0.009 | 0.257 | |||||
| FSS | Initial | 4.2±2.1 | 4.9±1.5 | 5.7±1.5 | 5.1±1.7 | 5.3±1.7 | 6.0±1.0 | 0.129 |
| p a) | 0.285 | 0.55 | &0.999 | |||||
| 1 Month | 2.8±1.7 | 3.2±1.9 | 3.7±1.8 | 3.6±1.6 | 3.8±2.0 | 5.1±1.1 | 0.054 | |
| p a) | 0.401 | 0.685 | 0.352 | |||||
| 3 Months | 2.8±1.8 | 2.3±1.6 | 2.6±1.8 | 2.8±1.5 | 2.9±2.1 | 4.7±1.1 | 0.035e) | |
| p a) | 0.307 | 0.765 | 0.171 | |||||
| HADS-anxiety | Initial | 8.7±5.2 | 9.1±6.0 | 11.4±4.9 | 10.2±4.7 | 9.0±5.9 | 12.8±5.1 | 0.185 |
| p a) | 0.836 | 0.441 | 0.476 | |||||
| 1 Month | 3.7±3.4 | 4.4±5.2 | 4.6±4.8 | 4.6±3.4 | 4.3±3.9 | 8.3±2.2 | 0.149 | |
| p a) | 0.941 | 0.499 | 0.114 | |||||
| 3 Months | 2.0±2.6 | 2.4±4.6 | 3.1±5.2 | 2.6±3.1 | 1.7±2.7 | 5.8±1.5 | 0.208 | |
| p a) | 0.503 | 0.445 | 0.038 | |||||
| HADS-depression | Initial | 9.4±5.2 | 9.2±7.1 | 11.4±6.2 | 10.6±4.5 | 9.5±7.4 | 13.8±5.3 | 0.197 |
| p a) | 0.51 | 0.732 | 0.476 | |||||
| 1 Month | 3.2±3.5 | 3.9±4.5 | 4.9±5.2 | 4.0±3.8 | 3.3±4.5 | 8.0±2.8 | 0.171 | |
| p a) | 0.795 | 0.966 | 0.067 | |||||
| 3 Months | 2.8±5.4 | 2.3±4.3 | 3.6±5.5 | 2.3±3.3 | 0.5±1.2 | 5.0±1.2 | 0.243 | |
| p a) | 0.826 | 0.991 | 0.010 | |||||
| SF-12 | 1 Month | 78.6±14.7 | 79.2±17.4 | 71.5±16.6 | 73.9±12.7 | 71.6±15.2 | 62.5±3.6 | 0.035e) |
| p a) | 0.894 | 0.685 | 0.476 | |||||
| 3 Months | 90.0±15.1 | 88.5±19.0 | 84.7±18.3 | 88.5±12.6 | 83.4±13.3 | 78.4±10.9 | 0.168 | |
| p a) | 0.903 | 0.654 | 0.762 | |||||
Data are presented as mean±standard deviation.
VAS, visual analog scale for measuring pain; FSS, fatigue severity scale; HADS, hospital anxiety depression scale; SF-12, short form-12; p, significance level of data between mild, moderate and severe disease groups without distinction between male and female.
Significance level of the comparison of the mean of male and female within the group;
significance value of the difference between the mean age of patients with mild and moderate disease;
significance value of the difference between the mean age of patients with mild and severe disease;
significance value of the difference between the mean age of patients with moderate and severe disease;
There was no difference between groups in post hoc analyses.
In the initial evaluations, VAS values and fatigue levels showed a moderately positive correlation (p<0.001, r=0.38). In the long-term follow-up at 3 months, the correlation continued (p<0.001, r=0.35).
A weak correlation was found between the severity of depression symptoms and the VAS scores (p=0.007, r=0.27). In the long-term (3-month) follow-up, the correlation was not maintained (p=0.058).
In the evaluations done at hospital admission and at 3 months, moderately positive correlations between fatigue and depression symptoms (p<0.001; r=0.52, r=0.35, respectively) and between fatigue and anxiety symptoms (p<0.001; r=0.61, r=0.44, respectively) were detected.
SF-12 scores were negatively and weakly correlated with VAS scores at 3 months (p<0.001, r=0.261). SF-12 scores showed moderately negative correlations with fatigue (r=0.607), depression (r=0.538), and anxiety (r=0.564) at 3 months.
Comparisons of age; sex; presence of comorbidities; and VAS, FSS, HADS-anxiety, and HADS-depression scores in patients with and without pain in the third month are shown in Table 4. Sex (p=0.018), initial VAS values (p<0.001), HADS-depression scores at 3 months (p=0.002), and FSS scores at3 months) (p<0.001) were statistically different between the patients with and without pain at 3 months (Table 4). Next, we analyzed sex, initial VAS values, HADS-depression scores (3-month), and FSS scores (3-month) using logistic regression analysis and found that high VAS values at hospital admission were predictive of pain in the 3-month follow-up (odds ratio [OR], 1.067; p<0.001) (Table 5).
Table 4.
Factors associated with the presence of pain at the 3-month evaluation of COVID-19 patients
| Variable | Patients with pain in the third month |
Patients without pain in the third month |
p a) | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Age (y) | 53.4±12.7 | 53.1±9.5 | 54.7±13.5 | 52.1±13.8 | 0.727 |
| p | 0.897 | 0.443 | |||
| Comorbidity | |||||
| Yes | 0.238 | 0.762 | 0.521 | 0.479 | 0.248 |
| No | 0.5 | 0.5 | 0.64 | 0.36 | |
| p | 0.215 | 0.330 | |||
| VAS (initial) | 69.3±12.9 | 72.8±12.7 | 15.7±27.6 | 16.7±30.1 | <0.001 |
| p | 0.938 | 0.966 | |||
| FSS (3 mo) | 3.9±1.4 | 3.5±1.7 | 2.0±1.7 | 2.2±1.3 | <0.001 |
| p | 0.559 | 0.376 | |||
| HADS-anxiety (3 mo) | 4.1±4.8 | 2.6±2.4 | 2.1±3.7 | 2.8±4.4 | 0.034 |
| p | 0.658 | 0.596 | |||
| HADS-depression (3 mo) | 6.8±8.2 | 2.6±1.9 | 2.1±4.4 | 2.3±4.4 | 0.002 |
| p | 0.585 | 0.753 | |||
Data are presented as mean±standard deviation.
COVID-19, coronavirus disease 2019; VAS, visual analog scale for measuring pain; FSS, fatigue severity scale; HADS, hospital anxiety depression scale.
Significance value of the parameters compared without male-female grouping between patients with and without pain at the third month.
Table 5.
Multivariate logistic regression model for variables associated with pain at the 3-month follow-up
| Variable | OR (95% CI) | p |
|---|---|---|
| Sex | 1.243 (0.869−16.943) | 0.076 |
| VAS (initial) | 1.067 (1.032−1.102) | <0.001 |
| FSS (3 mo) | 1.377 (0.854−2.222) | 0.190 |
| HADS-anxiety (3 mo) | 0.792 (0.562−1.116) | 0.182 |
| HADS-depression (3 mo) | 1.243 (0.923−1.673) | 0.152 |
OR, odds ratio; CI, confidence interval; VAS, visual analog scale for measuring pain; FSS, fatigue severity scale; HADS, hospital anxiety depression scale.
Comparisons of age, sex, and presence of comorbidities, as well as VAS, FSS, HADS-anxiety, and HADS-depression scores in patients with and without pain in the third month are given in Table 6. VAS (3-month, p=0.001), HADS-anxiety (3-month, p<0.001), and HADS-depression (3-month, p<0.001) scores showed a statistically significant difference between patients with and without severe fatigue in the third month. We then analyzed VAS (3 months), HADS-anxiety (3 months), and HADS-depression (3 months) scores using logistic regression analysis and found that high VAS scores (OR, 1.072; p=0.003) and anxiety levels (OR, 1.360; p=0.007) were predictive of severe fatigue in the third month (Table 7).
Table 6.
Factors associated with the presence of severe fatigue at the 3-month evaluation of COVID-19 patients
| Variable | Patients with severe fatigue in the third month |
Patients without severe fatigue in the third month |
p a) | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Age (y) | 53.2±11.8 | 51.6±12.7 | 55.2±14.1 | 53.2±12.2 | 0.381 |
| p | 0.753 | 0.5 | |||
| Comorbidity | |||||
| Yes (%) | 39.3 | 60.7 | 46.3 | 53.7 | 0.860 |
| No (%) | 50.0 | 50.0 | 68.4 | 31.6 | |
| p | 0.530 | 0.111 | |||
| VAS (3 mo) | 5±11.2 | 13.7±14.7 | 1.6±6.3 | 3.9±7.9 | 0.001 |
| p | 0.051 | 0.108 | |||
| HADS-anxiety (3 mo) | 4.4±5.5 | 4.3±4.4 | 1.4±2.4 | 1.5±2.7 | <0.001 |
| p | 0.761 | 0.653 | |||
| HADS-depression (3 mo) | 4.6±5.7 | 4±4.5 | 1.9±4.7 | 1.3±2.3 | <0.001 |
| p | >0.999 | 0.343 | |||
Data are presented as mean±standard deviation.
VAS, visual analog scale for measuring pain; HADS, hospital anxiety depression scale.
Significance value of the parameters compared without male-female grouping between patients with and without severe fatigue at the third month.
Table 7.
Multivariate logistic regression model for variables associated with severe fatigue at the 3-month follow-up evaluation of COVID-19 patients
| Variable | OR (95% CI) | p |
|---|---|---|
| VAS (3 mo) | 1.072 (1.024−1.123) | 0.003 |
| HADS-anxiety (3 mo) | 1.360 (1.088−1.701) | 0.007 |
| HAD-depression (3 mo) | 0.918 (0.767−1.098) | 0.349 |
COVID-19, coronavirus disease 2019; OR, odds ratio; CI, confidence interval; VAS, visual analog scale for measuring pain; HADS, hospital anxiety depression scale.
Discussion
Arthralgia and myalgia are not unique to COVID-19 and are common symptoms of flu-like syndromes [15]. Myalgia is seen in up to 50% of COVID-19 patients [16]. We found the frequency of pain to be 46% in hospitalized COVID-19 patients. In a Turkish study, the most common pain symptoms were myalgia and arthralgia (69.2%) [17]. In another study, 159 patients reported at least 1 type of pain syndrome, with a prevalence of 71.6% [18]. Hoong et al. [19] reported that 30% of 294 hospitalized COVID-19 patients reported musculoskeletal symptoms. Of these, 88 patients (37.5%) had myalgia, 5.7% had arthralgia, 6.8% had new-onset back pain, and 50% had diffuse body pain. In our study, a detailed pain examination was conducted according to regions of the body, and back pain was found to be the most prevalent site (77%). This was followed by lower extremity pain (68%), neck pain (66%), upper extremity pain (64%), and low back pain (55%).
In a study of 379 patients in our hospital, the frequency of myalgia was found to be higher among patients in the ICU than on the ward [20]. Studies have reported that the frequency of pain increases as the severity of the disease increases. For example, in one study, low lymphocyte and high D-dimer levels, the presence of back pain, computed tomography findings of COVID-19, longer hospital stays, and chronic disease were associated with post-COVID-19 musculoskeletal symptoms [21]. Disser et al. [16] concluded that myalgia could be a predictive factor in determining overall disease severity in patients with abnormal chest computed tomography and radiography findings. Furthermore, Tuzun et al. [22] reported a higher incidence of arthralgia in patients with severe COVID-19, whereas Hoong et al. [19] concluded that the presence of musculoskeletal symptoms was not related to the risk of developing pneumonia. In our study, there were no significant differences in VAS scores according to disease severity. As far as we know, our study is the first to evaluate the presence and severity of pain in COVID-19 patients using the VAS. Unlike other studies, our study investigated whether the severity of pain, rather than the subjective presence of pain, changed according to the severity of the disease. Pain severity did not differ significantly between groups based on disease severity. Myalgia is a common symptom in COVID-19. Its presence may vary according to the severity of the disease. However, there is not enough evidence to conclude that the severity of myalgia increases according to the severity of the disease.
Studies have shown that symptoms such as myalgia, anxiety, and fatigue are more persistent in females than in males in long-term follow-ups [23,24]. More fatigue, pain, anxiety, and depression were reported in female patients [25]. In our study, we also found that the severity of pain was higher in females, although anxiety and fatigue were similar in both sexes. Understanding why pain is more common and more severe in females requires further research.
Although we found that pain persisted, it decreased from an initial rate of 46% to 27% at the 3-month follow-up. It has been reported in the literature that symptoms of COVID-19 can persist for up to 35 days and that approximately 10% of those infected with COVID-19 will suffer from musculoskeletal symptoms for some time within the first year [26,27]. The study by Karaarslan et al. [23] showed that approximately 2 out of 5 patients had at least 1 musculoskeletal symptom at 6 months. Fatigue, arthralgia, and myalgia are the most common musculoskeletal symptoms, both acutely and long-term.
Fatigue, muscle weakness, shortness of breath, pain and discomfort, anxiety and depression, and impaired concentration have been shown to persist in more than 20% of patients with post-COVID syndrome, up to 47% in some studies [28]. In one systematic review the prevalence of post-COVID syndrome reached 80% and symptoms, particularly fatigue, persisted for up to 24 weeks [29]. According to a large Chinese longitudinal cohort study, most COVID-19 survivors had a significant improvement at the 1-year follow-up. Although improved, these patients still had more problems with movement, pain, anxiety, or depression than the control group [30].
Research to optimize the management of post-COVID syndrome, the long-lasting symptoms of COVID-19 and their influencing factors, is ongoing. In the current literature, post-COVID syndrome is independent of the acute disease severity and the humoral response [31,32]. However, there are also studies showing that severe acute COVID-19, hospitalization, and comorbidities affect the persistence of symptoms [28], and that myalgia in the initial phase is associated with musculoskeletal pain as a post-COVID sequela [27]. Supporting this hypothesis, we found that high initial VAS scores were associated with pain at the 3-month follow-up. However, post-COVID syndrome is still poorly understood, and more research is needed [32].
There are studies showing that muscle weakness and decreased physical performance after COVID-19 infection [33] and chronic fatigue syndrome are common with COVID-19 [34,35]. In our study, we determined the frequency of severe fatigue, as evaluated with the FSS, to be 89% in hospitalized COVID-19 patients. Similarly, fatigue was reported in 85% of cases in a case series of 7 acute-phase patients [36]. In the study by Tuzun et al. [22], the most common musculoskeletal symptom was fatigue (85.3%). The overall prevalence of fatigue symptoms in one review was 25.6% (range, 4%‒100%) [5]. Goertz et al. [37] found that 92.9% and 93.5% of hospitalized and non-hospitalized COVID-19 patients, respectively, reported continued fatigue up to 79 days after disease onset. In a group of 120 hospitalized COVID-19 patients studied by Garrigues et al. [7], symptoms such as fatigue and shortness of breath were still present 110 days after the patients were sent home [7].
Because fatigue is a common and persistent symptom of COVID-19, it is important to analyze the associated factors. In a study by Townsend et al. [38], there was no relationship between the severity of the disease (hospital admission, supplemental oxygen, or intensive care needs) and the severity of fatigue. This is supported by our study, which found that the severity of fatigue did not differ according to disease severity. Townsend et al. [38] also reported more fatigue in females than in males and in those previously diagnosed with depression and anxiety. A relationship between fatigue and anxiety has also been shown in different diseases, such as stroke [39]. We found that fatigue was correlated with pain, depression, and anxiety. The current literature suggests that pain and anxiety may be associated with fatigue in COVID-19 disease. Therefore, although fatigue was a persistent symptom in COVID-19 regardless of disease severity, it may be associated with female sex, anxiety, depression, and pain.
Depression, anxiety, and post-traumatic stress syndrome are all long-term sequelae of COVID-19 [34]. Ma et al. [40] reported a 43.1% prevalence of depression in COVID-19 patients, and that having a family member infected with COVID-19, having a severe COVID-19 infection, being male, and frequently using social media to obtain information about COVID-19 may be independently associated with depression. Cohorts with >20% of patients admitted to the ICU during acute COVID-19 outbreaks reported a higher prevalence of fatigue, anxiety, depression, and sleep disturbance than cohorts with ICU admissions <20% [41]. The reported risk factors for mental health symptoms include female sex and being a healthcare worker. While pain can be a risk factor for mental health symptoms, some studies have also reported no relationship between pain and anxiety or depression [10,42]. Initially, 47% of our patients had symptoms of anxiety and 73% had symptoms of depression. The proportion of patients with anxiety decreased to 9% at the 1-month follow-up and to 6% at the 3-month follow-up. Patients with symptoms of depression decreased to 17% and 12% at the 1-month and 3-month follow-ups, respectively. In our study, no significant differences were observed in the symptoms of anxiety or depression according to disease severity and sex. Only fatigue and symptoms of anxiety or depression were correlated. Although various risk factors for anxiety and depression after COVID-19 have been reported, studies with larger samples are needed on this subject.
Pain, fatigue, anxiety, and depression can significantly affect quality of life. Patients with COVID-19 have been reported to have a poor quality of life and suffer from significant physical and psychological impairments. Therefore, we need to follow up with patients to fully understand the long-term impact of COVID-19 and establish prompt and efficient interventions to mitigate its consequences [43]. In our study, we evaluated the quality of life at 1 month and 3 months after discharge from the hospital and found a significant improvement in the quality of life at 3 months. It was an expected result that the quality of life would improve as the disease improved. Although significant improvements were reported in patients’ quality of life, based on the patients’ degree of dyspnea at rest and during daily activities for 15 days after discharge from the hospital, the quality of life in COVID-19 patients was worse than the normal population at 4-week and 6-week follow-ups [43‒45]. Longer follow-up studies have also shown an overall lower quality of life for up to 3 months [25]. One study reported that people with severe COVID-19 demonstrated a low quality of life at their 6-month follow-ups [46].
Studies have shown that decreased muscle performance, functional capacity, and dyspnea after COVID-19 infection can lead to increased disability and decreased quality of life [47]. In an analysis of 420 patients, it was reported that sex, age, education level, employment status, diabetes, heart failure, and ICU admission were important independent predictors of quality of life [48]. In another study, a relationship was found between age, sex, the severity of clinical subtypes, length of hospital stay, lung function parameters, and some subscales of the SF-36 quality of life questionnaire [43]. In a cohort study of 251 patients followed for 3 months, female sex and the need for intensive care were independently associated with worsening quality of life [49]. It has also been reported that the incidence of low quality of life is higher among patients with a history of ICU admission and fatigue [50]. Quality of life was also worse in patients with persistent pain [10]. In our study, although there was no significant difference in quality of life based on disease severity and sex, quality of life was found to be negatively correlated with the severity of fatigue, anxiety, and depression symptoms. Therefore, it is important that rehabilitation be implemented to ease pain and fatigue so that patients can live better lives.
Conclusion
Pain, fatigue, anxiety, and depression appear to be long-term sequelae of COVID-19 and can significantly affect patients’ quality of life. Regardless of disease severity, a high initial VAS level may be a risk factor for long-term pain. High VAS and anxiety levels were also found to be associated with long-term fatigue.
At the time of our study, the pandemic was waning due to effective vaccines. However, the problems of patients with long-term COVID-19 should not be ignored. To improve the quality of life for people who have had COVID-19, further studies are needed that focus on treatment and rehabilitation.
Limitations
A limitation of our study is that COVID-19 patients who were not hospitalized were not examined.
Footnotes
Ethics Approval
This study was approved by the Research Ethics Commission of the Afyonkarahisar Health Sciences University (2021/57) and was performed in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent, which was confirmed by the Research Ethics Commission of the Afyonkarahisar Health Sciences University.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding
None.
Availability of Data
The datasets are not publicly available but are available from the corresponding author upon reasonable request.
Authors’ Contributions
Conceptualization: all authors; Data curation: AİA; Formal analysis: SA; Investigation: SA, PŞK; Methodology: SA, ÜD, ND; Project administration: SA, PŞK, AİA; Resources: all authors; Software: AİA; Supervision: ÜD, ND; Validation: SA, ÜD, ND; Visualization: SA, AİA; Writing–original draft: SA; Writing–review and editing: all authors. All authors read and approved the final manuscript.
Additional Contributions
We sincerely thank all those affiliated with Afyonkarahisar Health Sciences University Hospital for their hard work during the COVID-19 pandemic and all the patients that participated in the study.
References
- 1.Ochani R, Asad A, Yasmin F, et al. COVID-19 pandemic: from origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez Med. 2021;29:20–36. [PubMed] [Google Scholar]
- 2. World Health Organization (WHO). Weekly epidemiological update on COVID-19-17-06 2022 [Internet]. WHO; 2022 [cited 2022 Oct 12]. Available at: https://covid19.who.int/?mapFilter=cases.
- 3.Baj J, Karakula-Juchnowicz H, Teresinski G, et al. COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med. 2020;9:1753. doi: 10.3390/jcm9061753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cipollaro L, Giordano L, Padulo J, et al. Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients. J Orthop Surg Res. 2020;15:178. doi: 10.1186/s13018-020-01702-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdullahi A, Candan SA, Abba MA, et al. Neurological and musculoskeletal features of COVID-19: a systematic review and meta-analysis. Front Neurol. 2020;11:687. doi: 10.3389/fneur.2020.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93:1013–22. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 7.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi JI. Transition of COVID-19 to endemic phase and emergence of COVID-19 related neuropathic pain. Korean J Pain. 2022;35:237–9. doi: 10.3344/kjp.2022.35.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahin T, Ayyildiz A, Gencer-Atalay K, et al. Pain symptoms in COVID-19. Am J Phys Med Rehabil. 2021;100:307–12. doi: 10.1097/PHM.0000000000001699. [DOI] [PubMed] [Google Scholar]
- 11.Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S240–52. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 12.Gencay-Can A, Can SS. Validation of the Turkish version of the fatigue severity scale in patients with fibromyalgia. Rheumatol Int. 2012;32:27–31. doi: 10.1007/s00296-010-1558-3. [DOI] [PubMed] [Google Scholar]
- 13.Aydemir O, Küey L. Reliability and validity of the Turkish version of hospital anxiety and depression scale. Turkish J Psychiatry. 1997;8:280–7. [Google Scholar]
- 14. World Health Organization (WHO). Clinical management of COVID-19 [Internet]. WHO; 2020 [cited 2022 Jun 17]. Available at: https://apps.who.int/iris/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5eng.pdf?sequence=1&isAllowed=y.
- 15.Vacchiano V, Riguzzi P, Volpi L, et al. Early neurological manifestations of hospitalized COVID-19 patients. Neurol Sci. 2020;41:2029–31. doi: 10.1007/s10072-020-04525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Disser NP, De Micheli AJ, Schonk MM, et al. Musculoskeletal consequences of COVID-19. J Bone Joint Surg Am. 2020;102:1197–204. doi: 10.2106/JBJS.20.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murat S, Dogruoz Karatekin B, Icagasioglu A, et al. Clinical presentations of pain in patients with COVID-19 infection. Ir J Med Sci. 2021;190:913–7. doi: 10.1007/s11845-020-02433-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oguz-Akarsu E, Gullu G, Kilic E, et al. Insight into pain syndromes in acute phase of mild-to-moderate COVID-19: frequency, clinical characteristics, and associated factors. Eur J Pain. 2022;26:492–504. doi: 10.1002/ejp.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoong CW, Amin MN, Tan TC, et al. Viral arthralgia a new manifestation of COVID-19 infection? A cohort study of COVID-19-associated musculoskeletal symptoms. Int J Infect Dis. 2021;104:363–9. doi: 10.1016/j.ijid.2021.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koseoglu Toksoy C, Yavuz T, Orhan S, et al. Neurological symptoms and findings in COVID-19: a prospective clinical study. Neurol Res. 2022;44:1–6. doi: 10.1080/01616412.2021.1952740. [DOI] [PubMed] [Google Scholar]
- 21.Bakilan F, Gokmen IG, Ortanca B, et al. Musculoskeletal symptoms and related factors in postacute COVID-19 patients. Int J Clin Pract. 2021;75:e14734. doi: 10.1111/ijcp.14734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuzun S, Keles A, Okutan D, et al. Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. Eur J Phys Rehabil Med. 2021;57:653–62. doi: 10.23736/S1973-9087.20.06563-6. [DOI] [PubMed] [Google Scholar]
- 23.Karaarslan F, Guneri FD, Kardes S. Long COVID: rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin Rheumatol. 2022;41:289–96. doi: 10.1007/s10067-021-05942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113–9. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanbehzadeh S, Tavahomi M, Zanjari N, et al. Physical and mental health complications post-COVID-19: scoping review. J Psychosom Res. 2021;147:110525. doi: 10.1016/j.jpsychores.2021.110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One. 2020;15:e0243882. doi: 10.1371/journal.pone.0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-de-Las-Penas C, Rodriguez-Jimenez J, Fuensalida-Novo S, et al. Myalgia as a symptom at hospital admission by severe acute respiratory syndrome coronavirus 2 infection is associated with persistent musculoskeletal pain as long-term post-COVID sequelae: a case-control study. Pain. 2021;162:2832–40. doi: 10.1097/j.pain.0000000000002306. [DOI] [PubMed] [Google Scholar]
- 28.Kayaaslan B, Eser F, Kalem AK, et al. Post-COVID syndrome: a single-center questionnaire study on 1007 participants recovered from COVID-19. J Med Virol. 2021;93:6566–74. doi: 10.1002/jmv.27198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cabrera Martimbianco AL, Pacheco RL, Bagattini AM, et al. Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. Int J Clin Pract. 2021;75:e14357. doi: 10.1111/ijcp.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–58. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anaya JM, Rojas M, Salinas ML, et al. Post-COVID syndrome: a case series and comprehensive review. Autoimmun Rev. 2021;20:102947. doi: 10.1016/j.autrev.2021.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eccles JA, Davies KA. The challenges of chronic pain and fatigue. Clin Med (Lond) 2021;21:19–27. doi: 10.7861/clinmed.2020-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paneroni M, Simonelli C, Saleri M, et al. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am J Phys Med Rehabil. 2021;100:105–9. doi: 10.1097/PHM.0000000000001641. [DOI] [PubMed] [Google Scholar]
- 34.O'Connor CM. COVID-19 fatigue: not so fast. JACC Heart Fail. 2020;8:592–4. doi: 10.1016/j.jchf.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crook H, Raza S, Nowell J, et al. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 36.Ferraro F, Calafiore D, Dambruoso F, et al. COVID-19 related fatigue: which role for rehabilitation in post-COVID-19 patients? A case series. J Med Virol. 2021;93:1896–9. doi: 10.1002/jmv.26717. [DOI] [PubMed] [Google Scholar]
- 37.Goertz YM, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6:00542–2020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galligan NG, Hevey D, Coen RF, et al. Clarifying the associations between anxiety, depression and fatigue following stroke. J Health Psychol. 2016;21:2863–71. doi: 10.1177/1359105315587140. [DOI] [PubMed] [Google Scholar]
- 40.Ma YF, Li W, Deng HB, et al. Prevalence of depression and its association with quality of life in clinically stable patients with COVID-19. J Affect Disord. 2020;275:145–8. doi: 10.1016/j.jad.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Premraj L, Kannapadi NV, Briggs J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022;434:120162. doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Sousa Moreira JL, Barbosa SM, Vieira JG, et al. The psychiatric and neuropsychiatric repercussions associated with severe infections of COVID-19 and other coronaviruses. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110159. doi: 10.1016/j.pnpbp.2020.110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen KY, Li T, Gong FH, et al. Predictors of health-related quality of life and influencing factors for COVID-19 patients, a follow-up at one month. Front Psychiatry. 2020;11:668. doi: 10.3389/fpsyt.2020.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santus P, Tursi F, Croce G, et al. Changes in quality of life and dyspnoea after hospitalization in COVID-19 patients discharged at home. Multidiscip Respir Med. 2020;15:713. doi: 10.4081/mrm.2020.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Sar-van der Brugge S, Talman S, Boonman-de Winter L, et al. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir Med. 2021;176:106272. doi: 10.1016/j.rmed.2020.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carenzo L, Protti A, Dalla Corte F, et al. Short-term health-related quality of life, physical function and psychological consequences of severe COVID-19. Ann Intensive Care. 2021;11:91. doi: 10.1186/s13613-021-00881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akbarialiabad H, Taghrir MH, Abdollahi A, et al. Long COVID, a comprehensive systematic scoping review. Infection. 2021;49:1163–86. doi: 10.1007/s15010-021-01666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arab-Zozani M, Hashemi F, Safari H, et al. Health-related quality of life and its associated factors in COVID-19 patients. Osong Public Health Res Perspect. 2020;11:296–302. doi: 10.24171/j.phrp.2020.11.5.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Todt BC, Szlejf C, Duim E, et al. Clinical outcomes and quality of life of COVID-19 survivors: a follow-up of 3 months post hospital discharge. Respir Med. 2021;184:106453. doi: 10.1016/j.rmed.2021.106453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malik P, Patel K, Pinto C, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL): a systematic review and meta-analysis. J Med Virol. 2022;94:253–62. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]