Abstract
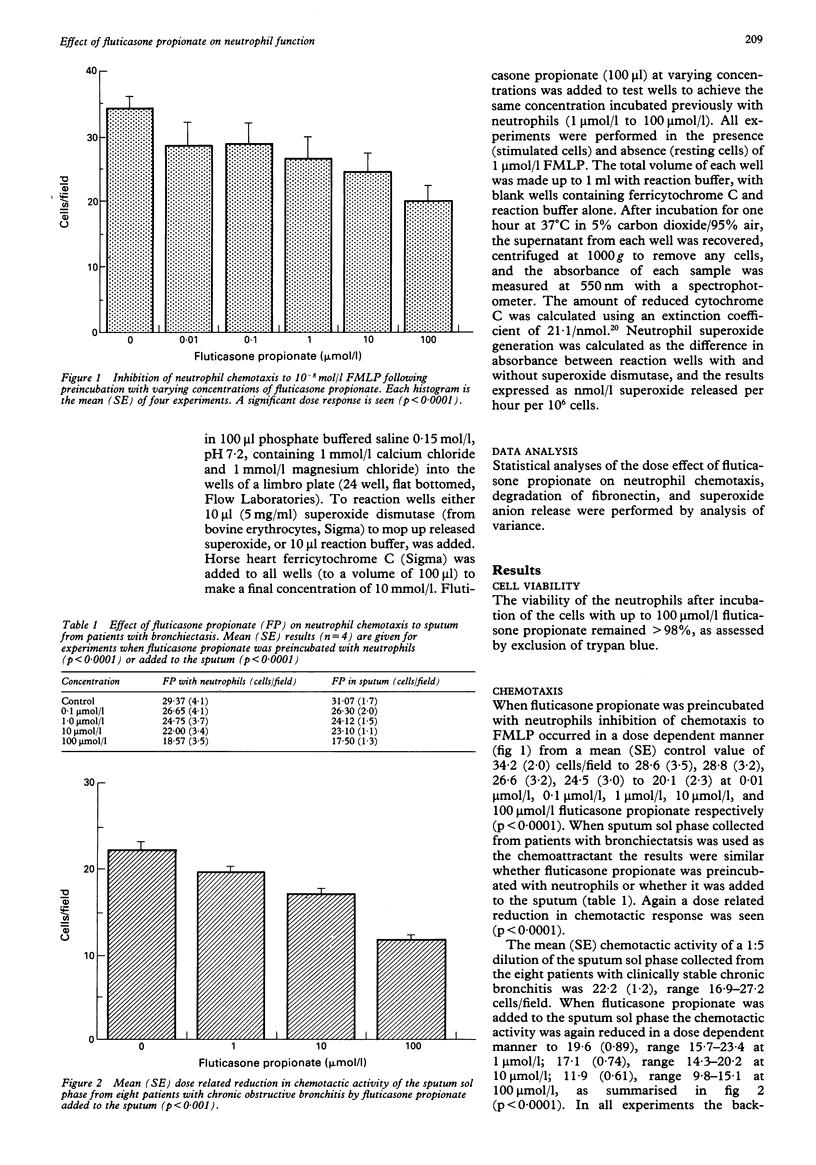
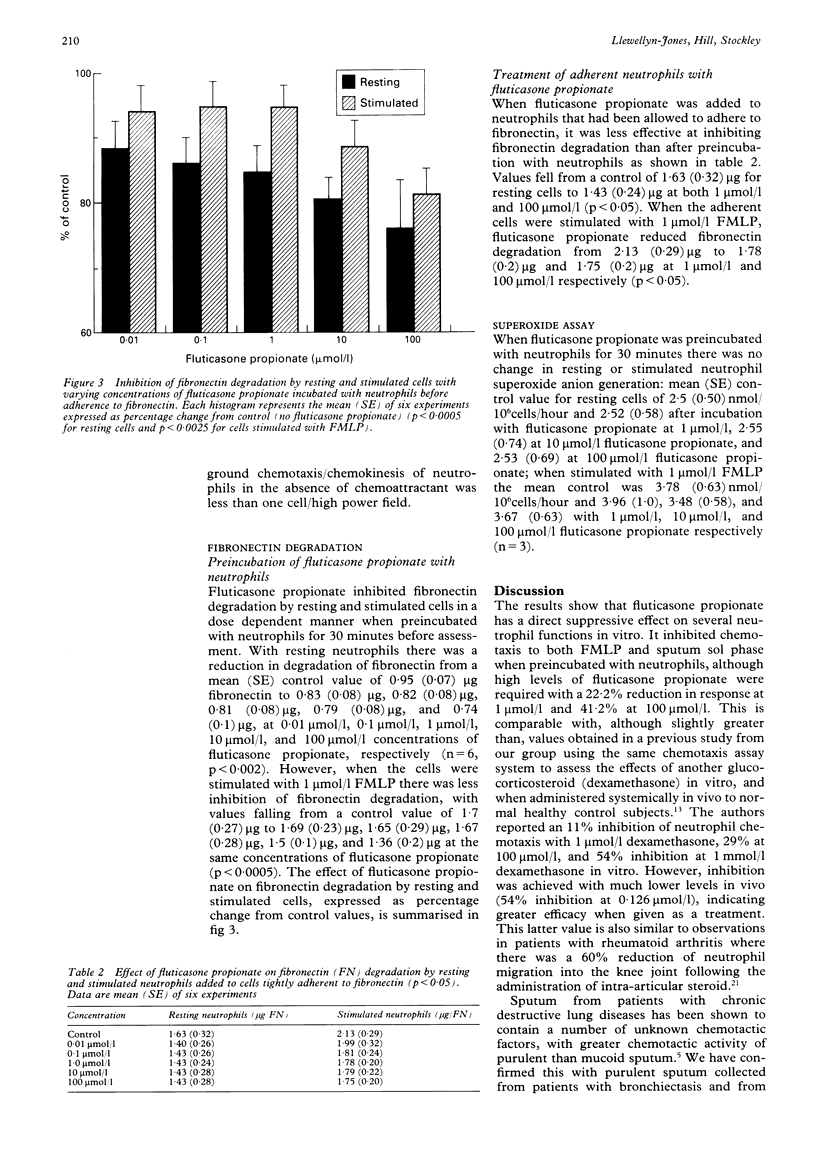
BACKGROUND--Corticosteroids are widely used in the treatment of many inflammatory conditions but the exact mode of action on neutrophil function is uncertain. Fluticasone propionate is a new topically active synthetic steroid which can be measured in body fluids and which undergoes first pass metabolism. METHODS--The effects of fluticasone propionate on the function of neutrophils isolated from normal, healthy control subjects and on the chemotactic activity of sputum sol phase were assessed. RESULTS--Preincubation of neutrophils with fluticasone propionate reduced the chemotactic response to 10(-8) mol/l F-Met-Leu-Phe (FMLP) and to a 1:5 dilution of sputum sol phase in a dose dependent manner. Furthermore, when fluticasone propionate was added to sputum from eight patients with stable chronic obstructive bronchitis the chemotactic activity of a 1:5 dilution of the sol phase fell from a mean (SE) value of 22.2 (1.21) cells/field to 19.6 (0.89), 17.1 (0.74), and 11.9 (0.6) cells field at 1 mumol/l, 10 mumol/l, and 100 mumol/l, respectively. In further experiments fluticasone propionate preincubated with neutrophils inhibited fibronectin degradation by resting cells and by cells stimulated by FMLP (15.2% inhibition of resting cells, 5.1% inhibition of stimulated cells with 1 mumol/l fluticasone propionate, 24% and 18.7% inhibition respectively at 100 mumol/l fluticasone propionate. Fluticasone propionate had no effect on generation of superoxide anion by resting or stimulated cells. CONCLUSIONS--These results indicate that fluticasone propionate has a direct suppressive effect on several aspects of neutrophil function and may suggest a role for this agent in the modulation of neutrophil mediated damage to connective tissue.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M. New mechanisms for effects of anti-inflammatory glucocorticoids. Biofactors. 1991 Jun;3(2):97–102. [PubMed] [Google Scholar]
- Burnett D., Chamba A., Hill S. L., Stockley R. A. Effects of plasma, tumour necrosis factor, endotoxin and dexamethasone on extracellular proteolysis by neutrophils from healthy subjects and patients with emphysema. Clin Sci (Lond) 1989 Jul;77(1):35–41. doi: 10.1042/cs0770035. [DOI] [PubMed] [Google Scholar]
- Burnett D., Chamba A., Hill S. L., Stockley R. A. Neutrophils from subjects with chronic obstructive lung disease show enhanced chemotaxis and extracellular proteolysis. Lancet. 1987 Nov 7;2(8567):1043–1046. doi: 10.1016/s0140-6736(87)91476-0. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Senior R. M., McDonald J. A., Cox D. L. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982 Oct;70(4):845–852. doi: 10.1172/JCI110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Fleming S. D., Edelman L. S., Chapes S. K. Effects of corticosterone and microgravity on inflammatory cell production of superoxide. J Leukoc Biol. 1991 Jul;50(1):69–76. doi: 10.1002/jlb.50.1.69. [DOI] [PubMed] [Google Scholar]
- Freischlag J. A., Colburn M. D., Quiñones-Baldrich W. J., Moore W. S. Alteration of neutrophil (PMN) function by heparin, dexamethasone, and enalapril. J Surg Res. 1992 May;52(5):523–529. doi: 10.1016/0022-4804(92)90322-q. [DOI] [PubMed] [Google Scholar]
- Fuenfer M. M., Carr E. A., Jr, Polk H. C., Jr The effect of hydrocortisone on superoxide production by leukocytes. J Surg Res. 1979 Jul;27(1):29–35. doi: 10.1016/0022-4804(79)90106-9. [DOI] [PubMed] [Google Scholar]
- Fukushima K., Ando M., Ito K., Suga M., Araki S. Stimulus- and cumulative dose-dependent inhibition of O2- production by polymorphonuclear leukocytes of patients receiving corticosteroids. J Clin Lab Immunol. 1990 Nov;33(3):117–123. [PubMed] [Google Scholar]
- Ip M., Lomas D. A., Shaw J., Burnett D., Stockley R. A. Effect of non-steroidal anti-inflammatory drugs on neutrophil chemotaxis--an in vitro and in vivo study. Br J Rheumatol. 1990 Oct;29(5):363–367. doi: 10.1093/rheumatology/29.5.363. [DOI] [PubMed] [Google Scholar]
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985 Aug;132(2):417–433. doi: 10.1164/arrd.1985.132.2.417. [DOI] [PubMed] [Google Scholar]
- Jepsen L. V., Skottun T. A rapid one-step method for the isolation of human granulocytes from whole blood. Scand J Clin Lab Invest. 1982 May;42(3):235–238. [PubMed] [Google Scholar]
- Jones A. K., al-Janabi M. A., Solanki K., Sobnack R., Greenwood A., Doyle D. V., Britton K. E., Huskisson E. C. In vivo leukocyte migration in arthritis. Arthritis Rheum. 1991 Mar;34(3):270–275. doi: 10.1002/art.1780340304. [DOI] [PubMed] [Google Scholar]
- Lomas D. A., Ip M., Chamba A., Stockley R. A. The effect of in vitro and in vivo dexamethasone on human neutrophil function. Agents Actions. 1991 Jul;33(3-4):279–285. doi: 10.1007/BF01986574. [DOI] [PubMed] [Google Scholar]
- Morrison H. M., Kramps J. A., Burnett D., Stockley R. A. Lung lavage fluid from patients with alpha 1-proteinase inhibitor deficiency or chronic obstructive bronchitis: anti-elastase function and cell profile. Clin Sci (Lond) 1987 Mar;72(3):373–381. doi: 10.1042/cs0720373. [DOI] [PubMed] [Google Scholar]
- Mowat A. G., Baum J. Chemotaxis of polymorphonuclear leukocytes from patients with rheumatoid arthritis. J Clin Invest. 1971 Dec;50(12):2541–2549. doi: 10.1172/JCI106754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin I., Foschi G. V., Rosen C. H. Inhibition of in vitro neutrophil chemotaxis and spontaneous motility by anti-inflammatory agents. Proc Soc Exp Biol Med. 1976 Nov;153(2):236–240. doi: 10.3181/00379727-153-39518. [DOI] [PubMed] [Google Scholar]
- Shakir K. M., O'Brian J. T., Gartner S. L. Enhanced phospholipase A2 activity in rat plasma, liver, and intestinal mucosa following endotoxin treatment: a possible explanation for the protective effect of indomethacin in endotoxic shock. Metabolism. 1985 Feb;34(2):176–182. doi: 10.1016/0026-0495(85)90129-5. [DOI] [PubMed] [Google Scholar]
- Stockley R. A. Proteolytic enzymes, their inhibitors and lung diseases. Clin Sci (Lond) 1983 Feb;64(2):119–126. doi: 10.1042/cs0640119. [DOI] [PubMed] [Google Scholar]
- Stockley R. A., Shaw J., Afford S. C., Morrison H. M., Burnett D. Effect of alpha-1-proteinase inhibitor on neutrophil chemotaxis. Am J Respir Cell Mol Biol. 1990 Feb;2(2):163–170. doi: 10.1165/ajrcmb/2.2.163. [DOI] [PubMed] [Google Scholar]
- Stockley R. A., Shaw J., Hill S. L., Burnett D. Neutrophil chemotaxis in bronchiectasis: a study of peripheral cells and lung secretions. Clin Sci (Lond) 1988 Jun;74(6):645–650. doi: 10.1042/cs0740645. [DOI] [PubMed] [Google Scholar]
- Thompson A. B., Mueller M. B., Heires A. J., Bohling T. L., Daughton D., Yancey S. W., Sykes R. S., Rennard S. I. Aerosolized beclomethasone in chronic bronchitis. Improved pulmonary function and diminished airway inflammation. Am Rev Respir Dis. 1992 Aug;146(2):389–395. doi: 10.1164/ajrccm/146.2.389. [DOI] [PubMed] [Google Scholar]
- Ward P. A. The chemosuppression of chemotaxis. J Exp Med. 1966 Aug 1;124(2):209–226. doi: 10.1084/jem.124.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. S., Roth J. A. Relationship of glucocorticoid suppression of arachidonic acid metabolism to alteration of neutrophil function. J Leukoc Biol. 1987 Feb;41(2):156–164. doi: 10.1002/jlb.41.2.156. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]


