Abstract
Objective:
Metastatic bladder cancer is an aggressive disease that can often be difficult to diagnose and stage with conventional cross-sectional imaging. The primary objective of this study was to determine the clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose (18F-FDG) PET/MRI for surveillance and restaging of patients with muscle-invasive, locally advanced, and metastatic bladder cancer compared to conventional imaging methods.
Materials and Methods:
This retrospective study enrolled patients with muscle-invasive, locally advanced and metastatic bladder cancer in a single institute evaluated with 18F-FDG PET/MRI. All patients also underwent conventional imaging with CT. Additional imaging may also have included 18F-FDG PET/CT (18F-FDG PET), or sodium fluoride (NaF) PET/CT in some patients. Images were reviewed by a diagnostic radiologist/nuclear medicine physician. Number of lesions and sites of disease were captured and compared between 18F-FDG PET/MRI and conventional imaging. Lesions were confirmed by sequential imaging or lesion biopsy. All patients were followed for survival.
Results:
Fifteen patients (4 for surveillance; 11 for restaging) underwent 34 18F-FDG PET/MRI scans. Each patient received a corresponding conventional CT around the time of the b18F-FDG PET/MRI (median 6 days). The 15 patients (11 male; 4 female) had a median age of 61.5 years (range 37–73) and histologies of urothelial carcinoma (n = 13) and small-cell carcinoma of the bladder (n = 2) diagnosed as stage 4 (n = 13), stage 3 (n = 1), or stage 2 (n = 1). 18F-FDG PET/MRI detected 82 metastatic malignant lesions involving lymph nodes (n = 22), liver (n = 10), lung (n = 34), soft tissue (n = 12), adrenal glands (n = 1), prostate (n = 1), and bone (n = 2) with a resultant advantage of 36% for lesion visibility in comparison with CT. Serial imaging or biopsy confirmed these lesions as malignant.
Conclusion:
18F-FDG PET/MRI can detect metastatic lesions which cannot be identified on conventional CT, and this can allow for better treatment planning and improved disease monitoring during therapy.
Keywords: Bladder cancer, 18F-FDG PET, 18F-FDG PET/MRI, PET/MRI, Urothelial cancer, Small-cell bladder cancer
1. Introduction
Bladder cancer is the 6th most commonly diagnosed cancer in the United States, and advanced stages convey a poor prognosis [1]. Multiple imaging techniques guide treatment, including computed tomography (CT), abdominal magnetic resonance imaging (MRI), 2-deoxy2-[fluorine-18]fluoro-D-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) and nuclear medicine bone scans when osseous metastasis is suspected [2, 3]. Chest, abdomen, and pelvis CTs are used in patients with muscle-invasive, locally advanced, and metastatic bladder cancer to assess disease burden. Iodinated contrast increases visualization of lymph nodes, especially in the pelvis [3].
Patients who are not eligible to receive iodinated contrast, for example those with mild/moderate renal impairment, can receive an MRI abdomen with group II/III gadolinium contrast agents in addition to a CT chest without iodinated contrast [3, 4]. Conventional MRI has the benefits of superior spatial and contrast resolution, no added radiation, functional sequences, a bladder cancer-specific standardizing scoring system, and more accurately detects primary bladder tumors and pelvic nodal disease than CT [5–7]. However, it still heavily relies on contrast enhancement and does not provide metabolic tumor activity.
Unlike conventional CT and MRI, 18F-FDG PET provides metabolic activity of the tumor with anatomical localization to distinguish malignant from benign lesions and is used to make clinical decisions in numerous malignancies [8, 9]. 18F-FDG PET can detect metastatic lymph nodes by their increased FDG uptake, even when they do not meet the CT-size criteria and can be utilized evaluating advanced bladder cancer [3, 6, 8, 10–13]. 18F-FDG PET/CT has disadvantages including radiation exposure, beam-hardening imaging artifact and is often paired with a CT images of lower imaging/non-diagnostic image quality [14–17]. Even when a diagnostic high-resolution CT is used, it is unlikely to produce the same soft tissue contrast as MRI [18].
Given the complementary advantages of both 18F-FDG PET and MRI, it is logical to combine these two technologies into the hybrid modality 2-deoxy-2-[fluorine-18]fluoro-D-glucose positron emission tomography magnetic resonance imaging (18F-FDG PET/MRI). 18F-FDG PET/MRI provides the anatomical imaging benefits of MRI over CT and the molecular imaging of 18F-FDG PET, without radiation or CT beam-hardening imaging artifacts. 18F-FDG PET/MRI could also potentially be used to follow disease in patients unable to receive gadolinium due to severe renal insufficiency, providing functional imaging in place of contrast enhancement. More information is needed on using 18F-FDG PET/MRI in bladder cancer patients, and data are limited in the advanced/metastatic setting. Because of this we compared the ability of 18F-FDG PET/MRI to detect metastatic disease with that of conventional imaging in patients with muscle-invasive and advanced/metastatic bladder cancer.
2. Materials and Methods
2.1. Patient selection and data acquisition
We identified patients with a history of muscle-invasive or advanced/metastatic urinary tract/bladder cancer who underwent 18F-FDG PET/MRI at the National Institutes of Health (NIH) between September 2014 and October 2016 for standard-of-care clinical evaluation. We retrospectively examined information extracted from the electronic medical record system and reviewed corresponding radiology images under an NIH Institutional Review Board-approved retrospective protocol.
2.2. 18F-FDG PET/MRI imaging protocol
18F-FDG PET/MRI scans were acquired according to NIH Radiology Department protocols. If present, the urinary bladder was imaged with a T2-weighted pulse sequence in 3 planes: 16-cm FOV; 3-mm slice thickness and voxel size of 0.7 mm x 0.7 mm x 3.0 mm; and TR/TE 4000 ms/120 ms. This was followed by an 18F-FDG PET/MRI scan: 6-minute bed position PET images in conjunction with coronal STIR (short tau inversion recovery) images; 40-cm FOV; 6-mm slice thickness and voxel size of 0.9 mm x 0.9 mm x 6.0 mm; and TR/TE/TI 4000 ms/60 ms/220 ms TI. We also obtained DWI whole-body scans with a single b-value of 800s/mm2.
2.3. Pre- and post-contrast-enhanced images
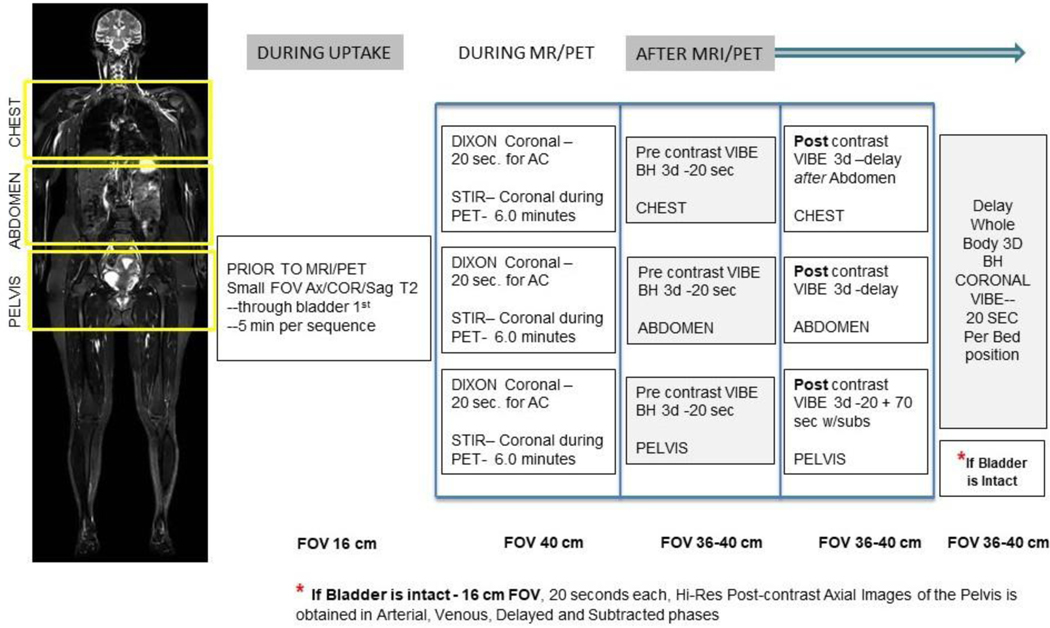
Pre-contrast, breath hold 3d VIBE (volumetric interpolated breath-hold examination) sequences were obtained in the chest, abdomen, and pelvis. Arterial, venous, and delayed images were obtained following administration of a single dose of gadobutrol. Automatic subtracted images were created to increase lesion conspicuity (Fig. 1). Attenuation correction was performed using 20-second Dixon coronal images of the chest, abdomen, and pelvis [19]. Acquired imaging data were transferred and stored in the NIH’s picture archiving and communication system (PACS).
Fig. 1.
18F-FDG PET/MRI bladder cancer protocol.
2.4. Evaluation of malignant lesions
Lesions were retrospectively classified by a board-certified diagnostic radiologist/nuclear medicine physician as malignant based on anatomic and metabolic characteristics on MRI and PET, respectively. Additionally, growth on follow-up imaging, or decrease in size in response to treatment was used to decide whether a lesion was metastatic or benign. Follow-up imaging included conventional CT, and may also have included 18F-FDG PET/MRI, FDG PET/CT, or sodium fluoride (NaF) PET.
3. Results
3.1. Demographics and scans
We identified 15 patients with bladder cancer who underwent 18F-FDG PET/MRI. The average patient age was 59 years, with a median age of 61.5 years (Table 1). 11 patients were male (73%) and 7 were female (27%). Most patients had stage 4 cancer (n = 13), while there was 1 stage 3 and 1 stage 2. Urothelial carcinoma was the predominant histology in 13 patients; 2 had small cell bladder cancer. 14 patients had primary bladder tumors; 1 patient had primary upper tract disease. 11 patients had prior urinary tract/bladder cancer-directed definitive surgery and 11 received systemic treatment for bladder cancer; 6 of those 11 had received multiple regimens. All 15 patients received a standard CT scan, which was correlated with 18F-FDG PET/MRI within a median of 6 days. Four initial 18F-FDG PET/MRI scans were performed for surveillance and 11 were acquired during restaging. In total we evaluated 34 18F-FDG PET/MRI (15 at baseline and 19 at follow-up), 30 CTs (15 at baseline and 15 at follow-up) among other additional imaging (Table 2).
Table 1.
Patient demographics and clinical characteristics.
| Age | N | |
|---|---|---|
| Mean (SD) | 59 | 11.7 |
| Median (range) | 61.5 | (37–73) |
| Sex | N | % |
| Male | 11 | 73 |
| Female | 4 | 27 |
| Diagnosis | N | % |
| Urothelial carcinoma | 13 | 87 |
| Small cell carcinoma | 2 | 13 |
| Primary Site | N | |
| Bladder | 14 | |
| Upper Tract | 1 | |
| Urinary Tract/Bladder Cancer Surgery | ||
| Cystectomy | 10 | |
| Nephroureterectomy | 1 | |
| None | 4 | |
| Prior Systemic Therapy | ||
| Any systemic cancer therapy | 11 | |
| Multiple prior systemic regimens | 6 | |
| Bevacizumab + cisplatin + gemcitabine | 1 | |
| Carboplatin + gemcitabine | 1 | |
| Cisplatin + etoposide | 2 | |
| Cisplatin + gemcitabine | 7 | |
| MVAC* | 3 | |
| N-paclitaxel | 1 | |
| Stage at Initial 8F-FDG PET/MRI | ||
| IV | 13 | |
| III | 1 | |
| II | 1 | |
| I | 0 | |
methotrexate + adriamycin (doxorubicin) + vinblastine + cisplatin
Table 2.
Radiographic study characteristics and rationale for imaging.
| Total 18F-FDG PET/MRI scans | 34 |
| Days Between Corresponding CT and Baseline 18F-FDG PET/MRI | |
| Average # of days | 21 |
| Median # of days | 6 |
| Range | 0–105 |
| Reason for Initial 18F-FDG PET/MRI | |
| Surveillance (off treatment) | 4 |
| Restaging (on treatment) | 11 |
| Additional Clinical Follow-Up Imaging Studies | |
| CT | 30 |
| MRI | 2 |
| 18F-FDG PET/CT | 1 |
| 18F-NaF PET/CT | 3 |
3.2. Lesion-based analysis
In the 15 patients studied, we detected metastatic disease in 9 patients, with 82 malignant lesions on initial 18F-FDG PET/MRI (Table 3). All 82 lesions were followed with at least one CT, 61 were followed with at least one 18F-FDG PET/MRI, 2 bone lesions were followed with NaF PET, and 7 lesions were confirmed by biopsy. All biopsies obtained by guided imaging were positive for tumor. In addition, 34 lung, 22 lymph node, 12 soft tissue, 10 liver, 2 bone, 1 adrenal and 1 prostate malignant lesion were detected on the baseline 18F-FDG PET/MRI (Table 3). In total, 60 of the 82 (73%) malignant lesions were seen on follow-up CT (33 lung (97%), 17 lymph node (77%), 1 soft tissue (8%), 5 liver (50%), 2 bone (100%), 1 adrenal (100%), and 1 prostate (100%)). Of the malignant lymph nodes 17 of the 22 (77%) were seen on follow-uo CT (8 neck/supraclavicular (88%), axial 0 (0%), 4 mediastinal/thoracic (100%), 0 abdominal (0%), 6 retroperitoneal/pelvic (75%)) (Supplemental Table 1). All biopsies were of lesions seen on both CT and PET/MRI confirming histologic diagnosis or were performed for correlative research for clinical trials, including 2 liver, 4 lung, and 1 lymph node metastasis.
Table 3.
Sites of metastatic disease along with 18F-FDG PET/MRI concordance with follow-up CT.
| Malignant Lesions Detected | ||||
|---|---|---|---|---|
| Disease Site | Baseline 18F-FDG PET/MRI (n) | Corresponding baseline CT (n) |
18F-FDG PET/MRI Lesions Detected on Corresponding CT (%) |
Lesion seen on NaF |
| Adrenal | 1 | 1 | 100 | 0 |
| Bone | 2 | 2 | 100 | 2 |
| Liver | 10 | 5 | 50 | 0 |
| Lung | 34 | 33 | 97 | 1 |
| Lymph node | 22 | 17 | 77 | 0 |
| Prostate | 1 | 1 | 100 | 0 |
| Soft tissue | 12 | 1 | 8 | 0 |
| Total | 82 | 60 | 73 | 3 |
4. Discussion
In this retrospective study we evaluated 18F-FDG PET/MRI’s ability to detect metastatic disease compared to standard CT scans in bladder cancer patients with various clinical stages, histologies, and treatments. At baseline, 18F-FDG PET/MRI identified 82 malignant lesions in 9 patients. These lesions were followed by standard CT, 18F-FDG PET/MRI, or biopsy and confirmed to be metastatic based on lesion growth or pathologic confirmation. 18F-FDG PET/MRI detected 27% more malignant lesions (82 vs. 60) than conventional CT alone, including at baseline 23% more lymph node lesions (22 vs. 17), 50% more liver lesions (10 vs. 5), and 92% more soft-tissue lesions (12 vs. 1). It also detected 33% more abdominal, retroperitoneal, and pelvic lymph nodes (9 vs. 6) (Supplemental Table 1). 18F-FDG PET/MRI’s superior ability to detect soft-tissue lesions compared to CT could help to guide clinical decisions such as initial staging of bladder cancer where lymph node metastasis determines surgical planning.
Four patients were categorized as having soft-tissue disease on 18F-FDG PET/MRI alone and not by CT, suggesting that PET/MRI may be better than CT at detecting early-phase soft-tissue disease. 18F-FDG PET/MRI findings led to a change in clinical management for one patient on the study. This patient had small-cell neuroendocrine carcinoma bladder cancer and was treated on a clinical trial with single-agent cabozantinib. 18F-FDG PET/MRI showed liver progression that was not seen on CT with IV done on the same day and led to a change in therapy.
Another advantage of 18F-FDG PET/MRI is its ability to follow disease in patients with renal impairment who are unable to receive iodinated or gadolinium contrast. A patient with a kidney transplant, a glomerular filtration rate less than 30 and unable to receive contrast underwent a radical cystectomy for a pT4a pN1 bladder cancer with positive microscopic invasive margins. She was subsequently followed on active surveillance with 18F-FDG PET/MRI without gadolinium for 3 years until disease recurred in her rectum.
Previous bladder cancer 18F-FDG PET/MRI research has focused on localized or locally advanced disease within the pelvis. An early study by Rosenkrantz et al. showed simultaneously acquired PET and MRI acquisition improves bladder tumor co-registration [20]. This was followed by a prospective pilot study that suggests 18F-FDG PET/MRI is more accurate at identifying primary bladder tumors and pelvic metastasis than MRI alone [21].
This study illustrates our experience at our clinical center with 18F-FDG PET/MRI in bladder cancer and its technological potential. Both the study and the technology have limitations. This was a small, retrospective study, and only a small group of patients had pathologic confirmation of lesions on 18F-FDG PET/MRI. Also, 5 patients (1/3 of the patients on this study) were on surveillance post-radical cystectomy and had no active disease or malignant lesions on baseline 18F-FDG PET/MRI.
As for the technology, 18F-FDG PET/MRI equipment costs 50% more than PET/CT equipment, which may limit its use to a small number of academic institutions. It also requires specialized operator training and longer scan times than standard CT with contrast [22]. Another issue is that simultaneous whole-body 18F-FDG PET/MRI scans have long patient in-scanner time, often more than 1 hour. However, with hardware and software advances and more disease-specific MRI protocols, it is expected that imaging time and cost will decrease significantly in the future.
5. Conclusion
Our hypothesis-generating, retrospective data show that 18F-FDG PET/MRI can potentially detect metastatic lesions not visible on CT. Prospective studies are required to determine 18F-FDG PET/MRI’s sensitivity/specificity and clinical benefit in bladder cancer.
Supplementary Material
Highlights.
PET/MRI combines the benefits of MRI with functional imaging.
PET/MRI can detect metastatic bladder cancer lesions not seen on CT.
PET/MRI may be an option for patients that cannot receive contrast.
PET/MRI may lead to improved treatment planning and monitoring for bladder cancer.
Funding
This research was made possible through the NIH Medical Research Scholars Program, a public/private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH by the Doris Duke Charitable Foundation (Grant #2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, and other private donors. For a complete list, see the foundation’s website at http://www.fnih.org.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Bagheri MH, Ahlman MA, Lindenberg L, Turkbey B, Lin J, Cahid Civelek A, et al. Advances in medical imaging for the diagnosis and management of common genitourinary cancers. Urol Oncol. 2017;35:473–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Network NCC. NCCN Clinical Practice Guidlines in Oncology Bladder Cancer Version 6.2020-July 16, 2020. 2020. [Google Scholar]
- [4].Weinreb JC, Rodby RA, Yee J, Wang CL, Fine D, McDonald RJ, et al. Use of Intravenous Gadolinium-Based Contrast Media in Patients With Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2021;3:142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Panebianco V, Narumi Y, Altun E, Bochner BH, Efstathiou JA, Hafeez S, et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur Urol. 2018;74:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Civelek A, Agarwal, Evers, Bluemke and Malayeri. 18F-FDG PET-MRI in the management of muscle invasive bladder cancer: Challenges in imaging and solutions. J Nucl Med. 2016;57. [Google Scholar]
- [7].Malayeri AA, Pattanayak P, Apolo AB. Imaging muscle-invasive and metastatic urothelial carcinoma. Current opinion in urology. 2015;25:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Apolo AB, Riches J, Schöder H, Akin O, Trout A, Milowsky MI, et al. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J Clin Oncol. 2010;28:3973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Farwell MD, Pryma DA, Mankoff DA. PET/CT imaging in cancer: current applications and future directions. Cancer. 2014;120:3433–45. [DOI] [PubMed] [Google Scholar]
- [10].Zattoni F, Incerti E, Dal Moro F, Moschini M, Castellucci P, Panareo S, et al. (18)F-FDG PET/CT and Urothelial Carcinoma: Impact on Management and Prognosis-A Multicenter Retrospective Study. Cancers. 2019;11:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kitajima K, Yamamoto S, Fukushima K, Yamakado K, Katsuura T, Igarashi Y, et al. FDG-PET/CT as a post-treatment restaging tool in urothelial carcinoma: Comparison with contrastenhanced CT. Eur J Radiol. 2016;85:593–8. [DOI] [PubMed] [Google Scholar]
- [12].Lu Y-Y, Chen J-H, Liang J-A, Wang H-Y, Lin C-C, Lin W-Y, et al. Clinical value of FDG PET or PET/CT in urinary bladder cancer: A systemic review and meta-analysis. European Journal of Radiology. 2012;81:2411–6. [DOI] [PubMed] [Google Scholar]
- [13].Al-Nabhani KZ, Syed R, Michopoulou S, Alkalbani J, Afaq A, Panagiotidis E, et al. Qualitative and quantitative comparison of PET/CT and PET/MR imaging in clinical practice. J Nucl Med. 2014;55:88–94. [DOI] [PubMed] [Google Scholar]
- [14].Tan H, Gu Y, Yu H, Hu P, Zhang Y, Mao W, et al. Total-Body PET/CT: Current Applications and Future Perspectives. American Journal of Roentgenology. 2020;215:325–37. [DOI] [PubMed] [Google Scholar]
- [15].Bagheri MH, Ahlman MA, Lindenberg L, Turkbey B, Lin J, Cahid Civelek A, et al. Advances in medical imaging for the diagnosis and management of common genitourinary cancers. Urologic Oncology: Seminars and Original Investigations. 2017;35:473–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Krishnasetty V, Bonab AA, Fischman AJ, Halpern EF, Aquino SL. Comparison of Standard-Dose vs Low-Dose Attenuation Correction CT on Image Quality and Positron Emission Tomographic Attenuation Correction. Journal of the American College of Radiology. 2008;5:579–84. [DOI] [PubMed] [Google Scholar]
- [17].de Haas RJ, Steyvers MJ, Fütterer JJ. Multiparametric MRI of the Bladder: Ready for Clinical Routine? American Journal of Roentgenology. 2014;202:1187–95. [DOI] [PubMed] [Google Scholar]
- [18].Berthelsen AK, Holm S, Loft A, Klausen TL, Andersen F, Højgaard L. PET/CT with intravenous contrast can be used for PET attenuation correction in cancer patients. Eur J Nucl Med Mol Imaging. 2005;32:1167–75. [DOI] [PubMed] [Google Scholar]
- [19].Costa DN, Pedrosa I, McKenzie C, Reeder SB, Rofsky NM. Body MRI using IDEAL. AJR Am J Roentgenol. 2008;190:1076–84. [DOI] [PubMed] [Google Scholar]
- [20].Rosenkrantz AB, Balar AV, Huang WC, Jackson K, Friedman KP. Comparison of Coregistration Accuracy of Pelvic Structures Between Sequential and Simultaneous Imaging During Hybrid PET/MRI in Patients with Bladder Cancer. Clinical Nuclear Medicine. 2015;40:637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rosenkrantz AB, Friedman KP, Ponzo F, Raad RA, Jackson K, Huang WC, et al. Prospective Pilot Study to Evaluate the Incremental Value of PET Information in Patients With Bladder Cancer Undergoing 18F-FDG Simultaneous PET/MRI. Clin Nucl Med. 2017;42:e8–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mayerhoefer ME, Prosch H, Beer L, Tamandl D, Beyer T, Hoeller C, et al. PET/MRI versus PET/CT in oncology: a prospective single-center study of 330 examinations focusing on implications for patient management and cost considerations. Eur J Nucl Med Mol Imaging. 2020;47:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.