Abstract
Background:
Pythium keratitis is a difficult-to-treat corneal infection.
Methods:
A meta-analysis of individual patient data from observational studies of Pythium keratitis was performed. The outcomes of interest were therapeutic penetrating keratoplasty (TPK) and globe removal (evisceration, enucleation, or exenteration); the main exposures were linezolid and azithromycin use.
Findings:
Of 46 eligible articles, individual patient data were available for 306 eyes (34 studies). Pythium keratitis was associated with high rates of TPK (80%, 95%CI 70–87%) and globe removal (25%, 95%CI 13–43). In multivariable models adjusting for age and country, fewer TPKs were performed in patients treated with azithromycin (RR=0.80, 95%CI 0.67–0.96; P=0.04) and linezolid (RR=0.82, 95%CI 0.67–0.99; P=0.02).
Conclusions:
Studies of Pythium keratitis reported high rates of TPK and globe removal. Use of azithromycin and linezolid was associated with a lower rate of TPK. While promising, these results should be interpreted with caution given the biases inherent to observational studies.
Keywords: Pythium, pythiosis, corneal ulcer, azithromycin, linezolid, penetrating keratoplasty, systematic review
INTRODUCTION
Pythium insidiosum is an aquatic oomycete that closely resembles fungus morphologically and histologically.1 P. insidiosum can infect both animals and humans, with published reports of cutaneous, vascular, systemic, gastrointestinal and ocular infections.2 Corneal infection secondary to Pythium, or Pythium keratitis, has been gaining attention in the past few decades due to its high virulence, challenging diagnosis, lack of effective treatment regimen, high recurrence rate, and poor visual prognosis.34 The majority of cases have been reported mainly in tropical and subtropical countries such as India and Thailand, although there have been scattered cases reported in areas with temperate conditions like the United States, France, New Zealand, Australia, Spain, Israel, and Japan.5–14 Pythium zoospores develop in swampy areas, and thus agriculture and water-based activities are considered the major predisposing risk factors.2
Due to its clinical, microbiological and histological resemblance to fungal keratitis, Pythium keratitis diagnosis is usually delayed, leading to severe complications, including corneal perforation, vision loss, evisceration, enucleation, exenteration and in rare cases, death.15 Ocular Pythiosis often necessitates early therapeutic penetrating keratoplasty (TPK) due to the high virulence, rapid proliferation of the pathogen, and limited response to medical management.16 In the past, cases of Pythium keratitis were often treated with antifungal therapy because Pythium was mistakenly grouped as a fungal species. However, several studies have shown that antifungals have limited efficacy against Pythium.16,17 In the past few years, antibiotics such as azithromycin and linezolid have been reported in some studies as an alternative medical treatment with some efficacy against Pythium.3,18 However, evidence from individual studies has been limited. Therefore, in this meta-analysis, we collected and analyzed individual patient data from published studies to assess the outcomes of Pythium keratitis and the efficacy of different medical treatment regimens.
METHODS
Literature search.
A literature search was performed on PubMed from inception to August 20, 2022 using the phrase (pythium[tiab] AND (keratitis[tiab] OR “corneal ulcer”[tiab])) OR “ocular pythiosis”[tiab]. Titles and abstracts were screened to select articles reporting cases of human ocular Pythiosis. Only manuscripts available in English were reviewed.
Data extraction.
Two co-authors (BC and VTG) independently assessed the full-text version of all the selected articles and extracted data onto a standardized electronic data collection form. Data was summarized at the individual patient level. If a study reported data only at the group level, the corresponding author was contacted to request the individual-level data. Discrepancies between the two data extractors were resolved by discussing each issue and coming to consensus.
Eligibility criteria.
Patients were included in the study if they had a corneal infiltrate accompanied by positive results for Pythium from at least one of the following tests: (1) microbiological testing (i.e., culture and microscopy), (2) molecular assay (i.e., PCR and sequence homology), or (3) histological assessment. If a patient had bilateral disease, the worst eye was chosen for analyses. Patients who failed to meet one of the above diagnostic criteria for Pythium keratitis were excluded.
Exposure definition.
The main exposures of interest were treatment with linezolid and azithromycin, defined as the use of these medications prior to any surgical therapy.
Outcome measures.
The main outcome measures were therapeutic penetrating keratoplasty (TPK) and the composite outcome of evisceration, enucleation, or exenteration, named globe removal in this report.
Definitions and conventions.
Visual acuity (VA) was converted from Snellen VA measurements to logarithm of the minimum angle of resolution (logMAR) equivalent for statistical analysis. Infiltrate size was calculated as the geometric mean of the reported height and width, assuming a corneal diameter of 11.5 mm to calculate infiltrate size of “total” infiltrates, and assigning the median value from the estimated tertiles when infiltrates were described as “small” or “large.” Medications were recorded as dichotomous variables, based on whether they were used before either of the outcomes had occurred.
Statistical methods.
Statistical analyses were performed using individual patient data in order to address potential confounders. Univariable robust Poisson regression models were created to provide estimates of relative risk, with TPK or globe removal as the response variable and various risk factors at presentation as well as instituted medical treatments as the explanatory variables. Similar multivariable models were constructed to explore the relationship between linezolid/azithromycin use and each of the outcomes, with adjustment for age, country, and visual acuity at presentation. Regression models were performed using the survey commands in Stata to account for the likelihood of intra-cluster correlation within each study (i.e., patients from the same study were likely to be more similar to each other compared with patients from a different study). All regression models were complete case analyses. P-values less than 0.05 were considered statistically significant. Analyses were performed with Stata 17.
RESULTS
Literature search and study characteristics.
A total of 84 articles were identified from the Pubmed search criteria, of which 46 contained data on the exposures and outcomes of interest (Figure 1). Of these manuscripts, 31 contained individual-level data of all participants, 1 contained individual data of some participants, and 6 had only group-level data. The authors of 3 group-level papers provided individual patient-level data upon request. From the resulting dataset, 8 eyes were excluded because of missing data for the outcomes of interest or bilateral disease. The final analysis population consisted of 306 patients (n=306 eyes) from 34 articles.19–52 Manuscripts were published from 23 different clinical sites of 10 different countries, with the majority coming from India (N=14) and Thailand (N=8) (Table 1). Studies were published between 1993 and 2022, with an increasing number of manuscripts over time (Figure 2). Most of the identified studies were small case series, with a median of 1 (IQR 1–10; range 1–67) patient per study.
Figure 1:
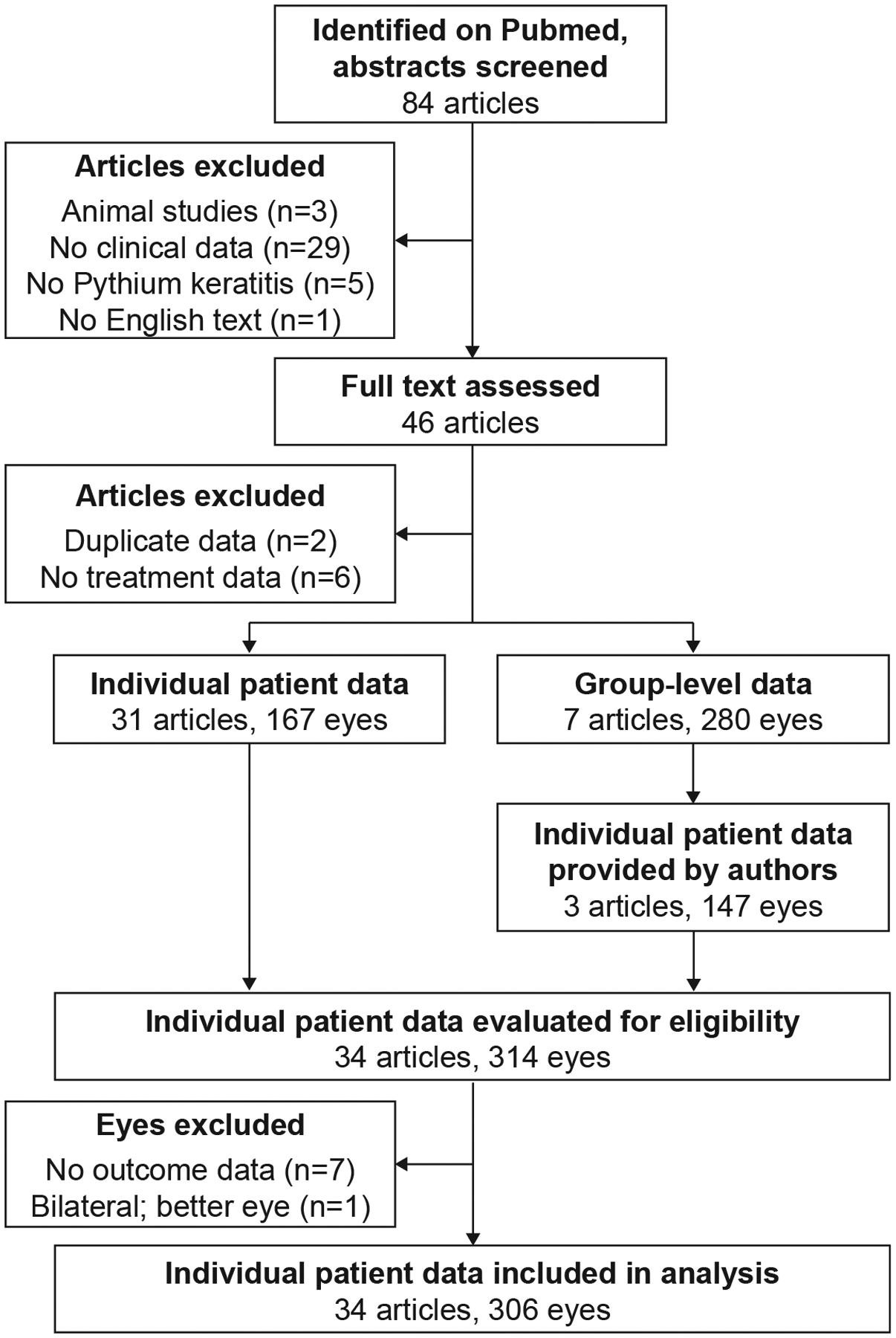
Study flow.
Table 1.
Demographic and clinical characteristics
| Individual-level data | Group-level data only | ||||
|---|---|---|---|---|---|
| Characteristics | Studies N = 34 (%) |
Patients N = 306 (%) |
Studies n=4 (%) |
Patients n=133 (%) |
|
| Countries | |||||
| India | 14 (41) | 209 (68) | 3 (75) | 103 (77) | |
| Thailand | 8 (24) | 83 (27) | 1 (25) | 30 (23) | |
| Others | 12 (35) | 14 (5) | 0 | 0 | |
| Sites | |||||
| Aravind (Madurai, India) | 1 (3) | 67 (22) | 0 | 0 | |
| Sitalakshmi (Tamil Nadu, India) | 3 (9) | 58 (19) | 0 | 0 | |
| Chulalongkorn (Bangkok, Thailand) | 2 (6) | 35 (11) | 1 (25) | 30 (23) | |
| Srinagarind (Khon Kaen, Thailand) | 3 (9) | 33 (11) | 0 | 0 | |
| LV Pradad (Hyderabad, India) | 5 (15) | 33 (11) | 2 (50) | 90 (68) | |
| Aravind (Pondicherry, India) | 1 (3) | 30 (10) | 0 | 0 | |
| LV Prasad (Bhubaneswar, India) | 1 (3) | 18 (6) | 1 (50) | 13 (9) | |
| Ramathibodi (Bangkok, Thailand) | 3 (9) | 15 (5) | 0 | 0 | |
| Others | 15 (44) | 17 (6) | 0 | 0 | |
Figure 2: Studies about Pythium keratitis over time.
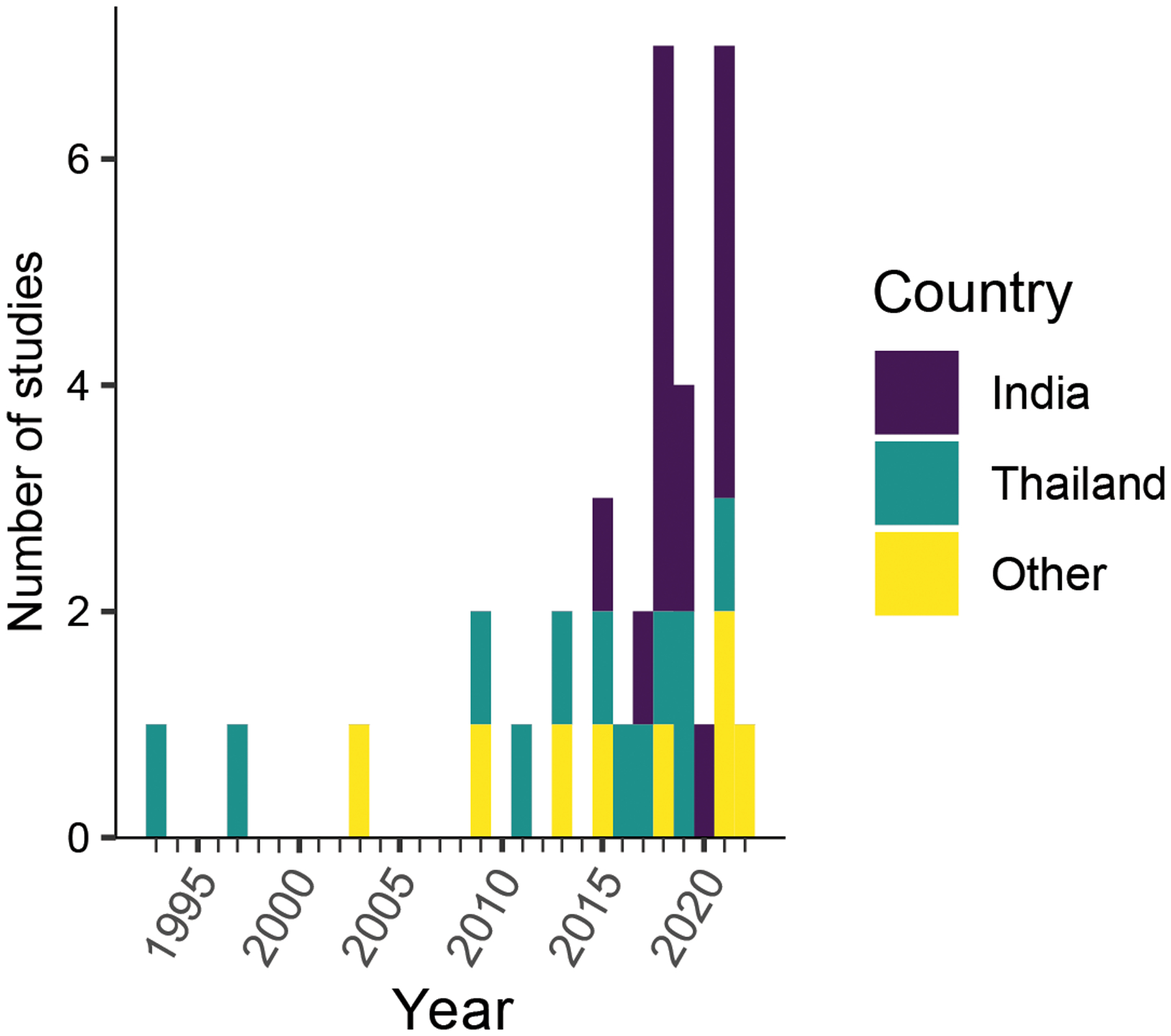
The number of studies published on Pubmed per year, identified with the search terms: (pythium[tiab] AND (keratitis[tiab] OR “corneal ulcer”[tiab])) OR “ocular pythiosis”[tiab]
Overall, the study population (n=306) had a mean age of 43 years (95%CI 41 – 44 years), and 37% (95%CI 32–42%) were female. The most commonly reported occupation was farming (42%, 95%CI 28–58%, from 166 records with available data), and the most commonly reported risk factor was exposure to vegetative matter (38%, 95%CI 24–54%, from 215 records with available data). Visual acuity was generally poor at presentation (mean logMAR 1.9, 95%CI 1.7–2.2, among 213 records with available data). The median length of follow-up among the 213 records with available data was 82 days (IQR 30–365). Other demographic and clinical data from the initial presentation are shown in Table 2, stratified by country (i.e., India, Thailand, or other).
Table 2.
Characteristics at presentation, Pythium keratitis
| India | Thailand | Other | ||||
|---|---|---|---|---|---|---|
| Number with data (n=209) | Mean or Proportion (95%CI) | Number with data (n=83) | Mean or Proportion (95%CI) | Number with data (n=14) | Mean or Proportion (95%CI) | |
| Age, years | 209 | 43 (41–45) | 83 | 44 (42–47) | 14 | 30 (19–41) |
| Female | 209 | 34% (27–42%) | 83 | 42% (34–51%) | 14 | 43% (21–68%) |
| Occupation | ||||||
| Farmer | 104 | 46% (37–56%) | 60 | 37% (10–75%) | 2 | 0% |
| Other | 104 | 54% (44–63%) | 60 | 63% (25–90%) | 2 | 100% |
| Risk factors | ||||||
| Ocular injury | 137 | 32% (16–60%) | 64 | 28% (13–50%) | 14 | 14% (3–46%) |
| Vegetative matter | 137 | 40% (22–62%) | 64 | 38% (21–57%) | 14 | 14% (4–39%) |
| Water exposure | 137 | 7% (3–16%) | 64 | 53% (33–72%) | 14 | 79% (53–92%) |
| Contact lens use | 137 | 1% (0–7%) | 64 | 11% (2–52%) | 14 | 50% (21–79%) |
| Days to presentation | 147 | 18 (13–24) | 64 | 13 (7–19) | 14 | 14 (6–22) |
| Ulcer size, mm | 129 | 6.3 (4.4–8.2) | 55 | 5.5 (4.4–6.6) | 7 | 4.6 (2.9–6.2) |
| VA at presentationa | 148 | 1.9 (1.6–2.2) | 57 | 2.1 (2.0–2.3) | 8 | 1.0 (0.5–1.6) |
| Days to last follow-up | 157 | 195 (16–374) | 42 | 407 (166–648) | 14 | 1240 (0–3141) |
Visual acuity converted into logMAR units
Medical therapy for Pythium keratitis was variable, and included many classes of antifungals, antibiotics, and antiparasitics (Table 3). Overall, the most commonly use medication was topical natamycin (76%), followed by a topical azole (61%). Use of an oral azole was also relatively common (31%). The most commonly used antibiotic among the entire study population was a topical fluoroquinolone (25%), although in India topical azithromycin and linezolid were more commonly used (29% each). Oral antibiotics were used less frequently than topical forms, and most commonly prescribed in India (oral azithromycin 14%, oral linezolid 4%). Topical antiparasitics were instituted less often than other medications. The proportion of patients treated with a biguanide agent was 3% in India and 5% in Thailand.
Table 3.
Medical treatment for Pythium keratitis prior to eye surgery
| India | Thailand | Other | |||||
|---|---|---|---|---|---|---|---|
| Medical treatment | Number with data n=209 | Proportion (95%CI) | Number with data n=83 | Proportion (95%CI) | Number with data n=14 | Proportion (95%CI) | |
| Topical antibiotic | |||||||
| Fluoroquinolone | 173 | 21% (6–51%) | 75 | 31% (9–66%) | 14 | 36% (15–63%) | |
| Vancomycin | 173 | 1% (0–5%) | 75 | 4% (2–9%) | 14 | 36% (17–61%) | |
| Cephalosporin | 173 | 1% (0–4%) | 75 | 16% (7–34%) | 14 | 36% (17–61%) | |
| Aminoglycoside | 173 | 5% (2–16%) | 75 | 12% (5–28%) | 14 | 36% (17–61%) | |
| Azithromycin | 209 | 29% (9–61%) | 75 | 7% (1–27%) | 14 | 0% | |
| Linezolid | 209 | 29% (7–67%) | 75 | 4% (1–17%) | 14 | 0% | |
| Topical antifungal | |||||||
| Natamycin | 165 | 80% (47–95%) | 83 | 75% (55–88%) | 14 | 43% (21–67%) | |
| Azole | 165 | 50% (25–75%) | 83 | 82% (61–93) | 14 | 71% (40–90%) | |
| Amphotericin B | 165 | 2% (0–7%) | 83 | 59% (45–72) | 14 | 21% (6–53%) | |
| Topical antiparasitic | |||||||
| Biguanide | 209 | 3% (2–5%) | 75 | 5% (2–17%) | 14 | 29% (10–30%) | |
| Diamidine | 209 | 0% | 75 | 4% (1–20%) | 14 | 14% (3–46%) | |
| Oral antibiotic | |||||||
| Oral azithromycin | 209 | 14% (3–45%) | 83 | 4% (1–11%) | 14 | 0% | |
| Oral linezolid | 209 | 4% (1–22%) | 83 | 1% (0–1%) | 14 | 7% (1–40%) | |
| Other oral antibiotics | 209 | 0% (2–11%) | 83 | 4% (1–11%) | 14 | 7% (1–40%) | |
| Oral antifungal | |||||||
| Oral azole | 209 | 10% (3–31%) | 83 | 78% (48–94%) | 14 | 57% (33–79%) | |
| Oral terbinafine | 209 | 0% | 83 | 37% (17–64%) | 14 | 0% | |
| Intrastromal injections | |||||||
| Intrastromal amphotericin | 209 | 0% | 83 | 39% (15–69%) | 14 | 10% (3–32%) | |
| Intrastromal azole | 209 | 0% (0–4%) | 83 | 6% (2–19%) | 14 | 14% (4–39%) | |
| Intravenous medication | |||||||
| Intravenous antibiotic | 209 | 0% (0–4%) | 83 | 0% | 14 | 21% (6–53%) | |
| Intravenous antifungal | 209 | 0% (0–4%) | 83 | 0% | 14 | 21% (6–53%) | |
| Oral and/or topical | |||||||
| Linezolid | 209 | 29% (7–67%) | 75 | 4% (1–17%) | 14 | 7% (1–41%) | |
| Azithromycin | 209 | 29% (9–61%) | 75 | 22% (9–46%) | 14 | 8% (2–25%) |
Outcomes were also variable, with an average of 80% (95%CI 70–87%) requiring TPK, and 25% (95%CI 13–43%) progressing to evisceration, enucleation, or exenteration (Figure 3). Univariable analyses provided evidence suggesting that disease severity at presentation was associated with subsequent need for TPK (Table 4) and globe removal (Table 5). For example, the mean infiltrate size at presentation was larger in patients who eventually required TPK (6.4mm vs. 4.6mm among 191 records with available data), and the presentation visual acuity worse (logMAR 2.0 vs 1.7). Use of linezolid and/or azithromycin decreased the need of TPK (Table 4) and globe removal (Table 5) in univariable analyses.
Figure 3: Fraction of patients in studies of Pythium keratitis undergoing therapeutic keratoplasty or globe removal.
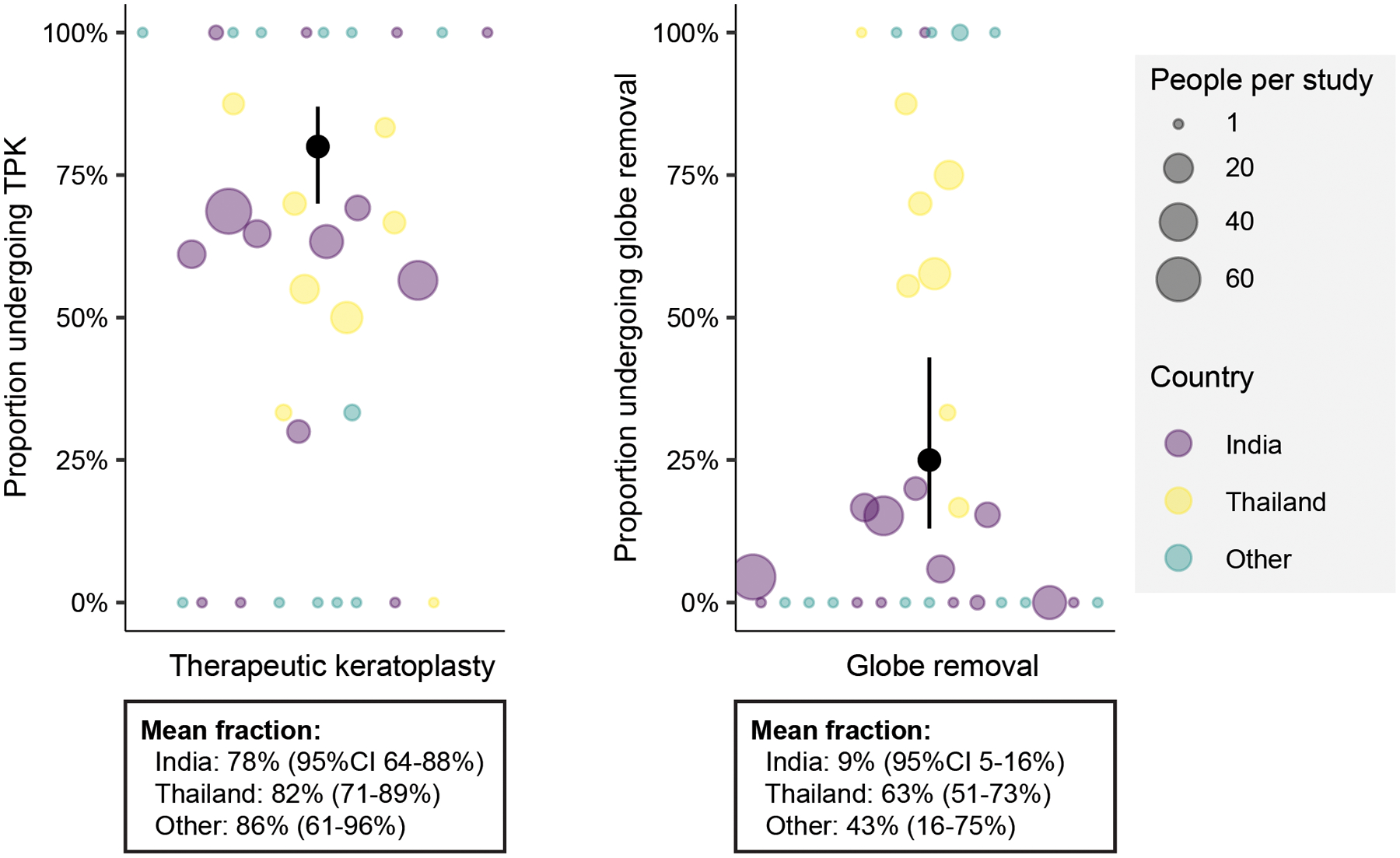
Each marker represents a different study, sized relative to the number of patients in the study, and colored by country. The black marker and bars represent the overall mean and confidence intervals.
Table 4.
Risk factors for therapeutic keratoplasty in Pythium keratitis, univariable analyses.
| TPK- | TPK+ | Univariable | ||
|---|---|---|---|---|
| Factor | n=62 | n=244 | Relative Risk | p-value |
| Feature at presentation | ||||
| Country | 0.77 | |||
| India | 45/62 (73%) | 164/244 (67%) | 0.92 (0.71–1.17) | 0.48 |
| Thailand | 15/62 (24%) | 68/244 (28%) | 0.96 (0.77–1.19) | 0.68 |
| Other | 2/62 (3%) | 12/244 (5%) | Reference | |
| Age, years | 43 ±18 | 43 ±16 | 1.00 (0.99–1.00) | 0.88 |
| Female | 22/62 (35%) | 90/244 (37%) | 0.99 (0.89–1.10) | 0.81 |
| Farmer | 17/40 (43%) | 53/126 (42%) | 1.00 (0.78–1.29) | 0.97 |
| Risk factor | ||||
| Ocular injury | 12/45 (27%) | 51/170 (30%) | 1.03 (0.85–1.26) | 0.73 |
| Vegetative matter | 20/45 (44%) | 61/170 (36%) | 0.93 (0.78–1.11) | 0.38 |
| Water | 13/45 (29%) | 41/170 (24%) | 0.95 (0.81–1.11) | 0.50 |
| Contact lens | 1/45 (2%) | 14/170 (8%) | 1.20 (1.00–1.43) | 0.05 |
| Days to presentation | 12 ±10 | 16 ±14 | 1.00 (1.00–1.01) | 0.04 |
| Ulcer size, mm | 4.6 ±2.0 | 6.4 ±3.0 | 1.05 (1.02–1.08) | 0.003 |
| logMAR vision | 1.7 ±0.9 | 2.0 ±0.7 | 1.10 (0.99–1.24) | 0.08 |
| Medical treatment | ||||
| Topical antibiotic | ||||
| Fluoroquinolone | 9/61 (15%) | 55/201 (27%) | 1.17 (1.01–1.34) | 0.03 |
| Vancomycin | 2/61 (3%) | 7/201 (3%) | 1.01 (0.69–1.48) | 0.94 |
| Cephalosporin | 3/61 (5%) | 16/201 (8%) | 1.11 (0.92–1.33) | 0.28 |
| Aminoglycoside | 5/61 (8%) | 18/201 (9%) | 1.02 (0.83–1.26) | 0.83 |
| Azithromycin | 21/61 (34%) | 44/237 (19%) | 0.82 (0.69–0.97) | 0.03 |
| Linezolid | 21/61 (34%) | 42/237 (18%) | 0.80 (0.70–0.93) | 0.004 |
| Topical antifungal | ||||
| Natamycin | 50/62 (81%) | 150/200 (75%) | 0.93 (0.77–1.12) | 0.44 |
| Azole | 32/62 (52%) | 128/200 (64%) | 1.13 (0.99–1.30) | 0.07 |
| Amphotericin B | 10/62 (16%) | 45/200 (23%) | 1.09 (0.98–1.22) | 0.11 |
| Topical antiparasitic | ||||
| Biguanide | 2/61 (3%) | 12/237 (5%) | 1.08 (0.91–1.28) | 0.35 |
| Diamidine | 0/61 (0%) | 5/237 (2%) | 1.26 (1.13–1.42) | <0.001 |
| Oral antibiotic | ||||
| Oral azithromycin | 11/62 (18%) | 21/244 (9%) | 0.81 (0.67–0.98) | 0.03 |
| Oral linezolid | 3/62 (5%) | 7/244 (3%) | 0.87 (0.67–1.15) | 0.32 |
| Other | 0/62 (0%) | 4/244 (2%) | 1.26 (1.13–1.41) | <0.001 |
| Oral antifungal | ||||
| Oral azole | 18/62 (29%) | 76/244(31%) | 1.02 (0.85–1.22) | 0.82 |
| Oral terbinafine | 6/62 (10%) | 25/244 (10%) | 1.01 (0.86–1.20) | 0.88 |
| Intrastromal injection | ||||
| Amphotericin | 6/62 (10%) | 26/244 (11%) | 1.02 (0.84–1.24) | 0.83 |
| Azole | 1/62 (2%) | 7/244 (3%) | 1.10 (0.79–1.54) | 0.56 |
| Intravenous | ||||
| Antibiotic | 0/62 (0%) | 4/244 (2%) | 1.26 (.13–1.41) | <0.001 |
| Antifungal | ||||
| Oral and/or topical | ||||
| Azithromycin | 21/61 (34%) | 45/237 (19%) | 0.82 (0.70–0.98) | 0.03 |
| Linezolid | 22/61 (36%) | 42/237 (18%) | 0.79 (0.68–0.91) | 0.002 |
Table 5.
Risk factors for globe removal in Pythium keratitis, univariable analyses.
| EEE- | EEE+ | Univariable | ||
|---|---|---|---|---|
| Factor | n=229 | n=77 | Risk Ratiob | p-value |
| Feature at presentation | ||||
| Country | <0.001 | |||
| India | 190/229 (83%) | 19/77 (25%) | 0.21 (0.08–0.57) | 0.003 |
| Thailand | 31/229 (14%) | 6/77 (8%) | 1.46 (0.64–3.32) | 0.35 |
| Other | 8/229 (3%) | 52/77 (67%) | Reference | |
| Age, years | 40 ±17 | 49 ±12 | 1.03 (1.01–1.04) | 0.001 |
| Female | 84/229 (37%) | 28/77 (36%) | 0.99 (0.69–1.42) | 0.95 |
| Farmer | 45/119 (38%) | 25/47 (53%) | 1.56 (0.39–6.2) | 0.50 |
| Risk factor | ||||
| Ocular injury | 43/157 (27%) | 20/58 (34%) | 1.27 (0.59–2.75) | 0.53 |
| Vegetative matter | 53/157 (34%) | 28/58 (48%) | 1.54 (0.78–3.07) | 0.21 |
| Water | 26/157 (17%) | 28/58 (48%) | 2.78 (1.80–4.30) | <0.001 |
| Contact lens | 11/157 (7%) | 4/58 (7%) | 0.99 (0.34–2.85) | 0.98 |
| Days to presentation | 16 ±14 | 15 ±12 | 1.00 (0.97–1.02) | 0.64 |
| Ulcer size, mm | 5.8 ±3.0 | 6.8 ±2.5 | 1.10 (0.95–1.26) | 0.18 |
| logMAR vision | 1.8 ±0.8 | 2.2 ±0.5 | 1.97 (1.26–3.09) | 0.005* |
| Medical treatment | ||||
| Topical antibiotic | ||||
| Fluoroquinolone | 48/198 (24%) | 16/64 (25%) | 1.03 (0.47–2.24) | 0.94 |
| Vancomycin | 5/198 (3%) | 4/64 (6%) | 1.87 (0.80–4.37) | 0.14 |
| Cephalosporin | 10/198 (5%) | 9/64 (14%) | 2.09 (1.02–4.28) | 0.04 |
| Aminoglycoside | 16/198 (8%) | 7/64 (11%) | 1.28 (0.68–2.39) | 0.43 |
| Azithromycin | 59/228 (26%) | 6/70 (9%) | 0.34 (0.08–1.39) | 0.13 |
| Linezolid | 59/228 (26%) | 4/70 (6%) | 0.23 (0.06–0.93) | 0.04 |
| Topical antifungal | ||||
| Natamycin | 142/192 (74%) | 58/70 (83%) | 1.50 (0.60–3.77) | 0.38 |
| Azole | 110/192 (57%) | 50/70 (71%) | 1.59 (0.83–3.06) | 0.16 |
| Amphotericin B | 23/192 (12%) | 32/70 (46%) | 3.17 (1.53–6.57) | 0.003 |
| Topical antiparasitic | ||||
| Biguanide | 10/228 (4.4%) | 4/70 (6%) | 1.23 (0.47–3.22) | 0.67 |
| Diamidine | 2/228 (1%) | 3/70 (4%) | 2.62 (1.09–6.29) | 0.03 |
| Oral antibiotic | ||||
| Oral azithromycin | 28/229 (12%) | 4/77 (5%) | 0.47 (0.15–1.44) | 0.18 |
| Oral linezolid | 8/229 (3%) | 2/77 (3%) | 0.79 (0.30–2.10) | 0.63 |
| Other | 4/229 (2%) | 0/77 (0%) | <0.01 | <0.001 |
| Oral antifungal | ||||
| Oral azole | 42/229 (18%) | 52/77 (68%) | 4.69 (2.19–10.07) | <0.001 |
| Oral terbinafine | 16/229 (7%) | 15/77 (19%) | 2.15 (1.07–4.31) | 0.03 |
| Intrastromal injection | ||||
| Amphotericin | 7/229 (3%) | 277 (32%) | 4.12 (2.04–8.31) | <0.001 |
| Azole | 5/229 (2%) | 3/77 (4%) | 1.51 (0.64–3.59) | 0.34 |
| Intravenous | ||||
| Antibiotic | 3/229 (1%) | 1/77 (1%) | 0.99 (0.15–6.59) | 0.99 |
| Antifungal | 3/229 (1%) | 1/77 (1%) | 0.99 (0.15–6.59) | 0.99 |
| Oral and/or topical | ||||
| Linezolid | 60/228 (26%) | 4/70 (6%) | 0.22 (0.05–0.90) | 0.04 |
| Azithromycin | 60/228 (26%) | 6/70 (9%) | 0.33 (0.08–1.36) | 0.12 |
Risk ratio (RR) assessed from log-binomial regression, weighted by number of observations per study. RR indicates the risk of the outcome for each additional 10% of the study population treated with the medication.
Although the amount of missing data limited the complexity of modeling, multivariable models adjusted for country and age found fewer TPKs performed in patients treated with azithromycin (RR 0.82, 95%CI 0.67–0.99; P=0.04) and linezolid (RR 0.80, 95%CI 0.67–0.96; P=0.02) (n=298 observations with non-missing data included in the analysis; Table 6). Fewer globe removal surgeries were also performed in patients treated with azithromycin and linezolid, although this association was not significant (Table 6). Sensitivity analyses restricted to India (i.e., where the vast majority of linezolid and azithromycin was prescribed) that were adjusted for age and presenting vision were consistent, finding fewer TPKs in those treated with azithromycin (RR 0.66, 95%CI 0.42–1.04; P=0.07) and linezolid (RR 0.79, 95%CI 0.58–1.07; P=0.12) (n=148 observations with non-missing data included in the analysis). Analyses adjusting for corneal ulcer size at presentation in addition to age and presenting vision demonstrated a similar, albeit weaker, association, with fewer TPKs in patients treated with azithromycin (RR=0.63, 95%CI 0.31–1.28; p=0.19) and linezolid (RR=0.8, 95%CI 0.53–1.21; p=0.28) (n=101 observations with non-missing data included in the analysis).
Table 6. Association between topical azithromycin and linezolid with poor outcomes in Pythium keratitis, multivariable analysis.
Values represent odds ratios with 95% confidence intervals.
| Risk Ratioa | ||||
|---|---|---|---|---|
| Factor | Azithromycin | Linezolid | ||
| Risk factor | TPK (n=298) |
Globe removal (n=298) |
TPK (n=298) |
Globe removal (n=298) |
| Relevant antibioticb | 0.82 (0.67–0.99) | 0.66 (0.23–1.85) | 0.80 (0.67–0.96) | 0.47 (0.14–1.51) |
| Country | ||||
| India | 0.98 (0.75–1.27) | 0.15 (0.06-.36) | 0.98 (0.75–1.27) | 0.16 (0.06–0.39) |
| Thailand | 0.97 (0.77–1.22) | 0.92 (0.51–1.67) | 0.96 (0.77–1.21) | 0.92 (0.51–1.65) |
| Other | Reference | Reference | Reference | Reference |
| Age, years | 1.00 (0.99–1.00) | 1.04 (1.02–1.05) | 1.00 (0.99–1.00) | 1.04 (1.02–1.05) |
TPK = therapeutic keratoplasty. Globe removal = evisceration, enucleation, or exenteration.
Risk ratio assessed from robust Poisson regression.
Relevant antibiotic refers to either topical azithromycin (results given in first two columns) or topical linezolid (results given in last two columns.
DISCUSSION
This review demonstrated that Pythium keratitis was associated with a poor prognosis, including high overall rates of TPK (80%) and globe removal (25%)—albeit with variability between studies, especially for the globe removal outcome. Topical antifungals were the most commonly reported medical therapies (e.g., natamycin in 76% and an azole in 61%), followed by oral antifungals (e.g., an oral azole in 31%) and topical antibiotics (e.g., a topical fluoroquinolone in 24%). Patients treated with linezolid and azithromycin were least likely to undergo TPK or globe removal, and multivariable models were consistent with a protective effect of these antibiotics, although models could not account for many possible confounders due to missing data. Linezolid/azithromycin therapy was used most commonly in India, and more complex multivariable models restricted to India were also consistent with a protective effect of linezolid/azithromycin, although did not meet criteria for statistical significance.
Surgical interventions have been more commonly reported for Pythium keratitis compared to prior reports of other forms of infectious keratitis. For example, the proportion of patients requiring TPK has been reported in other studies as approximately 6% for bacterial keratitis, 35–44% for fungal keratitis, and 9–43% for acanthamoeba keratitis.53–57 The proportion of patients requiring evisceration has been reported as approximately 6% for bacterial keratitis, 10% for fungal keratitis, and 5% for acanthamoeba keratitis.55,57 Although the rates reported in this meta-analysis may have been related to differential practice patterns at each of the study settings (e.g., globe removal more likely in Thailand than India), the findings of approximately 80% TPK and 25% globe removal confirm the clinical impression that Pythium is more difficult to control with medical therapy compared with other corneal infections.
The poor prognosis of Pythium infection reflects multiple challenges in diagnosis and treatment, including its variable clinical presentations, microbiological and histological resemblance to fungal keratitis, and especially, resistance to medical treatment. Although antifungals were commonly prescribed for Pythium keratitis due to the morphological similarities between Pythium and fungi, clinicians have found antifungals to have limited efficacy, and have sometimes resorted to methods not commonly used in other types of corneal infections, such as cryotherapy and alcohol.19–21,58 This meta-analysis confirmed that antifungal treatments were not associated with medical treatment success. Azithromycin and linezolid were originally shown to be effective against Pythium in vivo using a rabbit infection model.59,60 In 2017, azithromycin and linezolid were first used to treat Pythium keratitis, and patients treated with these antibiotics were noted to have a lower rate of TPK and higher rate of healing.17 The present study, which aggregated all individual patient data currently available, is consistent with these earlier reports, finding a lower rate of TPK among Pythium keratitis patients treated with linezolid and azithromycin. It is important to note that the association between linezolid/azithromycin and TPK was weaker in the multivariable analyses that included presentation visual acuity as a potential confounder. This suggests that there may be some unmeasured confounders that could account for some of the protective effect seen with linezolid/azithromycin, although it should also be noted that these multivariable models included only a subset of the study population due to missing data, and thus may not be representative of the total study population.
Azithromycin, a macrolide, and linezolid, an oxazolidinone, both inhibit protein synthesis by binding to the 50S subunit of the bacterial ribosome. It has been proposed that the likely mechanisms of azithromycin and linezolid against Pythium involve the inhibition of protein synthesis and their immunomodulatory effects.61 Both azithromycin and linezolid can suppress the production of pro-inflammatory cytokines and may reduce the inflammatory damage.61 Interestingly, linezolid was shown to have a superior efficacy and safety compared to azithromycin after prolonged treatment for more than 3–4 weeks in a rabbit model.60 However, since linezolid and azithromycin are used together in most cases reported in this study, it is hard to compare the efficacy of these two antibiotics when used clinically for Pythium keratitis. Further study into the antimicrobial mechanisms of action against Pythium is warranted, especially given the poor outcomes seen in Pythium keratitis.
Limitations of this review include its observational design, which increased the likelihood of biased assessment and reporting of exposure and outcome data, increased the potential for misclassified and missing data, and limited the opportunities to address potential confounding. The study is subject to reporting bias, since centers may not have been willing to report poor outcomes. The vast majority of cases treated with linezolid and azithromycin received both antibiotics together, so it is difficult to know if one of these antibiotics may be superior to the other. No randomized comparative trials were available, although trials may never be performed given the relative paucity of cases. The study drew mainly from Thailand and India, and the associations between linezolid/azithromycin therapy and outcomes derived mostly from the Indian reports. It is unclear if the results are generalizable to other settings.
In conclusion, this meta-analysis of individual patient data found high rates of TPK and globe removal in cases of Pythium keratitis. The study found that patients treated with azithromycin and linezolid had a lower frequency of TPK, suggesting these medications may be promising therapies for treatment of Pythium keratitis. Additional research is needed to determine the optimal treatments for Pythium keratitis.
Financial support:
This project was supported by the Fogarty International Center of the National Institutes of Health (NIH) under Award Number D43TW009343 and the University of California Global Health Institute (UCGHI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or UCGHI. The work was also supported by the All May See Foundation and Research to Prevent Blindness.
Footnotes
Conflict of interest: No conflicting relationship exists for any author.
REFERENCES
- 1.Gurnani B, Kaur K. Pythium Keratitis. In: StatPearls. StatPearls Publishing; 2022. AccessedMarch 31, 2022.http://www.ncbi.nlm.nih.gov/books/NBK573072/ [PubMed] [Google Scholar]
- 2.Gurnani B, Kaur K, Venugopal A, et al. Pythium insidiosum keratitis - A review. Indian Journal of Ophthalmology. 2022;70(4):1107–1120. doi: 10.4103/ijo.IJO_1534_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian J Ophthalmol. 2021;69(5):1095–1101. doi: 10.4103/ijo.IJO_1808_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurnani B, Kaur K, Agarwal S, et al. Pythium insidiosum Keratitis: Past, Present, and Future. Ophthalmol Ther. Published online; July 5, 2022. doi: 10.1007/s40123-022-00542-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badenoch PR, Mills RA, Chang JH, Sadlon TA, Klebe S, Coster DJ. Pythium insidiosum keratitis in an Australian child. Clinical & Experimental Ophthalmology. 2009;37(8):806–809. doi: 10.1111/j.1442-9071.2009.02135.x [DOI] [PubMed] [Google Scholar]
- 6.Barequet IS, Lavinsky F, Rosner M. Long-Term Follow-Up after Successful Treatment of Pythium insidiosum Keratitis in Israel. Seminars in Ophthalmology. 2013;28(4):247–250. doi: 10.3109/08820538.2013.788676 [DOI] [PubMed] [Google Scholar]
- 7.Bernheim D, Dupont D, Aptel F, et al. Pythiosis: Case report leading to new features in clinical and diagnostic management of this fungal-like infection. International Journal of Infectious Diseases. 2019;86:40–43. doi: 10.1016/j.ijid.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 8.Lelievre L, Borderie V, Garcia-Hermoso D, et al. Imported Pythium insidiosum Keratitis After a Swim in Thailand by a Contact Lens-Wearing Traveler. Am J Trop Med Hyg. 2015;92(2):270–273. doi: 10.4269/ajtmh.14-0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeno S, Oie Y, Sunada A, et al. Successful medical management of Pythium insidiosum keratitis using a combination of minocycline, linezolid, and chloramphenicol. Am J Ophthalmol Case Rep. 2019;15:100498. doi: 10.1016/j.ajoc.2019.100498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murdoch D, Parr D. Pythium insidiosum keratitis. Australian and New Zealand Journal of Ophthalmology. 1997;25(2):177–179. doi: 10.1111/j.1442-9071.1997.tb01304.x [DOI] [PubMed] [Google Scholar]
- 11.Neufeld A, Seamone C, Maleki B, Heathcote JG. Pythium insidiosum keratitis: a pictorial essay of natural history. Canadian Journal of Ophthalmology. 2018;53(2):e48–e50. doi: 10.1016/j.jcjo.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 12.Ros Castellar F, Sobrino-Jiménez C, del-Hierro-Zarzuelo A, Herrero-Ambrosio A, Boto-de-los-Bueis A. Intraocular minocycline for the treatment of ocular pythiosis. American Journal of Health-System Pharmacy. 2017;74(11):821–825. doi: 10.2146/ajhp160248 [DOI] [PubMed] [Google Scholar]
- 13.Tanhehco TY, Stacy RC, Mendoza L, Durand ML, Jakobiec FA, Colby KA. Pythium insidiosum Keratitis in Israel. Eye & Contact Lens. 2011;37(2):96–98. doi: 10.1097/ICL.0b013e3182043114 [DOI] [PubMed] [Google Scholar]
- 14.Virgile R, Perry HD, Pardanani B, et al. Human infectious corneal ulcer caused by Pythium insidiosum. Cornea. 1993;12(1):81–83. doi: 10.1097/00003226-199301000-00015 [DOI] [PubMed] [Google Scholar]
- 15.Rathi A, Chakrabarti A, Agarwal T, et al. Pythium Keratitis Leading to Fatal Cavernous Sinus Thrombophlebitis. Cornea. 2018;37(4):519–522. doi: 10.1097/ICO.0000000000001504 [DOI] [PubMed] [Google Scholar]
- 16.Hasika R, Lalitha P, Radhakrishnan N, Rameshkumar G, Prajna NV, Srinivasan M. Pythium keratitis in South India: Incidence, clinical profile, management, and treatment recommendation. Indian J Ophthalmol. 2019;67(1):42–47. doi: 10.4103/ijo.IJO_445_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagga B, Sharma S, Guda SJM, et al. Leap forward in the treatment of Pythium insidiosum keratitis. British Journal of Ophthalmology. 2018;102(12):1629–1633. doi: 10.1136/bjophthalmol-2017-311360 [DOI] [PubMed] [Google Scholar]
- 18.Permpalung N, Worasilchai N, Manothummetha K, et al. Clinical outcomes in ocular pythiosis patients treated with a combination therapy protocol in Thailand: A prospective study. Medical Mycology. 2019;57(8):923–928. doi: 10.1093/mmy/myz013 [DOI] [PubMed] [Google Scholar]
- 19.Agarwal S, Iyer G, Srinivasan B, Agarwal M, Panchalam Sampath Kumar S, Therese LK. Clinical profile of pythium keratitis: perioperative measures to reduce risk of recurrence. Br J Ophthalmol. 2018;102(2):153–157. doi: 10.1136/bjophthalmol-2017-310604 [DOI] [PubMed] [Google Scholar]
- 20.Agarwal S, Iyer G, Srinivasan B, et al. Clinical profile, risk factors and outcome of medical, surgical and adjunct interventions in patients with Pythium insidiosum keratitis. Br J Ophthalmol. 2019;103(3):296–300. doi: 10.1136/bjophthalmol-2017-311804 [DOI] [PubMed] [Google Scholar]
- 21.Agarwal S, Srinivasan B, Janakiraman N, et al. Role of Topical Ethanol in the Treatment of Pythium insidiosum Keratitis-A Proof of Concept. Cornea. 2020;39(9):1102–1107. doi: 10.1097/ICO.0000000000002370 [DOI] [PubMed] [Google Scholar]
- 22.Anutarapongpan O, Thanathanee O, Worrawitchawong J, Suwan-Apichon O. Role of Confocal Microscopy in the Diagnosis of Pythium insidiosum Keratitis. Cornea. 2018;37(2):156–161. doi: 10.1097/ICO.0000000000001466 [DOI] [PubMed] [Google Scholar]
- 23.Badenoch PR, Mills RAD, Chang JH, Sadlon TA, Klebe S, Coster DJ. Pythium insidiosum keratitis in an Australian child. Clin Exp Ophthalmol. 2009;37(8):806–809. doi: 10.1111/j.1442-9071.2009.02135.x [DOI] [PubMed] [Google Scholar]
- 24.Bagga B, Sharma S, Madhuri Guda SJ, et al. Leap forward in the treatment of Pythium insidiosum keratitis. Br J Ophthalmol. 2018;102(12):1629–1633. doi: 10.1136/bjophthalmol-2017-311360 [DOI] [PubMed] [Google Scholar]
- 25.Barequet IS, Lavinsky F, Rosner M. Long-term follow-up after successful treatment of Pythium insidiosum keratitis in Israel. Semin Ophthalmol. 2013;28(4):247–250. doi: 10.3109/08820538.2013.788676 [DOI] [PubMed] [Google Scholar]
- 26.Bernheim D, Dupont D, Aptel F, et al. Pythiosis: Case report leading to new features in clinical and diagnostic management of this fungal-like infection. Int J Infect Dis. 2019;86:40–43. doi: 10.1016/j.ijid.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee S, Agrawal D. Azithromycin in the Management of Pythium insidiosum Keratitis. Cornea. 2018;37(2):e8–e9. doi: 10.1097/ICO.0000000000001419 [DOI] [PubMed] [Google Scholar]
- 28.Gurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian J Ophthalmol. 2021;69(5):1095–1101. doi: 10.4103/ijo.IJO_1808_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasika R, Lalitha P, Radhakrishnan N, Rameshkumar G, Prajna NV, Srinivasan M. Pythium keratitis in South India: Incidence, clinical profile, management, and treatment recommendation. Indian J Ophthalmol. 2019;67(1):42–47. doi: 10.4103/ijo.IJO_445_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He H, Liu H, Chen X, Wu J, He M, Zhong X. Diagnosis and Treatment of Pythium Insidiosum Corneal Ulcer in a Chinese Child: A Case Report and Literature Review. Am J Case Rep. 2016;17:982–988. doi: 10.12659/ajcr.901158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou H, Wang Y, Tian L, Wang F, Sun Z, Chen Z. Pythium insidiosum keratitis reported in China, raising the alertness to this fungus-like infection: a case series. J Med Case Rep. 2021;15(1):619. doi: 10.1186/s13256-021-03189-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kate A, Bagga B, Ahirwar LK, Mishra DK, Sharma S. Unusual Presentation of Pythium Keratitis as Peripheral Ulcerative Keratitis: Clinical Dilemma. Ocul Immunol Inflamm. Published online August; 19, 2021:1–4. doi: 10.1080/09273948.2021.1952276 [DOI] [PubMed] [Google Scholar]
- 33.Kunavisarut S, Nimvorapan T, Methasiri S. Pythium corneal ulcer in Ramathibodi Hospital. J Med Assoc Thai. 2003;86(4):338–342. [PubMed] [Google Scholar]
- 34.Lekhanont K, Chuckpaiwong V, Chongtrakool P, Aroonroch R, Vongthongsri A. Pythium insidiosum keratitis in contact lens wear: a case report. Cornea. 2009;28(10):1173–1177. doi: 10.1097/ICO.0b013e318199fa41 [DOI] [PubMed] [Google Scholar]
- 35.Lelievre L, Borderie V, Garcia-Hermoso D, et al. Imported pythium insidiosum keratitis after a swim in Thailand by a contact lens-wearing traveler. Am J Trop Med Hyg. 2015;92(2):270–273. doi: 10.4269/ajtmh.14-0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeno S, Oie Y, Sunada A, et al. Successful medical management of Pythium insidiosum keratitis using a combination of minocycline, linezolid, and chloramphenicol. Am J Ophthalmol Case Rep. 2019;15:100498. doi: 10.1016/j.ajoc.2019.100498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittal R, Jena SK, Desai A, Agarwal S. Pythium Insidiosum Keratitis: Histopathology and Rapid Novel Diagnostic Staining Technique. Cornea. 2017;36(9):1124–1132. doi: 10.1097/ICO.0000000000001244 [DOI] [PubMed] [Google Scholar]
- 38.Murdoch D, Parr D. Pythium insidiosum keratitis. Aust N Z J Ophthalmol. 1997;25(2):177–179. doi: 10.1111/j.1442-9071.1997.tb01304.x [DOI] [PubMed] [Google Scholar]
- 39.Nonpassopon M, Jongkhajornpong P, Aroonroch R, Koovisitsopit A, Lekhanont K. Predisposing Factors, Clinical Presentations, and Outcomes of Contact Lens-Related Pythium Keratitis. Cornea. 2021;40(11):1413–1419. doi: 10.1097/ICO.0000000000002651 [DOI] [PubMed] [Google Scholar]
- 40.Permpalung N, Worasilchai N, Plongla R, et al. Treatment outcomes of surgery, antifungal therapy and immunotherapy in ocular and vascular human pythiosis: a retrospective study of 18 patients. J Antimicrob Chemother. 2015;70(6):1885–1892. doi: 10.1093/jac/dkv008 [DOI] [PubMed] [Google Scholar]
- 41.Puangsricharern V, Chotikkakamthorn P, Tulvatana W, et al. Clinical Characteristics, Histopathology, and Treatment Outcomes of Pythium Keratitis: A Retrospective Cohort Study. Clin Ophthalmol. 2021;15:1691–1701. doi: 10.2147/OPTH.S303721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raghavan A, Bellamkonda P, Mendoza L, Rammohan R. Pythium insidiosum and Acanthamoeba keratitis in a contact lens user. BMJ Case Rep. 2018;11(1):bcr-2018–226386. doi: 10.1136/bcr-2018-226386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramappa M, Nagpal R, Sharma S, Chaurasia S. Successful Medical Management of Presumptive Pythium insidiosum Keratitis. Cornea. 2017;36(4):511–514. doi: 10.1097/ICO.0000000000001162 [DOI] [PubMed] [Google Scholar]
- 44.Rathi A, Chakrabarti A, Agarwal T, et al. Pythium Keratitis Leading to Fatal Cavernous Sinus Thrombophlebitis. Cornea. 2018;37(4):519–522. doi: 10.1097/ICO.0000000000001504 [DOI] [PubMed] [Google Scholar]
- 45.Ros Castellar F, Sobrino Jiménez C, del Hierro Zarzuelo A, Herrero Ambrosio A, Boto de Los Bueis A. Intraocular minocycline for the treatment of ocular pythiosis. Am J Health Syst Pharm. 2017;74(11):821–825. doi: 10.2146/ajhp160248 [DOI] [PubMed] [Google Scholar]
- 46.Sharma S, Balne PK, Motukupally SR, et al. Pythium insidiosum keratitis: clinical profile and role of DNA sequencing and zoospore formation in diagnosis. Cornea. 2015;34(4):438–442. doi: 10.1097/ICO.0000000000000349 [DOI] [PubMed] [Google Scholar]
- 47.Sharma S, Rathi VM, Murthy SI, Garg P, Sharma S. Application of Trypan Blue Stain in the Microbiological Diagnosis of Infectious Keratitis-A Case Series. Cornea. 2021;40(12):1624–1628. doi: 10.1097/ICO.0000000000002725 [DOI] [PubMed] [Google Scholar]
- 48.Tanhehco TY, Stacy RC, Mendoza L, Durand ML, Jakobiec FA, Colby KA. Pythium insidiosum keratitis in Israel. Eye Contact Lens. 2011;37(2):96–98. doi: 10.1097/ICL.0b013e3182043114 [DOI] [PubMed] [Google Scholar]
- 49.Thanathanee O, Bhoomibunchoo C, Anutarapongpan O, Suwan-Apichon O, Charoensuk K, Chindamporn A. Role of Immunotherapy in Pythium insidiosum Keratitis. Am J Trop Med Hyg. 2022;107(1):110–112. doi: 10.4269/ajtmh.22-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thanathanee O, Enkvetchakul O, Rangsin R, Waraasawapati S, Samerpitak K, Suwan-apichon O. Outbreak of Pythium keratitis during rainy season: a case series. Cornea. 2013;32(2):199–204. doi: 10.1097/ICO.0b013e3182535841 [DOI] [PubMed] [Google Scholar]
- 51.Virgile R, Perry HD, Pardanani B, et al. Human infectious corneal ulcer caused by Pythium insidiosum. Cornea. 1993;12(1):81–83. doi: 10.1097/00003226-199301000-00015 [DOI] [PubMed] [Google Scholar]
- 52.Vishwakarma P, Mohanty A, Kaur A, et al. Pythium keratitis: Clinical profile, laboratory diagnosis, treatment, and histopathology features post-treatment at a tertiary eye care center in Eastern India. Indian J Ophthalmol. 2021;69(6):1544–1552. doi: 10.4103/ijo.IJO_2356_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogers GM, Goins KM, Sutphin JE, Kitzmann AS, Wagoner MD. Outcomes of Treatment of Fungal Keratitis at the University of Iowa Hospitals and Clinics: A 10-Year Retrospective Analysis. 2013;32(8):6. [DOI] [PubMed] [Google Scholar]
- 54.Jurkunas U, Behlau I, Colby K. Fungal Keratitis: Changing Pathogens and Risk Factors. 2009;28(6):6. [DOI] [PubMed] [Google Scholar]
- 55.Wong TY, Ng TP, Fong KS, Tan DT. Risk factors and clinical outcomes between fungal and bacterial keratitis: a comparative study. CLAO J. 1997;23(4):275–281. [PubMed] [Google Scholar]
- 56.Robaei D, Carnt N, Minassian DC, Dart JKG. The Impact of Topical Corticosteroid Use before Diagnosis on the Outcome of Acanthamoeba Keratitis. Ophthalmology. 2014;121(7):1383–1388. doi: 10.1016/j.ophtha.2014.01.031 [DOI] [PubMed] [Google Scholar]
- 57.Bouheraoua N, Gaujoux T, Goldschmidt P, Chaumeil C, Laroche L, Borderie VM. Prognostic Factors Associated With the Need for Surgical Treatments in Acanthamoeba Keratitis. Cornea. 2013;32(2):130–136. doi: 10.1097/ICO.0b013e31826429bd [DOI] [PubMed] [Google Scholar]
- 58.Chitasombat MN, Jongkhajornpong P, Lekhanont K, Krajaejun T. Recent update in diagnosis and treatment of human pythiosis. PeerJ. 2020;8:e8555. doi: 10.7717/peerj.8555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jesus FPK, Loreto ÉS, Ferreiro L, et al. In Vitro and In Vivo Antimicrobial Activities of Minocycline in Combination with Azithromycin, Clarithromycin, or Tigecycline against Pythium insidiosum. Antimicrob Agents Chemother. 2015;60(1):87–91. doi: 10.1128/AAC.01480-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahirwar LK, Kalra P, Sharma S, et al. Linezolid shows high safety and efficacy in the treatment of Pythium insidiosum keratitis in a rabbit model. Experimental Eye Research. 2021;202:108345. doi: 10.1016/j.exer.2020.108345 [DOI] [PubMed] [Google Scholar]
- 61.Medhasi S, Chindamporn A, Worasilchai N. A Review: Antimicrobial Therapy for Human Pythiosis. Antibiotics. 2022;11(4):450. doi: 10.3390/antibiotics11040450 [DOI] [PMC free article] [PubMed] [Google Scholar]


