Abstract
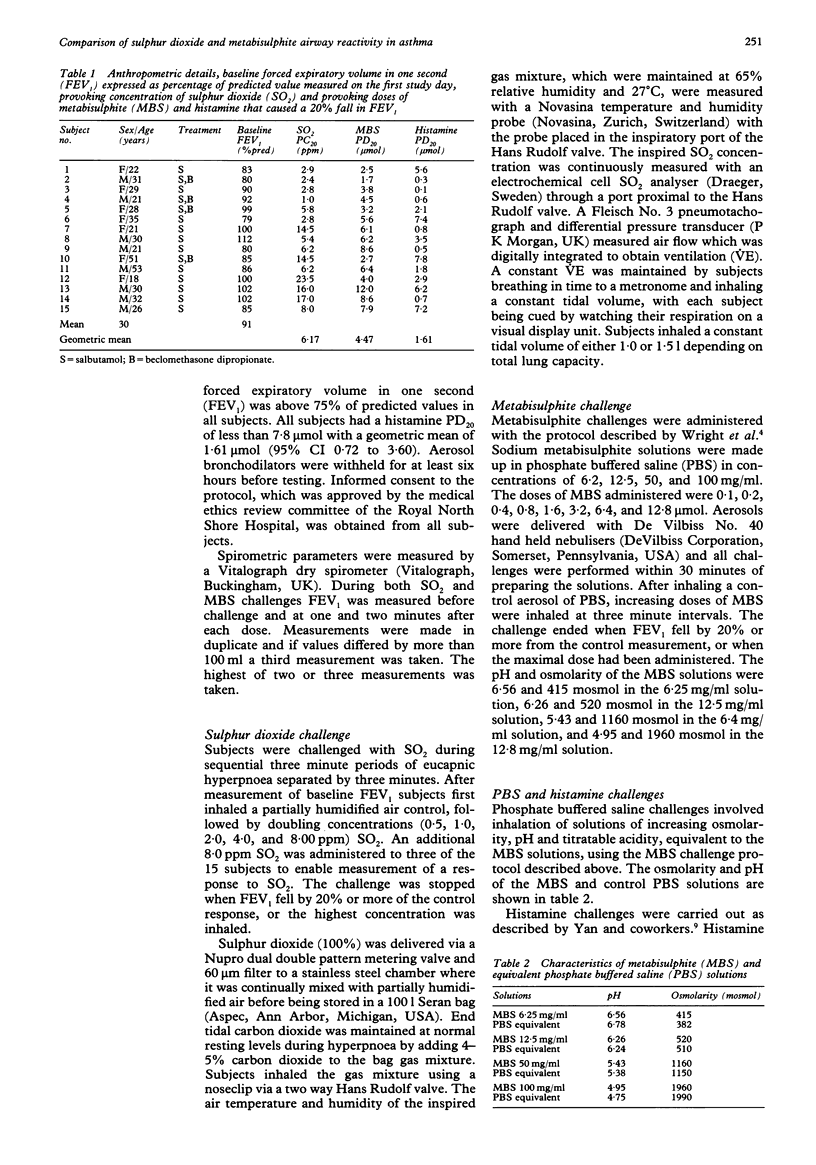
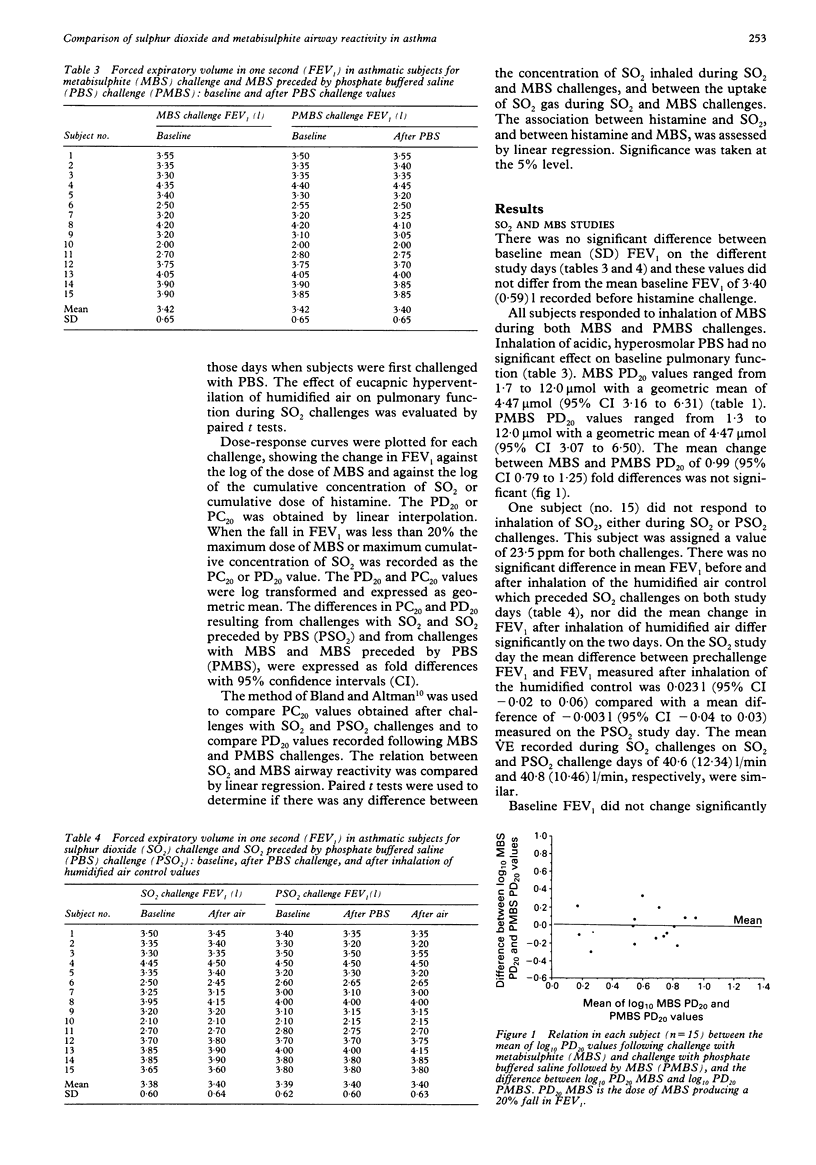
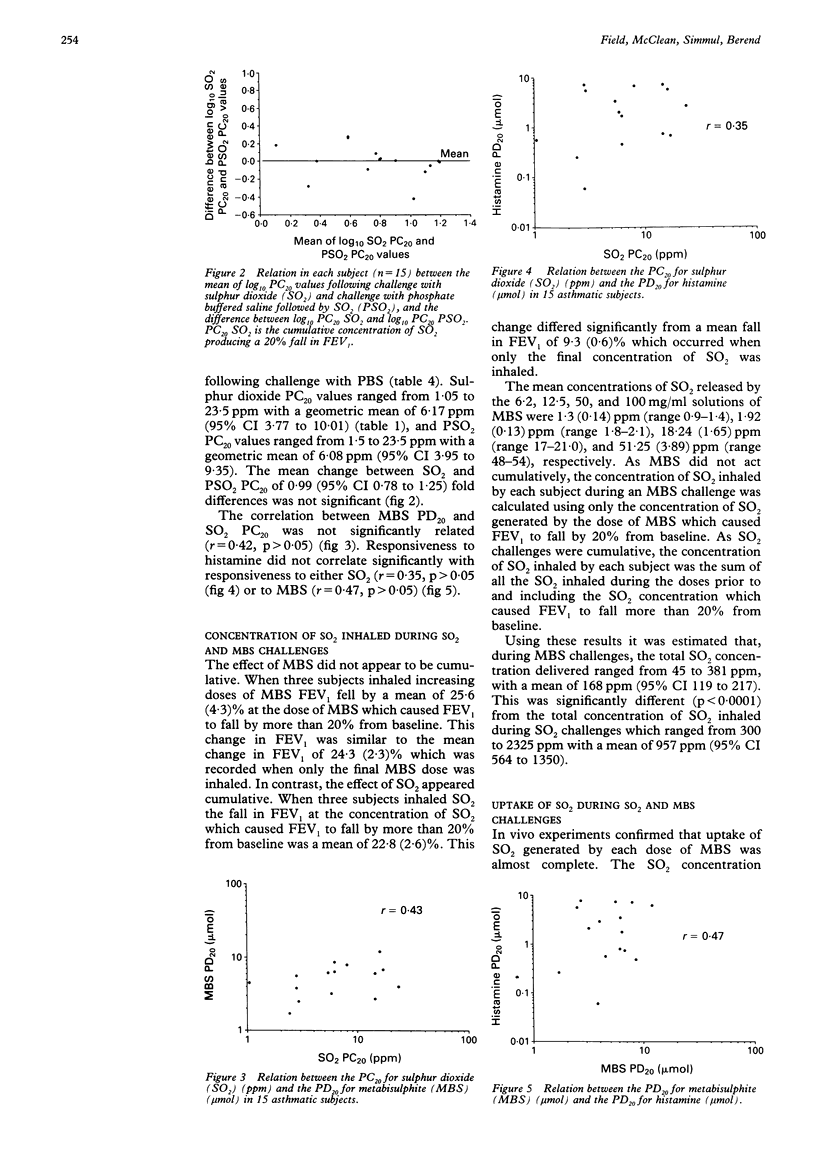
BACKGROUND--In asthmatic subjects bronchoconstriction is induced by inhalation of the common food preservatives sulphur dioxide (SO2) and metabisulphite (MBS). SO2 and MBS challenges share many similarities, but it is not known whether they are equivalent. In this study of subjects with mild clinical asthma equivalence was assessed by comparing SO2 and MBS reactivity by estimating the total dose of SO2 inhaled during SO2 and MBS challenges, and by calculating SO2 uptake during both challenges. In addition, as the MBS solutions inhaled were acidic and hyperosmolar, the effect of these factors on MBS responsiveness was investigated. METHODS--Fifteen subjects were challenged on separate days with doubling (0.5 to 8.0 ppm) concentrations of SO2 gas inhaled during three minute periods of isocapnic hyperventilation and MBS administered in doses ranging from 0.1 to 12.8 mumol using the Wright protocol. On two other days SO2 and MBS challenges were preceded by a challenge with phosphate buffered saline (PBS) solutions of pH and osmolarity similar to MBS solutions. Response was measured as the dose or concentration causing a 20% fall in FEV1 (PD20 or PC20). RESULTS--All subjects reacted to MBS and 14 responded to SO2. Geometric mean histamine PD20 was 1.61 mumol (95% confidence interval 0.72 to 3.60). MBS and SO2 airway responsiveness were not significantly related. Estimates of the mean concentration of SO2 inhaled during SO2 and MBS challenges differed, as did estimates of the mean SO2 uptake during both challenges. MBS and SO2 reactivity were not affected by prior challenge with PBS solutions. CONCLUSIONS--SO2 and MBS challenges are not comparable. MBS reactivity was not affected by the hyperosmolar, acidic nature of its solutions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bascom R., Bleecker E. R. Bronchoconstriction induced by distilled water. Sensitivity in asthmatics and relationship to exercise-induced bronchospasm. Am Rev Respir Dis. 1986 Aug;134(2):248–253. doi: 10.1164/arrd.1986.134.2.248. [DOI] [PubMed] [Google Scholar]
- Bland J. M., Altman D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–310. [PubMed] [Google Scholar]
- Bodem C. R., Lampton L. M., Miller D. P., Tarka E. F., Everett E. D. Endobronchial pH. Relevance of aminoglycoside activity in gram-negative bacillary pneumonia. Am Rev Respir Dis. 1983 Jan;127(1):39–41. doi: 10.1164/arrd.1983.127.1.39. [DOI] [PubMed] [Google Scholar]
- DALHAMN T., STRANDBERG L. Acute effect of sulphur dioxide on the rate of ciliary beat in the trachea of rabbit, in vivo and in vitro, with studies on the absorptional capacity of the nasal cavity. Int J Air Water Pollut. 1961 Sep;4:154–167. [PubMed] [Google Scholar]
- Delohery J., Simmul R., Castle W. D., Allen D. H. The relationship of inhaled sulfur dioxide reactivity to ingested metabisulfite sensitivity in patients with asthma. Am Rev Respir Dis. 1984 Dec;130(6):1027–1032. doi: 10.1164/arrd.1984.130.6.1027. [DOI] [PubMed] [Google Scholar]
- Dixon C. M., Fuller R. W., Barnes P. J. Effect of nedocromil sodium on sulphur dioxide induced bronchoconstriction. Thorax. 1987 Jun;42(6):462–465. doi: 10.1136/thx.42.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenbacher W. L., Bethel R. A., Boushey H. A., Sheppard D. Morphine sulfate inhibits bronchoconstriction in subjects with mild asthma whose responses are inhibited by atropine. Am Rev Respir Dis. 1984 Sep;130(3):363–367. doi: 10.1164/arrd.1984.130.3.363. [DOI] [PubMed] [Google Scholar]
- Fine J. M., Gordon T., Sheppard D. The roles of pH and ionic species in sulfur dioxide- and sulfite-induced bronchoconstriction. Am Rev Respir Dis. 1987 Nov;136(5):1122–1126. doi: 10.1164/ajrccm/136.5.1122. [DOI] [PubMed] [Google Scholar]
- Fine J. M., Gordon T., Thompson J. E., Sheppard D. The role of titratable acidity in acid aerosol-induced bronchoconstriction. Am Rev Respir Dis. 1987 Apr;135(4):826–830. doi: 10.1164/arrd.1987.135.4.826. [DOI] [PubMed] [Google Scholar]
- Frank N. R., Yoder R. E., Brain J. D., Yokoyama E. SO2 (35S labeled) absorption by the nose and mouth under conditions of varying concentration and flow. Arch Environ Health. 1969 Mar;18(3):315–322. doi: 10.1080/00039896.1969.10665414. [DOI] [PubMed] [Google Scholar]
- Nichol G. M., Nix A., Chung K. F., Barnes P. J. Characterisation of bronchoconstrictor responses to sodium metabisulphite aerosol in atopic subjects with and without asthma. Thorax. 1989 Dec;44(12):1009–1014. doi: 10.1136/thx.44.12.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petering D. H., Shih N. T. Biochemistry of bisulfite-sulfur dioxide. Environ Res. 1975 Feb;9(1):55–65. doi: 10.1016/0013-9351(75)90049-3. [DOI] [PubMed] [Google Scholar]
- Schoeffel R. E., Anderson S. D., Altounyan R. E. Bronchial hyperreactivity in response to inhalation of ultrasonically nebulised solutions of distilled water and saline. Br Med J (Clin Res Ed) 1981 Nov 14;283(6302):1285–1287. doi: 10.1136/bmj.283.6302.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz H. J., Chester E. H. Bronchospastic responses to aerosolized metabisulfite in asthmatic subjects: potential mechanisms and clinical implications. J Allergy Clin Immunol. 1984 Oct;74(4 Pt 1):511–513. doi: 10.1016/0091-6749(84)90387-7. [DOI] [PubMed] [Google Scholar]
- Sheppard D., Wong W. S., Uehara C. F., Nadel J. A., Boushey H. A. Lower threshold and greater bronchomotor responsiveness of asthmatic subjects to sulfur dioxide. Am Rev Respir Dis. 1980 Dec;122(6):873–878. doi: 10.1164/arrd.1980.122.6.873. [DOI] [PubMed] [Google Scholar]
- Sheppard D., Wong W. S., Uehara C. F., Nadel J. A., Boushey H. A. Lower threshold and greater bronchomotor responsiveness of asthmatic subjects to sulfur dioxide. Am Rev Respir Dis. 1980 Dec;122(6):873–878. doi: 10.1164/arrd.1980.122.6.873. [DOI] [PubMed] [Google Scholar]
- Speizer F. E., Frank N. R. The uptake and release of SO2 by the human nose. Arch Environ Health. 1966 Jun;12(6):725–728. doi: 10.1080/00039896.1966.10664471. [DOI] [PubMed] [Google Scholar]
- Wright W., Zhang Y. G., Salome C. M., Woolcock A. J. Effect of inhaled preservatives on asthmatic subjects. I. Sodium metabisulfite. Am Rev Respir Dis. 1990 Jun;141(6):1400–1404. doi: 10.1164/ajrccm/141.6.1400. [DOI] [PubMed] [Google Scholar]
- Yan K., Salome C., Woolcock A. J. Rapid method for measurement of bronchial responsiveness. Thorax. 1983 Oct;38(10):760–765. doi: 10.1136/thx.38.10.760. [DOI] [PMC free article] [PubMed] [Google Scholar]


