Abstract
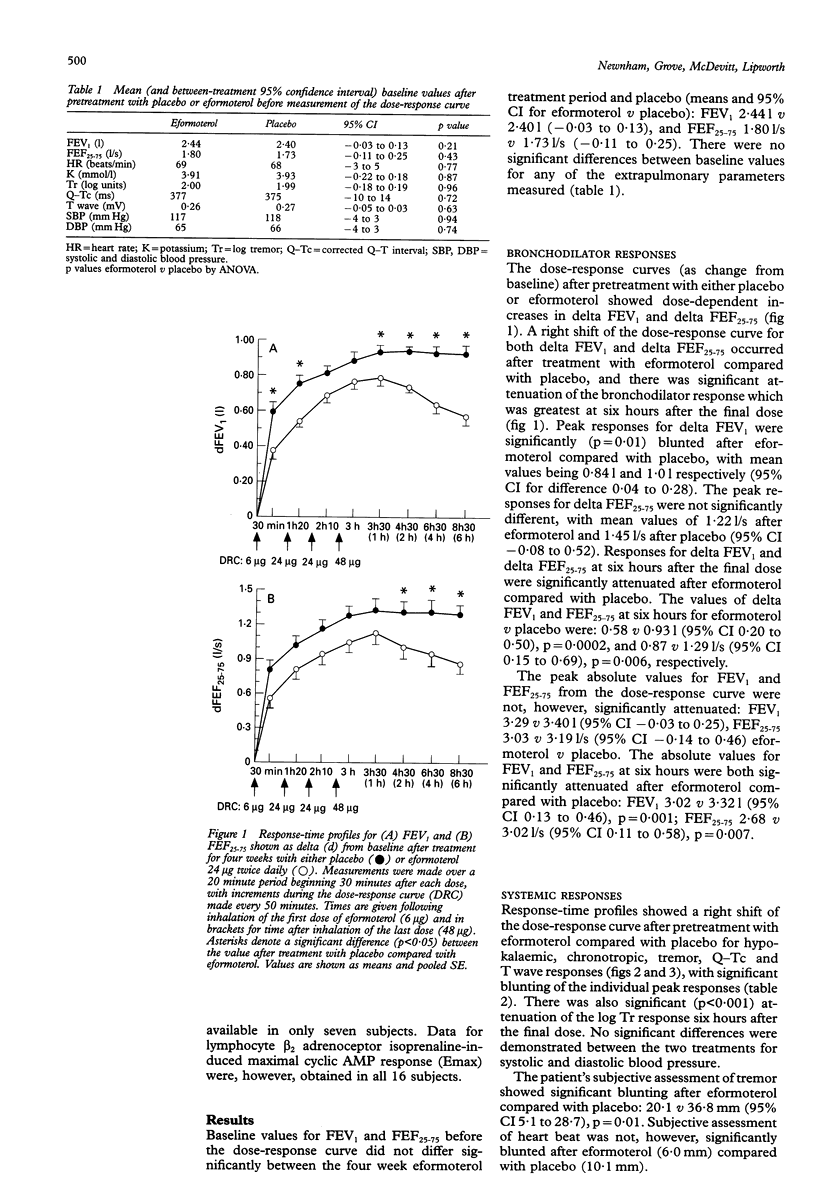
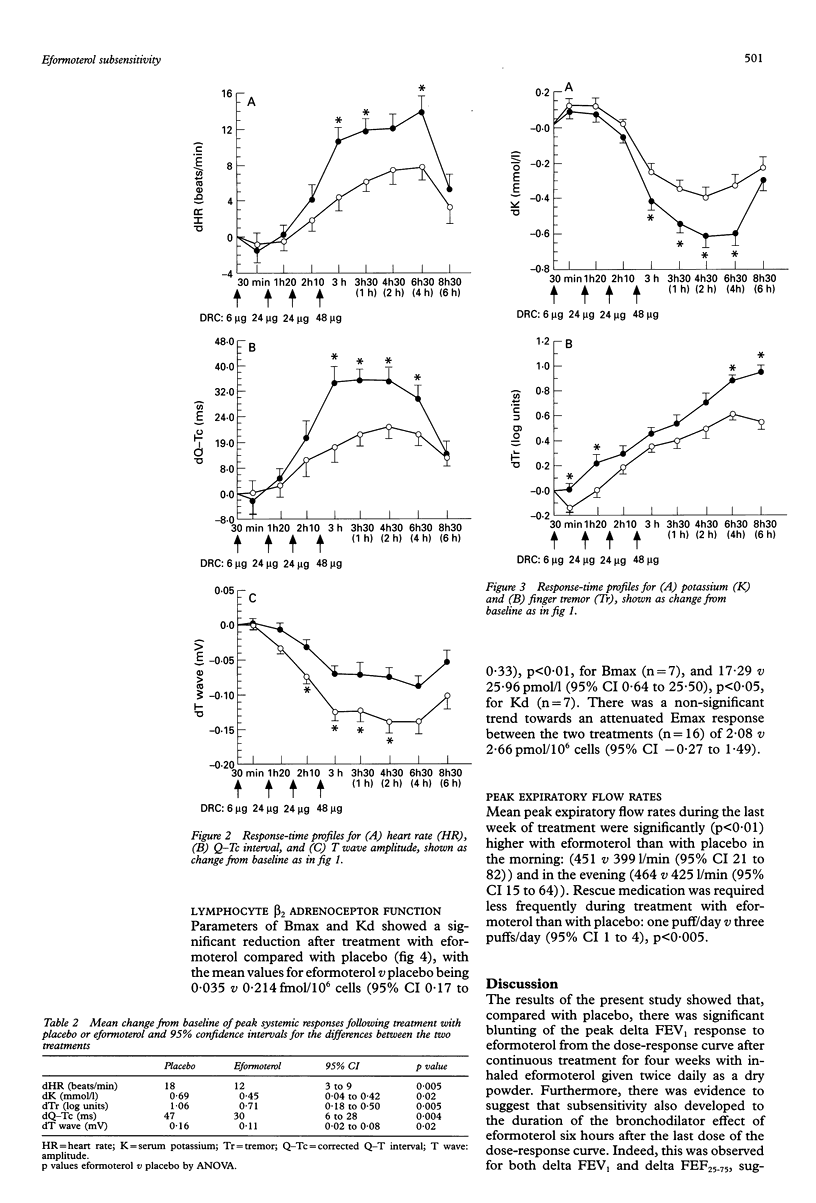
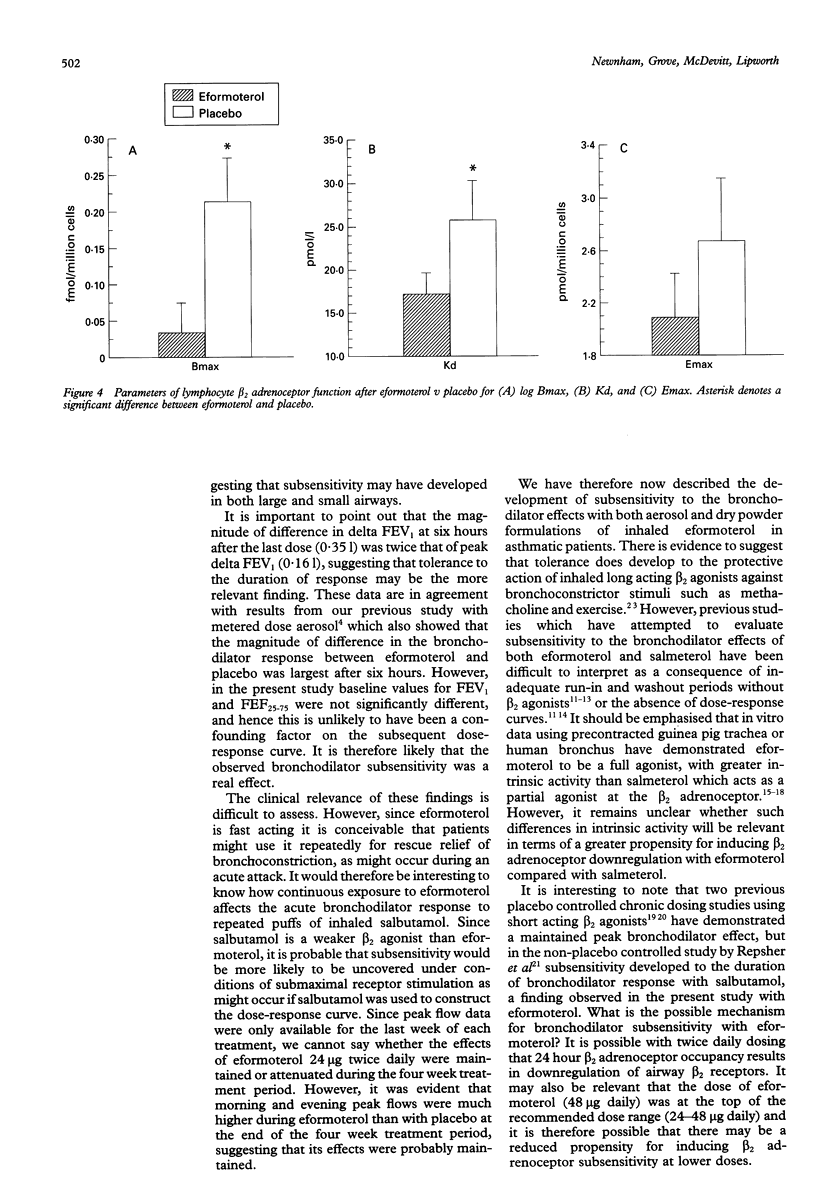
BACKGROUND--There is controversy as to the role of long acting beta 2 agonists such as eformoterol and, in particular, whether bronchodilator tolerance occurs during continuous therapy. The purpose of this study was to extend previous observations of bronchodilator subsensitivity with metered dose eformoterol aerosol in order to assess whether tolerance also occurs with a dry powder formulation of the same drug. METHODS--Sixteen asthmatic patients of mean age 33 (range 18-53) years and FEV1 (% predicted) of 64 (3)%, of whom 13 were receiving inhaled corticosteroids, received regular treatment with eformoterol 24 micrograms twice daily or placebo twice daily (without beta 2 agonists) given concurrently for four weeks in a randomised double blind cross-over design. An initial two week run-in was used when beta 2 agonists were withdrawn and substituted with ipratropium bromide. Dose-response curves to eformoterol (cumulative dose 6-102 micrograms) for airways and systemic beta 2 responses were constructed at the end of each treatment period. RESULTS--Baseline values for airways and systemic responses were similar. The peak delta FEV1 response from the dose-response curve (as change from baseline) and the delta response for FEV1 and FEF25-75 at six hours after the last dose were attenuated after eformoterol compared with placebo: peak delta FEV1 response 1.001 with placebo v 0.841 with eformoterol (95% CI 0.04 to 0.28); at six hours 0.931 with placebo v 0.581 with eformoterol (95% CI 0.20 to 0.50); and for delta FEF25-75 at six hours 1.29 1/s with placebo v 0.87 1/s with eformoterol (95% CI 0.15 to 0.69). Morning peak expiratory flow rate was significantly improved during treatment with eformoterol (451 1/min) compared with placebo (399 1/min) (95% CI 21 to 82). Systemic beta 2 responses were blunted after eformoterol, together with a reduction in lymphocyte beta 2 receptor binding density. CONCLUSIONS--Regular twice daily eformoterol dry powder may produce bronchodilator subsensitivity in terms of both peak and duration of response to cumulative repeated doses of eformoterol. Systemic beta 2-mediated adverse effects also showed tolerance, which was mirrored by downregulation of lymphocyte beta 2 adrenoceptors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidsson P., Larsson S., Löfdahl C. G., Melander B., Wåhlander L., Svedmyr N. Formoterol, a new long-acting bronchodilator for inhalation. Eur Respir J. 1989 Apr;2(4):325–330. [PubMed] [Google Scholar]
- Ball D. I., Brittain R. T., Coleman R. A., Denyer L. H., Jack D., Johnson M., Lunts L. H., Nials A. T., Sheldrick K. E., Skidmore I. F. Salmeterol, a novel, long-acting beta 2-adrenoceptor agonist: characterization of pharmacological activity in vitro and in vivo. Br J Pharmacol. 1991 Nov;104(3):665–671. doi: 10.1111/j.1476-5381.1991.tb12486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodde O. E., Brinkmann M., Schemuth R., O'Hara N., Daul A. Terbutaline-induced desensitization of human lymphocyte beta 2-adrenoceptors. Accelerated restoration of beta-adrenoceptor responsiveness by prednisone and ketotifen. J Clin Invest. 1985 Sep;76(3):1096–1101. doi: 10.1172/JCI112063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodde O. E., Howe U., Egerszegi S., Konietzko N., Michel M. C. Effect of prednisolone and ketotifen on beta 2-adrenoceptors in asthmatic patients receiving beta 2-bronchodilators. Eur J Clin Pharmacol. 1988;34(2):145–150. doi: 10.1007/BF00614551. [DOI] [PubMed] [Google Scholar]
- Cheung D., Timmers M. C., Zwinderman A. H., Bel E. H., Dijkman J. H., Sterk P. J. Long-term effects of a long-acting beta 2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med. 1992 Oct 22;327(17):1198–1203. doi: 10.1056/NEJM199210223271703. [DOI] [PubMed] [Google Scholar]
- Collier J. G., Dobbs R. J., Williams I. Salbutamol aerosol causes a tachycardia due to the inhaled rather than the swallowed fraction. Br J Clin Pharmacol. 1980 Mar;9(3):273–274. doi: 10.1111/j.1365-2125.1980.tb04837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi E., Borghi C., Cerchiari E. L., Della Puppa T., Francucci B. Analogue chromatic continuous scale (ACCS): a new method for pain assessment. Clin Exp Rheumatol. 1983 Oct-Dec;1(4):337–340. [PubMed] [Google Scholar]
- Hauck R. W., Böhm M., Gengenbach S., Sunder-Plassmann L., Fruhmann G., Erdmann E. Beta 2-adrenoceptors in human lung and peripheral mononuclear leukocytes of untreated and terbutaline-treated patients. Chest. 1990 Aug;98(2):376–381. doi: 10.1378/chest.98.2.376. [DOI] [PubMed] [Google Scholar]
- Küng M., Croley S. W., Phillips B. A. Systemic cardiovascular and metabolic effects associated with the inhalation of an increased dose of albuterol. Influence of mouth rinsing and gargling. Chest. 1987 Mar;91(3):382–387. doi: 10.1378/chest.91.3.382. [DOI] [PubMed] [Google Scholar]
- Lindén A., Bergendal A., Ullman A., Skoogh B. E., Löfdahl C. G. Salmeterol, formoterol, and salbutamol in the isolated guinea pig trachea: differences in maximum relaxant effect and potency but not in functional antagonism. Thorax. 1993 May;48(5):547–553. doi: 10.1136/thx.48.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipworth B. J., Clark R. A., Dhillon D. P., McDevitt D. G. Comparison of the effects of prolonged treatment with low and high doses of inhaled terbutaline on beta-adrenoceptor responsiveness in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990 Aug;142(2):338–342. doi: 10.1164/ajrccm/142.2.338. [DOI] [PubMed] [Google Scholar]
- Lipworth B. J., McDevitt D. G. Beta-adrenoceptor responses to inhaled salbutamol in normal subjects. Eur J Clin Pharmacol. 1989;36(3):239–245. doi: 10.1007/BF00558154. [DOI] [PubMed] [Google Scholar]
- Lipworth B. J., McDevitt D. G., Struthers A. D. Systemic beta-adrenoceptor responses to salbutamol given by metered-dose inhaler alone and with pear shaped spacer attachment: comparison of electrocardiographic, hypokalaemic and haemodynamic effects. Br J Clin Pharmacol. 1989 Jun;27(6):837–842. doi: 10.1111/j.1365-2125.1989.tb03447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipworth B. J. Risks versus benefits of inhaled beta 2-agonists in the management of asthma. Drug Saf. 1992 Jan-Feb;7(1):54–70. doi: 10.2165/00002018-199207010-00007. [DOI] [PubMed] [Google Scholar]
- Lipworth B. J., Struthers A. D., McDevitt D. G. Tachyphylaxis to systemic but not to airway responses during prolonged therapy with high dose inhaled salbutamol in asthmatics. Am Rev Respir Dis. 1989 Sep;140(3):586–592. doi: 10.1164/ajrccm/140.3.586. [DOI] [PubMed] [Google Scholar]
- Newnham D. M., Coutie W. J., McFarlane L. C., Lipworth B. J. Comparison of parameters of in vitro lymphocyte beta 2-adrenoceptor function in normal and asthmatic subjects. Eur J Clin Pharmacol. 1993;45(6):535–538. doi: 10.1007/BF00315310. [DOI] [PubMed] [Google Scholar]
- Newnham D. M., McDevitt D. G., Lipworth B. J. Bronchodilator subsensitivity after chronic dosing with eformoterol in patients with asthma. Am J Med. 1994 Jul;97(1):29–37. doi: 10.1016/0002-9343(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Newnham D. M., McDevitt D. G., Lipworth B. J. Comparison of the extrapulmonary beta2-adrenoceptor responses and pharmacokinetics of salbutamol given by standard metered dose-inhaler and modified actuator device. Br J Clin Pharmacol. 1993 Nov;36(5):445–450. doi: 10.1111/j.1365-2125.1993.tb00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman D. S., Chervinsky P., LaForce C., Seltzer J. M., Southern D. L., Kemp J. P., Dockhorn R. J., Grossman J., Liddle R. F., Yancey S. W. A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N Engl J Med. 1992 Nov 12;327(20):1420–1425. doi: 10.1056/NEJM199211123272004. [DOI] [PubMed] [Google Scholar]
- Ramage L., Lipworth B. J., Ingram C. G., Cree I. A., Dhillon D. P. Reduced protection against exercise induced bronchoconstriction after chronic dosing with salmeterol. Respir Med. 1994 May;88(5):363–368. doi: 10.1016/0954-6111(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Repsher L. H., Anderson J. A., Bush R. K., Falliers C. J., Kass I., Kemp J. P., Reed C., Siegel S., Webb D. R. Assessment of tachyphylaxis following prolonged therapy of asthma with inhaled albuterol aerosol. Chest. 1984 Jan;85(1):34–38. doi: 10.1378/chest.85.1.34. [DOI] [PubMed] [Google Scholar]
- Soediono P., Burnstock G. Contribution of ATP and nitric oxide to NANC inhibitory transmission in rat pyloric sphincter. Br J Pharmacol. 1994 Nov;113(3):681–686. doi: 10.1111/j.1476-5381.1994.tb17046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman A., Hedner J., Svedmyr N. Inhaled salmeterol and salbutamol in asthmatic patients. An evaluation of asthma symptoms and the possible development of tachyphylaxis. Am Rev Respir Dis. 1990 Sep;142(3):571–575. doi: 10.1164/ajrccm/142.3.571. [DOI] [PubMed] [Google Scholar]
- Wallin A., Melander B., Rosenhall L., Sandström T., Wåhlander L. Formoterol, a new long acting beta 2 agonist for inhalation twice daily, compared with salbutamol in the treatment of asthma. Thorax. 1990 Apr;45(4):259–261. doi: 10.1136/thx.45.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schayck C. P., Graafsma S. J., Visch M. B., Dompeling E., van Weel C., van Herwaarden C. L. Increased bronchial hyperresponsiveness after inhaling salbutamol during 1 year is not caused by subsensitization to salbutamol. J Allergy Clin Immunol. 1990 Nov;86(5):793–800. doi: 10.1016/s0091-6749(05)80185-x. [DOI] [PubMed] [Google Scholar]


