Abstract
Gene transfer to differentiated airway epithelia with existing viral vectors is very inefficient when they are applied to the apical surface. This largely reflects the polarized distribution of receptors on the basolateral surface. To identify new receptor-ligand interactions that might be used to redirect vectors to the apical surface, we investigated the process of infection of airway epithelial cells by human coronavirus 229E (HCoV-229E), a common cause of respiratory tract infections. Using immunohistochemistry, we found the receptor for HCoV-229E (CD13 or aminopeptidase N) localized mainly to the apical surface of airway epithelia. When HCoV-229E was applied to the apical or basolateral surface of well-differentiated primary cultures of human airway epithelia, infection primarily occurred from the apical side. Similar results were noted when the virus was applied to cultured human tracheal explants. Newly synthesized virions were released mainly to the apical side. Thus, HCoV-229E preferentially infects human airway epithelia from the apical surface. The spike glycoprotein that mediates HCoV-229E binding and fusion to CD13 is a candidate for pseudotyping retroviral envelopes or modifying other viral vectors.
While gene transfer is considered the most direct means to treat or prevent the lung disease associated with cystic fibrosis, several barriers prevent the practical application of this approach (36). A problem that currently limits efficient gene transfer to airway epithelia is that the receptor in most cases is localized to the basolateral surface. This has been demonstrated for several retroviral envelopes (32, 33), adenovirus (19, 31, 34), and adeno-associated virus (7). Thus, the mere fact that a viral vector is derived from a respiratory pathogen does not imply that it will efficiently transduce airway epithelia via the apical surface.
As a first step in identifying novel ligand-receptor interactions that might be exploited to direct vectors to the apical surface of airway epithelia, we studied the infection process of human coronavirus 229E (HCoV-229E) in well-differentiated airway epithelia. Human coronaviruses are enveloped, plus-stranded RNA viruses represented by the two serologically unrelated strains, HCoV-229E and HCoV-OC43, that cause mainly upper respiratory tract infections (3, 16). Epidemiological data demonstrate that the HCoV infections are responsible for approximately one-third of common colds (17, 35). HCoV-229E contains a genomic RNA of 27,277 nucleotides, a nucleocapsid (N) protein and a lipid envelope with three major membrane proteins. The three membrane proteins are the membrane (M) glycoprotein, the envelope (E) protein, and the surface spike (S) glycoprotein (11).
We selected HCoV-229E for our studies for several reasons. First, it is a common cause of respiratory infections in humans (16). Second, the viral proteins involved in cell binding and the host cell glycoprotein that serves as the receptor have been identified (20, 38). Third, infection by HCoV-229E involves both binding and membrane fusion events that are mediated by the S glycoprotein. These events are features common to the envelopes of recombinant retroviral vectors that we and others are currently investigating for gene transfer (10, 12, 18, 32, 33). Finally, human aminopeptidase N (hAPN), a membrane-bound metalloprotease, has been identified as the receptor for HCoV-229E (38). Identical to CD13, a glycoprotein surface marker on monocytes and granulocytes (15, 21, 38), this receptor is also expressed on neuronal cells, renal tubule epithelia, intestinal epithelia, and pulmonary epithelia (1, 13, 14, 27). The native function of this protein is to remove amino-terminal residues from short peptides in the gut and from neurotransmitter peptides in the brain (14).
Although HCoV-229E is an important respiratory pathogen, no studies have specifically investigated the polarity of infection in differentiated airway epithelia. In this report, we used primary cultures of human airway epithelia and human tracheal explants to study HCoV-229E entry. We found that the apical surface of differentiated airway epithelia expresses the CD13 receptor and that HCoV-229E infects differentiated airway cells preferentially from the apical surface. These results suggest that the HCoV-229E spike glycoprotein is a candidate for pseudotyping retroviral envelopes or modifying other viral vectors to target gene transfer to the apical surface of airway epithelia.
MATERIALS AND METHODS
Virus strain and antibodies.
HCoV-229E (VR-740) and the human lung fibroblast cell line MRC-5 (CCL-171) were used in these studies. Goat polyclonal antiserum was raised against HCoV-229E virions that had been propagated in WI-38 cells. Virions from the supernatant medium were purified by ultracentrifugation in sucrose density gradients as previously described for murine coronavirus, mouse hepatitis virus (MHV) (9). This antibody recognizes the viral structural proteins, including the S glycoprotein, the N protein, and the M glycoprotein of HCoV-229E. The anti-CD13 mouse monoclonal antibody was purchased from PharMingen (San Diego, Calif.). Monoclonal anti-goat or anti-mouse immunoglobulin G (IgG) with a fluorescein isothiocyanate (FITC) conjugate was purchased from Sigma (St. Louis, Mo.).
Virus production and titers.
HCoV-229E virus was grown in MRC-5 cells as previously reported (38). To determine the titers of the virus, serial dilutions of HCoV-229E were applied to a confluent cell layer of MRC-5 cells. After a 1-h infection, the virus was removed. The cells were kept in culture for an additional 16 h. The focus-forming units (FFU) were determined by an immunostaining approach described below (“Infection of human airway epithelia by HCoV-229E”).
Primary cultures of human airway epithelia.
Well-differentiated primary human airway epithelial cells were obtained from the Tissue Culture Core of the Cystic Fibrosis Center at the University of Iowa. Airway epithelia were isolated from nasal polyps, trachea, and bronchi and grown on collagen-coated permeable membranes at the air-liquid interface as previously described (40). All preparations used were polarized and well differentiated (>2 weeks old; transepithelial resistance, >1,000 Ω × cm2) (37, 40). In contrast to cells grown on tissue culture plastic, primary cultures of differentiated human airway epithelia morphologically resemble the human airways in vivo (37, 40). Similar to the human airways in vivo, they are relatively resistant to transduction by both viral and nonviral vectors applied to the apical surface (8, 19, 32–34, 39, 40). This study was approved by the Institutional Review Board at the University of Iowa.
Immunolocalization of CD13 on human airway epithelia.
Differentiated human airway epithelia were fixed for 20 min in 4% paraformaldehyde and rinsed twice in phosphate-buffered saline (PBS) for 10 min each time. No agents were used to permeabilize the cells. A blocking solution with 5% bovine serum albumin (BSA) was applied for 1 h. A 150-μl portion of mouse anti-human CD13 antibody at a concentration of 5 μg/ml was applied to the apical or basal surface for 1 h. The cells were then rinsed with PBS three times over a period of 60 min. A 150-μl portion of FITC-conjugated anti-mouse IgG antibody at a concentration of 5 μg/ml was applied to the apical or basal surface for 1 h. After the PBS washes, the cells were mounted on slides using Vectorshield mounting medium with 4′-6′-diamidino-2-phenylindole (DAPI; Vector Laboratory, Burlingame, Calif.) and were examined under a laser scanning confocal microscope (MRC 1024; Bio-Rad). To localize the receptor in lung tissue, human tracheal specimens were fixed in 4% paraformaldehyde for 1 h and rinsed in PBS. The tissues were then embedded in OCT and 10- to 15-μm cryosections were obtained. The sections were immunostained for CD13 proteins as described above.
Infection of human airway epithelia by HCoV-229E.
Well-differentiated airway cells were infected with HCoV-229E at a multiplicity of infection (MOI) of 0.1. Virus was applied to either the apical or the basal surface for 1 h at 37°C as previously described (32). At 10 to 16 h postinfection, the cells were fixed with 4% paraformaldehyde for 20 min and then rinsed twice with PBS for 20 min. A blocking solution with 5% BSA was applied for 1 h followed by incubation with the goat polyclonal anti-HCoV-229E antibody (dilution, 1:100) for 1 h. After the secondary antibody reaction, the slides were counterstained with DAPI, mounted in Vectorshield mounting medium, and examined microscopically. A total of 500 cells from each epithelium were counted to determine the percentage of cells expressing HCoV proteins. HCoV-infected control cells and noninfected control cells treated with normal goat serum were negative for HCoV-229E proteins, confirming the specificity of the antisera (data not shown).
Polarity of release of HCoV-229E from differentiated airway epithelial cells.
Well-differentiated human airway epithelial cells were infected from the apical or basolateral surface for 1 h with HCoV-229E (MOI, ∼0.1). Samples were collected at the indicated times from both surfaces following apical or basal infection. To investigate the polarity of virus release, the basal medium (500 μl) was collected at 48 h postinfection. Similarly, to determine viral release from the apical side, the apical surface was washed with 500 μl of saline. The virus titers of the collected solutions were then determined on MRC-5 cells.
Electron microscopy.
To confirm the virus release assays, virus egress from the apical and basal surfaces was examined using transmission electron microscopy. Ninety-six hours following inoculation with HCoV-229E from the apical surface, human airway epithelia were fixed in 2.5% glutaraldehyde (0.1 M sodium cacodylate buffer, pH 7.4) overnight at 4°C and then postfixed with 1% osmium tetroxide for 1 h. Following serial alcohol dehydration, samples were embedded in Eponate 12 (Ted Pella, Inc., Redding, Calif.). Sectioning and poststaining were performed using routine methods (2a). Samples were examined under a Hitachi H-7000 transmission electron microscope. Epithelia from three different human specimens were examined. Four to five grids from each preparation were studied.
Measurement of transepithelial resistance.
Transepithelial resistance was measured following HCoV-229E infection in differentiated airway epithelia. Transepithelial resistance was measured with an ohmmeter (EVOM; World Precision Instruments, Inc., Sarasota, Fla.) by adding cell culture media to the apical surface, and the values were compared to untreated controls. Virus was applied to the apical surface as described above, and serial resistance measurements were made.
Infection of human tracheal explants with HCoV-229E.
Human tracheal explants (∼0.5 cm2 in size) were placed in airway culture media in a 24-well culture dish and grown in short-term culture (n = 4). The mucosal surface of the explants was maintained above the media. A 240-μl portion of HCoV-229E (4 × 106 focus-forming units/ml) was applied to the mucosal surface. Repeated applications were required, as the virus solution remained on the apical surface of the explant only for short time intervals due to the unevenness of the tissue pieces. Two control explants were fixed in 4% paraformaldehyde immediately following the application of the virus, while the other explants remained in contact with the virus for 1 h and were then rinsed with cell culture medium to remove any unbound virus. Twenty-four hours later the samples were fixed in paraformaldehyde and embedded in OCT, and 15- to 20-μm-thick frozen sections were prepared. Immunofluorescent staining for HCoV-229E proteins was performed as described above.
RESULTS
CD13 expression on airway epithelia.
The interaction of a virus and its receptor initiates the infection process. The receptor for HCoV-229E has been identified as hAPN (38). Interestingly, hAPN is identical to CD13, a surface marker on granulocytes, monocytes, and their progenitors (21). Although this receptor has been detected on epithelia from several organs, including the lung, no studies of its distribution on well-differentiated pulmonary epithelia have been reported.
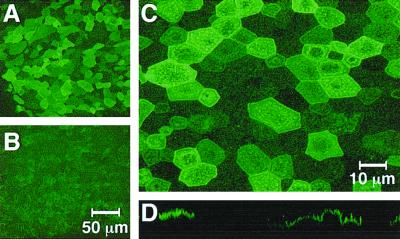
Confocal microscopy showed positive CD13 staining on the majority of airway epithelial cells when antibodies were applied to the apical surface (Fig. 1A and C). At a higher magnification, individual stained cells were clearly visible (Fig. 1C). The amount of CD13 expressed per cell differed somewhat, but most cells showed some expression. An x-z plane section confirmed the apical localization of the receptor (Fig. 1D). In contrast to the apical staining, CD13 expression on the basal surface was much weaker (Fig. 1B).
FIG. 1.
The HCoV-229E receptor aminopeptidase N is abundantly expressed on the apical surface of differentiated human airway epithelial cells. Nonpermeabilized, well-differentiated human airway epithelial cells were fixed and immunostained with a mouse anti-human CD13 antibody by directly applying the antibody to either the apical or basal surface. Samples were examined by confocal microscopy. (A) CD13 expression on apical surface; (B) CD13 expression on basal surface; (C) higher magnification view of apical surface demonstrating detailed stained cell boundaries; (D) x-z image construction showing characteristic apical cell surface staining pattern. When the primary antibody was omitted or mouse serum was substituted, no signal was detected (data not shown). Data shown are representative of two independent experiments done using cells derived from different donor lungs.
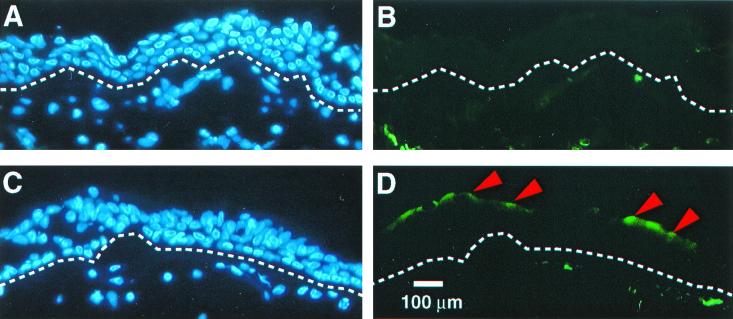
To confirm the findings from cultured airway cells, we also immunostained cryosections of human trachea. Figure 2 shows an apical expression pattern for CD13 (Fig. 2D), and labeling of the basal membrane was weaker (Fig. 2B). From these data we conclude that the receptor for HCoV-229E is preferentially expressed on the apical surface of differentiated airway epithelia.
FIG. 2.
The HCoV-229E receptor preferentially localizes to the apical surface of human trachea. Tracheal tissues were immunostained with the anti-human CD13 monoclonal antibody as described in Materials and Methods. For panels A and B, normal mouse serum (control) was substituted for the primary antibody. Nuclear staining of the epithelia with DAPI (blue); dotted lines indicate location of basement membrane. In all cells stained with anti-CD13 antibody (panels C and D), CD13 expression was seen along the apical surface of some of the tracheal cells (arrowheads). Results are representative of two independent experiments performed using cells from different donor tissues.
Polarity of HCoV-229E infection of airway epithelia.
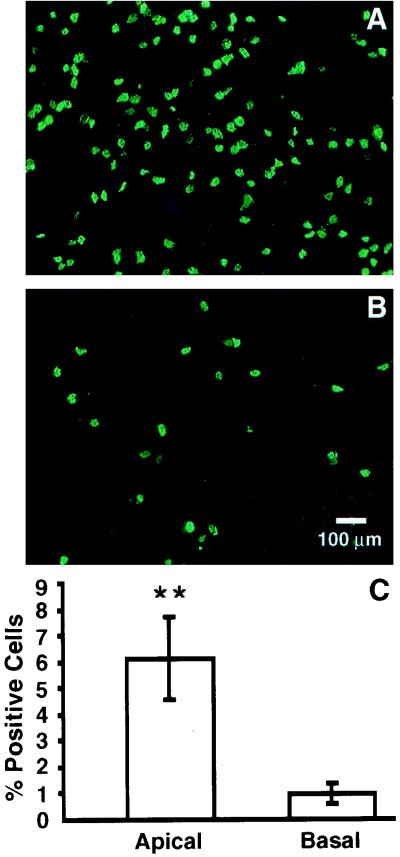
To investigate the polarity of HCoV-229E binding and infection, we applied the virus to well-differentiated human airway epithelial cells from the apical or basal side (MOI, 0.1). After 16 h, the infected cells were immunostained for expression of HCoV proteins. As shown in Fig. 3, HCoV-229E infects airway epithelia from the apical surface more efficiently than from the basal surface. Figure 3C shows that the efficiency of virus infection following apical inoculation with the virus was approximately five- to sixfold greater than that following basal inoculation.
FIG. 3.
HCoV-229E infects airway epithelial cells preferentially from the apical surface. Well-differentiated airway epithelial cells were inoculated with HCoV-229E at an MOI of 0.1 from the apical (A) or basal (B) surface for 16 h, and immunofluorescent staining for HCoV-229E proteins was performed to document the polarity of infection, as indicated by the percentage of HCoV-229E protein-expressing cells (C). The efficiency of infection was significantly greater from the apical surface (P < 0.05 by Student's t test).
Polarity of release of HCoV-229E in airway epithelia.
Polarity of virus release from airway epithelial cells may determine whether a virus will spread systemically or remain in the respiratory tract. Since coronaviruses tend to cause disease limited to the respiratory and or gastrointestinal systems, we hypothesized that virus would be released from the apical surface. We inoculated differentiated airway epithelia with HCoV-229E from the apical or basal surfaces as described above. At 48 h postinfection, infectious virus was collected from the apical or basal surfaces. The released virus was quantified by determining the titers on MRC-5 cells or human airway epithelia. The apical washes always contained more virus than the basal washes (the numbers of virus particles released were as follows [values are means ± the standard errors of the means from experiments performed in triplicate]: for apical collection, 1.05 × 104 ± 0.15 × 104 FFU/ml following apical infection and 5.67 × 102 ± 3.21 × 102 FFU/ml following basal infection; for basal collection, 9.33 × 102 ± 3.06 × 102 FFU/ml following apical infection and 1.00 × 102 ± 1.00 × 102 FFU/ml following basal infection). The data show that HCoV-229E preferentially releases its progeny viral particles to the apical side of the epithelial cells.
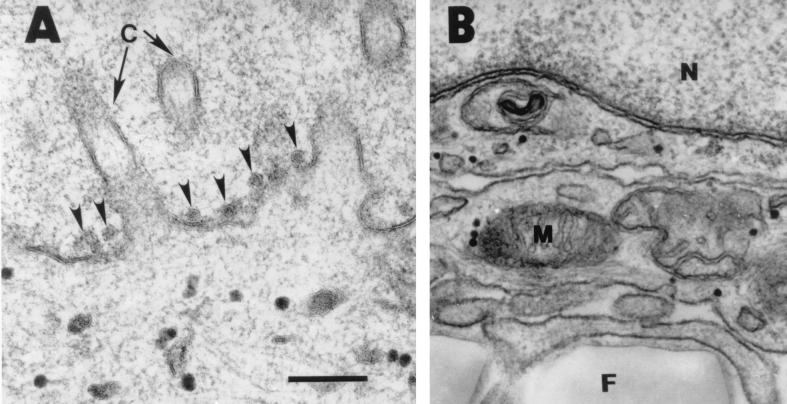
In additional experiments, transmission electron microscopy was used to assess virus egress from the apical and basolateral surfaces of airway epithelia. As shown in Fig. 4A, virions were readily observed on the apical surface of infected cells. In the same specimens, we also examined the basolateral surfaces of epithelia for virus, but similar viral particles were not visualized (Fig. 4B).
FIG. 4.
Transmission electron microscopy of airway epithelia infected with HCoV-229E. (A) HCoV virions were frequently detected on the apical surface of epithelia (arrowheads) (A), but individual virions were not seen in vesicles or released at the basolateral surface (B). The lower portion of panel B shows the permeable membrane on which the epithelia were growing. A total of 100 cells from three epithelial preparations were examined. N, nucleus; M, mitochondria; F, permeable filter. Bar = 200 nm.
Effect of HCoV-229E infection on transepithelial resistance.
We hypothesized that infection with HCoV-229E would cause a time-dependent fall in transepithelial resistance (Rte) across airway epithelia. However, when the Rte was measured both before infection and 4 days following infection with an MOI of 0.1 from the apical surface, there were no significant differences. The mean baseline Rte ± the standard error of the mean was 1,751 ± 72 Ω × cm2 for the control versus 1,572 ± 196 Ω × cm2 for infected epithelia. Four days following infection, the Rte was 1,920 ± 138 Ω × cm2 for the control versus 1,820 ± 94 × cm2 for infected epithelia (n = 6 epithelia/condition, performed on three different epithelial preparations). Daily resistance time course studies between baseline and day 4 also showed no significant changes on any day (data not shown).
Infection of human trachea by HCoV-229E in vitro.
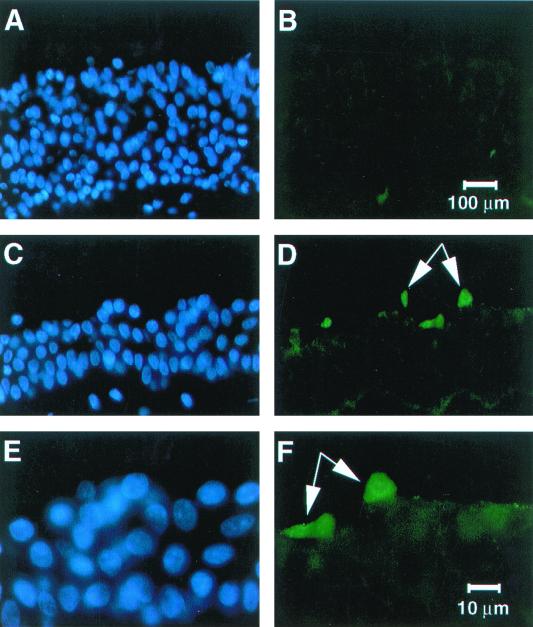
To further confirm the findings of the in vitro cell culture model, we also infected human tracheal explants with HCoV-229E (200 μl of virus [106 FFU/ml]). Twenty-four hours after infection, the explants were fixed and cryosections were prepared for immunostaining. As shown in Fig. 5, staining with the anti-HCoV-229E antibody demonstrated expression of viral proteins in tracheal epithelial cells at the apical surface of the explant. Higher magnification photos demonstrated an area of focal viral protein expression (Fig. 5D and F).
FIG. 5.
HCoV-229E infects human tracheal explants from the apical surface. Human tracheal explants were cultured overnight. HCoV-229E virus was applied to the mucosal surface, and 24 h later frozen sections of tissue were immunostained for HCoV-229E protein expression. The sections were counterstained with DAPI to identify cell nuclei (blue). Control specimens were processed immediately after application of the virus. No viral proteins were detected in controls (A and B), whereas samples infected with HCoV-229E for 24 h (C to F) showed scattered virus antigen-positive cells (indicated by arrows) at the apical surface (green).
DISCUSSION
One approach to overcoming current barriers to efficient gene transfer to airway epithelia is to identify attachment proteins of respiratory viruses that mediate binding and entry from the apical surface. Such proteins might then be exploited as novel ligands to retarget vectors for attachment and entry via the apical surface. We selected HCoV-229E as a candidate because it is a frequent cause of respiratory tract infections (3, 16). To our knowledge, this is the first time that the polarity of HCoV-229E infection and its receptor distribution have been investigated in differentiated human airway epithelia. We showed that HCoV-229E preferentially infects and leaves human airway epithelia from the apical side, which is also the site of most abundant CD13 expression.
Studies of several classes of viruses show that infection and release generally proceed from one side of polarized epithelia (30). For example, members of the paramyxovirus family, such as parainfluenza and measles, preferentially enter and exit epithelia via the apical surface (2, 22). In cultured epithelia, the orientation of coronavirus entry and release is also polarized to the apical or basal cell surface. The site of entry and exit depends upon the virus and host cell type. Transmissible gastroenteritis virus, a swine enteric coronavirus, also restricts its entry and release to the apical surface in porcine epithelial kidney cells (25). In contrast, mouse hepatitis virus strain A59, a well-studied murine coronavirus, preferentially infects from the apical surface and buds from the basolateral surface of porcine kidney or human colon carcinoma cells expressing the recombinant MHV receptor and murine kidney epithelial cells. However, the same mouse virus almost exclusively infects and is released from the apical membrane of MDCK cells expressing the recombinant MHV receptor, a canine kidney cell line (23, 24).
We found that HCoV-229E efficiently infected differentiated airway epithelia from the apical surface and also preferentially exited through the same surface. In polarized CaCo-2 intestinal epithelia, HCoV-229E also preferentially infects and exits via the apical surface (D. M. Blau and K. V. Holmes, unpublished data). The mechanism specifying directional release of virions that bud at intracellular membranes is unclear (23, 24). Perhaps through evolution the respiratory and enteric coronaviruses adopted an optimal means to spread to neighboring epithelial cells. Releasing viral particles to the lumen (apical side) in the airways has advantages over release via the basolateral membrane. As we demonstrated, the HCoV-229E receptor is predominantly localized on the apical surface of airway epithelia. This polar receptor distribution would facilitate the subsequent rounds of infection, if the newly produced virions were released to the apical surface. This direction of release would also tend to minimize exposure of viral antigens to the circulation, thereby reducing the strength of the host immune response and diminishing the likelihood of systemic infection.
The HCoV-229E S glycoprotein is the membrane glycoprotein responsible for the attachment of virions to the cell surface and the fusion of the envelope with cellular membranes (11, 20). Studies of related coronaviruses, such as transmissible gastroenteritis virus, MHV, and infectious bronchitis virus of chickens, also demonstrate a similar function for the S protein (5, 6, 28, 29). Several laboratories have shown that it is possible to modify the cell tropism of retroviral vectors through envelope pseudotyping. For example, pseudotyping with the vesicular stomatitis virus G protein greatly broadens the range of cell types that can be infected with murine leukemia virus or lentiviral vectors (4). Such strategies for modifying the host range properties of retroviral vectors were recently reviewed by Russell and Cosset (26). Based on the present studies, we propose that the HCoV-229E S protein is a candidate for pseudotyping retroviral vectors, including lentiviral vectors, and for targeting gene transfer to the apical surface of airway epithelia.
ACKNOWLEDGMENTS
We thank Phil Karp and Pary Weber for preparation of the human airway cell cultures and Randy Nessler for his technical assistance in confocal microscopy. We thank David Depew for critical reading of the manuscript.
We acknowledge the support of the Cell Morphology Core and Cell Culture Core, partially supported by the Cystic Fibrosis Foundation, NHLBI (PPG HL51670-05), and the Center for Gene Therapy for Cystic Fibrosis (NIH P30 DK-97-010). We acknowledge the support provided by NIH RO1HL61460 (P.B.M. and B.L.D.), the Cystic Fibrosis Foundation (WangG99GO), and NIH RO1-AI26075 (K.V.H. and D.M.B.).
REFERENCES
- 1.Arbour N, Ekande S, Cote G, Lachance C, Chagnon F, Tardieu M, Cashman N R, Talbot P J. Persistent infection of human oligodendrocytic and neuroglial cell lines by human coronavirus 229E. J Virol. 1999;73:3326–3337. doi: 10.1128/jvi.73.4.3326-3337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blau D M, Compans R W. Entry and release of measles virus are polarized in epithelial cells. Virology. 1995;210:91–99. doi: 10.1006/viro.1995.1320. [DOI] [PubMed] [Google Scholar]
- 2a.Bozzola J J, Russell L D. Electron microscopy. 2nd ed. Sudbury, Mass: Jones and Bartlett Publishers; 1998. [Google Scholar]
- 3.Bradburne A F, Bynoe M L, Tyrrell D A. Effects of a “new” human respiratory virus in volunteers. Br Med J. 1967;3:767–769. doi: 10.1136/bmj.3.5568.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J-K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavanagh D, Davis P J. Coronavirus IBV: removal of spike glycopolypeptide S1 by urea abolishes infectivity and haemagglutination but not attachment to cells. J Gen Virol. 1986;67:1443–1448. doi: 10.1099/0022-1317-67-7-1443. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh D, Davis P J, Darbyshire J H, Peters R W. Coronavirus IBV: virus retaining spike glycopolypeptide S2 but not S1 is unable to induce virus-neutralizing or haemagglutination-inhibiting antibody, or induce chicken tracheal protection. J Gen Virol. 1986;67:1435–1442. doi: 10.1099/0022-1317-67-7-1435. [DOI] [PubMed] [Google Scholar]
- 7.Duan D, Yue Y, McCray P B, Jr, Engelhardt J F. Polarity influences the efficiency of recombinant adeno-associated virus infection in differentiated airway epithelia. Hum Gene Ther. 1998;9:2761–2776. doi: 10.1089/hum.1998.9.18-2761. [DOI] [PubMed] [Google Scholar]
- 8.Fasbender A J, Zabner J, Welsh M J. Optimization of cationic lipid-mediated gene transfer to airway epithelia. Am J Physiol. 1995;269:L45–L51. doi: 10.1152/ajplung.1995.269.1.L45. [DOI] [PubMed] [Google Scholar]
- 9.Frana M F, Behnke J N, Sturman L S, Holmes K V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J Virol. 1985;56:912–920. doi: 10.1128/jvi.56.3.912-920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman M J, Lee P-S, Yang J-S, Wilson J M. Lentiviral vectors for gene therapy of cystic fibrosis. Hum Gene Ther. 1997;8:2261–2268. doi: 10.1089/hum.1997.8.18-2261. [DOI] [PubMed] [Google Scholar]
- 11.Holmes K V, Lai M M C. Coronaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1075–1093. [Google Scholar]
- 12.Johnson L G, Mewshaw J P, Ni H, Friedmann T, Boucher R C, Olsen J C. Effect of host modification and age on airway epithelial gene transfer mediated by a murine leukemia virus-derived vector. J Virol. 1998;72:8861–8872. doi: 10.1128/jvi.72.11.8861-8872.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenny A J, Maroux S. Topology of microvillar membrance hydrolases of kidney and intestine. Physiol Rev. 1982;62:91–128. doi: 10.1152/physrev.1982.62.1.91. [DOI] [PubMed] [Google Scholar]
- 14.Lachance C, Arbour N, Cashman N R, Talbot P J. Involvement of aminopeptidase N (CD13) in infection of human neural cells by human coronavirus 229E. J Virol. 1998;72:6511–6519. doi: 10.1128/jvi.72.8.6511-6519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Look A T, Ashmun R A, Shapiro L H, Peiper S C. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J Clin Investig. 1989;83:1299–1307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIntosh K. Coronaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1095–1103. [Google Scholar]
- 17.McIntosh K, Chao R K, Krause H E, Wasil R, Mocega H E, Mufson M A. Coronavirus infection in acute lower respiratory tract disease of infants. J Infect Dis. 1974;130:502–507. doi: 10.1093/infdis/130.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen J C, Johnson L G, Wong-Sun M L, Moore K L, Swanstrom R, Boucher R C. Retrovirus-mediated gene transfer to cystic fibrosis airway epithelial cells: effect of selectable marker sequences on long-term expression. Nucleic Acids Res. 1993;21:663–669. doi: 10.1093/nar/21.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raabe T, Schelle-Prinz B, Siddell S G. Nucleotide sequence of the gene encoding the spike glycoprotein of human coronavirus HCV 229E. J Gen Virol. 1990;71:1065–1073. doi: 10.1099/0022-1317-71-5-1065. [DOI] [PubMed] [Google Scholar]
- 21.Riemann D, Kehlen A, Langner J. CD13—not just a marker in leukemia typing. Immunol Today. 1999;20:83–88. doi: 10.1016/S0167-5699(98)01398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts S R, Compans R W, Wertz G W. Respiratory syncytial virus matures at the apical surfaces of polarized epithelial cells. J Virol. 1995;69:2667–2673. doi: 10.1128/jvi.69.4.2667-2673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossen J W, Strous G J, Horzinek M C, Rottier P J. Mouse hepatitis virus strain A59 is released from opposite sides of different epithelial cell types. J Gen Virol. 1997;78:61–69. doi: 10.1099/0022-1317-78-1-61. [DOI] [PubMed] [Google Scholar]
- 24.Rossen J W, Voorhout W F, Horzinek M C, van der Ende A, Strous G J, Rottier P J. MHV-A59 enters polarized murine epithelial cells through the apical surface but is released basolaterally. Virology. 1995;210:54–66. doi: 10.1006/viro.1995.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossen J W A, Bekker C P J, Voorhout W F, Strous G J A M, van der Ende A, Rottier P J M. Entry and release of transmissible gastroenteritis coronavirus are restricted to apical surfaces of polarized epithelial cells. J Virol. 1994;68:7966–7973. doi: 10.1128/jvi.68.12.7966-7973.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell S J, Cosset F-L. Modifying the host range properties of retroviral vectors. J Gene Med. 1999;1:300–311. doi: 10.1002/(SICI)1521-2254(199909/10)1:5<300::AID-JGM59>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 27.Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Annu Rev Cell Biol. 1986;2:255–313. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]
- 28.Sturman L S, Holmes K V. The molecular biology of coronaviruses. Adv Virus Res. 1983;28:35–112. doi: 10.1016/S0065-3527(08)60721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sune C, Jimenez G, Correa I, Bullido M J, Gebauer F, Smerdou C, Enjuanes L. Mechanisms of transmissible gastroenteritis coronavirus neutralization. Virology. 1990;177:559–569. doi: 10.1016/0042-6822(90)90521-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tucker S P, Compans R W. Virus infection of polarized epithelial cells. Adv Virus Res. 1993;42:187–247. doi: 10.1016/S0065-3527(08)60086-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 32.Wang G, Davidson B L, Melchert P, Slepushkin V A, van Es H H G, Bodner M, Jolly D J, McCray P B., Jr Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J Virol. 1998;72:9818–9826. doi: 10.1128/jvi.72.12.9818-9826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang G, Slepushkin V A, Zabner J, Keshavjee S, Johnston J C, Sauter S L, Jolly D J, Dubensky T, Davidson B L, McCray P B., Jr Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J Clin Investig. 1999;104:R49–R56. doi: 10.1172/JCI8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G, Zabner J, Deering C, Launspach J, Shao J, Bodner M, Jolly D J, Davidson B L, McCray P B., Jr Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo. Am J Respir Cell Mol Biol. 2000;22:129–138. doi: 10.1165/ajrcmb.22.2.3938. [DOI] [PubMed] [Google Scholar]
- 35.Wege H, Siddell S, ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- 36.Welsh M J. Gene transfer for cystic fibrosis. J Clin Investig. 1999;104:1165–1166. doi: 10.1172/JCI8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaya M, Finkbeiner W E, Chun S Y, Widdicombe J H. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 38.Yeager C L, Ashmun R A, Williams R K, Cardellichio C B, Shapiro L H, Look A T, Holmes K V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zabner J, Fasbender A J, Moninger T, Poellinger K A, Welsh M J. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- 40.Zabner J, Zeiher B G, Friedman E, Welsh M J. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]